Abstract
Background:
In recent years, prostate cancer prevails as one of the lead cancers affecting men. Currently, prostate cancer research involves the phytochemical study of plants with anti-tumour effects. This study compares the anti-tumour effects of three plant species indigenous to Iran and their interaction with cluster of differentiation (CD)-82 protein, a therapeutic target found in prostate cancer cells.
Methods:
The extracts of Hypericum perforatum, Achillea millefolium, and Aloe vera were prepared and their toxicological, cellular and gene expression responses were evaluated in PC-3 human prostate cancer cells and normal human chondrocyte cell line C28/I2. They were exposed to different concentrations of the plants (10 mg/mL, 5 mg/mL, 1 mg/mL, 100 µg/mL, 10 µg/mL, and 1 µg/mL) at three exposure time points (24, 48, 72 hours) to determine cancer cell cytotoxicity and gene expression profiles.
Results:
: Half-maximal inhibitory concentration (IC50) in PC-3 cells ranged from 0.6 to 8.5 mg/mL for H. perforatum extract, from 0.4 to 7.5 mg/mL for A. Millefolium extract, and from 0.2 to 8.0 mg/mL for A. vera extract in a time-dependent manner. A. vera extract caused the highest cell death levels in PC-3 cells (94%) and C28/I2 cells (57%) after 48 hours. A 1.97-, 3.00-, and 3.48-fold increase in relative gene expression of CD82 was observed for H. perforatum, A. millefolium, and A. vera extracts, respectively.
Conclusion:
A. vera and A. millefolium extracts are a selective inhibitor of prostate cancer cells and a potent activator of CD82 expression.
Key Words: CD82, Gene expression, Herbal medicine, Prostate cancer
Introduction
Prostate cancer is one of the leading causes of mortality amongst men in several developed nations, and across the globe. Several therapeutic approaches have been proposed to treat cancer, for example, chemotherapy. In most cases, however, the non-selective and non-specific toxicity of chemotherapy drugs can result in the destruction of healthy tissue. Therefore, scientific efforts are shifting towards alternative therapies that use plant extracts as an alternative to fighting cancer. Testing anti-tumour candidates and screening for plant-based extracts is very important prior to conducting clinical evaluation (3).
Hypericum perforatum L., known as St. John's Wort, belongs to the Hypericaceae species (4) and has been found to have anti-tumour properties due to the presence of complex compounds, namely hypericin, α-terpineol, β-carotene, caffeic acid, isoquercitrin, kaempferol, gallic acid, limonene, rutin, and vanillic acid (5). Our recent finding supports its antiproliferative activity against different cancer cells (5, 6). H. perforatum functions as a serotonin-reuptake inhibitor and serotonin antagonist in prostate tumors. Using the PC-3 human prostate adenocarcinoma cell line, its methanolic extract could successfully prevent cellular growth by 80% (7).
Achillea millefolium is another traditional plant, known as yarrow from the Asteraceae family that is widely distributed amongst Europe, Asia, North Africa and North America (8, 9). Several studies highlight the anti-tumour effects of A. millefolium extracts in human cancer cell lines (10, 11). It has been postulated that phenolic acid derivatives, and flavones are involved in its bioactivity (11).
Aloe vera (Aloe barbadensis), a popular herbal compound with an extensive medicinal history for several ailments, is known to have preventive and therapeutic impacts against a range of cancers (12-14). Lectin, aloin, barbaloin, aloe-emodin, and aloesin are some of its bioactive components substantiated for chemoprevention, immune potentiating properties, antimutagenic activity, anti-proliferation, apoptosis-inducing effects, antioxidant actions, and anti-metastatic functions (12, 13).
CD82, known as KAI-1, is a tetraspanin membrane protein and serves to inhibit metastasis in human cancers. Indeed, its expression is suppressed in many malignant cells (15, 16). This gene is located on chromosome 11p, and published studies have revealed its involvement in the dissemination of prostate tumor cells (17, 18). Moreover, it is involved in extensive physiological processes, such as detachment, motility/invasion, and cell survival (16). In this study, the potential of H. perforatum, A. millefolium, and A. vera to selectively inhibit cancer cell growth and interfere with CD82 function was investigated in PC-3 and C28/I2 cell lines.
Materials and Methods
Preparation of plant extracts
H. perforatum seeds, aerial parts of A. millefolium, and leaves of A. vera were collected from National Botanical Garden of Iran (NBGI).
