Abstract
Climate change is expected to impact across every domain of society, including health. The majority of the world's population is susceptible to pathological, infectious disease whose life cycles are sensitive to environmental factors across different physical phases including air, water and soil. Nearly all so-called neglected tropical diseases (NTDs) fall into this category, meaning that future geographic patterns of transmission of dozens of infections are likely to be affected by climate change over the short (seasonal), medium (annual) and long (decadal) term. This review offers an introduction into the terms and processes deployed in modelling climate change and reviews the state of the art in terms of research into how climate change may affect future transmission of NTDs. The 34 infections included in this chapter are drawn from the WHO NTD list and the WHO blueprint list of priority diseases. For the majority of infections, some evidence is available of which environmental factors contribute to the population biology of parasites, vectors and zoonotic hosts. There is a general paucity of published research on the potential effects of decadal climate change, with some exceptions, mainly in vector-borne diseases.
Keywords: Climate change, NTDs, Zoonoses, Vectors, Viruses, Helminths, Protozoans, Bacteria
1. Introduction
Much has been written about climate change and its potential impact on civilisations in the coming decades. The news is rarely positive—from predicting an increasing frequency of el Nino events (Cai et al., 2014) to reduction in biodiversity (Mantyka-Pringle et al., 2015) and reduced wheat production (Asseng et al., 2015). There are likely to be substantial effects on illness and mortality statistics, disproportionately affecting poorer regions (Patz et al., 2005). The consensus now rests with those who consider the anticipated change to be anthropogenic in nature. Despite this consensus, however, there is still much uncertainty about what the future holds for wider aspects of human health (Wardekker et al., 2012).
Within the wider domain of climate and health, the neglected tropical diseases (Hotez et al., 2006), often abbreviated to NTDs, are a collection of infectious diseases affecting hundreds of millions of individuals living in tropical countries. In recent years, there has been considerable increase in investment towards reducing the burden of several NTDs (Molyneux et al., 2017), but they still collectively contribute to productivity loss (reviewed by Conteh et al., 2010), illness and suffering in many countries, including several within the G20 (Hotez, 2014). Recent estimates of their overall burden suggest NTD kill over 350,000 people per annum and cause the loss of between 27 and 56 million disability-adjusted life years (Hotez et al., 2014).
Climate change projections are typically associated looking forward several decades, often reaching out as far as 100 years or more (Collins et al., 2013). NTDs are a contemporary issue and are subject to attempts to eliminate them as a public health problem, or even eradicate them from the planet. The timescale for these activities is typically around a decade. At the time of writing, the dates 2020 and 2030 feature prominently in documents including the WHO roadmaps (World Health Organization, 2012, World Health Organisation, 2013a, World Health Organization, 2016a).
The latest WHO documents regarding the roadmap for NTD control also mentions climate change in a number of places (World Health Organisation, 2017). There exists a ‘Climate and Health atlas’, published in 2012 (World Health Organisation, 2013b) that is referred to by the WHO literature, and ‘which explores the numerous and variable effects of climate change on infectious diseases, including NTDs’. On closer inspection, however, the Climate and Health Atlas contains only material on meningitis, dengue, malaria and diarrhoea.
Simultaneous to the technical and targeted approaches being recommended by WHO are much wider attempts at sustainable development, most visible through the lens of the sustainable development goals (Griggs et al., 2013). Aspects of the NTD impact on health and productivity permeate many SDG themes (Bangert et al., 2017), including Goal 3 (Health), which even contains a target for NTDs, namely Target 3.3: ‘By 2030, end the epidemics of AIDS, tuberculosis, malaria and neglected tropical diseases and combat hepatitis, water-borne diseases and other communicable diseases’ (Fitzpatrick and Engels, 2016).
Success in implementing the WHO plans and the SDGs could spell the end for some or all the NTDs. But considerable literature exists, primarily derived from studies of parasites affecting wildlife that global environmental change may lead to responses by hosts, vectors and parasites themselves that could affect the outcome of interventions (Cable et al., 2017). Thus, it will be important to consider not just meeting targets set within the SDG and WHO documents, but how those targets are met.
In reviewing how decadal climate change may impact on the future transmission of the NTDs, it is necessary to be somewhat pessimistic and assume that most NTDs are not going to be eliminated or eradicated by 2030. This includes assuming that policy change associated with SDG Goal 13 ‘Action on Climate’ does not result in returning the climate to preindustrial levels—a target that at the time of writing looked increasingly unreachable (UNEP, 2017).
The tension between contemporary knowledge and future projections can be resolved partially by deploying a universal caveat—namely by stating that future projections may be valid, ‘all other things being equal’. Given future uncertainties in terms of climate change scenarios (described below), it is highly unlikely that anything will remain equal over the coming decades. Anthropogenic activities connected to, or independent of, climate change will also have an impact, e.g., through early case detection combined with equal access to medicines. The point of existing research into climate change and health is therefore not to give definitive conclusions but to reach interim conclusions that feed into the next round of projections which can consider a range of natural and anthropogenic interventions.
The ‘precautionary principle’ as applied widely to environmental science (Kriebel et al., 2001), and specifically to climate change (Hallegatte, 2009) is also relevant in terms of understanding why climate change research is important. Under this principle, it is not necessary to fully understand the factors that underpin and contribute to a particular situation in order to take action. However, it is also important to recognise that (1) any particular action can have unintended consequences and (2) linearly scaled up solutions do not always work as intended across all scales of intervention (Mangham and Hanson, 2010). Thus, applying the precautionary principle in terms of action against climate change, or any other domain contained within the SDGs, may not sufficient to guarantee a future free of NTDs.
2. Aims
The main aims of this chapter are (1) to review crosscutting issues that are likely to affect future transmission of NTDs, (2) to provide information about the current state of the art with respect to investigations into climate change and NTD transmission and (3) to identify gaps in knowledge with a view to identifying potential areas of research activity. The review considers 34 different species of established or emerging public health importance, drawn from the WHO list of NTDs and the WHO blueprint list of priority diseases. Infections are listed in Table 1 along with brief descriptions of their climate-sensitive life stages.
Table 1.
List of Infections Considered Within This Chapter Drawn From the WHO R&D Blueprint Diseases (A) and the WHO NTD List (B), Together With Their Poikilothermic (Climate Sensitive) Stages and/or Vectors and/or Zoonotic Hosts
| A. WHO Priority Diseases | Vectors | Zoonotic or Intermediate Hosts | Poikilothermic Stages |
|---|---|---|---|
| Arenaviral haemorrhagic fevers (including Lassa fever) | — | Mastomys spp. | Mastomys urine, faeces |
| Crimean Congo Haemorrhagic Fever (CCHF) | Ioxid ticks | — | — |
| Filoviral diseases (including Ebola and Marburg) | — | Diverse taxa including bats and apes | — |
| Corona viruses (MERS-CoV and SARS) | — | Bats and palm civets | Bat excreta and aerosolised virus |
| Nipah and related henipaviral diseases | — | Fruit bats | Bat excreta |
| Rift Valley fever (RVF) | Various mosquitoes including Anopheles and Culex | — | — |
| Severe fever with thrombocytopenia syndrome (SFTS) | Ioxid ticks | — | — |
| Zika | Aedes | — | — |
| B. WHO NTD List | Vectors | Zoonotic or Intermediate Hosts | Poikilothermic Stages |
|---|---|---|---|
| Buruli ulcer | Naucoridae | Fish and shellfish | Unknown |
| Chagas disease | Triatominae | Dogs and other mammals | — |
| Dengue | Aedes | ||
| Echinococcosis | — | Canidae, farmed mammals | Eggs in soil |
| African trypanosomiasis | Glossina | — | — |
| Leishmaniasis | Phlebotominae | Dogs | |
| Leprosy | — | — | Bacterium in water |
| Lymphatic filariasis | Anopheles and Culex | — | — |
| Onchocerciasis | Simulium | — | — |
| Rabies | — | Dogs and bats | — |
| Schistosomiasis | — | Water snails | Miracidia and cercaria |
| Soil-transmitted helminthiasis | — | — | Eggs in soil |
| Guinea worm | Copepods | Larvae | |
| Cysticercosis | — | Swine | Eggs in soil |
| Trachoma | Musca | — | — |
| Fascioliasis | Freshwater snails | Eggs, miracidia and cercariae | |
| Paragonimus | Crustaceans | Eggs, miracidia and (meta)cercariae | |
| Clonorchiasis and Opisthorchiasis | Freshwater snails, fish and crustaceans | Eggs, miracidia and (meta)cercariae |
3. A Parasitologist's Guide to Climate Change
Detailed insights into the causes and drivers of climate change are available elsewhere (IPCC, 2013). To understand how climate change may change the landscape of transmission for NTDs, I begin with some macroscale considerations of the underpinning physics of climate change and a description of how we interpret the climate change vernacular.
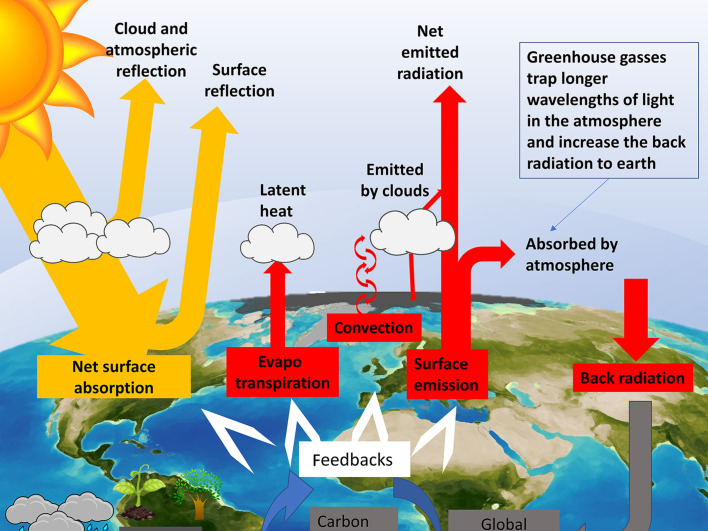
Historic observations support the basic tenet of climate change which is that increasing levels of so-called greenhouse gases have driven upwards the global mean surface temperature (Hartmann et al., 2013). The characteristic of a greenhouse gas is that it influences ‘radiative forcing’ towards a more positive value. Radiative forcing is defined as a rate of change in energy per unit area (measured in W/m2) of the upper atmosphere. As greenhouse gasses (also known as radiative forcing components or climate sensitivities) trap more of the incoming energy from the sun, so the ratio of incoming vs reflected energy gets greater and the radiative forcing value increases (Fig. 1 ).
Fig. 1.
The role of greenhouses in terms of climate change is to affect the balance between surface and atmospheric energy absorption and emission (the energy budget). Increasing the back radiation will affect global temperature changes, the carbon and water cycles and have both direct and indirect effects at various scales across multiple domains of organisation as illustrated in Fig. 2.
The Intergovernmental Panel on Climate Change (IPCC) has adopted several representative climate change scenarios throughout its working history. The aim of these scenarios has consistently been to present policymakers and research scientist possible outcomes associated with specific narratives which have then been modelled using methods summarised later. The first iteration of these scenarios consisted of so-called ‘Special Report on Emissions Scenarios’ (Nakicenovic et al., 2000). They are known as A1, B1, A2 and B2. The A1 scenario predicts a future world that converges in terms of rapid economic growth, and rapid introduction of more efficient technologies. The A2 scenario indicates a more fragmented world with slower rates of development. The B1 scenario describes a convergent world with a static population, and the B2 scenario describes a world with intermediate population growth and technological development.
The so-called relative concentration pathways (RCPs), rather than being based on socioeconomic scenarios, use radiative forcing narratives to project global warming trends over the coming decades. They differ from the SRES family by decoupling climate modelling from scenario development and thereby allow for independent modelling of specific interventions, rather than having them built in at the onset. These scenarios are known as RCP2.6 (van Vuuren et al., 2011a), RCP4.5 (Thomson et al. 2011), RCP6 (Masui et al., 2011) and RCP8.5 (Riahi et al., 2011). The nomenclature of these pathways corresponds specifically to the anticipated change in the global average level of radiative forcing in the year 2100 compared to preindustrial levels. So, for example, RCP2.6 represents a change of 2.6 W/m2 over this time period.
The RCPs have been developed over several years by research teams that modelled changes to the atmosphere based on projected anthropogenic drivers of greenhouse gasses. Each RCP imagines a particular future where levels of CO2 and other gasses are either reduced or increased by changes in the drivers of emissions. For example, RCP2.6 is the output of models that combine reforestation programmes, reduced methane emissions and moderate population growth. Conversely, RCP8.5 considers a future where methane emissions increase substantially, there is considerable population growth and continued heavy reliance on fossil fuels. RCP2.6 is considered to be ‘reversible’ and in the underpinning models it is suggested that emissions will peak in 2050 before returning to historically normal values by 2100. Conversely, RCP8.5 summarises a future of no climate policies and no possibility of return to historic levels of emission.
RCPs are associated with integrated assessment models (van Vuuren et al., 2011b) to produce time series data of emissions that act as inputs into more complex Atmosphere-Ocean Global Circulation models (AOGCMs). The next step in producing state-of-the-art projections is to develop earth system models (ESMs) that include both land and ocean biogeochemistry. Combining AOGCMs and ESMs leads to multimodel ‘experiments’ that project global temperature, precipitation and other variables over coming decades. The experiment known as CMIP5 is capable of producing dozens of different simulated output variables (Taylor et al., 2012) including snowfall flux, zooplankton carbon concentration, near-surface wind speed, evaporation, soil temperature and water content. The spatial and temporal resolutions of these outputs can be specified depending on need and within the limits of the available IT infrastructure. Daily estimates of precipitation and temperature are now available, for example through the NASA Earth Exchange Global Daily Downscaled Projections (NEX-GDDP) at a temporal resolution of 1 day and a spatial resolution of 0.25 degree (approximately 25 × 25 km). One of the criticisms of highly detailed models is that as they become more realistic, they become more uncertain (Maslin and Austin, 2012).
4. Crosscutting Environmental and Anthropocentric Issues
Evidence suggests that recent climate change is already affecting the phenology of a wide range of organisms across the globe (Walther et al., 2002). From the relatively simple concept of climate forcing springs a hugely complex and interactive web of interacting ecosystems that might impact on the ecology of hosts, parasites and vectors over both time and space. This idea has been previously and commonly referred to as chaos theory, or the butterfly effect, originally proposed by Lorenz (1963) in terms of long-term weather prediction. Below I summarise some important elements of anticipated change that evidence suggests may impact on NTD ecology.
4.1. Asynchrony
Life cycles of several parasitic infections, particularly those with a life cycle involving a vector or intermediate host, rely on circadian rhythms to ensure that transmission stages are available at the same time as the host is exposed to the intermediate host or vector. For example, it has been long established that malaria parasites exhibit circadian patterns in emergence from red-blood cells (Mideo et al., 2013), and also well known that schistosome cercariae exhibit a circadian pattern of emergence from snail hosts (Mintsa-Nguéma et al., 2014). Less well known is that humans excrete eggs of schistosome parasites in a circadian pattern, with peak excretion late morning (Doehring et al., 1983; Hawking, 1975). Climate change has the potential to create asynchrony by either changing host behaviour (e.g. time of faecal expulsion change as a result of abiotic and biotic pressures on host behaviour), or by disrupting the availability of hosts at the time required to complete the life cycle (e.g., by forcing a range shift). Evidence for this occurring already has emerged from studies of livestock carrying Nematodirus battus (Gethings et al., 2015).
The net result of asynchrony may be to reduce disease in the short term, but it may also place a selection pressure on the parasites, selecting those variants that induce the host to expel transmission stages at a time appropriate to the new system. Variation in the timing of peak output of schistosome cercariae from snails has been recorded across different species of definitive host (Théron, 2015)—indicating that selection pressures can alter circadian emergence patterns. It remains to be seen whether the selection pressure will be sufficiently strong to produce new timing peaks in the future, and whether the potential for reducing disease will be offset by increased abundance of both vectors and hosts.
4.2. Scale
NTD is so called because their geographical distribution is bounded by environmental conditions normally encountered between the lines of latitude denoted as the tropic of Cancer and Capricorn. There is some evidence that the width of the tropics, or at least the arid tropical edge, is increasing northwards and southwards at a rate of between 0.5 and 1 degrees latitude in each direction each decade (Lucas et al., 2014), possibly in part due to stratospheric ozone depletion at the poles (Kang et al., 2011). What remains unclear is not just how best to measure changes in the area constituting the tropics and subtropics (reviewed by Lucas et al., 2014), but also how individual vegetation and other zones within the geographic tropics will vary locally in their biotic and abiotic characteristics.
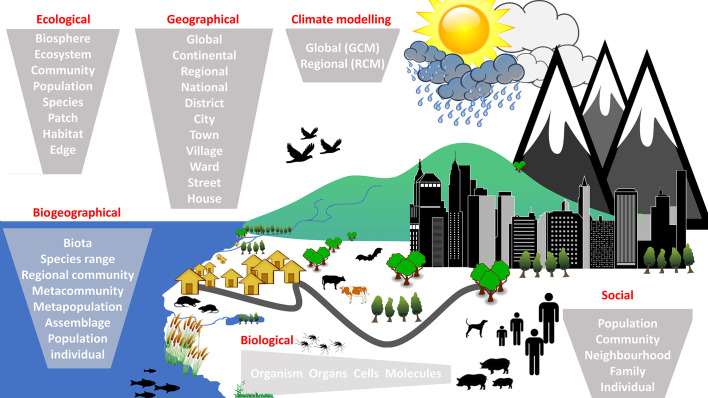
Climate change is likely to have an effect at every scale of biological, social, ecological and geographical organisation (Fig. 2 ). Local scale considerations are important in terms understanding the effects of climate change on NTDs because the life history traits of many species of organism involved in NTD life cycles are tied to a particular environmental envelope. Ecologists continue to debate whether or not heterogeneity in abiotic resources at a particular scale is associated with diversity of organisms (Lundholm, 2009), but it remains true that microclimatic variation is a driver of species abundance at a very local level. The availability of specific habitats is a requirement for many vector and intermediate host species. For example, eggs of helminth species require specific abiotic and biotic conditions to thrive. Intermediate snail hosts require vegetation that is anchored in a substrate that will supply appropriate nutrients.
Fig. 2.
Illustration of the range of scales within ecological, biogeographical, social and geographical domains of organisation that will be affected directly or indirectly by climate change. The terms ‘macro’ and ‘micro’ are relative to each domain.
The relationship between spatial diversity in abiotic, biotic resources, host availability and parasite distribution remains poorly understood in the NTD context. Studies of wildlife populations offer some insights into the drivers of current relationships (Ellis et al., 2015), pointing to a complex system of host-switching and localised adaption driven by host availability at specific locations.
As the local soil and water chemistry alters as a result of changes to the local climate, it can be expected that current patterns of heterogeneous transmission will change in the future. Areas that are currently unsuitable for transmission, perhaps because of a lack of suitable vegetation to support an intermediate or zoonotic host, may become more suitable at some point in the future.
4.3. Population Movement, Urbanisation and Growth
While considering the effects of changing temperature and precipitation patterns is a vital component of understanding climate change and NTDs, it is also necessary to take a step sideways to consider other anthropocentric aspects of global environmental change that are directly or indirectly connected to climate change.
Population movement for reasons ranging from tourism to labour migration is an important component in the epidemiology of several NTDs (Aagaard-Hansen et al., 2010). Urbanisation, as a major subdomain of population movement is now considered to be not just a driver of climate change (Kalnay and Cai, 2003), but also a consequence of climate change (Barrios et al., 2006). As people move to the cities because of, e.g., failing crops due to prolonged drought (Barrios et al., 2006), they will contribute to increased emissions and potentially expose themselves to NTDs that thrive in urban situations, including Dengue (Were, 2012).
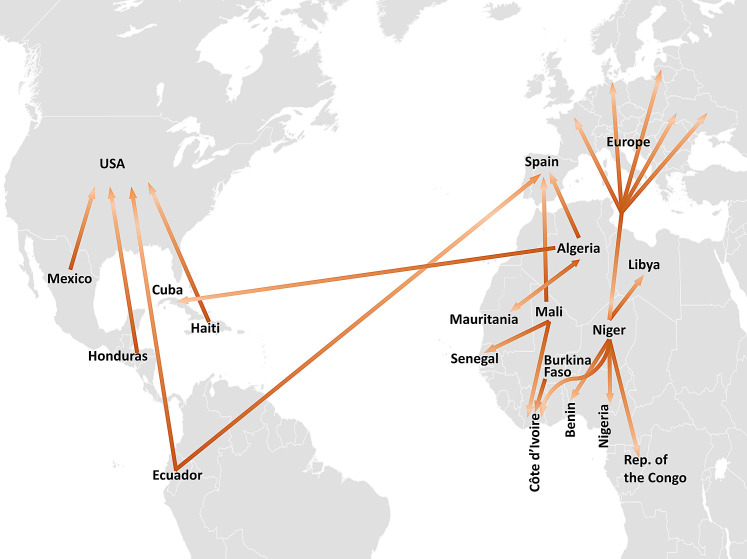
Water demand. Global water demand is projected to increase significantly, particularly in terms of water needs for irrigation (reviewed by Wang et al., 2017). Globally, water scarcity is expected to increase (Gosling and Arnell, 2016). The fragmented nature of the change (van Vliet et al., 2013) may lead to selective national or international population migration from at risk areas, as has been observed in various countries (reviewed by Obokata et al., 2014), including Ethiopia (Gray and Mueller, 2012), Mexico (Nawrotzki et al., 2015) and South Africa (Mastrorillo et al., 2016). Fig. 3 illustrates known international movements attributable to water-based climatic factors such as drought and flooding. This occurs alongside and in addition to internal and crossborder migration, often temporary, which has a multitude of environmental causes (Reuveny and Moore, 2009). Understanding the role of climate-associated migration is important as individuals who migrate may carry parasites and cause new outbreaks, as has been observed recently in Corsica, where Schistosoma hybrids have been observed and attributed to the mixing of imported Schistosoma haematobium and local Schistosoma bovis (Boissier et al., 2016).
Fig. 3.
Known population movements attributable to climate-based issues including drought and natural disasters. Points of departure and destination are country level and based on table 2 of Obokata et al. (2014).
Urbanisation is associated with population growth (Cohen, 2006), but not necessarily in a readily predictable or linear manner. Within Africa alone, the population is expected to double to 2 billion by 2050 (United Nations, 2015), but several models and observations suggest complex patterns of migration and countermigration depending on the motives and opportunities (Geyer and Geyer, 2015). Increasing levels of urbanisation associated with population increase (Satterthwaite, 2009) is nonetheless likely to impact on the climate substantially—e.g., as land-use changes are enacted (Pielke, 2005), as habitats are altered and fragmented (Haddad et al., 2015), as biodiversity decreases (Mooney et al., 2009). Human–wildlife interactions in both rural (Aryal et al., 2014) and urban (Becker et al., 2015) locations will inevitably change over the coming decades as a result of these and other changes.
4.4. Agriculture and Farming
A large fraction of people exposed to NTDs is smallholders or subsistence farmers dependent on natural water cycles to support crops and/or livestock. Evidence suggests that climate-associated events such as prolonged drought, delayed onset of rains, or above normal precipitation can adversely affect a range of livelihood assets (Ziervogel and Calder, 2003). Small holders and poorer farmers are more likely to be concerned about heavy rainfall, but may have no livelihood response due to a lack of assets and entitlements (Cooper and Wheeler, 2017). This effect may not be universal, as there is also evidence that farming communities have adapted to harsh environments over many generations (Kassie et al., 2013; Mortimore and Adams, 2001) to include allocating labour differentially across seasons to mitigate unpredictable precipitation patterns, increasing biodiversity and diversifying livelihoods.
Food demand and production are likely to change considerably in coming decades due to population growth, direct and indirect effects of climate change (Valin et al., 2014). Various large-scale effects have been speculated including risks to global food security (Wheeler and von Braun, 2013) through, e.g., loss of freshwater for irrigation (Elliott et al., 2014). Simultaneously, the demand for water for aquaculture is rising, bringing the potential for food-borne diseases to become a major issue in coming decades. Almost 60 species of fish-borne trematode have been described (Hung et al., 2013). Infections among farmed fish have been associated with aquaculture practices in several SE Asian countries (reviewed by Lima dos Santos and Howgate, 2011). Concerns have also been raised regarding potential spillover from wildlife populations into tilapia productions in China (Li et al., 2013). The encroachment of wildlife into human communities is also expected to increase with increased urbanisation, habitat encroachment, loss and fragmentation (Hassell et al., 2017.)
For NTDs with a zoonotic life cycle that can involve domesticated animals, the potential effects of climate change cannot be ignored. Livestock is a driver of climate change due to the emissions of greenhouse gasses within the system (Gill et al., 2010). Thornton et al. (2009) consider various potential effects including change in quality and quantity of feed, heat stress and water security. Potential effects on transmission of infections in livestock animals have been reviewed by Baylis and Githeko (2006), who suggest that climate change is likely to have been responsible for the introduction of several infectious diseases into new areas including bluetongue virus in the United Kingdom, but also suggest that Fasciola infections in the United Kingdom may decline due to lower levels of summer rainfall.
4.5. Exposure, Vulnerability and Risk
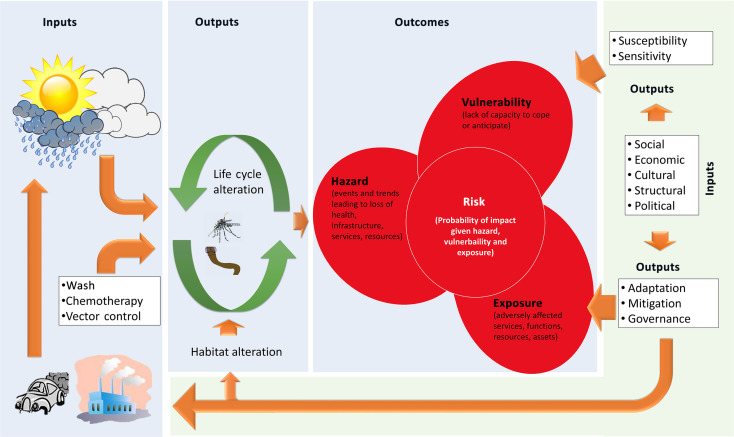
This review concentrates largely on the ecology and natural history of infections, as this where most of the literature on NTDS and environmental change is located. In the IPCC framework on vulnerability and adaptation (IPCC, 2014), the information contained herein would contribute to understanding future hazards, as distinct from exposures or vulnerability. The term ‘Hazard’, quoting directly from IPCC (2014), refers to ‘The potential occurrence of a natural or human-induced physical event or trend or physical impact that may cause loss of life, injury, or other health impacts, as well as damage and loss to property, infrastructure, livelihoods, service provision, ecosystems, and environmental resources’. The term ‘Exposure’ refers to ‘The presence of people, livelihoods, species or ecosystems, environmental functions, services, and resources, infrastructure’. The term ‘vulnerability’ refers to ‘The propensity or predisposition to be adversely affected. Vulnerability encompasses a variety of concepts and elements including sensitivity or susceptibility to harm and lack of capacity to cope and adapt’. The term ‘Risk’ refers to the ‘… probability of occurrence of hazardous events or trends multiplied by the impacts if these events or trends occur. Risk results from the interaction of vulnerability, exposure, and hazard …’. Fig. 4 illustrates how these issues are connected and interact in the context of climate change and NTDs.
Fig. 4.
A core concept of the IPCC (2014) report on vulnerability and adaptations is that risks to populations are formed by interactions between sources of hazard, vulnerability and exposure. In the context of NTDs, natural and anthropogenic inputs—including interventions such as WASH and vector control—combine to affect the life cycles of climate-sensitive stages (the hazard). Simultaneously a wide variety of societal inputs can affect vulnerability and exposure levels, and can lead to mitigations that modify emissions and habitats to affect the NTD-associated hazard. A lack of adaptive inputs is likely to lead to higher exposure, vulnerability, hazards and risk.
