Graphical abstract
Keywords: Phytochemistry, Pharmacology, Traditional usage, Toxicology, Research prospects
Abstract
Toona sinensis (Juss.) M.Roem, Meliaceae, a deciduous plant native to eastern and southeastern Asia, is widely used in Traditional Chinese Medicine. This paper was aimed to summarize the current advances in traditional usage, phytochemistry, pharmacology and toxicology of T. sinensis. In this review, various types of data of T. sinensis are discussed in the corresponding parts of this paper, and perspectives for possible future studies of this plant are discussed. The main constituents of T. sinensis are terpenoids, phenylpropanoids and flavonoids, etc., and its pharmacological activities include anti-tumor effects, antioxidant activities, anti-diabetic effects and anti-inflammatory effects. Although a series of phytochemical and pharmacological researches of this plant have been conducted, the active constituents and action mechanism of these activities should be also further explored. Furthermore, the present review also indicates that T. sinensis has potentials to develop into drugs for treating various diseases with high efficacy and low toxicity, particularly in cancer, diabetes and inflammatory disorders. In conclusion, the paper provides a full-scale profile of the traditional usage, phytochemistry, pharmacology and toxicology of T. sinensis, and also provides potential therapeutic uses and drug development prospects of this plant.
Introduction
Toona sinensis (Juss.) M.Roem [synonyms: Cedrela sinensis Juss, xiāngchūn (in Chinese)], belonging to Meliaceae family and popularly known as Chinese toon or Chinese mahogany, is a deciduous woody plant native to eastern and southeastern Asia (Liao et al., 2009). T. sinensis has a cultivation history more than 2000 years and is used as a vegetable source in China and Malaysia, and as animal fodder in India (Liao et al., 2007). T. sinensis is also widely used in Traditional Chinese Medicine (TCM) and various parts tissues of this plant have been used for a wide variety of diseases. The stems and leaves of TSR are traditionally used for the treatment of dysentery, enteritis, carminative and itchiness, etc. (Dong et al., 2013a, Dong et al., 2013b). The roots are used as correctives, the bark is used as astringent and depurative, and the fruits are used as astringent for the treatment of eye infections (Perry, 1980). Previous phytochemical investigations on this plant have revealed that the main constituents include terpenoids, phenylpropanoids, flavonoids and anthraquinones (Feng et al., 2007, Mu et al., 2007, Hsieh et al., 2008). Modern researches have also reported that T. sinensis possessed various pharmacological activities including anti-tumor effects, antioxidant effects, anti-diabetic effects, anti-inflammatory effects, antibacterial and antiviral effects. (Chen et al., 2007, Cheng et al., 2009, Wu et al., 2010) (Fig. 1 ).
Fig. 1.
Toona sinensis Roem. (A) Whole plant of Toona sinensis. (B) Leaves of T. sinensis.
Currently, T. sinensis has aroused considerable public interests in its medicinal and food uses, as well as novel terpenoids compounds. However, there is no systemic review on its recent traditional uses, chemical constituents, pharmacological activities and toxicological aspects; moreover, few current available literatures could suggest what working directions should be devoted to this plant in the future. Consequently, this paper was aimed to summarize the current advances in traditional usage, phytochemistry, pharmacology and toxicology of T. sinensis; furthermore, the present paper also provides some discussions to propose potential future development perspectives of this plant.
Traditional usage
Toona sinensis has been used as a natural herbal medicine for thousands years based on its reliable pharmacological effects. The medicinal use of this plant was firstly recorded in Tang materia medica which is a famous TCM monograph written in Tang dynasty in China (Anonymous, 1999, Wang et al., 2014a, Wang et al., 2014b). In Chinese folk medicine, T. sinensis was described as an herbal medicine with good anti-inflammatory, detoxifying and hemostatic effects, and thus this plant was commonly used to treat enteritis, dysentery, urinary tract infection, leukorrheal diseases and skin itch (Anonymous, 1977, Anonymous, 1999, Li et al., 2006). Furthermore, due to its special onion-like flavor and wealth of carotene and vitamins B and C, the edible leaves and young shoots of T. sinensis are also delicious and nutritious food stuff in China and other Southeast Asia countries (Anonymous, 1999, Dong et al., 2013a).
Phytochemistry
In the early 1970s, compounds such as toosendanin (1), sterol and vitamins have been reported from the leaves and barks of T. sinensis in China (Anonymous, 1972). From then on, the phytochemical constituents of T. sinensis have been comprehensively investigated. So far, over one hundred compounds have been isolated and identified from this plant, including terpenoids, phenylpropanoids, and flavonoids. In this section, we described the main chemical components of T. sinensis, the corresponding isolation parts of these compounds were also concluded in Box 1 .
Box 1.
Chemical compounds isolated from Toona sinensis.
| Classification | No. | Chemical component | Part of plant | References |
|---|---|---|---|---|
| Terpenoids | 1 | 3-Oxo-12-en-28-oic acid | Leaves | Yang et al. (2013) |
| 2 | α-Betulin | Roots |
Dong et al. (2013a) Yang et al. (2013) |
|
| 3 | Ursolic acid | Leaves | Yang et al. (2013) | |
| 4 | Betulonic acid | Leaves | Yang et al. (2013) | |
| 5 | Betulic acid | Leaves | Yang et al. (2013) | |
| 6 | 11α-Hydroxygedunin | Barks | Mitsui et al. (2006) | |
| 7 | 11β-Hydroxygedunin | Barks | Mitsui et al. (2006) | |
| 8 | 7-Deacetoxy-7α,11α-dihydroxygedunin | Barks | Mitsui et al. (2006) | |
| 9 | 7-Deacetoxy-7α,11β-dihydroxygedunin | Barks | Mitsui et al. (2006) | |
| 10 | Gedunin | Barks | Mitsui et al. (2006) | |
| 11 | 7-Deacetoxy-7α-hydroxygedunin | Barks | Mitsui et al. (2006) | |
| 12 | 7-Deacetylgedunin | Fruits | Chen et al. (2017a) | |
| 13 | 11-Oxo-gedunin | Barks | Mitsui et al. (2006) | |
| 14 | Toonins A | Roots | Dong et al. (2013a) | |
| 15 | Proceranone | Roots | Dong et al. (2013a) | |
| 16 | 6-Acetoxyobacunol acetate | Leaves | Luo et al. (2000) | |
| 17 | 7α-Acetoxy-dihydronomilin | Leaves | Luo et al. (2000) | |
| 18 | 11β-Hydroxy-7α-obacunyl acetate | Leaves | Mitsui et al. (2004) | |
| 19 | 11-Oxo-7α-obacunyl acetate | Leaves | Mitsui et al. (2004) | |
| 20 | 11-Oxo-7a-obacunol | Leaves | Mitsui et al. (2004) | |
| 21 | 11β-Hydroxycneorin G | Leaves | Mitsui et al. (2004) | |
| 22 | 11β-Oxocneorin G | Leaves | Mitsui et al. (2004) | |
| 23 | Cedrellin | Leaves | Luo et al. (2000) | |
| 24 | Toonins B | Roots | Dong et al. (2013a) | |
| 25 | Grandifoliolenone | Barks | Mitsui et al. (2007) | |
| 26 | Bourjotinolone A | Roots | Dong et al. (2013a) | |
| 27 | Toona triterpenoids A | Barks | Mitsui et al. (2007) | |
| 28 | Toona triterpenoids B | Barks | Mitsui et al. (2007) | |
| 29 | Piscidinol A | Barks | Mitsui et al. (2007) | |
| 30 | Hispidol B | Barks | Mitsui et al. (2007) | |
| 31 | 20-Hydroxy-24-dammaren-3-one | Barks | Tang et al. (2016) | |
| 32 | (20S)-3-oxo-tirucalla-25-nor-7-en-24-oic acid | Barks | Tang et al. (2016) | |
| 33 | (20S)-5α,8α-epidioxy-3-oxo-24-nor-6.9(11)-dien-23-oic acid | Barks | Tang et al. (2016) | |
| 34 | Ocotillone | Barks | Tang et al. (2016) | |
| 35 | (20S,24R)-epoxydammarane-12.25-diol-3-one | Barks | Tang et al. (2016) | |
| 36 | (20S,24R)-epoxydammarane-3β,25-diolmarane-3β,25-diol | Barks | Tang et al. (2016) | |
| 37 | Methyl shoreate | Barks | Tang et al. (2016) | |
| 38 | Shoreic acid | Barks | Tang et al. (2016) | |
| 39 | Richenone | Barks | Tang et al. (2016) | |
| 40 | Cabralealactone | Barks | Tang et al. (2016) | |
| 41 | Cylindrictone D | Barks | Tang et al. (2016) | |
| 42 | Hollongdione | Barks | Tang et al. (2016) | |
| 43 | 4,4,14-Trimethyl-3-oxo-24-nor-5α,13α,14β,17α,20S-chol-7-en-23-oic acid | Barks | Tang et al. (2016) | |
| 44 | (20S,24S)-dihydroxydammar-25-en-3-one | Barks | Tang et al. (2016) | |
| 45 | Bourjotinolone B | Barks | Tang et al. (2016) | |
| 46 | 21α-Methylmeliandiol | Barks | Mitsui et al. (2007) | |
| 47 | 21β-Methylmeliandiol | Barks | Mitsui et al. (2007) | |
| 48 | 3-O-acetyl-21R-O-methyltoosendanpentol | Barks | Mitsui et al. (2007) | |
| 49 | 3-O-acetyl-21S-O-methyltoosendanpentol | Barks | Mitsui et al. (2007) | |
