Abstract
Cellular and humoral aspects of the immune response develop sequentially in the fetus. During the ontogeny, the pluripotent stem cells emerge and differentiate into all hematopoietic lineages. Basic questions including the identification of the first lympho-hematopoietic sites, the origin of T and B lymphocytes, the development of different subpopulations of αβ T, γδ T and B lymphocytes as well as development of innate immunity and the acquisition of full immunological capacities are discussed here for swine and compared with other species. The description of related topics such as fertilization, morphogenesis, maternal-fetal-neonatal physiology and early neonatal development are also discussed.
Keywords: Ontogeny of the porcine immune system, Swine adaptive immunity, Development of αβ and γδ T cells, Maturation of B lymphocytes, Hematopoiesis, Lymphopoiesis, Lymphogenesis and morphogenesis, Primary and secondary lymphoid tissues, Bone marrow, Thymus
1. Fertilization and gestation periods
Most sows and boars become sexually active between 6 and 12 months after birth. A mature boar is generally ready to mate at any time but a sow will accept it only in estrus. The beginning of estrus is called the time of the first acceptance. As ovulation and fertilization takes place from 1 to 3 days after the first acceptance, true embryonic age can be overestimated [1]. The age of various fetuses in the same litter also differs. A fetal age difference of about 1 day is the most probable and this fact may slightly affect the experimental data. The full gestation period of pigs have a mean about 114 days [1]. The day or date of gestation is referred hereafter as DG.
2. Morphogenesis in the fetal pig
Based on cleavage, primary cavitation, lamination, tubulation, organ formation, regionalization, time of establishment of the body axis, time when male and female can first be distinguished and organogenesis is finished, morphogenesis of pigs can be arbitrarily divided into 3 intervals (for the main stages see schematic Fig. 1 ):
-
1.
The initial development of the zygote (DG0–DG12).
-
2.
The embryonic period of embryogenesis (DG12–DG36).
-
3.
The fetal period of embryogenesis (DG36–DG114).
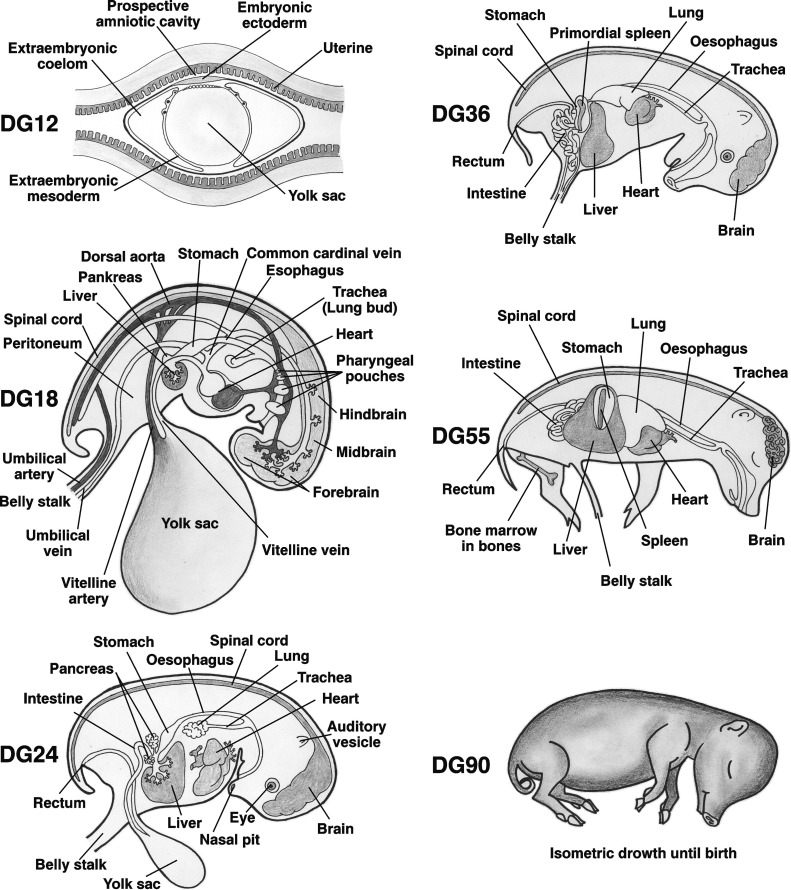
Fig. 1.
Schematic illustration of fetal development of porcine organs.
The initial development of the zygote includes cell divisions of individual blastomeres and ongoing maturation to the morula and thereafter to blastula (blastocyst) stage. Fertilized ova pass down oviduct and enter the uterus at about DG2–5. By DG11, the embryo shows signs of attachment to endometrium.
At the beginning of the embryonic period, the blastocyst initiates gastrulation to form the gastrula with three discernable germ cell layers. This stage is thereafter followed by organogenesis. Basic primordial organs like lung buds, liver, stomach, pancreas, mesonephros, uteri bud, primordial intestine or primordial pouches are easily distinguishable at about DG18–20 (Fig. 1). Principal organogenesis is finished at about DG35 with the fetal liver constituting a major tissue over embryo. From DG12, the embryo attached to the uterus also forms the placenta that is not complete until DG18. The fetal part of the placenta is formed by extra-embryonic membranes that include the amnion, chorion, allantois and yolk sac.
The fetal period starting from DG36 can be further divided by focusing attention on two phases of growth: (a) from DG36 to DG55 the growth is allometric and (b) from DG55 until birth it becomes predominantly isometric. Although the lymphatic system is physiologically formed at the end of organogenesis by DG35, lymphoid elements and lymphocytes in different organs are infrequent until the bone marrow starts its hematopoietic activity at about DG45. Lymphatic nodes including mesenteric lymph nodes are thereby negligible until DG70; this is connected to the expansion of the peripheral lymphatic pool occurring between DG60 and DG90.
At the birth, the porcine fetus has reached on elementary level of immunocompetence, especially innate immunity. Due to type of placentation in swine, the adaptive immune system of newborns remains virgin and their blood is devoid of maternal antibodies and transferred maternal immune components.
After the birth, the expansion of lymphocytes in secondary lymphoid organs occurs and appears related to colonization of the gastrointestinal tract and encounter with neonatal pathogens. In that time, intraepithelial lymphocytes, mesenteric lymph nodes, lamina propria cells and Peyer's patches expand and increase in size. Early neonatal development of immune system is briefly discussed below.
3. Porcine placenta and maternal-fetal-neonatal physiology
The pig possesses a noninvasive epitheliochorial form of placentation [2]. Chorionic villi are loosely opposed to the maternal epithelium by microvillus interdigitation or even separated from it at areas known as areolae. In the early stages, there are as many as six layers separating the fetus from the maternal bloodstream. This includes maternal tissue (uterine endothelium, connective tissue and epithelium) and fetal chorion (trophoblasts, connective tissue and endothelium). With advancing gestation, however, some fetal capillaries penetrate the trophoblast and bring the vascular beds closer. Antibodies and large organic molecules, however, have never been shown to cross the placenta in the pig and the failure of the trophoblast to invade maternal tissues probably means that even small peptides including cytokines are not transferred to the fetus [3]. For these reasons, the immune system of the porcine fetus, colostrum-deprived newborns and colostrum-deprived piglets reared in a germ-free (GF) environment, can be considered to be immunologically virgin compared to rodents and humans and with blood devoid of maternal antibodies [4].
