Abstract
Steam enhanced extraction (SEE) is an in-situ thermal remediation technique used to remove and recover polycyclic aromatic hydrocarbons (PAHs) from contaminated soils. However, limited studies have been conducted on the formation of PAH derivatives during and after SEE of PAH contaminated soils. Creosote contaminated soil samples collected from the Wyckoff-Eagle Harbor Superfund site were remediated with laboratory scale SEE. The samples were quantified for unsubstituted PAHs and their derivatives, and assessed for developmental toxicity, pre- and post-SEE. Following SEE, unsubstituted PAH concentrations decreased, while oxygenated PAH concentrations increased in soil and aqueous extracts. Differences in developmental toxicity were also measured and linked to the formation of PAH derivatives. Additive toxicity was measured when comparing unfractionated extracts to fractionated extracts in pre- and post-SEE samples. SEE is effective in removing unsubstituted PAHs from contaminated soil, but other, potentially more toxic, PAH derivatives are formed.
Graphical Abstract
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are environmental contaminants that contain two or more fused benzene rings. These compounds are predominantly formed from incomplete combustion and pyrolysis of organic matter.1 In the environment, PAHs occur mostly in complex mixtures, containing both a range of unsubstituted PAHs, including high molecular weight PAHs (HMW-PAHs) and PAH derivatives. HMW-PAHs are studied as a subgroup of unsubstituted PAHs and have a molecular weight (MW) ≥ 278 g mol−1.2 PAH derivatives can be formed from the same processes as PAHs and contain functional groups, such as: hydroxy-PAHS (OHPAHs), oxy-PAHS (OPAHs) and nitro-PAHs (NPAHs).
PAH derivatives are widespread in the environment, and have been measured in atmospheric particulate matter,3,4-7 industrial waste,8 sediments,9-15 and soils.15,16 However, in comparison to unsubstituted PAHs, there is limited knowledge on the environmental and biological effects of PAH derivatives. Due to their hydrophobicity, PAHs are among the most common contaminants of concern17 in soils at United States Environmental Protection Agency (U.S. EPA) Superfund sites and are present at many other contaminated sites not regulated under federal programs.18-20 Potential transformation products of the oxidation of unsubstituted PAHs in the environment include OHPAHs and OPAHs. Some of these oxygenated PAHs are more persistent and mobile in the environment due to their increased polarity, as well as more toxic to humans, soil organisms and plants, compared to their corresponding unsubstituted PAHs.16,21-23 As degradation of unsubstituted PAHs occurs during biotic or abiotic processes, OH- and OPAHs may form. Several studies have noted the formation and/or accumulation of OH- and OPAHs during biodegradation, chemical oxidation, or thermal remediation of PAH contaminated soils.1,24-27
Thermal remediation technologies are aggressive and robust remedial techniques, which use heat to remove PAHs from contaminated soil. These technologies have been shown to be efficient and economical methods to remediate soil and water systems contaminated with PAHs.1,28,29 As described in the 2017 Superfund Remedy Report by the U.S. EPA, thermal treatment encompasses 12% of in-situ remediation performed at Superfund sites.30
Steam enhanced extraction (SEE) is a thermal treatment method used for contaminated soils that introduces steam (>105 °C) into the subsurface. SEE is most applicable at large sites where the contamination is at depth, and the hydraulic conductivity of the formation is greater than 10−5 centimeters per second. SEE can be used to recover both VOCs and SVOCs both above and below the water table. The steam will flow preferentially in more permeable strata, and lower permeability zones will be heated by heat conduction from the steam zones. The preferred approach for SEE is to surround the contaminated area with steam injection wells, which then displaces the contaminants to the central extraction wells, preventing loss of contaminants outside the treatment area.
With SEE, the steam reduces the viscosity of liquids within the pore spaces, and displaces non-aqueous phase liquids (NAPLs) and dissolved phase contaminants toward recovery wells. The increase in temperature will also increase the vapor pressure of volatile (VOCs) and semi-volatile (SVOCs) organic compounds in the soil. Volatilized compounds become part of the air/water vapor phase and are displaced to extraction wells, facilitating extraction and collection of the compounds.31 SEE was first used in 1930s as an enhanced oil recovery method for the petroleum industry,31,32 and, for example, is used in the Tar Sands of Alberta, Canada33,34 and the Kern River field in Bakersfield, CA.35,36 Between May 1997 and June 2000, approximately 300 million kilograms of steam were injected at depths ranging from 30 to 40 meters below ground surface at Southern California Edison’s Visalia Pole Yard Superfund Site to recover approximately 1.3 million pounds of creosote.37,38 Most of the creosote recovered was as an emulsion. Steam injection was terminated at this site when recovery of the emulsion ended, and groundwater extraction continued until 2003. By 2010, the groundwater cleanup standards for pentachlorophenol, dioxins, and benzo(a)pyrene, which had been set in the Record of Decision, had been met, and the site was de-listed from the National Priorities List.37
While unsubstituted PAHs have been studied in relation to thermal treatment,24,39 there is limited information and research available regarding the formation of PAH derivatives during and after thermal remediation. PAH derivatives, including oxygenated PAHs, are emerging contaminants of concern, and interest in these compounds has increased in the past decade due to their widespread presence in the environment, together with the increased toxicity of some oxygenated PAHs.22,40 Previous research in zebrafish (Danio rerio) and eukaryotic cell lines, indicates that oxygenated PAHs may induce oxidative stress, disrupt the endocrine system and cause cytotoxic effects.22,40,41 Furthermore, the mechanisms underlying the toxicity of oxygenated PAHs are complex and far from fully understood.22 The increasing environmental and toxicological concerns regarding PAH derivatives in soils, particularly oxygenated PAHs, coupled with the lack of research on these compounds, demonstrate an urgent need to determine if SEE is an effective remediation strategy for removal of PAH derivatives, as well as to establish if chemical processes that occur during SEE transform unsubstituted PAHs to PAH derivatives.
The objectives of this research were to determine the effectiveness of SEE in remediating creosote contaminated soil by identifying and quantifying unsubstituted PAHs, HMW-PAHs, NPAHs, OPAHs, and OHPAHs in pre- and post-remediation soil and aqueous samples, as well as assessing differences in developmental toxicity pre- and post-SEE. Laboratory-scale SEE experiments were performed at the Robert S. Kerr Environmental Research Center (U.S. EPA). Gas chromatography, coupled with mass spectrometry (GC/MS), was used for wide-ranging identification and measurement of PAHs in soil and aqueous samples, pre- and post-SEE and developmental toxicity was assessed in these samples using the embryonic zebrafish bioassay. To our knowledge, we are the first to study the developmental toxicity and formation of PAH derivatives in soil and leachate samples, pre- and post-SEE.
MATERIALS AND METHODS
Chemicals and materials.
Native stock solutions of unsubstituted PAHs (n = 21), OHPAHs (n = 38), OPAHs (n = 24), HMW-PAHs (n = 14), NPAHs (n = 23), and isotopically labeled standards were purchased from different vendors. All 120 PAHs studied and isotopically labeled standards, their abbreviations, and vendors are listed in the Supporting Information (Table S1 and S2). The solid-phase extraction (SPE) Bond Elut Si (20 g, 60 mL) cartridges were purchased from Agilent Technologies (New Castle, DE) and the ISOLUTE ENV+ cartridges (100 mg, 6mL) were purchased from Biotage (Charlotte, NC). All solvents (methanol (MetOH), hexane (Hex), ethyl acetate (EA), acetonitrile (ACN), acetone (Ace), and dichloromethane (DCM); all optima grade) and 20 mL clear glass vials were purchased from Thermo Fisher Scientific (Santa Clara, CA). Toluene (Tol) (≥ 99.9%) and the derivatizing agent N-methyl-N-(tert-butyldimethylsilyl) trifluoroacetamide (MTBSTFA) (>97%) were purchased from Sigma-Aldrich (Milwaukee, WI).
Study area and soil samples.