| 50 | Sapellin E acetate | Barks | Mitsui et al. (2007) | |
| 51 | Azadirone | Barks | Mitsui et al. (2007) | |
| 52 | Toosendanin | Barks | Anonymous (1972) | |
| 53 | Phytol | Leaves | Luo et al. (2000) | |
| 54 | 2,6,10-Phytatriene-1,14,15-triol | Leaves | Luo et al. (2000) | |
| 55 | (2E, 6E, 10E)-3,7,11,15-tetramethylhexadeca-2,6-10-triene-1, 14,15-triol | Fruits | Hou et al. (2011) | |
| 56 | Eudesm-4(15)-ene-1β,6α-diol | Fruits | Hou et al. (2011) | |
| Phenylpropanoids | 57 | Cedralins A | Leaves | Lee et al. (2010) |
| 58 | Cedralins B | Leaves | Lee et al. (2010) | |
| 59 | Toonins C | Root | Dong et al. (2013a) | |
| 60 | Matairesinol | Root | Dong et al. (2013a) | |
| 61 | Lyoniresinol | Root | Dong et al. (2013a) | |
| 62 | Scopoletin | Leaves |
Luo et al. (2001) Shen et al. (2013) |
|
| 63 | 4, 7-Dimethoxy-5-methylcoumarin | Leaves | Shen et al. (2013) | |
| 64 | Ficusesquilignans A | Fruits | Hou et al. (2011) | |
| 65 | Ficusesquilignans B | Fruits | Hou et al. (2011) | |
| Flavonoids | 66 | (+)-Catechin | Rachis | Park et al. (1996) |
| 67 | (−)-Epicatechin | Rachis | Park et al. (1996) | |
| 68 | Procyanidin B3 | Leaves Stems |
Kakumu et al. (2014) Zhao et al. (2009) |
|
| 69 | Procyanidin B4 | Stems | Zhao et al. (2009) | |
| 70 | Quercetin | Leaves |
Zhang et al. (2001) Zhan and Zhang (2000) |
|
| 71 | Quercitrin | Leaves Rachis |
Zhang et al. (2001) Park et al. (1996) |
|
| 72 | Isoquercitrin | Rachis Bark Leaves |
Park et al. (1996) Li et al. (2006) Zhang et al. (2001) |
|
| 73 | Rutin | Rachis | Park et al. (1996) | |
| 74 | Kaempferol | Leaves | Luo et al. (2001) | |
| 75 | Kaempferol-3-O-α-l-rhamopyranoside | Fruits |
Hou et al. (2011) Shen et al. (2013) |
|
| 76 | Astragalin | Leaves | Shen et al. (2013) | |
| 77 | Myricetin | Bark | Li et al. (2006) | |
| 78 | Myricitrin | Bark | Li et al. (2006) | |
| 79 | Quercetin-3-O-(2″-O-galloyl)-β-d-glucopyranoside | Leaves | Cheng et al. (2009) | |
| 80 | Astragalin-2″-O-gallate | Leaves | Kakumu et al. (2014) | |
| 81 | Loropetalin D | Leaves | Kakumu et al. (2014) | |
| 82 | 6,7,8,2′-Tetramethoxy-5,6′-dihydroxy-flavone | Leaves | Luo et al. (2001) | |
| 83 | 5,7-Dihydroxy-8-methoxy flavone | Leaves | Luo et al. (2001) | |
| Others | 84 | bis-(p-Hydroxyphenyl) ether | Rachis | Park et al. (1996) |
| 85 | Gallic acid | Leaves |
Chen et al. (2009b) Yang et al. (2013) |
|
| 86 | Methyl gallate | Rachis | Park et al. (1996) | |
| 87 | Ethyl gallate | Leaves |
Luo et al. (2001) Shen et al. (2013) [17] |
|
| 88 | Syringic acid | Roots | Dong et al. (2013a) | |
| 89 | 3,5-Dihydroxy-phenyl ether | Fruits | Hou et al. (2011) | |
| 90 | 4-Methoxy-6-(2′,4′-dihydroxy-6′-methylphenyl)-pyran-2-one | Roots | Dong et al. (2013a) | |
| 91 | 4-Hydroxy-3-methoxybenzene-ethanol | Roots | Dong et al. (2013a) | |
| 92 | 3α-Hydroxy-5,6-epoxy-7-megastigmen-9-one | Leaves | Luo et al. (2001) | |
| 93 | Aloeemodin | Roots | Dong et al. (2013a) | |
| 94 | β-Sitosterol | Bark |
Li et al. (2006) Dong et al. (2013a) Anonymous (1972) |
|
| 95 | Daucosterol | Leaves | Yang et al. (2013) | |
| 96 | 1,2,6-Tri-O-galloyl-β-d-glucopyranose | Leaves | Cheng et al. (2009) | |
| 97 | 1,2,3,4,6-Penta-O-galloyl-β-d-glucopyranose | Leaves | Shen et al. (2013) | |
| 98 | 1-O-methyl-2,3,4,6-tetra-O-galloyl-β-d-glucopyanose | Seeds | Zhao et al. (2011) | |
| 99 | (S,S)-γ-glutamyl-(cis-S-1-propenyl)thioglycine | Shoots | Li et al. (2013) | |
| 100 | (S,S)-γ-glutamyl-(trans-S-1-propenyl)thioglycine | Shoots | Li et al. (2013) | |
| 101 | γ-Glutamyl-(cis-S-1-propenyl)-cysteine | Shoots | Li et al. (2013) | |
| 102 | γ-Glutamyl-(trans-S-1-propenyl)-cysteine | Shoots | Li et al. (2013) | |
| 103 | cis-S-1-propenyl-l-cysteine | Shoots | Li et al. (2013) | |
| 104 | trans-S-1-Propenyl-l-cysteine | Shoots | Li et al. (2013) | |
| 105 | Adenosine | Rachis | Park et al. (1996) | |
Volatile oils
As well known that, special perfume is one of the characteristics of T. sinensis plant, thus previous researchers have investigated the volatile oils of this plant. For extraction of volatile oils from T. sinensis, the hydro-distillation and headspace solid-phase microextraction (HS-SPME) are commonly used, and gas chromatography coupled to mass spectrometry (GC–MS) is often used to identify the composition of volatile oil (Chen et al., 2009a, Li and Wang, 2014). Nowadays, over forty volatile components were isolated and identified from the tender shoots and leaves of T. sinensis. These constituents are mainly sesquiterpenes hydrocarbons, including caryophyllenes, β-caryophyllenes, copaenes and β-eudesmenes (Chen et al., 2009a, Dong et al., 2013b, Wu et al., 2014).
Terpenoids
Natural products is a large resource for finding novel structures for candidate drugs, and more than 40% of the marketed drugs are derived from the secondary metabolites in plant. Among them, the terpenoids are prominent secondary metabolites for discovering new candidate drugs with wide spectrum of activities, including hepatoprotective, antiviral, anti-bacterial, anti-inflammatory, and anti-tumor agents (James and Dubery, 2009, Zhou et al., 2017).
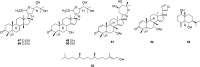
It is reported that this plant contains abundant terpenoids, and toosendanin (1) is the first triterpenoid isolated from this plant in 1972 (Anonymous, 1972). Till now, 59 terpenoids (including triterpenoids, diterpenes and sesquiterpenes) have been isolated from the leaves, shoots, barks and roots of T. sinensis, and limonoids triterpenoids are the characteristic constituents of T. sinensis (Box 1). The predominant terpenoids of this plant are the triterpenoids and include 3-oxo-12-en-28-oic acid (2) (Yang et al., 2013), α-betulin (3) (Dong et al., 2013a), ursolic acid (4), betulonic acid (5), betulic acid (6) (Yang et al., 2013), 11α-hydroxygedunin (7), 11β-hydroxygedunin (8), 7-deacetoxy-7α,11α-dihydroxygedunin (9), 7-deacetoxy-7α,11β-dihydroxygedunin (10), gedunin (11), 7-deacetoxy-7α-hydroxygedunin (12) (Mitsui et al., 2006), 7-deacetylgedunin (13) (Chen et al., 2017a, Chen et al., 2017b), 11-oxo-gedunin (14) (Mitsui et al., 2006), toonins A (15), proceranone (16) (Dong et al., 2013a), 6-acetoxyobacunol acetate (17), 7α-obacunyl acetate, 7α-acetoxy-dihydronomilin (18) (Luo et al., 2000), 11β-hydroxy-7α-obacunyl acetate (19), 11-oxo-7α-obacunyl acetate (20), 11-oxo-7α-obacunol (21), 11β-hydroxycneorin G (22), 11β-oxocneorin G (23) (Mitsui et al., 2004), cedrellin (24) (Luo et al., 2000), toonins B (25) (Dong et al., 2013a), grandifoliolenone (26) (Mitsui et al., 2007), bourjotinolone A (27) (Dong et al., 2013a), toona triterpenoids A (28), B (29), piscidinol A (30), hispidol B (31) (Mitsui et al., 2007), 20-hydroxy-24-dammaren-3-one (32), (20S)-3-oxo-tirucalla-25-nor-7-en-24-oic acid (33), (20S)-5α,8α-epidioxy-3-oxo-24-nor-6.9(11)-dien-23-oic acid (34), ocotillone (35), (20S,24R)-epoxydammarane-12.25-diol-3-one (36), (20S,24R)-epoxydammarane-3β,25-diolmarane-3β,25-diol (37), methyl shoreate (38), shoreic acid (39), richenone (40), cabralealactone (41), cylindrictone D (42), hollongdione (43), 4,4,14-trimethyl-3-oxo-24-nor-5α,13α,14β,17α,20S-chol-7-en-23-oic acid (44), (20S,24S)-dihydroxydammar-25-en-3-one (45), bourjotinolone B (46) (Tang et al., 2016), 21α-methylmeliandiol (47), 21β-methylmeliandiol (48), 3-O-acetyl-21R-O-methyltoosendanpentol (49), 3-O-acetyl-21S-O-methyltoosendanpentol (50), toona triterpenoids C, D, F, sapellin E acetate (51), azadirone (52) (Mitsui et al., 2007), and toosendanin (1) (Anonymous, 1972).
Furthermore, there are also other terpenoids reported in this T. sinensis, including phytol (53), 2,6,10-phytatriene-1,14,15-triol (Luo et al., 2000), (2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6-10-triene-1,14,15-triol, eudesm-4(15)-ene-1β,6α-diol (54) (Hou et al., 2011).


Phenylpropanoids
Phenylpropanoids, including lignins and coumarins, commonly exist in natural plants, and these compounds often have some interesting activities such as antiviral, antibacterial, anti-inflammatory and antitumor activities (de Souza et al., 2016, Hassan et al., 2016, Figueiredo et al., 2017).
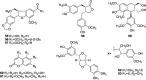
So far, nine phenylpropanoids have been found in the leaves, fruits and roots of T. sinensis, and these constituents were identified as cedralins A (55), B (56) (Lee et al., 2010), toonins C (63), matairesinol (58), lyoniresinol (59) (Dong et al., 2013a), scopoletin (60) (Luo et al., 2001), 4,7-dimethoxy-5-methylcoumarin (61) (Shen et al., 2013), and ficusesquilignans A (62), B (63) (Hou et al., 2011).
