Abstract
The giant panda, with an estimated population size of 2239 in the world (in 2015), is a global symbol of wildlife conservation that is threatened by habitat loss, poor reproduction and limited resistance to some infectious diseases. Of these factors, some diseases caused by parasites are considered as the foremost threat to its conservation. However, there is surprisingly little published information on the parasites of the giant panda, most of which has been disseminated in the Chinese literature. Herein, we review all peer-reviewed publications (in English or Chinese language) and governmental documents for information on parasites of the giant pandas, with an emphasis on the intestinal nematode Baylisascaris schroederi (McIntosh, 1939) as it dominates published literature. The purpose of this chapter is to: (i) review the parasites recorded in the giant panda and describe what is known about their biology; (ii) discuss key aspects of the pathogenesis, diagnosis, treatment and control of key parasites that are reported to cause clinical problems and (iii) conclude by making some suggestions for future research. This chapter shows that we are only just ‘scratching the surface’ when it comes to parasites and parasitological research of the giant panda. Clearly, there needs to be a concerted research effort to support the conservation of this iconic species.
Keywords: Giant panda, Ailuropoda melanoleuca, Conservation, Parasites, Baylisascaris schroederi, Acarina
1. Introduction
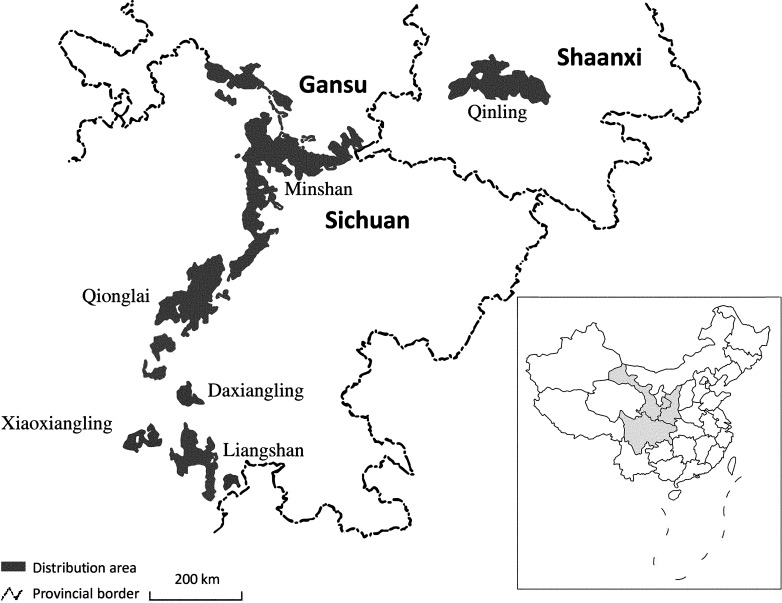
The giant panda, Ailuropoda melanoleuca, is one of the world's most recognised and rarest animals. It is a solitary bear of the subfamily Ailuropodinae in the family Ursidae, whose diet is mainly bamboo (Swaisgood et al., 2006). Giant pandas usually live for 14–20 years in the wild and up to 38 years in captivity (China Conservation and Research Centre for the Giant Panda, CCRCGP, unpublished data; Schaller et al., 1985). From the 16th to the 19th centuries, this panda was distributed widely in Western China (Gansu, Hubei, Hunan, Shaanxi and Sichuan provinces; Zhu and Long, 1983). However, today, this species is restricted to 30–40 populations in six isolated mountain ranges at the eastern edge of the Tibetan plateau in China, i.e., the Minshan, Qionglai, Qinling, Daxiangling, Xiaoxiangling and Liangshan mountains (Fig. 1 ), with a total estimated population size of 1864 in the wild (The State Forestry Administration of China, 2015). To protect this iconic and threatened animal, more than 375 giant pandas have been raised in captivity in conservation centres and zoos (The State Forestry Administration of China, 2015).
Fig. 1.
Distribution of wild giant pandas in six mountain regions (Qinling, Minshan, Qionglai, Liangshan, Daxiangling and Xiaoxiangling) in China.
Adapted from Zhang, L., Wu, Q., Hu, Y., Wu, H., Wei, F., 2015. Major histocompatibility complex alleles associated with parasite susceptibility in wild giant pandas. Heredity (Edinb) 114, 85–93.
Many factors threaten this endangered species, including habitat loss, degradation and fragmentation, poor reproduction and limited resistance to some infectious diseases (Feng et al., 1985; Wei et al., 2015; cf. Tables 1 and 2 ). Of these factors, diseases caused by parasites are reported to be a major threat to the conservation of the giant panda. In particular, a disease (baylisascariasis) caused by the ascaridoid nematode Baylisascaris schroederi (McIntosh, 1939) is a leading cause of deaths in wild populations (Zhang et al., 2008). Indeed, between 2001 and 2005, visceral larva migrans (VLM) linked to B. schroederi infection was reported to be responsible for 50% (12/24) of mortalities in the giant panda (Zhang et al., 2008).
Table 1.
Viruses and Bacteria Which Have the Potential to Threaten the Health and Conservation of the Giant Panda
| Pathogens | Classification | Location | Signs or Problem | Comments | References |
|---|---|---|---|---|---|
| Viruses | |||||
| Canine distemper virus | Paramyxoviridae | Nervous, digestive and respiratory systems | Fever and inflammation | The most dangerous virus to the giant pandas | Hvistendahl (2015) and Zhao et al. (2017) |
| Canine adenovirus | Adenoviridae | Liver and brain | Qin et al. (2011) | ||
| Canine coronavirus | Coronaviridae | Stomach and intestine | Mental depression, vomiting, flatulence and watery diarrhoea | There is no comprehensive knowledge on CCV of giant pandas | Mainka et al. (1994) |
| Canine parvovirus | Parvoviridae | Unknown | Watery diarrhoea and vomiting | Mainka et al. (1994) and Qin et al. (2011) | |
| Rotavirus | Reoviridae | Stomach and small intestine | Depression, anorexia vomiting and diarrhoea | Wang et al. (2008a) | |
| Bacteria | |||||
| Clostridium perfringens (Clostridium welchii) | Bacillaceae | Intestine | Subclinical | Opportunistic pathogen; sudden death | Pan et al. (2001) |
| Escherichia coli | Enterobacteriaceae | Intestinal tract and vagina | Diarrhoea, hemorrhagic enterocolitis and intestinal mucosal inflammation | Opportunistic pathogen | Wang et al. (2013b) |
| Klebsiella pneumoniae | Enterobacteriaceae | Respiratory and intestinal tracts | Lethargy, mental depression, diarrhoea, inappetence, emaciation vomiting, haematochezia and haemorrhagic enterocolitis | Yang et al. (2016) | |
| Proteus mirabilis | Enterobacteriaceae | Urogenital tract | Urinary urogenital infections | Opportunistic pathogen; might cause reproductive problems in females | Wang et al. (2007a) |
Table 2.
Parasites Recorded in the Giant Panda
| Parasite | Classification | Location | First Report | Complications | Comments | References |
|---|---|---|---|---|---|---|
| Helminths | ||||||
| Ancylostoma ailuropodae sp. nov. | Ancylostomatidae | Small intestine | First reported as Ancylostoma caninum in Sichuan province, China (2005). Renamed as Ancylostoma ailuropodae, based on morphological study (Xie et al., 2017) | n/a | Validity needs to be confirmed | Lai et al. (1991) and Xie et al. (2017) |
| Baylisascaris schroederi sp. nov. | Ascarididae | Small intestine | First reported in the Bronx Zoo, USA (1939) | Intestinal obstruction, emaciation, inflammation (enteritis and pneumonia), sometimes death | The most studied parasite of the giant panda | McIntosh (1939) and Sprent (1968) |
| Lungworm | n/a | n/a | First reported in Sichuan province, China (1991) | n/a | No genus and species names Identification based on egg examination |
Lai et al. (1991) |
| Strongyloides sp. | Strongyloididae | Small intestine | First reported in Sichuan province, China (1991) | n/a | Identification based on egg examination. Parasite name misspelled as ‘Storonglata’ | Lai et al. (1991) |
| Toxascaris seleactis | Ascarididae | Small intestine | First reported in Sichuan province, China (1991) | n/a | Identification based on egg examination | Lai et al. (1991) |
| Ogmocotyle sikae | Notocotylidae | Small intestine | First reported from an informal publication by He et al. (1987) in Shanxi province, China (without morphological description) | n/a | Life cycle and pathogenesis unknown | He et al. (1987) and Song et al. (2016) |
| First detailed morphological and phylogenetic studies conducted by Song et al. (2016) | ||||||
| Protists | ||||||
| Cryptosporidium sp. | Cryptosporidiidae | Gastrointestinal tract | First reported with a prevalence of 1.75% (1/57) in Sichuan province, China (2013), with morphological and phylogenetic analysis | No associated clinical signs | Potential novel genotype | Liu et al. (2013) |
| Cryptosporidium andersoni | Cryptosporidiidae | Gastrointestinal tract | First reported with a prevalence of 15.6% (19/122) and 0.5% (1/200) in captive and wild giant pandas, Sichuan province, China (2015) | No associated clinical signs | Wang et al. (2015) | |
| Enterocytozoon bieneusi | Microsporidia | Small intestine | First reported with a prevalence of 8.70% (4/46) in the northwest of China (2015) | No associated clinical signs | Potential novel genotype | Tian et al. (2015) |
| Toxoplasma gondii | Sarcocystidae | Liver, spleen, lungs, kidneys and intestines | First reported in a dead captive panda in Henan province, China (2015) | Fatal toxoplasmosis associated with serious respiratory and gastroenteritis signs | Potential novel genotype | Ma et al. (2015) |
| Sarcocystis sp. | Sarcocystidae | Muscle | Described in two reviews of parasites of the giant panda (Yang, 1998, Zhang et al., 2010); however, original literature is not available from either review | n/a | n/a | |
| Ectoparasites | ||||||
| Blowfly | Unknown | The gastroenteritis maggots were found in a giant panda (2007), without morphological description | n/a | No genus and species names | Li et al. (2007) | |
| Dermacentor taiwanensis | Ixodidae | Skin surface | Described in two reviews of parasites of the giant panda (Yang, 1998, Zhang et al., 2010); however, original literature is not available from either review | n/a | n/a | |
| Haemaphysalis aponommoides | Ixodidae | Skin surface | First reported in Sichuan province, China (1985) | n/a | Lai et al. (1990) | |
| H. flava | Ixodidae | Skin surface | First reported in Sichuan province, China (1990) without morphological description; first detailed morphological and phylogenetic analysis were conducted by Cheng et al. (2013) | n/a | Cheng et al. (2013) and Lai et al. (1990) | |
| H. hystricis | Ixodidae | Skin surface | Described in two reviews of parasites of the giant panda (Yang, 1998, Zhang et al., 2010); however, original literature is not available from either review | n/a | n/a | |
| H. kitaotai | Ixodidae | Skin surface | Described in two reviews of parasites of the giant panda (Yang, 1998, Zhang et al., 2010); however, original literature is not available from either review | n/a | n/a | |
| H. longicornis | Ixodidae | Skin surface | First reported in Sichuan province, China (1992) with detailed morphological description | n/a | Chen and Shi (1992) | |
| H. megaspinosa | Ixodidae | Skin surface | First reported in Gansu province, China (1987) without morphological description | n/a | Ma (1987) | |
| H. montgomeryi | Ixodidae | Skin surface | First reported in Gansu province, China (1987) without morphological description | n/a | Ma (1987) | |
| H. warburtoni | Ixodidae | Skin surface | First reported in Sichuan province, China (1985) with detailed morphological description | n/a | Wu and Hu (1985a) | |
| H. ailuropodae sp. nov. | Ixodidae | Skin surface | First reported in Shanxi province, China (1998) with detailed morphological description; no associated clinical signs reported | n/a | Validity needs to be confirmed | Yu et al. (1998) |
| Ixodes acutitarsus | Ixodidae | Skin surface | First reported in Gansu province, China (1987) without morphological description | n/a | Ma (1987) | |
| I. granulatus | Ixodidae | Skin surface | First reported in Gansu province, China (1987) without morphological description | n/a | Ma (1987) | |
| I. ovatux | Ixodidae | Skin surface | First reported in Gansu province, China (1987) without morphological description | n/a | Ma (1987) | |
| Chorioptes panda sp. nov. | Psoroptidae | Skin surface | First reported in Paris Zoo, France (1975) | Mild alopecia, erythema and crusting affecting sleep and appetite | Fain and Leclerc (1975) | |
| Chaetopsylla ailuropodae | Vermipsyllidae | Skin surface | First reported in Sichuan province, China (1991) with detailed morphological description | n/a | Qiu et al. (1991) | |
| C. mikado | Vermipsyllidae | Skin surface | First reported in Sichuan province, China (1990) with detailed morphological description | n/a | Lai et al. (1990) | |
| Demodex ailuropodae | Demodicidae | Hair follicles, sebaceous gland | First reported in Shanghai, China (1985), with detailed morphological description | n/a | Xu et al. (1986) | |
n/a, no information available.