All three plants were compared with those species in the Medical Plant Farm, Jahad Daneshgah, Islamic Republic of Iran. Their names have been checked with www.theplantlist.org
As for H. perforatum, 180 g powder of the plant material was extracted with 100 mL methanol. Following 24 hours, the mixture was filtered and then evaporated under vacuum. Thereafter, 10 mg of H. perforatum extract was put into a flask, and 70 mL of methanol was added in a shaking water bath at 60 °C.
The aerial parts of A. millefolium were initially rinsed and dried before boiling in water for 10 minutes. The extract was incubated for 30 minutes at room temperature and then passed through a filter. A 10 mg/mL of the extract was solved in 5 mL of de-ionized water in a shaking water bath at 60 °C.
The whole leaves of A. vera were cut into thin pieces and dried at shade and room temperature. After grinding in a mixer, distilled water was added, and the resultant mixture underwent centrifugation at 1000 ×g, followed by filtration. A 10 mg of A. vera extract was solved in 5 mL of de-ionized water at ambient temperature. The final solutions of each plant at a concentration of 2 mg/mL were prepared in RPMI medium prior to the next use.
Determination of phenolic content
The Folin–Ciocalteu reagent assay was used to measure the total phenolic content (19). Briefly, 2 N Folin–Ciocalteu reagent was mixed with 0.5 mL of EOs. After five minutes, 2 mL of 75 g/L sodium carbonate was added to the mixture. Afterward, absorbance was determined at 760 nm.
Cell culture
There were two cell lines in this study obtained from the Pasteur Institute of Iran: human prostate cancer cell line PC-3 and normal human chondrocyte cell line C28/I2. They were cultured in RPMI medium supplemented with 10% fetal bovine serum (FBS), 2% L-glutamine, 2 g/L bicarbonate, 100 U/mL penicillin, and 100 µg/L streptomycin at 37 °C in a 5% CO2 humidified atmosphere.
Determination of cell viability
The tetrazolium (MTT) test was employed to calculate cell viability. Briefly, PC-3 and C28/I2 cells (20,000 cells/mL) were cultured in 96-well plates (BD Biosciences, USA), each well of which contained 200 μL of supplemented RPMI medium. They were incubated for 24 hours before treating with the different concentrations of the plant extracts at 1 µg/mL, 10 µg/mL, 100 µg/mL, 1 mg/mL, 5 mg/mL, and 100 mg/mL for 24, 48, 72 hours. Thereafter, 5 mg/mL MTT (Sigma, USA) solution was added to the wells, and the cells underwent an extra incubation for 4 hours at 37 °C. Following the addition of 100 μL of dimethyl sulfoxide (DMSO; Sigma, USA), the optical density was recorded at 540 nm.
Estimation of IC50 values
The IC50 values were determined by ELISA and estimated from concentration–cell viability curves through linear regression. Measurements were conducted at six concentrations (1, 10 and 100 µg/mL; 1, 5 and 100 mg/mL).
Analysis of CD82 gene expression
The cells were exposed to the plant extracts at twice dose of IC50 as followed: PC-3 + H. perforatum (S1); PC-3 + A. millefolium (S2); PC-3 + A. vera (S3); C28/I2 + H. perforatum (S4); C28/I2 + A. millefolium (S5); C28/I2 + A. vera (S6); PC-3 (S7); C28/I2 (S8). The RNA was extracted from the cell lines by Trizol reagent (Invitrogen, USA), and spectroscopy was utilized to photometrically detect the purity and concentration of the RNA samples by a NanoPhotometer® (Implen GmbH, Munich, Germany). Total RNA was reversed transcribed by cDNA synthesis kit (Roche Applied Science, Germany). Determination of CD82 expression was carried out by quantitative real-time polymerase chain reaction (PCR) using a SYBR Green assay (Applied Biosystems, USA). In this study, these primers were used: CD82 forward, 5'-GCTCATTCGAGACTACA ACAGC-3' and reverse, 5'-GTGACCTCAGGGCGAT TCA-3'; GAPDH forward, 5' GGATGCTGGAGGTCTGCGAGGAAC 3' and reverse, 5' GAGAGGAAGCGTGTGAGGCGGTAG 3'.