In some cases, the fetal maternal barrier can be disrupted and/or crossed by infections. For example, some strains of the porcine parvovirus or the porcine reproductive and respiratory syndrome virus have been reported to cross the placenta by an unknown mechanism [5]. Another example is Toxoplasma gondii that may infect fetuses via congenital infection [6]. In many cases, the infection of the immunologically virgin fetuses leads to death. Whether these results from their immature immune system or a maternal immune response that gains access to the fetus due to a damaged placental barrier is not entirely clear. Antibody repertoire studies indicated that the former is most probable because the immune response of survivors is fetus-specific and does not appear to involve maternal antibodies [5]. In any case, the fetal immune response in survivors is mostly weak and will only rise near birth when the fetal immune system is more competent.
4. Hematopoiesis
The earliest source of multipotent hematopoietic cells in the embryo is the mesoblast, an area called the para-aortic splanchnopleure or aorta-gonad-mesonephros [7]. The hematopoietic system develops from those common stem cells that migrate into different location and seed primary hematopoietic organs. Hematopoietic activity in pigs occurs basically in three phases that are characterized by different locations that correspond to fetal morphogenesis. The first primary hematopoietic organ where stem cells develop is the yolk sac; this moves then to the fetal liver and finally to the bone marrow [8]. In the pig, hematopoiesis in the yolk sac can be detected as soon as at the 17 somite stage at DG16. By DG18, approximately 3 million hemocytoblasts and 10 million erythroid cells can be found in the yolk sac. However, the yolk sac is no longer functional by DG24–27 and hematopoietic activity afterwards is supported by the fetal liver where hematopoietic activity can be detected from DG20. Later on DG40, the bone marrow starts its hemapoietic activity and this organ serves from that time as a major hematopoietic organ throughout life [4], [8], [9]. The basic information about hematopoietic and lymphopoietic activities in different lymphoid organs during prenatal ontogeny is summarized in Table 1 .
Table 1.
The basic information about hematopoietic and lymphopoietic activities in different lymphoid organs during prenatal ontogeny
| Type of tissue | Establishment | Hemopoietic activity | First αβ T cells | First γδ T cells | First B cells | Basic germ layers | References |
|---|---|---|---|---|---|---|---|
| Yolk sack | 12 DG - primordial | From DG16 to DG27 | Not detected | Not detected | Only VDJH rearrangement detected | Endoderm | [8], [9], [25], [34] |
| 24 DG - functional capacity is finishing | Mesoderm | ||||||
| Liver | 15 DG - primordial | From DG30 to the birth (decreasing) | DG58 | DG45 | DG30 | Endoderm | [8], [9], [14], [25], [34] |
| 30–39 DG - invaded by hepatic cells | Mesoderm | ||||||
| Cord blood | 15 DG - heart starts with pumping | – | DG58 | DG45 | DG40 | – | [10], [34] |
| 28 DG - definitive coronary circulation | |||||||
| 55 DG - RBC are almost entirely enucleated | |||||||
| Spleen | 20 DG - primordial | – | DG58 | DG45 | DG40 | Mesoderm | [9], [10], [34] |
| 36 DG - complete formed | |||||||
| 36–114 DG - allometric growth | |||||||
| Thymus | 22 DG - primordial | – | DG55 | DG40 | DG55 | Endoderm | [10], [14], [25], [33], [34], [43] |
| 36 DG - complete formed | Ectoderm | ||||||
| 36–114 DG - allometric growth | |||||||
| MLN | 55 DG - intestine complete in essential form | – | DG60 | DG70 (always minor) | DG50 | Mesoderm | [10], [34] |
| Bone marrow | 18 DG - basically formed skeleton | From 45 DG to the birth (increasing) | DG60 (always minor) | DG60 (always minor) | DG55 | Mesoderm | [6], [8], [9], [14], [25], [34] |
| 28 DG - ossification of bones | |||||||
Occurrence of the first lymphocytes in different organs is based on flow cytometry studies detected by the expression of T and B cell receptors.
5. Development of innate immunity
The information about ontogeny and development of innate immunity in swine is scarce. The components of porcine innate immunity are often studied in infection models using for example classical swine fever virus (CSFV), porcine reproductive and respiratory syndrome virus (PRRSV), swine influenza virus (SIV), foot-and-mouth disease virus (FMDV), E. coli, Salmonella or coronavirus infections (see other articles and chapters of this issue). This approach is applied because the frequency and function of different components of innate immunity in healthy animals is mostly independent of age. In any case, innate immunity of pigs consists of the similar components described for other mammals. The effector functions are realized through two major mechanisms: (a) the recruitment and activation of cellular components; including macrophages, neutrophils, natural killer (NK) cells, and dendritic cells (DCs) and (b) the release of a broad spectrum of extracellular mediators such as cytokines, chemokines, complement, and antimicrobial peptides (AMPs).
The cellular components of innate immunity such as polymorphonuclear leukocytes, macrophages and DCs appear together with the hematopoietic activity of the primary lymphoid organs. Cells with the phenotype of NK cells (CD3−CD8βα+CD2+) are also observed quite early in ontogeny [10] and are first found at DG45 in umbilical blood and spleen. The proportion of NK cells in various tissues is between 1 and 10% with a tendency to increase in number during fetal ontogeny [10]. The occurrence and the frequency of NK cells stabilize at about DG70 and remains approximately the same through birth into postnatal life. NK cells in adult conventional pigs represent maximally 15% of all lymphocytes. Functional studies show that killing is not observed before birth and is delayed in germ-free piglets [11], [12]. This suggests that NK cells exhibit some sort of maturation and probably need colonization and microbial flora for the development of their full functional capabilities.
γδ T cells are also often categorized as a part of innate immunity because they are involved in innate immune efficiency. The ontogeny of γδ T cells is well recognized and is discussed below under T cell section.
Extracellular mediators of innate immunity and inflammatory proteins are found very early during fetal ontogeny. For example, the presence of IFN-α and IFN-α secreting cells can be detected in the fetal liver as early as at DG26 [13]. At that time, lymphoid cells cannot be detected in fetal liver. IFN-α secreting cells at later stages of gestation are found in different fetal tissues like the blood, spleen or bone marrow and their occurrence is associated with hematopoietic organs. Antimicrobial proteins (AMPs), also called host defense peptides (HDPs), include a wide range of proteins that can be classified into defensins (best known in swine is β-defensin 1) and cathelicidins (best known in swine is PR-39 and protegrin 1). Both categories of HDPs have direct antimicrobial activity as well as the ability to modulate immune responses. Unfortunately, there is no relevant information about prenatal ontogeny of these innate components that would show whether they are present during fetal period or need some sort of induction by microbial flora or the presence of Pathogen-Associated Molecular Patterns (PAMPs).
6. Lymphopoiesis and development of adaptive immunity
Lymphopoiesis is closely related to hematopoiesis because B cells in pigs develop in primary hematopoietic organs. The thymus is populated by pro-T cell progenitors from the same hematopoietic centers so the onset and course of B and T lymphopoietic activity is closely related. The earliest source of lymphopoietic activity in the porcine embryo is the yolk sac. Although very infrequent, the first lymphocytes that can be detected at DG20 are B lineage cells [8]. These first B cells were detected based on complete VDJH gene rearrangement in the heavy chain locus. The absence of transcripts for light chain genes, inability to detect expression of specific genes for rearrangement such as TdT and RAG, and the absence of lymphocytes with B cells markers confirm that the number of cells involved in VDJH rearrangement is extremely low [8]. Therefore, although the yolk sac is the first site of lymphogenesis in pigs, the contribution of generated cells to overall lymphocyte pool is marginal.
The yolk sac in the fetal pig involutes after DG24–27 so termination of its role in lymphogenesis afterward would be expected. Lymphopoietic activity and development of the pre-immune B cell receptor repertoire can be thereafter detected in the fetal liver (Fig. 2A) which is the major site of B cell lymphogenesis in the porcine embryo from DG30 until at about DG45 when the bone marrow becomes active (Fig. 2B) [8].