However, surprisingly, there is limited published information on the parasites of the giant panda (n = 91 peer-reviewed publications and archived governmental reports; 14 January 2017 ), most of which have been published in the Chinese literature (n = 55 publications). Unpublished information from zoos, animal parks and breeding centres (sources available from authors) suggests that parasites of the giant panda continue to be a persistent and chronic issue, adversely impacting the health and conservation of this iconic animal. Therefore, we consider it appropriate and timely to review the literature on parasites of the giant panda, with an emphasis on B. schroederi because it dominates published literature. The purpose of this chapter is to: (i) review the parasites recorded in the giant panda and describe what is known about their life cycles; (ii) discuss key aspects of the pathogenesis, diagnosis, treatment and control of common parasites and (iii) conclude by making some suggestions for future research.
2. Parasite Records for the Giant Panda
Since B. schroederi (originally named Ascaris schroederi) (McIntosh, 1939) was found in the small intestine from a giant panda in the New York Zoological Park (now the Bronx Zoo) in 1939, an increasing number of parasites have been identified in this animal species. To date, at least 29 parasite taxa, including 11 endoparasites (5 nematode, 1 trematode and 5 protozoan taxa) and 18 ectoparasites (13 tick, 2 mite, 2 flea taxa and 1 ‘blow fly’) have been recorded in the giant panda (Table 2). However, many of these records are in the Chinese literature (publications or archival documents), often with very limited descriptions of morphology and/or other aspects. Beyond these records, reports of clinical cases often involve B. schroederi, the mite Chorioptes panda and ixodid ticks.
2.1. Key Parasites Reported to Cause Clinical Problems
2.1.1. Baylisascaris schroederi
B. schroederi (Nematoda: Ascaridoidea) is a large parasitic nematode inhabiting the small intestine of giant pandas and belongs to the genus Baylisascaris, of which there are 11 species, namely, B. procyonis of raccoons, B. columnaris of skunks, B. potosis of kinkajous, B. ailuri of red pandas, B. transfuga of bears, B. melis of badgers, B. laevis of marmots and ground squirrels, B. devosi of marten and fishers, B. tasmaniensis of Tasmanian devils and quolls and B. venezuelensis of spectacled bears (Kazacos, 2008; Pérez Mata et al., 2016; Sprent, 1968; Tokiwa et al., 2014; Tranbenkova and Spiridonov, 2017; Table 3 ). There is no evidence that B. schroederi affects other animal species or humans under natural conditions. Despite its massive health impact on wild giant panda populations in the early 20th century (Qiu and Mainka, 1993; Zhang et al., 2008), B. schroederi was first described in 1939 as Ascaris schroederi (McIntosh, 1939), before being renamed B. schroederi in 1968 (Sprent, 1968). Presently, baylisascariasis is considered the most harmful parasitic disease of the giant panda (Feng et al., 1985; Zhang et al., 2015). Thus, B. schroederi has been the most studied parasite of this animal. Most published studies have investigated important aspects of this parasite's biology, epidemiology, pathogenesis, diagnosis, treatment and control.
Table 3.
Recognized Species of Baylisascaris
| Speciesa | Primary Definitive Host(s)b |
|---|---|
| Baylisascaris ailuri (Wu et al., 1987) | Red panda |
| Baylisascaris columnaris (Leidy, 1856) | Skunks |
| Baylisascaris devosi (Sprent, 1952) | Marten, fisher |
| Baylisascaris melis (Gedoelst, 1920) | European badger |
| Baylisascaris potosis (Tokiwa et al., 2014) | Kinkajou |
| Baylisascaris procyonis (Stefanski and Zarnowski, 1951) | Raccoon |
| Baylisascaris schroederi (McIntosh, 1939) | Giant panda |
| Baylisascaris transfuga (Rudolphi, 1819) | Bears |
| Baylisascaris laevis (Leidy, 1856) | Ground hog, ground squirrels |
| Baylisascaris tasmaniensis (Sprent, 1970) | Tasmanian devil, quolls |
| Baylisascaris venezuelensis (Pérez Mata et al., 2016) | Spectacled bear |
Adapted from Kazacos, K.R., 2008. Baylisascaris procyonis and related species. In: Samuel, W.M., Margo, J.P., Kocan A.A. (Eds.), Parasitic of Wild Mammals. Iowa State University Press, Ames, USA, pp. 301–341.
List of definitive hosts is not extensive due to the numerous species recorded; see Sprent (1968) and Kazacos (2016) for extensive list.
2.1.1.1. Life Cycle
Similar to many other ascaridoids, B. schroederi has a complex life cycle in a single host, involving larval moults and development in several organ systems. Although not all aspects of this cycle have been proven, based on postmortem findings for giant pandas (CCRCGP, unpublished data) and experimentally infection studies in mice (Li, 1989, Li, 1990a, Li, 1990b, Li, 1993), evidence indicates that giant pandas become infected via the faecal–oral route, and larvae undergo hepatopulmonary and somatic migration prior to establishing as dioecious adults in the small intestine. Currently, there is no evidence of vertical or transmammary transmission of B. schroederi.
The current understanding is that, after eggs hatch in the intestine, the infective larvae penetrate the mucosa of the intestine. Subsequently, based on current knowledge, the larvae migrate via the mesenteric and portal blood system to the liver and then the lungs, and eventually return (via the trachea) to the intestinal lumen, where they mature to adults, mate and reproduce. Unembryonated eggs are released from female worms into chyme and faeces into the environment, where they can remain viable in moist soil for many years (Hou et al., 2012; Li, 1988; Yang and Zhang, 2013). Previous studies have shown that eggs of B. schroederi survive better than those of some other ascaridoids, such as Ascaris lumbricoides and Ascaris suum, and are characterised by rapid embryonation and resistance to low temperatures (4–12°C) (Li, 1988; Wu and Hu, 1985b). It usually takes 2–4 weeks for fertile eggs of B. schroederi to become infective at 22°C in moist and shaded soil (Wu et al., 1985), whereas 6–10 weeks are required by Ascaris eggs under the same conditions (Maung, 1978). Li (1988) found that 43% of B. schroederi eggs contained infective larvae within 30 days at − 10°C.
2.1.1.2. Epidemiology
Since Baylisascaris eggs are highly resistant to environmental pressures (e.g. temperature and desiccation), it is difficult for panda individuals to avoid exposure once the environment is contaminated (Zhang and Wang, 2003). According to the limited, but valuable reports on the prevalence of B. schroederi infection (Feng et al., 1985; Lai et al., 1991; Li et al., 2014; Peng et al., 1989; Wang et al., 2001, Wang et al., 2013a; Yang, 1993; Ye, 1989; Yu et al., 1998; Zhang et al., 2011, Zhang et al., 2015; Zhou et al., 2013b), baylisascariasis commonly occurs in wild and captive populations of the giant panda (Loeffler et al., 2006). It is noteworthy that B. schroederi infection has been frequently detected in giant pandas upon arrival at international zoos, such as Adelaide Zoo (Australia), Chiang Mai Zoo (Thailand), Edinburgh Zoo (UK), Kebecity Oji Zoo (Japan), Pairi Daiza Zoo (Belgium), San Diego Zoo (USA), Smithsonian National Zoo (USA), Ueno Zoo (Japan) and Zoo Negara (Malaysia) (personal communication, Chengdong Wang, 10 February 2017).
Table 4 shows up-to-date, published prevalence information for B. schroederi infection in giant pandas (in English or Chinese language). Although there are questions surrounding the validity of some information in some Chinese publications, due to incomplete descriptions of methodologies and/or result sections, it is reassuring to see that independent studies, applying different methodologies, reveal similar findings, namely (1) that B. schroederi infection is commonly found in the wild giant panda populations across all six isolated mountain ranges in China (Fig. 1), (2) that the prevalence of infection in captive giant pandas is high (7%–88%) in most of breeding centres and zoos, (3) that the infection rate has not changed significantly over the last four decades in both captive and wild populations and (4) that there is no significant difference in the prevalence of B. schroederi infection among different age groups of giant pandas or between the sexes from previous large-scale surveys (n = 2680, Lai et al., 1991; n = 336, Yang, 1993). In this context, it is noteworthy to mention that Zhang et al. (2011) employed a genetic testing technique to link each stool sample to individual wild giant pandas, when estimating the prevalence of B. schroederi infection. The use of this noninvasive, genetic approach simultaneously provides the precise number of individual pandas sampled as well as the B. schroederi infection status (Zhang et al., 2011).
Table 4.
Published Prevalence Information for Baylisascaris schroederi of the Giant Panda
| Year | Locationa | Population | Techniqueb | Reported Prevalence | References |
|---|---|---|---|---|---|
| 1974–1986 | Minshan and Qionglai | Wild | n/a | 100% (50/50) | Ye (1989) |
| 1984 | Shanghai Zoo | Captive | Sedimentation–flotation technique | 67% (2/3) | Peng et al. (1989) |
| 1985 | Minshan and Qionglai | Wild | Necropsy | 100% (13/13) | Feng et al. (1985) |
| 1985–1988 | Minshan, Qionglai, Daxiangling, Xiaoxiangling and Liangshan | Wild | Sedimentation–flotation technique | 56% (1050/2680) | Lai et al. (1991) |
| 1985–1988 | Minshan | Wild | Sedimentation–flotation technique | 78% (262/336) | Yang (1993) |
| Qionglai | 83% (160/194) | ||||
| Liangshan | 60% (81/135) | ||||
| Daxiangling and Xiaoxiangling | 47% (15/32) | ||||
| 1998 | Qinling mountain | Wild | Sedimentation–flotation technique | 100% (2/2) | Yu et al. (1998) |
| 2001 | Chengdu Zoo | Captive | Sedimentation–flotation technique | 7% (1/14) | Wang et al. (2001) |
| 2006–2008 | Qinling | Wild | Sedimentation–flotation technique | 66% (31/47) | Zhang et al. (2011) |
| Minshan | 44% (10/23) | ||||
| Qionglai | 48% (14/29) | ||||
| Liangshan | 57% (8/14) | ||||
| Daxiangling | 80% (4/5) | ||||
| Xiaoxiangling | 13% (1/8) | ||||
| 2009–2010 | Minshan | Wild | PCR/CE-SSCP | 48% (15/31) | Zhang et al. (2012) |
| 2012 | Ya’an, CCRCGP | Captive | PCR (cox2) | 68% (34/50) | Wang et al. (2013a) |
| 2013 | Ya’an, CCRCGP | Captive | PCR (12S rRNA) | 88% (44/50) | Zhou et al. (2013b) |
| 2014 | Ya’an, CCRCGP and Chengdu, CRBGP | Captive | Sedimentation–flotation technique | 26% (54/210) | Li et al. (2014) |
| 2014 | Minshan, Qionglai, Qinling, Xiaoxiangling and Liangshan | Wild | McMaster method | 55% (48/87) | Zhang et al. (2015) |
CCRCGP, China Conservation and Research Centre for the Giant Panda; CRBGP, Chengdu Research Base of Giant Panda.
12S rRNA, mitochondrial 12S ribosomal RNA gene; cox2, mitochondrial cytochrome c oxidase subunit 2 gene; n/a, not available; PCR/CE-SSCP, PCR-based capillary electrophoretic single-strand conformation polymorphism analysis.