Statistical analysis
All measurements were performed in three replicates. The Kolmogorov–Smirnov tests were performed to ensure normal data distribution. Given normal distribution, student’s t-test was initially conducted to determine any significant difference at P-values <0.05 (SPSS 19.0 software Package, IBM Inc., Chicago IL, USA). If normal distribution evaded, the non-parametric equivalent was used.
Results
iThe yield was 13.94 ± 2.63 %wt/wt for the methanol extract of H. perforatum, 29.76 ± 2.21 %wt/wt for the aqueous extract of A. millefolium, and 3.41 ± 0.69 %wt/wt for the aqueous extract of A. vera. The ordering of the extracts in terms of total phenol content from highest to lowest was H. perforatum (95.28 ± 7.54 mg GAEs/g extract), A. millefolium (39.67 ± 1.05 mg GAEs/g extract), and A. vera (0.106 ± 0.007 mg GAEs/g extract).
PC-3 cells were incubated with six different concentrations of the herbal extracts for 24, 48, and 72 hours. Their effects on cell viability are presented in Figure 1. The treatment of PC-3 cells with H. perforatum considerably reduced growth in a dose- and time-dependent manner (p < 0.05). Concentrations of H. perforatum below 1 mg/mL carried no significant effect after 24 hours (p > 0.05), whereas a marked decline of at least 30% was found at 1, 5, and 10 mg/mL as compared with the control (p < 0.05). The 48- and 72-hour post-exposure to H. perforatum caused notable reductions in the cell counts at 100 μg/mL, 1, 5, and 10 mg/mL (p < 0.05). As for A. millefolium, Figure 1B shows a dose- and time-dependent decrease in the number of the treated cells(Fig. 1B). The concentrations of A. millefolium extract at 1, 5, and 10 mg/mL resulted in statistically significant growth inhibitions after 24 hours as opposed to the control (p < 0.05). Longer exposure of the cells to A. millefolium extract lowered the minimum concentration associated with substantial changes in the cell count to 100 μg/mL for 48 hours and 10 μg/mL for 72 hours (p < 0.05). As can be seen in Figure 1C, significant declines in the cell growth started with 1 mg/mL (33%), 100 μg/mL (40%), and 10 μg/mL (38%) after 24-, 48-, and 72-hour treatment with A. vera extract (p < 0.05)(Fig. 1C).
Fig. 1.
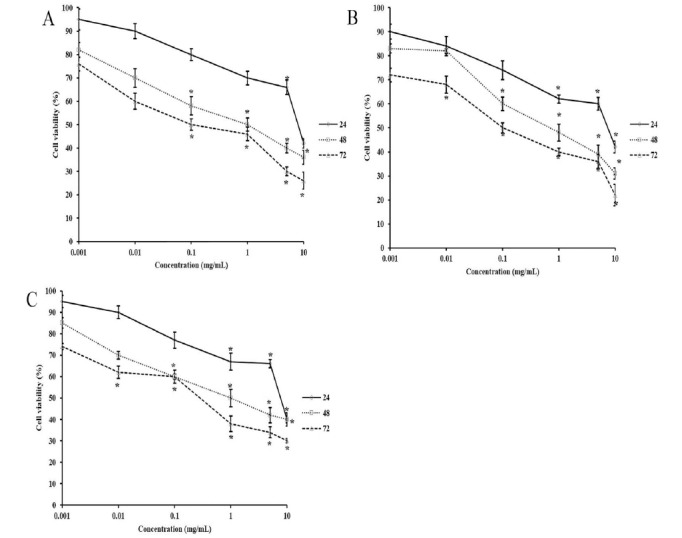
The effect of H. perforatum (A), A. millefolium (B), and A. vera (C) extracts on the growth of PC-30 cells at different exposure time points (* p < 0.05).
Figure 2 displays IC50 for each herbal extract at different time points(Fig. 2). It was figured out that IC50 changed at different exposure time points, and H. perforatum extract showed the highest values. With changing the cellular model from PC-3 cells to C28/I2 cells, different herbal extracts induced selective cell death in different cell lines. For example, 82% of PC-3 cells underwent death upon 48-hour treatment with H. perforatum extract at 2.720 mg/mL (∼ twice dose of IC50), whereas C28/I2 cells remained viable up to 65% at similar conditions (Fig. 3). The significant elevation of CD82 expression was observed following exposure of PC-3 cells to A. vera and A. millefolium extracts as compared with the control. A 1.97-, 3.00-, and 3.48-fold increase in the relative gene expression of CD82 was determined for H. perforatum, A. millefolium, and A. vera extracts, respectively. Moreover, it was found that CD82 expression levels in the normal cells considerably reduced in reference to the cancerous cells (p < 0.05)(Fig. 4).