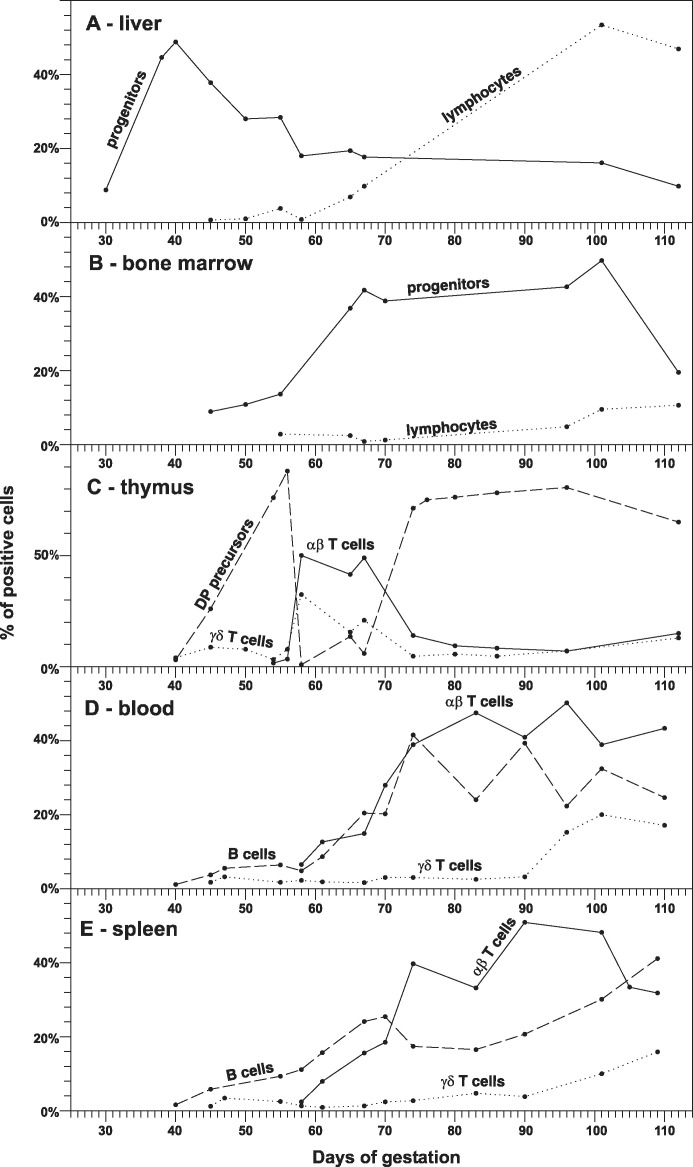
Fig. 2.
The ontogeny of primary lymphopoietic activity in fetal liver (A), bone marrow (B) and thymus (C). The proportions of putative lymphoid progenitors (CD45loCD172a− cells, solid line) and mature lymphocytes (CD45hiCD172a− cells, dotted line) in fetal liver (A) and bone marrow (B) demonstrate the shift of lymphopoietic activity between these hematopoietic centers. The thymus (C) is populated by pro-T cell progenitors from the same hematopoietic centers that can be easily observed by two waves of developing immature DP precursors (large CD4+CD8+ cells that are CD3−TCRαβ−TCRγδ−, dashed line). Relationship to the development of mature αβ (small CD3hiTCRαβ+ cells, solid line) and γδ (small CD3hiTCRγδ+ cells, dotted line) T lymphocytes in the thymus is also shown. The ontogeny of occurrence for peripheral mature αβ T cells (solid line), γδ T cells (dotted line) and B cells (dashed line) is shown for fetal blood (D) and spleen (E). Data are based on results previously published by Sinkora et al. [8], [10], [14], [33], [43].
T lymphopoietic activity is directly correlated with the hematopoietic activity of the fetal liver. The first T cells can be detected in thymus at about DG40 (Fig. 2C), i.e., with about 10 days delay in comparison with B cell lymphogenesis in the fetal liver [10], [14]. In the same time, the first sIgM+ B cells appear in the periphery (Fig. 2D–E) and remain the major lymphocyte population until at least DG55 [8], [10], [14]. The first peripheral T cells (Fig. 2D–E) appear about 5 days after they are detectable in the thymus (Fig. 2C); all of which are γδ T cells [14]. αβ thymocytes require about 15–20 days to express TCRαβ on their surface while γδ thymocytes do so for TCRγδ in less then 3 days. In agreement with this, the first αβ T cells appear in the periphery at about DG55 (Fig. 2D–E) [14]. The period of lymphopoietic activity from DG20 to DG45 generates the first wave of progenitors because all their lymphocyte progeny originate from the yolk sack and fetal liver.
The onset of B cell lymphogenesis in the bone marrow starting from DG45 (Fig. 2B) is followed by a rapid expansion of B cells in the periphery (Fig. 2D–E) [10]. The bone marrow from that time also serves as a major hematopoietic organ where early T cell progenitors are derived from stem cells. These progenitors migrate to the thymus where they cause the second wave of colonization (Fig. 2C) [14]. Lymphopoietic activity in the liver after this period fades and become marginal (Fig. 2A). Lymphopoietic activity in the bone marrow peaks between DG60 and DG80 (Fig. 2B) and during this period, most peripheral B and T cells are generated (Fig. 2D–E). Although γδ T cells are the first T lymphocyte subset appearing early in ontogeny, the ratio of porcine αβ/γδ lymphocytes gradually increased later in ontogeny, resulting in a predominance of αβ T cells in both the thymus and the periphery up to end of gestation [10], [14].
6.1. Development of B cells
6.1.1. B cell development at molecular level
Differentiation of B cell precursors from multipotent stem cell to immature B cells can be divided into a series of stages based on the status of immunoglobulin (Ig) gene rearrangement and the expression of certain genes and proteins [15], [16], [17], [18], [19]. The first step in the generation of the antibody repertoire involves combinatorial joining of VH, DH and JH gene segments in the Ig heavy chain (HC) locus of precursor pro-B cells, a process called VDJH rearrangement. Cells that have productively rearranged their VDJs may further differentiate to the early pre-B cell stage (preB-I) in which the pre-B cell receptor (pre-BCR), consisting of the VDJ spliced to the IgM HC (called μHC) in association with surrogate light chain (SLC consisting λ5 plus VpreB) and the signaling components Ig-α and Ig-β, are expressed on the cell surface [20]. The functional pre-BCR delivers signals that stop further rearrangement in the HC locus and thus assure allelic exclusion. Further cellular differentiation to the late pre-B cell stage (preB-II) involves Ig light chain (LC) gene segment rearrangement (Vλ and Vκ jointed to JL) and replacement of the SLC with authentic an LC [16], [17]. This authentic B cell receptor is the defining marker of the immature B cell stage, the direct predecessor of transitional stage T-1 and T-2 B cells [21].
Development of a functional B cell in all known species requires that VDJH rearrangement be productive, i.e., generate an open reading frame or “in-frame” sequence that can lead to a translatable μHC that does not contain an internal stop codon(s). Unlike LC locus, the HC locus can be rearranged only once because of the loss of recombination signal sequences during VDJH rearrangement and the non-tandem arrangement of VH, DH and JH segments in the HC locus. This phenomenon is particularly demonstrated in chickens and swine that have only a single JH [22], [23], [24]. B cells precursors that do not succeed in making a productive rearrangement using the first chromosome (theoretically 2/3 of all cells if the process is random) have only one more chance to generate a translatable μHC by using the second chromosome, again with a 67% chance to failure. B cell precursors that do not attain the pre-BI cell stage due to lack of a productive rearrangement are presumably eliminated. Thus, less than 55% of all precursor B cells that start the recombination process can express a μHC and these survivors will have frequencies of in-frame and out-of-frame rearrangements of ∼71% and ∼29%, respectively.