Creosote contaminated soil cores were collected in 1998 from the Wyckoff/Eagle Harbor Superfund site in Bainbridge Island, Washington at borehole 2 and 3 (B2 and B3) (Figure S1).24 The cores were shipped on ice to the Robert S. Kerr (Kerr Lab) Environmental Research Center (U.S. EPA) in Ada, Oklahoma, and stored at 4 °C. In 2015, the cores were combined and homogenized following US EPA’s standard operating procedure.42 The homogenized soil was treated in a laboratory scale steam enhanced extraction (SEE) system under the same conditions, but in three different experiments, at the Kerr Lab during the summer of 2015. Soil characterization was performed by the Central Analytical Laboratory in the Crop and Soil Science Department at Oregon State University. The detailed results of soil characterization are shown in the Supporting Information (Table S3) and include % moisture, % carbon (C), % nitrogen (N), % organic matter (OM), C:N ratio, pH, electrical conductivity (EC), nutrients (Ca, Cu, Fe, K, Mg, Mn, P, Zn) and cation exchange capacity (CEC). The soil texture was classified as marine sand and gravel.
Laboratory scale steam enhanced extraction experiments.
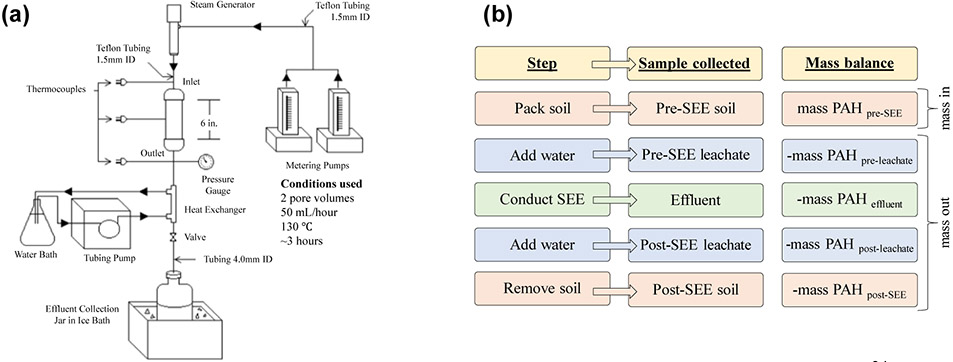
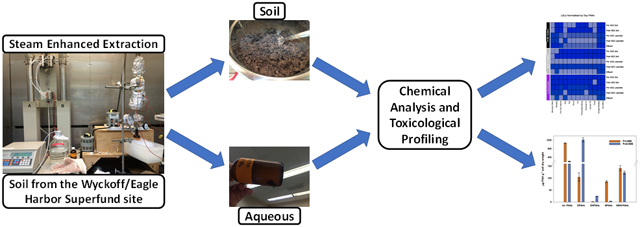
The equipment for laboratory scale SEE has been described previously and is shown in Figure 1a.24 Approximately 250 g of contaminated soil was packed into a galvanized carbon steel column that was connected to a steam generator on the top and to a heat exchanger on the bottom. Thermocouples were inserted at the top, middle and bottom of the steel column to monitor temperature. Metering pumps delivered a set flow rate of water (50 mL/hr) to the steam generator. The steam generator produced steam at a temperature of approximately 130°C. Steam was injected at the top of the column, producing a vertical downward flow through the column, until reaching two pore volumes. Pore volume refers to the volume in the cell that is available for steam to occupy if it was condensed. Previous research as shown that two pore volumes are sufficient to remove PAHs from soil.24 Effluent (~500 mL/SEE) was collected in amber sample bottles immersed in an ice bath (Figure 1a).
Figure 1:
(a) Diagram of laboratory scale steam enhanced extraction experimental setup,24 modified with conditions used in this study. (b) Flowchart of steps conducted during SEE, samples collected before, during and after SEE, and mass losses through SEE steps.
Prior to SEE, room temperature deionized water was added to the steel column containing the contaminated soil and allowed to equilibrate with the contents of the column for ~12 hours. The water was then drained from the column and collected as “pre-leachate” sample. Identical procedures were conducted after completion of SEE, and the water was collected as “post-leachate” (Figure 1a and 1b). The purpose of the pre- and post-SEE leachate samples was to mimic water flow through the soils.24
The following samples were collected: pre and post SEE soil, effluent, and pre and post SEE leachate. The contaminated soil before treatment was labeled as “pre-SEE” and the soil after treatment as “post-SEE”. Aqueous samples obtained from experiments were labeled as “pre-SEE leachate”, “effluent” and “post-SEE leachate”.
Sample extraction.
Figure S2 shows the process used to prepare the chemical and toxicology fractions for soil samples (pre- and post-SEE) and aqueous samples (pre and post SEE leachate and effluent).
Soil samples.
Approximately 5 g of wet weight soil, from either pre- or post-SEE samples, was extracted in 33 mL cells using Accelerated Solvent Extraction (ASE) (Dionex ASE 350) first with Hex:Ace (3:1, v/v) followed by DCM:Ace:MetOH (6:3:1, v/v) (1500 psi, 100 °C, 2 cycles, 240 s purge). The extract was then gravimetrically split 80% for toxicity testing and 20% for chemical analysis, and the portion undergoing chemical analysis was spiked with isotopically labeled surrogate standards (Table S2). Surrogates were only added to the chemical analysis split fraction, and not the toxicity split fraction, to prevent potentially toxic labeled PAHs from being present in the toxicity fraction. This process has been previously used27,43 and results in accurate measurement of concentrations in the chemical analysis and toxicity extracts, but underestimates slightly the concentration in the soil samples because of potential analyte loss during extraction (estimated at 10-20%)27. The dry weights of soil and percent moistures were measured after drying the soil at 105 °C for 24 hours. All soil PAH concentrations are reported on a dry weight soil basis.
Aqueous samples.
A 105 mL aqueous sample, either pre-SEE leachate, effluent or post-SEE leachate, was extracted using solid phase extraction (SPE), with a modified version of a previously published method.44 Isolute ENV+ (100 mg, 6 mL) cartridges were preconditioned with 25 mL MetOH, followed by 25 mL deionized water. Aqueous samples were loaded onto the cartridges, target analytes were retained on the sorbent, and the eluent was collected as waste and discarded. Target analytes were eluted, and collected together, with 30 mL EA:Ace, 25 mL DCM and 20 mL Hex. The extract was then gravimetrically split 95% for toxicity testing and 5% for chemical analysis, and the portion undergoing chemical analysis was spiked with isotopically labeled surrogate standards as described above with the soil samples (Table S2).
Sample fractionation and preparation.
The soil and aqueous extracts undergoing chemical and toxicity analysis were solvent exchanged to Hex using a TurboVap evaporation system (nitrogen gas, 30 °C water bath), and fractionated with silica SPE. Bond Elut Silica (20 g, 60 mL) cartridges were preconditioned with 50 mL EA:Ace (1:1, v/v), followed by 50 mL DCM, and lastly with 50 mL Hex. Extracts for chemical analysis were loaded onto the silica cartridges and target analytes were retained to the sorbent. Target analytes were eluted, and fractions were collected separately for chemical analysis, with 50 mL Hex (alkanes and alkenes and other non-polar compounds), 100 mL DCM (mid polarity compounds, PAHs, NPAHs, OPAHs, and HMW-PAHs), and 100 mL EA:Ace (1:1, v/v) (polar compounds, OHPAHs) (Table S1).
After SPE, the chemical analysis fractions were solvent exchanged to EA and concentrated to 300 μL. The concentrated samples were split to allow for chemical analysis of all PAHs. The first portion, consisting of 225 μL of sample extract, was spiked with isotopically labeled internal standards for a final volume of 300 μL, and was used for the analysis of PAHs, NPAHs, OPAHs and HMW-PAHs. For the analysis of OHPAHs, extracts were derivatized to make them suitable for GC analysis (i.e. increase volatility and decrease boiling point). For this, 75 μL of the prepared sample were transferred into a 300 μL spring insert containing 100 μL of ACN:Tol (5:1) and 25 μL of isotopically labeled internal standards, and concentrated to 20 μL using a nitrogen fine stream. The derivatizing agent, N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide (MTBSTFA), was added to the extract (30 μL), the mixture was placed in the oven at 65 °C for 25 min, vortexed, and analyzed with GC/MS (described in Chemical Analyses).