Flavonoids
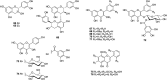
Flavonoids are common constituents in various plants all over the world. There were thirteen flavonoids in different parts of T. sinensis which were isolated and identified as (+)-catechin (64), (−)-epicatechin (65) (Park et al., 1996), procyanidin B3 (66) (Kakumu et al., 2014), procyanidin B4 (Zhao et al., 2009), quercetin, quercitrin (Zhang et al., 2001), isoquercitrin (67), rutin (Park et al., 1996), kaempferol (Luo et al., 2001), kaempferol-3-O-α-l-rhamopyranoside (68) (Hou et al., 2011), astragalin (69) (Shen et al., 2013), myricetin (70), myricitrin (71) (Li et al., 2006), quercetin-3-O-(2″-O-galloyl)-β-d-glucopyranoside (72) (Cheng et al., 2009), astragalin-2″-O-gallate (73), loropetalin D (74) (Kakumu et al., 2014), 6,7,8,2′-tetramethoxy-5,6′-dihydroxy-flavone (75), and 5,7-dihydroxy-8-methoxy flavone (76) (Luo et al., 2001).
Other compounds
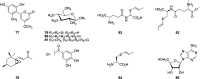
Besides these compounds mentioned above, there are also other compounds, including phenols, sterols, anthraquinones, tannins, and sulfocompounds reported in T. sinensis. These constituents are identified as bis-(p-hydroxyphenyl) ether (Park et al., 1996), gallic acid (Chen et al., 2009a, Chen et al., 2009b), methyl gallate (Park et al., 1996), ethyl gallate (Luo et al., 2001), syringic acid (Dong et al., 2013a), 3,5-dihydroxy-phenylether (Hou et al., 2011), 4-methoxy-6-(2′,4′-dihydroxy-6′-methylphenyl)-pyran-2-one (77), 4-hydroxy-3-methoxybenzene-ethanol (Dong et al., 2013a), 3α-hydroxy-5, 6-epoxy-7-megastigmen-9-one (78) (Luo et al., 2001), aloeemodin (Dong et al., 2013a), β-sitosterol (Li et al., 2006), daucosterol (Yang et al., 2013), 1,2,6-tri-O-galloyl-β-d-glucopyranose (79) (Cheng et al., 2009), 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose (80) (Shen et al., 2013), (S,S)-γ-glutamyl-(cis-S-1-propenyl) thioglycine (81), (S,S)-γ-glutamyl-(trans-S-1-propenyl)thioglycine (82), γ-glutamyl-(cis-S-1-propenyl)-cysteine (83), γ-glutamyl-(trans-S-1-propenyl)-cysteine, cis-S-1-propenyl-l-cysteine (84), trans-S-1-propenyl-l-cysteine (Li et al., 2013), and adenosine (85) (Park et al., 1996).
Pharmacology
Previous investigations have comprehensively considered the pharmacological activities of T. sinensis and reported that this plant possesses various pharmacological activities including anti-tumor, hypoglycemic, antioxidant, anti-inflammatory, protecting effect on ischemia–reperfusion injury, hepatoprotective, antibacterial and antiviral, anti-gout effect, male reproductive system protection, and anticoagulation effects (Box 2 ).
Box 2.
The pharmacological activities of Toona sinensis.
| Pharmacological effects | Detail | Tested substance | Doses/concentrations | References |
|---|---|---|---|---|
| Antitumor effect | Leukemia (K562 cell line) | ACTSL | IC50 was 102.53 μg/ml | Chen et al. (2011a) |
| Leukemia (WEHI-3 cell line) | TSL | 50 mg/kg on WEHI-3 cell bearing mice | Yang et al. (2017) | |
| Leukemia (HL-60 cell line) | TSL | 10–75 μg/ml | Huang et al. (2012) | |
| Gallic acid | 5, 10 μg/ml |
Huang et al. (2012) Kakumu et al. (2014) |
||
| Leukemia (HL-60 and K562 cell lines) | Cedralins A | IC50 were 26.2 and 22.4 μg/ml | Lee et al. (2010) | |
| Leukemia (HL-60 cell line) | Loropetalin D | 50 μM | Kakumu et al. (2014) | |
| Quercetin | ||||
| Quercitrin | ||||
| Afzelin | ||||
| Astragalin 2′′-O-gallate | ||||
| (+)-Catechin | ||||
| Lung cancer (H441, H520, H661 cell lines) | TSL | 0.125–1.0 mg/ml for 24 or 48 h, IC50 were 1.2, 0.73 and 0.29 mg/ml for H441, H520 and H661 cells |
Wang et al. (2010); Yang et al., 2010a, Yang et al., 2010b |
|
| Oral carcinoma | TSL | 1.0 g/kg | Wang et al. (2016) | |
| Gallic acid | 250, 500 μg/ml | Chia et al. (2010) | ||
| Gastric cancer (MGC-803 cell line) | Betulonic acid | IC50 was 17.7 μM | Yang et al. (2013) | |
| 3-Oxo-12-en-28-oic acid | IC50 was 13.6 μM | |||
| Gastric cancer (SGC-7901 cell line) | ACTSL | IC50 was 168.47 μg/ml | Chen et al. (2011a) | |
| Prostatic cancer (DU145 cell line) | Gallic acid | 25–100 μg/ml | Chen et al., 2009a, Chen et al., 2009b | |
| Prostatic cancer (PC3 cell line) | Betulonic acid | IC50 was 26.5 μM | Yang et al. (2013) | |
| 3-Oxo-12-en-28-oic acid | IC50 was 21.9 μM | |||
| Ovarian cancer (SKOV3 cell line) | TSL | 0–1000 μg/ml | Chang et al. (2006) | |
| Cervical carcinoma (Hela cell line) | TSL | 25, 50 μg/ml | Zhen et al. (2014) | |
| Renal carcinoma (ccRCC cell line) | TSL | – | Chen et al. (2016) | |
| Rsteosarcoma (Saos-2 cell line) | TSL | IC50 was 42.8–52.3 μg/ml in vitro 1 g/kg and 5 g/kg on Saos-2 cell bearing mice |
Chen et al. (2017b) | |
| Colon cancer Caco-2 cell | TSL | IC50 was 4.0 μg/ml | Liu et al. (2012) | |
| Liver cancer HepG2 cell | TSL | IC50 was 153.16 μg/ml | ||
| Breast cancer MCF-7 cell | TSL | IC50 was 193.46 μg/ml | ||
| Hypoglycemic effects | Glucose uptake-enhancing effect | ETSL | 0.001, 0.01, 0.1 mg/ml, for 60 min | Yang et al. (2003) |
| Inhibiting LDL glycation induced by glucose and glyoxal | TSL | 0.5 mg/ml | Hsieh et al. (2005) | |
| Alleviating hyperglycemia via altering adipose glucose transporter 4 | TSL | 0.5, 1, 2 g/kg/14 days | Wang et al. (2008a) | |
| Hypoglcemic effects on diabetic mice | FTSL | 0.6, 0.12 mg/kg | Zhang et al., 2008, Zhang et al., 2011 | |
| Protective effects on hepatic injury at early stage in diabetic rats | TPST | 60, 80, 100 mg/kg, for 14 days | Xing and Chen (2011) | |
| Hypoglycemic effect in diabetic rats | STSL | 15 mg/kg, for 15 days | Du et al. (2011) | |
| kidney protecting effects in diabetic rats | STSL | 50 mg/kg, for 10 weeks | Li et al. (2016) | |
| Preventing the progression of diabetes | NPTSL | 12.5–100 μg/ml in vitro; 150 mg/kg for 8 weeks in vivo |
Hsieh et al. (2012) | |
| Stimulating glucose uptake and ameliorating insulin resistance | ETSL | 0, 10, 50, 70, and 95% | Liu et al. (2015) | |
| α-Glucosidase inhibitory activities | Gallic acid | IC50 was 24.3 μM | ||
| (+)-Catechin | IC50 was 190.7 μM | Zhao et al. (2009) | ||
| (−)-Epicatechin | IC50 was 189.0 μM | |||
| Procyanidin B3 | IC50 was 111.0 μM | |||
| Procyanidin B4 | IC50 was 89.0 μM | |||
| Reducing the risk of diabetes and its secondary complications via reducing oxidative stress in the liver | Quercetin | 200 mg/kg | Zhang et al. (2016) | |
| Antioxidant effects | Against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells | Methyl gallate | 100 μM | Hsieh et al. (2004) |
| In vitro antioxidant experiments | TSL | 0.1–1.6 mg/ml | Zhang et al. (2007) | |
| TSL | 25–100 μg/ml | Hseu et al. (2008) | ||
| Gallic acid | 50 μg/ml | |||
| TPST | _ |
Wang et al., 2008a, Wang et al., 2008b |
||
| Antioxidant properties that protect endothelial cells from oxidative stress | TSL | 50–100 μg/ml | Yang et al. (2011) | |
| Up-regulating antioxidant enzymes in SD rats | FETSL | 0.5 and 1.0 mg/kg | Chen et al. (2013) | |
| Anti-inflammatory effects | Inhibiting carrageenin-induced paw edema in rats | TSL | 0.5 and 1.0 mg/kg (p.o.) | Ruan et al. (2010) |
| Treating adjuvant-induced arthritis in rats | TPST | 35 and 70 mg/kg (p.o.) | Yang and Chen (2012) | |
| Inhibiting LPS-induced inflammation in mice via suppressing NF-κB pathway | TSL | 100 mg/kg (p.o.) | Hsiang et al. (2013) | |
| Gallic acid | 5 mg/kg (p.o.) | |||
| Inhibiting LPS-induced inflammation in vascular smooth muscle cells (A7r5 cell line) via suppressing NF-κB pathway | TSL | 25–100 μg/ml | Yang et al. (2014) | |
| Gallic acid | 5 μg/ml | |||
| Inhibiting inflammatory responses in RAW264.7 cells via activating Keap1/Nrf2/HO-1 pathway | 7-deacetylgedunin | 1–25 μM | Chen et al. (2017a) | |
| Protecting effects on ischemia–reperfusion | Protective effect on myocardial ischemia/reperfusion injury in rats | TPST | 50, 100, 200 mg/kg/d (p.o., for 7 days) | Li and Chen, 2011a, Li and Chen, 2011b, Li and Chen, 2012 |
| protective effects on MODS caused by brain ischemia–reperfusion in rats | BUST | 20,30 mg/kg/d (p.o., for 7 days) | Yuan et al. (2013) | |
| Hepatoprotective effect | Alleviating thioacetamide induced liver fibrosis | TSL | 1 g/kg/d (p.o., for 7 days) | Fan et al. (2007) |
| Ameliorating antioxidant enzymes activity in H2O2 induced oxidative rats liver | TSL | 0.013– 1.88 g/kg/d (p.o., for 8 weeks) | Yu et al. (2012a) | |
| Protective effects on hepatic injury at early stage in diabetic rats | TPST | 60, 80, 100 mg/kg (p.o., for 14 days) | Xing and Chen (2011) | |
| Attenuating acetaminophen induced acute liver toxicity in HepG2 cells and mice via inducing antioxidant machinery and inhibiting inflammation | Quercitrin | 25, 50 μg/ml; 10, 50 mg/kg/d (p.o., for 7 days) |
Truong et al. (2016) | |
| Antiviral and antibacterial effects | Antiviral activity against SARS-CoV | TSL | IC50 = 30 μg/ml | Chen et al. (2008) |