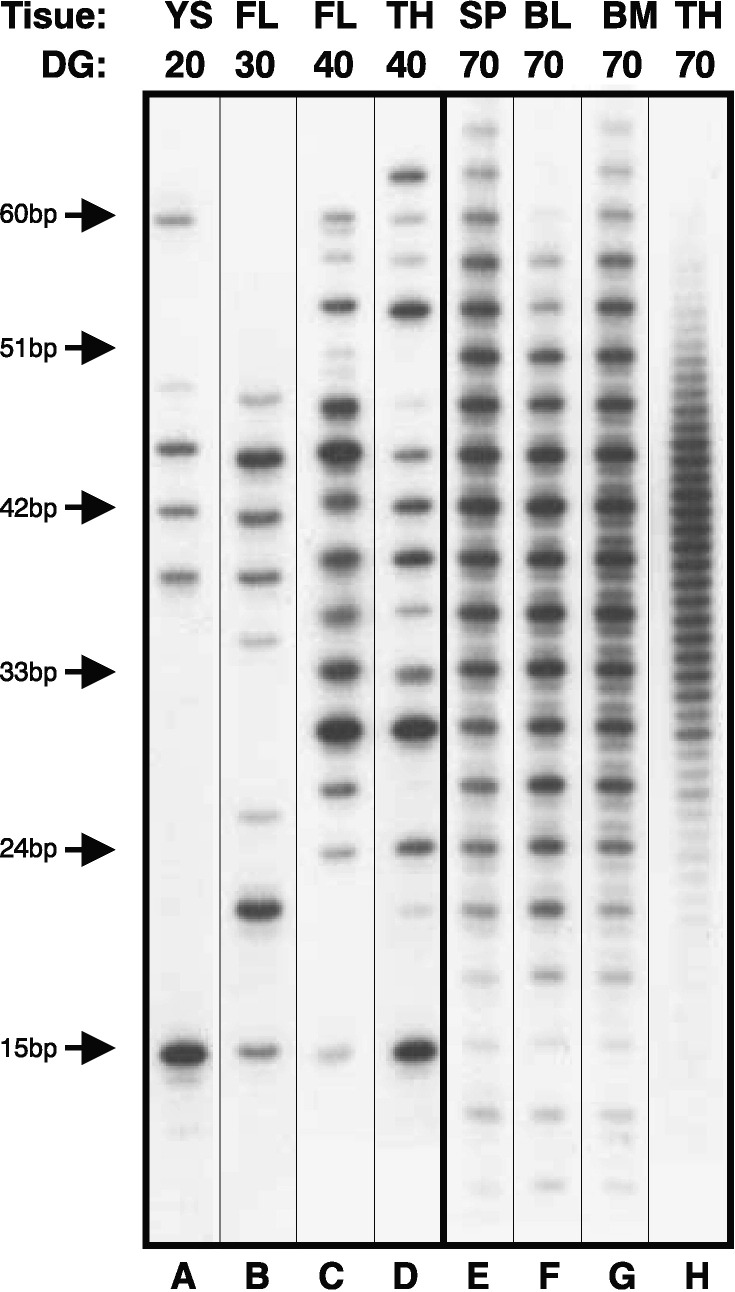
It is generally accepted that B cell lymphogenesis operates by the same mechanism regardless of the type of tissue where primary B cell lymphogenesis occurs. Data from swine counter this conviction and show that certain B cell populations from fetal tissues do not fulfill the textbook description of B cell lymphogenesis that yields 71% productive rearrangements [8], [25]. Rather, fetal B cells coming from the yolk sac and fetal liver in the first wave of progenitors result in B cells in which 100% carry productive rearrangement on one allele and no rearrangement on the second (Fig. 3A–D). On the other hand, B cells arising from the second wave of progenitors that originates in the bone marrow follow the established paradigm and are not limited in progressive VDJ rearrangement on second chromosome (Fig. 3E–G). These observations suggest that either different rearrangement mechanisms or different selection mechanisms operate outside the bone marrow [8]. Based on in vitro experiments, the influence of the bone marrow environment on selection of productive heavy chain rearrangements has also been suggested in mice [26]. However, no direct proof has been provided from studies in mice because maturation of mice B cells in fetal liver coincides with maturation in bone marrow and therefore mixture of cells originating from both sources contributes to the overall B cell pool. This makes further analysis of VDJ status on different chromosomes impossible. In any case, detailed sequence analysis of the fetal VDJH repertoire in swine does not reveal any significant age- or tissue-dependent changes in VDJH diversity during fetal life (i.e., VH and DH usage, number of N-region additions, number of somatic mutation and extend of CDR3 diversity). The limited single VDJH rearrangement on one allele is the only difference that distinguishes early B cells from yolk sac and fetal liver from those later recovered from bone marrow. Whether these represent developmentally distinct B cell subsets as described for B-1 and B-2 cells in mice remains to be determined because porcine B cells cannot yet be distinguished by flow cytometry. In any event, the limitation in progressive VDJ rearrangement on the second chromosome in early fetal B cells as evidenced in swine, is similar to development of B cells in chickens and rabbits that have all their VDJ rearrangements in-frame. All B cells in these species are generated during a narrow window in fetal life and are thereafter maintained through life by mitosis and somatic diversification in the chicken bursa [23] and the rabbit appendix [27].
Fig. 3.
Length analysis of the HCDR3 repertoire recovered from DNA amplifications of VDJH in representative samples coming from yolk sac (YS), fetal liver (FL), thymus (TH), spleen (SP), blood (BL) and bone marrow (BM) during different days of gestation (DG). The left panels (A–D) represent samples recovered from fetuses before the bone marrow starts its hemapoietic and lymphopoietic activity (before at about DG45) while right panels (E–H) represent samples isolated from fetuses after the bone marrow become active. HCDR3 length markers are indicated on the left. Note that HCDR3s isolated from B cells coming from the first wave of progenitors (panels A–D) present a pattern in which there are no out-of-frame rearrangements (no minor bands in lengths between in-frame rearrangements). On the other hand, B cells arising from the second wave of progenitors that originate in the bone marrow (panels E–G) contain out-of-frame rearrangements that represent non-productive rearrangements. The VDJH rearrangements isolated from thymus after DG55 (panel H) displays the total absence of selection for in-frame rearrangements.
All VH genes in swine are VH3 family genes and 29 variants (genes or alleles) have been identified [28]. 80% or more of the fetal pre-immune repertoire uses just four VH genes (VHA, VHB, VHC, VHE) and by including three additional VH genes (VHF, VHX, VHY), can account for >95% of the fetal repertoire [29]. Given: (a) the limited combinatorial diversity in this swine where 7 major VH genes are used in fetal development and only two DH (DHA and DHB) and one JH segments exist and (b) the nearly identical framework regions of its VH3-like genes, >95% of the pre-immune repertoire in swine is determined by junctional diversity in CDR3. The light chain repertoire is also combinatorially restricted and shows little or no junctional diversity [30]. Lambda is preferentially used in early B cell development; presumably because of λ5. Otherwise the expressed κ:λ ratio is very similar to humans and therefore very different from the heavily skewed ratios in rodents, rabbits, cattle and horses. The significance of pre-immune VH, Vκ and Vλ repertoires is discussed in more detail in chapter “Immunoglobulins, antibody repertoire and B cell development” in this issue.
6.1.2. B cell development at cellular level
Although the development of B cells on a molecular level generally operates by similar mechanisms across different species, the monitoring of this development by expression of cell surface markers differs markedly. This inconsistency is apparent namely in species that are extensively studied like humans and mice. However, it also becomes evident for other species like swine where investigators are limited in the number of monoclonal antibodies available to recognize cell surface markers.