Vulnerability is a contested term in the risk-reduction community (Füssel, 2007) but has its roots in geography and social sciences, often referring to indicators such as socioeconomic status, the political economy, human agency and social capital. In relation to infectious diseases, vulnerability has been historically assessed in these contexts for HIV, TB and Malaria in Europe (Bates et al., 2004). The EU funded Healthy Futures programme (www.healthyfutures.eu) adopted the IPCC vulnerability–hazard-risk framework to produce risk maps related to decadal climate change in the context of Rift Valley Fever, Malaria and S. mansoni in East Africa (Taylor et al., 2016). In that project, stakeholder analysis and expert consultation were deployed to provide weighted indicators that could be included in the vulnerability domain. A comparative approach to estimating vulnerability that compared the expert-weighting approach to statistical modelling found high concordance in the context of modelling vulnerability to Dengue (Hagenlocher et al., 2013).
5. Environmental Phases
5.1. Soil
Soil is the upper covering layer of the earth, consisting of three subphases (water, gas and solids) which combine to describe the overall mechanics and other properties. Typically, soil properties vary in terms of texture (particle size and distribution), chemical and mineralogical properties, surface area and particle aggregation (related to aeration, compaction, temperature and water retention). The overall structure, mechanics and properties of a soil matrix are also influenced by a range of other factors such as the amount and properties of organic matter (detritus), oxides, clays, living vegetation, bacteria and fungi.
Recent theoretical and empirical studies have improved understanding of soil processes, mainly from the viewpoint of ensuring ‘soil security’ (Amundson et al., 2015). However, there are still many gaps in knowledge of how a changing climates might affect ‘soil health’—defined as ‘the capacity of a specific kind of soil to function, within natural or managed ecosystem boundaries, to sustain plant and animal productivity, maintain or enhance water and air quality, and support human health and habitation’ (Doran and Zeiss, 2000).
Soil is essential to the natural history of many parasites and/or their vectors; examples include eggs of geohelminths (Steinbaum et al., 2016), larvae of Tsetse flies (Leak, 1999) and burrows of mammals fed on by triatominae insects (Miles et al., 1981). An understanding of how changes to the soil phase will affect the survival of these and other life stages is an essential component of understanding the wider impact of climate change on transmission. Part of this process is likely to include a better understanding of how microgeographical variation in soil chemistry affects herb-layer vegetation (Bruelheide and Udelhoven, 2005).
5.2. Water
A central tenet of climate change is rising temperatures in the water phase, including freshwater and marine domains (see above). Temperature change, combined with population growth, and many other factors related to future aspects of landscape, hydrology, human behaviour, water and sanitation infrastructure, water- and land-use, is likely to have profound effects on many aspects of the water budget. The global hydrological cycle is thereby expected to undergo potentially profound changes (Held and Soden, 2006). Here I summarise some of the key aspects that are relevant to NTD natural history.
5.2.1. Precipitation
It is likely that anthropogenic influences differentially affected precipitation during the 20th century depending on region—with increase precipitation in (very broadly speaking) northern latitudes and decreased precipitation in southern latitudes (Zhang et al., 2007). Projecting precipitation patterns into the 21st century has proved more challenging than temperature (Schaller et al., 2011). Consensus is emerging that extremes of precipitation are likely to increase in frequency (Knapp et al., 2015). Also of concern is increased frequency of drought (Dewes et al., 2017) and flooding (Hirabayashi et al., 2013), although there is still considerable debate on the role of anthropogenic drivers underpinning these changes (Kundzewicz et al., 2014). This alteration in hydrological stability may nonetheless impact on not just aquatic habitats (Marino et al., 2017), groundwater and streamflow (Taylor et al., 2013) but also the carbon cycle (Haverd et al., 2017), soil moisture and vegetation phenology (Richardson et al., 2013). Effects may include fragmented changes to freshwater systems, due to, for example, changes to location-specific river discharges (Schewe et al., 2014) and recharges (Hartmann et al., 2017).
Increased precipitation in urban areas without adequate capacity to adapt or mitigate the situation has been associated with outbreaks of Dengue in several countries, including India (Mutheneni et al., 2017) and Bangladesh (Karim et al., 2012). Similarly, abundance of freshwater snails acting as intermediate hosts in the schistosome life cycle, as well as transmission of the parasite, are known to peak at specific times of year, depending on location (reviewed by Rollinson, 2011) and driven by climatic factors including rainfall (e.g. Moser et al., 2014).
Climate change-driven changes to the water balance, such that affect soil moisture conditions, are also likely to affect suitability of specific habitats for soil-transmitted helminths. Seasonality of hookworm transmission in several countries including South Africa (Mabaso et al., 2003), Nigeria (Nwosu and Anya, 1980) and Timor Leste (Wardell et al., 2017) has been at least partly attributed to seasonal precipitation.
5.2.2. Thermal Tolerance
Thermal tolerance may be a critical issue for many water-based, or semiaquatic organisms involved in the life cycles of NTDS—including insects, freshwater snails, fish, crabs, copepods, crayfish and insects. Poikilothermic ectotherms such as these consume oxygen based on the water or temperature until some threshold temperature where ATP supply and demand is overwhelmingly disrupted and the organism dies (Poertner, 2001). Tropical species may have relatively wide tolerances, but may also be more vulnerable to increases in temperature due to already inhabiting water bodies with temperatures close to their thermal limits (Sunday et al., 2012). Whether mean increases in temperature are more important than changes in diurnal variation is being debated in the literature (Vasseur et al., 2014).
5.2.3. Stratification
The life cycles of several NTDs including Schistosoma, the food-borne trematodes and Dracuncula involve intermediate hosts that may inhabit and reproduce in water bodies with thermal stratification, such as lakes. Analysis of historic data indicates that global warming is associated with changes to lake stratification that are dependent on lake morphometry (Kraemer et al., 2015a). How the intermediate hosts will respond over the coming decades is unclear, but evidence suggests evolution may have led to divergent populations of copepods that have adapted to warmer or colder conditions (Wallace et al., 2014).
5.2.4. Sea Levels
Rising sea levels are expected to impact significantly on coastal areas, not just in terms of flood risk but also in terms of the influx of salt water into coastal fresh water systems. Evidence of the effects on ecosystems is emerging in the literature. For example, saltwater intrusion into tropical rivers can affect the bacteria of floodplain soils by altering both the salinity and pH (Nelson et al., 2016). Increase in the influx of brackish water in coastal areas due to sea level changes has been implicated as the reason behind an increase in the abundance of salinity-tolerant Aedes mosquitoes in the Sri Lankan context (Ramasamy and Surendran, 2012).
5.2.5. Mitigation
Mitigating the challenges outlined above through providing sustainable water resources forms part of SDG Goal 6—the other key component for NTDs being access to sanitation and hygiene ‘for all’ by 2030. This latter aspect, commonly termed water, sanitation and hygiene (WASH) is considered crucial in reducing transmission of STH (Freeman et al., 2013), trachoma (Stocks et al., 2014), schistosomiasis (Grimes et al., 2014) and Entamoeba (Speich et al., 2016).
Our warning from history on this subject is quite clear. Development projects with all good intentions, related to water infrastructure in particular, have themselves been associated with increased transmission of parasitic infections including malaria in unstable areas (Ijumba and Lindsay, 2001; Kibret et al., 2017), filariasis (Erlanger et al., 2005) and schistosomiasis (e.g. N’Goran et al., 1997). In the latter case, concerns have been raised recently about how a large-scale water conservation project could translocate Oncomelania hupensis (an intermediate host snail of S. japonica) in China (Liang et al., 2012; Zhu et al., 2017), how migration of seasonal workers related to dam construction might have led to admixture of S. mansoni populations in Senegal (Van den Broeck et al., 2015), how dam construction could affect the transmission of Schistosoma mekongi in Laos (Attwood and Upatham, 2012) and prevent the migration of snail-eating prawns across sub-Saharan Africa (Sokolow et al., 2017). These and other examples remind us of the importance of implementing health in all policies (Rudolph et al., 2013) when undertaking sustainable development projects.
5.3. Air
The central tenet of climate change is the forcing effects of so-called ‘greenhouse’ gasses including CO2 and aerosols. Forcing in this context means the impact that production of these gasses has on the balance of energy in the atmosphere. As greenhouse gasses increase in density, they tip the energy balance positively and positive forcing ensues. All climate models derive from this process and then simulate how varying degrees of forcing will affect global air and land surface temperatures.
Tropical climates are typically governed by the Intertropical Convergent Zone (ITCZ). The ITCZ is a belt of low pressure surrounding the earth close to the equator that moves between the tropics of cancer and capricorn at different times of year. It is this movement that generates the characteristic dry and rainy seasons in countries located within the tropics (for animation click here): http://www2.palomar.edu/users/pdeen/animations/23_weatherpat.swf.
The dynamics and positioning of the ITCZ are highly sensitive to small changes in the global energy balance (Sachs et al., 2009; Schneider et al., 2014). Models struggle to predict the future dynamics of the ITCZ (Bony et al., 2015), and until the models can project the future of the ITCZ in relation to climate scenarios it will be challenging to model the transmission of NTDs effectively.
The relationship between surface air temperature (as predicted by climate projections) is generally assumed to be correlated, over decadal scales, with the ground surface temperature, but over shorter time scales there may be considerable variability. Soil acts as a heatsink and conducts heat from the air on a daily timescale, resulting in some level of phase shifting that depends on location and other variables including precipitation (Smerdon et al., 2004). Abiotic changes to the soil as a result to changes in air temperature may affect the natural history of a wide range of NTDS as diverse as trypanosomes and cestodes.
6. Crosscutting Modelling Issues
6.1. Scale
Downscaling is a recently developed process, derived from subnational weather forecasting, to improve the spatial resolution of GCMs over limited areas (Dickinson et al., 1989). A broad and accessible overview of the methodologies is available elsewhere (USAID, 2014). Here I summarise some of the key methods.
Statistical downscaling is a two-step process that involves understanding statistical relationships between observations at one point over time and GCM outputs at that location over the same time period, and correcting the GCM output to more closely resemble the observations (also known as bias correction). Statistical downscaling is computationally inexpensive but has low utility if the observations are scarce over time and space, and/or if the relationship between GCM and observations changes over time.
Dynamical downscaling (also known as generating regional climate models or RCM) is a process whereby a GCM is run and the lateral boundary outputs at the edge of the RCM region are used as the initial conditions of an RCM using the same physics-based model as the GCM but at a higher spatial resolution and over a relatively small area. The output of this computationally intensive process is a climate model at relatively high spatial resolution compared to the GCM (typically less than 0.5 degree).
In the context of understanding how climate change might affect local scale transmission of NTDs, RCMs arguably have greater utility than statistical models as they can be run on relatively sparse observations. Their disadvantage, apart from computing costs, is that each individual model can output widely differentiated products in regions with complex climates and widely varying but sparse observations (e.g. the tropical regions). For this reason, RCMs are often combined into ensembles with multiple outputs summarised into a single model that represents the average of all models in the ensemble. RCM ensembles, made available through the coordinated regional climate downscaling experiment have been used to model future precipitation over the African continent (Nikulin et al., 2012).
Despite recent advances, none of the current models predict or project temperature at a microgeographical scale, which is considered a major limitation in estimating how a particular organism may be vulnerable to a future climate (Scheffers et al., 2014; Storlie et al., 2014). Furthermore, the choice of RCP tends to be arbitrary as there are many possible future climate scenarios (Fuss et al., 2014), and a more objective approach is therefore needed when selecting a particular scenario (Casajus et al., 2016). Estimating precipitation continues to challenge the modelling community, due partly to the complex interaction between temperature and rainfall (Zhang et al., 2007), including the influence of fine-scale drivers of cloud formation and rainfall.
6.2. Absence vs Missing Data
All models are limited by the absence of data. There is a need to distinguish between absence and missing data in order to reduce potential bias. In their attempts to address this issue when working on leishmaniasis, Carvalho et al. (2015) tested several ecological niche modelling algorithms and concluded that the inclusion of absence data improved model performance. A range of modelling approaches benefit from inclusion of absence data (Li and Guo, 2013) if a survey has been undertaken in the area and the absence has been confirmed by direct observation. If there has been no survey in a particular area the data are missing and cannot be used in place of absence data. This is one of the major factors preventing accurate mapping of several NTDs or their vectors. The Maxent approach, as used by authors researching various parasites including leishmaniasis Peterson and Shaw (2003) and lymphatic filariasis (Slater and Michael, 2012) is a valuable tool for analysing presence-only data. Process-based mapping issue, as used by Stensgaard et al. (2016), can ameliorate the problem to an extent by predicting where the environment may be suitable for a vector or intermediate host based on the results of experimental observations.
6.3. Uncertainty and Bias
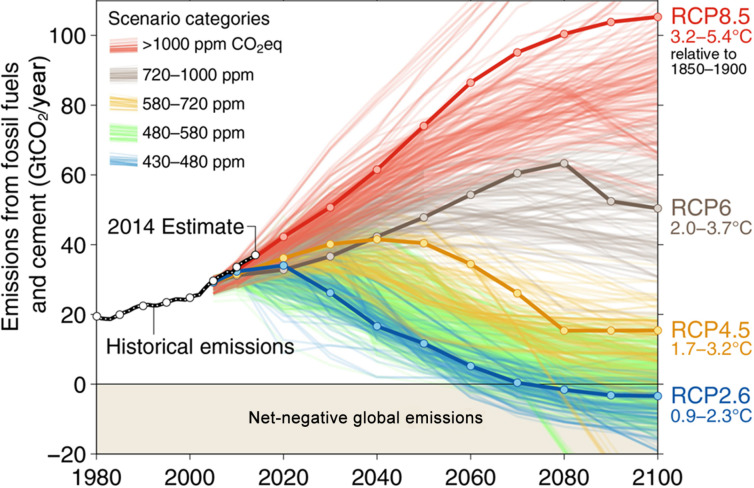
Uncertainty is a fact of climate projections from which it is difficult to escape. It is not possible to draw data from the future and there are many possible intermediate scenarios as depicted in Fig. 5 . We could end up in 2100 at any one or none of the points on this chart. The RCPs offer useful touch points for comparative purposes, but every projection of the impact of climate change must acknowledge the inherent uncertainty.
Fig. 5.
Historical and potential future CO2 emission scenarios to the year 2100, with four representative concentration pathways (RCP2.6, RCP4.5, RCP6 and RCP8.5).
Reproduced with permission from Fuss, S., et al., 2014. Betting on negative emissions. Nature Climate Change 4 (10), 850–853. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. Available at: https://doi.org/10.1038/nclimate2392.
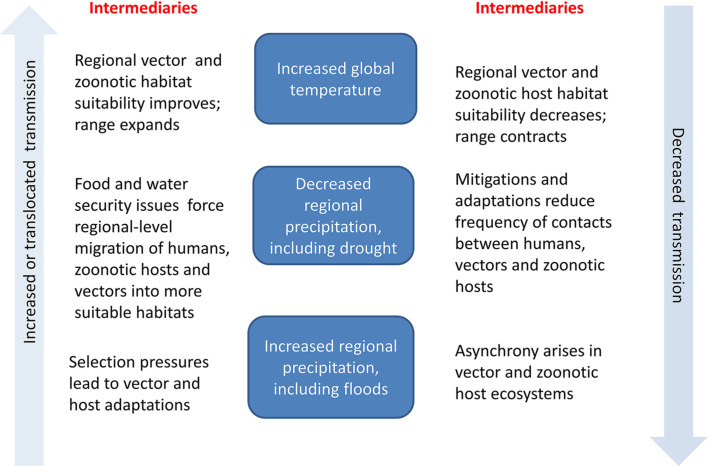
To illustrate the uncertainty regarding estimating the potential impact of climate change on NTDs, consider Fig. 6 . As surface temperatures warm there may be differential effects on transmission of NTD infections depending on regional environmental changes. For example, in some places the regional climate may become too extreme to support vectors or zoonotic hosts, whereas in others an extreme flooding event may translocate vectors or zoonotic hosts from one part of a river system to another. This level of uncertainty places additional challenges on modelling future scenarios of not just schistosomes but all NTDs due to their tight association with specific environments.
Fig. 6.
Generalised framework of how increases in global temperatures and regional changes to precipitation patterns may lead to increased, translocated or decreased transmission of NTDs. Central column describes particular temperature and precipitation changes associated with climate change. Left column describes intermediary steps that might be expected to increase or translocate transmission. Right column describes intermediary steps that could lead to reduced transmission.
Bias is another universal feature of climate models. Future trends in warming and precipitation are based on simulations of historic events. Simulations of those past events produce results that are different to observations. This is referred to as bias, and the bias is then carried over into future projections. Bias correction involves correcting the future simulations so that they more accurately predict the future. The simplest method is the so-called ‘delta’ method (e.g. Hay et al., 2000). This approach requires calculating the difference between observed and simulated climate from the past and applying the difference to simulations of the future. There are several different types of bias correction available, which are detailed elsewhere (e.g. Teutschbein and Seibert, 2012).
7. Crosscutting Vectors and Zoonotic Hosts
7.1. Mosquitoes
Several genera of mosquitoes are involved in the life cycles of vector-borne NTDs. Here, I briefly describe factors affecting the life history traits of three of the main vectors—Aedes, Culex and Anopheles. For a more comprehensive review of oviposition, see Day (2016).
7.1.1. Aedes
This urban dwelling mosquito is perhaps the most sensitive indicator of how environmental change can affect transmission of vector-borne diseases. Many different NTDs are transmitted by various species of Aedes. Two of the most prominent Aedes species in terms of the number of diseases that can be transmitted via their feeding mechanism are Aedes albopictus and Aedes aegypti. A. albopictus has been observed to carry Yellow fever virus (YFV), Chikungunya viruses, West Nile virus, Eastern equine encephalitis, Japanese encephalitis. It can also transmit dog heartworm. A. aegypti can transmit both Dengue and YFV as well as Chikungunya, Zika virus and Mayaro virus.
Some information is available on how Aedes mosquitoes respond to pressures exerted by short-term environmental change. For example, it is now known that gene flow is higher in wet than dry seasons due to transient selection pressures (Sayson et al., 2015) and that interaction between vectors and viruses that alters the carrying capacity of the mosquito to vary over time and space also likely to be important determinants of future transmission in particular locations (Lambrechts et al., 2009; Yee et al., 2012).
A second important factor may be diapause (Jia et al., 2016, Jia et al., 2017). This feature of Aedes mosquitoes natural history allows them to suspend development during adverse environmental conditions, such as cold weather (Brady et al., 2013). Both temperature and photoperiodicity affect the length of dormancy (Yee et al., 2012). Photoperiodicity in particular has been identified as a target for potential intervention, with efforts underway to identify genes that produce potential targets for genetic or chemical disruption (Huang et al., 2015).
A number of reasons have been cited as responsible for the lack of effectiveness of campaigns to eradicate Aedes mosquitoes historically, even after initial success. Aedes spp. oviposit in a wide range of man-made containers (Tun-Lin et al., 2009), adapt oviposition rates to local water conditions (Wong et al., 2012) and rest after feeding in places that are difficult to reach with insecticides, including storm drains (Paploski et al., 2016), and on dark wall surfaces across different rooms of houses (Chadee, 2013; Perich et al., 2000). From a climate change perspective, it will be important for modelling efforts to accommodate these extremely successful adaptations of the target mosquitoes to available environments. One of the key challenges will be to consider how future rates of urbanisation and climate change connect with issues such as precipitation, water collection and drainage (Moore et al., 2016; Semadeni-Davies et al., 2008).
7.1.2. Culex
As with other mosquito vectors, Culex spp. all life stages are ectothermic and therefore climate sensitive. Species of Culex are currently distributed across the globe. Culex pipiens complex is the most widely distributed, with mosquitoes inhabiting latitudes as far apart as Northern Europe and the South Island of New Zealand (Farajollahi et al., 2011). In terms of the NTDs covered within this chapter, C. pipiens complex is responsible for transmission of Rift Valley fever and lymphatic filariasis. Culex quinquefasciatus is distributed across the tropics and subtopics and is responsible for the transmission of lymphatic filariasis and possibly Zika virus (Diallo et al., 2014).
The development of Culex mosquitoes has been demonstrated to correlate with temperature in a number of studies. Gunay et al. (2011) observed that body size of inbred C. quinquefasciatus decreased with increasing temperature (covering the range 20–27°C). This result echoed earlier work by Rueda et al. (1990) who also observed C. quinquefasciatus body size parameters (including head capsule width, larval body widths and weight) decreased with temperature (covering the range 15–34°C). The parabolic nature of the relationship between temperature and survival was demonstrated also in that study, with the peak emergence occurring at temperatures between 20 and 30°C and high levels of mortality recorded at 15 and 34°C. A more comprehensive review of the relationship between temperature and Culex life history traits is available elsewhere (Ciota et al., 2014).
Observations on the relationship between temperature and development of Culex mosquitoes have been used to inform investigations into the possible effects of climate change on the geographic distribution of Culex species. Morin and Comrie (2013), focusing on the southern United States, applied their dynamic mosquito simulation model (Morin and Comrie, 2010) to project the distribution of the mosquitoes up to 2050 under a downscaled A2 climate scenario. The results of their analysis suggest a pattern of regional changes that reflect the complex topography of the location under study, but also an overall trend towards a lengthier mosquito breeding season combined with a lower abundance in summer months.
Focusing on a more global picture, Samy et al. (2016) combined observed occurrence data of the contemporary distribution of C. quinquefasciatus with climatic projections of temperature based on the RCPs (covering RCP2.6, RCP4.5, RCP6.0 and RCP8.5) and a set of biolimactic variables containing monthly temperature and rainfall data. Current potential distribution of the mosquito was then estimated by first estimating, using an ecological niche model, which bioclimatic variables were contemporaneously associated with the distribution of Culex. From this model, it was then possible to predict how different RCP scenarios may affect future geographical distribution. The conclusion from this work was that the limits of the geographical distribution would increase by up to 4.9% in the future (no specific date given) between RCP2.6 and RCP6.0 and then decrease under RCP8.5.
7.1.3. Anopheles
Mosquitoes of the Anopheles genus are responsible for the transmission of malaria and as such have been studied relatively extensively in terms of their biology and life history. Information on this vector is included here due to its role in transmission of Wuchereria bancrofti and Brugia malayi (Bockarie et al., 2008), but with the caveat that species-specific observations may not translate across species.
Anophelene mosquitoes generally lay their eggs singly onto water, and on hatching the larvae float horizontally to allow breathing. Exceptionally, viable Anopheles gambiae eggs have been observed in both moist soil (Minakawa et al., 2001), and dry soil (Bier et al.,) and treeholes (Omlin et al., 2007). The larvae are amphibious and will move towards water (Miller et al., 2007).
As all stages of all species are poikilothermic, the life history traits of the organism are tightly controlled by environmental conditions from egg laying onwards (Davies et al., 2016; Lyons et al., 2013). Several other environmental factors including pH, water flow and presence of algae are also important drivers of egg, larval, pupal and adult stage survival, larval feeding behaviour, larval—adult development time, gonotrophic cycle rate and population abundance (e.g. Araújo et al., 2012; Gouagna et al., 2012; Kamara et al., 2015).
Understanding the individual life history traits of individual species is necessary but not sufficient for modelling purposes. For example, a study in Nigeria by Lenhart et al. (2007) established that in Nigeria the relative contribution of A. gambiae, Anopheles arabiensis and Anopheles funestus to Wuchereria bancrofi transmission is likely to vary over a 12-month period. Competition between sibling Anopheles species (Paaijmans et al., 2009) may be partly responsible for the dominance of particular species at different times, combine with changes to water and/or soil phases that favour the development of one species over another.
Taking into account all the possible abiotic and biotic factors that influence Anopheles life history, understanding how climate change might affect the populations of Anopheles mosquitoes and transmission of filarial infections is therefore challenging. Evidence is nonetheless emerging that a mixture of anthropogenic activities related to land cover change, combined with increased temperatures, is shifting the species range in specific areas (e.g. Fuller et al., 2012; Kulkarni et al., 2016) and may either cause local extinction (Escobar et al., 2016), or an overall increase in environmental suitability combined with seasonal and range shifts (e.g. Ryan et al., 2015).
7.1.4. Bats
Bats are either known, or suspected, to be vectors of many zoonotic infections (Olival et al., 2017) including several filoviruses and hepanaviruses (Moratelli and Calisher, 2015; Olival and Hayman, 2014). Infections of humans via bats infected with filoviruses have tended to occur in outbreaks, which has been hypothesised to occur as a consequence of within-host dynamics (Plowright et al., 2016). Outbreaks of emerging viral infections, including filoviruses, have been increasing in recent decades (Smith et al., 2014) correlating with global environmental change.
Like many nonhuman mammals, bats are acutely susceptible to the impacts of environmental change, including climate change (Aguiar et al., 2016; Sherwin et al., 2013). The direction of travel is less certain than the anticipation of change. Published species–distribution models suggest that the fruit bat Pipistrellus kuhlii has extended its range over recent decades as global temperatures have increased (Ancillotto et al., 2016). Extreme temperatures (> 42°C) have been identified as fatal to flying foxes (Welbergen et al., 2008). A more complex, and localised, situation may exist across all species, given evidence that the call frequency is affected by temperature and humidity (Mutumi et al., 2016) and that efficiency of echolocation (and hence foraging success) is affected by temperature in a convex manner (Luo et al., 2014).
Known bat vectors of filoviruses include flying foxes and fruit bats (Table 1). An attempt to predict which of the other existing 1116 bat species could possibly host filoviruses suggests that candidate species tend to produce more than 1 L per year of relatively large neonates, inhabit relatively large geographic ranges of high mammal density and live in larger roosts (Han et al., 2016). One of the challenges in identifying bat vectors is the lack of pathology attributable to the viruses themselves, a situation that has led to the hypothesis that metabolic and internal temperature increases caused by flight may have led to the evolution of tolerance (O’Shea et al., 2014).
8. Direct Life Cycle Parasites
8.1. Goehelminths
At the time of writing this chapter, there were no original research articles retrievable through PubMed specifically referring to climate change and hookworm infections, climate change and geohelminths, climate change and Ascaris lumbricoides infections or climate change and Trichuris trichiura infections. I therefore summarise here what is known about the climate-sensitive stages of their life cycles to inform future efforts at statistical and/or dynamic modelling.
8.1.1. Trichuris
The climate-sensitive stages of the Trichuris spp. life cycle are eggs deposited onto the ground within faecal matter. Embryonation occurs at a pace dependent on temperature (Beer, 1973), with an optimum rate of development at approximately 34°C. At this temperature, embryonation and development to infective stages take approximately 2 weeks. These and other experiments of temperature on embryonation of Trichuris suis indicate a lower threshold of 20°C and an upper threshold of approximately 40°C for development of the organism (Beer, 1973; Vejzagic et al., 2016). Typically, the soil needs to be moist (Spindler, 1929). Increasing the pH of the soil can reduce the survivorship of eggs (O’Donnell et al., 1984), in a temperature-dependent manner with a rapid reduction in survivorship under alkaline conditions (Ghiglietti et al., 1995).
Egg survival periods of 2–6 years have been suggested in temperate conditions (Beer, 1973). The upper temperature boundary of approximately 40°C for development to infective stages is similar to that reported in prevalence studies among school-aged children, where evidence of transmission has been observed in communities with land surface temperatures up to 45°C (Brooker et al., 2004). Other factors related to soil chemistry, including the level of quartz, may also be important in determining egg survival and viability—sandy soil channels may form down which the eggs fall to become incorporated into the subsoil and protected from environmental hazards (Brooker et al., 2004).