Fig. 2.
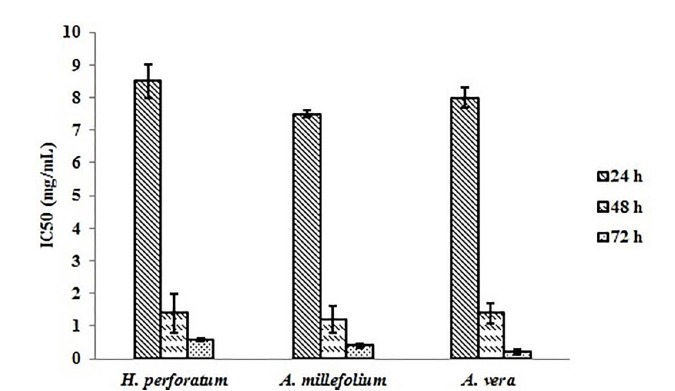
The IC50 values of H. perforatum, A. millefolium, and A. vera extracts at different exposure time points.
Fig. 3.
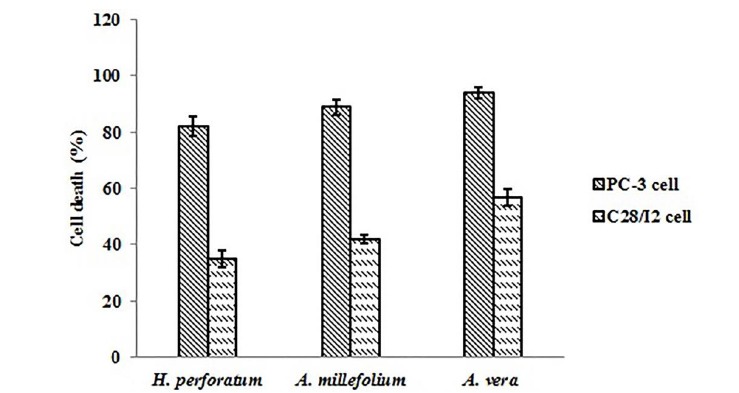
The cytotoxicity of H. perforatum, A. millefolium, and A. vera extracts against PC-30 and C28/I2 cell lines.
Fig. 4.
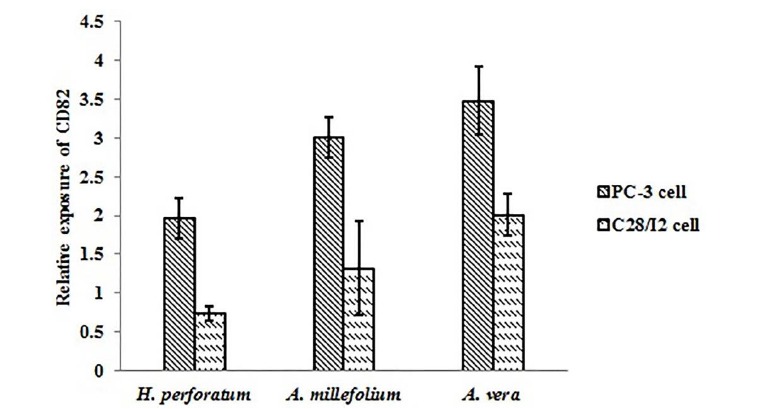
The relative expression of CD82 after the administration of H. perforatum, A. millefolium, and A. vera extracts to PC-30 and C28/I2 cell lines (* p < 0.05).
Discussion
The extraction yield and phenolic content of H. perforatum were 13.94 ± 2.63 %wt/wt and 95.28 ± 7.54 mg GAEs/g extract, respectively. There have been three published papers about the methanolic extract of H. perforatum outside of Iran. Stamenković et al. prepared the methanol extract from H. perforatum collected from Leskovac in July with a yield of 20.16%. Upon re-extraction, the yield increased to 27.13% (20). Spiridon et al. with H. perforatum from Iasi, Romania developed a methanolic extract containing 50 mg GAEs/g extract (21). Another study by Öztürk et al. obtained the methanol extract using H. perforatum collected in Eskişehir, Turkey, in June. They found a yield of 39.6 %w/w and total phenol content of 319.34 ± 2.46 mg GAEs/g extract (22), which were inconsistent with our findings.