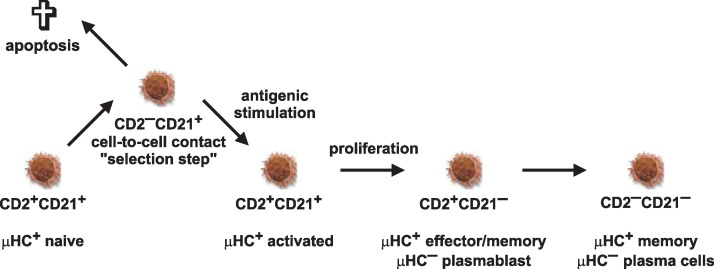
There is no pan-B cell marker for swine other than intracellular CD79α detected by cross-reactive anti-human monoclonal antibodies [31]. Because identification is restricted to intracellular staining, many studies are done using anti-μHC antibodies that detect all Ig positive cells, thus omitting plasma cells, some switched B cells and B cell precursors from detection. Analysis by this approach showed that all μHC+ B cells in swine are MHC-II+, CD25lo and CD45RC+ [10]. Fetal studies showed that virtually all immature μHC+ B cells are also CD21+ and CD2lo [8], [10], [32]. CD2 and CD21 molecules can be expressed differentially so the mature B cells are subdivided into four subpopulations that include CD2+CD21+, CD2−CD21+, CD2+CD21− and CD2−CD21− cells [33]. Because the numbers of CD2− and CD21− B lymphocytes significantly increases in germ-free animals that were colonized by a complex intestinal microflora or just with the age of conventional animals [32], these markers were considered a B cell differentiation marker in pigs. Our recent data showed that CD2 but not CD21 can be re-expressed on the surface of B cells so that only CD21 can be considered a maturation marker (Fig. 4 ). The function of CD2 on the surface of B cells is not known but CD2 is an adhesion molecule that acts during intercellular interactions so it can be down-regulated by cell-to-cell contact. Once B cells recover from such interactions, CD2 expression on B cells is re-established in a short period. CD2 may also be regarded as a developmental marker because porcine B cell precursors express CD2 earlier than the BCR during B lymphopoiesis [32], [34]. In this respect, expression of CD25, CD79α and CD172a can be also used for identification of B cell precursors in primary lymphopoietic centers [23]. Cross-reactive CD20 and CD86 were also recently showed to be useful for phenotyping of porcine B cells [35].
Fig. 4.
Proposed model of peripheral B cell maturation and diversification. CD2+CD21+ B cells represent naive mature B cells that down-regulate CD2 following cell-to-cell contact and activation. A portion of the activated CD2−CD21+ B cells probably died while survivors re-establish CD2 expression to become again CD2+CD21+. Further maturation of activated B cells includes down-regulation of CD21. Resultant CD2+CD21− B cells can subsequently generate μHC− large proliferating plasmablasts or small non-dividing plasma cells that eventually become CD2−CD21−. This schematic representation is deduced from recent findings [32], [33], [34]. The phenotype and functional status of individual subsets of B cells is given.
B1 and B2 cells cannot currently be discriminated by CD5, IgD or other CD marker expression in swine [36]. Although porcine B cells were shown to be heterogeneous for CD5 expression, other markers do not correspond with the expression profiles found in mice or humans. If the phenomenon of B1 and B2 cells is applicable to pig, the discrimination of the cell types responsible may have to be done on the molecular level by differences in VDJ rearrangement patterns during fetal ontogeny (see above). Although no mAb exists to recognize porcine IgD, the transcript for this Ig does not appear until B cells reach secondary lymphoid tissues [37]. IgD is nearly absent from the bone marrow but is prominently expressed in the periphery. The structural features [38] and expression pattern of porcine IgD therefore differs from IgD in mice and humans and does not appear to be a useful candidate for monitoring of B cell development in swine.
Although the precise step-by-step description of porcine B cell development using cell surface markers remains to be determined, the available evidence indicates that putative B cell precursors are μHC− MHC-II+ CD2lo CD25lo and CD21− and mature to the μHClo CD21+ stage and thereafter into immature B cells that are μHChi CD21+ [8]. Further development after activation or priming (Fig. 4) involves down-regulation of CD21. The resultant CD2+CD21− B cells can subsequently generate large proliferating μHC− plasmablasts and small non-dividing μHC− plasma cells which may eventually lose CD2 expression [34], [39].
6.1.3. Fetal class switch recombination and thymic B cells
A remarkable feature of B cell development in swine is the early onset of class switch recombination (CSR) of Ig genes that occurs in utero without contact with environmental antigen. Transcripts for IgG1, IgG2, and IgA and of course IgM are presented at midgestation [5], [28]. Furthermore, de novo synthesized IgG, IgM and IgA are present in fetal sera at low levels during the second half of gestation [5]. Remarkably, IgG and IgA plasma cells are present in the thymic medulla during the same period [5]. In newborn piglets, IgE is also transcribed in thymus [37]. Probably, the CSR is a programmed event in fetal piglets, independent of antigen and perhaps the result of an intrinsic stochastic phenomenon that occurs spontaneously. The porcine thymus in these regards plays a considerable role and is a prominent site for the transcription of IgG and IgA switched isotypes during prenatal life [5], [40]. Whether this is a result of preferential accumulation of plasma cells in the thymus or whether the thymus environment is responsible for spontaneous switching, remains to be determined. The ratio of IgG to IgA secreting cells in thymus is nearly equal, whereas the serum IgG to IgA ratio from the same animals is ∼10:1. Thus, it appears that IgG: (a) must be secreted at a greater rate, (b) accumulates in the blood vascular system because IgA is preferentially transported to mucosal sites, (c) has a longer half-life because of protection by FcRn, (d) is in part derived from maternal blood or (e) is synthesized in large amounts outside of thymus. In any case, the reason why the epicenter for isotype switch recombination is the fetal thymus and why plasma cells accumulate in this organ and why serum IgG and IgA levels do not agree with IgG and IgA cell numbers is unexplained.
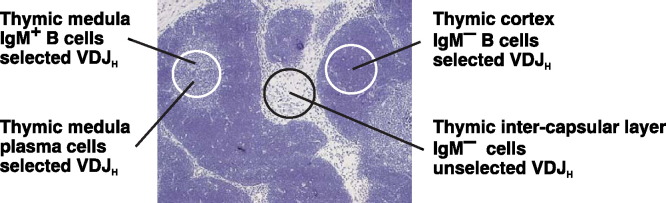
The porcine thymus is interesting also by occurrence of VDJ rearrangements that are apparently not selected for productive rearrangement. CDR3 length analysis of crude thymus lysate (Fig. 3H) expresses the total absence of selection for productive (in-frame) rearrangements [23], [29]. IgM+ B cells (Fig. 5 ) are present within porcine thymus in the same frequencies as known for other species and constitute ∼1% from all thymocytes [14]. Recent findings showed that these B cells are similar to IgM+ B cells found in the periphery and other lymphatic organs being CD2lo MHC-II+ CD45RC+ and CD25lo. Because thymic IgM+ B cells express functional IgM on the cell surface and therefore are selected for productive rearrangements, these B cells cannot contribute to unselected VDJ repertoire found in the thymus. The same conclusion applies for plasma cells (Fig. 5) that produce switched IgG and IgA. Both, plasma cells and μHC+ B cells are found exclusively in the thymic medulla (Fig. 5). Site-specific PCR on clusters of cells together with CDR3 length analysis also described a small population of developing B cells that can be found in thymic cortex (Fig. 5). These cells are Ig negative and have the characteristics of B cells that have gone through a selection process, similar to B cell in the bone marrow. These cells may be indicative of lymphogenesis in the thymus which also does not contribute to the unselected B cell repertoire. Micromanipulation experiments and single-cell PCR found a specific and abundant population of cells in thymic inter-capsular layer that are responsible for unselected VDJH repertoire (Fig. 5). These cells contains rearranged VDJH that are entirely unaffected by the mechanism governing the selection of in-frame VDJH rearrangement. Although thymus is not remarkable producer of B cells at all, different population of thymic cells containing VDJH rearrangement clearly demonstrate that differences in the microenvironment at different sites control B cell selection. The thymus is a specific example of this process and its architecture provides specific microenvironments that could allow for distinctive selection and differentiation of B cells.