| Antiviral activity against H1N1 | TSL | 10–100 μg/ml | You et al. (2013) | |
| Antibacterial activity against E. coli C83902 | TSL | MIC = 0.25 g/ml | Chen et al. (2011b) | |
| Antibacterial activity against E. coli K88 | TSL | MIC = 0.125 g/ml | ||
| Antibacterial activity against Salmonella C500 | TSL | MIC = 0.25 g/ml | ||
| Antibacterial activity against Staphylococcus CAU0183 | TSL | MIC = 0.25 g/ml | ||
| Anti-gout activity | Inhibiting XO | TSL | IC50 = 151.6 μg/ml | Liang et al. (2011) |
| Inhibiting COX- 2 | TSL | IC50 = 2.26 μg/ml | ||
| Hypouricemic effects on hyperuricemic mice | FTSL | 50, 100, 200 mg/kg/d (p.o., for 7 days) | Wang et al. (2011) | |
| Male reproductive system protection | Suppressing steroidogenesis, cAMP-PKA pathway and steroidogenic enzymes activities in normal mouse leydig cells | TSL | 0.005, 0.05, 0.5 mg/ml | Poon et al. (2005) |
| TSL could improves the functions of sperm and testes | TSL | 13 mg/kg/d (p.o., for 8 weeks) | Yu et al. (2012a) | |
| Anticoagulation effect | Anticoagulation effect of on adrenaline induced hypercoagulable rats via prolonging RT, APTT, TT and PT, and increasing AT-III activity | BUST | 10, 20 mg/kg/d (p.o., for 7 days) | Jin and Chen (2011) |
| Enhancing fibrinolysis of topical FeCl3 induced carotid artery thrombosis rats via increasing t-PA, PL G and DD. | BUST | 40 mg/kg/d (p.o., for 7 days) | Jin and Chen (2011) | |
| Other pharmacological effects | Lipolytic effect in differentiated 3T3-L1 adipocytes via protein kinase C pathway | TSL | 0.001, 0.01, 0.1 mg/ml | Hsu et al. (2003) |
| Suppressing BV-2 microglia mediated neuroinflammation | TSL | 5, 10, 50 mg/ml | Wang et al. (2014a) | |
| Antinociceptive effect on acetic acid induced writhing in mice | TSL | 0.003–1 g/kg (p.o.) | Su et al. (2015) | |
| Anticomplementary activity on complement-injured SH-SY5Y cells | 1-O-methyl-2,3,4,6-tetra-O- galloyl-β-d-glucopyanose |
100, 200 mg/ml | Zhao et al. (2011) | |
| Improving capacities of stress resistance and delaying senescence for Caenorhabditis elegans | FTSL | 100 μg/ml | Yang et al. (2010) | |
ACTSL, ethyl acetate extracts of T. sinensis leaf; APTT, activated partial thromboplastin time; AT-III, increasing the activity of antithrombin III; BUST, n-butanol extract of the seeds of T. sinensis; CLP, cecal ligation and puncture; COX-2, cyclooxygenase-2; DD, D-dimer; FETSL, anaerobic fermented leaves extract of T. sinensis; ETSL, ethanol extracts of T. sinensis leaf; FTSL, total flavonoids of T. sinensis leaf; IC50, half maximal inhibitory concentration; PLG, plasminogen; MODS, multiple organ dysfunction syndrome; ROS, reactive oxygen species; RT, cation time; STSL, water extracts of the seeds of T. sinensis; TSL, water extracts of T. sinensis leaf; t-PA, tissue plasminogen activator; TPST, total polyphenols from the seeds of T. sinensis; TT, thrombin time; PT, prothrombin time; XO, xanthine oxidase.
Anti-tumor effect
Toona sinensis is a known TCM with the function of heat-clearing and detoxifying, and anti-tumor effects are important pharmacological activities of T. sinensis which have been comprehensively investigated, including leukemia, lung cancer, oral carcinoma, cervical carcinoma, osteosarcoma, ovarian cancer, prostate cancer, renal carcinoma, gastric cancer, colon cancer, and breast cancer (Hseu et al., 2011).
By using MTT assays in vitro, Chen et al. (2011a) found that ethyl acetate extracts of T. sinensis (ACTSL) significantly inhibited the proliferation of leukemia K562 cell line with the IC50 was 102.53 μg/ml. Later in 2012, it is reported that water extracts of T. sinensis (TSL) could arrest leukemia HL-60 cells at G1–S transition phase and down-regulate VEGF (Huang et al., 2012). In 2016, research of Yang et al. (2017) found that TSL (50 mg/kg) had notable antitumor effect against leukemia in WEHI-3 cells bearing mice. Furthermore, it is reported that many interesting compounds isolated from the T. sinensis possess promising antitumor effects against leukemia, including cedralin A (55) (IC50 was 26.2 μg/ml), loropetalin D (74) (IC50 was 22.4 μg/ml) (Lee et al., 2010), quercetin, quercitrin, afzelin, astragalin 2′′-O-gallate (73), (+)-catechin (64) (Kakumu et al., 2014) and gallic acid (Huang et al., 2012, Kakumu et al., 2014).
In 2010, using lung cancer cell lines including human lung adenocarcinoma H441 cell line, human lung squamous cell carcinoma H520 cell line, and human lung large cell carcinoma cell line H661, Wang et al. and Yang et al. reported that TSL possess notable antitumor potentials against lung cancer cell lines via cell cycle arrest and apoptosis, and the IC50 values were 1.2, 0.73 and 0.29 mg/ml for H441, H520 and H661 cells, respectively (Wang et al., 2010, Yang et al., 2010a, Yang et al., 2010b).
In 2011, Chen et al., 2011a, Chen et al., 2011b reported that ACTSL had the antitumor potential against gastric cancer SGC-7901 cell line with the IC50 value of 168.47 μg/ml. Later in 2013, Yang et al. (2013) reported betulonic acid (5) and 3-oxo-12-en-28-oic acid (2) isolated from T. sinensis inhibited the proliferation of gastric cancer MGC-803 (IC50 were 17.7 and 13.6 μM) and prostatic cancer PC3 cell lines (IC50 were 26.5 and 21.9 μM). Interestingly, gallic acid isolated from T. sinensis is also an important agent against prostatic cancer DU145 cells via inducing generation of reactive oxygen species (ROS) and mitochondria-mediated apoptosis; furthermore, gallic acid also showed a synergistic effect with doxorubicin in inhibiting DU145 cells’ growth (Chen et al., 2009a, Chen et al., 2009b).
Water extracts of T. sinensis and gallic acid were also reported to be active agents against oral carcinoma via inducing apoptosis. Chang et al. (2006) reported that TSL induced apoptosis of human ovarian cancer SKOV3 cells and inhibits tumor growth in SKOV3 cells xenograft model. Zhen et al. (2014) found that TSL could induce cell cycle arrest in human cervical carcinoma HeLa cells via apoptosis. In 2016, it is reported that TSL inhibited the growth and migration of renal carcinoma ccRCC cells via inducing apoptosis (Chen et al., 2016). Recently, Chen et al. (2017b) revealed that TSL caused significant cytotoxicity in osteosarcoma Saos-2 cell in vivo and in vitro via inducing apoptosis (IC50 was 42.8–52.3 μg/ml in vitro, 1 g/kg and 5 g/kg on Saos-2 cell bearing mice).
Additionally, TSL was also reported to be an active agent against colon cancer Caco-2 cell, human liver cancer HepG2 cell and breast cancer MCF-7 cell lines, and the IC50 values were 4.0, 153.16 and 193.46 μg/ml, respectively (Liu et al., 2012).
Hypoglycemic effect
Currently, increasing researches have demonstrated that extracts/constituents from the T. sinensis have promising hypoglycemic potentials, which would be beneficial for the diabetes patients. In 2003, the research team of Yang et al. (2003) reported that ethanol extracts of T. sinensis leaf (ETSL) could enhance the cellular glucose uptake in basal and insulin stimulated 3T3-L1 adipocytes. In 2015, Liu et al. (2015) revealed that the mechanisms of TSL stimulating glucose uptake and ameliorating insulin resistance might be related to AMPK activation in skeletal muscles and up-regulation of PPARγ and normalized adiponectin in adipose tissues. In 2005, the inhibitory effect of TSL on LDL glycation induced by glucose and glyoxal was reported (Hsieh et al., 2005). It was also indicated that TSL could alleviate hyperglycemia via altering adipose glucose transporter 4 (Wang et al., 2008a, Wang et al., 2008b), and results of Zhang et al., 2008, Zhang et al., 2011 indicated that total flavonoids of T. sinensis (FTSL) might be the active constituents corresponding to the hypoglycemic effects of this plant. Furthermore, it is reported that extracts of the seeds of T. sinensis (STSL) has hypoglycemic and kidney protecting effects in diabetic rats (Du et al., 2011, Li et al., 2016), and Xing and Chen found that total polyphenols from the seeds of T. sinensis (TPST) could inhibit hepatic injury at early stage in diabetic rats (Xing and Chen, 2011). In 2012, Hsieh et al. (2012) indicated the supercritical-CO2 fluid extracted non-polar leaves extract of T. sinensis (NPTSL) could prevent the progression of type 2 diabetes. Besides, Zhao et al. (2009) reported that in this plant, gallic acid, (+)-catechin (64), (−)-epicatechin (65, and procyanidin B3 (66), and B4 showed α-glucosidase inhibitory activities with IC50 of 24.3, 190.7, 189.0, 111.0, and 89.0 μM, respectively. Zhang et al. (2016) indicated that quercetin is also an active agent in T. sinensis could reduce the risk of diabetes and its secondary complications via reducing oxidative stress in the liver.