After SPE, the toxicity fractions were blown down to dryness under a stream of nitrogen in pre-weighed vials. The mass of the dry residue was measured using an analytical balance, and the residue was reconstituted in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) to a concentration of 10 mg extract residue per mL DMSO.
Chemical Analysis.
For the chemical analysis fractions, Gas chromatography/mass spectrometry (GC/MS) analysis for unsubstituted PAHs, HMW-PAHs, OPAHs, and OHPAHs was performed using an Agilent 7890B GC system coupled to an Agilent 5977A mass spectrometer (MS) detector with electron impact (EI) ionization. NPAHs were analyzed with an Agilent 6890 GC system coupled to an Agilent 5973N MS detector with negative chemical ionization (NCI) with methane as the reagent gas and with a programmed temperature vaporization inlet (Gerstel, Germany). All analyses were run in selected ion monitoring (SIM) mode.
GC/MS analyses for all analytes, except HMW-PAHs, were performed using an Agilent DB-5MS (30 m × 0.25 mm I.D. × 0.25 μm film thickness) capillary column. For the HMW-PAHs analysis, an Agilent DB-17MS (60 m × 0.25 mm I.D. × 0.25 μm film thickness) capillary column was used. PAHs, NPAHs and HMW-PAHs were analyzed following previously described methods.45 OHPAHs and OPAHs were analyzed with optimized methods from previously published methods,46,47 and are described in the Supporting Information (Table S4 and S5).
Estimated detection limits (EDLs) were calculated following section 7.3 of the EPA Method 828048, and ranged from 0.05 to 2.734 pg μL−1 for unsubstituted PAHs, 0.40 to 498.61 pg μL−1 for OPAHs, 0.11 to 77.61 pg μL−1 for NPAHs, 0.07 to 5.68 pg μL−1 for OHPAHs, and 0.005 to 2.09 pg μL−1 for HMW-PAHs (Table S6).
Toxicity Analysis.
Zebrafish Husbandry.
For the toxicity fractions, The Sinnhuber Aquatic Research Laboratory (SARL) standard procedures were used with adult fish for a wildtype (Tropical 5D) that were maintained at 28±1 °C on a recirculating system, with a 14 h light/10 h dark cycle.49 Embryos were collected from group spawns of adult zebrafish50 and enzymatically dechorionated at 4 hours post fertilization (hpf).51 Embryos were then mechanically placed into 100 μl of embryo medium in individual wells of a 96-well plate51,52 and dilutions of all pre- and post-SEE soil, leachate, and effluent samples were dispensed using the HP D300 digital dispenser (Mannerdorf, Switzerland) at 6 hpf. Embryos were exposed to 5 concentrations (10, 7.5, 5, 2.5, 1, 0 μg mL−1). Treatment and control groups (n=32 embryos/group) were equally distributed across replicate 96-well plates and a final DMSO concentrations of 1%. All experiments were conducted with fertilized embryos according to Oregon State University Animal Care and Use Protocols.
Developmental Toxicity Screen.
Following embryo exposure at 6 hpf, the 96-well plates were sealed with parafilm to prevent evaporation, wrapped in aluminum foil to prevent photodegradation, and placed on an orbital shaker at 235 rpm overnight; plates were stored at 28 °C.49 Embryos were maintained in these static conditions throughout the exposure period (6 −120 hpf). Mortality and morphological outcomes were visually assessed at 24 and 120 hpf using a dissecting microscope as previously described.53 Morphological assessments consisted of 22 endpoints including malformations, edema (i.e. yolk sac, pericardial), and changes in pigmentation. Custom R scripts from an in-house laboratory information management system, called the Zebrafish Acquisition and Analysis Program (ZAAP), were utilized for data collection and processing. Statistical comparisons were as previously reported.49
Statistical Analysis.
Statistical analyses were completed using Microsoft® Excel 2016 and SigmaPlot v14.0 (Systat Software Inc., San Jose, CA) software. Differences and significance between triplicate means for changes in PAH concentrations pre- and post-SEE treatment were evaluated using Student t-tests with statistical significance at p-value ≤ 0.05 and/or ≤ 0.001. For the embryonic zebrafish assay, lowest effect levels were determined as previously reported with custom R scripts in ZAAP.49 Heatmaps were created in RStudio (Boston, MA) using the gplots heatmap.2 package.54 Lowest Effect Levels (LELs) from the toxicology reports were normalized by dividing the LEL by the sum of total quantified PAHs (unsubstituted PAHs, HMW-PAHs, and derivatives) and summed values from individual classes.
RESULTS AND DISCUSSION
Soil characterization.
Table S3 shows that post-SEE soil had statistically significantly (p < 0.05) lower concentrations of C, OM, C:N, EC, Ca, Mg, and Mn than pre-SEE soil. However, post-SEE soil had statistically significantly (p < 0.05) higher concentrations of P, Zn, Fe, and pH. The SEE column used for SEE experiments were made of galvanized carbon steel, which contains Zn and Fe. Zinc is used to protect steel from corrosion, but can be corroded over time and exhaustive conditions (such as the high temperature and pressure used during SEE experiments).55 This may explain the increased Zn and Fe concentrations in post-SEE soil, relative to pre-SEE soil. Both galvanized carbon steel and stainless steel are used in field SEE. Lastly, N, Cu, and K concentrations did not change significantly in post-SEE soil relative to pre-SEE soil.
Chemical analysis.
All individual PAH, HMW-PAH, OPAH, NPAH, and OHPAH mean concentrations and standard errors for soil and aqueous samples, as well as percent changes (increase or decrease) for pre- and post-SEE comparison in both soil and leachate samples, are available in the Supporting Information Spreadsheet (SI Spreadsheet),
Soil and Effluent Samples.
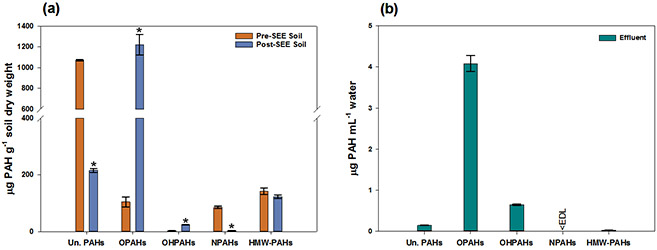
There was a statistically significant decrease (79.7%, p-value ≤ 0.001) in mean total unsubstituted PAH concentrations between pre- and post-SEE soil samples, and individual unsubstituted PAH concentrations decreased from 14.9% to 99.9%. This decrease is comparable to a previous SEE treatability study performed with the same soil samples, where a 73.0% decrease in total unsubstituted PAH concentrations was measured.24 This demonstrates that SEE is effective for removal of unsubstituted PAHs from soil. The unsubstituted PAHs with higher molecular weights had lower percent removals (e.g. BbFlt and I(1,2,3-cd)Pyr had 14.9% and 28.7% removal, respectively).
While we measured 21 unsubstituted PAHs in this study, only 14 were measured in the previous treatability study24 performed by the U.S. EPA in 2002. In general, our pre-SEE soil samples had lower concentrations than previously measured. For example, the BaP concentration in pre-SEE soil was 11 μg g−1 soil in 2002 and we measured 5.71 μg g−1 soil. Potential reasons for the difference in measured concentrations include the potential loss of these compounds during long term storage at 4 °C and differences in the analytical methods. However, because we are comparing pre and post-SEE concentrations and toxicity on the soil samples as of 2015, this difference does not impact our interpretation.