2.1.1.3. The Disease: Baylisascariasis
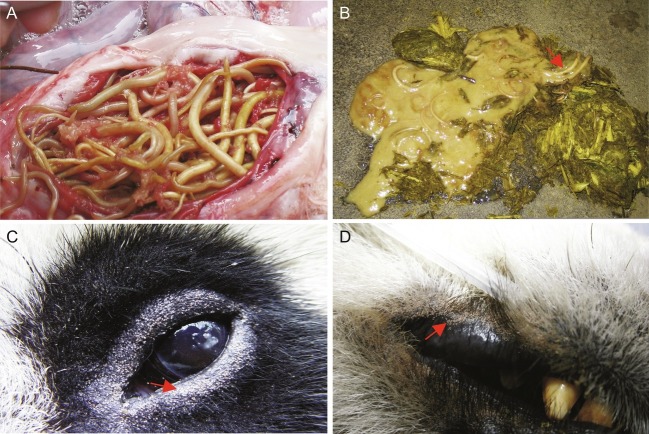
Most pathological and clinical features of baylisascariasis in the giant panda are caused by the migration of B. schroederi larvae in various tissues and by adult worms in the gastrointestinal tract (Feng et al., 1985; Ye, 1989). When the Baylisascaris larvae migrate through the tissues of their host, significant tissue damage relates predominantly to extensive inflammation and (subsequent) scarring in the intestinal wall as well as in the parenchymata of the liver and lungs (Li, 1990a, Li, 1990b; Loeffler et al., 2006). In some extreme cases, in addition to inflammation, entangled or clumped adult worms can lead to mechanical intestinal obstruction, which can be life-threatening (Fig. 2A) (Li, 1990b; Wang et al., 2007b). Moreover, although not yet reported, it is possible that pulmonary injury caused by migrating Baylisascaris larvae may enable or exacerbate bacterial infections (cf. Yang and Zhang, 2013). To date, there have been no reports of neural larva migrans associated with B. schroederi as seen for B. procyonis in raccoons (Procyon lotor) in North America (Graeff-Teixeira et al., 2016; Kazacos, 2016).
Fig. 2.
Selected parasites of the giant panda. (A) The parasitic nematode Baylisascaris schroederi in the gastrointestinal tract of the giant panda, causing obstruction. (B) Baylisascaris schroederi expelled from an infected giant panda following anthelmintic treatment. (C) The mite Chorioptes panda and its typical predilections sites on the eyelid and/or lips (D). Red arrows indicating B. schroederi (B) or skin affected by C. panda (C and D).
Morbidity and mortality associated with baylisascariasis are reported to relate directly to the intensity of B. schroederi infection (Qiu and Mainka, 1993), whereas individual pandas harbouring small numbers of worms tend to be asymptomatic. In captive giant panda populations, where there is a focus on controlling B. schroederi, this nematode rarely causes specific clinical symptoms (CCRCGP, unpublished clinical records), although the migration of larvae through lungs can cause acute symptoms, such as coughing and wheezing, particularly in young cubs (Yang and Zhang, 2013). Nonetheless, it is commonly observed that captive giant pandas, especially juveniles, can pass whole adult worms in the faeces or vomit (CCRCGP, unpublished data; Loeffler et al., 2006). Nevertheless, B. schroederi infection is presently recognised as the biggest threat to free-ranging panda populations (Qiu and Mainka, 1993). Based on the literature (including publications in Chinese scientific journals, governmental reports and websites) between 1971 and 2005, Zhang et al. (2008) studied the causes of death in 789 adult wild giant pandas in natural habitats. These authors concluded that VLM caused by B. schroederi appeared to be the most significant threat of the three major factors of wild giant panda mortality during that period, the other two factors being food shortage (i.e. flowering and ‘die-off’ of bamboo; Reid et al., 1989) and poaching (Li et al., 2003). Surprisingly, baylisascariasis was reported to be responsible for 50% (12/24) of all deaths in free-ranging giant pandas between 2001 and 2005 (Zhang et al., 2008).
2.1.1.4. Diagnosis
Although the presence of adult worms in the faeces or vomit indicates infection in giant pandas, the diagnosis of B. schroederi infection is commonly performed using the quantitative McMaster test or a semiquantitative sedimentation–flotation method in most breeding centres and zoos (Loeffler et al., 2006; Zhang and Zhang, 2002). However, due to the large amount of undigested bamboo fibers in giant panda's faeces, B. schroederi eggs may be challenging to detect using a microscopy upon routine laboratory examination. In some instances, giant pandas with repeatedly ‘negative’ faecal test results have been reported to suddenly vomit bundles of worms. Hence, test sensitivity appears to be relatively low, in spite of the high reproductive index of B. schroederi (see Yang and Zhang, 2013), suggesting that ‘false negative’ results might relate to the presence of immature worms in the intestines.
Polymerase chain reaction (PCR)-based techniques can overcome this issue. For instance, some researchers (Wang et al., 2013a; Zhou et al., 2013b) have developed a PCR-based tool to directly amplify parts of the mitochondrial 12S ribosomal RNA or cytochrome c oxidase subunit 2 (cox2) gene from stool DNA samples. Their results showed that this tool is able to detect genomic DNA amounts that are at least equivalent to that from a single egg of B. schroederi and are more sensitive (0.5–1 time) than traditional coproscopic methods. In addition, no ‘cross-reactivity’ with DNA from other nematodes (i.e. Ancylostoma caninum, B. transfuga or B. procyonis) was found using this molecular diagnostic approach. In addition, other workers (Zhang et al., 2012) employed a combined PCR and capillary electrophoretic-based single-strand conformation polymorphism analysis (PCR-based CE-SSCP) using the mitochondrial gene cox2 to screen for B. schroederi DNA in stool samples from wild giant pandas from the Minshan mountains. Using this approach, these authors concluded that they were able to establish the prevalence and intensity of B. schroederi infection.
Apart from conventional and molecular tests, some progress has been made on developing serological detection methods. For instance, an antibody detection enzyme-linked immunosorbent assay (ELISA) employing a B. schroederi glutathione S-transferase antigen was established for the detection anti-B. schroederi serum antibody (IgG) in experimentally infected mice, with a sensitivity of 79.1% and a specificity of 82.0% (Xie et al., 2015a). However, such an assay has not yet been assessed for the diagnosis of baylisascariasis or B. schroederi infection in giant pandas. In the meantime, preliminary experiments are being planned at Ghent University (Belgium) and CCRCGP to assess an ELISA using A. suum antigen (Vlaminck et al., 2012) for the serological monitoring of B. schroederi infection in pandas.
2.1.1.5. Treatment and Control
The transmission of B. schroederi infection within and among captive giant panda populations is dependent on various factors, including the housing system, hygiene, management practices and anthelmintic treatment. However, to accomplish the short-term goal of reducing infection intensity and transmission potential, current control strategies rely mainly on monthly coprological examination (for eggs) and a mass anthelmintic treatment strategy (all individuals, including those with possible false-negative results for B. schroederi) (Loeffler et al., 2006; Wu and Hu, 1988). Anthelmintics used in practice include pyrantel pamoate; albendazole, fenbendazole, mebendazole; ivermectin, milbemycin oxime, doramectin and selamectin (CCRCGP, unpublished data; Loeffler et al., 2006)—the dosages of these compounds are indicated in Table 5 . Usually, multiple (2–4) treatments are given until an individual panda ceases to expel worms and/or eggs in the faeces (Fig. 2B) (Wu and Hu, 1988; CCRCGP, unpublished clinical records; for limitations of conventional diagnostic methods, see Section 2.1.1.4). However, the efficacies of these anthelmintics at the doses routinely used against B. schroederi have not yet been critically assessed in captive giant pandas. Thus, there is a need to evaluate and compare the efficacies of these anthelmintics using a standardised protocol (cf. International Harmonisation of Anthelmintic Efficacy Guidelines; Vercruysse et al., 2001, Vercruysse et al., 2002).
Table 5.
Suggested Efficacy of Different Anthelmintic Drugs Tested Against Baylisascaris schroederi
| Anthelmintics | Formulation | Dosage | Effectb | Referencesc |
|---|---|---|---|---|
| Albendazolea | Oral | 6 mg/kg, once | Negative of eggs in faecal floatation examination after 9 days of treatment | Liu and Yang (1994) |
| Doramectina | Pour on | 0.5 mg/kg, once | n/a | CCRCGP (unpublished data) |
| Febantel | Oral | 20 mg/kg, once | Reported as effective without supporting data | Wu and Hu (1985b) |
| Fenbendazolea | Oral | 5 mg/kg, once | n/a | CCRCGP (unpublished data) |
| Levamisole | Oral | 7–8 mg/kg per 2 months, four times | Negative of worm or eggs in faecal examination after four times of treatment | Wu and Hu (1988) |
| Oral/in feed | 5–10 mg/kg, once | Negative of worm in faecal examination after 4 days of treatment | Ye and Zhang (1981) | |
| Unknown | 8–10 mg/kg per 2 weeks | Reported as effective without supporting data | Qiu (1990) | |
| Mebendazolea | Unknown | 3.2–6.4 mg/kg | Reported as effective without supporting data | Qiu (1990) |
| Methylimidazole compound | Unknown | Unknown | Reported as effective without supporting data | Zhang and Zhang (2002) |
| Milbemycina | 0.5 mg/kg, once | n/a | CCRCGP (unpublished data) | |
| Ivermectina | Usually oral | 0.2 mg/kg per day, twice | None reduction of egg counts in faecal flotation examination after 10–15 days of treatment (without negative control) | Li et al. (2015) |
| Pyrantel pamoatea | Oral | 10 mg/kg per day, twice | 80.0% (ointment) reduction of egg counts in faecal floatation examination after 10–15 days of treatment | Li et al. (2015) |
| Piperazine citrate | Oral | 150 mg/kg, once | Significant reduction of worms in faecal examination after 5 days of treatment | Ye and Zhang (1981) |
| Unknown | 140–160 mg/kg per 2 weeks | Reported as effective without supporting data | Qiu (1990) | |
| Selamectina | Spot-on | 6–12 mg/kg, once | n/a | CCRCGP (unpublished data) |
| Trichlorfon | Unknown | 55 mg/kg | Negative of worm in faecal examination after 5 days of treatment; significant side effect was observed | Ye and Zhang (1981) |
Anthelmintics used in practice currently.
None of these studies followed International Harmonisation of Anthelmintic Efficacy Guidelines (Vercruysse et al., 2001, Vercruysse et al., 2002); n/a, not available.
CCRCGP, China Conservation and Research Centre for the Giant Panda; CRBGP, Chengdu Research Base of Giant Panda.
Due to the relatively short activity of anthelmintics in the host and the spread of large numbers of resilient B. schroederi eggs from infected animals in the environment, reinfection can occur rapidly (usually within 20–40 days; CCRCGP, unpublished records). Given that resistance to anthelmintics is increasing in a number of nematode species of both animals and humans (e.g. Kaplan, 2004; Vercruysse et al., 2011), it is readily possible that resistance could develop in Baylisascaris as anthelmintics are routinely (excessively) administered to giant pandas at varying or imperfect dosages. Given the implementation of reintroduction programmes for captive giant pandas to the wild in recent years (Shan et al., 2014), drug resistance genes carried by Baylisascaris in released giant pandas could spread to and through wild populations.
Therefore, the possibility or likelihood that drug resistance in Baylisascaris could emerge as a problem has stimulated the search for alternative methods of prevention and control. One possibility could be to develop a vaccine against baylisascariasis. Inspired by research towards developing a vaccine against A. suum (see Islam et al., 2005; Matsumoto et al., 2009; Tsuji et al., 2001, Tsuji et al., 2002, Tsuji et al., 2003, Tsuji et al., 2004), considerable attention and research effort have been directed towards a recombinant subunit vaccine against baylisascariasis. It is encouraging to know that pigs can mount some protection (58%) following vaccination with recombinant antigens against challenge infection in a pig-Ascaris model (Tsuji et al., 2004), suggesting that vaccination against B. schroederi might be feasible. The first vaccination trial of a recombinant antigen, Bs-Ag3 (37 kDa), against B. schroederi infection in laboratory mice was reported in 2008 (Wang et al., 2008b). Repeated subcutaneous administration (three times at 2-weekly intervals) of recombinant Bs-Ag3 with adjuvant in mice (BALB/c) resulted in a 63% reduction in the number of larvae collected from the lungs and a significant increase in total IgG in serum, in comparison with nonimmunised control mice. Subsequently, similar results were observed when mice were immunised with other recombinant antigens, Bs-Ag1 (64% reduction in number of lung larvae) and Bs-Ag2 (69% reduction), using same experimental design (He et al., 2009, He et al., 2012). However, to date, there has been no investigation of the IgG subclasses in the sera from immunised mice or of the precise mechanism(s) by which protection is achieved against B. schroederi.