Antioxidant effect
By using a series in vitro experiment, previous researches indicated that TSL and gallic acid are potential natural antioxidant agents (Zhang et al., 2007, Hseu et al., 2008, Cheng et al., 2009, Liu et al., 2012). In addition, Hsieh et al. (2004) reported the antioxidant effects of methyl gallate isolated from T. sinensis against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. In addition, the antioxidant effects of phenolic compounds in T. sinensis have been widely proved by using DPPH scavenging assays (Wang et al., 2008a, Wang et al., 2008b, Xing and Chen, 2010). In 2011, Yang et al. (2011) indicated that TSL could protect endothelial cells from oxidative stress which is beneficial for treating atherosclerosis. Later in 2013, another study reported the anaerobic fermented leaves extract of T. sinensis (FETSL) could up-regulate the expression of antioxidant enzymes in SD rats (Chen et al., 2013).
Anti-inflammatory effect
To date, natural derived anti-inflammatory agents play important and indispensable roles in preventing and treating inflammatory diseases (Wang et al., 2013). In addition, many natural products (including extracts and monomers) isolated form the T. sinensis have been reported to possess notable anti-inflammatory effects. Ruan et al. (2010) reported that TSL could inhibit the carrageenin- induced paw edema in rats via suppressing inflammatory mediators. Later in 2012, a report demonstrated that total polyphenols from the seeds of T. sinensis (TPST) had therapeutic effects on adjuvant-induced arthritis rats (Yang and Chen, 2012). Furthermore, using NF-κB transgenic mice and bioluminescence imaging, another two investigations revealed that TSL and gallic acid could inhibit LPS-induced inflammation via suppressing NF-κB pathway in vivo and in vitro (Hsiang et al., 2013; Yang et al., 2014). Recently, Chen et al., 2017a, Chen et al., 2017b reported that the 7-deacetylgedunin (13) isolated form the T. sinensis suppresses LPS induced inflammatory responses in RAW264.7 cells through activating Keap1/Nrf2/HO-1 pathway.
Protecting effect on ischemia–reperfusion injury
Ischemia–reperfusion injury is one of the leading reasons for the death in the rescue and treatment of ischemic disease, in particularly the myocardial and brain tissues. In 2011, the protective effect of total polyphenols extracted from T. sinensis (TPST) on myocardial ischemia/reperfusion injury in rats were reported, and the possible mechanism might be correlated to decreasing creatine kinase (CK), cardiac troponin (cTn) I, malonaldehyde (MDA), thromboxane (TXB2), whereas increasing superoxide dismutase (SOD) and 6-keto-PGF1 (Li and Chen, 2011a, Li and Chen, 2011b). Later in 2012, another paper by Li and Chen reported that the protective effect of TPST on myocardial ischemia/reperfusion injury is also related to alleviating inflammatory reactions via decreasing pro-inflammatory cytokines such as TNF-α & IL-6 and suppressing NF-κB pathway (Li and Chen, 2012). Besides, Yuan et al. (2013) revealed that n-butanol extract of the seeds of T. sinensis (BUST) had protective effects on multiple organ dysfunction syndrome (MODS) caused by brain ischemia–reperfusion in rats via suppressing oxidative stress.
Hepatoprotective effect
Nowadays, increasing evidences have demonstrated that herbal medicines are good resources for finding hepatoprotective drugs. Interestingly, Fan et al. (2007) found that TSL could alleviate thioacetamide induced liver fibrosis via reducing TGFβR1 and collagen. In addition, another investigation in 2012 reported that TSL could ameliorate the antioxidant enzymes activity in H2O2 induced oxidative rats liver which would be beneficial for the hepatic detoxification (Yu et al., 2012a, Yu et al., 2012b). Recently, Truong et al. (2016) reported that the quercitrin extracted from the T. sinensis attenuated acetaminophen-induced acute liver toxicity in HepG2 Cells and mice, and the related mechanisms is correlated to activating defensive genes and inhibiting pro-inflammatory mediators via suppressing JNK and p38 pathway.
Antiviral and antibacterial effect
Currently, the antiviral and antibacterial effects of T. sinensis have aroused researchers’ attention. In 2008, Chen et al. (2008) found that TSL had antiviral activity against SARS-CoV in vitro with an IC50 value of 30 μg/ml. Later in 2013, another report revealed that TSL could be used an alternative treatment and prophylaxis against H1N1 virus (You et al., 2013). Besides, it is reported that TSL also possessed promising antibacterial potential against E. coli C83902, E. coli K88, Salmonella C500, and Staphylococcus CAU0183, and the minimum inhibitory concentration (MIC) were 0.25, 0.125, 0.25 and 0.25 g/ml (Chen et al., 2011a, Chen et al., 2011b).
Anti-gout effect
Gout is a common painful diseases caused by accumulation of uric acid crystals in joints which is closely related to chronic purine metabolic disorder. In 2011, Liang et al. (2011) reported that TSL possessed significant inhibitory activities in vitro against xanthine oxidase (XO, IC50 was 151.6 μg/ml), cyclooxygenase (COX)-2 (IC50 was 2.26 μg/ml). In addition, Wang et al. (2011) reported the total flavonoids of T. sinensis leaf (FTSL) had notable hypouricemic effects on hyperuricemic mice in vivo. These results above indicate that T. sinensis possesses significant inhibitory effect on the progression of gout.
Male reproductive system protection
In 2005, results of Poon et al. (2005) suggested that TSL could increase the motility of sperms via suppressing steroidogenesis, cAMP-PKA pathway and steroidogenic enzymes activities in normal mouse leydig cells. Furthermore, another paper also reported TSL could improves the functions of sperm and testes via down-regulation of glutathione transferase mu6, heat shock protein 90 kDa-β, cofilin 2 and cyclophilin A, whereas up-regulation of crease3-hydroxy-3-methylglutaryl-coenzyme A synthase 2, heat shock glycoprotein 96, and pancreatic trypsin 1 (Yu et al., 2012b). These findings suggest T. sinensis is a valuable agent for men to ameliorate functions of sperm and testes under oxidative stress.
Anticoagulation effect
Using the topical FeCl3 induced carotid artery thrombosis rats model, Liu and Chen (2009) reported that n-butanol extract of the seeds of T. sinensis (BUST) could obviously enhance the fibrinolysis of carotid artery thrombosis rats, and the main mechanism is involved in increasing tissue plasminogen activator (t-PA), plasminogen (PL G) and D-dimer (DD). Later in 2011, Jin and Chen (2011) reported that BUST had anticoagulation effect of on adrenaline induced hypercoagulable rats, which is might be correlated to prolonging the re-calcification time (RT), activated partial thromboplastin time (APTT), thrombin time (TT) and prothrombin time (PT), and increasing the activity of anti-thrombin III (AT-III).
Other pharmacological effects
Besides these pharmacological activities, it is also reported that TSL possesses lipolytic effect in differentiated 3T3-L1 adipocytes via protein kinase C pathway (Hsu et al., 2003), and Liu et al. (2014) reported that TSL could inhibit lipid accumulation through up-regulating genes related to lipolysis and fatty acid oxidation in adipocytes. Furthermore, it is reported that TSL could suppress BV-2 microglia mediated neuroinflammation (Wang et al., 2014a, Wang et al., 2014b), and have anti-nociceptive effect on acetic acid induced writhing in mice (Su et al., 2015). Besides, 1-O-methyl-2,3,4,6-tetra-O-galloyl-β-d-glucopyanose isolated from the T. sinensis had anti-complementary activity on complement-injured SH-SY5Y cells (Zhao et al., 2011), and FTSL could improve the capacities of stress resistance and delaying senescence for Caenorhabditis elegans (Yang et al., 2010).
Toxicology
T. sinensis is a plant could be used both as drug and food in China for thousands years. Generally, T. sinensis was commonly considered to be as a safe herbal drug, in particular the tender shoots of T. sinensis is delicious and nutritious food stuff. However, in some ancient books of TCM, such as the Tang materia medica, the bark of this plant is reported to be an herbal medicine with mild toxicity (Anonymous, 1999).
Currently, the systematic toxicity and safety evaluations of T. sinensis were still lacking, and only few reports had been documented. In 2007, Liao et al. (2007) evaluated the safety of the water extracts of T. sinensis leaf (TSL) using Ames test, and no obvious mutagenicity was found for all testing strains of Salmonella typhimurium TA98, TA100, TA102 and TA1535. In addition, Liao et al. (2007) also evaluated the acute oral toxicity of TSL (5000 mg/kg/day, for 14 days) and sub-acute oral toxicity of TSL (1000 mg/kg/day, for 28 days) in mice. The results indicated that TSL (5000 mg/kg/day, for 14 days) might decrease the food intake and kidney relative weight of female mice in acute oral toxicity test, and TSL (5000 mg/kg/day, for 28 days) could decrease the body weight gain, food intake and lung relative weight in sub-acute toxicity test. In another research, Liao et al. (2009) reported that no significant mutagenicity of water extract of fermented T. sinensis leaves (FTSL) was found in Ames test with the strains of S. typhimurium TA98, TA100, TA102 and TA1535; also, no obvious orally acute or subacute toxicity of FTSL (1000 mg/kg) was observed in mice. Moreover, the safety of a health care tea made by T. sinensis leaves was also evaluated on acute toxicity test in mice, ames test in S. typhimurium, micronucleus test of bone marrow PCE cell in mice, sperm shape abnormality test in mice, and the results revealed that no acute toxicity, genetic toxicity was observed (Cheng et al., 2007).
Conclusion
The present review provides a full-scale profile of the traditional usage, phytochemistry, pharmacology and toxicology of T. sinensis. In the present review, 109 compounds from different parts of this plant were summarized, and these compounds mainly concluded terpenoids, phenylpropanoids, and flavonoids, etc. Additionally, the existing pharmacological investigations have revealed that agents or extracts from this plant have a wide spectrum of pharmacological effects which is beneficial for the health of human being, in particular for its anti-tumor and hypoglycemic activities. Emerging evidences from animal experiments and in vitro studies have demonstrated some traditional uses of T. sinensis; however, new drug development for this plant is still require lots of detailed studies in both the preclinical and clinical works.