8.1.2. Ascaris
A. lumbricoides eggs, deposited onto soil within faecal matter, are the climate-sensitive stage of this roundworm parasite. They are more robust to environmental perturbation than either Trichuris spp. eggs or hookworm larvae due to being coated in chitin (Meng et al., 1981). Some studies have suggested the eggs may survive for several years in soil (Rudolfs in Storey and Phillips, 1985). Statistical analysis of bioclimatic data suggests that relatively moderate amounts of rain are associated with peak infection (Schüle et al., 2014).
Changes in relative humidity (RH) under experimental conditions reflect expected seasonal changes at certain latitudes, with much greater mortality under simulated field conditions that combined prolonged periods of simulated sunlight and dry soil conditions (Gaasenbeek and Borgsteede, 1998).
In experimental studies, a major determinant of egg longevity has been reported to be the level of ammonia in the faecal matter (Jensen et al., 2009; Pecson et al., 2007). A combination of ammonia, temperature and pH is also deterministic, with the majority of eggs able to survive at pH 7 in low ammonia conditions at 20°C for several 100 days, whereas conditions combining high pH with high ammonia at 40°C kill the eggs within minutes (Pecson et al., 2007). In separate studies, it has been reported that egg survival is also sharply determined by relative survival, with a fall from almost 100% 8-week survival at 100% RH to almost 0% 8-week survival at 7.5% humidity (Gaasenbeek and Borgsteede, 1998).
8.1.3. Hookworms
The climate-sensitive stages of hookworm infection are the eggs and larval stages. Much of our current understanding of how abiotic and biotic variables affect the natural history of these stages comes from research undertaken many decades in the past (e.g. Chandler, 1929). Like the other geohelminths the picture is still very incomplete, but nonetheless yields some relevant information.
The presence of hookworm infection is associated with particular bioclimatic variables related to temperature and moisture. Soil types are important with larvae thriving in particularly sandy soils (Mabaso et al., 2003). Efforts to map the ecological niche suggest arid areas and minimum temperatures of < 20°C are inhibitory (Mudenda et al., 2012), as are temperatures above 30°C (Udonsi and Atata, 1987). The dependence on environmental cues for the behaviour of the juvenile worms indicates that a changing environment which is predisposed to longer periods of dryness is likely to be detrimental. Under stable and ideal soil conditions, the larvae may live for several weeks (Augustine, 1923). If the soil becomes more clay like due to perturbation the larvae are not likely to survive (Payne, 1923). If there is rapid alteration of drying and moistening, the larvae will likewise not thrive (Beaver, 1953).
8.1.4. Toxocara
Eggs of Toxocara species are deposited onto land by canids and other animals upon excretion of faecal matter. The eggs are therefore directly sensitive to climate factors. Like eggs of other soil-transmitted nematodes, the eggs of Toxocara spp. have evolved to withstand a range of changes in the abiotic and biotic features of the soil phase. Specifically in the case of Toxocara canis, the eggs are able withstand extremes of temperature ranging from below freezing (O’Lorcain, 1995) to over 30°C (Azam et al., 2012). Toxocara leonis, which rarely affects humans, has been historically present in artic foxes at very high latitudes of the Canadian Artic (Elmore et al., 2013), indicating how this species of this genus have adapted to extreme environmental conditions before through selection pressures.
The microgeographical distribution of Toxocara eggs is affected by conditions including soil texture (Mizgajska, 1997) and oxygenation. Lower temperatures, lower humidity and low levels of oxygenation slow development times (Azam et al., 2012; Gamboa, 2005) leading to diversity in the rate of maturation depending on geographical location—typically maturation times are lower in tropical regions with all year transmission as a result (Macpherson, 2013).
Evidence for changing patterns of Toxocara infection globally is emerging from studies of zoonotic infections in northern latitudes lying well beyond tropical regions. North-west Canada is sited at the southern limit of the discontinuous permafrost zone, overlapping with the Arctic circle (> 60 degree latitude). High levels of parasitic infection in Artic regions, excluding Toxocara, have been historically recorded (Hotez, 2010). This changed in 2006 when a survey of dogs recorded a prevalence of 5%—up from a prevalence of 0 in previous surveys at the same latitude (Salb et al., 2008). More recent studies have confirmed that the prevalence of Toxocara in adults living in regions above 60 degree latitude is below 5% (Messier et al., 2012). In Northern Saskatchewan, however, the prevalence of T. canis in humans was recently recorded at 13.4% (Schurer et al., 2013).
Northern Canada is facing a disproportionate increase in temperature changes and is therefore considered as a sentinel site for understanding how climate change might affect parasitic disease transmission (Jenkins et al., 2011). In that review, Jenkins and colleagues suggest that a combination of migrating animal populations, including arctic fox, combined with increased survival of eggs over the winder period will result in a net increase in transmission despite the potential for higher summer temperatures to affect the eggs negatively. There are no published studies that have projected how climate change may affect transmission and this issue remains to be investigated further through a combination of epidemiological surveys and modelling projects.
8.2. Bacterial
8.2.1. Leprosy
The cause of leprosy, Mycobacterium leprae, is another example of an organism of public health importance for which there is a paucity of information available on its association with environmental factors (Franco-Paredes and Rodriguez-Morales, 2016). Long considered to be transmitted directly among individuals through nasal discharges and droplets, the role of the wider environment, including vector-borne transmission, remains cryptic (Franco-Paredes and Rodriguez-Morales, 2016). Some climate-sensitive factors are considered below.
8.2.1.1. Soil
M. leprae bacteria survive in soil under specific laboratory conditions (Desikan and Sreevatsa, 1995) and have been found in soil in the natural environment close to human habitation (Turankar et al., 2012). It is therefore important to consider how soil conditions may be affected under conditions of a changing climate (see above).
8.2.1.2. Water
M. leprae have been detected in water samples, using PCR, in India (Matsuoka et al., 1999). The same study also reported an association between the presence of leprosy in water and the prevalence of leprosy in the population.
At the time of writing, there were no original research articles available on how climate change might affect future incidence of Leprosy.
9. Parasites With Intermediate Hosts
9.1. Trematodes
9.1.1. Schistosomes (S. mansoni, S. haematobium)
The breadth of research undertaken on each aspect of this lesser-neglected NTD is sufficient to fill several books (Evans, 2015; Mahmoud, 2001; Secor and Colley, 2004). Here, I focus on describing recent research relevant to modelling decadal climate change.
The complex life cycles of schistosome species contain several climate-sensitive stages (miracidia, sporocysts, cercaria and intermediate host snails). Each of these components is affected temperature (reviewed by Kalinda et al., 2017) and a wide range of other environmental and physiochemical factors, depending on species, but which includes substrate type, flow velocity, water turbidity, metal content and chlorophyll content (Monde et al., 2016).
An early attempt at modelling the future transmission of Schistosomiasis in the African context used a deterministic model of the entire life cycle, suggesting that higher temperatures (> 30°C) could substantially reduce both prevalence and intensity of transmission (Mangal et al., 2008). While it is clear that the temperature and the state of the aquatic ecosystem are a critical factor for snail and parasite development (Morley and Lewis, 2013), the question of whether climate change alone will have a noticeably existential impact on the future transmission of schistosomiasis is still very uncertain (McCreesh and Booth, 2013; Stensgaard et al., 2016).
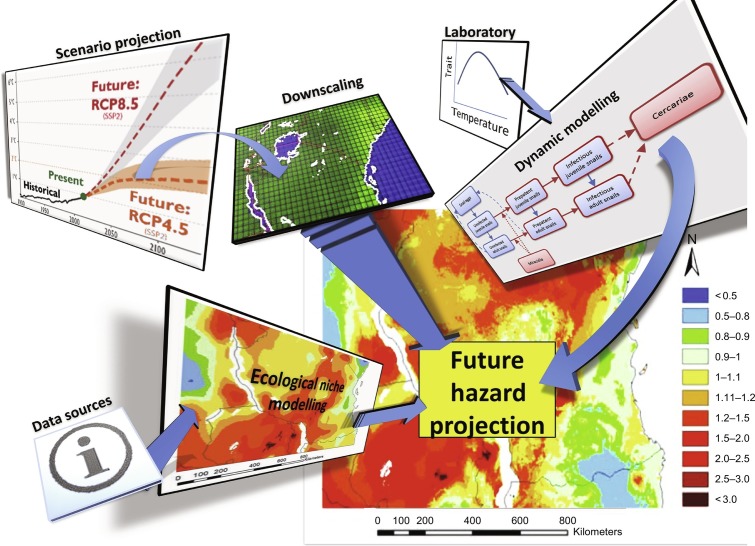
Pedersen et al. (2014) and Stensgaard et al. (2013) used an ecological niche model to estimate potential changes to the macrodistribution of two snail species (Biomphalaria pfeifferi and Biomphalaria sudanica) in the African context. The results of their studies hinted at the both the underlying complexity and uncertainty of this type of projection. Assuming all else remains equal; Stensgaard et al. (2013) predicted that snail range of B. pfeifferi could either contract by 43% by 2080 under the SRES A2 scenario while the range of B. sudanica could increase by 14% under the same scenario. Their conclusion, that climate change is unlikely to have a uniform and unilateral effect, was borne out in a later study of S. mansoni in East Africa (McCreesh et al., 2015). Functional relationships drawn from the literature on B. pfeifferi were first used to model how a long-term trend towards warmer water may affect snail population biology (McCreesh and Booth, 2014). Virtual miracidia were added into the model, with the output being virtual cercariae. Using this output as a measure of ‘infection risk’, combined with downscaled climate change projections for specific RCP scenarios allowed for mapping future transmission potential up to 2050 in Tanzania, Kenya, Uganda, Rwanda, Burundi and Zambia (McCreesh et al., 2015). Owing to the nature of the functional relationships contained within the model it was observed that some areas are likely to become unsuitable either for the parasite or the host, whereas other areas would become more suitable. A later collaboration that combined the ecological niche and functional trait modelling reached a similar conclusion (Stensgaard et al., 2016). Fig. 7 summarises the steps taken in this project, which represented an application of a generalisable approach to assessing the effect climate change through considering how abiotic changes may affect functional traits of both intermediate hosts and parasites (Cizauskas et al., 2017).
Fig. 7.
Illustration of the steps taken to map future transmission potential of S. mansoni in East Africa, combining functional trait knowledge on the relationship between temperature and Biomphalaria fecundity with dynamic agent-based models, downscaled climate projections and ecological niche modelling. The results of combining the outputs of these models and experiments resulted in a high-resolution hazard map which was then used to underpin a risk map that incorporated a vulnerability layer as observable in the Healthy Futures Atlas.
Elements of the figure reproduced from McCreesh, N., Booth, M., 2014. The effect of increasing water temperatures on Schistosoma mansoni transmission and Biomphalaria pfeifferi population dynamics: an agent-based modelling study. PLoS ONE 9 (7). doi: 10.1371/journal.pone.0101462; Stensgaard, A.-S., et al., 2016. Combining process-based and correlative models improves predictions of climate change effects on Schistosoma mansoni transmission in eastern Africa. Geospatial Health 11 (1 Suppl), 406. doi: 10.4081/gh.2016.406.
9.1.2. Schistosoma japonicum
Early models of the potential for transmission of S. japonicum transmission to be altered by climate change focused on how projected changes in average temperature with China may affect the potential transmission area (e.g. Yang et al., 2005). A later effort extended this approach to include statistical modelling of the relationship between temperature and snail natural history to produce risk maps that illustrated the potential range shift in 2030 and 2050 (Zhou et al., 2008). An assessment by Moore et al. (2012) of the degree-day modelling approach used by Zhou et al. (2008)—as well as other publications (see table 2 Moore et al., 2012) cautioned against the use of the use of degree-day models due to their inability to deal effectively with parametric uncertainty.
9.2. Food-Borne Trematodes
This is an important group of zoonotic trematodes transmitted to humans via poorly processed food, particularly fish, crustaceans and plants. Included in this group is Clonorchiasis, Fascioliasis, Opisthorchiasis and Paragonimiasis (Keiser and Utzinger, 2009). The epidemiology, pathology and control of the parasites causing the first three diseases in the above list have been reviewed, in the SE Asia context, elsewhere (Sripa et al., 2010). For Paragonimiasis, a recent comprehensive review is available (Blair, 2014), and aspects of the evolution and phylogeography of all four genera have also been reviewed (Attwood, 2010). At the time of writing, there was little published literature regarding decadal climate change and transmission of these infections. The discussion below summarises what is known about the relationship between environmental change and the climate-sensitive stages of the life cycles.
9.2.1. Clonorchiasis and Opisthorchiasis
The life cycles of Clonorchis sinensis (endemic in Asia), Opisthorchis viverrini (endemic in SE Asia) and Opisthorchis felineus (endemic in Europe and Asia) involve open defaecation by a definitive host into fresh water, typically small ponds, containing vegetation and compatible snail hosts. There are numerous species of snail that support the infections (Tang et al., 2016), each of which may be restricted to specific habitats and possess its own set of attributes affected by abiotic and biotic characteristic properties of the water body (Petney et al., 2012). Water temperature is crucial in terms of snail population size and dynamics—e.g., Parafossarulus manchouricus abundance–temperature relationship follows a convex curve, with the highest seasonal abundance associated with temperatures of between 24 and 26°C, and lowest abundance below 10–13°C (Chung et al., 1980).
The second intermediate host is a freshwater (often a cyprinid) fish or crustacean that predates on the snail host. Again, there are multiple species of fish or crustacean involved, depending on location (Tang et al., 2016). The natural history of cyprinid fish, which are poikilothermic, is also affected by water temperature and hence likely to be affected by climate change (Ficke et al., 2007). Research into the effects of climate-induced changes suggest that population turnover (Buisson et al., 2008), recruitment into rivers (Nunn et al., 2007), species range (Comte et al., 2013), body size and growth rates (Ruiz-Navarro et al., 2016) are all likely to be affected depending on species and habitats (Buisson and Grenouillet, 2009).
The natural histories of both miracidia, cercariae and metacercariae of Clonorchis and Opisthoricis, and their relationship with snail and fish intermediate hosts, are acutely affected by the abiotic properties of the water bodies they inhabit. For example, field and experimental observations of the Opisthorchis intermediate host, Bithynia siamensis goniomphalos, indicate that infection rate by miracidia of O. viverrini is minimised at water temperature of 16°C, maximised at 30–34°C, declines rapidly thereafter, and is more common in relatively small snails (Echaubard et al., 2017; Prasopdee et al., 2015). The convex nature of infectivity and survival of both snails and free-living parasites indicates that water bodies which exceed the upper temperature threshold over the coming years may become unsuitable habitats, whereas those water bodies that move from below 16°C towards 20°C or higher may become more suitable.
Evidence of the downstream effect of warmer waters on transmission of the infections to humans is very limited, but one published study on Clonorchis from Guangzou city, China suggests a link (Li et al., 2014). In that study, annual average 1°C increase was associated with an average 1.18% rise in monthly incidence from 2006 to 2012, a 1 mm change in rainfall was associated with 0.03% increase in incidence, and 1% rise in RH was associated with a 1.5% decrease in incidence. In contrast, projected impacts over several decades have been explored for Opisthorchis, specifically in Thailand (Suwannatrai et al., 2017), where Maxent was used to model a potential future hazard distribution, using IPPC A2 scenarios to 2070. The conclusion from that study was that northern regions may become unsuitable for transmission.
9.2.1.1. Fascioliasis
Fasciola hepatica is an important food-borne parasite with a global distribution (Mas-Coma et al., 2009). Although mainly an infection among livestock, human cases are regularly reported (World Health Organisation. Foodborne Disease Burden Epidemiology Reference Group, 2015). Risk factors include a list of anthropogenic behaviours that lead to ingestion of contaminated vegetation (Ashrafi et al., 2014).
The parasite life cycle is similar to other food-borne trematodes and schistosoma with one main difference. The snail intermediate hosts are air-breathing freshwater mollusks of the ‘fossarine’ group (family Lymnaeidae), most importantly Galba (formerly Lymnaea) trunculata, from which metacercaria emerge onto vegetation. This vegetation is ingested by the definitive host, which may include humans. The snails typically inhabit slow-moving or standing water bodies, often within marshy or muddy habitats, where they feed and lay eggs. They can aestivate by burying into the substrate to survive drought conditions, with the period of aestivation decreasing with altitude (Goumghar et al., 2001).
The global spread of the infection has been attributed to anthropocentric activities, related to domestication and transport of livestock, stretching back thousands of years (Mas-Coma et al., 2009). Habitats with temperatures as low as 10°C have been recorded as suitable for several intermediate host species including Fossaria bulimoides (Cruz-Mendoza et al., 2004) and G. trunculata (Rapsch et al., 2008). Adaptations of snails and parasite to conditions associated with high altitude (~ 4000 m) have also been observed (Mas-Coma et al., 2001). Temperature above 30°C is associated with rapid reduction in egg survival, with the ‘lethal’ temperature depending on species (Harris and Charleston, 1977).
Miracidial development and hatching have been observed to depend on temperature of the water body, with early studies indicating that no development was possible at temperatures lower than 9°C or above 30°C (Kendall). Cercarial shedding is also temperature dependent, with minimal shedding at 9°C (Kendall and McCullough, 1951). There is some disagreement over the optimal temperature. The studies of Kendall and McCullough (1951) suggested ongoing shedding at 26°C. Subsequent observations have suggested not just that 20°C may be optimal, but also that the magnitude of the shedding may depend on susceptibility of the snail species and the degree of diurnal variation in temperature (Rondelaud et al., 2013).
As a zoonotic infection affecting livestock of economic importance, Fasciola has received relatively more attention, in terms of environmental change research, than the other food-borne infections. It is known, for example, that contemporary weather patterns shape exposure among cattle in the European context, with the number of relatively warm days per annum, combined with relatively high average levels of within-farm precipitation (excluding within-year spikes) are positively associated with milk-seropositivity (Charlier et al., 2016; Munita et al., 2016). Within-country spatial clustering is thereby associated with spatial variation in these meteorological factors (Selemetas and de Waal, 2015). Enhanced vegetation (assessed remotely) has also been associated with increased risk of transmission in areas as regionally distant as Colombia (Valencia-López et al., 2012) and Pakistan (Afshan et al., 2014).
Soil type, slope and treatment, along with the characteristics of the underlying water budget contribute significantly to determining spatial distribution. In the tropical US context, hydric soils with poor drainage (Malone et al., 1992) or chenier soil with accumulated water that seeps out to provide a habitat for snails (Zukowski et al., 1993) is positively associated with exposure. In the tropical African context, arid and/or acidic soils (< 5.5 pH) are negatively associated with endemicity (Malone et al., 1998).
Exposure within human communities is also associated with seasonal variation, as observed in Pakistan (Qureshi et al., 2016). Historic climate data have been used to map risk of human fascioliasis in Iran (Halimi et al., 2015), with conclusions on the role of rainfall, temperature and vegetation that align with studies of the parasite in animal populations.
The wide window of available temperatures and habitat characteristics, as well as anthropocentric activities such as global travelling, highlights the potential for continued transmission of Fasciola infections under many climate change scenarios. At the time of writing, the majority of literature related to environmental change consisted of articles as described earlier, mapping spatial heterogeneity in exposure or levels of infection through analysis of environmental covariates. In terms of future projections, only a few articles have been published. These include modelling snail habitat suitability in Zimbabwe up to 2099 using the SRES A1B scenario (Pedersen et al., 2014); modelling decadal exposure up to 2089 in the United Kingdom based on the SRES 1B scenario (Fox et al., 2011) and modelling infection risk in New Zealand up to 2090 using SRES A1B, A2 and B1 scenarios (Haydock et al., 2016).
9.2.1.2. Paragonimus
This food-borne parasite consists of over 40 species that infect a wide range of intermediate and definitive hosts (Blair et al., 1999). Nine species or species–complexes are known to affect humans (Blair, 2014; Chai, 2013). The main focus of infection with species of the Paragonimus westermani complex is SE Asia, where multiple individual species within the complex infect intermediate hosts with low specificity (Doanh et al., 2013). Other foci, consisting of highly distinct parasite species are located in West Africa (Paragonimus africanus and Paragonimus uterobilateralis) and (sub)-tropical parts of North, Central and South America (Paragonimus mexicanus and Paragonimus kellicotti).
In common with the other food-borne trematodes, the climate-sensitive stages of the Paragonimus life cycle are those contained in the water phase. The first intermediate hosts are freshwater snails from the super families Rissoidea and Cerithioidea, but the full range is still unknown (Attwood, 2010). The second intermediate hosts are decapod crustaceans consisting drawn from several genera, depending on location and including Cambraoides crayfish (Kim et al., 2009), the freshwater crabs Liberonautes and Sudanonautes (Aka et al., 2008) and Tehuana (Vargas-Arzola et al., 2014). This is not an exhaustive list, as many other genera have been identified specific to regions including Ecuador (Calvopiña et al., 2014) and the United States (Fischer et al., 2011). The list of susceptible hosts in these and other papers point to the extreme generalisation of this parasite, and thus indicate a major challenge to modelling future trends in transmission.
In the absence of available literature on climate change and paragonimiasis per se, it is nonetheless plausible to suggest that the contemporaneous distribution of Paragonimus species is shaped by a wide range of factors as described in previous sections. Future transmission of the infection may also be affected by climate-related changes to local ecosystems, food webs, zoonotic reservoirs, farming practices, consumption patterns, human behaviours related to sanitation and translocation of humans, intermediate and zoonotic hosts. Long-distance translocation may have occurred to bring the parasite into South Africa, for example (Appleton, 2014).
9.3. Nematodes
9.3.1. Guinea Worm
Although scheduled for eradication due to the low number of recent cases, guinea worm may reemerge as a public health problem in the future due to the recent observations of infections in various animal hosts including dogs, fish and frogs (Eberhard et al., 2016; Ruiz-Tiben et al., 2014). The climate-sensitive stages of Dracuncula are the L1 larvae released by the adult female worm, along with the L2 and L3 larvae contained within the poikilothermic copepods of the Metacyclops, Mesocylops and Thermocylops genera that act as the intermediate host.
There were no published papers available on climate change and guinea worm at the time of writing. Historically, guinea worm infections are known to occur seasonally (Bloch and Simonsen, 1998), with peak infection tied to rainy seasons in countries at the southern fringe of the Sahara desert, and tied to dry seasons in those countries close to the Gulf of Guinea (reviewed by Ruiz-Tiben and Hopkins, 2006). In both regions, this has been attributed to the presence of stagnant freshwater used for drinking purposes rather than the population biology of the copepods or changes in ambient water temperature.
The thermal tolerance of warm-water copepods was established several decades ago. For example, the eggs of Thermocyclops spp. develop at a linear rate from 15 to 30°C, after which point there is a rapid downturn until 40°C, whereupon development ceases (Burgis, 1970). Studies on the phenology of warm-water copepods suggest that increased temperatures can lead to increased fecundity and thereby increase population size at specific times of year (Gerten and Adrian, 2002; Wagner and Adrian, 2011). Concern has been raised that the frequency of diurnal changes in the physiochemical environment could overwhelm the adaptability of copepods (Almén et al., 2014), and freshwater scarcity (see above) may also play a role in determining future abundance.
Given that cases among humans are an historically low point, but that copepod populations may be put under stress by environmental change, it is challenging to speculate how climate change will affect future Dracuncula transmission. History tells us that reduced efforts at controlling parasites can cause increases in transmission of parasites previously controlled (Ekwanzala et al., 1996). Calls have been made for continued financing into field-based research (Molyneux and Sankara, 2017). This will need to be accompanied by continued awareness of the potential dangers of drinking copepod-infested water to ensure sustainable interruption of the parasite life cycle.
9.4. Cestodes
9.4.1. Echinococcosis
Key to understanding how climate change might affect the distribution of Echinococcus spp. in the future is a detailed understanding of all climate-sensitive stages of the life cycle. The life cycle of Echinococcus spp. involves homeotherm mammals (including humans). Only one life stage—the eggs deposited with faecal matter from the definitive host—is essentially poikilothermic. Typically, these eggs are deposited onto soil through open defecation of dogs and other canidae. Eggs are therefore most likely to be directly affected by abiotic or biotic factors that affect the soil.
Echinococcus multilocularis is confined to northern latitudes and its eggs can survive freezing temperatures, with an optimal temperature range of 0–10°C and are viable only for a few hours at temperatures > 25°C (Veit et al., 1995). Echinococcus granulosus is responsible for the majority of morbidity in humans and has a wider distribution including tropical and temperate regions (McManus et al., 2003). It is clear from this latter fact alone that the eggs of E. granulosus must have historically adapted to a wide range of temperatures above 0°C. This has been reported by a very small number of studies at the time of writing. Wachira et al. (1991) tested the viability of E. granulosus eggs under various conditions in a semiarid region of Kenya. After placing eggs in suspension, samples were positioned on open ground, near a house or near a water hole. Only the eggs near the water hole were observed to be viable after 10 days, where the average temperature was approximately 29°C. Most recently, Thevenet et al. (2005) observed that eggs of the parasite kept outdoors on deposited stool samples from canines were viable after 41 months exposure to the elements in an arid region of Pategonia. During the exposure period, the eggs were subject to a temperature variation of − 3 to + 37°C and low rainfall (< 300 mm per annum). At the end of the exposure period, the eggs were still viable in terms of producing cysts in Texel ovines. Together, these studies, combined with a few earlier studies cited by Wachira et al. (1991), suggest that eggs of E. granulosus may be viable when deposited onto the ground at temperatures not yet reached by more temperate areas.
At the time of writing, there have been no published studies in terms of modelling the potential future transmission of Echinococcus spp. under specific climate change scenarios. Atkinson et al. (2013) offer a wide-ranging narrative review of how environmental change, including climate change, might affect future transmission. Much research has covered the myriad environmental factors that shape the contemporary geographic distribution (Atkinson et al., 2013). It is also made clear in that review that there is a lack of evidence on the role of anthropogenic factors.
Anthropogenic changes that are likely to affect the contemporary transmission of Echinococcus are those which modify the habitat, range and/or behaviour of predator and prey animals. Atkinson et al. (2013) review the possibilities regarding urbanisation, deforestation and land use change. The modifying effects of climate change on anthropogenic influences presents a further, as yet unexplored, challenge.
9.4.2. Taeniasis–Cysticercosis
Cysticercosis is a disease, mainly endemic in low-to-middle income countries (LMICs), which is considered to be responsible for approximately 30% of all epilepsy cases in endemic countries (Ndimubanzi et al., 2010). The primary risk factor is consumption of pigs infected by Taenia solium. The WHO updated its map of T. solium distribution in 2015 (Donadeu et al., 2016), highlighting how the import of infected pigs leads to cases in countries without comprehensive screening protocols.
The life cycle of Taenia parasites includes a climate-sensitive stage and its future transmission potential may therefore depend partly on how soil phases change in the future. At the time of writing, there have been no papers published that model future transmission scenarios. This review is therefore restricted to reviewing existing literature that has explored how environmental variables affect the survival and viability of the eggs shed by the definitive hosts.
In common with other parasites, the majority of work related to the natural history of taenid eggs was comprehensively investigated several decades ago (reviewed Lawson and Gemmell, 1983; see also Willis and Herbert, 1984 regarding Taenia multiceps). The aim of these studies was to understand the abiotic and biotic factors that affect the survival of eggs either under in vitro or field conditions, covering a range of species including Taenia pisiformis, Taenia ovis, Taenia hydatigena and T. multiceps. The in vitro experiments examined the effects of varying temperature and/or humidity or surface moisture on egg survival. Field experiments have focused more on the distribution and survival of eggs (e.g. Wachira et al., 1991) and uptake of eggs by animals under controlled conditions.