More recently, aside from these immunogens, more attention has focused on targets that play essential roles in the survival of the parasite. For example, Xie et al. (2013) identified PYP-1, a new homologue of inorganic pyrophosphatases (PPases) (Kajander et al., 2013), which likely plays critical roles in nematode development and moulting (Islam et al., 2003; Ko et al., 2007) and is distributed widely in the body wall, gut epithelium, ovary and uterus of adult female Baylisascaris. Two separate vaccination experiments in mice (BALB/c) showed that recombinant PYP-1 induced 69%–71% reductions in the number of liver-stage and lung-stage larvae 7 days following challenge infection (Xie et al., 2013). An investigation of the IgG subclasses in the sera of immunised mice showed that the level of IgG1 was significantly higher than IgG2a, with increased levels of IL-4 and IL-10, indicating a type 2 protective immune response (Xie et al., 2013).
Apart from work directed towards a vaccine against B. schroederi, efforts have also been made to understand aspects of the molecular biology and genetics of this parasite. In another study (Zhao et al., 2013), the microRNA profile of B. schroederi via high-throughput sequencing and real-time quantitative PCR suggested that chitinases, ovarian or egg development related proteins and ribosomes were the targets of large numbers of microRNAs for the regulation of genes at the posttranscriptional level. This study also highlighted the potential of (at least some of) these microRNAs as intervention targets against baylisascariasis (Zhao et al., 2013).
2.1.1.6. Genetics
Following the publication of the complete mitochondrial genome of B. schroederi (see Xie et al., 2011), Li et al. (2012) undertook the first molecular characterisation of this parasite using nuclear ribosomal (r)RNA genes (18S and 28S) and a mitochondrial gene (12S) (Li et al., 2012). Importantly, Li et al. (2012) showed that 18S was not a suitable candidate marker for assessing variation within the genus Baylisascaris, but that 28S and 12S sequences were capable of distinguishing B. schroederi from B. ailuri and B. transfuga.
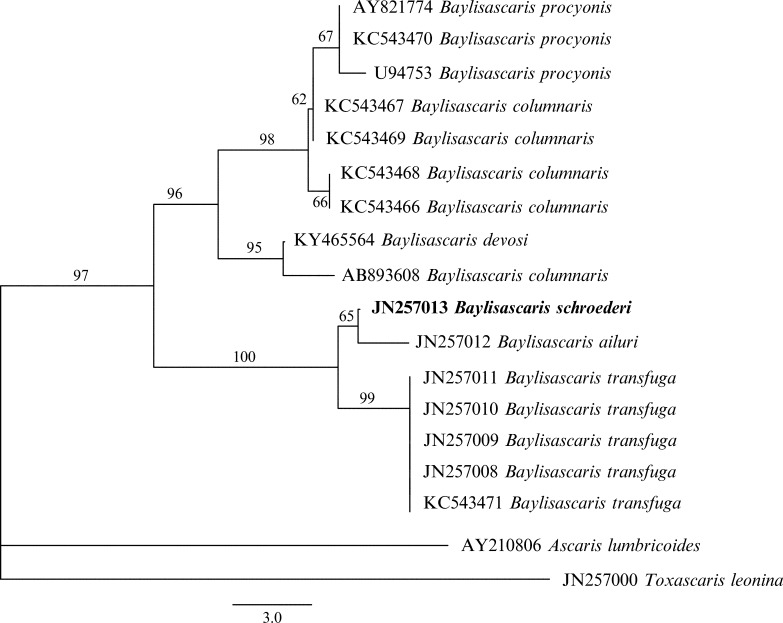
Current and published phylogenetic trees for members of the genus Baylisascaris using 28S and mitochondrial cox1 gene data sets (Tokiwa et al., 2014) consistently show two main groupings: one containing B. schroederi, B. ailuri from the red panda and B. transfuga from a variety of bear hosts, and the other comprising Baylisascaris species from raccoon, skunk and the South American kinkajou (see Table 3). An analysis using recent sequence data (accession no. KY465564) places B. devosi from mustelids with B. potosis from this kinkajou (Fig. 3 ). Additionally, a new species of Baylisascaris, called B. venezuelensis, from the South American spectacled bear (which is closely related to the giant panda; Talbot and Shields, 1996) was described recently (Pérez Mata et al., 2016). Clearly, further sequence data from other members of the genus Baylisascaris are needed to gain a better understanding of the phylogeographic history of B. schroederi, which likely involves ‘host-switching' and ‘ecological fitting' (Araujo et al., 2015).
Fig. 3.
A phylogenetic tree showing the position of Baylisascaris schroederi in relation to all other species of Baylisascaris for which nucleotide sequence data are available, using Ascaris lumbricoides (from human) and Toxascaris leonina as outgroups. This tree is based on the analysis of 28S rRNA gene sequence data using the neighbour joining method. All data were obtained from the GenBank database, and accession numbers precede species names in the phylogenetic tree.
Adapted from fig. 3A from Tokiwa, T., Nakamura, S., Taira, K., Une, Y., 2014. Baylisascaris potosis n. sp., a new ascarid nematode isolated from captive kinkajou, Potos flavus, from the Cooperative Republic of Guyana. Parasitol. Int. 63, 591–596.
Based on a morphological study by Wan et al. (2003), giant pandas from the Qinling mountain range represent a distinct subspecies (Ailuropoda melanoleuca qinlingensis) from Ailuropoda melanoleuca melanoleuca and are thought to be genetically distinct from all other populations of pandas (Wei et al., 2012). Although the draft nuclear genome of the giant panda has been published (Li et al., 2010), there is no study describing the use of large nuclear genomic sequence data sets to explore the population genetics of this panda. Several studies (see later) have attempted to search for genetic differences between B. schroederi of the two subspecies of giant panda. However, no genetic variation has been found between worms of the two subspecies of giant panda using first and second internal transcribed spacers (ITS-1 and ITS-2 = ITS) of nuclear ribosomal DNA (Lin et al., 2012; Zhao et al., 2012). Other studies attempted to resolve the same issue using various mitochondrial gene markers. First, Zhou et al. (2013a) used the cytb gene and concluded that there was a high rate of gene flow among three populations of B. schroederi representing the two recognised subspecies. Second, Xie et al., 2014, Xie et al., 2015b used portions of the mitochondrial cox1, atp6 and 12S rRNA genes, which displayed very few parsimony informative sites. Again, these authors found no discernable genetic difference in worms among the populations from distinct habitats of the giant panda (i.e. the Minshan, Qionglai and Qinling mountain ranges). A low level of genetic diversity but a high level of gene flow in worms suggested the potential for a rapid spread of drug resistance in B. schroederi (see Xie et al., 2014). Third, Zhao et al. (2014) did not find support for the genetic substructuring within B. schroederi among samples from the Qinling mountains and one sample from Sichuan province using portions of the mitochondrial genes cytb, cox3 and nad5. Unfortunately, to date, only mitochondrial genes, whose validity as a population genetic markers has been questioned (Galtier et al., 2009), have been used to explore structuring and substructuring in B. schroederi populations. Therefore, future studies should be conducted using multiple neutral nuclear genomic markers. A project in China is now underway to sequence, assemble and annotate the nuclear genome of B. schroederi, which, if successful, would underpin such a focus.
2.1.2. Chorioptes panda
Chorioptes spp. (Acariformes: Psoroptidae) are skin mites that cause mange in domestic and wild animals. These mites are commonly found in herbivorous hosts, including cattle, sheep, goats, horses, camelids and moose (Yeruham et al., 1999). Chorioptes was first found in the ears of captive giant pandas in the Paris Zoo, France and named Chorioptes panda (Fain and Leclerc, 1975), and subsequently reported in China in 1986 (Ye, 1986). However, C. panda was considered an invalid species by some researchers (Zahler et al., 2001). In ensuing years, other researchers reappraised the morphology of the mite, undertook phylogenetic analyses of mitochondrial cox1 and nuclear 18S rRNA gene data sets, and concluded that C. panda is a valid species (Bochkov et al., 2014; Hestvik et al., 2007; Wang et al., 2012).
Although no epidemiological data are available, C. panda is commonly found in captive populations of the giant panda (Qiu et al., 1984; Wang et al., 2000; Xu and Zhang, 2002; Yang et al., 2001; Zhou et al., 1989), particularly in late spring and early autumn (CCRCGP, unpublished records). However, to date, there is only one published record of C. panda infection in wild populations of the giant panda (Wang et al., 2012), although obtaining such information for such populations is a considerable challenge. In addition, wild giant pandas are usually solitary animals, and only occasionally interact with each other, mainly during their short mating season (Schaller et al., 1985). Thus, the probability of direct cross-transmission of this mite appears to be low.
Typically, C. panda is found on the eyelids, lips and ears of giant pandas (Fig. 2), causing mild alopecia, erythema and crusting, and affecting the sleep and appetite of giant pandas (Qiu et al., 1984). The control of Chorioptes mange relies mainly on chemotherapeutic treatment. Macrocyclic lactones (e.g. ivermectin and selamectin) have been found to be effective when routinely administered on a monthly basis (CCRCGP, unpublished records). Closantel (Wang et al., 2000) and deltamethrin (Xu and Zhang, 2002) have also been proposed to be effective against C. panda (see Wang et al., 2000; Xu and Zhang, 2002), but well-controlled experiments are needed to verify the authors’ claims.
2.1.3. Ixodidae (Hard Ticks)
Other ectoparasites that can affect the health of the giant panda are (blood-feeding) hard ticks. Since the first description of tick infection by Haemaphysalis warburtoni (Wu and Hu, 1985a), an increasing number of hard tick species have been identified on giant pandas in the last two decades (Lai et al., 1990; Ma, 1987; Qiu and Zhu, 1987; Yu et al., 1998). To date, 13 species representing 3 genera of hard ticks have been proposed as recorded (from rescued, sheltered or dead, wild giant pandas). These ticks include members of the genera Haemaphysalis (9 species), Ixodes (3 species) and Dermacentor (1 species) (Table 2). Of these ticks, Haemaphysalis flava has been most commonly reported in giant panda populations (Cheng et al., 2013; Ma, 1987; Qiu and Zhu, 1987). Recently, molecular tools have also been employed for the genetic characterisation of ticks from the giant panda. Using mitochondrial and ribosomal DNA markers as well as key morphological characters, H. flava was identified to predominate on giant pandas in the Qinling mountain range (Cheng et al., 2013). Although there is no report on mortality caused by such hard ticks, morbidity involving dermatitis and/or weight loss has been recorded in infested giant pandas (CCRCGP, unpublished records). Similar to the treatment of C. panda, ivermectin and selamectin are the compounds most commonly used against ticks in breeding centres and zoos (CCRCGP, unpublished clinical records). To date, there are no reports of any associated tick-borne diseases in the giant panda.
2.2. Protists: An Emerging Issue?
Using molecular diagnostic tools, such as PCR, Cryptosporidium spp. (Liu et al., 2013; Wang et al., 2015), Enterocytozoon bieneusi (see Tian et al., 2015) and Toxoplasma gondii (see Ma et al., 2015) have been detected and characterised from giant pandas. During a routine coprological examination (flotation) of 57 faecal samples at CCRCGP (Liu et al., 2013), one sample from an 18-year-old male captive giant panda was test-positive for Cryptosporidium, with oocysts being 4–4.6 μm in size. A phylogenetic analysis of partial 18S rRNA (786 bp), 70 kDa heat shock protein (1879 bp) and actin (1044 bp) gene sequence data (GenBank accession nos. JF970610, JN588571 and JN969985) showed that Cryptosporidium from the giant panda is genetically similar to that of genotype of Cryptosporidium from a black bear (Ursus americanus) (98%–99.5%; GenBank accession nos. AF247535, AF247536 and AF382339). Due to its sequence divergence (0.5%–2.0%) from other nucleotide sequences available in the GenBank database, the Cryptosporidium taxon from the giant panda was inferred to be a novel genotype, designated Cryptosporidium ‘giant panda genotype’ (Liu et al., 2013). Subsequently, a study of a larger sample size (n = 322) confirmed the presence of Cryptosporidium infection in captive and wild giant panda populations, recording prevalences of 15.6% (19/122) and 0.5% (1/200), respectively (Wang et al., 2015). Interestingly, a genetic analysis of partial 18S rRNA gene sequence data revealed that Cryptosporidium from these pandas was more similar genetically (94.1%–99.8%) to C. andersoni from cattle than to the Cryptosporidium ‘giant panda genotype' (84.6%–89.8% similarity) (Liu et al., 2013), even though most test-positive samples (n = 17) were collected from the same conservation centre (i.e. CCRCGP). This finding raises a question about the spectrum of Cryptosporidium genotypes that might infect the giant panda in different environments, geographical localities and times of the year. However, the authors (Wang et al., 2015) suggested that seasonal variation in the distribution of Cryptosporidium might be a possible reason for this finding. In addition, these authors reported a higher prevalence of Cryptosporidium infection in captive than in wild giant panda populations. Whether this difference relates to a high degree of transmission of Cryptosporidium among giant pandas or between other animals and pandas in a captive (high population density) environment remains to be elucidated. Although no clinical signs, such as diarrhoea (often associated with cryptosporidiosis), were observed in either of these two studies (Liu et al., 2013; Wang et al., 2015), systematic investigations should be performed to gain a better understanding of the pathogenicity/virulence of Cryptosporidium in the giant panda as a basis for improved prevention or control in the event of clinical outbreaks.