Firstly, currently there are few systemically ADME (absorption, distribution, metabolism, and excretion) and toxicities data of the compounds/extracts derived from T. sinensis, which is an important reason for the delay of new drug development of this plant. Thus, more works should be done on the toxicities and pharmacokinetic profile of T. sinensis. Secondly, previous researches have reported various pharmacological effects of T. sinensis, however most of the researches only focused on the crude extract and gallic acid in this plant. Gallic acid have obvious biological activities, however, it's not a characteristic compound of T. sinensis. Due to this plant contains abundant terpenoids, extensive researches are required to investigate the pharmacological properties of monomers belonging to terpenoids in this plant. Third, as a traditional delicious food and nutritious food stuff, previous researches have revealed that this plant possesses good anti-tumor, hypoglycemic and antioxidant effects, the tender shoots and leaves of T. sinensis also have the huge potential for functional food development. Fourth, the T. sinensis was traditional used to treat dysentery, enteritis, carminative, itchiness, and eye infections in Chinese folk medicine. However, not all of these traditional uses above were demonstrated by current pharmacological experiments; thus, more possible medicinal potentials of this plant might be investigated in the future. Lastly, there is no clinic trial of this plant, thus more works should be devoted to do some systemic clinic trials for T. sinensis in the future.
In conclusion, this paper systemic reviewed the traditional usage, phytochemistry, pharmacology and toxicology of T. sinensis, which might highlight the importance of this plant and provides some directions for the future development of T. sinensis.
Authors’ contributions
QWH and CJW contributed in conceiving this review; WP, YJL, MBH and MMZ contributed in collecting and analysis the references and data; JY and FL contributed in editing the manuscript; WP and YJL contributed in writing the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors are grateful to the Jian-Guo Wu (Department of pharmacy, Fujian University of Traditional Chinese Medicine, Fuzhou, China) for the pictures of T. sinensis. This work was supported by the China Postdoctoral Science Foundation (no. 2018M631071), and Project of Administration of Traditional Chinese Medicine of Sichuan Province of China (no. 2018JC001).
References
- Anonymous . vol. 1. People's Medical Publishing House; Beijing: 1972. p. 445. (Study on the Active Constituents of Chinese Herbal Medicine). [Google Scholar]
- Anonymous . vol. 2. Science and Technology Press of Shanghai; Shanghai: 1977. pp. 2429–2431. (Dictionary of Chinese Materia Medica). [Google Scholar]
- Anonymous . vol. 5. Science and Technology Press of Shanghai; Shanghai: 1999. pp. 45–48. (Chinese Material Medica). [Google Scholar]
- Chen C.H., Li C.J., Tai I.C., Lin X.H., Hsu H.K., Ho M.L. The fractionated Toona sinensis leaf extract induces apoptosis of human osteosarcoma cells and inhibits tumor growth in a murine xenograft model. Integr. Cancer Ther. 2017;16:397–405. doi: 10.1177/1534735416675951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.J., Michaelis M., Hsu H.K., Tsai C.C., Yang K.D., Wu Y.C., Cinatl J., Jr., Doerr H.W. Toona sinensis Roem tender leaf extract inhibits SARS coronavirus replication. J. Ethnopharmacol. 2008;120:108–111. doi: 10.1016/j.jep.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.J., Yang G.E., Yuan L.J., Huang K.Y. Analysis of volatile components from Toona sinensis (A. Juss) Roem buds and leaves by headspace-solid-phase micro-extraction gas chromatography–mass spectrometry. Fine Chem. 2009;26:1080–1084. [Google Scholar]
- Chen G.H., Huang F.S., Lin Y.C., Hsu C.K., Chung Y.C. Effects of water extract from anaerobic fermented Toona sinensis Roemor on the expression of antioxidant enzymes in the Sprague–Dawley rats. J. Funct. Foods. 2013;5:773–780. [Google Scholar]
- Chen J.Y., Zhu G.Y., Su X.H., Wang R., Liu J., Liao K., Ren R., Li T., Liu L. 7-Deacetylgedunin suppresses inflammatory responses through activation of Keap1/Nrf2/HO-1 signaling. Oncotarget. 2017;8:55051–55063. doi: 10.18632/oncotarget.19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Chien L.H., Huang B.M., Chia Y.C., Chiu H.F. Aqueous extracts of Toona sinensis leaves inhibit renal carcinoma cell growth and migration through JAK2/stat3, Akt, MEK/ERK, and mTOR/HIF-2α pathways. Nutr. Cancer. 2016;68:654–666. doi: 10.1080/01635581.2016.1158292. [DOI] [PubMed] [Google Scholar]
- Chang H.L., Hsu H.K., Su J.H., Wang P.H., Chung Y.F., Chia Y.C., Tsai L.Y., Wu Y.C., Yuan S.S. The fractionated Toona sinensis leaf extract induces apoptosis of human ovarian cancer cells and inhibits tumor growth in a murine xenograft model. Gynecol. Oncol. 2006;102:309–314. doi: 10.1016/j.ygyno.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Cheng D., Du G.S., Han X.Y., Zhang T.L. Study of toxicity of Toona sinensis health care tea. Chin. Prev. Med. 2007;18:576–579. [Google Scholar]
- Cheng K.W., Yang R.Y., Tsou S.C., Lo C.S., Ho C.T., Lee T.C., Wang M. Analysis of antioxidant activity and antioxidant constituents of Chinese toon. J. Funct. Foods. 2009;1:253–259. [Google Scholar]
- Chen H.M., Wu Y.C., Chia Y.C., Chang F.R., Hsu H.K., Hsieh Y.C., Chen C.C., Yuan S.S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009;286:161–171. doi: 10.1016/j.canlet.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Chen H.Y., Lin Y.C., Hsieh C.L. Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs. Food Chem. 2007;104:1418–1424. [Google Scholar]
- Chen Y.L., Ruan Z.P., Lin L.S., Li C.L. Anti-tumor activity of extracts of Toona sinensis in vitro. J. Fujian Univ. TCM. 2011;21:30–32. [Google Scholar]
- Chen Y.K., Ou H.P., Fang C.L., Yang H.H., Liu S.B. Antibacterial test of cortex Toonae and cortex Ailanthi in vitro. Chin. Anim. Health. 2011;13:24–26. [Google Scholar]
- Chia Y.C., Rajbanshi R., Calhoun C., Chiu R.H. Anti-neoplastic effects of gallic acid, a major component of Toona sinensis leaf extract, on oral squamous carcinoma cells. Molecules. 2010;15:8377–8489. doi: 10.3390/molecules15118377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza L.G., Rennã M.N., Figueroa-Villar J.D. Coumarins as cholinesterase inhibitors: a review. Chem. Biol. Interact. 2016;254:11–23. doi: 10.1016/j.cbi.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Dong X.J., Zhu Y.F., Bao G.H., Hu F.L., Qin G.W. New limonoids and a dihydrobenzofuran norlignan from the roots of Toona sinensis. Molecules. 2013;18:2840–2850. doi: 10.3390/molecules18032840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Yang W.Q., Wang M., Ma Y.H. Analysis of characteristic aroma components of Toona sinensis fruits grown in Yuxi, Yunnan province. Food Sci. 2013;34:217–220. [Google Scholar]
- Du C.H., Yan Y., Song Q., Guan A.P., Wang Y., Zhao B., Ma C., Fu Y.W. Preliminary study of hypoglycemic effect of aqueous extract from fructus Toonae sinensis. J. Shanxi Coll. TCM. 2011;12:2–4. [Google Scholar]
- Fan S., Chen H.N., Wang C.J., Tseng W.C., Hsu H.K., Weng C.F. Toona sinensis Roem (Meliaceae) leaf extract alleviates liver fibrosis via reducing TGF-β1 and collagen. Food Chem. Toxicol. 2007;45:2228–2236. doi: 10.1016/j.fct.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Feng W., Wang M., Cao J., Sun J., Jiang W. Regeneration of denatured polyphenol oxidase in Toona sinensis (A. Juss.) Roam. Process Biochem. 2007;42:1155–1159. [Google Scholar]
- Figueiredo P., Lintinen K., Hirvonen J.T., Kostiainen M.A., Santos H.A. Properties and chemical modifications of lignin: towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2017;93:233–269. [Google Scholar]
- Hassan M.Z., Osman H., Ali M.A., Ahsan M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016;123:236–255. doi: 10.1016/j.ejmech.2016.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Fu Y.H., Tang G.H., Hao X.J., Zhao Q., He H.P. Studies on chemical constituents of the fruits of Toona sinensis var. schensiana. J. Yunnan Univ. TCM. 2011;34:21–27. [Google Scholar]