There was no statistically significant difference in HMW-PAH mean concentrations pre- and post-SEE soil (Figure 2a), and HMW-PAHs were measured at low concentrations in the effluent because the analytes remained in the soil post-SEE. Higher temperatures may be needed to remove HMW-PAHs from the soil particles, which would increase their concentrations in the effluent. However, steam injection temperatures in the lab are limited by the maximum pressure that can be safely used, while the steam injection temperature in the field are limited by the depth of injection in soil.32 A statistically significant decrease (95.3%, p-value < 0.001) in NPAHs concentration in post-SEE soil was measured (Figure 2a), and all NPAH concentrations were below the EDLs in the effluent samples (Figure 2b and Table S6).
Figure 2:
(a) Mean concentrations in soil dry weight (with standard error bars, n = 3) pre- and post-SEE. Statistically significant differences between pre- and post –SEE are indicated by “*” (p ≤ 0.001). (b) Mean concentrations in effluent (with standard error bars, n = 3) collected during SEE. “Un. PAHs” signifies unsubstituted PAHs; <EDL indicates that the measured concentrations were below estimated detection limits.
Statistically significant increases (p-value < 0.001) in OHPAH mean concentrations (826%) and OPAH mean concentrations (1,068%) were measured in the post-SEE soil compared to pre-SEE soil (Figure 2a). Individual increase percentages for OHPAHs and OPAHs ranged from 140% to 3340%, and from 69.5% to 2774%, respectively. Of all 120 compounds measured, 1,4-PheQ and 9,10-AntQ had the highest mean concentration in post-SEE soil (6.83x102 μg g−1 dry soil and 1.09x102 μg g−1 dry soil). Of the OPAHs, 9,10-PheQ and 1,6-BaPyrQ were newly formed during SEE and were present in soil post-SEE. Of the OHPAHs 1,6-OH-Nap, 2,7-OH-Nap, 3-OH-BaPyr, 11-OH-BbFlt, and 11-OH-BgChr were newly formed and were present in soil post-SEE. Although some soil bioremediation studies have measured increased concentrations of some OPAHs like 9-Flon,56,57 this is the first study to assess OPAH concentrations in SEE, and measure an increase in 9-Flon concentration post-SEE.
The measured increase in OHPAH and OPAH soil concentrations post-SEE may be due to the transformation of unsubstituted PAHs to OHPAH and OPAH during SEE, since pressure and high temperature can accelerate oxidation reactions.58 Previous research has showed that these reactions are temperature dependent and that temperature changes from 25°C to 130°C accelerate the oxidation of unsubstituted PAHs in both gas and aqueous phase.58-60 A previous SEE study observed the consumption of oxygen in an enclosed system, potentially indicating the formation of oxygenated analytes after SEE.24,39 An additional explanation may be that oxygenated PAHs are more available for extraction in the post-SEE soil due to steam being introduced in the soil.27,40,61,62 The elevated temperature of SEE may enhance the desorption of oxygenated PAHs from soil particles and allow them to be transported from soil to groundwater.63,64 Overall, total PAH mass measured in the effluent accounted for 0.361% of the total PAH mass measured in soil pre-SEE.
Leachate.
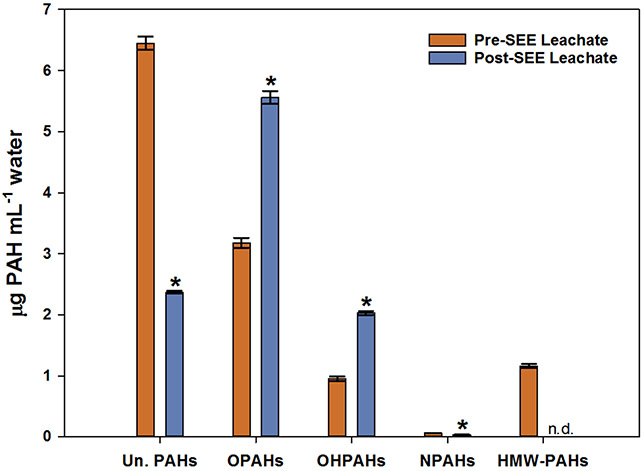
In this laboratory-scale SEE, the leachate was used to mimic leaching to groundwater. There was a statistically significant decrease (63.3%, p-value < 0.001) in mean unsubstituted PAH concentrations in the post-SEE leachate samples, relative to pre-SEE leachate samples (Figure 3). Individual PAH concentrations decreased by 3.65% to 98.2% between pre and post-SEE leachate samples. We investigated the relationship of the percent decrease in unsubstituted PAH concentrations as a function of vapor pressure and molecular weight and did not find any statistically significant trends. Our results suggest that the amount of unsubstituted PAHs that could reach groundwater and/or surface waters from soils are reduced following SEE.
Figure 3:
Mean concentrations in leachate (with standard error bars, n = 3) pre- and post-SEE. Statistically significant differences between concentrations pre- and post -SEE (p ≤ 0.001) are indicated by “*”. n.d. signifies not detected. “Un. PAHs” signifies unsubstituted PAHs.
HMW-PAHs were detected and quantified in pre-SEE leachate samples. However, no HMW-PAHs were identified or quantified in post-SEE leachate samples. A statistically significant decrease (42.1%, p-value < 0.001) was measured in mean NPAH concentrations in post-SEE leachate samples (Figure 3), with 2-+3-N-Flt, 9-N-Ant, 1-N-Pyr, and 7-N-BaA showing percent decreases higher than 80%. There were statistically significant increases (p-value < 0.001) in OHPAHs mean concentrations (113%) and OPAHs concentrations (74.9%), in post-SEE leachate samples relative to pre-SEE leachate (Figure 3). Individual OHPAH concentrations increased by 18.4% to 3,621%, while OPAH concentrations increased by 9.51% to 1,017%, in post-SEE leachate relative to pre-SEE leachate. Some of the OPAHs that increased significantly in post-SEE leachate include 9-OH-Phen, AceaQ, 1,4-BQ, 1-OH-Nap, 4-OH-Phen, 5,12-NQ, among others (SI Spreadsheet).
The statistically significant increase in the oxygenated PAH (OH- and OPAH) concentrations in post-SEE leachate samples supports the hypothesis that transformation reactions occur during and/or after SEE in soil. This is the first time, to our knowledge, that these derivatives have been measured in soil and leachate samples pre- and post-SEE. Thus, direct comparison of these results to previous SEE pilot or treatability studies is not possible. However, previous work59 on pressurized hot water (100 – 350 °C) (not steam) spiked with unsubstituted PAHs concluded that degradation of unsubstituted PAHs occurs and that different oxidation products are formed, including ketones and quinones (e.g. 9,10-AntQ and 9-Flon). The decreased concentration of unsubstituted PAHs in post-SEE leachates, coupled with increased concentration of oxygenated PAHs in post-SEE leachates can be interpreted as transformation of unsubstituted PAHs to oxygenated PAHs. Additionally, the decrease in NPAH concentrations in post-SEE leachate suggests that NPAHs are not being formed and SEE may be effective in removing these analytes from soils and reducing leaching into groundwater. Our results suggest that, after SEE, the amount of oxygenated PAHs that could reach groundwater from soils is increased.
Chemical Mass Balance.
Percent mass balances were calculated for all individual unsubstituted PAHs by comparing the unsubstituted PAH mass in the pre-SEE soil to unsubstituted PAH mass in all of the potential loss points shown in Figure 1b (including pre-leachate, effluent, post-leachate, and post-SEE soil). A mass balance less than 100% suggests an unaccounted loss of mass and/or not accounting for all transformation products and a mass balance greater than 100% suggests an unaccounted increase of mass and/or not accounting for all reactants. For unsubstituted PAHs, most of percentages were below 100% ± 30%. (Table S7), indicating mass balance was not achieved and that there was net loss of unsubstituted PAHs. This may be attributed to transformation of the unsubstituted PAH into PAH derivatives, and/or losses during SEE (i.e. slow recondensation) and sample preparation.