Recently, E. bieneusi was identified in captive giant pandas in Shanxi province, with an estimated prevalence of 9% (4/46) (Tian et al., 2015). Subsequent phylogenetic analysis based on the nuclear ITS rDNA sequences of E. bieneusi suggested that a novel genotype I-like E. bieneusi occurs in giant pandas. Although E. bieneusi is known as an emerging and opportunistic enteric pathogen, causing diarrhoea in humans and animals, and progress has been made in our knowledge of the epidemiology of E. bieneusi, the transmission routes of this pathogen are still unclear (Matos et al., 2012; Santín, 2015). Given the possibility of transmission via respiratory secretions (Mathis et al., 2005) and the potentially broad host range of this pathogen (Santín and Fayer, 2009), whether this new genotype I-like E. bieneusi has a specific host affiliation to the giant panda remains to be explored. Similar to the reports of Cryptosporidium infection in giant pandas, no associated clinical signs were observed in E. bieneusi-infected animals (Tian et al., 2015).
Meanwhile, other than asymptomatic Cryptosporidium and Enterocytozoon infections in giant pandas, there is one report of an acute, fatal toxoplasmosis case, characterised by serious respiratory and gastroenteritis symptoms (Ma et al., 2015). Here, a 7-year-old giant panda was found dead at Zhengzhou Zoo, China (Ma et al., 2015). The necropsy findings, and serological results from an immunofluorescence assay and a modified agglutination test as well as PCR results for DNA from tissue biopsy samples (from liver, spleen, lungs, kidneys and intestines) all indicated that the giant panda had died from acute toxoplasmosis. Additional multilocus, nested PCR–RFLP analysis, using 10 genetic markers (SAG1, SAG2, SAG3, BTYB, GRA6, c22-8, c29-2, L358, PK1 and Apico; see Dubey et al., 2007), provided evidence that the T. gondii isolate causing this panda's death represented an atypical genotype (with reference to strains GT1, PTG, CTG, MAS, TgCgCal, TgCatBr5, TaCatBr40, TgCatBr64 and TgRsCr1). The findings suggested that a T. gondii-infected stray cat or rodents in the zoo might have been the source of infection in this fatal case (Ma et al., 2015).
3. Conclusions and a Perspective on Future Research
This chapter has reviewed current information on parasites of the giant panda, but it seems that we have only just ‘scratched the surface’ when it comes to research of the giant panda. Therefore, in our opinion, there is a need to conduct research in the following areas: (1) detailed genetic comparison of the two subspecies of giant panda using advanced genomic sequencing and analytical approaches employing the genomic resources available for the panda (cf. Li et al., 2010); (2) more morphological and molecular studies of parasites of the giant panda, improved (genetic) classification of known taxa, as well as detailed molecular epidemiological studies to assess the prevalence and distribution of parasites in captive and wild populations; (3) identification and characterisation of any emerging parasites and other pathogens using noninvasive sampling and PCR-based molecular or next-generation sequencing tools (cf. Korhonen et al., 2016); (4) studies to improve our understanding of the fundamental biology and molecular biology of B. schroederi; (5) investigations to assess whether anthelmintic resistance is emerging in B. schroederi and whether it is spread in the field and (6) work towards improved methods for the prevention or control of baylisascariasis (e.g. vaccination).
Without an accurate catalogue of which parasite species exist in wildlife, and a deep understanding of their life cycles, biology and the diseases that they cause, it is challenging to evaluate their risk to animal health (Colwell et al., 2009). Although numerous parasite taxa (n = 29) have been detected/recorded in the giant panda (Table 2), many of them, such as Sarcocystis, Strongyloides and lungworm (see Table 2), have not been described in any detail, and, to our knowledge, no voucher specimens are readily accessible in China. In our opinion, it should be a priority to classify known parasite taxa using international taxonomic rules. In addition, studies are needed to understand the biology and the epidemiology of these parasites, to guide conservation decisions.
Previous observations of B. schroederi infection in baby giant pandas (< 2 months) indicate the possibility of transplacental transmission (Yang, 1993), but there is no direct evidence of this mode. Thus, it would be interesting to undertake studies to critically assess whether B. schroederi can undergo transplacental and/or transmammary transmission. In addition, a major impediment to large-scale epidemiological investigations of giant panda parasites is the difficulty of assessing infection status in free-ranging populations. This limitation seems to have been somewhat overcome by the development of a PCR-based diagnostic approach (see Section 2.1.1.4; Wang et al., 2013a) for the simultaneous genetic ‘fingerprinting’ of individual pandas (see Section 2.1.1.2; Zhang et al., 2011) and the detection of their parasites in faecal samples, which could be used for field studies, in order to explore the distribution and dynamics of parasitic infections/diseases. Moreover, it would not be surprising to detect new parasite species using PCR-based or high-throughput DNA sequencing technology, considering the recent detection of new genotypes of protists (i.e. Cryptosporidium, Enterocytozoon and Toxoplasma) in the giant panda (Liu et al., 2013; Ma et al., 2015; Tian et al., 2015; Wang et al., 2015), which could not be characterised using conventional parasitological methods.
There is no doubt that baylisascariasis continues to cause serious health problems in the giant panda and will likely remain one of the biggest challenges for the conservation of this animal. Although modern anthelmintics appear to be reasonably effective for the treatment of baylisascariasis, the dissemination of large numbers of eggs into the environment and the resilience of these thick-shelled eggs make this disease/infection challenging to control B. schroederi without the implementation of an integrated approach, including management components (pen cleaning protocols and housing infrastructure) and regular monitoring for infection in different age groups of panda. Because of the potential for anthelmintic resistance to develop in B. schroederi, as a consequence of routine and excessive use of anthelmintics in captive animals, an integrated approach for baylisascariasis control needs to be explored. Such approaches might include the use of effective disinfectants (against the egg stage) to block transmission, new drugs with different modes of action and/or vaccination. Early work showed that the disinfectant neopredisan (active constituent: chlorocresol or p-chloro-m-cresol) has a high efficacy (100%) against A. suum eggs under laboratory conditions (Mielke and Hiepe, 1998). Whether this chemical has similar efficacy against B. schroederi eggs remains to be determined.
On the other hand, lessons learned from previous attempts to develop vaccines against ascaridoid nematodes (Matsumoto et al., 2009; Tsuji et al., 2004) indicate that it is critical to gain knowledge of the immunobiology of the parasite and to ensure that any vaccine candidate consistently induces high-level and long-lasting protective immunity (Geldhof et al., 2007). Also, a deep knowledge of parasite-derived molecules involved in vital developmental processes in Baylisascaris, host–parasite interactions and mechanism by which this nematode develops and survives within the host might assist in defining novel candidate vaccine targets. Clearly, developing an anti-Baylisascaris vaccine for an endangered animal, such as the giant panda, presents considerable challenges, such that immunogens might need to be selected based on consistent protection and immunorecognition in different laboratory (paratenic) hosts (e.g. mice, rodents and/or rabbits) prior to any well-controlled trials (without experimental challenge infection) in captive panda populations in which B. schroederi is known to be highly endemic, to assess whether a reduction in prevalence and intensity of infection is achievable.
In the last three decades, multidrug resistance has emerged across five continents in endoparasites and ectoparasites of animals and, to some extent, those of humans (Blake and Coles, 2007; Coles et al., 1994; Geary, 2005; Kaplan, 2004; McNair, 2015; Srivastava and Misra-Bhattacharya, 2015; Vercruysse et al., 2011). Given that anthelmintics are often used routinely and excessively in a suppressive manner in captive animal populations, there is a probability that drug resistance is emerging (cf. Geary, 2005). If this is the case for B. schroederi, what are the implications of this when captive animals are released into the wild? In the first instance, it seems pertinent to assess B. schroederi for resistance to commonly used anthelmintics using a faecal egg count reduction testing (FECRT) protocol similar to that recommended by the World Association for the Advancement of Veterinary Parasitology (WAAVP) for livestock animals (Coles et al., 2006). The presence of anthelmintic resistance in B. schroederi would make immunoprevention via vaccination even more attractive.
Apart from addressing some of these issues, there is an opportunity to pool expertise from a range of key experts in parasitology, in order to address some of the salient parasite problems and management issues to ensure that the health of captive giant pandas is maximised. By sharing new knowledge and information, this would not only significantly increase the utilisation of available data and clinical information but also enhance research opportunities to ensure the conservation of the giant panda. Although this animal is critically endangered, this review indicates, surprisingly, that parasitological research of this animal is in its infancy. Thus, much can be done to contribute towards the conservation of this iconic species. Although major public and scientific attention has focused on the conservation of the giant panda, with injections of funding from a number of bodies including the Giant Panda Conservation Foundation, it seems that very little funding is spent on the research of infectious (including parasitic) diseases of this species. Therefore, we strongly recommend that more attention be paid to the diseases that are likely to threaten this animal's conservation. We strongly believe that there is little room for error at this time point in history.
Acknowledgement
The support from the International Cooperation Project of Sichuan Science and Technology Department (Program No. 2014HH0047; T.W.) is gratefully acknowledged.