Fig. 5.
The localization of thymic cell populations that have rearranged their immunoglobulin VDJH genes. One population can be found exclusively in thymic medulla and consists mainly of immature and mature sIgM+ B cells and plasma cells, all of which have productively rearranged their VDJH genes. The second population found mainly in thymic cortex are sIgM− cells that possess characteristics of a selected population, similar to B cells arising from the bone marrow. The third population is found in the thymic inter-capsular layer and shows no evidence of selection for in-frame VDJH rearrangement. These cells do not express a BCR and do not bear other known swine B cell surface markers.
6.2. Development of T cells
Two types of CD3-associated T cell receptor (TCR) molecules have been identified in all vertebrates studied so far, consisting of either a TCRαβ or TCRγδ heterodimer. In some species like human, mouse and rat, TCRαβ is expressed on >95% of all T cells. Some other species like pigs, ruminants and chickens have a higher proportion of γδ T cells in the peripheral blood and lymphoid organs and these may account for more than 80% of the peripheral T cell pool [10]. The reason for abundance of γδ T cells in these species is still unknown and speculation that it is an indication of higher utilization of γδ T cells in cellular immunity was not clearly confirmed. In any case, the ontogeny and development of T cells in different species are comparable and follow similar pattern. This uniformity is in agreement with the findings of very strong evolutionary conservation of TCR genes that have been conserved since the emergence of jawed vertebrates more than 450 million years ago.
Similar to B cell development, T cells are also generated in at least two waves during prenatal ontogeny of swine. This phenomenon is because early T cell progenitors (pro-T cells) are derived from stem cells in primary hemopoietic centers (where also B lymphogenesis occurs) and migrate to the thymus where further T cell differentiation takes place. Similar to the most frequently studied species, e.g., mice, human and chickens [41], [42], porcine γδ cells are the first T cells to develop [8], [14], [33], [34], [43]. Ontogenic studies showed that γδ T cells require less than 3 days to express the TCR receptor while the αβ thymocytes do so in about 15–20 days [14]. The shorter period for expression of TCR receptor in γδ T cells and development without any CD3ɛlo or TCRγδlo transitional stage, suggests that development of γδ T cells does not include stringent selection for TCRγδ specificity as for αβ T cells [10], [14]. This supposition is further supported by findings that all porcine TCRγδ-bearing thymocytes are large medullar cells so there is no cortex-dependent maturation and/or selection [14]. In fact, our very recent data demonstrate that the maturation and phenotypic alteration of γδ thymocytes occurs after the full expression of TCRγδ [33]. Assuming that there are different hemopoietic centers during prenatal ontogeny, the influx of thymocyte progenitors to the thymus is discontinuous, which results in various waves of thymic colonization and further waves of lymphocyte migration to the periphery. The rapid maturation rate of γδ versus αβ T lymphocytes in thymus is associated with asynchronous migration from the thymus so in each of the individual waves, γδ T cells populate the periphery before αβ T cells (Fig. 2D–E). This difference in developmental and maturation rate is particularly evident in early ontogeny [14], [33], [43]: The first triple-negative lymphoid elements (CD3−CD4−CD8− pro-T cells coming from the fetal liver) could be observed in the thymic rudiments on DG38. The resultant γδ T cells from these progenitors appear in the thymus 2 day later at DG40 (Fig. 2C) and consecutively migrate to the periphery where they appeared at about DG45 (Fig. 2D–E). Differentiating thymocytes belonging to the αβ lineage in the meantime undergo positive and negative selection through CD3−CD4+CD8+ double-positive stage so the first CD3lo thymocytes appeared in the thymus at DG45 and the first TCRαβ+ cells are detectable at DG55 (Fig. 2C). The mature αβ T cells are subsequently exported from the thymus so the earliest peripheral αβ T lymphocytes can be detected on DG58 (Fig. 2D–E). The same developmental and maturation pathway can be expected when the hemopoiesis shifts from the fetal liver to the bone marrow at about DG45. This wave is not so easily observed in the periphery where mixture of cells from different sources overlap but can be clearly observed in the thymus according to the phenotype of individual αβ and γδ developmental stages [33], [43].
6.2.1. Development of γδ T cells in the thymus
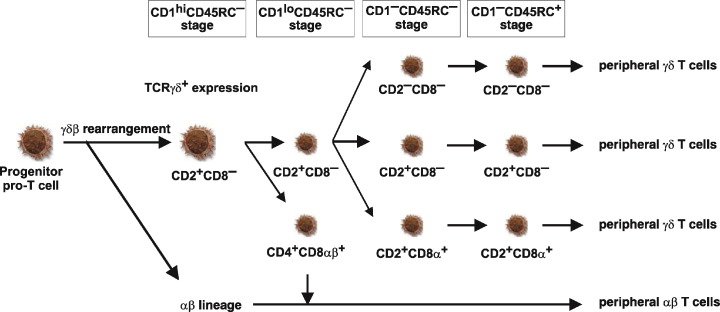
Developing porcine γδ T cells in the thymus can be subdivided into two major groups: (a) numerous CD4− γδ thymocytes that could be further subdivided according to the CD2/CD8α phenotype into CD2−CD8−, CD2+CD8− and CD2+CD8α+ cells and (b) sparse CD4+ γδ thymocytes bearing always CD2 and CD8αβ and possessing certain unusual features [33], [43].
The CD4− thymocytes give rise to all peripheral γδ T cells [33], [43]. Maturation within this group begins with proliferation of the CD2+CD8−CD1+CD45RC− γδ common precursor (Fig. 6 ). This developmental stage is followed by diversification into the CD2−CD8−, CD2+CD8− and CD2+CD8α+ subsets (Fig. 6). The further maturation of each subset is accompanied by a loss of expression of CD1 and by increased expression of CD45RC in a mutually exclusive fashion (Fig. 6). Therefore, individual γδ subsets develop from immature CD1+CD45RC− through intermediate CD1−CD45RC− into mature CD1−CD45RC+ cells. This indicates that each of the three CD4− γδ thymocyte subsets defined by CD2 and CD8α expression develop in thymus through separate differentiation pathways and are exported from the thymus independently [33], [43]. It follows that all TCRγδ+ T cells subsets develop in the thymus and that expression of CD2 and/or CD8 are not useful differentiation markers for γδ thymocyte lineage. The last two subpopulation of intermediate and mature γδ T cells are exported from thymus into the periphery where the final maturation step from CD1−CD45RC− into CD1−CD45RC+ cells may also occur. All of these findings indicate that the maturation and phenotype alteration of γδ thymocytes occurs after successful expression of TCRγδ.
Fig. 6.
Proposed model of γδ T cell development and diversification in the thymus. Large cell icons demonstrate differentiation stages of large, mitotically active thymocytes while small cell icons represent small, non-dividing populations. The phenotype of individual subsets is given. Developmental stages determined using CD1 and CD45RC expression are summarized at the top. This schematic representation is deduced from recent findings [33], [43].