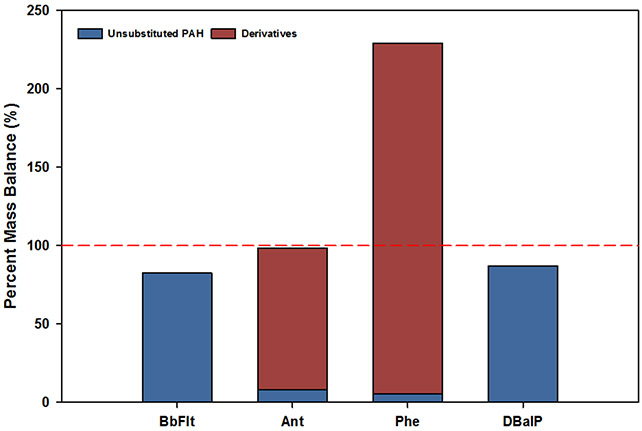
In order to account for the transformation of unsubstituted PAHs into PAH derivatives, mass balances were then calculated by accounting for the oxygenated derivatives of the unsubstituted PAHs with commercial standards in our inventory. The percent mass balances obtained for individual unsubstituted PAHs for which there were homolog oxygenated derivatives in the chemical analysis (13 of the 21 unsubstituted PAHs) are shown in Table S8. Figure 4 shows representative examples of all observed scenarios in mass balance for this study. The various mass balance scenarios include (Table S7, S8, and S9): 1) below 100 % mass balance for an individual unsubstituted PAH with no derivatives (e.g., BbFlt, Figure 4), 2) near 100 % mass balance for an individual PAH with no derivatives (e.g., DBalP, Figure 4), 3) near 100 % mass balance for individual unsubstituted PAH and its commercially available oxygenated derivatives (e.g., Ant, Figure 4), and 4) above 100 % mass balance for individual unsubstituted PAH and its commercially available oxygenated derivatives (e.g., Phe, Figure 4). Mass balances were also estimated on a ‘per mole’ basis, also called molecular balances, where the mass was divided by the respective compound molecular weight to obtain number of moles (Table S10). These results were not significantly different to the traditional mass balance calculations.
Figure 4:
Representative examples of all observed scenarios in calculated percent mass balances. Four scenarios are presented: below 100 % mass balance for BbFlt, 100 % mass balance when considering Ant derivatives, mass balance above 100%, indicating increased Phe derivatives after SEE, and close to 100 % mass balance for DBalP.
Overall, 6 out of 13 mass balances for unsubstituted PAHs were below 100% (Flo, Ant, Pyr, Flt, Chr+TriPh, and I(1,2,3-cd)Pyr) and 7 were above 100% (Nap, Acy+Ace, Phe, Bb+BkFlt, Ba+BePyr, BcFlo, and BaAnt+(Dah+DacAnt)). Advancement of the chemical analysis capabilities, including non-targeted analysis, is needed to ensure more accurate identification and quantification of PAH derivatives, in order to ‘close’ the mass balance. Further research is needed to better understand what occurs during and after SEE, and better assess mass balances in such a complex system, including the tracking of isotopically labeled individual PAHs and their resulting transformation products.
Toxicity Analysis.
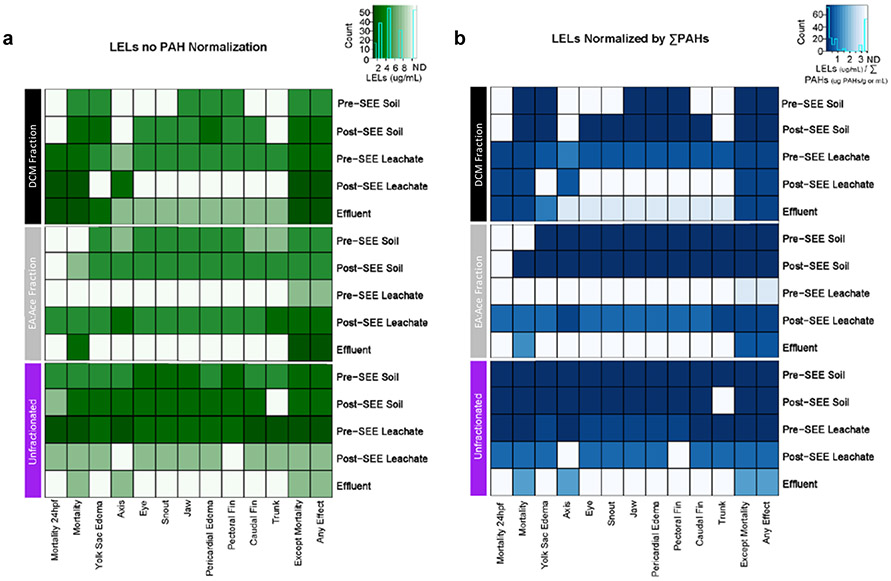
The embryonic zebrafish bioassay was used to study the developmental toxicity of the soil and leachate extracts pre- and post-SEE. Lowest effect levels (LELs) for mortality and morphological endpoints were obtained following developmental exposures to a concentration range of the extracts (Figure 5). Unfractionated samples and fractionated samples (Hex, DCM, and EA:Ace fractions) were tested to identify which fractions were contributing to the observed toxicity. As per the chemical analysis, Hex fraction contains alkanes and alkenes, and other non-polar compounds; DCM fraction contains mid polarity compounds like PAHs, NPAHs, OPAHs, and HMW-PAHs; and EA:Ace fraction contains the most polar compounds, OHPAHs.
Figure 5.
Lowest Effect Levels for Fractionated and Unfractionated Soil, Leachate, and Effluent Samples Pre- and Post-SEE. Embryos are exposed to 6 concentrations of each sample from 6 to 120 hpf. A Lowest Effect Levels (LELs, μg/mL) is calculated for mortality (24, 120 hpf), 9 morphological endpoints (120 hpf) and 2 summary endpoints are reported for all samples (a) and for all samples with LELs normalized by ∑PAHs (μg PAHs/g soil or mL leachate, effluent) measured in each sample (b). Increasing color intensity indicates increased toxicity, “N.D.” indicates no detected developmental response. Fraction 1 data was not displayed as there was no observed developmental responses in any of the samples following exposure starting at 6 hpf.
Across all extracts, the Hex fraction did not induce toxicity and was excluded from visual representation of the data for both chemical and toxicity analysis. This fraction contains non-polar analytes, such as alkanes and alkenes, present in the soil, as well as other less polar organic compounds in the complex soil matrix, that would act as interferences in the analysis. Previous research has also shown that this fraction is nontoxic to embryonic zebrafish and does not contain the PAH analytes in this study.27,45
DCM and EA:Ace fractions appeared to have increased additive toxicity when comparing to LELs in the unfractionated extracts for the pre- and post-SEE soil and pre-SEE leachate. Conversely, toxicity decreased (increased LELs) for most endpoints in the unfractionated samples when compared to the individual DCM and EA:Ace fraction LELs in the post-SEE leachate and effluent samples. One potential reason for this finding is that the bioavailability of compounds in these fractions may be increased compared to the unfractionated sample. Bioavailability of oxygenated PAHs is variable and dependent on the physical-chemical properties of PAHs. For example, 1,4-naphthoquinone, a lower molecular weight OPAH, will degrade faster than 9,10-anthraquinone, a higher molecular weight OPAH,57,21,65 which means some OPAHs might be more bioavailable than others.
When considering the LELs normalized by the total concentration of PAHs in each sample (Figure 5b) similar trends were observed compared to non-normalized LELs (Figure 5a). However, more toxic responses (darker color intensity) were observed when adjusting for the concentration of PAHs in the sample. This data suggests that PAHs play a role in the observed toxicity, particularly in the fractionated pre- and post-SEE soil samples. Furthermore, when adjusting for specific classes of PAHs (Figure S3) clear trends in toxicity were observed, such as the toxicity being driven by OPAHs (Figure S3b) as compared to unsubstituted PAHs (Figure S3a). This highlights the important roles of OH- and OPAHs in toxicity across biological endpoints and sample types. These findings agree with previous studies that have observed how some OPAHs and OHPAHs exhibit greater toxicity in humans, soils, organisms, and plants than their corresponding parent PAH precursors, even without requiring enzymatic activation.22,43,66,67 Furthermore, the toxicity effects associated with OPAHs vary between individual compounds and there are limited studies that have assessed the toxicity of OPAHs as a mixture and with co-contaminants other than PAHs.