In terms of temperature, eggs have recorded to withstand freeing conditions of up to − 20°C without any effect on hatching (Willis and Herbert, 1984), while surviving for just 4 days at 21°C (Gemmell, 1977) and for just a few hours, if at all at 37°C (Coman, 1975; Gemmell, 1977). In terms of humidity and surface water, the evidence suggests that conditions of low RH can dramatically affect the survival of eggs. Laws (1968) reported that only T. hydatigena eggs could survive RH levels below 60%, while Gemmell (1977) observed that a lack of surface moisture reduced hatching to almost 0% after 4 days, irrespective of species and storage temperature. Similar observations were made by Sánchez Thevenet et al. (2017) who again concluded from laboratory studies of T. hydatigena that low RH values are inimical to eggs of this parasite. The inferences from all these studies are that dryer environments cause higher rates of mortality, possibly due to the desiccation of the keratin shell leading to shrinkage and increased hydrostatic pressure on the embryo, leading to its demise (Laws, 1968).
Risks to humans as a result of climate change are currently challenging to estimate. There are two routes to infection—either the consumption of infected pork or ingestion of eggs, e.g., by eating food stuffs fertilised with contaminated faeces. Pigs can be treated and meat can be screened to prevent that particular route of infection (Gabriel et al., 2015), and there is no a priori reason to expect that would be directly affected by a changing climate.
It is more likely that changing climate conditions will affect the ingestion of eggs. Changes to the soil phase that supports the eggs may have localised effects depending on a combination of soil temperature and soil moisture. Given the evidence from the studies summarised earlier, it is possible only to speculate that areas where a combination of increased humidity and only moderate increases in temperature may experience a greater percentage of egg survival. This may translate into a localised hazard function for modelling purposes, but this function will need to be modified by other risk factors associated with the vulnerability of associated human populations that may or may not alter depending on which climate change scenario is realised.
Another factor that will need to be considered is the potential for insects to distribute the eggs over extended distances through coming into contact with contaminated faecal matter. It has been previously suggested that blow flies could distribute eggs (Lawson and Gemmell, 1990) but this has not been demonstrated other than experimentally with dead flies being ingested by lambs (Lawson and Gemmell, 1990). More recently, Ammophorus rubripes, a dung beetle, has been implemented as a potential carrier through experiments that demonstrated carriage of viable eggs by the beetles for up to 24 days (Gomez-Puerta et al., 2014). A broad range of synergistic and competing factors is likely to affect the ecology of any organism capable of dispersing eggs, leading perhaps to localised extinction (Brook et al., 2008), among other effects related to anthropogenic disruption of established ecosystems (Cable et al., 2017).
10. Zoonotic Viruses
10.1. Coronoviruses
Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome (SARS) are two coronaviruses on the list of WHO priority diseases due to the lack of preparedness for future outbreaks. Bats are the major suspects in terms of zoonotic origin of both SARS and MERS-CoV (Anthony et al., 2017; Drexler et al., 2014).
Person-to-person transmission of aerosolised virus is the predominant risk factor for infection post-spillover. As with all other respiratory viruses, a range of environmental factors facilitate or inhibit the transmission, including temperature, precipitation, RH and airflow (reviewed by Pica and Bouvier, 2012). Relatively few investigations have been undertaken on SARS or MERS-CoV specifically. From the available literature, it is possible to deduce that both SARS and MERS-CoV persistence in the environment decreases at temperatures > 20°C, at a rate dependent on a convex humidity–temperature interaction (Casanova et al., 2010; Chan et al., 2011; van Doremalen et al., 2013).
At the time of writing, there were no original research papers available on the potential for climate change to affect transmission. Given the earlier observations, it may be possible to speculate that both MERS-CoV and SARS are likely to be less common in tropical and tropical areas in the future.
10.2. Henipavirus
10.2.1. Nipah and Hendra
Nipah henipavirus is a recently discovered member of the Henipavirus genus and is closely related to Hendra henipavirus (Ksiazek et al., 2011). Both Hendra and Nipah are on the WHO list of priority diseases, are highly pathogenic, affect populations in southeast Asia and are zoonotic. Fruit bats have been confirmed as the main zoonotic hosts of Nipah (Yob et al., 2001) and Hendra (Halpin et al., 2000). Food-borne transmission is possible given observations of cases who reported drinking palm sap from containers previously accessed by fruit bats in Bangladesh (Islam et al., 2016; Luby et al., 2006).
At the time of writing, there were no original papers in the literature on the potential for decadal climate change to affect Henipavirus transmission. Some environmental cues are nonetheless available. Under experimental conditions, both Nipah and Hendra can tolerate conditions with pH of 3–4 to 11, can survive more than 4 days in bat urine kept at 22°C, but are rapidly inactivated under conditions of desiccation (Fogarty et al., 2008).
In epidemiological studies, seasonality of Nipah spillover into human populations has been observed in Bangladesh (Luby et al., 2009) outside the typhoon season. In terms of understanding which environmental factors are associated with Nipah spillover risk, Walsh (2015) identified bat density and a derived variable termed ‘human footprint’ as key factors, but excluded vegetation cover and pig density. Seasonality has also been observed in terms of viral antibody dynamics in bats (Baker et al., 2014). Taken together, these observations hint that the risk of spillover events is determined by an interacting combination of bioclimatic factors and bat ecology.
10.3. Filoviruses
10.3.1. Ebola and Marburg
The filoviruses (Ebola and Marburg) are highly pathogenic NTDs (MacNeil and Rollin, 2012) that affect human populations in outbreaks associated with a spillover event from zoonotic hosts. Bats are speculated to be primarily responsible for these spillover events, where the infections persist, possibly due to biannual birth pulses (Hayman, 2014). Epidemiological evidence of spillover phenomenon comes from observations of seasonal variation in the prevalence of Marburg virus coinciding with the onset of outbreaks (Amman et al., 2012). The majority of human infection after the spillover is through direct contact with cases.
At the time of writing, there were no original research papers available that have modelled future climate change-related projections of either of these filoviruses. All attempts at modelling the risk of infection has instead focused on the near term. Bats have received some attention in the literature in relation to both Ebola and Marburg, particularly in terms of modelling interactions between bat ecology and environmental factors. Taken together, the current models suggest that infected bats migrate to resource rich areas (Buceta and Johnson, 2017) but are limited in their geographic distribution by a combination of temperature, evapotranspiration and elevation (Pigott et al., 2014, Pigott et al., 2015). Areas that combine relatively high human population density with high vegetation coverage are at relatively high risk of outbreak (Walsh and Haseeb, 2015), presumably as this increases the rate of contact between bats and humans. It remains to be seen whether climate change will significantly alter the interactions between these risk factors.
10.4. Arenaviruses
10.4.1. Lassa Fever
Lassa mammarenavirus (the aetiological agent of Lassa Fever) is a member of the Arenaviridae family of viruses (Yun and Walker, 2012). Up to 37 million people, mainly living in West Africa, may currently live in areas where the environment is suitable to support the main zoonotic host of Lassa Fever, the Natal multimammate rat, Mastomys natalensis (Fichet-Calvet et al., 2009; Mylne et al., 2015). Human-to-human transmission is considered to be account for between 5% and 20% of all cases, possibly due to ‘super-spreaders’ (Lo Iacono et al., 2015). Transmission occurs when humans come into close contact with the mouse specifically through ingestion or inhalation of mouse urine, faeces or blood. Risk factors include living in close proximity to the mice, butchering and consumption (Bonwitt et al., 2016, Bonwitt et al., 2017).
As with other zoonotic NTDs, a consideration of the life history traits and population biology of the zoonotic host is essential to understanding how climate change may affect future transmission patterns. M. natalensis is an endotherm and as such its gestation period is not directly affected by external temperatures. In contrast, the population abundance of this organism is characterised by clearly definable and high-amplitude fluctuations. Published field studies have consistently observed that these fluctuations are predictably associated with seasonal rainfall patterns (Coetzee, 1975; Makundi et al., 2007; Sluydts et al., 2007; Stenseth et al., 1997). Typically, M. natalensis breed during rainy seasons and are most abundant in the proceeding dry season (Christensen, 1993). Specific rainfall events, including so-called ‘short rains’ have been associated with rapid growth in population, termed outbreaks (Mwanjabe et al., 2002). This phenomenon is the result of more rapid development of the pups (Leirs et al., 1990), a process triggered by the rains and the consequential lifting of food restrictions (Christensen, 1993), and leads to both parents and their offspring breeding within relatively short periods of time.
Although known as an opportunistic species able to occupy a wide range of habitats, several natural environment factors nonetheless limit the spatial distribution of M. natalensis. The type of soil appears important, with higher abundance observed in regions with high vegetation cover and sandy (Massawe et al., 2005, Massawe et al., 2008). Both these environmental factors can be naturally and anthropogenically determined, thus farming practice and land use are also likely to affect the population biology of the rodent (Massawe et al., 2007).
Survival of the virus in the environment is also key to completing the transmission cycle. Once an infected mouse has urinated or defecated, there is a finite period over which the virus is viable. Laboratory investigations indicate that aerosolised virus is inactivated within 60 min at temperatures > 24°C (Stephenson et al., 1984) and that dark, dry conditions (Sagripanti et al., 2010) are associated with rapid decay of the virus.
The potential effects of climate change on the transmission of Lassa fever to humans have so far received limited attention in the literature. Given the well-known propensity of the mouse to undergo population outbreaks, historic attention has been given to either mapping contemporary risk (Fichet-Calvet et al., 2009) or forecasting over short periods (Leirs et al., 1996). One study has focused on the issue of estimating the effect of global change, including climate change (Redding et al., 2016). In that particular study, the authors created a spatially stratified mechanistic model for West Africa and modelled the effects of three climate change scenarios drawn from the HADGem3 AOGCM. The results of this simulation suggest that more extreme scenarios of both temperature increase and land-use change are likely to increase the rate of spillover events from animals to humans.
10.5. Lyssavirus
10.5.1. Rabies
Dog-transmitted rabies are present in 150 countries including all of Africa, parts of Latin America and all of Asia (Hampson et al., 2015). As a vaccine preventable, zoonotic disease, most research is focused on contemporary issues related to these two domains of enquiry. Efforts to understand the environmental component of transmission are geared towards operational aspects of vaccine delivery, e.g., in relation to landscape features (Russell et al., 2006), the delivery of vaccines to wildlife (Rupprecht et al., 2004). There is little attention in the literature on how environmental change (including climate change) might affect transmission decades in to the future. To date, the focus on climate change and rabies has been confined to arctic areas (Huettmann et al., 2017; Kim et al., 2014).
11. Vector-Borne Infections
In contrast to direct life cycle infections, there has been more attention given to the vector-borne diseases (e.g. Parham et al., 2015; Rogers and Randolph, 2006). This reflects both the larger number of actors working in the field of vector-borne disease, and a relatively early realisation that climate can have a profound impact on the distribution of vector species, particularly mosquitoes.
11.1. Helminths
11.1.1. Lymphatic Filariasis
W. bancrofti, the parasite that causes LF, is transmitted by mosquitoes of the Anopheles, Culex and Aedes genera. The potential effects of climate change on the transmission of this infection (assuming all else remains equal) therefore depends primarily on how the vectors respond to the biotic and abiotic shifts associated with changing climates. See sections earlier for more information on each of these species.
The potential impact of climate change on the parasite itself has not been comprehensively studied. There is some evidence that ambient temperature can affect the density of the symbiotic Wolbachia density (Mouton et al., 2007), particularly in terms of the interaction between host genotype and Wolbachia strain. This may reflect local adaptability to changing temperatures over short timescales. A less direct effect may be possible through competition for resources as the availability of food changes over a longer period of time (Ross et al., 2016), with evidence that certain Wolbachia strains (wMel, wMelPop and wAlbB) can reduce the survival times of Aedes larvae under starvation conditions. In the context of using Wolbachia to control populations of Aedes mosquitoes, high temperatures (e.g. 30–40°C over several days) have been observed to reduce bacterial levels, at least in the short term (Ulrich et al., 2016). Again, this points to adaptability but tells us little about how a sustained pressure will shift the host–symbiont relationship. Such information may emerge from longer-term studies over multiple generations of vector kept at constantly higher temperatures.
11.1.2. Onchocerciasis
Onchocerca is transmitted by the blackfly Simulium damnosum complex and is therefore a parasite with a life cycle that is sensitive to climate change. Reviews of blackfly ecology and the relation between vector and parasite are available in specific contexts (e.g. see Cheke et al., 2015 for a modelling perspective; Takaoka et al., 1982 for a review specific to Guatemala). Here I summarise work that has been conducted on the potential impact of climate change in particular.
The fact that over 60 sibling species or cytoforms have been identified (Adler et al., 2010), each with different life history parameters, makes any attempt at projecting the effects of climate change highly challenging. Nonetheless it has been possible, as with other NTDs, to estimate how changing temperatures can affect the development of each stage of the vector, the development of Onchocerca within the fly, fly fecundity and the mortality rate of the fly (Cheke et al., 2015; Takaoka et al., 1982). Unlike some other vector species, there appears to be no threshold temperature (within the range 15–32°C) at which point either fly mortality increases or the rate of development of the parasite decreases (Cheke et al., 2015). Fluctuations in daily temperatures may affect the overall development time, corresponding to seasonal fluctuations in fly abundance (Zarroug et al., 2016).
Importantly, temperature is not the only driver of the ecology of Simulium. A crucial feedback mechanism may lie in the phenomenon of aggregated oviposition, which is the process by which gravid Simulium females select existing egg masses to lay their eggs upon (McCall et al., 1994). The stabilising effect on the population of the vector possibly comes from a higher rate of egg mortality with increasing egg mass (Kyorku and Raybould, 1987). When incorporated into a mathematical model, this feedback produced a sharply convex curve when plotting temperature against the abundance of both parous and nulliparous flies, peaking at 29°C (Cheke et al., 2015).
At the time of writing, there were no original research publications available that considered the potential for long-term climate change to influence future transmission of this NTD.
11.2. Viruses
11.2.1. Dengue
Of all the NTDs, Dengue infections have been considered more often in terms of climate than all other infections, with over 200 publications available at the time of writing. A recent review on the subject by Ebi and Nealon (2016) revealed that most of this literature is concerned with identifying risk factors for contemporary transmission, with only a handful of studies devoted specifically to estimating the effects of decadal-scale climate change on Dengue transmission. These studies have employed a range of modelling techniques (reviewed by Messina et al., 2015), with varying results in terms of where in the world Dengue infections are likely or not to be transmitted under particular scenarios. Messina et al. (2015) attempted to unify the field by writing a statistical modelling framework for future projections that relies on capturing spatially explicit information at high spatial resolution to map the contemporary distribution before replacing the existing values of covariates (e.g. temperature, precipitation) with projected values based on different RCPs.
Most of the existing models project that Dengue is likely to increase its range in the coming decades as global temperatures rise. Where they differ is the amount of uncertainty in their outputs (Messina et al., 2015). Neither the individual projects nor any unifying frameworks consider the possibility that selection pressures acting on the Aedes vector or the dengue virus itself could affect future transmission. General evolutionary features of the dengue virus in relation to climate change and urban vs sylvatic cycles of the Aedes mosquitoes are reviewed elsewhere (Tabachnick, 2016), with the conclusion that past evidence of evolution warns us to expect future adaptation.
Studies of how temperature change affects host–virus interactions are few in number, with most evidence emerging in the context of investigating how Wolbachia bacteria may be used to control populations of dengue-transmitting mosquitoes. In that context, ambient temperature has been observed to affect DENV infection rate in A. aegypti mosquitoes (Sgrò et al., 2016).
11.2.2. Yellow Fever
YFV is a member of the Flaviviridae family, closely related to Dengue and also transmitted by Aedes mosquitoes. It is predominantly observed in Africa and South America. Unlike other most other VBDs, yellow fever has been historically partially controlled through successful vaccination campaigns (Rogers et al., 2006), combined with visa restrictions on travellers who have not been vaccinated. Control efforts have not been entirely successful, with recent estimates putting the death toll at approximately 78,000 in Africa during 2013 alone (Garske et al., 2014).
Outbreaks of yellow fever have highlighted a number of issues, predominantly related to lack of vaccination campaigns in East and Central Africa, and large-scale urbanisation (Kraemer et al., 2017). One expressed fear is that this outbreak will facilitate world-wide distribution of the virus due to the establishment of direct air travel routes from east Africa into areas with susceptible mosquito populations (Wasserman et al., 2016). Notable in this regard is the recent observation that Australian strains of A. aegypti are susceptible to YFV infection (Higgs et al., 2011) that European populations of A. albopictus can carry YFV (Amraoui et al., 2016), and that either A. albopictus or A. aegypti is present in numerous US states (Kraemer et al., 2015b).
The reasons for the absence of YFV in SE Asia are unknown. Hypotheses have been established that include a lack of carrying capacity among Asian strains of Aedes, an historic lack of travel from endemic areas to Asia, crossimmunity related to Dengue infection, a lack of a sylvatic cycle in Asia and the lack of a slave trade in Asia (Cathey and Marr, 2014; Rogers et al., 2006; Wasserman et al., 2016). Given uncertainty about current reasons for lack of transmission, it is even more uncertain how climate change might affect future transmission of YVF in Asia. Aedes mosquitoes are susceptible to selection pressures as a result of climate change (see above), and may therefore disseminate due to certain environments becoming more suitable. The establishment of susceptible Aedes from Australia into parts of SE Asia where the environment has become more suitable is possible through international trade (Benedict et al., 2007).
The same hypothesis can be applied to other areas where the environment may become suitable for species or strains of Aedes that are capable of carrying YFV, contemporaneously with the transport of the virus through globalised air travel of people and cargo. For example, as the tropics expand (see above), it may be expected that some areas will become more humid and vegetative cover may increase.
Like Dengue, YFV transmission may be affected by climate change due to the fact that Aedes mosquitoes are the vectors. It cannot be assumed, however, that the direction of change will be the same for all these infections. This is due to the fact that each virus has at least two types of transmission cycle involving partly overlapping combinations of vectors or nonhuman hosts (Rogers et al., 2006; Tabachnick, 2016). The sylvatic cycle is particularly important in YFV epidemiology, contrasting with the urban cycle being predominant in terms of Dengue transmission. Thus, we have to consider how the different cycles of each infection may be affected by global environmental change.
Rogers et al. (2006) offer some insights into the differential effects of environmental variation on transmission of Dengue and YFV. They attempted to map the global risk of Dengue and YBF by considering which environmental variables (including land surface temperature and normalised difference vegetation index) are associated with known outbreaks. They concluded that YFV transmission is predominantly associated with changes in vegetation cover and humidity, whereas Dengue virus transmission is more closely associated with changes in temperature. The maps produced by Rogers et al. (2006) also suggest that environmental conditions are suitable for transmission for YFV in SE Asia despite an absence of the virus in that region.
The effects of climate change on sylvatic cycles of either Dengue or Yellow fever transmission have not been directly studied. Again, we must infer the likelihood of climate having a significant impact based on what is already known about the ecology and the natural history of the organisms within each cycle. For example, toque macaques (Macaca sinica) are known hosts of Dengue in Sri Lanka. The genus Macaca is also known for its plastic characteristics in terms of its ability to adapt to changing food availability caused by anthropogenic activities (Riley, 2007). The macaques adapt their foraging strategy by moving beyond their home range if food becomes scarce in one particular area. This range extension could occur as a result of decreasing availability of natural foodstuffs in areas with altered patterns of agriculture, forcing the monkeys into new areas (including urban settings) where food is more available. Toque macaques are already found in urban settings in Sri Lanka and are exploited in a number of ways—as pets, performer and for ritual activities (Radhakrishna et al., 2014).
11.2.3. Rift Valley Fever (RVF)
RVF is a vaccine preventable, epizootic and zoonotic disease that has a complex natural history involving dozens of mosquito species as vectors (Pepin et al., 2010). Aedes and Culex spp. have been implicated as the main vector genera, with the former acting to transmit RVF in the interepidemic period and the latter acting as an amplifier species during epidemics (Pepin et al., 2010).
Epidemics of RVF are closely associated with excessive rainfall and El Nino events (Linthicum et al., 1999). Additional large-scale determinants include the presence of irrigation schemes, a high level of cultivation, higher population density (Redding et al., 2017). At a local scale, a critical factor is the nature of the local habitats—they must be suitable for supporting mosquito populations. In this context, Sang et al. (2010) collected both Aedes and Culex mosquitoes associated with RVF from a diverse range of habitats in Garissa, Kenya, including human settlements near flooded wetlands, mixed forest close to mangrove swamps and livestock holding areas. Elsewhere, Culex tritaeniorhynchus—a vector of RVF in Saudi Arabia, prefers wet, muddy substrates with a low total dissolved salts content (Sallam et al., 2013).
Mathematical models of RVF typically focus on identifying factors that can be used to forecast or mitigate the next epidemic (e.g. Leedale et al., 2016; Mpeshe et al., 2011; Pedro et al., 2016). The repeated observation of the importance of precipitation and water availability at specific locations, combined with the differentiated natural history of the main vectors, means that attempting to predict longer-term transmission of RVF under projected climate change scenarios is inherently more challenging than for a disease that has only one vector. Additional to the bioclimatic variable of importance are other factors related to the overall vulnerability of a population. This aspect of disease has been explored by Taylor et al. (2016) who constructed an overall risk map of future RVF outbreaks in East Africa that comprised the deterministic RVF model of Leedale et al. (2016), downscaled RCP projections and several expert-weighted indicators of social vulnerability.
Like all attempts to capture future transmission potential of an NTD, a number of assumptions have to be made. In the case of Taylor et al. (2016), one of the main assumptions was that the weight of certain social indicators (e.g. the relative importance of the percentage of homes with a mobile phone as an indicator of capacity to anticipate) would remain constant over the coming decades. It was also assumed that neither the mosquito vectors nor the virus itself will evolve as a result of selection pressures from climate change.
11.2.4. Crimean Congo Haemorrhagic Fever (CCHF)
A tick-borne infection, the CCHF virus is a member of the Bunyaviridae family. Many tick species, especially members of the Hylalomma genus, can act as vectors. Both domestic animals and wildlife, particularly birds, can act as reservoir populations.
The geographic distribution of infection is currently limited to the countries within Africa, the Middle East, Southern-Eastern Europe and Southern Asia (Dreshaj et al., 2016; Ergönül, 2006). Not all populations within each country affected by CCHF are at risk due to factors such as limited environmental suitability for the ticks (Messina et al., 2015) or the restricted distribution of zoonotic reservoirs including cattle and goats (Mostafavi et al., 2013). Concerns have been raised that countries such as Turkey and those in former Yugoslavia can act as portals for exporting cases into neighbouring countries (Mahzounieh et al., 2012; Mild et al., 2010), due to the ability of the vectors to be transported across boundaries.
Retrospective analyses of incidence data from human cases in Europe have observed distinct seasonality and correlations with environmental factors including increased vegetation (grass, scrubland and herbaceous) cover, medium–high level fragmentation of landscapes and warmer temperatures (Vescio et al., 2012). Similar conclusions regarding high temperatures were reached in a study of cases in Pakistan (Abbas et al., 2017).
Efforts to estimate the potential impact of climate change on future transmission of CCHF have, to date, focused on the role of migratory birds spreading the infection further into livestock of Europe (Gale et al., 2012). In that context, it has been concluded that climate change will not have a substantial impact by 2084.
The absence of a contribution from birds does not preclude other organisms from affecting potential future transmission. The role of climate in affecting tick ecology is likely to be highly significant given the sensitivity of tick development and mortality to temperature, water availability and vapour deficit (Estrada-Peña et al., 2015). In common with other infections included in this review, the relationship between success of the organism and temperature is nonlinear due to the differential effects on development, transovarial transmission, egg survival and mortality of the adult ticks (Estrada-Peña et al., 2013).
11.2.5. Severe Fever With Thrombocytopenia Syndrome (SFTS)
SFTS is caused by a phlebovirus from the Bunyaviridae family, first reported in 2009 in China (Yu et al., 2011). Haemaphysalis longicornis ticks are reservoir hosts of SFTS (Luo et al., 2015). Antibodies to the virus have been detected in goats, cattle, dogs and chickens (Ding et al., 2014; Zhao et al., 2012). The virus is on the list of WHO priority diseases. Elderly agricultural workers inhabiting rural, shrub or forested areas are at the greatest risk at specific times of year, according to epidemiological surveys (Liu et al., 2014).
At the time of writing, there were no original research publications on the potential for climate change to affect transmission in the future. A current hypothesis is that ticks have historically distributed the infection around China, South Korea and Japan by attachment to migrating birds (Li et al., 2016; Zhang and Xu, 2016).
11.2.6. Zika Virus
Zika virus is an arbovirus of the Flaviviridae family, known to be transmitted to humans through the bite of Aedes mosquitoes (Marchette et al., 1969). Culex has been assessed as a vector but appears refractory (Huang et al., 2016). Historically, the infection has been known to be endemic in parts of Africa (particularly Nigeria) and parts of southeast Asia (including Borneo and the Philippines). It was the emergence of Zika virus as a public health problem in Latin America in 2015 that created disquiet among global public health practitioners. The potential importance of the infection was recognised by WHO, which declared a public health emergency in February 2016 as the infection was observed to spread across 69 countries and territories, causing thousands of cases of microcephaly (World Health Organisation, 2016b). The designation was removed in November 2016, with WHO maintaining that Zika was a high priority issue. At the time of writing, Zika virus was placed on the list of priority diseases for research and development to prevent epidemics.
It has been speculated that climate variation was partly responsible for the rapid expansion of Zika during 2015 (Ali et al., 2017; Paz and Semenza, 2016). The reason for the rapid rise from 2015 onwards has been attributed to the El Nino event of 2015 (Caminade et al., 2017), due to the rise in temperatures increasing the biting rate of the mosquito vectors.
A few modelling studies have been published to estimate the contemporary risk of transmission of Zika virus, using ecological niche models to estimate environmental suitability for the two main vectors, A. albopictus and A. aegypti (Messina et al., 2016); or estimates of R0 for South America (Perkins et al., 2016). At the time of writing, there were no published original research studies on the potential for decadal changes in climate to affect transmission of Zika virus on a local or global scale. As with other vector-borne diseases, a key factor will be the effect of environmental change on the ecology of the vectors (see above), modified by the vectoral capacity of the two vectors (Gardner et al., 2017).
11.3. Bacteria
11.3.1. Trachoma
There have been several papers published that have considered whether or not transmission of trachoma (Chlamydia trachomatis) is associated with abiotic factors including temperature, rainfall, RH and sunshine fraction (reviewed by Ramesh et al., 2013). Of the various factors considered, rainfall and temperature (or altitude as a proxy for temperature) were most consistently reported (in mainly cross-sectional studies) to be associated with variation in the prevalence of active Trachoma. None of the eight papers reviewed by Ramesh et al. (2013) modelled prospective transmission under specific climate change scenarios. Clements et al. (2010) incorporated land cover and land use in a geostatistical model with the conclusion that transmission is higher in savannah and grassland compared to areas with higher precipitation or high water-table (wetlands).
C. trachomatis can be transmitted directly from person to person or through fomites. From a climate change perspective, it will be important to identify all potential fomites and consider how they may be affected in the future. The most widely implicated are fabrics that can come into contact with faces—towels, bedlinen, etc. But exudates from the eyes are often wiped away with fingers which then come into contact with a wide range of surfaces and objects touched by others. Some of these may be associated with climate-sensitive factors in ways that are poorly understood.
For example, there is a known link between inclusion conjunctivitis and genital chlamyidia (caused also by C. trachomatis), whereby contaminated genital secretions are transferred by hand to the eye either through autoinoculation or from a partner. Contaminated fingers may or may not be washed depending on the availability of water. Water availability is a climate-sensitive issue. In areas of water scarcity, hand washing is likely to be less frequent. Blinding trachoma is a major problem in countries with a high level of desertification, including Egypt and Chad. Although speculative, it is possible that blinding trachoma in such areas is partly associated with both the presence of genital chlamydia and lack of water for hand washing.