The extraction yield and phenolic content of A. millefolium were 29.76 ± 2.21 %wt/wt and 39.67 ± 1.05 mg GAEs/g extract, respectively. There has been three published papers about the aqueous extract of A. millefolium; two of which has been reported from Iran. Noureddini and Rasta obtained an aqueous extract from a plant found in Kashan, Iran. Their study yielded 36% phenolic content in their aqueous extract (23). Further, Eghdami and Sadeghi investigated the chemical composition and biological properties of A. millefolium extract obtained from Nowbaran, a district located in Saveh County in Markazi Province, Iran. The resultant extract had a phenolic content of 48.4 ± 2.7 mg GAEs/g extract (24). A. millefolium from a different region, Rhodope Mountains (Bulgaria), was used as an aqueous extract. In this study, the yield of the dried extract was 20 %w/w (25).
The extraction yield and phenolic content of A. vera were 3.41 ± 0.69 %wt/wt and 0.106 ± 0.007 mg GAEs/g extract, respectively. There have been five published papers about the aqueous extract of A. vera, however, no study has been performed on the plant species indigenous to Iran, yet. Regarding the aqueous extract yield of A. vera, previous evidence reports that 1.2% from Boksburg, South Africa (26), 5.4% from Kogi State (27), Nigeria, and 11.5% from Assam, India (28). Hęś et al et al. showed a total phenol content of 17.85 mg GAE/g dry matter for its aqueous extract (29). Kammoun et al. tested the aqueous extract obtained from a plant indigenous to Kairouan, Tunisia. The extraction yield and phenolic content were 66.67% and 2.072 ± 0.002 mg GAE/g of extract, respectively (30). Such differences found for each extract with the mentioned studies could be due to various collection times, environmental conditions, and geographic locations.
In this work, the administration of H. perforatum extract negatively affected PC-3 cells in a concentration and time dependent manner. This anti-proliferative effect in prostate cancer was corroborated by Martarelli et al., whose in vitro study showed an IC50 of 0.42 mg/mL after a 3-day treatment (7). Consistently, the IC50 in the present study was 0.60 mg/mL at 72 hours. The inhibitory impact of H. perforatum extract against prostate cancer cells was confirmed by Toros et al., as well (31). In another study by Colasanti et al., it was found that hypericin, one of the biologically active compounds of H. perforatum, induced phototoxic effects on PC-3 (32).
In contrast, exposure to A. millefolium extract led to considerable declines of PC-3 cells in a time and concentration dependent fashion. Comparing the methanolic extract of H. perforatum with aqueous extract of A. millefolium, it was found that the latter carried more destructive influences on PC-3 cells at the same exposure time. The pertinent literature reveals the antimicrobial, antioxidant, antitumor, and cytotoxic activities of the Achillea species (33, 34). A. wilhelmsii, a member of the Achillea genus, is widely distributed in Iran, has been found to have beneficial effects on breast cancer cell lines (34). The methanolic extract of A. millefolium elevated the antiproliferative activity induced by bleomycin in the prostate cancer cell (DU-145) without any significant toxicity on normal cells (human normal skin cell; HFFF2) (35). The growth of various human cancer cell lines, including PC-3 cells, was inhibited by the methanol and chloroform extracts of A. millefolium obtained from the root, stem, and floral parts (36). Only one study shows the antitumor potential of the aqueous extract of A. millefolium (37).
The treatment of PC-3 cells with A. vera extract produced detrimental effects on cellular growth in a time and concentration dependent fashion by affecting CD82 function. A number of laboratory and clinical studies support the anticancer properties of A. vera (38-40). In a study on PC-3 cells, aloe emodin, an ingredient in A. vera, appeared to repress the growth of prostate cancer cells that act through the mammalian target, rapamycin complex 2 (41).
In reference to H. perforatum extract, A. millefolium and A. vera extracts demonstrated increasing potentcy and selective anti-proliferative activities by promoting CD82 function in both physiological and disease conditions. Also, it can be concluded that A. vera and A. millefolium extracts act as a potent activator of CD82 expression in human prostate cancer cells. Studies find that phosphoinositide 3-kinase (PI3K) activation, and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) loss are principally involved in prostate tumorigenesis. These two changes stimulate Akt (also known as protein kinase B or PKB), whichhas implications for cell survival and cell growth. Phosphorylation of Akt at Ser473 plays role in the complete stimulation of mammalian target of rapamycin 2 (mTOR2).