References
- Araujo S.B., Braga M.P., Brooks D.R., Agosta S.J., Hoberg E.P., von Hartenthal F.W., Boeger W.A. Understanding host-switching by ecological fitting. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake N., Coles G. Flock cull due to anthelmintic-resistant nematodes. Vet. Rec. 2007;161:36. doi: 10.1136/vr.161.1.36-b. [DOI] [PubMed] [Google Scholar]
- Bochkov A.V., Klimov P.B., Hestvik G., Saveljev A.P. Integrated Bayesian species delimitation and morphological diagnostics of chorioptic mange mites (Acariformes: Psoroptidae: Chorioptes) Parasitol. Res. 2014;113:2603–2627. doi: 10.1007/s00436-014-3914-9. [DOI] [PubMed] [Google Scholar]
- Chen K.L., Shi X.Q. Identification of Haemaphysalis longicornis and H. megaspinosa in giant panda. Shanghai Commun. Anim. Husb. Vet. Med. 1992;1:8. (In Chinese) [Google Scholar]
- Cheng W.Y., Zhao G.H., Jia Y.Q., Bian Q.Q., Du S.Z., Fang Y.Q., Qi M.Z., Yu S.K. Characterization of Haemaphysalis flava (Acari: Ixodidae) from Qingling subspecies of giant panda (Ailuropoda melanoleuca qinlingensis) in Qinling Mountains (Central China) by morphology and molecular markers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles G.C., Borgsteede F.H., Geerts S. Recommendations for the control of anthelmintic resistant nematodes of farm animals in the EU. Vet. Rec. 1994;134:205–206. doi: 10.1136/vr.134.9.205. [DOI] [PubMed] [Google Scholar]
- Coles G.C., Jackson F., Pomroy W.E., Prichard R.K., von Samson-Himmelstjerna G., Silvestre A., Taylor M.A., Vercruysse J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006;136:167–185. doi: 10.1016/j.vetpar.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Colwell D.D., Otranto D., Stevens J.R. Oestrid flies: eradication and extinction versus biodiversity. Trends Parasitol. 2009;25:500–504. doi: 10.1016/j.pt.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Sundar N., Gennari S.M., Minervino A.H.H., Farias N.A.D.R., Ruas J.L., Dos Santos T.R.B., Cavalcante G.T., Kwok O.C.H., Su C. Biologic and genetic comparison of Toxoplasma gondii isolates in free-range chickens from the northern Para state and the southern state Rio Grande do Sul, Brazil revealed highly diverse and distinct parasite populations. Vet. Parasitol. 2007;143:182–188. doi: 10.1016/j.vetpar.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Fain A., Leclerc M. A case of mange in a giant panda caused by a new species of Chorioptes (Acarina: Psoroptidae) Acarologia. 1975;17:177–182. (In French) [PubMed] [Google Scholar]
- Feng W.H., Hu T.Q., Bi F.Z., Chui Y.T., He G.X., Ye Z.Y. A study on the endangering causes of giant panda. Anim. World. 1985;2:1–7. (In Chinese) [Google Scholar]
- Galtier N., Nabholz B., Glémin S., Hurst G.D. Mitochondrial DNA as a marker of molecular diversity: a reappraisal. Mol. Ecol. 2009;18:4541–4550. doi: 10.1111/j.1365-294X.2009.04380.x. [DOI] [PubMed] [Google Scholar]
- Geary T.G. Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol. 2005;21:530–532. doi: 10.1016/j.pt.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Geldhof P., De Maere V., Vercruysse J., Claerebout E. Recombinant expression systems: the obstacle to helminth vaccines? Trends Parasitol. 2007;23:527–532. doi: 10.1016/j.pt.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Graeff-Teixeira C., Morassutti A.L., Kazacos K.R. Update on baylisascariasis, a highly pathogenic zoonotic infection. Clin. Microbiol. Rev. 2016;2016(29):375–399. doi: 10.1128/CMR.00044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.D., Liu S.X., Chen X.H. First report of parasites of giant panda from Qinling; Selected Acadmic Papers of Giant Panda Disease Control; Beijing, China: China Forestry Press; 1987. pp. 25–29. (In Chinese) [Google Scholar]
- He G., Wang T., Yang G., Fei Y., Zhang Z., Wang C., Yang Z., Lan J., Luo L., Liu L. Sequence analysis of Bs-Ag2 gene from Baylisascaris schroederi of giant panda and evaluation of the efficacy of a recombinant Bs-Ag2 antigen in mice. Vaccine. 2009;27:3007–3011. doi: 10.1016/j.vaccine.2009.02.077. [DOI] [PubMed] [Google Scholar]
- He G., Chen S., Wang T., Yan Y., Zhang Z., Li D., Yu H., Xie Y., Wang C., Gu X., Wang S., Peng X., Yang G. Sequence analysis of the Bs-Ag1 gene of Baylisascaris schroederi from the giant panda and an evaluation of the efficacy of a recombinant Baylisascaris schroederi Bs-Ag1 antigen in mice. DNA Cell Biol. 2012;31:1174–1181. doi: 10.1089/dna.2011.1395. [DOI] [PubMed] [Google Scholar]
- Hestvik G., Zahler-Rinder M., Gavier-Widen D., Lindberg R., Mattsson R., Morrison D., Bornstein S. A previously unidentified Chorioptes species infesting outer ear canals of moose (Alces alces): characterization of the mite and the pathology of infestation. Acta Vet. Scand. 2007;49:21. doi: 10.1186/1751-0147-49-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z.J., He S.W., Zhang C.S., Ma J.Z. Reseach advances in the knowledge of Baylisascaris schroederi of the giant panda. Jiangsu Agr. Sci. 2012;40:215–217. (In Chinese) [Google Scholar]
- Hvistendahl M. Captive pandas succumb to killer virus. Science. 2015;347:700–701. doi: 10.1126/science.347.6223.700. [DOI] [PubMed] [Google Scholar]
- Islam M.K., Miyoshi T., Kasuga-Aoki H., Isobe T., Arakawa T., Matsumoto Y., Tsuji N. Inorganic pyrophosphatase in the roundworm Ascaris and its role in the development and molting process of the larval stage parasites. Eur. J. Biochem. 2003;270:2814–2826. doi: 10.1046/j.1432-1033.2003.03658.x. [DOI] [PubMed] [Google Scholar]
- Islam M.K., Miyoshi T., Tsuji N. Vaccination with recombinant Ascaris suum 24-kilodalton antigen induces a Th1/Th2-mixed type immune response and confers high levels of protection against challenged Ascaris suum lung-stage infection in BALB/c mice. Int. J. Parasitol. 2005;35:1023–1030. doi: 10.1016/j.ijpara.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Kajander T., Kellosalo J., Goldman A. Inorganic pyrophosphatases: one substrate, three mechanisms. FEBS Lett. 2013;587:1863–1869. doi: 10.1016/j.febslet.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kazacos K.R. Baylisascaris procyonis and related species. In: Samuel W.M., Margo J.P., Kocan A.A., editors. Parasitic of Wild Mammals. Iowa State University Press; Ames, USA: 2008. pp. 301–341. [Google Scholar]
- Kazacos K.R. 2016. Baylisascaris Larva Migrans: U.S. Geological Survey Circular 1412. 122 p. [Google Scholar]
- Ko K.M., Lee W., Yu J.R., Ahnn J. PYP-1, inorganic pyrophosphatase, is required for larval development and intestinal function in C. elegans. FEBS Lett. 2007;581:5445–5453. doi: 10.1016/j.febslet.2007.10.047. [DOI] [PubMed] [Google Scholar]
- Korhonen P.K., Young N.D., Gasser R.B. Making sense of genomes of parasitic worms: tackling bioinformatic challenges. Biotechnol. Adv. 2016;34:663–686. doi: 10.1016/j.biotechadv.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Lai C.L., Yang M.L., Wang Q. Ectoparasites of the giant panda. Sichuan J. Zool. 1990;9:7. (In Chinese) [Google Scholar]
- Lai C.L., Qiu X.M., Luo X.F., Fan W.A. The internal parasitic infection in wild giant pandas (Ailuropoda melanoleuca) Expl. Nat. 1991;35:68–71. (In Chinese) [Google Scholar]
- Li J.H. Effects of cold on the development of Baylisascaris schroederi eggs. Chinese J. Vet. Sci. Technol. 1988;9:41–42. (In Chinese) [Google Scholar]
- Li J.H. The development of Baylisascaris schroederi larvae in mice. Chinese J. Vet. Sci. Technol. 1989;8:24–25. (In Chinese) [Google Scholar]
- Li J.H. Migration, distribution and development of larvae of panda ascarid, Baylisascaris schroederi, in mice. Acta Zool. Sin. 1990;36:236–243. (In Chinese) [Google Scholar]
- Li J.H. Observations on the pathogenicity of Baylisascaris schroederi in experimental mice. Chinese J. Zoooses. 1990;9:32–34. (In Chinese) [Google Scholar]
- Li J.H. Morphological observations on the larvae of panda Ascarid, Baylisascaris schroederi, in experimental host. J. Guiyang Med. Coll. 1993;18:184–187. (In Chinese) [Google Scholar]
- Li Y., Guo Z., Yang Q., Wang Y., Niemelä J. The implications of poaching for giant panda conservation. Biol. Conserv. 2003;111:125–136. [Google Scholar]
- Li D.S., Wang C.D., Yang G.Y. A case report describing the diagnosis and treatment of gastrointestinal myiasis in a giant panda. Progr. Vet. Med. 2007;28:115–116. (In Chinese) [Google Scholar]
- Li R., Fan W., Tian G., Zhu H., He L., Cai J., Huang Q., Cai Q., Li B., Bai Y., Zhang Z., Zhang Y., Wang W., Li J., Wei F., Li H., Jian M., Li J., Zhang Z., Nielsen R., Li D., Gu W., Yang Z., Xuan Z., Ryder O.A., Leung F.C., Zhou Y., Cao J., Sun X., Fu Y., Fang X., Guo X., Wang B., Hou R., Shen F., Mu B., Ni P., Lin R., Qian W., Wang G., Yu C., Nie W., Wang J., Wu Z., Liang H., Min J., Wu Q., Cheng S., Ruan J., Wang M., Shi Z., Wen M., Liu B., Ren X., Zheng H., Dong D., Cook K., Shan G., Zhang H., Kosiol C., Xie X., Lu Z., Zheng H., Li Y., Steiner C.C., Lam T.T., Lin S., Zhang Q., Li G., Tian J., Gong T., Liu H., Zhang D., Fang L., Ye C., Zhang J., Hu W., Xu A., Ren Y., Zhang G., Bruford M.W., Li Q., Ma L., Guo Y., An N., Hu Y., Zheng Y., Shi Y., Li Z., Liu Q., Chen Y., Zhao J., Qu N., Zhao S., Tian F., Wang X., Wang H., Xu L., Liu X., Vinar T., Wang Y., Lam T.W., Yiu S.M., Liu S., Zhang H., Li D., Huang Y., Wang X., Yang G., Jiang Z., Wang J., Qin N., Li L., Li J., Bolund L., Kristiansen K., Wong G.K., Olson M., Zhang X., Li S., Yang H., Wang J., Wang J. The sequence and de novo assembly of the giant panda genome. Nature. 2010;463:311–317. doi: 10.1038/nature08696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Niu L., Wang Q., Zhang Z., Chen Z., Gu X., Xie Y., Yan N., Wang S., Peng X., Yang G. Molecular characterization and phylogenetic analysis of ascarid nematodes from twenty-one species of captive wild mammals based on mitochondrial and nuclear sequences. Parasitology. 2012;139:1329–1338. doi: 10.1017/S003118201200056X. [DOI] [PubMed] [Google Scholar]
- Li D.S., He Y., Wu H.L., Wang C.D., Li C.W., Lan J.C., Chen Z.Q., Xie Y., Han H.Y., Yang G.Y., Wang C.D. Prevalence of helminths in captive giant pandas. J. Econ. Anim. 2014;4:214–216. (In Chinese) [Google Scholar]
- Li D.S., He Y., Deng L.H., Chen Z.Q., Cheng Y.X., Xie Y., Han H.Y., Yang G.Y., Wang C.D. Comparison of the efficacy of ivermectin and pyrantel pamoate for the control of Baylisascaris schroederi infection in the giant panda. Anim. Husb. Vet. Med. 2015;47:87–90. (In Chinese) [Google Scholar]
- Lin Q., Li H.M., Gao M., Wang X.Y., Ren W.X., Cong M.M., Tan X.C., Chen C.X., Yu S.K., Zhao G.H. Characterization of Baylisascaris schroederi from Qinling subspecies of giant panda in China by the first internal transcribed spacer (ITS-1) of nuclear ribosomal DNA. Parasitol. Res. 2012;110:1297–1303. doi: 10.1007/s00436-011-2618-7. [DOI] [PubMed] [Google Scholar]
- Liu W.Z., Yang G.C. Dignosis and treatment of baylisascariasis in the giant panda. Henan Anim. Husb. Vet. Med. 1994;15:48. (In Chinese) [Google Scholar]