- Hseu Y.C., Chang W.H., Chen C.S., Liao J.W., Huang C.J., Lu F.J., Chia Y.C., Hsu H.K., Wu J.J., Yang H.L. Antioxidant activities of Toona sinensis leaves extracts using different antioxidant models. Food Chem. Toxicol. 2008;46:105–114. doi: 10.1016/j.fct.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Hseu Y.C., Chen S.C., Lin W.H., Hung D.Z., Lin M.K., Kuo Y.H., Wang M.T., Cho H.J., Wang L., Yang H.L. Toona sinensis (leaf extracts) inhibit vascular endothelial growth factor (VEGF)-induced angiogenesis in vascular endothelial cells. J. Ethnopharmacol. 2011;134:111–121. doi: 10.1016/j.jep.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Hsiang C.Y., Hseu Y.C., Chang Y.C., Kumar K.J., Ho T.Y., Yang H.L. Toona sinensis and its major bioactive compound gallic acid inhibit LPS-induced inflammation in nuclear factor-κB transgenic mice as evaluated by in vivo bioluminescence imaging. Food Chem. 2013;136:426–434. doi: 10.1016/j.foodchem.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Hsieh C.L., Lin Y.C., Ko W.S., Peng C.H., Huang C.N., Peng R.Y. Inhibitory effect of some selected nutraceutic herbs on LDL glycation induced by glucose and glyoxal. J. Ethnopharmacol. 2005;102:357–363. doi: 10.1016/j.jep.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Hsieh T.J., Liu T.Z., Chia Y.C., Chern C.L., Lu F.J., Chuang M.C., Mau S.Y., Chen S.H., Syu Y.H., Chen C.H. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. Food Chem. Toxicol. 2004;42:843–850. doi: 10.1016/j.fct.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Hsieh T.J., Wang J.C., Hu C.Y., Li C.T., Kuo C.M., Hsieh S.L. Effects of rutin from Toona sinensis on the immune and physiological responses of white shrimp (Litopenaeus vannamei) under vibrio alginolyticus challenge. Fish Shellfish Immunol. 2008;25:581–588. doi: 10.1016/j.fsi.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Hsieh T.J., Tsai Y.H., Liao M.C., Du Y.C., Lien P.J., Sun C.C., Chang F.R., Wu Y.C. Anti-diabetic properties of non-polar Toona sinensis Roem extract prepared by supercritical-CO2 fluid. Food Chem. Toxicol. 2012;50:779–789. doi: 10.1016/j.fct.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Hsu H.K., Yang Y.C., Hwang J.H., Hong S.J. Effects of Toona sinensis leaf extract on lipolysis in differentiated 3T3-L1 adipocytes. Kaohsiung J. Med. Sci. 2003;19:385–390. doi: 10.1016/S1607-551X(09)70481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.J., Hseu Y.C., Lee M.S., Senthil K.J., Wu C.R., Hsu L.S., Liao J.W., Cheng I.S., Kuo Y.T., Huang S.Y. In vitro and in vivo activity of gallic acid and Toona sinensis leaf extracts against HL-60 human premyelocytic leukemia. Food Chem. Toxicol. 2012;50:3489–3497. doi: 10.1016/j.fct.2012.06.046. [DOI] [PubMed] [Google Scholar]
- James J.T., Dubery I.A. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) urban. Molecules. 2009;14:3922–3941. doi: 10.3390/molecules14103922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G.L., Chen C. Experimental study on anticoagulation activity of n-butanol extract of Toona sinensis seeds. Chin. Hosp. Pharm. J. 2011;31:913–914. [Google Scholar]
- Kakumu A., Ninomiya M., Efdi M., Adfa M., Hayashi M., Tanaka K., Koketsu M. Phytochemical analysis and antileukemic activity of polyphenolic constituents of Toona sinensis. Bioorg. Med. Chem. Lett. 2014;24:4286–4290. doi: 10.1016/j.bmcl.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Lee I.S., Kim H.J., Youn U.J., Chen Q.C., Kim J.P., Ha D.T., Ngoc T.M., Min B.S., Lee S.M., Jung H.J. Dihydrobenzofuran norlignans from the leaves of Cedrela sinensis A. Juss. Helv. Chim. Acta. 2010;3:272–276. [Google Scholar]
- Liang N., Wang C.L., Luo C., Chen M.H., Wang Y.R., Li F.J. Effect of Toona sinensis leaves extract against to gout. Acad. Period. Farm. Prod. Process. 2011;7:12–14. [Google Scholar]
- Liao J.W., Yeh J.Y., Lin Y.C., Wei M.M., Chung Y.C. Mutagenicity and safety evaluation of water extract of fermented Toona sinensis Roemor leaves. J. Food Sci. 2009;74:T7–T13. doi: 10.1111/j.1750-3841.2008.01007.x. [DOI] [PubMed] [Google Scholar]
- Liao J.W., Chung Y.C., Yeh J.Y., Lin Y.C., Lin Y.G., Wu S.M., Chan Y.C. Safety evaluation of water extracts of Toona sinensis Roemor leaf. Food Chem. Toxicol. 2007;45:1393–1399. doi: 10.1016/j.fct.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Li H.Y., Chen C. Interventional effects of pretreatment with total polyphenols extracted from Toona sinensis on the injury induced by myocardial ischemia–reperfusion in rats. Med. J. Chin. PLA. 2011;36:58–60. [Google Scholar]
- Li H.Y., Chen C. Protective effects of total polyphenols extracted from Toona sinensis on myocardial ischemia/reperfusion-induced injury in rats. Chin. J. Exp. Trad. Med. Formul. 2011;17:117–119. [Google Scholar]
- Li H.Y., Chen C. Mechanism of total polyphenols extracted from Toona sinensis Roem on acute inflammation during myocardial ischemia–reperfusion in rats. Chin. J. Exp. Trad. Med. Formul. 2012:187–190. [Google Scholar]
- Li J.X., Eidman K., Gan X.W., Haefliger O.P., Carroll P.J., Pika J. Identification of (S,S)-γ-glutamyl-(cis-S-1-propenyl) thioglycine, a naturally occurring norcysteine derivative, from the Chinese vegetable Toona sinensis. J. Agric. Food Chem. 2013;61:7470–7476. doi: 10.1021/jf401946d. [DOI] [PubMed] [Google Scholar]
- Liu H.W., Huang W.C., Yu W.J., Chang S.J. Toona sinensis ameliorates insulin resistance via AMPK and PPARγ pathways. Food Funct. 2015;6:1855–1864. doi: 10.1039/c5fo00056d. [DOI] [PubMed] [Google Scholar]
- Liu H.W., Tsai Y.T., Chang S.J. Toona sinensis leaf extract inhibits lipid accumulation through up-regulation of genes involved in lipolysis and fatty acid oxidation in adipocytes. J. Agric. Food Chem. 2014;62:5887–5896. doi: 10.1021/jf500714c. [DOI] [PubMed] [Google Scholar]
- Liu J., You L., Wang C., Liu R. Antioxidization and antiproliferation of extract from leaves of Toona sinensis. J. Cent. South Univ. 2012;37:42–47. doi: 10.3969/j.issn.1672-7347.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Li G.J., Wang F. The analysis of chemical composition of volatile oil from Toona sinensis Roem by GC–MS. Anhui Chem. Ind. 2014;40:85–88. [Google Scholar]
- Li G.C., Yu X.X., Liao R.F., Wang D.Y. Chemical constituents of the bark of Toona sinensis. Chin. J. Hosp. Pharm. 2006;26:949–952. [Google Scholar]
- Li W.Z., Wang X.H., Zhang H.X., Mao S.M., Zhao C.Z. Protective effect of the n-butanol Toona sinensis seed extract on diabetic nephropathy rat kidneys. Genet. Mol. Res. 2016;15 doi: 10.4238/gmr.15017403. [DOI] [PubMed] [Google Scholar]
- Liu Z.Q., Chen C. Effect of n-butanol extract from Toona sinensis on the body fibrinolysis system. Shaanxi J. TCM. 2009;30:1256–1257. [Google Scholar]
- Luo X.D., Wu S.H., Ma Y.B., Wu D.G. Limonoids and phytol derivatives from Cedrela sinensis. Fitoterapia. 2000;71:492–496. doi: 10.1016/s0367-326x(00)00158-1. [DOI] [PubMed] [Google Scholar]
- Luo X.D., Wu S.H., Ma Y.B., Wu D.G. Studies on chemical constituents of Toona sinensis. Chin. Trad. Herb. Drugs. 2001;32:390–391. [Google Scholar]
- Mitsui K., Maejima M., Fukaya H., Hitotsuyanagi Y., Takeya K. Limonoids from Cedrela sinensis. Phytochemistry. 2004;65:3075–3081. doi: 10.1016/j.phytochem.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Mitsui K., Saito H., Yamamura R., Fukaya H., Hitotsuyanagi Y., Takeya K. Apotirucallane and tirucallane triterpenoids from Cedrela sinensis. Chem. Pharm. Bull. 2007;55:1442–1447. doi: 10.1248/cpb.55.1442. [DOI] [PubMed] [Google Scholar]
- Mitsui K., Saito H., Yamamura R., Fukaya H., Hitotsuyanagi Y., Takeya K. Hydroxylated gedunin derivatives from Cedrela sinensis. J. Nat. Prod. 2006;69:1310–1314. doi: 10.1021/np068021f. [DOI] [PubMed] [Google Scholar]
- Mu R., Wang X., Liu S., Yuan X., Wang S., Fan Z. Rapid determination of volatile compounds in Toona sinensis (A. Juss.) Roem. by MAE-HS-SPME followed by GC–MS. Chromatographia. 2007;65:463–467. [Google Scholar]
- Park J.C., Yu Y.B., Lee J.H., Choi J.S., Ok K.D. Phenolic compounds from the rachis of Cedrela sinensis. Korean J. Pharmacogn. 1996;27:219–223. [Google Scholar]
- Perry L.M. MIT Press; Cambridge, MA, USA: 1980. Medical Plants of East and Southeast Asia: Attributed Properties and Uses; p. 263. [Google Scholar]
- Poon S.L., Leu S.F., Hsu H.K., Liu M.Y., Huang B.M. Regulatory mechanism of Toona sinensis on mouse leydig cell steroidogenesis. Life Sci. 2005;76:1473–1487. doi: 10.1016/j.lfs.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Ruan Z.P., Chen Y.L., Lin S.S. Anti-inflammation effect and its mechanism of aqueous extract from Toona sinensis. Chin. J. Public Health. 2010;26:334–335. [Google Scholar]
- Shen Y.P., Zhong X.X., Yu X.J., Zhou C.S., Yang H., Jia X.B. Chemical constituents of Toona Sinensis leaves. Chin. Pharm. J. 2013;48:22–24. [Google Scholar]
- Su Y.F., Yang Y.C., Hsu H.K., Hwang S.L., Lee K.S., Lieu A.S., Chan T.F., Lin C.L. Toona sinensis leaf extract has antinociceptive effect comparable with non-steroidal anti-inflammatory agents in mouse writhing test. BMC Compl. Alt. Med. 2015 doi: 10.1186/s12906-015-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Xu J., Zhang J., Liu W.Y., Xie N., Chen L., Feng F., Qu W. Novel tirucallane triterpenoids from the stem bark of Toona sinensis. Fitoterapia. 2016;112:97–103. doi: 10.1016/j.fitote.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Truong V.L., Ko S.Y., Jun M., Jeong W.S. Quercitrin from Toona sinensis (Juss.) M. Roem. attenuates acetaminophen-induced acute liver toxicity in HepG2 cells and mice through induction of antioxidant machinery and inhibition of inflammation. Nutrients. 2016;8:431. doi: 10.3390/nu8070431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.C., Tsai Y.J., Hsieh Y.C., Lin R.J., Lin C.L. The aqueous extract from Toona sinensis leaves inhibits microglia-mediated neuro-inflammation. Kaohsiung J. Med. Sci. 2014;30:73–81. doi: 10.1016/j.kjms.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.L., Li Z.J., Jiang S.H., Li F.J., Chen M.H., Wang Y.R., Li Z., Luo C. Hypouricemic action of total flavonoids from Toona sinensis leaves. Liaoning J. TCM. 2011;38:1933–1935. [Google Scholar]