Additionally, mTOR2 is responsible for transforming human prostate epithelial cells deficient in PTEN causing tumor formation (42, 43). Liu et al. observed that Akt is up-regulated in PC-3 cells (41). In contrast, it has been previously reported that KAI1/CD82 attenuated the metastatic phenotype of H1299 lung carcinoma cells via the down-regulation of Rac1 expression (Rho guanosine triphosphatase; GTPase) through the PI3K/Akt/mTOR pathway (44). Currently, there is no evidence regarding the effect of KAI1/CD82 on the PI3K/Akt/mTOR pathway in prostate cancer cells warranting future investigation.
Acknowledgements
The authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
References
- 1.de Souza JA, Hunt B, Asirwa FC, Adebamowo C, Lopes G. Global Health Equity: Cancer Care Outcome Disparities in High-, Middle-, and Low-Income Countries. J Clin Oncol. 2016 ;34(1):6–13. doi: 10.1200/JCO.2015.62.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pakzad R, Rafiemanesh H, Ghoncheh M, Sarmad A, Salehiniya H, Hosseini S, et al. Prostate Cancer in Iran: Trends in Incidence and Morphological and Epidemiological Characteristics. Asian Pac J Cancer Prev. 2016;17(2):839–43. doi: 10.7314/apjcp.2016.17.2.839. [DOI] [PubMed] [Google Scholar]
- 3.Rafieian-Kopaie M, Nasri H. On the Occasion of World Cancer Day 2015; the Possibility of Cancer Prevention or Treatment with Antioxidants: The Ongoing Cancer Prevention Researches. Int J Prev Med. 2015;6(108):2008–7802. doi: 10.4103/2008-7802.169077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soleymani S, Bahramsoltani R, Rahimi R, Abdollahi M. Clinical risks of St John’s Wort (Hypericum perforatum) co-administration. Expert Opin Drug Metab Toxicol. 2017;13(10):1047–62. doi: 10.1080/17425255.2017.1378342. [DOI] [PubMed] [Google Scholar]
- 5.Amjadi I, Rabiee M, Hosseini M, Sefidkon F, Mozafari M. Nanoencapsulation of Hypericum perforatum and doxorubicin anticancer agents in PLGA nanoparticles through double emulsion technique. Micro Nano Lett. 2013;8(5):243–7. [Google Scholar]
- 6.Amjadi I, Mohajeri M, Borisov A, Hosseini MS. Antiproliferative effects of free and encapsulated Hypericum perforatum L. extract and its potential interaction with doxorubicin for esophageal squamous cell carcinoma. J Pharmacopuncture. 2019;22(2):102–8. doi: 10.3831/KPI.2019.22.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martarelli D, Martarelli B, Pediconi D, Nabissi MI, Perfumi M, Pompei P. Hypericum perforatum methanolic extract inhibits growth of human prostatic carcinoma cell line orthotopically implanted in nude mice. Cancer Lett. 2004;210(1):27–33. doi: 10.1016/j.canlet.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Potrich FB, Allemand A, da Silva LM, Dos Santos AC, Baggio CH, Freitas CS, et al. Antiulcerogenic activity of hydroalcoholic extract of Achillea millefolium L.: involvement of the antioxidant system. J Ethnopharmacol. 2010;130(1):85–92. doi: 10.1016/j.jep.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Vitalini S, Beretta G, Iriti M, Orsenigo S, Basilico N, Dall'Acqua S, et al. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim Pol. 2011;58(2):203–9. [PubMed] [Google Scholar]
- 10.Csupor-Loffler B, Hajdu Z, Zupko I, Rethy B, Falkay G, Forgo P, et al. Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium s.l. on cultured human tumour cell lines. . Phytother Res. 2009;23(5):672–6. doi: 10.1002/ptr.2697. [DOI] [PubMed] [Google Scholar]
- 11.Dias MI, Barros L, Duenas M, Pereira E, Carvalho AM, Alves RC, et al. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013;141(4):4152–60. doi: 10.1016/j.foodchem.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Joseph B, Raj SJ. Pharmacognostic and phytochemical properties of Aloe vera linn an overview. Int J Pharm Sci Rev Res. 2010;4(2):106–10. [Google Scholar]