- Liu X., He T., Zhong Z., Zhang H., Wang R., Dong H., Wang C., Li D., Deng J., Peng G., Zhang L. A new genotype of Cryptosporidium from giant panda (Ailuropoda melanoleuca) in China. Parasitol. Int. 2013;62:454–458. doi: 10.1016/j.parint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Loeffler K., Montali R.J., Rideout B.A. Diseases and pathology of giant pandas. In: Wildt D.E., Zhang A., Zhang H., Janssen D.L., Ellis S., editors. Giant Pandas: Biology, Veterinary Medicine and Management. Cambridge University Press; New York, USA: 2006. pp. 377–409. [Google Scholar]
- Ma G.Y. Records of Baylisascaris schroederi and ticks in the giant panda from Wenxian, Gansu province. Sichuan J. Zool. 1987;6:34. (In Chinese) [Google Scholar]
- Ma H., Wang Z., Wang C., Li C., Wei F., Liu Q. Fatal Toxoplasma gondii infection in the giant panda. Parasite. 2015;22:30. doi: 10.1051/parasite/2015030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainka S.A., Qiu X., He T., Appel M.J. Serologic survey of giant pandas (Ailuropoda melanoleuca), and domestic dogs and cats in the Wolong Reserve, China. J. Wildl. Dis. 1994;30:86–89. doi: 10.7589/0090-3558-30.1.86. [DOI] [PubMed] [Google Scholar]
- Mathis A., Weber R., Deplazes P. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 2005;18:423–445. doi: 10.1128/CMR.18.3.423-445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos O., Lobo M.L., Xiao L. Epidemiology of Enterocytozoon bieneusi infection in humans. J. Parasitol. Res. 2012;2012 doi: 10.1155/2012/981424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Suzuki S., Nozoye T., Yamakawa T., Takashima Y., Arakawa T., Tsuji N., Takaiwa F., Hayashi Y. Oral immunogenicity and protective efficacy in mice of transgenic rice plants producing a vaccine candidate antigen (As16) of Ascaris suum fused with cholera toxin B subunit. Transgenic Res. 2009;18:185–192. doi: 10.1007/s11248-008-9205-4. [DOI] [PubMed] [Google Scholar]
- Maung M. The occurrence of the second moult of Ascaris lumbricoides and Ascaris suum. Int. J. Parasitol. 1978;8:371–378. doi: 10.1016/0020-7519(78)90035-8. [DOI] [PubMed] [Google Scholar]
- McIntosh A. A new nematode, Ascaris schroederi, from a giant panda, Ailuropoda melanoleuca. Fortschr. Zool. 1939;24:355–357. [Google Scholar]
- McNair C.M. Ectoparasites of medical and veterinary importance: drug resistance and the need for alternative control methods. J. Pharm. Pharmacol. 2015;67:351–363. doi: 10.1111/jphp.12368. [DOI] [PubMed] [Google Scholar]
- Mielke D., Hiepe T. The effectiveness of different disinfectants based on p-chloro-m-cresol against Ascaris suum eggs under laboratory conditions. Berl. Munch. Tierarztl. Wochenschr. 1998;111:291–294. (In German) [PubMed] [Google Scholar]
- Pan X.W., Yan Y.X., Lv J.Y., Ma Q., Yuan Y.H. Studies on the pathogens of sudden death in panda. Chinese J. Zoonoses. 2001;17:53–55. (In Chinese) [Google Scholar]
- Peng D.W., Huang S., Wang J., Wei F., Xue G.F. Investigation of helminths of rare wild animals in the Shanghai Zoo. Chinese J. Zoonoses. 1989;5:62–63. (In Chinese) [Google Scholar]
- Pérez Mata A., García Pérez H., José Gauta Parra J. Morphological and molecular description of Baylisascaris venezuelensis, n. sp. from a natural infection in the South American spectacled bear Tremarctos ornatus Cuvier, 1825 in Venezuela. Neotrop. Helminthol. 2016;10:85–103. (In Spainsh) [Google Scholar]
- Qin Q., Li D., Zhang H., Hou R., Zhang Z., Zhang C., Zhang J., Wei F. Serosurvey of selected viruses in captive giant pandas (Ailuropoda melanoleuca) in China. Vet. Microbiol. 2011;142:199–204. doi: 10.1016/j.vetmic.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X.M. The giant panda's treatment and prevention from diseases [sic. Approaches for the treatment and prevention of diseases of the giant panda] J. Sichuan Teach. Coll. 1990;11:195–199. (In Chinese) [Google Scholar]
- Qiu X.M., Mainka S.A. Review of mortality of the giant panda (Ailuropoda melanoleuca) J. Zoo Wildl. Med. 1993;24:425–429. (In Chinese) [Google Scholar]
- Qiu M.H., Zhu C.J. Parasites of giant panda and their control; Selected Acadmic Papers of Giant Panda Disease Control; Beijing, China: China Forestry Press; 1987. pp. 1–9. (In Chinese) [Google Scholar]
- Qiu M.H., Zhou Y.H., Xiang P.L. Observations of mites from the giant panda. Sichuan J. Zool. 1984;2:36–37. (In Chinese) [Google Scholar]
- Qiu M.H., Wang D.Q., Li K.Z. A new flea of the genus Chaetopsylla from giant panda. Sichuan J. Zool. 1991;10:7–9. (In Chinese) [Google Scholar]
- Reid D.G., Hu J., Dong S., Wang W., Huang Y. Giant panda Ailuropoda melanoleuca behaviour and carrying capacity following a bamboo die-off. Biol. Conserv. 1989;49:85–104. [Google Scholar]
- Santín M. Enterocytozoon bieneusi. In: Xiao L., Ryan U., Feng Y., editors. Biology of Foodborne Parasites. CRC Press; Boca Raton, USA: 2015. pp. 149–174. [Google Scholar]
- Santín M., Fayer R. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: a consensus. J. Eukaryot. Microbiol. 2009;56:34–38. doi: 10.1111/j.1550-7408.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- Schaller G.B., Hu J.C., Pan W.S., Zhu J. University of Chicago Press; Chicago, USA: 1985. The Giant Pandas of Wolong. [Google Scholar]
- Shan L., Hu Y., Zhu L., Yan L., Wang C., Li D., Jin X., Zhang C., Wei F. Large-scale genetic survey provides insights into the captive management and reintroduction of giant pandas. Mol. Biol. Evol. 2014;31:2663–2671. doi: 10.1093/molbev/msu210. [DOI] [PubMed] [Google Scholar]
- Song J.K., Wang Z.H., Wang H.B., Peng X.Q., Wang X.T., Ren G.J., Zhao G.H. Identification and phylogenetic analysis of Ogmocotyle sp. from giant panda in Qinling Mountain. Chinese J. Vet. Sci. 2016;36:563–566. (In Chinese) [Google Scholar]
- Sprent J.F. Notes on Ascaris and Toxascaris, with a definition of Baylisascaris gen.nov. Parasitology. 1968;58:185–198. doi: 10.1017/s0031182000073534. [DOI] [PubMed] [Google Scholar]
- Srivastava M., Misra-Bhattacharya S. Overcoming drug resistance for macro parasites. Future Microbiol. 2015;10:1783–1789. doi: 10.2217/fmb.15.73. [DOI] [PubMed] [Google Scholar]
- Swaisgood R.R., Zhang G.Q., Zhou X.P., Zhang H.M. The science of behavioural management: creating biologically relevant living environments in captivity. In: Wildt D.E., Zhang A., Zhang H., Janssen D.L., Ellis S., editors. Giant Pandas: Biology, Veterinary Medicine and Management. Cambridge University Press; New York, USA: 2006. pp. 377–409. [Google Scholar]
- Talbot S.L., Shields G.F. A phylogeny of the bears (Ursidae) inferred from complete sequences of three mitochondrial genes. Mol. Phylogenet. Evol. 1996;5:567–575. doi: 10.1006/mpev.1996.0051. [DOI] [PubMed] [Google Scholar]
- The State Forestry Administration of China, 2015. The results of the fourth national giant panda survey (Report). (In Chinese)
- Tian G.R., Zhao G.H., Du S.Z., Hu X.F., Wang H.B., Zhang L.X., Yu S.K. First report of Enterocytozoon bieneusi from giant pandas (Ailuropoda melanoleuca) and red pandas (Ailurus fulgens) in China. Infect. Genet. Evol. 2015;34:32–35. doi: 10.1016/j.meegid.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Tokiwa T., Nakamura S., Taira K., Une Y. Baylisascaris potosis n. sp., a new ascarid nematode isolated from captive kinkajou, Potos flavus, from the Cooperative Republic of Guyana. Parasitol. Int. 2014;63:591–596. doi: 10.1016/j.parint.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Tranbenkova N.A., Spiridonov S.E. Molecular characterization of Baylisascaris devosi Sprent, 1952 (Ascaridoidea, Nematoda) from Kamchatka sables. Helminthologia. 2017;54:105–112. [Google Scholar]
- Tsuji N., Suzuki K., Kasuga-Aoki H., Matsumoto Y., Arakawa T., Ishiwata K., Isobe T. Intranasal immunization with recombinant Ascaris suum 14-kilodalton antigen coupled with cholera toxin B subunit induces protective immunity to A. suum infection in mice. Infect. Immun. 2001;69:7285–7292. doi: 10.1128/IAI.69.12.7285-7292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji N., Kasuga-Aoki H., Isobe T., Arakawa T., Matsumoto Y. Cloning and characterisation of a highly immunoreactive 37 kDa antigen with multi-immunoglobulin domains from the swine roundworm Ascaris suum. Int. J. Parasitol. 2002;32:1739–1746. doi: 10.1016/s0020-7519(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Tsuji N., Suzuki K., Kasuga-Aoki H., Isobe T., Arakawa T., Matsumoto Y. Mice intranasally immunized with a recombinant 16-kilodalton antigen from roundworm Ascaris parasites are protected against larval migration of Ascaris suum. Infect. Immun. 2003;71:5314–5323. doi: 10.1128/IAI.71.9.5314-5323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji N., Miyoshi T., Islam M.K., Isobe T., Yoshihara S., Arakawa T., Matsumoto Y., Yokomizo Y. Recombinant Ascaris 16-Kilodalton protein-induced protection against Ascaris suum larval migration after intranasal vaccination in pigs. J. Infect. Dis. 2004;190:1812–1820. doi: 10.1086/425074. [DOI] [PubMed] [Google Scholar]
- Vercruysse J., Holdsworth P., Letonja T., Barth D., Conder G., Hamamoto K., Okano K., World Organization for Animal Health International harmonisation of anthelmintic efficacy guidelines. Vet. Parasitol. 2001;96:171–193. doi: 10.1016/s0304-4017(00)00443-x. [DOI] [PubMed] [Google Scholar]
- Vercruysse J., Holdsworth P., Letonja T., Conder G., Hamamoto K., Okano K., Rehbein S., Veterinary International Co-operation on Harmonisation Working Group on anthelmintic guidelines International harmonisation of anthelmintic efficacy guidelines (Part 2) Vet. Parasitol. 2002;103:277–297. doi: 10.1016/s0304-4017(01)00615-x. [DOI] [PubMed] [Google Scholar]
- Vercruysse J., Behnke J.M., Albonico M., Ame S.M., Angebault C., Bethony J.M., Engels D., Guillard B., Nguyen T.V., Kang G., Kattula D., Kotze A.C., McCarthy J.S., Mekonnen Z., Montresor A., Periago M.V., Sumo L., Tchuente L.A., Dang T.C., Zeynudin A., Levecke B. Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl. Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaminck J., Nejsum P., Vangroenweghe F., Thamsborg S.M., Vercruysse J., Geldhof P. Evaluation of a serodiagnostic test using Ascaris suum haemoglobin for the detection of roundworm infections in pig populations. Vet. Parasitol. 2012;189:267–273. doi: 10.1016/j.vetpar.2012.04.024. [DOI] [PubMed] [Google Scholar]
- Wan Q.H., Fang S.G., Wu H., Fujihara T. Genetic differentiation and subspecies development of the giant panda as revealed by DNA fingerprinting. Electrophoresis. 2003;24:1353–1359. doi: 10.1002/elps.200390174. [DOI] [PubMed] [Google Scholar]
- Wang C.D., Yang G.Y., Wang Q., Yu X.M., Zhao B., Zhong S.T. Experimental by closantel treatment of Chorioptes panda in the giant panda. Chinese J. Vet. Sci. Technol. 2000;30:39. (In Chinese) [Google Scholar]
- Wang C.D., Yang G.Y., Wang Q., Yu X.M., Zhao B., Lan J.C. Investigation of parasitic infections of rare mammals in Chengdu Zoo. Chinese J. Vet. Sci. 2001;37:21–22. (In Chinese) [Google Scholar]
- Wang C.D., Li D.S., Tan C.X., Deng L.H., Huang Z., Han H.Y., Zhang Y. Proteus mirabilis infection of the reproductive tract of a giant panda. Sichuan J. Zool. 2007;26:167. (In Chinese) [Google Scholar]