An understanding of the ecology of the vector-borne route is of critical importance when considering climate change. Musca sorbens, a major vector of trachoma, lays its eggs on (preferably human) faeces (Emerson et al., 2001). The larvae develop and emerge if conditions are suitable. Factors affecting the probability of emergence include whether or not the environment around the faeces leads to crust formation (making it harder to emerge), whether or not the faeces is removed by dung beetles, or whether fly predators such as the dermapateran Labidura riparia or histerid beetles such as Atholus rothkirchi Berkhardt, predate the larvae prior to emergence (Toyama and Ikeda, 1981). When considering how climate change may affect trachoma transmission it is therefore necessary to consider how dung beetles, and arthropod fly predators will also be affected. Furthermore, although M. sorbens appears to prefer human faeces, the fly will also settle and lay eggs on dung of animals such as cows. So it also important to consider how humans and animals will deposit faeces, and what happens to the faeces once deposited, under conditions of a changing climate which may include increasingly long periods of extreme conditions.
In a recent attempt to understand better how climate affects trachoma transmission, Ramesh et al. (2013) reviewed studies of abiotic factors associated with M. sorbens ecology. First implicated as a vector at the turn of the 21st century (Emerson et al., 2000), the fly is known to be affected by temperature and humidity, but the limited number of relevant studies makes modelling of the future transmission potential highly challenging (Ramesh et al., 2013).
The lack of empirical data to support dynamic models is of particular concern. The WHO strategy of combining mass antibiotic distribution, facial cleanliness and environmental hygiene (known as SAFE), has been scaled up in recent years, and blinding trachoma is one of the conditions scheduled for global elimination by 2020. Given the paucity of information on the epidemiology of blinding trachoma, its links to genital chlamydia, water insecurities, land use change and other potential ecological variances, it is still an uncertain future.
11.3.2. Buruli Ulcer
Buruli ulcer is a debilitating and disfiguring infection of tropical regions caused by Mycobacterium ulcerans. For a recent review of the pathology of Buruli ulcer, see Yotsu et al. (2015). Within that review is a list of known or suspected vectors of M. ulcerans that includes insects, mammals, fish and shellfish. One of the key suspects is Naucoridae water bugs (Portaels et al., 2008), which have been demonstrated to be able to transfer the bacterium to mice through biting—the bacterium being located in the salivary glands (Marsollier et al., 2002). There is also evidence that certain types of water body (e.g. swamps) are associated with increased risk of exposure to the bacterium, but no evidence that direct water contact is a risk factor (Williamson et al., 2012). The reason for proximity to water being a risk factor may be partly due to the presence of the water bugs. Linking the abundance of water bugs to the risk of infection has proven challenging—various studies have identified that carriage of the bacterium in in the mouthparts of the water bugs changes at specific times of year (Marion et al., 2010), but also that unidentified environmental factors can better explain spatiotemporal variation in disease when compared to spatiotemporal variation of M. ulcerans presence in the environment (Garchitorena et al., 2016).
Cases of the Buruli ulcer are predominantly found in West Africa (Yotsu et al., 2015), but also in other tropical countries including Japan (Yotsu et al., 2012) and northern parts of Australia (Lavender et al., 2012). Anthropocentric activities such as dam building have been implicated in terms of establishing new sites of infection (Marion et al., 2011). A recent case report suggests the bacterium is also found in Honduras (Southern, 2016). Buruli ulcer is classified as an emerging disease. The number of cases per country fluctuates and there is no clear temporal trend over the previous decade (Yotsu et al., 2015).
Evidence is emerging of landscape factors driving spatiotemporal variation in abundance of M. ulcerans cases—(Aboagye et al., 2017—presence of bacterial DNA in the environment; Landier et al., 2014; Merritt et al., 2010). At the time of writing, there were no original research articles on the potential for decadal climate change to affect transmission patterns.
11.4. Protozoa
11.4.1. Leishmania
For a review of papers published on this subject before 2008, see Ready (2008).
Several species of Phlebotomus and Lutzomyia sandflies, distributed and segregated geographically across the tropics, have been identified as major vectors of leishmanial parasites (Bates, 2007). Each species of sandfly occupies a specific ecological niche and has a climate-sensitive life cycle and population biology governed by a mixture of abiotic and biotic factors acting independently and through interactions. While it has been recognised since the turn of the century that climate change may have a significant impact on the distribution of both the vectors and the disease (Peterson and Shaw, 2003), the general direction of travel is less well specified due to the fact that sandfly populations are affected on a microgeographical scale.
In the European context, it has been demonstrated that the density of Phlebotomus ariasi at a particular sampling location is affected by both minimum and maximum temperatures and to some extent by RH (Prudhomme et al., 2015). South facing slopes, wall vegetation, soil type and neighbouring land cover precipitation may also be important factors in determining local abundance of this and other species (Ballart et al., 2014). Effects of individual explanatory factors disaggregate among species of sandfly, indicating a need to consider not just how climate change might affect individual ecological drivers of abundance and site-specific vector density, but how individual species of vector might be affected.
In the South American context, the relatively early models of Peterson and Shaw (2003), using a combination of ecological niche modelling and a general circulation model, predicted that by 2055 conditions in parts of Brazil may become more favourable for transmission due largely to anticipated increases in temperature. A more refined conclusion was reached by Carvalho et al. (2015), working with an ensemble of ecological niche models and downscaled (344 km2) general circulation models, projected to 2041–60. In that study, the simulations indicate that southern and eastern parts of Brazil are likely to become more suitable in terms of the environment a simulation model of climate change scenarios on the distribution of leishmanial vectors in the Colombian context, it was suggested that spatial range could decrease under minimally disruptive scenarios through to maximally disruptive scenarios (González et al., 2014).
11.4.2. Chagas Disease
The organism responsible for Chagas disease is Trypanasoma cruzi. This protozoan parasite is distributed widely across Latin America and is estimated to infect 6–8 million people of all ages (Rassi et al., 2010). The climate-sensitive stage of the parasite is in the insect vector, members of the Triatominae subfamily.
The evolution, natural history and ecology of many Triatominae vectors are described in detail elsewhere (Abad-Franch et al., 2015; Galvao and Justi, 2015; Teixeira et al., 2009). What has emerged from these and other studies is that many Triaotominae species have evolved to be closely associated with specific natural environments involving vertebrates such as birds, bats and rodents (Galvao and Justi, 2015) from which they derive blood meals. The large number of species means that there are a corresponding large number of potential niches, each of which may respond differently to changing climates.
It is becoming increasingly apparent that these traditional niches are being disturbed by anthropogenic changes affecting life history traits, species composition in specific locations and habitat availability (Gottdenker et al., 2012). Several studies have reported that normally sylvatic species are increasingly invading urban domestic properties, for example in Bolivia (Rojas-Cortez et al., 2016) and Brazil (Ribeiro et al., 2015). Reasons for expansion of the vector range into human habitations have speculated to include the increasing availability of electrical light. Many insect species are attracted to the blue light fraction of electrical light and several studies have observed clustering of Triatomine species around artificial light sources, both in sylvatic (Castro et al., 2010) and urban (Pacheco-Tucuch et al., 2012) settings. Economic activities associated with the availability of electric light have also been suggested to underlie recent epidemics of T. cruzi in new areas.
The anthropogenic influences above demonstrate that Triatominae adapt rapidly to new opportunities and/or pressures. The potential for climate change to affect transmission patterns of T. cruzi over much longer periods, through long-term effects on the soil and water phases, is less well understood but has received some attention in the literature, specifically through attempts to model the future suitability of habitats to support vectors. Two separate studies have focused on how projected warming scenarios may affect the geographic distribution of Rhodnius prolixus and Triatoma infestans in Venezuala and Argentina (Medone et al., 2015), and the geographic distribution of Eratyrus mucronatus, Panstrongylus geniculatus, R. prolixus, Rhodnius robustus and Triatoma maculata in Venezuala (Ceccarelli et al., 2015).
A common output of these studies was that the distribution of infection is likely to change over the coming decades. Specifically, the distribution of infection is expected to reduce in the countries studied due to environments becoming unsuitable for the vectors. Neither of these studies considered other changes to the niches that will be affected by climate change. Palm trees, bromeliads, bird and rodent nests, hollow trees and mammal burrows are among the sylvatic ecotopes occupied by the vectors of T. cruzi. Climate change is likely to impact on each ecotype in ways that we do not yet fully understand and which will be challenging to project given the potential for multidimensional interactions among biotic and abiotic domains.
11.4.3. Human African Trypanosomiasis (HAT)
The life cycle of HAT involves humans, wildlife and over 20 species of Glossina, the Tsetse fly. The most climate-sensitive stage of the lifecycle is in the flies. It is well established that Tsetse natural history dynamics are shaped by environmental factors—as far back as 1940 studies were being undertaken into the role of humidity on the population of Glossina pallidipes, Glossina austeni and Glossina brevipalpis in Kenya (Moggridge, 1949). Readers are referred to Leak (1999) for a comprehensive overview of Tsetse biology and ecology, and Rogers (2000) for an introduction into the use of satellite imagery to map the distribution of Tsetse flies.
More recently, Pagabeleguem et al. (2016) have investigated the fecundity and survival of three strains of G.p. gambiensis under conditions of varying temperature at constant RH (25–35°C at 60% RH) and varying RH at constant temperature (40%–75% RH at 25°C). From these two experiments, it was observed that temperature was more important than humidity at affecting survival. The effect of changing temperature on the survival was dramatic. Peak survival of all three strains was estimated to occur at 25°C, with rapid declines thereafter and a median survival time of less than 5 days at 32°C.
In terms of the potential for climate change to alter current transmission patterns, there has been one published study. Moore et al. (2012) used a deterministic model based on a series of ordinary differential equations that described the rate at which tsetse flies become susceptible, exposed, infected and recovered. The effect of changing temperature on the basic reproductive rate (R0) was then estimated from published studies on the effect of temperature change on mortality, biting rate, parasite-biting rate and population growth rate. Changes in temperature over future decades were drawn from the IPCC scenarios B1 and A2 to 2090. Population was assumed to remain constant.
Moore et al. (2012) predicted that rather than expansion of the tseste fly population under conditions set by the IPCC scenarios, there may be a shift of up to 60% in the geographical extent of the range. Under the A2 scenario, there is likely to be range contraction by 2090 due to some regions of eastern Africa becoming too hot to support the fly population.
In recognising the limits of their study, Moore et al. (2012) highlight a point relevant to all modelling studies—namely that the modelling exercise itself sets a framework for future projects rather than making any definitive statements.
12. Conclusions
In writing this review, I examined the published peer-reviewed literature for articles that represent interests in climate change and NTDs. Climate change in this context primarily concerns forward looking, decadal-scale changes and is therefore distinct from near or mid-term forecasting which typically concerns within-decade timescales.
It is clear from reviewing the published literature that there is a relative abundance of literature from previous decades, as well as more recently, regarding the environmental factors that underpin the natural history of several NTDs. In contrast, there is a paucity of forward-looking research being conducted on the decadal timescale for the majority of infections. This document therefore serves as a gap analysis as much as a review of the state of the art.
One conclusion that is nonetheless shared among all research outputs is that a changing climate is associated with spatiotemporal variation in exposure and transmission of each species of infection. Along with other aspects of global change (reviewed by Cable et al., 2017), it can be concluded that there are likely to be profound yet hard-to-discern changes to global patterns of NTD transmission in the near, mid- and long term. Some of these changes may be extreme enough to cause elimination or extinction of parasites, vectors and zoonotic hosts in localised, regional or global contexts (Cable et al., 2017; Cizauskas et al., 2017).
The future can only be imagined and modelled, as we cannot draw data down from the future. No model can capture every potential interaction, and the increasingly fragmented nature of ecosystems (Haddad et al., 2015) is an ongoing challenge in terms of providing mitigation and/or adaptation (Villard and Metzger, 2014).
In the absence of sureties regarding the future, combined with recognition of the inherent complexities facing humanity, a viable response may be to increase intersectoral collaboration so that, e.g., emerging knowledge in one domain can be assessed for its utility in another domain. The ‘One Health’ concept is a potential avenue down which colleagues working on NTDs from many disciplines could travel together (Webster et al., 2016). One Health joins several other attempts to synthesise an integrated, multidisciplinary, multisectoral framework, including blue marble health (Hotez et al., 2016) and planetary health (Horton et al., 2014). A complementary approach may be to improve surveillance and adaptation efforts (Ebi et al., 2013; Parham et al., 2015; Wilby and Dessai, 2010). It has been advocated that these new approaches should be community-based (Ebi and Semenza, 2008) and combine capacity building in modelling with decision support tools that are sufficiently flexible and adaptive to emerging conditions (Booth and Clements, 2018).
Acknowledgements
I would like to first acknowledge the contribution of all the scientists who undertook research many decades ago into the natural history of what are now known as NTDs. Their work has arguably more relevance than ever given the future uncertainties that we face. Those scientists who have more recently advanced our knowledge of environmental change and its impact on ecosystems have provided complementary knowledge that will significantly enhance the potential for mitigation. I also thank the editors for the invitation to review this important, yet still neglected, subject; an anonymous referee for several constructive comments, Henry J. Booth for helpful advice on the figures, and Anna Fruehauf for assistance with the bibliography.
References
- Aagaard-Hansen J., Nombela N., Alvar J. Population movement: a key factor in the epidemiology of neglected tropical diseases. Trop. Med. Int. Health. 2010;15(11):1281–1288. doi: 10.1111/j.1365-3156.2010.02629.x. [DOI] [PubMed] [Google Scholar]
- Abad-Franch F. On palms, bugs, and Chagas disease in the Americas. Acta Trop. 2015;151:126–141. doi: 10.1016/j.actatropica.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Abbas T. Seasonality in hospital admissions of Crimean-Congo hemorrhagic fever and its dependence on ambient temperature—empirical evidence from Pakistan. Int. J. Biometeorol. 2017;61:1893–1897. doi: 10.1007/s00484-017-1359-4. Springer, Berlin/Heidelberg. [DOI] [PubMed] [Google Scholar]
- Aboagye S.Y. Seasonal pattern of Mycobacterium ulcerans, the causative agent of Buruli ulcer, in the environment in Ghana. Microb. Ecol. 2017;74(2):350–361. doi: 10.1007/s00248-017-0946-6. Springer, USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler P.H., Cheke R.A., Post R.J. Evolution, epidemiology, and population genetics of black flies (Diptera: Simuliidae) Infect. Genet. Evol. 2010;10(7):846–865. doi: 10.1016/j.meegid.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Afshan K. Impact of climate change and man-made irrigation systems on the transmission risk, long-term trend and seasonality of human and animal fascioliasis in Pakistan. Geospat. Health. 2014;8(2):317. doi: 10.4081/gh.2014.22. [DOI] [PubMed] [Google Scholar]
- Aguiar L.M.S. Should I stay or should I go? Climate change effects on the future of Neotropical savannah bats. Glob. Ecol. Conserv. 2016;5:22–33. [Google Scholar]
- Aka N.A. Human paragonimiasis in Africa. Ann. Afr. Med. 2008;7(4):153–162. doi: 10.4103/1596-3519.55660. [DOI] [PubMed] [Google Scholar]
- Ali S. Environmental and social change drive the explosive emergence of Zika virus in the Americas. PLoS Negl. Trop. Dis. 2017;11(2) doi: 10.1371/journal.pntd.0005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almén A.-K. Coping with climate change? Copepods experience drastic variations in their physicochemical environment on a diurnal basis. J. Exp. Mar. Biol. Ecol. 2014;460:120–128. doi: 10.1016/J.JEMBE.2014.07.001. [DOI] [Google Scholar]
- Amman B.R. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 2012;8(10) doi: 10.1371/journal.ppat.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amraoui F., Vazeille M., Failloux A.B. French Aedes albopictus are able to transmit yellow fever virus. Euro. Surveill. 2016;21(39):30361. doi: 10.2807/1560-7917.ES.2016.21.39.30361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundson R. Soil and human security in the 21st century. Science. 2015;348(6235):1261071. doi: 10.1126/science.1261071. [DOI] [PubMed] [Google Scholar]
- Ancillotto L. Extraordinary range expansion in a common bat: the potential roles of climate change and urbanisation. Sci. Nat. 2016;103(3–4):15. doi: 10.1007/s00114-016-1334-7. [DOI] [PubMed] [Google Scholar]
- Anthony S.J. Further evidence for bats as the evolutionary source of Middle East respiratory syndrome coronavirus. mBio. 2017;8(2):e00373–17. doi: 10.1128/mBio.00373-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton C.C. Paragonimiasis in KwaZulu-Natal province, South Africa. J. Helminthol. 2014;88(1):123–128. doi: 10.1017/S0022149X12000831. [DOI] [PubMed] [Google Scholar]
- Araújo M.d.-S., Gil L.H.S., e-Silva A.d.-A. Larval food quantity affects development time, survival and adult biological traits that influence the vectorial capacity of Anopheles darlingi under laboratory conditions. Malar. J. 2012;11:261. doi: 10.1186/1475-2875-11-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal A., Brunton D., Raubenheimer D. Impact of climate change on human–wildlife–ecosystem interactions in the Trans-Himalaya region of Nepal. Theor. Appl. Climatol. 2014;115(3):517–529. [Google Scholar]
- Ashrafi K. Fascioliasis: a worldwide parasitic disease of importance in travel medicine. Travel. Med. Infect. Dis. 2014;12(6):636–649. doi: 10.1016/J.TMAID.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Asseng S. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015;5(2):143–147. [Google Scholar]
- Atkinson J.-A.M. Environmental changes impacting Echinococcus transmission: research to support predictive surveillance and control. Glob. Chang. Biol. 2013;19(3):677–688. doi: 10.1111/gcb.12088. [DOI] [PubMed] [Google Scholar]
- Attwood S.W. Studies on the parasitology, phylogeography and the evolution of host–parasite interactions for the snail intermediate hosts of medically important trematode genera in Southeast Asia. Adv. Parasitol. 2010;73:405–440. doi: 10.1016/S0065-308X(10)73013-X. [DOI] [PubMed] [Google Scholar]
- Attwood S.W., Upatham E.S. Observations on Neotricula aperta (Gastropoda: Pomatiopsidae) population densities in Thailand and central Laos: implications for the spread of Mekong schistosomiasis. Parasit. Vectors. 2012;5:126. doi: 10.1186/1756-3305-5-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine D.L. Investigations on the control of hookworm disease. XXIII. Experiments on the factors determining the length of life of infective hookworm larvae. Am. J. Epidemiol. 1923;3(4):420–443. doi: 10.1093/oxfordjournals.aje.a118945. [DOI] [Google Scholar]
- Azam D. Temperature and the development and survival of infective Toxocara canis larvae. Parasitol. Res. 2012;110(2):649–656. doi: 10.1007/s00436-011-2536-8. [DOI] [PubMed] [Google Scholar]
- Baker K.S. Viral antibody dynamics in a chiropteran host. J. Anim. Ecol. 2014;83:415–428. doi: 10.1111/1365-2656.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballart C. Importance of individual analysis of environmental and climatic factors affecting the density of Leishmania vectors living in the same geographical area: the example of Phlebotomus ariasi and P. perniciosus in northeast Spain. Geospat. Health. 2014;8(2):389. doi: 10.4081/gh.2014.28. [DOI] [PubMed] [Google Scholar]
- Bangert M. The cross-cutting contribution of the end of neglected tropical diseases to the sustainable development goals. Infect. Dis. Poverty. 2017;6(1):73. doi: 10.1186/s40249-017-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios S., Bertinelli L., Strobl E. Climatic change and rural–urban migration: the case of sub-Saharan Africa. J. Urban Econ. 2006;60(3):357–371. [Google Scholar]
- Bates P.A. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int. J. Parasitol. 2007;37(10):1097–1106. doi: 10.1016/j.ijpara.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates I. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet Infect. Dis. 2004;4(5):267–277. doi: 10.1016/S1473-3099(04)01002-3. [DOI] [PubMed] [Google Scholar]
- Baylis M., Githeko A.K. Office of Science and Innovation, UK Government; 2006. T7.3: The Effects of Climate Change on Infectious Diseases of Animals.http://webarchive.nationalarchives.gov.uk/20140108145827/http://www.bis.gov.uk/assets/foresight/docs/infectious-diseases/t7_3.pdf Available at. [Google Scholar]
- Beaver P. Persistence of hookworm larvae in soil. Am. J. Trop. Med. Hyg. 1953;2(1):102–108. doi: 10.4269/ajtmh.1953.2.102. [DOI] [PubMed] [Google Scholar]
- Becker D.J., Streicker D.G., Altizer S. Linking anthropogenic resources to wildlife-pathogen dynamics: a review and meta-analysis. Ecol. Lett. 2015;18(5):483–495. doi: 10.1111/ele.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer R.J.S. Studies on the biology of the life-cycle of Trichuris suis Schrank, 1788. Parasitology. 1973;67(3):253–262. doi: 10.1017/S0031182000046497. 2009/04/01. [DOI] [PubMed] [Google Scholar]
- Benedict M.Q. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7(1):76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. Advances in Experimental Medicine and Biology. Springer International Publishing AG; 2014. Paragonimiasis; pp. 115–152. [Google Scholar]
- Blair D., Xu Z.B., Agatsuma T. Paragonimiasis and the genus Paragonimus. Adv. Parasitol. 1999;42:113–222. doi: 10.1016/s0065-308x(08)60149-9. [DOI] [PubMed] [Google Scholar]
- Bloch P., Simonsen P.E. Immunoepidemiology of Dracunculus medinensis infections II. Variation in antibody responses in relation to transmission season and patency. Am. J. Trop. Med. Hyg. 1998;59(6):985–990. doi: 10.4269/ajtmh.1998.59.985. [DOI] [PubMed] [Google Scholar]
- Bockarie M.J. Role of vector control in the global program to eliminate lymphatic filariasis. Annu. Rev. Entomol. 2008;54(1):469–487. doi: 10.1146/annurev.ento.54.110807.090626. [DOI] [PubMed] [Google Scholar]
- Boissier J. Outbreak of urogenital schistosomiasis in Corsica (France): an epidemiological case study. Lancet Infect. Dis. 2016;16(8):971–979. doi: 10.1016/S1473-3099(16)00175-4. [DOI] [PubMed] [Google Scholar]
- Bonwitt J. Rat-atouille: a mixed method study to characterize rodent hunting and consumption in the context of Lassa fever. Ecohealth. 2016;13(2):234–247. doi: 10.1007/s10393-016-1098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonwitt J. At home with Mastomys and Rattus: human–rodent interactions and potential for primary transmission of Lassa virus in domestic spaces. Am. J. Trop. Med. Hyg. 2017;96(4):935–943. doi: 10.4269/ajtmh.16-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bony S. Clouds, circulation and climate sensitivity. Nat. Geosci. 2015;8(4):261–268. [Google Scholar]
- Booth M., Clements A.C. Neglected tropical disease control—the need for adaptive solutions. Trends Parasitol. 2018 doi: 10.1016/j.pt.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Brady O.J. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit. Vectors. 2013;6(1):351. doi: 10.1186/1756-3305-6-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook B., Sodhi N., Bradshaw C. Synergies among extinction drivers under global change. Trends Ecol. Evol. 2008;23(8):453–460. doi: 10.1016/j.tree.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Brooker S. Spatial analysis of the distribution of intestinal nematode infections in Uganda. Epidemiol. Infect. 2004;132(6):1065–1071. doi: 10.1017/s0950268804003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruelheide H., Udelhoven P. Correspondence of the fine-scale spatial variation in soil chemistry and the herb layer vegetation in beech forests. For. Ecol. Manage. 2005;210(1):205–223. doi: 10.1016/j.foreco.2005.02.050. [DOI] [Google Scholar]
- Buceta J., Johnson K. Modeling the ebola zoonotic dynamics: interplay between enviroclimatic factors and bat ecology. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0179559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson L., Grenouillet G. Contrasted impacts of climate change on stream fish assemblages along an environmental gradient. Divers. Distrib. 2009;15(4):613–626. doi: 10.1111/j.1472-4642.2009.00565.x. [DOI] [Google Scholar]
- Buisson L. Climate change hastens the turnover of stream fish assemblages. Glob. Chang. Biol. 2008;14(10):2232–2248. doi: 10.1111/j.1365-2486.2008.01657.x. [DOI] [Google Scholar]
- Burgis M.J. The effect of temperature on the development time of eggs of Thermocyclops sp., a tropical cyclopoid copepod from Lake George, Uganda. Limnol. Oceanogr. 1970;15(5):742–747. [Google Scholar]
- Cable J. Global change, parasite transmission and disease control: lessons from ecology. Philos. Trans. R Soc. Lond. B Biol. Sci. 2017;372(1719) doi: 10.1098/rstb.2016.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. Increasing frequency of extreme El Nino events due to greenhouse warming. Nat. Clim. Chang. 2014;4(2):111–116. [Google Scholar]