- 13.Niciforovic A, Adzic M, Spasic SD, Radojcic MB. Antitumor effects of a natural anthracycline analog (Aloin) involve altered activity of antioxidant enzymes in HeLaS3 cells. Cancer Biol Ther. 2007;6(8):1211–6. doi: 10.4161/cbt.6.8.4383. [DOI] [PubMed] [Google Scholar]
- 14.Tabolacci C, Rossi S, Lentini A, Provenzano B, Turcano L, Facchiano F, et al. Aloin enhances cisplatin antineoplastic activity in B16-F10 melanoma cells by transglutaminase-induced differentiation. Amino acids. 2013;44(1):293–300. doi: 10.1007/s00726-011-1166-x. [DOI] [PubMed] [Google Scholar]
- 15.Lee HA, Park I, Byun HJ, Jeoung D, Kim YM, Lee H. Metastasis suppressor KAI1/CD82 attenuates the matrix adhesion of human prostate cancer cells by suppressing fibronectin expression and beta1 integrin activation. Cell Physiol Biochem. 2011;27(5):575–86. doi: 10.1159/000329979. [DOI] [PubMed] [Google Scholar]
- 16.Yang YG, Sari IN, Zia MF, Lee SR, Song SJ, Kwon HY. Tetraspanins: Spanning from solid tumors to hematologic malignancies. Exp Hematol. 2016;44(5):322–8. doi: 10.1016/j.exphem.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, et al. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. . 1995;268(5212):884–6. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Iiizumi-Gairani M, Okuda H, Kobayashi A, Watabe M, Pai SK, et al. KAI1 gene is engaged in NDRG1 gene-mediated metastasis suppression through the ATF3-NFkappaB complex in human prostate cancer. J Biol Chem. 2011;286(21):18949–59. doi: 10.1074/jbc.M111.232637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azimi M, Sharifan A, Ghiasi Tarzi B. he Use of Pistacia khinjuk Essential Oil to Modulate Shelf-Life and Organoleptic Traits of Mechanically Deboned Chicken Meat. J Food Process Preserv. 2017;41:e12814. [Google Scholar]
- 20.Stamenković J, Radojković I, Đorđević A, Jovanović O, Petrović G, Stojanović G, et al. Optimization of HPLC method for the isolation of Hypericum perforatum L. methanol extract. Biol Nyssana. 2013;4(1-2):81–5. [Google Scholar]
- 21.Spiridon I, Bodirlau R, Teaca C-A. Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Cent Eur J Biol. 2011;6(3):388–96. [Google Scholar]
- 22.Öztürk N, Tunçel M, Potoğlu-Erkara İ. Phenolic compounds and antioxidant activities of some Hypericum species: A comparative study with H. perforatum. Pharm Biol. 2009;47(2):120–7. [Google Scholar]
- 23.Noureddini M, Rasta V-r. Analgesic Effect of Aqueous Extract of Achillea Millefolium L. on Rat’s Formalin Test. . Pharmacologyonline. 2008;3:659–64. [Google Scholar]
- 24.Eghdami A, Sadeghi F. Determination of total phenolic and flavonoids contents in methanolic and aqueous extract of Achillea millefolium. Org Chem J. 2010;2:81–4. [Google Scholar]
- 25.Burk DR, Cichacz ZA, Daskalova SM. Aqueous extract of Achillea millefolium L.(Asteraceae) inflorescences suppresses lipopolysaccharide-induced inflammatory responses in RAW 264.7 murine macrophages. J Med Plants Res. 2010;4(3):225–34. [Google Scholar]
- 26.Beya W, Davidson B, Erlwanger KH. The effects of crude aqueous and alcohol extracts of Aloe vera on growth and abdominal viscera of suckling rats. Afr J Tradit Complement Altern Med. 2012;9(4):553–60. [PMC free article] [PubMed] [Google Scholar]
- 27.Raphael E. Phytochemical constituents of some leaves extract of Aloe vera and Azadirachta indica plant species. Glob Adv Res J Environ Sci Toxicol. 2012;1(2):014–7. [Google Scholar]
- 28.Choudhury D, Gairola B, Roy D, Sikidar P. valuation of analgesic activity of aqueous extract of Aloe vera [AEAV] in albino Wistar rats. . Int J Pharm Sci Res. 2017;8(4):1850–7. [Google Scholar]