It is also important to mention, that like other remedial techniques, SEE has limitations that need to be considered when deciding if it is an appropriate remediation technique. These limitations include: (1) inefficient removal of contaminants due to differences in permeability in the area of interest (subsurface heterogeneities), causing residual saturation of steam/water with contaminants; (2) possible mobilization and loss of contaminants through unanticipated channels and vapor loss through the surface; and/or (3) difficulty recovering contaminants at significant depths (>150 meters).68
This laboratory scale research demonstrates that unsubstituted PAHs and PAH derivatives may be present in soils and groundwater after SEE remediation of PAH-contaminated soils, either due to transformation reactions or increased availability for extraction. Currently, these PAH derivatives are not considered priority pollutants, and do not have environmental standards associated with them. To understand the impact of remedial activities on the toxicity of the remediated soils and aquifers, future studies (pilot and treatability studies) should include the measurements of PAH derivatives which were identified in this study with increased concentrations post-SEE, as well as increased toxicity, and the potential catalytic effects of ZnO when galvanized carbon steel is used during remediation. In particular, studies are needed to determine the long-term fate of these compounds, such as, the effect of prolonged elevated temperatures (on the order of years rather than hours) on the formation and potential degradation of these compounds, and their partitioning between the soil and groundwater phases. The higher water solubility, bioavailability and reactivity of oxygenated PAHs suggests that they may degrade rapidly in soils; however, previous studies have shown that some oxygenated PAHs may be persistent.57 Previous work has predominantly focused solely on the regulated, unsubstituted PAHs and therefore lacks the assessment of these potentially influential analytes. This research also highlights the need for oxygenated PAHs, such as 1,4-PheQ and 9,10-AntQ, and HMW-PAHs to be considered in policies, risk assessments, and regulations for monitoring and quantification at contaminated sites, especially when the derivatives are identified as more toxic than the unsubstituted PAHs.
Supplementary Material
ACKNOWLEDGEMENTS
This publication was made possible in part by grant number P42 ES016465, and P30 ES000210 from the National Institute of Environmental Health Sciences (NIEHS), NIEHS Training Grant Fellowship T32 ES007060, NIEHS KC Donnelly Externship Supplement 3 P42 ES016465-0852. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIEHS and NIH. We thank Jackson P. Wiley for the method modification for the extraction of aqueous samples; Anna Chlebowski and the toxicity screening team of OSU Sinnhuber Aquatic Research Laboratory for data collection of the zebrafish developmental bioassay. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Footnotes
SUPPORTING INFORMATION
Chemicals, chemical analysis of OHPAHs and OPAHs, estimated detection limits, mass balances, Tables S1-S10, and Figures S1-S3.
REFERENCES
- (1).Gan S; Lau EV; Ng HK Remediation of Soils Contaminated with Polycyclic Aromatic Hydrocarbons (PAHs). J. Hazard. Mater 2009, 172 (2–3), 532–549. 10.1016/j.jhazmat.2009.07.118. [DOI] [PubMed] [Google Scholar]
- (2).Wei S; Liu M; Huang B; Bi X; Sheng G; Fu J Polycyclic Aromatic Hydrocarbons with Molecular Weight 302 in PM2.5 at Two Industrial Sites in South China. J. Environ. Monit 2011, 13 (9), 2568–2574. 10.1039/C1EM10320B. [DOI] [PubMed] [Google Scholar]
- (3).Albinet A; Leoz-Garziandia E; Budzinski H; ViIlenave E Polycyclic Aromatic Hydrocarbons (PAHs), Nitrated PAHs and Oxygenated PAHs in Ambient Air of the Marseilles Area (South of France): Concentrations and Sources. Sci. Total Environ 2007, 384 (1–3), 280–292. 10.1016/j.scitotenv.2007.04.028. [DOI] [PubMed] [Google Scholar]
- (4).Wang W; Primbs T; Tao S; Simonich SLM Atmospheric Particulate Matter Pollution during the 2008 Beijing Olympics. Environ. Sci. Technol 2009, 43 (14), 5314–5320. 10.1021/es9007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wang W; Jariyasopit N; Schrlau J; Jia Y; Tao S; Yu T-W; Dashwood RH; Zhang W; Wang X; Simonich SLM Concentration and Photochemistry of PAHs, NPAHs, and OPAHs and Toxicity of PM2.5 during the Beijing Olympic Games. Environ. Sci. Technol 2011, 45 (16), 6887–6895. 10.1021/es201443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wang W; Simonich S; Giri B; Chang Y; Zhang Y; Jia Y; Tao S; Wang R; Wang B; Li W; Cao J; Lu X Atmospheric Concentrations and Air-Soil Gas Exchange of Polycyclic Aromatic Hydrocarbons (PAHs) in Remote, Rural Village and Urban Areas of Beijing-Tianjin Region, North China. Sci. Total Environ 2011, 409 (15), 2942–2950. 10.1016/j.scitotenv.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Albinet A; Leoz-Garziandia E; Budzinski H; Villenave E; Jaffrezo J-L Nitrated and Oxygenated Derivatives of Polycyclic Aromatic Hydrocarbons in the Ambient Air of Two French Alpine Valleys - Part 1: Concentrations, Sources and Gas/Particle Partitioning. Atmos. Environ 2008, 42 (1), 43–54. 10.1016/j.atmosenv.2007.10.009. [DOI] [Google Scholar]
- (8).Mosi AA; Reimer KJ; Eigendorf GK Analysis of Polyaromatic Quinones in a Complex Environmental Matrix Using Gas Chromatography Ion Trap Tandem Mass Spectrometry. Talanta 1997, 44 (6), 985–1001. 10.1016/S0039-9140(96)02172-8. [DOI] [PubMed] [Google Scholar]
- (9).Fernandez P; Grifoll M; Solanas AM; Bayona JM; Albaiges J Bioassay-Directed Chemical Analysis of Genotoxic Components in Coastal Sediments. Environ. Sci. Technol 1992, 26 (4), 817–829. 10.1021/es00028a024. [DOI] [Google Scholar]
- (10).Grifoll M; Solanas AM; Bayona JM Characterization of Genotoxic Components in Sediments by Mass Spectrometric Techniques Combined WithSalmonella/Microsome Test. Arch. Environ. Contam. Toxicol 1990, 19 (2), 175–184. 10.1007/BF01056084. [DOI] [PubMed] [Google Scholar]
- (11).Machala M; Ciganek M; Bláha L; Minksová K; Vondráck J Aryl Hydrocarbon Receptor-Mediated and Estrogenic Activities of Oxygenated Polycyclic Aromatic Hydrocarbons and Azaarenes Originally Identified in Extracts of River Sediments. Environ. Toxicol. Chem 2001, 20 (12), 2736–2743. [PubMed] [Google Scholar]
- (12).McKinney RA; Pruell RJ; Burgess RM Ratio of the Concentration of Anthraquinone to Anthracene in Coastal Marine Sediments. Chemosphere 1999, 38 (10), 2415–2430. 10.1016/S0045-6535(98)00435-4. [DOI] [PubMed] [Google Scholar]
- (13).Wang X; Yuan K; Yang L; Lin L; Tam NFY; Chen B; Luan T Characterizing the Parent and Oxygenated Polycyclic Aromatic Hydrocarbons in Mangrove Sediments of Hong Kong. Marine Pollution Bulletin 2015, 98 (1–2), 335–340. 10.1016/j.marpolbul.2015.06.033. [DOI] [PubMed] [Google Scholar]
- (14).Witter AE; Nguyen MH Determination of Oxygen, Nitrogen, and Sulfur-Containing Polycyclic Aromatic Hydrocarbons (PAHs) in Urban Stream Sediments. Environmental Pollution 2016, 209, 186–196. 10.1016/j.envpol.2015.10.037. [DOI] [PubMed] [Google Scholar]
- (15).Rodgers-Vieira EA; Zhang Z; Adrion AC; Gold A; Aitken MD Identification of Anthraquinone-Degrading Bacteria in Soil Contaminated with Polycyclic Aromatic Hydrocarbons. Appl. Environ. Microbiol 2015, 81 (11), 3775–3781. 10.1128/AEM.00033-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Rivas FJ Polycyclic Aromatic Hydrocarbons Sorbed on Soils: A Short Review of Chemical Oxidation Based Treatments. Journal of Hazardous Materials 2006, 138 (2), 234–251. 10.1016/j.jhazmat.2006.07.048. [DOI] [PubMed] [Google Scholar]
- (17).ATSDR – Priority List of Hazardous Substances http://www.atsdr.cdc.gov/SPL/index.html (accessed Jan 5, 2015).