- Wang C.D., Tang C.X., Deng L.H., Li D.S. Case report of rectal prolapse associated with intussusception in a wild giant panda. Chinese J. Vet. Med. 2007;43:64–65. (In Chinese) [Google Scholar]
- Wang C.D., Yan Q.G., Zhang Z.H., Luo L., Fan W.Q., Yang Z., Lan J.C., Huang X.M., Li M.X. Isolation and identification of rotavirus from giant panda cubs. Acta Theriol. Sin. 2008;28:87–91. (In Chinese) [Google Scholar]
- Wang T., He G., Yang G., Fei Y., Zhang Z., Wang C., Yang Z., Lan J., Luo L., Liu L. Cloning, expression and evaluation of the efficacy of a recombinant Baylisascaris schroederi Bs-Ag3 antigen in mice. Vaccine. 2008;26:6919–6924. doi: 10.1016/j.vaccine.2008.09.079. [DOI] [PubMed] [Google Scholar]
- Wang S., Gu X., Fu Y., Lai S., Wang S., Peng X., Yang G. Molecular taxonomic relationships of Psoroptes and Chorioptes mites from China based on COI and 18S rDNA gene sequences. Vet. Parasitol. 2012;184:392–397. doi: 10.1016/j.vetpar.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Wang N., Li D.S., Zhou X., Xie Y., Liang Y.N., Wang C.D., Yu H., Chen S.J., Yan Y.B., Gu X.B., Wang S.X., Peng X.R., Yang G.Y. A sensitive and specific PCR assay for the detection of Baylisascaris schroederi eggs in giant panda feces. Parasitol. Int. 2013;62:435–436. doi: 10.1016/j.parint.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Wang X., Yan Q., Xia X., Zhang Y., Li D., Wang C., Chen S., Hou R. Serotypes, virulence factors, and antimicrobial susceptibilities of vaginal and fecal isolates of Escherichia coli from giant pandas. Appl. Environ. Microbiol. 2013;79:5146–5150. doi: 10.1128/AEM.01367-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Chen Z., Xie Y., Hou R., Wu Q., Gu X., Lai W., Peng X., Yang G. Prevalence and molecular characterization of Cryptosporidium in giant panda (Ailuropoda melanoleuca) in Sichuan province, China. Parasit. Vectors. 2015;8:344. doi: 10.1186/s13071-015-0953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F., Hu Y., Zhu L., Bruford M.W., Zhan X., Zhang L. Black and white and read all over: the past, present and future of giant panda genetics. Mol. Ecol. 2012;21:5660–5674. doi: 10.1111/mec.12096. [DOI] [PubMed] [Google Scholar]
- Wei F., Swaisgood R., Hu Y., Nie Y., Yan L., Zhang Z., Qi D., Zhu L. Progress in the ecology and conservation of giant pandas. Conserv. Biol. 2015;29:1497–1507. doi: 10.1111/cobi.12582. [DOI] [PubMed] [Google Scholar]
- Wu J., Hu H.G. Giant panda—a new host for Haemaphysalis warburtoni. Chinese J. Vet. Sci. 1985;8:10. (In Chinese) [Google Scholar]
- Wu J., Hu H.G. Recent advances in parasitic diseases of giant panda. Wildlife. 1985;5:42–43. (In Chinese) [Google Scholar]
- Wu J., Hu H.G. Reports of regular deworming of Baylisascaris schroederi. Anim. Husb. Vet. Med. 1988;3:118–119. (In Chinese) [Google Scholar]
- Wu J., Jiang Y.K., He G.Z., Wu G.Q., Zhang D.H., Hu H.G. Research of the life cycle of Baylisascaris schroederi of the giant panda. Chinese J. Vet. Sci. Technol. 1985;6:21–23. (In Chinese) [Google Scholar]
- Xie Y., Zhang Z., Wang C., Lan J., Li Y., Chen Z., Fu Y., Nie H., Yan N., Gu X., Wang S., Peng X., Yang G. Complete mitochondrial genomes of Baylisascaris schroederi, Baylisascaris ailuri and Baylisascaris transfuga from giant panda, red panda and polar bear. Gene. 2011;482:59–67. doi: 10.1016/j.gene.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Xie Y., Chen S., Yan Y., Zhang Z., Li D., Yu H., Wang C., Nong X., Zhou X., Gu X., Wang S., Peng X., Yang G. Potential of recombinant inorganic pyrophosphatase antigen as a new vaccine candidate against Baylisascaris schroederi in mice. Vet. Res. 2013;44:90. doi: 10.1186/1297-9716-44-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Zhou X., Zhang Z., Wang C., Sun Y., Liu T., Gu X., Wang T., Peng X., Yang G. Absence of genetic structure in Baylisascaris schroederi populations, a giant panda parasite, determined by mitochondrial sequencing. Parasit. Vectors. 2014;7:606. doi: 10.1186/s13071-014-0606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Zhou X., Chen L., Zhang Z., Wang C., Gu X., Wang T., Peng X., Yang G. Cloning and characterization of a novel sigma-like glutathione S-transferase from the giant panda parasitic nematode, Baylisascaris schroederi. Parasit. Vectors. 2015;8:44. doi: 10.1186/s13071-014-0629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Zhou X., Sun Y., Gu X., Yang G. Mitochondrial 12S-based analysis of genetic diversity of Baylisascaris schroederi in giant pandas from two mountain ranges in China. Acta Theriol. Sin. 2015;35:328–335. (In Chinese) [Google Scholar]
- Xie Y., Hoberg E.P., Yang Z., Urban J.F., Jr., Yang G. Ancylostoma ailuropodae n. sp. (Nematoda: Ancylostomatidae), a new hookworm parasite isolated from wild giant pandas in Southwest China. Parasit. Vectors. 2017;10:277. doi: 10.1186/s13071-017-2209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R.H., Zhang R.Y. Efficacy of drug treatment against mite infections in the giant panda. Fujian J. Anim. Husb. Vet. Med. 2002;24:55–56. (In Chinese) [Google Scholar]
- Xu Y.H., Xie H.X., Liu S.L., Zhou Z.Y., Shi X.Q. A new species of the genus Demodex (Acariformes: Demodicidae) Acta Zool. Sin. 1986;32:163–167. (In Chinese) [Google Scholar]
- Yang X.Y. Discussion of the relationship between Baylisascaris prevalence and habitat of wild giant pandas. Sichuan Forest. Sci. Technol. 1993;14:70–73. (In Chinese) [Google Scholar]
- Yang G.Y. Research advances in the parasite and parasitic disease of the giant panda. Chinese J. Vet. Sci. 1998;18:206–209. (In Chinese) [Google Scholar]
- Yang G.Y., Zhang Z.H. Science Press; Beijing, China: 2013. Wildlife Parasitology; pp. 458–465. (In Chinese) [Google Scholar]
- Yang W., Xu G.J., Lan J.C. Diagnosis and treatment of skin mite of the giant panda. Sichuan J. Zool. 2001;20:56–60. (In Chinese) [Google Scholar]
- Yang X., Yang J., Wang H., Li C., He Y., Jin S., Zhang H., Li D., Wang P., Xu Y., Xu C., Fan C., Xu L., Huang S., Qu C., Li G. Normal vaginal bacterial Flora of giant pandas (Ailuropoda melanoleuca) and the antimicrobial susceptibility patterns of the isolates. J. Zoo Wildl. Med. 2016;47:374–378. doi: 10.1638/2015-0203.1. [DOI] [PubMed] [Google Scholar]
- Ye Z.Y. Report of the treatment of itch mites in the giant panda. Chinese J. Vet. Sci. 1986;1:28–29. (In Chinese) [Google Scholar]
- Ye Z.Y. Fifty case reports on diseases and their treament in wild giant pandas. Chinese J. Vet. Sci. 1989;15:30–31. (In Chinese) [Google Scholar]
- Ye Z.Y., Zhang A.J. Discussion regarding the elimination of Baylisascaris schroederi. J. Zool. 1981;2:48–49. (In Chinese) [Google Scholar]
- Yeruham I., Rosen S., Hadani A. Chorioptic mange (Acarina: Psoroptidae) in domestic and wild ruminants in Israel. Exp. Appl. Acarol. 1999;23:861–869. doi: 10.1023/a:1006217016688. [DOI] [PubMed] [Google Scholar]
- Yu S.K., Feng N., Yuan W., Pu P., Li Y.Z., Ma Q.Y. Investigation of parasites of the giant panda, and a record of a new species. J. Chin. Agr. Univ. 1998;S2:117–118. (In Chinese) [Google Scholar]
- Zahler M., Hendrikx W.M., Essig A., Rinder H., Gothe R. Taxonomic reconsideration of the genus Chorioptes Gervais and van Beneden, 1859 (Acari: Psoroptidae) Exp. Appl. Acarol. 2001;25:517–523. doi: 10.1023/a:1011857802317. [DOI] [PubMed] [Google Scholar]
- Zhang H.M., Wang P.Y. China Forestry Publishing; Beijing, China: 2003. Breeding Research of Giant Panda; pp. 121–174. (In Chinese) [Google Scholar]
- Zhang H., Zhang Z.L. Diagnosis and treatment of giant panda ascarids. Gansu Anim. Vet. Sci. 2002;32:25–26. (In Chinese) [Google Scholar]
- Zhang J.S., Daszak P., Huang H.L., Yang G.Y., Kilpatrick A.M., Zhang S. Parasite threat to panda conservation. Ecohealth. 2008;5:6–9. doi: 10.1007/s10393-007-0139-8. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang X.H., Fan W.A., Yuan M. Literature review of the giant panda parasitic diseases. Gansu Anim. Vet. Sci. 2010;40:40–43. (In Chinese) [Google Scholar]
- Zhang L., Yang X., Wu H., Gu X., Hu Y., Wei F. The parasites of giant pandas: individual-based measurement in wild animals. J. Wildl. Dis. 2011;47:164–171. doi: 10.7589/0090-3558-47.1.164. [DOI] [PubMed] [Google Scholar]
- Zhang W., Yie S., Yue B., Zhou J., An R., Yang J., Chen W., Wang C., Zhang L., Shen F., Yang G., Hou R., Zhang Z. Determination of Baylisascaris schroederi infection in wild giant pandas by an accurate and sensitive PCR/CE-SSCP method. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wu Q., Hu Y., Wu H., Wei F. Major histocompatibility complex alleles associated with parasite susceptibility in wild giant pandas. Heredity (Edinb) 2015;114:85–93. doi: 10.1038/hdy.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G.H., Li H.M., Ryan U.M., Cong M.M., Hu B., Gao M., Ren W.X., Wang X.Y., Zhang S.P., Lin Q., Zhu X.Q., Yu S.K. Phylogenetic study of Baylisascaris schroederi isolated from Qinling subspecies of giant panda in China based on combined nuclear 5.8S and the second internal transcribed spacer (ITS-2) ribosomal DNA sequences. Parasitol. Int. 2012;61:497–500. doi: 10.1016/j.parint.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Zhao G.H., Xu M.J., Zhu X.Q. Identification and characterization of microRNAs in Baylisascaris schroederi of the giant panda. Parasit. Vectors. 2013;6:216. doi: 10.1186/1756-3305-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.H., Bian Q.Q., Ren W.X., Cheng W.Y., Jia Y.Q., Fang Y.Q., Zhao G.H. Genetic variability of Baylisascaris schroederi from the Qinling subspecies of the giant panda in China revealed by sequences of three mitochondrial genes. Mitochondrial DNA. 2014;25:212–217. doi: 10.3109/19401736.2013.792074. [DOI] [PubMed] [Google Scholar]
- Zhao N., Li M., Luo J., Wang S., Liu S., Wang S., Lyu W., Chen L., Su W., Ding H., He H. Impacts of canine distemper virus infection on the giant panda population from the perspective of gut microbiota. Sci. Rep. 2017;4 doi: 10.1038/srep39954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.H., Yang F.X., Zhu C.J., Zhao G.L. Study on the etiology, treatment and prevention of skin infection of baby panda. J. Chongqing Med. Univ. 1989;14:134–135. (In Chinese) [Google Scholar]
- Zhou X., Xie Y., Zhang Z.H., Wang C.D., Sun Y., Gu X.B., Wang S.X., Peng X.R., Yang G.Y. Analysis of the genetic diversity of the nematode parasite Baylisascaris schroederi from wild giant pandas in different mountain ranges in China. Parasit. Vectors. 2013;6:233. doi: 10.1186/1756-3305-6-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Yu H., Wang N., Xie Y., Liang Y.N., Li D.S., Wang C.D., Chen S.J., Yan Y.B., Gu X.B., Wang S.X., Peng X.R., Yang G.Y. Molecular diagnosis of Baylisascaris schroederi infections in giant panda (Ailuropoda melanoleuca) feces using PCR. J. Wildl. Dis. 2013;49:1052–1055. doi: 10.7589/2012-07-175. [DOI] [PubMed] [Google Scholar]
- Zhu L., Long Z. The vicissitudes of the giant panda. Acta Zool. Sin. 1983;29:90–104. (In Chinese) [Google Scholar]