- 29.Hęś M, Dziedzic K, Thanh-Blicharz JL, Kmiecik D, Górecka D. Antioxidant activity of true aloe (Aloe vera) extract in model systems. . Nauka Przyroda Technologie. . 2016;10(4):53. [Google Scholar]
- 30.Kammoun M, Miladi S, Ali YB, Damak M, Gargouri Y, Bezzine S. In vitro study of the PLA2 inhibition and antioxidant activities of Aloe vera leaf skin extracts. Lipids Health Dis. 2011;10(1):30. doi: 10.1186/1476-511X-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toros P, Şen B, Sönmez PK, Özkut M, Öztürk Ş, Çöllü F, et al. The Effect of Herbal Medicine on Prostate Cancer Cells in Culture. Proceedings. 2017;1:10. [Google Scholar]
- 32.Colasanti A, Kisslinger A, Liuzzi R, Quarto M, Riccio P, Roberti G, et al. Hypericin photosensitization of tumor and metastatic cell lines of human prostate. J Photochem Photobiol B. 2000;54(2-3):103–7. doi: 10.1016/s1011-1344(99)00149-9. [DOI] [PubMed] [Google Scholar]
- 33.Zengin G, Aktumsek A, Ceylan R, Uysal S, Mocan A, Guler GO, et al. Shedding light on the biological and chemical fingerprints of three Achillea species (A. biebersteinii, A. millefolium and A. teretifolia). . Food Funct. 2017 Mar 22;8(3):1152–65. doi: 10.1039/c6fo01847e. [DOI] [PubMed] [Google Scholar]
- 34.Galavi HR, Saravani R, Shahraki A, Ashtiani M. Anti-proliferative and apoptosis inducing potential of hydroalcoholic Achillea wilhelmsii C. Koch extract on human breast adenocarcinoma cell lines MCF-7 and MDA-Mb-468. Pak J Pharm Sci. 2016 ;29(6 Suppl):2397–403. [PubMed] [Google Scholar]
- 35.Shahani S, Hamzkanlu N, Zakeri N, Hosseinimehr SJ. Synergistic anti-tumoral effect of Achillea millefolium combined with bleomycin on prostate cancer cells. Res Mol Med. 2015;3(1):12–7. [Google Scholar]
- 36.Bhat HM-u-d, Bhat KA, Prabha S, Hamid A. Antioxidant and cytotoxic activities of Achillea millefolium from Kashmir. . Journal of Academia and Industrial Research. 2014;2(8):487–91. [Google Scholar]
- 37.Köngül E, Taş Ö, Paşayeva L, Karatoprak GŞ. Analysis of the Cytotoxic Effects of Achillea millefolium L. Extracts on MCF7 Cell Line. Proceedings. 2017;1:10. [Google Scholar]
- 38.Tomasin R, Gomes-Marcondes MC. Oral administration of Aloe vera and honey reduces Walker tumour growth by decreasing cell proliferation and increasing apoptosis in tumour tissue. . Phytother Res. 2011;25(4):619–23. doi: 10.1002/ptr.3293. [DOI] [PubMed] [Google Scholar]
- 39.Sanders B, Ray AM, Goldberg S, Clark T, McDaniel HR, Atlas SE, et al. Anti-cancer effects of aloe-emodin: a systematic review. J Clin Transl Res. 2017;3(3):283–96. [PMC free article] [PubMed] [Google Scholar]
- 40.Liu K, Park C, Li S, Lee KW, Liu H, He L, et al. Aloe-emodin suppresses prostate cancer by targeting the mTOR complex 2. Carcinogenesis. 2012;33(7):1406–11. doi: 10.1093/carcin/bgs156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11(6):859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Hietakangas V, Cohen SM. Re-evaluating AKT regulation: role of TOR complex 2 in tissue growth. Genes Dev. 2007;21(6):632–7. doi: 10.1101/gad.416307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi UJ, Jee BK, Lim Y, Lee KH. KAI1/CD82 decreases Rac1 expression and cell proliferation through PI3K/Akt/mTOR pathway in H1299 lung carcinoma cells. Cell Biochem Funct. 2009;27(1):40–7. doi: 10.1002/cbf.1532. [DOI] [PubMed] [Google Scholar]
- 44. Lu Q, Longo FM, Zhou H, Massa SM, Chen YH. Signaling through Rho GTPase pathway as viable drug target. Curr Med Chem. 2009;16(11):1355–65. doi: 10.2174/092986709787846569. [DOI] [PMC free article] [PubMed] [Google Scholar]