- Calvopiña M. Current status of Paragonimus and paragonimiasis in Ecuador. Mem. Inst. Oswaldo Cruz. 2014;109(7):849–855. doi: 10.1590/0074-0276140042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminade C. Global risk model for vector-borne transmission of Zika virus reveals the role of El Nino 2015. Proc. Natl. Acad. Sci. 2017;114(1):119–124. doi: 10.1073/pnas.1614303114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho B.M. Ecological niche modelling predicts southward expansion of Lutzomyia (Nyssomyia) flaviscutellata (Diptera: Psychodidae: Phlebotominae), vector of Leishmania (Leishmania) amazonensis in South America, under climate change. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0143282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casajus N. An objective approach to select climate scenarios when projecting species distribution under climate change. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0152495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L.M. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010;76(9):2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M.C. Attraction of Chagas disease vectors (Triatominae) to artificial light sources in the canopy of primary Amazon rainforest. Mem. Inst. Oswaldo Cruz. 2010;105(8):1061–1064. doi: 10.1590/S0074-02762010000800019. [DOI] [PubMed] [Google Scholar]
- Cathey J.T., Marr J.S. Yellow fever, Asia and the East African slave trade. Trans. R. Soc. Trop. Med. Hyg. 2014;108(5):252–257. doi: 10.1093/trstmh/tru043. [DOI] [PubMed] [Google Scholar]
- Ceccarelli S. Global climate change effects on Venezuela's vulnerability to Chagas disease is linked to the geographic distribution of five Triatomine species. J. Med. Entomol. 2015;52(6):1333–1343. doi: 10.1093/jme/tjv119. [DOI] [PubMed] [Google Scholar]
- Chadee D.D. Resting behaviour of Aedes aegypti in Trinidad: with evidence for the re-introduction of indoor residual spraying (IRS) for dengue control. Parasit. Vectors. 2013;6(1):255. doi: 10.1186/1756-3305-6-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.-Y. Vol. 114. Elsevier; 2013. Paragonimiasis; pp. 283–296. (Handbook of Clinical Neurology). [Google Scholar]
- Chan K.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011;2011:1–7. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler A.C. Macmillan; London: 1929. Hookworm Disease Its Distribution, Biology, Epidemiology, Pathology, Diagnosis, Treatment and Control. [Google Scholar]
- Charlier J. Climate-driven longitudinal trends in pasture-borne helminth infections of dairy cattle. Int. J. Parasitol. 2016;46(13–14):881–888. doi: 10.1016/J.IJPARA.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Cheke R.A. Potential effects of warmer worms and vectors on onchocerciasis transmission in West Africa. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015;370(1665) doi: 10.1098/rstb.2013.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J.T. The seasonal variation in breeding and growth of Mastomys natalensis (Rodentia: Muridae): evidence for resource limitation. Afr. J. Ecol. 1993;31(1):1–9. doi: 10.1111/j.1365-2028.1993.tb00513.x. [DOI] [Google Scholar]
- Chung B.J., Joo C.Y., Choi D.W. Seasonal variation of snail population of Parafossarulus manchouricus and larval trematode infection in river Kumho, Kyungpook province, Korea. Kisaengchunghak Chapchi. 1980;18(1):54–64. doi: 10.3347/kjp.1980.18.1.54. [DOI] [PubMed] [Google Scholar]
- Ciota A.T. The effect of temperature on life history traits of Culex mosquitoes. J. Med. Entomol. 2014;51(1):55–62. doi: 10.1603/me13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizauskas C.A. Parasite vulnerability to climate change: an evidence-based functional trait approach. R. Soc. Open Sci. 2017;4(1):160535. doi: 10.1098/rsos.160535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements A.C.A. Targeting trachoma control through risk mapping: the example of southern Sudan. PLoS Negl. Trop. Dis. 2010;4(8) doi: 10.1371/journal.pntd.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee C.G. The biology, behaviour, and ecology of Mastomys natalensis in southern Africa. Bull. World Health Organ. 1975;52(4–6):637–644. [PMC free article] [PubMed] [Google Scholar]
- Cohen B. Urbanization in developing countries: current trends, future projections, and key challenges for sustainability. Technol. Soc. 2006;28(1):63–80. doi: 10.1016/j.techsoc.2005.10.005. [DOI] [Google Scholar]
- Collins M. Long-term climate change: projections, commitments and irreversibility. In: Stocker T., editor. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge: Cambridge University Press; 2013. Available at: [Google Scholar]
- Coman B.J. The survival of Taenia pisiformis eggs under laboratory conditions and in the field environment. Aust. Vet. J. 1975;51(12):560–565. doi: 10.1111/j.1751-0813.1975.tb09381.x. [DOI] [PubMed] [Google Scholar]
- Comte L. Climate-induced changes in the distribution of freshwater fish: observed and predicted trends. Freshw. Biol. 2013;58(4):625–639. doi: 10.1111/fwb.12081. [DOI] [Google Scholar]
- Conteh L., Engels T., Molyneux D.H. Socioeconomic aspects of neglected tropical diseases. Lancet. 2010;375(9710):239–247. doi: 10.1016/S0140-6736(09)61422-7. [DOI] [PubMed] [Google Scholar]
- Cooper S.J., Wheeler T. Rural household vulnerability to climate risk in Uganda. Reg. Environ. Chang. 2017;17(3):649–663. [Google Scholar]
- Cruz-Mendoza I. Dynamics of Fasciola hepatica infection in two species of snails in a rural locality of Mexico. Vet. Parasitol. 2004;121(1–2):87–93. doi: 10.1016/J.VETPAR.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Davies C., Coetzee M., Lyons C.L. Effect of stable and fluctuating temperatures on the life history traits of Anopheles arabiensis and An. quadriannulatus under conditions of inter- and intra-specific competition. Parasit. Vectors. 2016;9(1):342. doi: 10.1186/s13071-016-1630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J.F. Mosquito oviposition behavior and vector control. Insects. 2016;7(4) doi: 10.3390/insects7040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan K.V., Sreevatsa Extended studies on the viability of Mycobacterium leprae outside the human body. Lepr. Rev. 1995;66(4):287–295. [PubMed] [Google Scholar]
- Dewes C.F. Drought risk assessment under climate change is sensitive to methodological choices for the estimation of evaporative demand. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo D. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson R.E. A regional climate model for the western United States. Clim. Change. 1989;15(3):383–422. [Google Scholar]
- Ding S. A cross-sectional survey of severe fever with thrombocytopenia syndrome virus infection of domestic animals in Laizhou City, Shandong Province, China. Jpn. J. Infect. Dis. 2014;67(1):1–4. doi: 10.7883/yoken.67.1. [DOI] [PubMed] [Google Scholar]
- Doanh P.N., Horii Y., Nawa Y. Paragonimus and paragonimiasis in Vietnam: an update. Korean J. Parasitol. 2013;51(6):621–627. doi: 10.3347/kjp.2013.51.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehring E., Feldmeier H., Daffalla A.A. Day-to-day variation and circadian rhythm of egg excretion in urinary schistosomiasis in the Sudan. Ann. Trop. Med. Parasitol. 1983;77(6):587–594. doi: 10.1080/00034983.1983.11811757. [DOI] [PubMed] [Google Scholar]
- Donadeu M. Taenia solium: WHO endemicity map update. Wkly. Epidemiol. Rec. 2016;91:585–600. [PubMed] [Google Scholar]
- Doran J.W., Zeiss M.R. Soil health and sustainability: managing the biotic component of soil quality. Appl. Soil Ecol. 2000;15(1):3–11. doi: 10.1016/S0929-1393(00)00067-6. [DOI] [Google Scholar]
- Dreshaj S. Current situation of Crimean-Congo hemorrhagic fever in Southeastern Europe and neighboring countries: a public health risk for the European Union? Travel Med. Infect. Dis. 2016;14(2):81–91. doi: 10.1016/j.tmaid.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Drexler J.F., Corman V.M., Drosten C. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Res. 2014;101:45–56. doi: 10.1016/j.antiviral.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard M.L. Possible role of fish and frogs as paratenic hosts of Dracunculus medinensis, Chad. Emerg. Infect. Dis. 2016;22(8):1428–1430. doi: 10.3201/eid2208.160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebi K.L., Nealon J. Dengue in a changing climate. Environ. Res. 2016;151:115–123. doi: 10.1016/j.envres.2016.07.026. [DOI] [PubMed] [Google Scholar]
- Ebi K.L., Semenza J.C. Community-based adaptation to the health impacts of climate change. Am. J. Prev. Med. 2008;35(5):501–507. doi: 10.1016/j.amepre.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Ebi K.L. Adaptation to the infectious disease impacts of climate change. Clim. Change. 2013;118(2):355–365. doi: 10.1007/s10584-012-0648-5. [DOI] [Google Scholar]
- Echaubard P. Experimental and modelling investigations of Opisthorchis viverrini miracidia transmission over time and across temperatures: implications for control. Int. J. Parasitol. 2017;47(5):257–270. doi: 10.1016/j.ijpara.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwanzala M. In the heart of darkness: sleeping sickness in Zaire. Lancet. 1996;348(9039):1427–1430. doi: 10.1016/S0140-6736(96)06088-6. [DOI] [PubMed] [Google Scholar]
- Elliott J. Constraints and potentials of future irrigation water availability on agricultural production under climate change. Proc. Natl. Acad. Sci. U.S.A. 2014;111(9):3239–3244. doi: 10.1073/pnas.1222474110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis V.A. Local host specialization, host-switching, and dispersal shape the regional distributions of avian haemosporidian parasites. Proc. Natl. Acad. Sci. 2015;112(36):11294–11299. doi: 10.1073/pnas.1515309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S.A. Endoparasites in the feces of arctic foxes in a terrestrial ecosystem in Canada. Int. J. Parasitol. Parasites Wildl. 2013;2:90–96. doi: 10.1016/j.ijppaw.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson P.M. Transmission ecology of the fly Musca sorbens, a putative vector of trachoma. Trans. R. Soc. Trop. Med. Hyg. 2000;94(1):28–32. doi: 10.1016/S0035-9203(00)90427-9. [DOI] [PubMed] [Google Scholar]
- Emerson P.M. Human and other faeces as breeding media of the trachoma vector Musca sorbens. Med. Vet. Entomol. 2001;15(3):314–320. doi: 10.1046/j.0269-283x.2001.00318.x. [DOI] [PubMed] [Google Scholar]
- Ergönül Ö. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 2006;6(4):203–214. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger T.E. Effect of water resource development and management on lymphatic filariasis, and estimates of populations at risk. Am. J. Trop. Med. Hyg. 2005;73(3):523–533. [PubMed] [Google Scholar]
- Escobar L.E. Declining prevalence of disease vectors under climate change. Sci. Rep. 2016;6 doi: 10.1038/srep39150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A. Factors driving the circulation and possible expansion of Crimean-Congo haemorrhagic fever virus in the western Palearctic. J. Appl. Microbiol. 2013;114(1):278–286. doi: 10.1111/jam.12039. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A. The impact of climate trends on a tick affecting public health: a retrospective modeling approach for Hyalomma marginatum (Ixodidae) PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. ML Books International—IPS; Delhi: 2015. Parasitic Diseases: Schistosomiasis. [Google Scholar]
- Farajollahi A. Bird biting mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011;11(7):1577–1585. doi: 10.1016/j.meegid.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichet-Calvet E. Risk maps of Lassa fever in West Africa. PLoS Negl. Trop. Dis. 2009;3(3) doi: 10.1371/journal.pntd.0000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficke A.D., Myrick C.A., Hansen L.J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007;17(4):581–613. doi: 10.1007/s11160-007-9059-5. [DOI] [Google Scholar]
- Fischer P.U. Molecular characterization of the North American lung fluke Paragonimus kellicotti in Missouri and its development in Mongolian gerbils. Am. J. Trop. Med. Hyg. 2011;84(6):1005–1011. doi: 10.4269/ajtmh.2011.11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick C., Engels D. Leaving no one behind: a neglected tropical disease indicator and tracers for the sustainable development goals: box 1. Int. Health. 2016;8(Suppl. 1):i15–i18. doi: 10.1093/inthealth/ihw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty R. Henipavirus susceptibility to environmental variables. Virus Res. 2008;132(1–2):140–144. doi: 10.1016/j.virusres.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.J. Predicting impacts of climate change on Fasciola hepatica risk. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Paredes C., Rodriguez-Morales A.J. Unsolved matters in leprosy: a descriptive review and call for further research. Ann. Clin. Microbiol. Antimicrob. 2016;15(1):33. doi: 10.1186/s12941-016-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M.C. Integration of water, sanitation, and hygiene for the prevention and control of neglected tropical diseases: a rationale for inter-sectoral collaboration. PLoS Negl. Trop. Dis. 2013;7(9) doi: 10.1371/journal.pntd.0002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller D.O. Linking land cover and species distribution models to project potential ranges of malaria vectors: an example using Anopheles arabiensis in Sudan and Upper Egypt. Malar. J. 2012;11(1):264. doi: 10.1186/1475-2875-11-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss S. Betting on negative emissions. Nat. Clim. Chang. 2014;4(10):850–853. doi: 10.1038/nclimate2392. [DOI] [Google Scholar]
- Füssel H.-M. Vulnerability: a generally applicable conceptual framework for climate change research. Glob. Environ. Chang. 2007;17(2):155–167. [Google Scholar]
- Gaasenbeek C.P., Borgsteede F.H. Studies on the survival of Ascaris suum eggs under laboratory and simulated field conditions. Vet. Parasitol. 1998;75(2–3):227–234. doi: 10.1016/s0304-4017(97)00198-2. [DOI] [PubMed] [Google Scholar]
- Gabriel S. Human migration and pig/pork import in the European Union: what are the implications for Taenia solium infections? Vet. Parasitol. 2015;213(1–2):38–45. doi: 10.1016/j.vetpar.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Gale P. Impact of climate change on risk of incursion of Crimean-Congo haemorrhagic fever virus in livestock in Europe through migratory birds. J. Appl. Microbiol. 2012;112(2):246–257. doi: 10.1111/j.1365-2672.2011.05203.x. [DOI] [PubMed] [Google Scholar]
- Galvao C., Justi S.A. An overview on the ecology of Triatominae (Hemiptera:Reduviidae) Acta Trop. 2015;151:116–125. doi: 10.1016/j.actatropica.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Gamboa M.I. Effects of temperature and humidity on the development of eggs of Toxocara canis under laboratory conditions. J. Helminthol. 2005;79(4):327–331. doi: 10.1079/JOH2005287. [DOI] [PubMed] [Google Scholar]
- Garchitorena A. Environmental transmission of Mycobacterium ulcerans drives dynamics of Buruli ulcer in endemic regions of Cameroon. Sci. Rep. 2016;5(1):18055. doi: 10.1038/srep18055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner L., Chen N., Sarkar S. Vector status of Aedes species determines geographical risk of autochthonous Zika virus establishment. PLoS Negl. Trop. Dis. 2017;11(3) doi: 10.1371/journal.pntd.0005487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garske T. Yellow fever in Africa: estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Negl. Trop. Dis. 2014;11(5) doi: 10.1371/journal.pmed.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell M.A. Taeniidae: modification to the life span of the egg and the regulation of tapeworm populations. Exp. Parasitol. 1977;41(2):314–328. doi: 10.1016/0014-4894(77)90104-7. [DOI] [PubMed] [Google Scholar]
- Gerten D., Adrian R. Species-specific changes in the phenology and peak abundance of freshwater copepods in response to warm summers. Freshw. Biol. 2002;47(11):2163–2173. doi: 10.1046/j.1365-2427.2002.00970.x. [DOI] [Google Scholar]
- Gethings O.J. Asynchrony in host and parasite phenology may decrease disease risk in livestock under climate warming: Nematodirus battus in lambs as a case study. Parasitology. 2015;142(10):1306–1317. doi: 10.1017/S0031182015000633. [DOI] [PubMed] [Google Scholar]
- Geyer H.S., Geyer H.S. Disaggregated population migration trends in South Africa between 1996 and 2011: a differential urbanisation approach. Urban Forum. 2015;26(1):1–13. [Google Scholar]
- Ghiglietti R. Viability of Ascaris suum, Ascaris lumbricoides and Trichuris muris eggs to alkaline pH and different temperatures. Parassitologia. 1995;37(2–3):229–232. [PubMed] [Google Scholar]
- Gill M., Smith P., Wilkinson J.M. Mitigating climate change: the role of domestic livestock. Animal. 2010;4(3):323–333. doi: 10.1017/S1751731109004662. 2009/05/22. [DOI] [PubMed] [Google Scholar]
- Gomez-Puerta L.A. Longevity and viability of Taenia solium eggs in the digestive system of the beetle Ammophorus rubripes. Rev. Bras. Parasitol. Vet. 2014;23(1):94–97. doi: 10.1590/s1984-29612014014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González C., Paz A., Ferro C. Predicted altitudinal shifts and reduced spatial distribution of Leishmania infantum vector species under climate change scenarios in Colombia. Acta Trop. 2014;129:83–90. doi: 10.1016/j.actatropica.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Gosling S.N., Arnell N.W. A global assessment of the impact of climate change on water scarcity. Clim. Change. 2016;134(3):371–385. [Google Scholar]
- Gottdenker N.L. Host life history strategy, species diversity, and habitat influence Trypanosoma cruzi vector infection in Changing landscapes. PLoS Negl. Trop. Dis. 2012;6(11) doi: 10.1371/journal.pntd.0001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouagna L.C. Abiotic and biotic factors associated with the presence of Anopheles arabiensis immatures and their abundance in naturally occurring and man-made aquatic habitats. Parasit. Vectors. 2012;5:96. doi: 10.1186/1756-3305-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumghar M.D. Influence of aestivation on the survival of Galba truncatula (Mollusca: Gasteropoda) populations according to altitude. Ann. Limnol. 2001;37(3):211–217. [Google Scholar]
- Gray C., Mueller V. Drought and population mobility in rural Ethiopia. World Dev. 2012;40(1):134–145. doi: 10.1016/j.worlddev.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs D. Policy: sustainable development goals for people and planet. Nature. 2013;495(7441):305–307. doi: 10.1038/495305a. [DOI] [PubMed] [Google Scholar]
- Grimes J.E.T. The relationship between water, sanitation and schistosomiasis: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2014;8(12) doi: 10.1371/journal.pntd.0003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunay F., Alten B., Ozsoy E.D. Narrow-sense heritability of body size and its response to different developmental temperatures in Culex quinquefasciatus (Say 1923) J. Vector Ecol. 2011;36(2):348–354. doi: 10.1111/j.1948-7134.2011.00175.x. [DOI] [PubMed] [Google Scholar]
- Haddad N.M. Habitat fragmentation and its lasting impact on Earth's ecosystems. Sci. Adv. 2015;1(2) doi: 10.1126/sciadv.1500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenlocher M. Assessing socioeconomic vulnerability to dengue fever in Cali, Colombia: statistical vs expert-based modeling. Int. J. Health Geogr. 2013;12(1):36. doi: 10.1186/1476-072X-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halimi M. Developing a climate-based risk map of fascioliasis outbreaks in Iran. J. Infect. Public Health. 2015;8(5):481–486. doi: 10.1016/J.JIPH.2015.04.024. [DOI] [PubMed] [Google Scholar]
- Hallegatte S. Strategies to adapt to an uncertain climate change. Glob. Environ. Chang. 2009;19(2):240–247. doi: 10.1016/J.GLOENVCHA.2008.12.003. [DOI] [Google Scholar]
- Halpin K. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J. Gen. Virol. 2000;81(8):1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- Hampson K. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015;9(4) doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B.A. Undiscovered bat hosts of filoviruses. PLoS Negl. Trop. Dis. 2016;10(7) doi: 10.1371/journal.pntd.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.E., Charleston W.A.G. Some temperature responses of Lymnaea tomentosa and L. columella (Mollusca: Gastropoda) and their eggs. N. Z. J. Zool. 1977;4(1):45–49. doi: 10.1080/03014223.1977.9517935. [DOI] [Google Scholar]
- Hartmann A. Enhanced groundwater recharge rates and altered recharge sensitivity to climate variability through subsurface heterogeneity. Proc. Natl. Acad. Sci. U. S. A. 2017;114(11):2842–2847. doi: 10.1073/pnas.1614941114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann D.L. No observations: atmosphere and surface. In: Stocker T.F., editor. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge: Cambridge University Press; 2013. [Google Scholar]
- Hassell J.M. Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface. Trends Ecol. Evol. 2017;32(1):55–67. doi: 10.1016/J.TREE.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverd V. Carbon cycle responses of semi-arid ecosystems to positive asymmetry in rainfall. Glob. Chang. Biol. 2017;23(2):793–800. doi: 10.1111/gcb.13412. [DOI] [PubMed] [Google Scholar]
- Hawking F. Circadian and other rhythms of parasites. Adv. Parasitol. 1975;13:123–182. [PubMed] [Google Scholar]
- Hay L.E., Wilby R.L., Leavesley G.H. A comparison of delta change and downscaled GCM scenarios for three mountainous basins in the United States. J. Am. Water Res. Assoc. 2000;36(2):387–397. doi: 10.1111/j.1752-1688.2000.tb04276.x. [DOI] [Google Scholar]
- Haydock L.A.J. A growing degree-day model for determination of Fasciola hepatica infection risk in New Zealand with future predictions using climate change models. Vet. Parasitol. 2016;228:52–59. doi: 10.1016/J.VETPAR.2016.05.033. [DOI] [PubMed] [Google Scholar]
- Hayman D.T.S. Biannual birth pulses allow filoviruses to persist in bat populations. Proc. R. Soc. B. 2014;282(20142591) doi: 10.1098/rspb.2014.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held I.M., Soden B.J. Robust responses of the hydrological cycle to global warming. J. Climate. 2006;19(21):5686–5699. doi: 10.1175/JCLI3990.1. [DOI] [Google Scholar]
- Higgs S. Vector competence of Australian mosquitoes for yellow fever virus. Am. J. Trop. Med. Hyg. 2011;85(3):446–451. doi: 10.4269/ajtmh.2011.11-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y. Global flood risk under climate change. Nat. Clim. Chang. 2013;3(9):816–821. [Google Scholar]
- Horton R. From public to planetary health: a manifesto. Lancet. 2014;383(9920):847. doi: 10.1016/S0140-6736(14)60409-8. [DOI] [PubMed] [Google Scholar]
- Hotez P.J. Neglected infections of poverty among the indigenous peoples of the arctic. PLoS Negl. Trop. Dis. 2010;4(1) doi: 10.1371/journal.pntd.0000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J. Ten global “hotspots” for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014;8(5) doi: 10.1371/journal.pntd.0002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P. The neglected tropical diseases: the ancient afflictions of stigma and poverty and the prospects for their control and elimination. In: Pollard A.J., Finn A., editors. Hot Topics in Infection and Immunity in Children III. Boston, MA; Springer: 2006. pp. 23–33. [DOI] [PubMed] [Google Scholar]
- Hotez P.J. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014;8(7) doi: 10.1371/journal.pntd.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Damania A., Naghavi M. Blue marble health and the global burden of disease study 2013. PLoS Negl. Trop. Dis. 2016;10(10) doi: 10.1371/journal.pntd.0004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. Global transcriptional dynamics of diapause induction in non-blood-fed and blood-fed Aedes albopictus. PLoS Negl. Trop. Dis. 2015;9(4) doi: 10.1371/journal.pntd.0003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-J.S. Culex species mosquitoes and Zika virus. Vector Borne Zoonotic Dis. 2016;16(10):673–676. doi: 10.1089/vbz.2016.2058. [DOI] [PubMed] [Google Scholar]
- Huettmann F., Magnuson E.E., Hueffer K. Ecological niche modeling of rabies in the changing Arctic of Alaska. Acta Vet. Scand. 2017;59(1):18. doi: 10.1186/s13028-017-0285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung N., Madsen H., Fried B. Global status of fish-borne zoonotic trematodiasis in humans. Acta Parasitol. 2013;58(3):231–258. doi: 10.2478/s11686-013-0155-5. [DOI] [PubMed] [Google Scholar]
- Ijumba J.N., Lindsay S.W. Impact of irrigation on malaria in Africa: paddies paradox. Med. Vet. Entomol. 2001;15(1):1–11. doi: 10.1046/j.1365-2915.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- IPCC In: Stocker T.F., editor. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge: Cambridge University Press; 2013. [Google Scholar]
- IPCC . In: Climate Change 2014 Impacts, Adaptation, and Vulnerability. Field C.B., editor. Cambridge University Press; Cambridge: 2014. [Google Scholar]
- Islam M.S. Nipah virus transmission from bats to humans associated with drinking traditional liquor made from date palm sap, Bangladesh, 2011–2014. Emerg. Infect. Dis. 2016;22(4):664–670. doi: 10.3201/eid2204.151747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins E.J., Schurer J.M., Gesy K.M. Old problems on a new playing field: helminth zoonoses transmitted among dogs, wildlife, and people in a changing northern climate. Vet. Parasitol. 2011;182(1):54–69. doi: 10.1016/j.vetpar.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Jensen P.K.M. Survival of Ascaris eggs and hygienic quality of human excreta in Vietnamese composting latrines. Environ. Health. 2009;8:57. doi: 10.1186/1476-069X-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P. A climate-driven mechanistic population model of Aedes albopictus with diapause. Parasit. Vectors. 2016;9(1):175. doi: 10.1186/s13071-016-1448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P. How does the dengue vector mosquito Aedes albopictus respond to global warming? Parasit. Vectors. 2017;10(1):140. doi: 10.1186/s13071-017-2071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinda C., Chimbari M., Mukaratirwa S. Implications of changing temperatures on the growth, fecundity and survival of intermediate host snails of schistosomiasis: a systematic review. Int. J. Environ. Res. Public Health. 2017;14(1) doi: 10.3390/ijerph14010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnay E., Cai M. Impact of urbanization and land-use change on climate. Nature. 2003;423(6939):528–531. doi: 10.1038/nature01675. [DOI] [PubMed] [Google Scholar]
- Kamara A., Koroma B.M., Gogra A.B. Seasonal changes in vegetation and land use in Lassa-fever-prone areas (Kenema and Kailahun districts) in eastern Sierra Leone. Nat. Res. 2015;6(7):450–456. doi: 10.4236/nr.2015.67043. [DOI] [Google Scholar]
- Kang S.M. Impact of polar ozone depletion on subtropical precipitation. Science. 2011;332(6032):951–954. doi: 10.1126/science.1202131. [DOI] [PubMed] [Google Scholar]
- Karim M.N. Climatic factors influencing dengue cases in Dhaka city: a model for dengue prediction. Indian J. Med. Res. 2012;136(1):32–39. http://www.ncbi.nlm.nih.gov/pubmed/22885261 Available at: [PMC free article] [PubMed] [Google Scholar]
- Kassie B.T. Adapting to climate variability and change: experiences from cereal-based farming in the central rift and kobo valleys, Ethiopia. Environ. Manag. 2013;52(5):1115–1131. doi: 10.1007/s00267-013-0145-2. [DOI] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Food-borne trematodiases. Clin. Microbiol. Rev. 2009;22(3):466–483. doi: 10.1128/CMR.00012-09. American Society for Microbiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall S.B., McCullough F.S. The emergence of the cercariae of fasciola hepatica from the snail limnaea truncatula. J. Helminthol. 1951;25(1–2):77. doi: 10.1017/S0022149X0001899X. Cambridge University Press. [DOI] [Google Scholar]
- Kibret S. The influence of dams on malaria transmission in sub-Saharan Africa. Ecohealth. 2017;14(2):408–419. doi: 10.1007/s10393-015-1029-0. [DOI] [PubMed] [Google Scholar]
- Kim E.-M. Infection status of freshwater crabs and crayfish with metacercariae of Paragonimus westermani in Korea. Korean J. Parasitol. 2009;47(4):425–426. doi: 10.3347/kjp.2009.47.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.I. A conceptual model for the impact of climate change on fox rabies in Alaska, 1980-2010. Zoonoses Public Health. 2014;61(1):72–80. doi: 10.1111/zph.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp A.K. Characterizing differences in precipitation regimes of extreme wet and dry years: implications for climate change experiments. Global Change Biol. 2015;21(7):2624–2633. doi: 10.1111/gcb.12888. [DOI] [PubMed] [Google Scholar]
- Kraemer B.M. Morphometry and average temperature affect lake stratification responses to climate change. Geophys. Res. Lett. 2015;42(12):4981–4988. [Google Scholar]