In contrast to the classical CD4− γδ thymocytes, γδ thymocytes within the CD4+ family follow a different developmental pathway (Fig. 6), have no counterpart in the periphery and possesses unusual features in comparison with all other γδ thymocytes: (a) they bear exclusively CD8αβ which is unique for any γδ T cells in any other organ, (b) they are always CD1+ indicating they do not mature to the terminal stage of T cell development and are not exported from the thymus, (c) they have no counterpart in the periphery because no CD4+ and/or CD8αβ+ and/or CD1+ γδ T cells exist in the periphery, (d) they may co-express CD45RC that indicates some degree of maturation and (e) they are actively dividing that suggests synchronization during development. CD4+CD8αβ+ γδ thymocytes therefore develop independently of other γδ thymocytes. Recent data show that this group of γδ thymocytes represents a transient and independent subpopulation that extinguish their TCRγδ expression and differentiate along the αβ lineage program [33]. Thus, these cells may alter their lineage program by re-entering the cell cycle and re-activating TCRαβ gene rearrangement. It is noteworthy that CD4+CD8+ γδ thymocytes also occur infrequently in humans [44], and early and transiently during fetal development in mice [45], [46]. A very minor subpopulation has also been reported in chickens [47]. CD4+ γδ T cells can also be obtained in substantial numbers from human fetal liver after in vitro stimulation [48], [49]. Perhaps CD4+ γδ thymocytes are not unique for swine and differentiation and maturation pathways described in swine also apply in many species.
6.2.2. Development of γδ T cells in the periphery
Peripheral γδ T cells in swine may be subdivided into CD2−CD8−, CD2+CD8− and CD2+CD8+ subsets [10], [51], correspondingly to the group of CD4− γδ thymocytes. There are no CD4+ and/or CD1+ and/or CD8αβ+ γδ T cells in the periphery [43]. Individual subsets of peripheral γδ T cells differ in their homing characteristic which is evident in early ontogeny and extends up to adulthood; CD2+CD8+ and CD2+CD8− γδ T cells preferentially accumulate in the spleen while CD2−CD8− are enriched in the circulation [10], [50], [51]. The same phenotype-dependent pattern of tissue distribution has not been identified in mice or humans although CD2+CD8− γδ T cells as well as γδ T cells expressing CD8 or lacking CD2 molecules have been observed and mucosal γδ T cells show a distinctive pattern of TCRγδ gene usage and phenotype that distinguishes them from the peripheral blood pool [44], [45], [46]. The porcine CD2+CD8α+ subset has been postulated to be the progeny of peripheral CD2+CD8− γδ T lymphocytes. This conclusion is based on the observation that: (a) some TCRγδ+CD8− cells may acquire CD8 upon activation [52], (b) that CD2+CD8α+ γδ T cells are potentially cytotoxic while other TCRγδ+ cell subsets are not [53], [54] and that (c) CD2+CD8α+ γδ T cell are scarce in porcine fetuses [10]. CD8α is expressed on αβ and γδ T cells following activation, on a subset of NK cells that are directly cytotoxic, on a subset of effector dendritic cells, and on many intra-epithelial lymphocytes from conventional but not from germ-free animals [55], [56], [57], [58]. In swine, in contrast to humans and mice, CD8α and/or MHC-II are not down-regulated so the αβ and γδ T cell maintain the expression of these entities after activation [33], [43], [59]. This eventually leads to two developmentally distinct subsets of CD2+CD8α+ γδ T cells in the periphery. One develops in the thymus while the second acquires CD8α extrathymically as a result of activation of CD8− thymus-dependent precursors [33]. These two developmentally distinct population of CD2+CD8α+ γδ T cells can be monitored by MHC-II expression, so that thymic-derived CD8α+ γδ T lymphocytes are MHC-II− while those derived in the periphery are MHC-II+. It should be mentioned that the expression of CD8α and MHC-II molecules after activation is mutually independent thus CD2−CD8− as well as CD2+CD8− γδ T cells may also express MHC-II after activation. Absence of activation of γδ T cell during fetal life is a reason for almost exclusive absence of MHC-II+ γδ T cells in embryos [33], [43]. The activation of CD8α expression on γδ T cells in the periphery cannot be ascribed and should not be confused with extra-thymic maturation of γδ T cells because this secondary peripheral maturation does not involve the rearrangement of TCRγδ genes and the genesis of γδ T cells de novo. More likely, γδ T cells just alter their accessory molecules to change the surface phenotype and function.
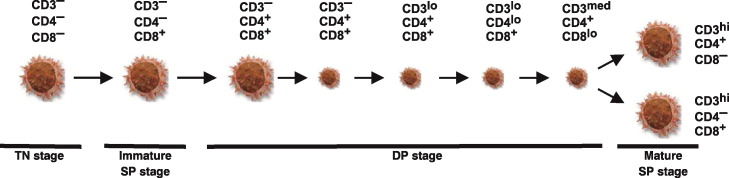
6.2.3. Development of αβ T cells in the thymus
Maturation of porcine αβ T lymphocytes in thymus follows the generally accepted model of intrathymic T cell differentiation derived from studies in other species (Fig. 7 ). Differentiating thymocytes belonging to the αβ lineage follow a progression from less differentiated, large triple-negative (CD3−CD4−CD8−) precursors through a CD8+CD3− immature stage to CD3−CD4+CD8+ double-positive (DP) thymocytes (Fig. 7). The latter subset proliferates vigorously to form large numbers of small cortical CD3− DP thymocytes that follow a progression through the CD3lo DP stage with different level of CD4 and CD8 expression to final populations of either mature CD4+CD8− or CD4−CD8+ single-positive T cells expressing CD3 at high level [14], [60].
Fig. 7.
Proposed model of αβ T cell development. Large cell icons demonstrate differentiation stages of large, mitotically active thymocytes while small cell icons represent small, non-dividing populations. The phenotype of individual subsets is given. This schematic representation is deduced from recent findings [14].
6.2.4. Development of αβ T cells in the periphery
Mature αβ T cells exported from thymus are composed of classical homogenous CD4−CD8αβ+ cytotoxic and CD4+CD8− helper αβ T cells subsets [14]. However, activation of CD4+CD8− helper αβ T cells with various antigens in the periphery leads to expression of CD8α that plays an important role in signal transduction associated with cell effector function. As mentioned for γδ T cells [33], [43], the expression of CD8α and MHC-II molecules in pigs is permanent and these activation molecules are not down-regulated after activation [59]. This results in the occurrence so-called “peripheral double-positive αβ T cells” that form the effector/memory T cell pool [10], [14], [53], [61], [62]. Again, it is important to emphasize that these extrathymically derived subsets are not connected and should not be confused with transitional DP stages of intrathymic development because the former are always CD8α+ [53], CD1− [61], mostly CD29+ [62], MHC-II+ [10], [61] and are generated from their CD8− counterparts upon stimulation [62]. The occurrence of peripheral CD4+CD8αβ+ αβ T cells is dependent on external antigenic stimuli and thus this subset is absent or very rare before birth and among newborns [10], [14].
7. The immunological status of newborns and early neonatal development
There is considerable evidence that the immune system of neonates is functionally different from that of adults. Noninvasive epitheliochorial form of placentation results in fetal pigs and newborns that are devoid of maternal antibodies. Limited or no external antigenic stimuli during fetal life results in the absence or very rare occurrence of effector/memory T cells [10], [14], [33], [43]. The B cell pool is also immature and consists of a uniform batch of unprimed B cells that are CD2+CD21+ [8], [10], [23]. All of these factors result in a systemic as well as a mucosal adaptive immune system in the neonatal piglet that is primitive compared to adult pigs [63]. This is consistent with the low level of peripheral lymphocytes, underdeveloped lymphoid nodes, rudimentary jejunal Peyers patches and the low numbers of effector/memory immune cells.