- Wang C.L., Ren L., Chen Z.Q., Jiang S.H., Liu C.J., Xia L.F. Study on antioxidant effects of polyphenols from old leaves of Toona sinensis (A. Juss) Roem. Chem. Ind. Forest Prod. 2008;28:89–92. [Google Scholar]
- Wang C.Y., Lin K.H., Yang C.J., Tsai J.R., Hung J.Y., Wang P.H., Hsu H.K., Huang M.S. Toona sinensis extracts induced cell cycle arrest and apoptosis in the human lung large cell carcinoma. Kaohsiung J. Med. Sci. 2010;26:68–75. doi: 10.1016/S1607-551X(10)70010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.H., Tsai M.J., Hsu C.Y., Wang C.Y., Hsu H.K., Weng C.F. Toona sinensis Roem (Meliaceae) leaf extract alleviates hyperglycemia via altering adipose glucose transporter 4. Food Chem. Toxicol. 2008;46:2554–2560. doi: 10.1016/j.fct.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Wang Q., Kuang H., Su Y., Sun Y., Feng J., Guo R., Chan K. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J. Ethnopharmacol. 2013;146:9–39. doi: 10.1016/j.jep.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Wang X.B., Gu Q.Y., Shen Y.P., Qin D., Yang H., Jia X.B. Research progress on the chemical constituents of Toona sinensis. J. Nanjing Univ. TCM. 2014;30:396–400. [Google Scholar]
- Wang W.C., Chen C.Y., Hsu H.K., Lin L.M., Chen Y.K. Chemopreventive effect of Toona sinensis leaf extract on 7,12-dimethylbenz[α]anthracene-induced hamster buccal pouch squamous cell carcinogenesis. Arch. Oral Biol. 2016;70:130–142. doi: 10.1016/j.archoralbio.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Wu C.C., Liu C.H., Chang Y.P., Hsieh S.L. Effects of hot-water extract of Toona sinensis on immune response and resistance to Aeromonas hydrophila in oreochromis mossambicus. Fish Shellfish Immunol. 2010;29:258–263. doi: 10.1016/j.fsi.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Wu J.G., Peng W., Yi J., Wu Y.B., Chen T.Q., Wong K.H., Wu J.Z. Chemical composition, antimicrobial activity against Staphylococcus aureus and a pro-apoptotic effect in SGC-7901 of the essential oil from Toona sinensis (A. Juss.) Roem. leaves. J. Ethnopharmacol. 2014;154:198–205. doi: 10.1016/j.jep.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S.S., Chen C. Study on the antioxidation of polyphenols from the seeds of Toona sinensis (A. Juss) Roem in vitro. J. Anhui Agric. Sci. 2010;38:7285–7287. [Google Scholar]
- Xing S.S., Chen C. Hypoglycemic effect of total polyphenols from seeds of Toona sinensis in diabetic mice. Chin. J. Exp. Trad. Med. Formul. 2011;17:169–171. [Google Scholar]
- Yang H.L., Huang P.J., Liu Y.R., Kumar K.J., Hsu L.S., Lu T.L., Chia Y.C., Takajo T., Kazunori A., Hseu Y.C. Toona sinensis inhibits LPS-induced inflammation and migration in vascular smooth muscle cells via suppression of reactive oxygen species and NF-κB signaling pathway. Oxid. Med. Cell Longev. 2014;2014:901315. doi: 10.1155/2014/901315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.J., Huang Y.J., Wang C.Y., Wang P.H., Hsu H.K., Tsai M.J., Chen Y.C., Bharath K.V., Huang M.S., Weng C.F. Antiproliferative effect of Toona sinensis leaf extract on non-small-cell lung cancer. Transl. Res. 2010;155:305–314. doi: 10.1016/j.trsl.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.J., Huang Y.J., Wang C.Y., Wang C.S., Wang P.H., Hung J.Y., Wang T.H., Hsu H.K., Huang H.W., Kumar S.P., Huang M.S., Weng C.F. Antiproliferative and antitumorigenic activity of Toona sinensis leaf extracts in lung adenocarcinoma. J. Med. Food. 2010;13:54–61. doi: 10.1089/jmf.2009.1166. [DOI] [PubMed] [Google Scholar]
- Yang H.L., Chen S.C., Lin K.Y., Wang M.T., Chen Y.C., Huang H.C., Cho H.J., Wang L., Kumar K.J., Hseu Y.C. Antioxidant activities of aqueous leaf extracts of Toona sinensis on free radical-induced endothelial cell damage. J. Ethnopharmacol. 2011;137:669–680. doi: 10.1016/j.jep.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Yang H.L., Thiyagarajan V., Liao J.W., Chu Y.L., Chang C.T., Huang P.J., Hsu C.J., Hseu Y.C. Toona sinensis inhibits murine leukemia WEHI-3 cells and promotes immune response in vivo. Integr. Cancer Ther. 2017;16:308–318. doi: 10.1177/1534735416642863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Zhao Q., Xiang H., Liu M., Zhang Q., Xue W., Song B., Yang S. Antiproliferative activity and apoptosis-inducing mechanism of constituents from Toona sinensis on human cancer cells. Cancer Cell Int. 2013;13:12. doi: 10.1186/1475-2867-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.X., Wang C.L., Cui G.Y., Chen M.H., Wang Y.R., Han H.J. Retarding ageing effect of Toona sinensis leaf flavonoids on Caenorhabditis elegans. Modern Food Sci. Technol. 2010;26:931–937. [Google Scholar]
- Yang Y.C., Hsu H.K., Hwang J.H., Hong S.J. Enhancement of glucose uptake in 3T3-L1 adipocytes by Toona sinensis leaf extract. Kaohsiung J. Med. Sci. 2003;19:327–333. doi: 10.1016/S1607-551X(09)70433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.L., Chen C. Effects of total polyphenols from seeds of Toona Sinensis in treating adjuvant-induced arthritis rats. Chin. J. Modern Appl. Pharm. 2012;29:1073–1077. [Google Scholar]
- You H.L., Chen C.J., Eng H.L., Liao P.L., Huang S.T. The effectiveness and mechanism of Toona sinensis extract inhibit attachment of pandemic influenza A (H1N1) virus. Evid. Based Complement. Altern. Med. 2013 doi: 10.1155/2013/47971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C., Chen C., You Y., Fu C., Li H., Pan N., He Z. Protection of n-butanol extract from seeds of Toon sinensis on multiple organ dysfunction syndrome caused by brain ischemia–reperfusion in rats. Chin. Trad. Herbal Drugs. 2013;44:323–326. [Google Scholar]
- Yu W.J., Chang C.C., Kuo T.F., Tsai T.C., Chang S.J. Toona sinensis Roem leaf extracts improve antioxidant activity in the liver of rats under oxidative stress. Food Chem. Toxicol. 2012;50:1860–1865. doi: 10.1016/j.fct.2012.03.068. [DOI] [PubMed] [Google Scholar]
- Yu B.C., Yu W.J., Huang C.Y., Chen Y.H., Tsai Y.C., Chang C.C., Chang S.J. Toona sinensis leaf aqueous extract improves the functions of sperm and testes via regulating testicular proteins in rats under oxidative stress. Evid. Based Complement. Altern. Med. 2012 doi: 10.1155/2012/681328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Q., Zhang Z.P. The isolation and identification of quercetin from leaves of Toona sinensis (A. Juss) Roem. J. Shandong Univ. TCM. 2000;24 F003. [Google Scholar]
- Zhang J.F., Wang D.M., Zhou L., Lu G., Tian L.W. Studies on antioxidative activities in vitro of different polarity fractions of extract from Toona Sinensis leaves. J. Chin. Inst. Food Sci. Technol. 2007;7:12–17. [Google Scholar]
- Zhang D., Jiang F.L., Huang L., Chen Y.L., Shao L.Q., Chai C.B., Wang F.X., Zuo X.C. Effects of total flavonoid of Toona sinensis leaves on the blood glucose of diabetes mice. Northwest Pharm. J. 2011;26:270–271. [Google Scholar]
- Zhang J.F., Yang J.Y., Wen J., Wang D.Y., Yang M., Liu Q.X. Experimental studies on hypoglycemic effects of total favonoid from Toona sinensis. J. Chin. Med. Mater. 2008;31:1712–1714. [PubMed] [Google Scholar]
- Zhang Z.P., Niu C., Sun Y. The isolation and identification of flavonoids from Toona sinensis. J. Chin. Med. Mater. 2001;24:725–726. [Google Scholar]
- Zhang Y.L., Dong H.H., Wang M.M., Zhang J.F. Quercetin isolated from Toona sinensis leaves attenuates hyperglycemia and protects hepatocytes in high-carbohydrate/high-fat diet and alloxan induced experimental diabetic mice. J. Diabetes Res. 2016;2016:8492780. doi: 10.1155/2016/8492780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhou X.W., Chen X.B., Wang Q.X. α-Glucosidase inhibitory constituents from Toona sinensis. Chem. Nat. Compd. 2009;45:244–246. [Google Scholar]
- Zhao Q., Sun Q.Y., Yang Q.X. Effects of an anticomplementary polyphenol from the seed of Toona sinensis on complement-injured SH-SY5Y cells. Chin. Pharm. Bull. 2011;27:1086–1090. [Google Scholar]
- Zhou M., Zhang R.H., Wang M., Xu G.B., Liao S.G. Prodrugs of triterpenoids and their derivatives. Eur. J. Med. Chem. 2017;131:222–236. doi: 10.1016/j.ejmech.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Zhen H., Zhang Y., Fang Z., Huang Z., You C., Shi P. Toona sinensis and moschus decoction induced cell cycle arrest in human cervical carcinoma HeLa cells. Evid. Based Complement. Altern. Med. 2014;2014:121276. doi: 10.1155/2014/121276. [DOI] [PMC free article] [PubMed] [Google Scholar]