- Kraemer M.U. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife. 2015;4 doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer M.U.G. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015-16: a modelling study. Lancet Infect. Dis. 2017;17(3):330–338. doi: 10.1016/S1473-3099(16)30513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel D. The precautionary principle in environmental science. Environ. Health Perspect. 2001;109(9):871–876. doi: 10.1289/ehp.01109871. National Institute of Environmental Health Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Rota P.A., Rollin P.E. A review of Nipah and Hendra viruses with an historical aside. Virus Res. 2011;162(1–2):173–183. doi: 10.1016/j.virusres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Kulkarni M.A. 10 years of environmental change on the slopes of Mount Kilimanjaro and its associated shift in malaria vector distributions. Front. Public Health. 2016;4:281. doi: 10.3389/fpubh.2016.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundzewicz Z.W. Flood risk and climate change: global and regional perspectives. Hydrol. Sci. J. 2014;59(1):1–28. doi: 10.1080/02626667.2013.857411. [DOI] [Google Scholar]
- Kyorku C.A., Raybould J.N. Preliminary studies on egg-mass development in the Simulium damnosum Theobald complex (Diptera: Simuliidae) Insect Sci. Appl. 1987;8(3):311–316. doi: 10.1017/S1742758400005294. 2011/09/01. [DOI] [Google Scholar]
- Lambrechts L. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evol. Biol. 2009;9(1):160. doi: 10.1186/1471-2148-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landier J. Spatio-temporal patterns and landscape-associated risk of Buruli ulcer in Akonolinga, Cameroon. PLoS Negl. Trop. Dis. 2014;8(9) doi: 10.1371/journal.pntd.0003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender C.J. Buruli ulcer disease in travelers and differentiation of Mycobacterium ulcerans strains from northern Australia. J. Clin. Microbiol. 2012;50(11):3717–3721. doi: 10.1128/JCM.01324-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws G.F. Physical factors influencing survival of taeniid eggs. Exp. Parasitol. 1968;22(2):227–239. doi: 10.1016/0014-4894(68)90097-0. [DOI] [PubMed] [Google Scholar]
- Lawson J.R., Gemmell M.A. Hydatidosis and cysticercosis: the dynamics of transmission. Adv. Parasitol. 1983;22:261–308. doi: 10.1016/s0065-308x(08)60464-9. [DOI] [PubMed] [Google Scholar]
- Lawson J.R., Gemmell M.A. Transmission of taeniid tapeworm eggs via blowflies to intermediate hosts. Parasitology. 1990;100(1):143–146. doi: 10.1017/S0031182000060224. [DOI] [PubMed] [Google Scholar]
- Leak S.G.A. CABI Publishing; Wallingford: 1999. Tsetse Biology and Ecology: Their Role in the Epidemiology and Control of Trypanosomosis. [Google Scholar]
- Leedale J. A dynamic, climate-driven model of Rift Valley fever. Geospat. Health. 2016;11(1s):394. doi: 10.4081/gh.2016.394. [DOI] [PubMed] [Google Scholar]
- Leirs H. Seasonal variation in growth of Mastomys natalensis (Rodentia: Muridae) in Morogoro, Tanzania. Afr. J. Ecol. 1990;28(4):298–306. doi: 10.1111/j.1365-2028.1990.tb01164.x. [DOI] [Google Scholar]
- Leirs H. Forecasting rodent outbreaks in Africa: an ecological basis for Mastomys control in Tanzania. J. Appl. Ecol. 1996;33(5):937. doi: 10.2307/2404675. [DOI] [Google Scholar]
- Lenhart A. Contributions of different mosquito species to the transmission of lymphatic filariasis in central Nigeria: implications for monitoring infection by PCR in mosquito pools. Filaria J. 2007;6(1):14. doi: 10.1186/1475-2883-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Guo Q. How to assess the prediction accuracy of species presence-absence models without absence data? Ecography. 2013;36(7):788–799. doi: 10.1111/j.1600-0587.2013.07585.x. [DOI] [Google Scholar]
- Li K. Risks for fishborne zoonotic trematodes in Tilapia production systems in Guangdong province, China. Vet. Parasitol. 2013;198(1–2):223–229. doi: 10.1016/j.vetpar.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Li T., Yang Z., Wang M. Correlation between clonorchiasis incidences and climatic factors in Guangzhou, China. Parasit. Vectors. 2014;7:29. doi: 10.1186/1756-3305-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Ecology of the tick-borne Phlebovirus causing severe fever with thrombocytopenia syndrome in an endemic area of China. PLoS Negl. Trop. Dis. 2016;10(4) doi: 10.1371/journal.pntd.0004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.-S. The South-to-North Water Diversion Project: effect of the water diversion pattern on transmission of Oncomelania hupensis, the intermediate host of Schistosoma japonicum in China. Parasit. Vectors. 2012;5:52. doi: 10.1186/1756-3305-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima dos Santos C.A.M., Howgate P. Fishborne zoonotic parasites and aquaculture: a review’. Aquaculture. 2011;318(3–4):253–261. doi: 10.1016/J.AQUACULTURE.2011.05.046. [DOI] [Google Scholar]
- Linthicum K.J. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science. 1999;285(5426) doi: 10.1126/science.285.5426.397. [DOI] [PubMed] [Google Scholar]
- Liu K. Epidemiologic features and environmental risk factors of severe fever with thrombocytopenia syndrome, Xinyang, China. PLoS Negl. Trop. Dis. 2014;8(5) doi: 10.1371/journal.pntd.0002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Iacono G. Using modelling to disentangle the relative contributions of zoonotic and anthroponotic transmission: the case of Lassa fever. PLoS Negl. Trop. Dis. 2015;9(1) doi: 10.1371/journal.pntd.0003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz E.N. Deterministic nonperiodic flow. J. Atmos. Sci. 1963;20(2):130–141. [Google Scholar]
- Luby S.P. Foodborne transmission of Nipah virus, Bangladesh. Emerg. Infect. Dis. 2006;12(12):1888–1894. doi: 10.3201/eid1212.060732. Centers for Disease Control and Prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby S.P. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg. Infect. Dis. 2009;15(8):1229–1235. doi: 10.3201/eid1508.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C., Timbal B., Nguyen H. The expanding tropics: a critical assessment of the observational and modeling studies. Wiley Interdiscip. Rev. Clim. Change. 2014;5(1):89–112. doi: 10.1002/wcc.251. [DOI] [Google Scholar]
- Lundholm J.T. Plant species diversity and environmental heterogeneity: spatial scale and competing hypotheses. J. Veg. Sci. 2009;20(3):377–391. doi: 10.1111/j.1654-1103.2009.05577.x. [DOI] [Google Scholar]
- Luo J. Global warming alters sound transmission: differential impact on the prey detection ability of echolocating bats. J. R. Soc. Interface. 2014;11(91) doi: 10.1098/rsif.2013.0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L.-M. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg. Infect. Dis. 2015;21(10):1770–1776. doi: 10.3201/eid2110.150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons C.L., Coetzee M., Chown S.L. Stable and fluctuating temperature effects on the development rate and survival of two malaria vectors, Anopheles arabiensis and Anopheles funestus. Parasit. Vectors. 2013;6(1):104. doi: 10.1186/1756-3305-6-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabaso M.L.H. The effect of soil type and climate on hookworm (Necator americanus) distribution in KwaZulu-Natal, South Africa. Trop. Med. Int. Health. 2003;8(8):722–727. doi: 10.1046/j.1365-3156.2003.01086.x. [DOI] [PubMed] [Google Scholar]
- MacNeil A., Rollin P.E. Ebola and Marburg hemorrhagic fevers: neglected tropical diseases? PLoS Negl. Trop. Dis. 2012;6(6) doi: 10.1371/journal.pntd.0001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson C.N.L. The epidemiology and public health importance of toxocariasis: a zoonosis of global importance. Int. J. Parasitol. 2013;43(12 − 13):999–1008. doi: 10.1016/j.ijpara.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Mahmoud A.F. Imperial College Press; London: 2001. Schistosomiasis (Tropical Medicine: Science and Practice) [Google Scholar]
- Mahzounieh M. Relationship between Crimean-Congo hemorrhagic fever virus strains circulating in Iran and Turkey: possibilities for Transborder transmission. Vector Borne Zoonotic Dis. 2012;12(9):782–785. doi: 10.1089/vbz.2011.0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makundi R.H., Massawe A.W., Mulungu L.S. Reproduction and population dynamics of Mastomys natalensisSmith, 1834 in an agricultural landscape in the Western Usambara Mountains, Tanzania. Integr. Zool. 2007;2(4):233–238. doi: 10.1111/j.1749-4877.2007.00063.x. [DOI] [PubMed] [Google Scholar]
- Malone J.B. Use of LANDSAT MSS imagery and soil type in a geographic information system to assess site-specific risk of fascioliasis on red river basin farms in Louisiana. Ann. N. Y. Acad. Sci. 1992;653(1 Tropical Vete):389–397. doi: 10.1111/j.1749-6632.1992.tb19667.x. [DOI] [PubMed] [Google Scholar]
- Malone J. A geographic information system on the potential distribution and abundance of Fasciola Hepatica and F. gigantica in east Africa based on food and agriculture organization databases. Vet. Parasitol. 1998;78(2):87–101. doi: 10.1016/S0304-4017(98)00137-X. [DOI] [PubMed] [Google Scholar]
- Mangal T.D., Paterson S., Fenton A. Predicting the impact of long-term temperature changes on the epidemiology and control of schistosomiasis: a mechanistic model. PLoS One. 2008;3(1) doi: 10.1371/journal.pone.0001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangham L.J., Hanson K. Scaling up in international health: what are the key issues? Health Policy Plan. 2010;25(2):85–96. doi: 10.1093/heapol/czp066. [DOI] [PubMed] [Google Scholar]
- Mantyka-Pringle C.S. Climate change modifies risk of global biodiversity loss due to land-cover change. Biol. Conserv. 2015;187:103–111. [Google Scholar]
- Marchette N.J., Garcia R., Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969;18(3):411–415. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- Marino N.A.C. Rainfall and hydrological stability alter the impact of top predators on food web structure and function. Global Change Biol. 2017;23(2):673–685. doi: 10.1111/gcb.13399. [DOI] [PubMed] [Google Scholar]
- Marion E. Seasonal and regional dynamics of M. ulcerans transmission in environmental context: deciphering the role of water bugs as hosts and vectors. PLoS Negl. Trop. Dis. 2010;4(7) doi: 10.1371/journal.pntd.0000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion E. Geographic expansion of Buruli ulcer disease, Cameroon. Emerg. Infect. Dis. 2011;17(3):551–553. doi: 10.3201/eid1703.091859. Centers for Disease Control and Prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsollier L. Aquatic insects as a vector for Mycobacterium ulcerans. Appl. Environ. Microbiol. 2002;68(9):4623–4628. doi: 10.1128/aem.68.9.4623-4628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas-Coma S., Funatsu I.R., Bargues M.D. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology. 2001;123(7):115–127. doi: 10.1017/S0031182001008034. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S., Valero M.A., Bargues M.D. Chapter 2 Fasciola, Lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 2009;69:41–146. doi: 10.1016/S0065-308X(09)69002-3. [DOI] [PubMed] [Google Scholar]
- Maslin M., Austin P. Uncertainty: climate models at their limit? Nature. 2012;486(7402):183–184. doi: 10.1038/486183a. [DOI] [PubMed] [Google Scholar]
- Massawe A. Influence of land preparation methods and vegetation cover on population abundance of Mastomys natalensis in Morogoro, Tanzania. Belg. J. Zool. 2005;135(Suppl):187–190. [Google Scholar]
- Massawe A.W. Do farming practices influence population dynamics of rodents? A case study of the multimammate field rats, Mastomys natalensis, in Tanzania. Afr. J. Ecol. 2007;45(3):293–301. doi: 10.1111/j.1365-2028.2006.00709.x. [DOI] [Google Scholar]
- Massawe A.W. Soil type limits population abundance of rodents in crop fields: case study of the multimammate rat Mastomys natalensis Smith, 1834 in Tanzania. Integr. Zool. 2008;3(1):27–30. doi: 10.1111/j.1749-4877.2008.00070.x. [DOI] [PubMed] [Google Scholar]
- Mastrorillo M. The influence of climate variability on internal migration flows in South Africa. Glob. Environ. Chang. 2016;39:155–169. [Google Scholar]
- Masui T. An emission pathway for stabilization at 6 Wm − 2 radiative forcing. Clim. Change. 2011;109(1):59–76. [Google Scholar]
- Matsuoka M. Mycobacterium leprae DNA in daily using water as a possible source of leprosy infection. Indian J. Lepr. 1999;71(1):61–67. [PubMed] [Google Scholar]
- McCall P.J. Aggregated oviposition in the Simulium damnosum complex is mediated by eggs in a laboratory bioassay. Med. Vet. Entomol. 1994;8(1):76–80. doi: 10.1111/j.1365-2915.1994.tb00390.x. [DOI] [PubMed] [Google Scholar]
- McCreesh N., Booth M. Challenges in predicting the effects of climate change on Schistosoma mansoni and Schistosoma haematobium transmission potential. Trends Parasitol. 2013;29(11):548–555. doi: 10.1016/j.pt.2013.08.007. [DOI] [PubMed] [Google Scholar]
- McCreesh N., Booth M. The effect of increasing water temperatures on Schistosoma mansoni transmission and Biomphalaria pfeifferi population dynamics: an agent-based modelling study. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreesh N., Nikulin G., Booth M. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit. Vectors. 2015;8:4. doi: 10.1186/s13071-014-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus D.P. Echinococcosis. Lancet. 2003;362(9392):1295–1304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- Medone P. The impact of climate change on the geographical distribution of two vectors of Chagas disease: implications for the force of infection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015;370(1665) doi: 10.1098/rstb.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.Q. The membranous structure of eggs of Ascaris lumbricoides as revealed by scanning electron microscopy. Scan. Electron. Microsc. 1981;(Pt. 3):187–190. [PubMed] [Google Scholar]
- Merritt R.W. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl. Trop. Dis. 2010;4(12) doi: 10.1371/journal.pntd.0000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier V. Seroprevalence of seven zoonotic infections in Nunavik, Quebec (Canada) Zoonoses Public Health. 2012;59(2):107–117. doi: 10.1111/j.1863-2378.2011.01424.x. [DOI] [PubMed] [Google Scholar]
- Messina J.P. The global distribution of Crimean-Congo hemorrhagic fever. Trans. R. Soc. Trop. Med. Hyg. 2015;109(8):503–513. doi: 10.1093/trstmh/trv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina J.P. Mapping global environmental suitability for Zika virus. eLife. 2016;5 doi: 10.7554/eLife.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mideo N. The Cinderella syndrome: why do malaria-infected cells burst at midnight? Trends Parasitol. 2013;29(1):10–16. doi: 10.1016/j.pt.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mild M. Towards an understanding of the migration of Crimean-Congo hemorrhagic fever virus. J. Gen. Virol. 2010;91(1):199–207. doi: 10.1099/vir.0.014878-0. [DOI] [PubMed] [Google Scholar]
- Miles M.A., de Souza A.A., Povoa M. Chagas’ disease in the Amazon Basin: III. Ecotopes of ten triatomine bug species (Hemiptera: Reduviidae) from the vicinity of Belem, Para State, Brazil. J. Med. Entomol. 1981;18(4):266–278. doi: 10.1093/jmedent/18.4.266. [DOI] [PubMed] [Google Scholar]
- Miller J.R. Life on the edge: African malaria mosquito (Anopheles gambiae s. l.) larvae are amphibious. Naturwissenschaften. 2007;94(3):195–199. doi: 10.1007/s00114-006-0178-y. [DOI] [PubMed] [Google Scholar]
- Minakawa N. Anopheline mosquito survival strategies during the dry period in western Kenya. J. Med. Entomol. 2001;38(3):388–392. doi: 10.1603/0022-2585-38.3.388. [DOI] [PubMed] [Google Scholar]
- Mintsa-Nguéma R. Cercarial emergence pattern of Schistosoma haematobium from Libreville, Gabon. Parasite. 2014;21:3. doi: 10.1051/parasite/2014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizgajska H. The role of some environmental factors in the contamination of soil with Toxocara spp. and other geohelminth eggs. Parasitol. Int. 1997;46(1):67–72. [Google Scholar]
- Moggridge Y.J. No climate and the activity of the Kenyan coastal Glossina. Bull. Entomol. Res. 1949;40:307–321. doi: 10.1017/s0007485300024585. [DOI] [PubMed] [Google Scholar]
- Molyneux D.H., Savioli L., Engels D. Neglected tropical diseases: progress towards addressing the chronic pandemic. Lancet. 2017;389(10066):312–325. doi: 10.1016/S0140-6736(16)30171-4. [DOI] [PubMed] [Google Scholar]
- Molyneux D., Sankara D.P. Guinea worm eradication: progress and challenges—should we beware of the dog? PLoS Negl. Trop. Dis. 2017;11(4) doi: 10.1371/journal.pntd.0005495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monde C., Syampungani S., van den Brink P.J. Natural and human induced factors influencing the abundance of Schistosoma host snails in Zambia. Environ Monit Assess. 2016;188(6):370. doi: 10.1007/s10661-016-5351-y. Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney H. Biodiversity, climate change, and ecosystem services. Curr. Opin. Environ. Sustain. 2009;1(1):46–54. doi: 10.1016/j.cosust.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. Predicting the effect of climate change on African trypanosomiasis: integrating epidemiology with parasite and vector biology. J. R. Soc. Interface. 2012;9(70):817–830. doi: 10.1098/rsif.2011.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T.L. Stormwater management and climate change: vulnerability and capacity for adaptation in urban and suburban contexts. Clim. Change. 2016;138(3):491–504. [Google Scholar]
- Moratelli R., Calisher C.H. Bats and zoonotic viruses: can we confidently link bats with emerging deadly viruses? Mem. Inst. Oswaldo Cruz. 2015;110(1):1–22. doi: 10.1590/0074-02760150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C.W., Comrie A.C. Modeled response of the West Nile virus vector Culex Quinquefasciatus to changing climate using the dynamic mosquito simulation model. Int. J. Biometeorol. 2010;54(5):517–529. doi: 10.1007/s00484-010-0349-6. [DOI] [PubMed] [Google Scholar]
- Morin C.W., Comrie A.C. Regional and seasonal response of a West Nile virus vector to climate change. Proc. Natl. Acad. Sci. U.S.A. 2013;110(39):15620–15625. doi: 10.1073/pnas.1307135110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley N.J., Lewis J.W. Thermodynamics of cercarial development and emergence in trematodes. Parasitology. 2013;140(10):1211–1224. doi: 10.1017/S0031182012001783. [DOI] [PubMed] [Google Scholar]
- Mortimore M.J., Adams W.M. Farmer adaptation, change and “crisis” in the Sahel. Glob. Environ. Chang. 2001;11(1):49–57. doi: 10.1016/S0959-3780(00)00044-3. [DOI] [Google Scholar]
- Moser W. The spatial and seasonal distribution of Bulinus truncatus, Bulinus forskalii and Biomphalaria pfeifferi, the intermediate host snails of schistosomiasis, in N’Djamena, Chad. Geospat. Health. 2014;9(1) doi: 10.4081/gh.2014.9. [DOI] [PubMed] [Google Scholar]
- Mostafavi E. Spatial analysis of Crimean Congo hemorrhagic fever in Iran. Am. J. Trop. Med. Hyg. 2013;89(6):1135–1141. doi: 10.4269/ajtmh.12-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton L. Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol. Lett. 2007;3(2):210–213. doi: 10.1098/rsbl.2006.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpeshe S.C., Haario H., Tchuenche J.M. A mathematical model of Rift valley fever with human host. Acta Biotheor. 2011;59(3–4):231–250. doi: 10.1007/s10441-011-9132-2. [DOI] [PubMed] [Google Scholar]
- Mudenda N.B. Modelling the ecological niche of hookworm in Brazil basedon climate. Geospat. Health. 2012;6(3):111–123. doi: 10.4081/gh.2012.129. [DOI] [PubMed] [Google Scholar]
- Munita M.P. Six-year longitudinal study of Fasciola hepatica bulk milk antibody ELISA in the dairy dense region of the republic Ireland. Prev. Vet. Med. 2016;134:16–25. doi: 10.1016/J.PREVETMED.2016.09.024. [DOI] [PubMed] [Google Scholar]
- Mutheneni S.R. Dengue burden in India: recent trends and importance of climatic parameters. Emerg. Microbes Infect. 2017;6(8) doi: 10.1038/emi.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutumi G.L., Jacobs D.S., Winker H. Sensory drive mediated by climatic gradients partially explains divergence in acoustic signals in two horseshoe bat species, rhinolophus swinnyi and rhinolophus simulator. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0148053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwanjabe P.S., Sirima F.B., Lusingu J. Crop losses due to outbreaks of Mastomys natalensis (Smith, 1834) Muridae, Rodentia, in the Lindi region of Tanzania. Int. Biodeter. Biodegr. 2002;49(2–3):133–137. [Google Scholar]
- Mylne A.Q.N. Mapping the zoonotic niche of Lassa fever in Africa. Trans. R. Soc. Trop. Med. Hyg. 2015;109(8):483–492. doi: 10.1093/trstmh/trv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Goran E.K. Changes in human schistosomiasis levels after the construction of two large hydroelectric dams in central Côte d’Ivoire. Bull. World Health Organ. 1997;75(6):541–545. [PMC free article] [PubMed] [Google Scholar]
- Nakicenovic N. Cambridge University Press; Cambridge: 2000. Special Report on Emissions Scenarios (SRES), A Special Report of Working Group III of the Intergovernmental Panel on Climate Change.http://pure.iiasa.ac.at/6101/2/sres-en.pdf Available at: [Google Scholar]
- Nawrotzki R.J. Climate change as a migration driver from rural and urban Mexico. Environ. Res. Lett. 2015;10(11):114023. doi: 10.1088/1748-9326/10/11/114023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndimubanzi P.C. A systematic review of the frequency of Neurocyticercosis with a focus on people with epilepsy. PLoS Negl. Trop. Dis. 2010;4(11) doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T.M. Bacteria in tropical floodplain soils are sensitive to changes in saltwater. Mar. Freshw. Res. 2016 doi: 10.1071/MF16033. [DOI] [Google Scholar]
- Nikulin G. Precipitation climatology in an ensemble of CORDEX-Africa regional climate simulations. J. Climate. 2012;25(18):6057–6078. doi: 10.1175/JCLI-D-11-00375.1. [DOI] [Google Scholar]
- Nunn A.D. Fish, climate and the Gulf stream: the influence of abiotic factors on the recruitment success of cyprinid fishes in lowland rivers. Freshw. Biol. 2007;52(8):1576–1586. doi: 10.1111/j.1365-2427.2007.01789.x. [DOI] [Google Scholar]
- Nwosu A.B., Anya A.O. Seasonality in human hookworm infection in an endemic area of Nigeria, and its relationship to rainfall. Tropenmed. Parasitol. 1980;31(2):201–208. [PubMed] [Google Scholar]
- Obokata R., Veronis L., McLeman R. Empirical research on international environmental migration: a systematic review. Popul. Environ. 2014;36(1):111–135. doi: 10.1007/s11111-014-0210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell C.J. Survival of parasite eggs upon storage in sludge. Appl. Environ. Microbiol. 1984;48(3):618–625. doi: 10.1128/aem.48.3.618-625.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Lorcain P. The effects of freezing on the viability of Toxocara canis and T. cati embryonated eggs. J. Helminthol. 1995;69(2):169–171. doi: 10.1017/s0022149x00014073. [DOI] [PubMed] [Google Scholar]
- O’Shea T.J. Bat flight and zoonotic viruses. Emerg. Infect. Dis. 2014;20(5):741–745. doi: 10.3201/eid2005.130539. Centers for Disease Control and Prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival K., Hayman D. Filoviruses in bats: current knowledge and future directions. Virus. 2014;6(4):1759–1788. doi: 10.3390/v6041759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival K.J. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546(7660):646–650. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omlin F.X. Anopheles gambiae exploits the treehole ecosystem in western Kenya: a new urban malaria risk? Am. J. Trop. Med. Hyg. 2007;77(6 Suppl.):264–269. https://doi.org/77/6_Suppl/264 [pii] [PubMed] [Google Scholar]
- Paaijmans K.P. Competitive interactions between larvae of the malaria mosquitoes Anopheles arabiensis and Anopheles gambiae under semi-field conditions in western Kenya. Acta Trop. 2009;109(2):124–130. doi: 10.1016/j.actatropica.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Pacheco-Tucuch F.S. Public street lights increase house infestation by the Chagas disease vector Triatoma dimidiata. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0036207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagabeleguem S. Influence of temperature and relative humidity on survival and fecundity of three tsetse strains. Parasit. Vectors. 2016;9(1) doi: 10.1186/s13071-016-1805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paploski I.A.D. Storm drains as larval development and adult resting sites for Aedes aegypti and Aedes albopictus in Salvador, Brazil. Parasit. Vectors. 2016;9(1) doi: 10.1186/s13071-016-1705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P.E. Climate change and vector-borne diseases of humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015;370(1665) doi: 10.1098/rstb.2014.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz J.A. Impact of regional climate change on human health. Nature. 2005;438(7066):310–317. doi: 10.1038/nature04188. [DOI] [PubMed] [Google Scholar]
- Payne F.K. Investigations on the control of hookworm disease. XXX. Studies on factors involved in migration of hookworm larvae in soil. Am. J. Epidemiol. 1923;3(5):547–583. doi: 10.1093/oxfordjournals.aje.a118955. [DOI] [Google Scholar]
- Paz S., Semenza J.C. El Niño and climate change—contributing factors in the dispersal of Zika virus in the Americas? Lancet. 2016;387(10020) doi: 10.1016/S0140-6736(16)00256-7. [DOI] [PubMed] [Google Scholar]
- Pecson B.M. The effects of temperature, pH, and ammonia concentration on the inactivation of Ascaris eggs in sewage sludge. Water Res. 2007;41(13):2893–2902. doi: 10.1016/j.watres.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Pedersen U.B. Modelling climate change impact on the spatial distribution of fresh water snails hosting trematodes in Zimbabwe. Parasit. Vectors. 2014;7(1) doi: 10.1186/s13071-014-0536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedro S.A. Predicting Rift Valley fever inter-epidemic activities and outbreak patterns: insights from a stochastic host-vector model. PLoS Negl. Trop. Dis. 2016;10(12) doi: 10.1371/journal.pntd.0005167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin M. Rift Valley fever virus(Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 2010;41(6) doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perich M.J. Behavior of resting Aedes aegypti (Culicidae: Diptera) and its relation to ultra-low volume adulticide efficacy in Panama City, Panama. J. Med. Entomol. 2000;37(4):541–546. doi: 10.1603/0022-2585-37.4.541. [DOI] [PubMed] [Google Scholar]
- Perkins T.A. Model-based projections of Zika virus infections in childbearing women in the Americas. Nat. Microbiol. 2016;1 doi: 10.1038/nmicrobiol.2016.126. [DOI] [PubMed] [Google Scholar]
- Peterson A.T., Shaw J. Lutzomyia vectors for cutaneous leishmaniasis in Southern Brazil: ecological niche models, predicted geographic distributions, and climate change effects. Int. J. Parasitol. 2003;33(9):919–931. doi: 10.1016/s0020-7519(03)00094-8. [DOI] [PubMed] [Google Scholar]
- Petney T. The ecology of the Bithynia first intermediate hosts of Opisthorchis viverrini. Parasitol. Int. 2012;61(1):38–45. doi: 10.1016/j.parint.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Pica N., Bouvier N.M. Environmental factors affecting the transmission of respiratory viruses. Curr. Opin. Virol. 2012;2(1):90–95. doi: 10.1016/j.coviro.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielke R.A. Land use and climate change. Science. 2005;310(5754):1625–1626. doi: 10.1126/science.1120529. [DOI] [PubMed] [Google Scholar]
- Pigott D.M. Mapping the zoonotic niche of Ebola virus disease in Africa. eLife. 2014;3 doi: 10.7554/eLife.04395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott D.M. Mapping the zoonotic niche of Marburg virus disease in Africa. Trans. R. Soc. Trop. Med. Hyg. 2015;109(6):366–378. doi: 10.1093/trstmh/trv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright R.K. Transmission or within-host dynamics driving pulses of zoonotic viruses in reservoir–host populations. PLoS Negl. Trop. Dis. 2016;10(8) doi: 10.1371/journal.pntd.0004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poertner H. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88(4):137–146. doi: 10.1007/s001140100216. [DOI] [PubMed] [Google Scholar]
- Portaels F. First cultivation and characterization of Mycobacterium ulcerans from the environment. PLoS Negl. Trop. Dis. 2008;2(3) doi: 10.1371/journal.pntd.0000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasopdee S. Temperature dependence of Opisthorchis viverrini infection in first intermediate host snail, Bithynia siamensis goniomphalos. Acta Trop. 2015;141(Pt. A):112–117. doi: 10.1016/j.actatropica.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Prudhomme J. Ecology and spatiotemporal dynamics of sandflies in the Mediterranean Languedoc region (Roquedur area, Gard, France) Parasit. Vectors. 2015;8:642. doi: 10.1186/s13071-015-1250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A.W., Tanveer A., Mas-Coma S. Epidemiological analysis of human fascioliasis in northeastern Punjab, Pakistan. Acta Trop. 2016;156:157–164. doi: 10.1016/J.ACTATROPICA.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Radhakrishna S., Huffman M.A., Sinha A. In: The Macaque Connection: Cooperation and Conflict between Humans and Macaques (Developments in Primatology: Progress and Prospects) Radhakrishna S., Huffman M.A., Sinha A., editors. Springer; Heidelberg: 2014. [Google Scholar]