- (18).Abdel-Shafy HI; Mansour MSM A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egyptian Journal of Petroleum 2016, 25 (1), 107–123. 10.1016/j.ejpe.2015.03.011. [DOI] [Google Scholar]
- (19).ATSDR. Toxicity of Polycyclic Aromatic Hydrocarbons (PAHs): Where are PAHs Found? ∣ ATSDR - Environmental Medicine & Environmental Health Education - CSEM https://www.atsdr.cdc.gov/csem/csem.asp?csem=13&po=5 (accessed Mar 18, 2017).
- (20).Latimer JS; Zheng J The Sources, Transport, and Fate of PAHs in the Marine Environment In PAHs: An Ecotoxicological Perspective; Douben PET, Ed.; John Wiley & Sons, Ltd, 2003; pp 7–33. 10.1002/0470867132.ch2. [DOI] [Google Scholar]
- (21).Meyer S; Cartellieri S; Steinhart H Simultaneous Determination of PAHs, Hetero-PAHs (N, S, O), and Their Degradation Products in Creosote-Contaminated Soils. Method Development, Validation, and Application to Hazardous Waste Sites. Anal. Chem 1999, 71 (18), 4023–4029. 10.1021/ac990136j. [DOI] [Google Scholar]
- (22).Knecht AL; Goodale BC; Truong L; Simonich MT; Swanson AJ; Matzke MM; Anderson KA; Waters KM; Tanguay RL Comparative Developmental Toxicity of Environmentally Relevant Oxygenated PAHs. Toxicol. Appl. Pharmacol 2013, 271 (2), 266–275. 10.1016/j.taap.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Lundstedt S; Bandowe BAM; Wilcke W; Boll E; Christensen JH; Vila J; Grifoll M; Faure P; Biache C; Lorgeoux C; et al. First Intercomparison Study on the Analysis of Oxygenated Polycyclic Aromatic Hydrocarbons (Oxy-PAHs) and Nitrogen Heterocyclic Polycyclic Aromatic Compounds (N-PACs) in Contaminated Soil. TrAC Trends in Analytical Chemistry 2014, 57, 83–92. 10.1016/j.trac.2014.01.007. [DOI] [Google Scholar]
- (24).Davis EL Final Report: Wyckoff/Eagle Harbor Superfund Site, Steam Injection Treatability; Report to Region X; U.S. Environmental Protection Agency, 2002. [Google Scholar]
- (25).Kuhlman MI Analysis of the Steam Injection at the Visalia Superfund Project with Fully Compositional Nonisothermal Finite Difference Simulations. J. Hazard. Mater 2002, 92 (1), 1–19. 10.1016/S0304-3894(01)00369-7. [DOI] [PubMed] [Google Scholar]
- (26).Davie-Martin CL; Stratton KG; Teeguarden JG; Waters KM; Simonich SLM Implications of Bioremediation of Polycyclic Aromatic Hydrocarbon-Contaminated Soils for Human Health and Cancer Risk. Environ. Sci. Technol 2017, 51 (17), 9458–9468. 10.1021/acs.est.7b02956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Chibwe L; Geier MC; Nakamura J; Tanguay RL; Aitken MD; Simonich SLM Aerobic Bioremediation of PAH Contaminated Soil Results in Increased Genotoxicity and Developmental Toxicity. Environ. Sci. Technol 2015, 49 (23), 13889–13898. 10.1021/acs.est.5b00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).U.S. EPA ORD. Thermal remediation https://archive.epa.gov/ada/web/html/thermal.html (accessed Dec 22, 2014).
- (29).US EPA. In Situ Treatment Technologies for Contaminated Soil; EPA/542/F-06/013, 35 pp; 2006.
- (30).US EPA. Superfund Remedy Report, 15th Edition; EPA-542-R-17-001; 2017. [Google Scholar]
- (31).Udell KS; Stewart LD Jr. Combined Steam Injection and Vacuum Extraction for Aquifer Cleanup In Subsurface Contamination by Immiscible Fluids; Balkema AA: Rotterdam, Netherlands, 1992; pp 327–336. [Google Scholar]
- (32).Davis E Steam Injection for Soil and Aquifer Remediation; Ground Water Issue; EPA 540/S-97/505; U.S. Environmental Protection Agency: Cincinnati, OH, 1998. [Google Scholar]
- (33).James LA; Rezaei N; Chatzis I VAPEX, Warm VAPEX, and Hybrid VAPEX-The State of Enhanced Oil Recovery for In Situ Heavy Oils in Canada; Petroleum Society of Canada, 2007. 10.2118/2007-200. [DOI] [Google Scholar]
- (34).Abramov OV; Abramov VO; Myasnikov SK; Mullakaev MS Extraction of Bitumen, Crude Oil and Its Products from Tar Sand and Contaminated Sandy Soil under Effect of Ultrasound. Ultrasonics Sonochemistry 2009, 16 (3), 408–416. 10.1016/j.ultsonch.2008.10.002. [DOI] [PubMed] [Google Scholar]
- (35).Masnadi MS; Brandt AR Climate Impacts of Oil Extraction Increase Significantly with Oilfield Age. Nature Climate Change 2017, 7 (8), nclimate3347. 10.1038/nclimate3347. [DOI] [Google Scholar]
- (36).Kovscek AR Emerging Challenges and Potential Futures for Thermally Enhanced Oil Recovery. Journal of Petroleum Science and Engineering 2012, 98–99 (Supplement C), 130–143. 10.1016/j.petrol.2012.08.004. [DOI] [Google Scholar]
- (37).US EPA Region 9. Second Five Year Review Report for Southern California Edison Company, Visalia Pole Yard Superfund Site, Visalia, Tulare County, California; SDMS DOCID #1123681; U.S. Environmental Protection Agency, 2010. [Google Scholar]
- (38).Suk W; Heacock M; Trottier B; Amolegbe S; Avakian M; Henry H; Carlin D; Reed L Assessing the Economic and Societal Benefits of SRP-Funded Research. Environ Health Perspect 2018, 126 (6). 10.1289/EHP3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Davis EL Final Report: McCormick & Baxter Superfund Site Stockton, California, Steam Injection Treatability; Report to Region 9; U.S. Environmental Protection Agency, 2003. [Google Scholar]
- (40).Lundstedt S; White PA; Lemieux CL; Lynes KD; Lambert IB; Oberg L; Haglund P; Tysklind M Sources, Fate, and Toxic Hazards of Oxygenated Polycyclic Aromatic Hydrocarbons (PAHs) at PAH-Contaminated Sites. Ambio 2007, 36 (6), 475–485. [DOI] [PubMed] [Google Scholar]
- (41).Hu J; Nakamura J; Richardson SD; Aitken MD Evaluating the Effects of Bioremediation on Genotoxicity of Polycyclic Aromatic Hydrocarbon-Contaminated Soil Using Genetically Engineered, Higher Eukaryotic Cell Lines. Environ. Sci. Technol 2012, 46 (8), 4607–4613. 10.1021/es300020e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).U.S., E. SESD Operating Procedure Soil Sampling; SESDPROC-300-R3; U.S. EPA: Athens, Georgia, 2014. [Google Scholar]
- (43).Schrlau JE; Kramer AL; Chlebowski A; Truong L; Tanguay RL; Simonich SLM; Semprini L Formation of Developmentally Toxic Phenanthrene Metabolite Mixtures by Mycobacterium Sp. ELW1. Environ. Sci. Technol 2017, 51 (15), 8569–8578. 10.1021/acs.est.7b01377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Motorykin O; Schrlau J; Jia Y; Harper B; Harris S; Harding A; Stone D; Kile M; Sudakin D; Massey Simonich SL Determination of Parent and Hydroxy PAHs in Personal PM2.5 and Urine Samples Collected during Native American Fish Smoking Activities. Science of The Total Environment 2015, 505, 694–703. 10.1016/j.scitotenv.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Titaley IA; Chlebowski A; Truong L; Tanguay RL; Massey Simonich SL Identification and Toxicological Evaluation of Unsubstituted PAHs and Novel PAH Derivatives in Pavement Sealcoat Products. Environ. Sci. Technol. Lett 2016. 10.1021/acs.estlett.6b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Motorykin O; Santiago-Delgado L; Rohlman D; Schrlau JE; Harper B; Harris S; Harding A; Kile ML; Massey Simonich SL Metabolism and Excretion Rates of Parent and Hydroxy-PAHs in Urine Collected after Consumption of Traditionally Smoked Salmon for Native American Volunteers. Science of The Total Environment 2015, 514, 170–177. 10.1016/j.scitotenv.2015.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).O’Connell SG; Haigh T; Wilson G; Anderson KA An Analytical Investigation of 24 Oxygenated-PAHs (OPAHs) Using Liquid and Gas Chromatography–Mass Spectrometry. Anal Bioanal Chem 2013, 405 (27), 8885–8896. 10.1007/s00216-013-7319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).U.S. EPA OEI. Polychlorinated Dibenzodioxins/Polychlorinated Dibenzofurans SW-846 Method 8280 Data Validation https://www.epa.gov/quality/polychlorinated-dibenzodioxinspolychlorinated-dibenzofurans-sw-846-method-8280-data (accessed Nov 28, 2017).