- Ramasamy R., Surendran S.N. Global climate change and its potential impact on disease transmission by salinity-tolerant mosquito vectors in coastal zones. Front Physiol. 2012;3 doi: 10.3389/fphys.2012.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh A. The impact of climatic risk factors on the prevalence, distribution, and severity of acute and chronic trachoma. PLoS Negl. Trop. Dis. 2013;7(11) doi: 10.1371/journal.pntd.0002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapsch C. An interactive map to assess the potential spread of Lymnaea Truncatula and the free-living stages of Fasciola hepatica in Switzerland. Vet. Parasitol. 2008;154(3–4):242–249. doi: 10.1016/J.VETPAR.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Rassi A., Rassi A., Marin-Neto J.A. Chagas disease. Lancet. 2010;375(9723):1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- Ready P.D. Leishmaniasis emergence and climate change. Rev. Sci. Tech. 2008;27(2):399–412. [PubMed] [Google Scholar]
- Redding D.W. Environmental-mechanistic modelling of the impact of global change on human zoonotic disease emergence: a case study of Lassa fever. Methods Ecol. Evol. 2016;7(6):646–655. doi: 10.1111/2041-210X.12549. [DOI] [Google Scholar]
- Redding D.W. Spatial, seasonal and climatic predictive models of Rift Valley fever disease across Africa. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372(1725) doi: 10.1098/rstb.2016.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny R., Moore W.H. Does environmental degradation influence migration? Emigration to developed countries in the late 1980s and 1990s. Soc. Sci. Q. 2009;90(3):461–479. [Google Scholar]
- Riahi K. RCP 8.5—a scenario of comparatively high greenhouse gas emissions. Clim. Change. 2011;109(1):33. [Google Scholar]
- Ribeiro G. Frequent house invasion of Trypanosoma cruzi-infected triatomines in a suburban area of Brazil. PLoS Negl. Trop. Dis. 2015;9(4) doi: 10.1371/journal.pntd.0003678. Public Library of Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A.D. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013;169:156–173. [Google Scholar]
- Riley E.P. Flexibility in diet and activity patterns of Macaca tonkeana in response to anthropogenic habitat alteration. Int. J. Primatol. 2007;28(1):107–133. [Google Scholar]
- Rogers D.J. Satellites, space, time and the African trypanosomiases. Adv. Parasitol. 2000;47:129–171. doi: 10.1016/s0065-308x(00)47008-9. [DOI] [PubMed] [Google Scholar]
- Rogers D.J. The global distribution of yellow fever and dengue. Adv. Parasitol. 2006;62:181–220. doi: 10.1016/S0065-308X(05)62006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D.J., Randolph S.E. Climate change and vector-borne diseases. Advances in Parasitology. 2006:345–381. doi: 10.1016/S0065-308X(05)62010-6. [DOI] [PubMed] [Google Scholar]
- Rojas-Cortez M. Trypanosoma cruzi-infected Panstrongylus geniculatus and Rhodnius robustus adults invade households in the tropics of Cochabamba region of Bolivia. Parasit. Vectors. 2016;9(1):158. doi: 10.1186/s13071-016-1445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollinson D. Biomphalaria Snails and Larval Trematodes. Springer; New York, NY: 2011. Biomphalaria: natural history, ecology and schistosome transmission; pp. 57–79. [Google Scholar]
- Rondelaud D. Consequence of temperature changes on cercarial shedding from Galba truncatula infected with Fasciola hepatica or Paramphistomum daubneyi. Parasite. 2013;20:10. doi: 10.1051/parasite/2013009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P.A., Endersby N.M., Hoffmann A.A. Costs of three Wolbachia infections on the survival of Aedes aegypti larvae under starvation conditions. PLoS Negl. Trop. Dis. 2016;10(1) doi: 10.1371/journal.pntd.0004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph L. American Public Health Institute; Washington, DC: 2013. Health in All Policies: A Guide for State and Local Governments. [Google Scholar]
- Rueda L.M. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 1990;27(5):892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- Ruiz-Navarro A., Gillingham P.K., Britton J.R. Shifts in the climate space of temperate cyprinid fishes due to climate change are coupled with altered body sizes and growth rates. Glob. Chang. Biol. 2016;22(9):3221–3232. doi: 10.1111/gcb.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Tiben E. The peculiar epidemiology of Dracunculiasis in Chad. Am. J. Trop. Med. Hyg. 2014;90(1):61–70. doi: 10.4269/ajtmh.13-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Tiben E., Hopkins D.R. Dracunculiasis (Guinea worm disease) eradication. Adv. Parasitol. 2006;61:275–309. doi: 10.1016/S0065-308X(05)61007-X. [DOI] [PubMed] [Google Scholar]
- Rupprecht C.E., Hanlon C.A., Slate D. Oral vaccination of wildlife against rabies: opportunities and challenges in prevention and control. Dev. Biol. 2004;119:173–184. [PubMed] [Google Scholar]
- Russell C.A., Real L.A., Smith D.L. Spatial control of rabies on heterogeneous landscapes. PLoS One. 2006;1(1) doi: 10.1371/journal.pone.0000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S.J. Mapping physiological suitability limits for malaria in Africa under climate change. Vector Borne Zoonotic Dis. 2015;15(12):718–725. doi: 10.1089/vbz.2015.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J.P. Southward movement of the Pacific intertropical convergence zone AD 1400-1850. Nat. Geosci. 2009;2(7):519–525. [Google Scholar]
- Sagripanti J.-L., Rom A.M., Holland L.E. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol. 2010;155(12):2035–2039. doi: 10.1007/s00705-010-0791-0. [DOI] [PubMed] [Google Scholar]
- Salb A.L. Dogs as sources and sentinels of parasites in humans and wildlife, Northern Canada. Emerg. Infect. Dis. 2008;14(1):60. doi: 10.3201/eid1401.071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam M.F. Ecological niche modeling and land cover risk areas for rift valley fever vector, Culex tritaeniorhynchus giles in Jazan, Saudi Arabia. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samy A.M. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0163863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Thevenet P., Alvarez H.M., Basualdo J.A. Survival, physical and physiological changes of Taenia hydatigena eggs under different conditions of water stress. Exp. Parasitol. 2017;177:47–56. doi: 10.1016/j.exppara.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Sang R. Rift Valley fever virus epidemic in Kenya, 2006/2007: the entomologic investigations. Am. J. Trop. Med. Hyg. 2010;83(2 Suppl):28–37. doi: 10.4269/ajtmh.2010.09-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite D. The implications of population growth and urbanization for climate change. Environ. Urban. 2009;21(2):545–567. doi: 10.1177/0956247809344361. [DOI] [Google Scholar]
- Sayson S.L. Seasonal genetic changes of Aedes aegypti (Diptera: Culicidae) populations in selected sites of Cebu City, Philippines. J. Med. Entomol. 2015;52(4):638–646. doi: 10.1093/jme/tjv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller N. Analyzing precipitation projections: a comparison of different approaches to climate model evaluation. J. Geophys. Res. 2011;116(D10):D10118. [Google Scholar]
- Scheffers B.R. Microhabitats in the tropics buffer temperature in a globally coherent manner. Biol. Lett. 2014;10(12):20140819. doi: 10.1098/rsbl.2014.0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schewe J. Multimodel assessment of water scarcity under climate change. Proc. Natl. Acad. Sci. U. S. A. 2014;111(9):3245–3250. doi: 10.1073/pnas.1222460110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T., Bischoff T., Haug G.H. Migrations and dynamics of the intertropical convergence zone. Nature. 2014;513(7516):45–53. doi: 10.1038/nature13636. [DOI] [PubMed] [Google Scholar]
- Schüle S.A. Ascaris lumbricoides infection and its relation to environmental factors in the Mbeya region of Tanzania, a cross-sectional, population-based study. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0092032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurer J.M. Parasitic zoonoses: one health surveillance in northern Saskatchewan. PLoS Negl. Trop. Dis. 2013;7(3) doi: 10.1371/journal.pntd.0002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secor W.E., Colley D.G. Springer; New York: 2004. Schistosomiasis (World Class Parasites) [Google Scholar]
- Selemetas N., de Waal T. Detection of major climatic and environmental predictors of liver fluke exposure risk in Ireland using spatial cluster analysis. Vet. Parasitol. 2015;209(3–4):242–253. doi: 10.1016/J.VETPAR.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Semadeni-Davies A. The impacts of climate change and urbanisation on drainage in Helsingborg, Sweden: combined sewer system. J. Hydrol. 2008;350(1–2):100–113. [Google Scholar]
- Sgrò C.M. The effect of temperature on Wolbachia-mediated dengue virus blocking in Aedes aegypti. Am. J. Trop. Med. Hyg. 2016;94(4):812–819. doi: 10.4269/ajtmh.15-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin H.A., Montgomery W.I., Lundy M.G. The impact and implications of climate change for bats. Mammal Rev. 2013;43(3):171–182. [Google Scholar]
- Slater H., Michael E. Predicting the current and future potential distributions of lymphatic Filariasis in Africa using maximum entropy ecological niche modelling. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluydts V. Survival and maturation rates of the African rodent, Mastomys natalensis: density-dependence and rainfall. Integr. Zool. 2007;2(4):220–232. doi: 10.1111/j.1749-4877.2007.00065.x. [DOI] [PubMed] [Google Scholar]
- Smerdon J.E. Air-ground temperature coupling and subsurface propagation of annual temperature signals. J. Geophys. Res. Atmos. 2004;109(D21) [Google Scholar]
- Smith K.F. Global rise in human infectious disease outbreaks. J. R. Soc. Interface. 2014;11(101) doi: 10.1098/rsif.2014.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolow S.H. Nearly 400 million people are at higher risk of schistosomiasis because dams block the migration of snail-eating river prawns. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372(1722) doi: 10.1098/rstb.2016.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P.M. Probable Buruli ulcer disease in Honduras. Open Forum Infect Dis. 2016;3(2) doi: 10.1093/ofid/ofv189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speich B. Effect of sanitation and water treatment on intestinal protozoa infection: a systematic review and meta-analysis. Lancet Infect. Dis. 2016;16(1):87–99. doi: 10.1016/S1473-3099(15)00349-7. [DOI] [PubMed] [Google Scholar]
- Spindler L.A. The relation of moisture to the distribution of human trichuris and ascaris. Am. J. Epidemiol. 1929;10(2):476–496. doi: 10.1093/oxfordjournals.aje.a112764. [DOI] [Google Scholar]
- Sripa B. Food-Borne Trematodiases in Southeast Asia. Advances in Parasitology. 2010:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- Steinbaum L. Soil-transmitted helminth eggs are present in soil at multiple locations within households in rural Kenya. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth N.C. Stochastic seasonality and nonlinear density-dependent factors regulate population size in an African rodent. Nature. 1997;389(6647):176–180. doi: 10.1038/38271. [DOI] [PubMed] [Google Scholar]
- Stensgaard A.-S. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: does climate matter? Acta Trop. 2013;128(2):378–390. doi: 10.1016/j.actatropica.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Stensgaard A.-S. Combining process-based and correlative models improves predictions of climate change effects on Schistosoma mansoni transmission in eastern Africa. Geospat. Health. 2016;11(1S):406. doi: 10.4081/gh.2016.406. [DOI] [PubMed] [Google Scholar]
- Stephenson E.H., Larson E.W., Dominik J.W. Effect of environmental factors on aerosol-induced Lassa virus infection. J. Med. Virol. 1984;14(4):295–303. doi: 10.1002/jmv.1890140402. [DOI] [PubMed] [Google Scholar]
- Stocks M.E. Effect of water, sanitation, and hygiene on the prevention of trachoma: a systematic review and meta-analysis. PLoS Med. 2014;11(2) doi: 10.1371/journal.pmed.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey G.W., Phillips R.A. The survival of parasite eggs throughout the soil profile. Parasitology. 1985;91(3):585–590. doi: 10.1017/S003118200006282X. [DOI] [PubMed] [Google Scholar]
- Storlie C. Stepping inside the niche: microclimate data are critical for accurate assessment of species’ vulnerability to climate change. Biol. Lett. 2014;10(9):20140576. doi: 10.1098/rsbl.2014.0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday J.M., Bates A.E., Dulvy N.K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2012;2(9):686–690. doi: 10.1038/nclimate1539. [DOI] [Google Scholar]
- Suwannatrai A. Modeling impacts of climate change on the potential distribution of the carcinogenic liver fluke, Opisthorchis viverrini, in Thailand. Parasitol. Res. 2017;116(1):243–250. doi: 10.1007/s00436-016-5285-x. [DOI] [PubMed] [Google Scholar]
- Tabachnick W.J. Climate change and the arboviruses: lessons from the evolution of the dengue and yellow fever viruses. Annu. Rev. Virol. 2016;3(1):125–145. doi: 10.1146/annurev-virology-110615-035630. [DOI] [PubMed] [Google Scholar]
- Takaoka H. Effects of temperature on development of Onchocerca volvulus in Simulium ochraceum, and longevity of the Simuliid vector. J. Parasitol. 1982;68(3):478. doi: 10.2307/3280961. [DOI] [PubMed] [Google Scholar]
- Tang Z.-L., Huang Y., Yu X.-B. Current status and perspectives of Clonorchis sinensis and clonorchiasis: epidemiology, pathogenesis, omics, prevention and control. Infect. Dis. Poverty. 2016;5(1) doi: 10.1186/s40249-016-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. Environmental change and Rift Valley fever in eastern Africa: projecting beyond healthy futures. Geospat. Health. 2016;11(1s) doi: 10.4081/gh.2016.387. [DOI] [PubMed] [Google Scholar]
- Taylor K.E. An overview of CMIP5 and the experiment design. Bull. Am. Meteorol. Soc. 2012;93(4):485–498. doi: 10.1175/BAMS-D-11-00094.1. [DOI] [Google Scholar]
- Taylor R.G. Ground water and climate change. Nat. Clim. Chang. 2013;3(4):322–329. [Google Scholar]
- Teixeira A.R.L. Environment, interactions between Trypanosoma cruzi and its host, and health. Cad. Saude Publica. 2009;25(Suppl. 1):S32–S44. doi: 10.1590/S0102-311X2009001300004. [DOI] [PubMed] [Google Scholar]
- Teutschbein C., Seibert J. Bias correction of regional climate model simulations for hydrological climate-change impact studies: review and evaluation of different methods. J. Hydrol. 2012;456:12–29. doi: 10.1016/j.jhydrol.2012.05.052. [DOI] [Google Scholar]
- Théron A. Chronobiology of trematode Cercarial emergence: from data recovery to epidemiological, ecological and evolutionary implications. Adv. Parasitol. 2015;88:123–164. doi: 10.1016/bs.apar.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Thevenet P.S. Viability and infectiousness of eggs of Echinococcus granulosus aged under natural conditions of inferior arid climate. Vet. Parasitol. 2005;133(1):71–77. doi: 10.1016/j.vetpar.2005.05.048. [DOI] [PubMed] [Google Scholar]
- Thornton P.K. The impacts of climate change on livestock and livestock systems in developing countries: a review of what we know and what we need to know. Agr. Syst. 2009;101(3):113–127. doi: 10.1016/j.agsy.2009.05.002. [DOI] [Google Scholar]
- Thomson A.M. RCP4.5: a pathway for stabilization of radiative forcing by 2100. Clim. Change. 2011;109(1):77. [Google Scholar]
- Toyama G.M., Ikeda J.K. Predation as a factor in seasonal abundance of Musca sorbens Wiedemann (Diptera: Muscidae) Proc. Hawaiian Entomol. Soc. 1981;23:447–454. http://hdl.handle.net/10125/11131 [Google Scholar]
- Tun-Lin W. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Trop. Med. Int. Health. 2009;14(9):1143–1153. doi: 10.1111/j.1365-3156.2009.02341.x. [DOI] [PubMed] [Google Scholar]
- Turankar R.P. Dynamics of Mycobacterium leprae transmission in environmental context: deciphering the role of environment as a potential reservoir. Infect. Genet. Evol. 2012;12(1):121–126. doi: 10.1016/j.meegid.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Udonsi J.K., Atata G. Necator americanus: temperature, pH, light, and larval development, longevity, and desiccation tolerance. Exp. Parasitol. 1987;63(2):136–142. doi: 10.1016/0014-4894(87)90154-8. [DOI] [PubMed] [Google Scholar]
- Ulrich J.N. Heat sensitivity of wMel wolbachia during Aedes aegypti development. PLoS Negl. Trop. Dis. 2016;10(7) doi: 10.1371/journal.pntd.0004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNEP The Emissions Gap Report 2017 A UN Environment Synthesis Report. 2017. https://wedocs.unep.org/bitstream/handle/20.500.11822/22070/EGR_2017.pdf?sequence=1&isAllowed=y Nairobi. Available at:
- United Nations . United Nations; New York: 2015. World Population Prospects: The 2017 Revision, Key Findings & Advance Tables, Working Paper No. ESA/P/WP/248.https://esa.un.org/unpd/wpp/publications/Files/WPP2017_KeyFindings.pdf Available at: [Google Scholar]
- USAID A Review of Downscaling Methods for Climate Change Projections. 2014. http://www.ciesin.org/documents/Downscaling_CLEARED_000.pdf Available at:
- Valencia-López N. Climate-based risk models for Fasciola hepatica in Colombia. Geospat. Health. 2012;6(3):75. doi: 10.4081/gh.2012.125. [DOI] [PubMed] [Google Scholar]
- Valin H. The future of food demand: understanding differences in global economic models. Agric. Econ. 2014;45(1):51–67. [Google Scholar]
- Van den Broeck F. Reconstructing colonization dynamics of the human parasite Schistosoma mansoni following anthropogenic environmental changes in Northwest Senegal. PLoS Negl. Trop. Dis. 2015;9(8) doi: 10.1371/journal.pntd.0003998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Munster V.J. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro. Surveill. 2013;18(38) doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- van Vliet M.T.H. Global river discharge and water temperature under climate change. Glob. Environ. Chang. 2013;23(2):450–464. [Google Scholar]
- van Vuuren D.P., Lowe J. How well do integrated assessment models simulate climate change? Clim. Change. 2011;104(2):255–285. [Google Scholar]
- van Vuuren D.P., Stehfest E. RCP2.6: exploring the possibility to keep global mean temperature increase below 2°C. Clim. Change. 2011;109(1):95. [Google Scholar]
- Vargas-Arzola J. Detection of Paragonimus mexicanus (Trematoda) metacercariae in crabs from Oaxaca, Mexico. Acta Trop. 2014;137:95–98. doi: 10.1016/J.ACTATROPICA.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Vasseur D.A. Increased temperature variation poses a greater risk to species than climate warming. Proc. Biol. Sci. 2014;281(1779) doi: 10.1098/rspb.2013.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit P. Influence of environmental factors on the infectivity of Echinococcus multilocularis eggs. Parasitology. 1995;110(Pt. 1):79–86. doi: 10.1017/s0031182000081075. [DOI] [PubMed] [Google Scholar]
- Vejzagic N. Temperature dependent embryonic development of Trichuris suis eggs in a medicinal raw material. Vet. Parasitol. 2016;215:48–57. doi: 10.1016/j.vetpar.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Vescio F.M. Environmental correlates of crimean-congo haemorrhagic fever incidence in Bulgaria. BMC Public Health. 2012;12(1):1116. doi: 10.1186/1471-2458-12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard M.-A., Metzger J.P. Review: beyond the fragmentation debate: a conceptual model to predict when habitat configuration really matters. J. Appl. Ecol. 2014;51(2):309–318. doi: 10.1111/1365-2664.12190. [DOI] [Google Scholar]
- Wachira T.M., Macpherson C.N.L., Gathuma J.M. Release and survival of Echinococcus eggs in different environments in Turkana, and their possible impact on the incidence of hydatidosis in man and livestock. J. Helminthol. 1991;65(1):55–61. doi: 10.1017/S0022149X00010440. [DOI] [PubMed] [Google Scholar]
- Wagner C., Adrian R. Consequences of changes in thermal regime for plankton diversity and trait composition in a polymictic lake: a matter of temporal scale. Freshw. Biol. 2011;56(10):1949–1961. doi: 10.1111/j.1365-2427.2011.02623.x. [DOI] [Google Scholar]
- Wallace G.T., Kim T.L., Neufeld C.J. Interpopulational variation in the cold tolerance of a broadly distributed marine copepod. Conserv Physiol. 2014;2(1) doi: 10.1093/conphys/cou041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M.G. Mapping the risk of Nipah virus spillover into human populations in South and Southeast Asia. Trans. R. Soc. Trop. Med. Hyg. 2015;109(9):563–571. doi: 10.1093/trstmh/trv055. [DOI] [PubMed] [Google Scholar]
- Walsh M.G., Haseeb M. The landscape configuration of zoonotic transmission of Ebola virus disease in West and Central Africa: interaction between population density and vegetation cover. Peer J. 2015;3 doi: 10.7717/peerj.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther G.-R. Ecological responses to recent climate change. Nature. 2002;416(6879):389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Wang X. Impacts of climate variability and changes on domestic water use in the Yellow River Basin of China. Mitig. Adapt. Strat. Glob. Chang. 2017;22(4):595–608. [Google Scholar]
- Wardekker J.A. Health risks of climate change: an assessment of uncertainties and its implications for adaptation policies. Environ. Health. 2012;11(1):67. doi: 10.1186/1476-069X-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell R. An environmental assessment and risk map of Ascaris lumbricoides and Necator americanus distributions in Manufahi District, Timor-Leste. PLoS Negl. Trop. Dis. 2017;11(5) doi: 10.1371/journal.pntd.0005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S., Tambyah P.A., Lim P.L. Yellow fever cases in Asia: primed for an epidemic. Int. J. Infect. Dis. 2016;48:98–103. doi: 10.1016/j.ijid.2016.04.025. [DOI] [PubMed] [Google Scholar]
- Webster J.P. One health - an ecological and evolutionary framework for tackling Neglected Zoonotic Diseases. Evol. Appl. 2016;9(2):313–333. doi: 10.1111/eva.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbergen J.A. Climate change and the effects of temperature extremes on Australian flying-foxes. Proc. R. Soc. Lond. B Biol. Sci. 2008;275(1633) doi: 10.1098/rspb.2007.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Were F. The dengue situation in Africa. Paediatr. Int. Child Health. 2012;32(Suppl. 1):18–21. doi: 10.1179/2046904712Z.00000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T., von Braun J. Climate change impacts on global food security. Science. 2013;341(6145):508–513. doi: 10.1126/science.1239402. [DOI] [PubMed] [Google Scholar]
- Wilby R.L., Dessai S. Robust adaptation to climate change. Weather. 2010;65(7):180–185. doi: 10.1002/wea.543. [DOI] [Google Scholar]
- Williamson H.R. Detection of Mycobacterium ulcerans in the environment predicts prevalence of Buruli ulcer in Benin. PLoS Negl. Trop. Dis. 2012;6(1) doi: 10.1371/journal.pntd.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis J.M., Herbert I.V. Some factors affecting the eggs of Taenia multiceps: their transmission onto pasture and their viability. Ann. Trop. Med. Parasitol. 1984;78(3):236–242. doi: 10.1080/00034983.1984.11811808. [DOI] [PubMed] [Google Scholar]
- Wong J. Linking oviposition site choice to offspring fitness in Aedes aegypti: consequences for targeted larval control of dengue vectors. PLoS Negl. Trop. Dis. 2012;6(5) doi: 10.1371/journal.pntd.0001632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . World Health Organisation; Geneva: 2012. Accelerating work to overcome the global impact of neglected tropical diseases a roadmap for implementation.http://apps.who.int/iris/bitstream/10665/70809/1/WHO_HTM_NTD_2012.1_eng.pdf Available at: [Google Scholar]
- World Health Organisation . World Health Organisation; Geneva: 2013. WHO | Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases.http://www.who.int/neglected_diseases/9789241564540/en/ Available at: [Google Scholar]
- World Health Organisation . World Health Organisation; Geneva: 2013. WHO | Atlas of health and climate.http://www.who.int/globalchange/publications/atlas/en/ Available at: [Google Scholar]
- World Health Organisation . World Health Organisation; 2016. WHO | Investing to Overcome the Global Impact of Neglected Tropical Diseases, WHO.http://www.who.int/neglected_diseases/9789241564861/en/ Available at: [Google Scholar]
- World Health Organisation . World Health Organisation; 2016. WHO | WHO Director-General Summarizes the Outcome of the Emergency Committee Regarding Clusters of Microcephaly and Guillain-Barré Syndrome.http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/ Available at: [Google Scholar]
- World Health Organisation . World Health Organisation; Geneva: 2017. Fourth WHO Report on Neglected Tropical Diseases: Integrating Neglected Tropical Diseases into Global Health and Development.http://www.who.int/neglected_diseases/resources/9789241565448/en/ Available at: [Google Scholar]
- World Health Organisation, Foodborne Disease Burden Epidemiology Reference Group . World Health Organisation; Geneva: 2015. WHO Estimates of the Global Burden of Foodborne Diseases.http://apps.who.int/iris/bitstream/10665/199350/1/9789241565165_eng.pdf Available at: [Google Scholar]
- Yang G.J. A potential impact of climate change and water resource development on the transmission of Schistosoma japonicum in China. Parassitologia. 2005;47(1):127–134. [PubMed] [Google Scholar]
- Yee D.A., Juliano S.A., Vamosi S.M. Seasonal photoperiods alter developmental time and mass of an invasive mosquito, Aedes albopictus (Diptera: Culicidae), across its north-south range in the United States. J. Med. Entomol. 2012;49(4):825–832. doi: 10.1603/me11132. [DOI] [PubMed] [Google Scholar]
- Yob J.M. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg. Infect. Dis. 2001;7(3):439–441. doi: 10.3201/eid0703.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsu R.R. Buruli ulcer and current situation in Japan: a new emerging cutaneous mycobacterium infection. J. Dermatol. 2012;39(7):587–593. doi: 10.1111/j.1346-8138.2012.01543.x. [DOI] [PubMed] [Google Scholar]
- Yotsu R.R. Revisiting Buruli ulcer. J. Dermatol. 2015;42(11):1033–1041. doi: 10.1111/1346-8138.13049. [DOI] [PubMed] [Google Scholar]
- Yu X.-J. Fever with thrombocytopenia associated with a novel Bunyavirus in China. N. Engl. J. Med. 2011;364(16):1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun N.E., Walker D.H. Pathogenesis of Lassa fever. Viruses. 2012;4(12):2031–2048. doi: 10.3390/v4102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarroug I.M.A. Seasonal variation in biting rates of Simulium damnosum sensu lato, vector of Onchocerca volvulus, in two Sudanese foci. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Detection of human influence on twentieth-century precipitation trends. Nature. 2007;448(7152):461–465. doi: 10.1038/nature06025. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-Z., Xu J. The emergence and cross species transmission of newly discovered tick-borne Bunyavirus in China. Curr. Opin. Virol. 2016;16:126–131. doi: 10.1016/j.coviro.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Zhao L. Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerg. Infect. Dis. 2012;18(6):963–965. doi: 10.3201/eid1806.111345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.-N. Potential impact of climate change on schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 2008;78(2):188–194. doi: 10.4269/ajtmh.2008.78.188. [DOI] [PubMed] [Google Scholar]
- Zhu G., Fan J., Peterson A.T. Schistosoma japonicum transmission risk maps at present and under climate change in mainland China. PLoS Negl. Trop. Dis. 2017;11(10) doi: 10.1371/journal.pntd.0006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziervogel G., Calder R. Climate variability and rural livelihoods: assessing the impact of seasonal climate forecasts in Lesotho. Area. 2003;35(4):403–417. doi: 10.1111/j.0004-0894.2003.00190.x. [DOI] [Google Scholar]
- Zukowski S.H., Wayne Wilkerson G., Malone J.B. Fasciolosis in cattle in Louisiana. II. Development of a system to use soil maps in a geographic information system to estimate disease risk on Louisiana coastal marsh rangeland. Vet. Parasitol. 1993;47(1–2):51–65. doi: 10.1016/0304-4017(93)90175-M. [DOI] [PubMed] [Google Scholar]