- (49).Truong L; Gonnerman G; Simonich MT; Tanguay RL Assessment of the Developmental and Neurotoxicity of the Mosquito Control Larvicide, Pyriproxyfen, Using Embryonic Zebrafish. Environ. Pollut 2016, 218, 1089–1093. 10.1016/j.envpol.2016.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Varga ZM Aquaculture and Husbandry at the Zebrafish International Resource Center. Methods Cell Biol. 2011, 104, 453–478. 10.1016/B978-0-12-374814-0.00024-0. [DOI] [PubMed] [Google Scholar]
- (51).Mandrell D; Truong L; Jephson C; Sarker MR; Moore A; Lang C; Simonich MT; Tanguay RL Automated Zebrafish Chorion Removal and Single Embryo Placement: Optimizing Throughput of Zebrafish Developmental Toxicity Screens. J Lab Autom 2012, 17 (1), 66–74. 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Noyes PD; Haggard DE; Gonnerman GD; Tanguay RL Advanced Morphological - Behavioral Test Platform Reveals Neurodevelopmental Defects in Embryonic Zebrafish Exposed to Comprehensive Suite of Halogenated and Organophosphate Flame Retardants. Toxicol. Sci 2015, 145 (1), 177–195. 10.1093/toxsci/kfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Truong L; Harper SL; Tanguay RL Evaluation of Embryotoxicity Using the Zebrafish Model. Methods Mol. Biol 2011, 691, 271–279. 10.1007/978-1-60761-849-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).R: The R Project for Statistical Computing. https://www.r-project.org/ (accessed Mar 28, 2018).
- (55).Marder AR The Metallurgy of Zinc-Coated Steel. Progress in Materials Science 2000, 45 (3), 191–271. 10.1016/S0079-6425(98)00006-1. [DOI] [Google Scholar]
- (56).Lundstedt S; Haglund P; Öberg L Degradation and Formation of Polycyclic Aromatic Compounds during Bioslurry Treatment of an Aged Gasworks Soil. Environmental Toxicology and Chemistry 2003, 22 (7), 1413–1420. 10.1002/etc.5620220701. [DOI] [PubMed] [Google Scholar]
- (57).Wilcke W; Kiesewetter M; Musa Bandowe BA Microbial Formation and Degradation of Oxygen-Containing Polycyclic Aromatic Hydrocarbons (OPAHs) in Soil during Short-Term Incubation. Environmental Pollution 2014, 184, 385–390. 10.1016/j.envpol.2013.09.020. [DOI] [PubMed] [Google Scholar]
- (58).Keyte IJ; Harrison RM; Lammel G. Chemical Reactivity and Long-Range Transport Potential of Polycyclic Aromatic Hydrocarbons – a Review. Chemical Society Reviews 2013, 42 (24), 9333–9391. 10.1039/C3CS60147A. [DOI] [PubMed] [Google Scholar]
- (59).Andersson T; Hartonen K; Hyötyläinen T; Riekkola M-L Stability of Polycyclic Aromatic Hydrocarbons in Pressurised Hot Water. Analyst 2003, 128 (2), 150–155. 10.1039/B211447J. [DOI] [PubMed] [Google Scholar]
- (60).Ananthula R; Yamada T; Taylor PH Kinetics of OH Radical Reaction with Phenanthrene: New Absolute Rate Measurements and Comparison with Other PAHs. International Journal of Chemical Kinetics 2007, 39 (11), 629–637. 10.1002/kin.20278. [DOI] [Google Scholar]
- (61).Reid BJ; Jones KC; Semple KT Bioavailability of Persistent Organic Pollutants in Soils and Sediments—a Perspective on Mechanisms, Consequences and Assessment. Environmental Pollution 2000, 108 (1), 103–112. 10.1016/S0269-7491(99)00206-7. [DOI] [PubMed] [Google Scholar]
- (62).Löser C; Seidel H; Hoffmann P; Zehnsdorf A Bioavailability of Hydrocarbons during Microbial Remediation of a Sandy Soil. Appl Microbiol Biotechnol 1999, 51 (1), 105–111. 10.1007/s002530051370. [DOI] [PubMed] [Google Scholar]
- (63).Davis EL How Heat Can Enhance In Situ Soil and Aquifer Remediation: Important Chemical Properties and Guidance on Choosing the Appropriate Technique; Ground Water Issue; EPA/540/S-97/502; U.S. Environmental Protection Agency: Cincinnati, OH, 1997. [Google Scholar]
- (64).Davis EL; Akladiss N; Hoey R; Brandon B; Nalipinski M; Carroll S; Heron G; Novakowski K; Udell K Steam Enhanced Remediation Research for DNAPL in Fractured Rock Loring Air Force Base, Limestone, Maine; EPA 540/R-05/010; US EPA Office of Research and Development: Cincinnati, OH, 2005. [Google Scholar]
- (65).Burgess RM; Ahrens MJ; Hickey CW Geochemistry of PAHs in Aquatic Environments: Source, Persistence and Distribution In PAHs: An Ecotoxicological Perspective; Douben PET, Ed.; John Wiley & Sons, Ltd, 2003; pp 35–45. 10.1002/0470867132.ch3. [DOI] [Google Scholar]
- (66).Flowers-Geary L; Bleczinski W; Harvey RG; Penning TM Cytotoxicity and Mutagenicity of Polycyclic Aromatic Hydrocarbon O-Quinones Produced by Dihydrodiol Dehydrogenase. Chemico-Biological Interactions 1996, 99 (1), 55–72. 10.1016/0009-2797(95)03660-1. [DOI] [PubMed] [Google Scholar]
- (67).Arey J; Harger WP; Helmig D; Atkinson R Bioassay-Directed Fractionation of Mutagenic PAH Atmospheric Photooxidation Products and Ambient Particulate Extracts. Mutat. Res 1992, 281 (1), 67–76. [DOI] [PubMed] [Google Scholar]
- (68).National Research Council. Alternatives for Ground Water Cleanup; The National Academies Press: Washington, DC, 1994. 10.17226/2311 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.