Changes in activation of immune system by antigenic stimuli by commensal microbial flora, pathogens and environmental influences result in the appearance of conventional, activated T and B cells and the influx and expansion of mucosal immune cells and those in the peripheral lymphoid pool. This phenomenon is particularly evident in experiments using isolator piglets raised in germ-free conditions. In such animals, relatively little development of the immune system occurs in comparison with conventional littermates of the same age [10], [39], [64], [65]. Moreover, germ-free animals are even unable to mount antibody responses to model T-dependent or T-independent antigens in contrast to colonized isolator piglets [66]. These observations further demonstrate the necessity of pathogen-associated molecular patterns (PAMPs) to act as the adjuvant for promoting adaptive immunoresponsiveness.
Maturation of adaptive immunity as a consequence of birth, delivery of maternal protective immunity by colostrum and milk, and interaction with environmental antigens, results in appearance of primed T and B cells that further develop into effector and memory progeny. Primed T cells are mostly manifested by elevated level of CD25 expression while their effector/memory offspring include MHC-II+ positive subsets, namely CD4+CD8α+ αβ T cell and CD2+CD8α+ γδ T cells [10], [14], [33], [43]. At the B cells level, priming results in loss of CD2 expression [32], [34]. The resultant CD2− CD21+ B cells without further stimulation rapidly re-express CD2 on their surface but once stimulated, they proliferate and mature into CD21− B cell compartment. CD21− B cells, either CD2+ or CD2− are able to generate proliferating uHC− plasmablast or non-proliferating uHC− plasma cells that are terminal stages of B cell maturation [34], [65]. Also remarkable is the divergence of the pattern antibody repertoire in that ∼80% of all VH genes in conventional pigs are no longer identifiable as one of the seven VH genes that comprise >95% of the pre-immune repertoire because they have undergone somatic hyper-mutations [28].
Acknowledgment
The studies described here have been mostly carried out in the Department of Immunology and Gnotobiology of the Institute of Microbiology v.v.i., Academy of Sciences of the Czech Republic and were financially supported by a grant 524/07/0087 and 523/07/0088 from the Grant Agency of the Czech Republic and the Institutional Research Concept no. AV0Z50200510.
References
- 1.Marrable A.W. Pitman Medical Publishing; Great Britain: 1971. The embryonic pig: a chronological account. [Google Scholar]
- 2.Sterzl J., Silverstein A.M. Developmental aspects of immunity. Adv Immunol. 1967;6(1):337–371. doi: 10.1016/s0065-2776(08)60525-8. [DOI] [PubMed] [Google Scholar]
- 3.Butler J.E., Brown W.R. The immunoglobulins and immunoglobulin genes of swine. Vet Immunol Immunopathol. 1994;43(1-3):5–12. doi: 10.1016/0165-2427(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 4.Tlaskalova-Hogenova H., Mandel L., Trebichavsky I., Kovaru F., BArot R., Sterzl J. Development of immune responses in early pig ontogeny. Vet Immunol Immunopathol. 1994;43(1-3):135–142. doi: 10.1016/0165-2427(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 5.Butler J.E., Sun J., Weber P., Ford S.P., Rehakova Z., Sinkora J. Antibody repertoire development in fetal and neonatal piglets. IV. Switch recombination, primarily in fetal thymus occurs independent of environmental antigen and is only weakly associated with repertoire diversification. J Immunol. 2001;167(6):3239–3249. doi: 10.4049/jimmunol.167.6.3239. [DOI] [PubMed] [Google Scholar]
- 6.Jungersen G., Bille-Hansen V., Jensen L., Lind P. Transplacental transmission of Toxoplasma gondii in minipigs infected with strains of different virulence. J Parasitol. 2001;87(1):108–113. doi: 10.1645/0022-3395(2001)087[0108:TTOTGI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Muller A., Medvinsky A., Strouboulis J., Grosveld F., Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1(4):291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 8.Sinkora M., Sun J., Sinkorova J., Christenson R.K., Ford S.P., Butler J.E. Antibody repertoire development in fetal and neonatal piglets. VI. B-cell lymphogenesis occurs at multiple sites with differences in the frequency of in-frame rearrangements. J Immunol. 2003;170(4):1781–1788. doi: 10.4049/jimmunol.170.4.1781. [DOI] [PubMed] [Google Scholar]
- 9.Trebichavsky I., Tlaskalova H., Cukrowska B., Splichal I., Sinkora J., Rehakova Z. Early ontogeny of immune cells and their functions in the fetal pig. Vet Immunol Immunopathol. 1996;54(1-4):75–81. doi: 10.1016/s0165-2427(96)05707-8. [DOI] [PubMed] [Google Scholar]
- 10.Sinkora M., Sinkora J., Rehakova Z., Splichal I., Yang H., Parkhouse R.M. Prenatal ontogeny of lymphocyte subpopulations in pigs. Immunology. 1998;95(4):595–603. doi: 10.1046/j.1365-2567.1998.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huh N.D., Kim Y.B., Koren H.S., Amos D.B. Natural killing and antibody-dependent cellular cytotoxicity in specific-pathogen-free miniature swine and germ-free piglets. II. Ontogenic development of NK and ADCC. Int J Cancer. 1981;28(2):175–178. doi: 10.1002/ijc.2910280210. [DOI] [PubMed] [Google Scholar]
- 12.Yang W.C., Schultz R.D. Ontogeny of natural killer cell activity and antibody dependent cell mediated cytotoxicity in pigs. Dev Comp Immunol. 1986;10(3):405–418. doi: 10.1016/0145-305x(86)90030-3. [DOI] [PubMed] [Google Scholar]
- 13.Splichal I., Bonneau M., Charley B. Ontogeny of interferon alpha secreting cells in the porcine fetal hematopoietic organs. Immunol Lett. 1994;43(3):203–208. doi: 10.1016/0165-2478(94)90224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinkora M., Sinkora J., Rehakova Z., Butler J.E. Early ontogeny of thymoctes in pigs: sequential colonization of the thymus by T cell progenitors. J Immunol. 2000;165(4):1832–1839. doi: 10.4049/jimmunol.165.4.1832. [DOI] [PubMed] [Google Scholar]
- 15.Hardy R.R., Carmack C.E., Shinton S.A., Kemp J.D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehlich A., Schaal S., Gu H., Kitamura D., Müller W., Rajewsky K. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993;72(5):695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- 17.Ghia P., ten Boekel E., Sanz E., de la Hera A., Rolink A., Melchers F. Ordering of human bone marrow B lymphocyte precursors by single-cell polymerase chain reaction analyses of the rearrangement status of the immunoglobulin H and L chain gene loci. J Exp Med. 1996;184(6):2217–2229. doi: 10.1084/jem.184.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolink A.G., ten Boekel E., Yamagami T., Ceredig R., Andersson J., Melchers F. B cell development in the mouse from early progenitors to mature B cells. Immunol Lett. 1999;68(1):89–93. doi: 10.1016/s0165-2478(99)00035-8. [DOI] [PubMed] [Google Scholar]
- 19.Hardy R.R., Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 20.Melchers F., Karasuyama H., Haasner D., Bauer S., Kudo A., Sakaguchi N. The surrogate light chain in B-cell development. Immunol Today. 1993;14(2):60–68. doi: 10.1016/0167-5699(93)90060-X. [DOI] [PubMed] [Google Scholar]
- 21.King L.B., Monroe J.G. Immunobiology of the immature B cell: plasticity in the B-cell antigen receptor-induced response fine tunes negative selection. Immunol Rev. 2000;176(1):86–104. doi: 10.1034/j.1600-065x.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- 22.Butler J.E., Sun J., Navarro P. The swine Ig heavy chain locus has a single JH and no identifiable IgD. Int Immunol. 1996;8(12):1897–1904. doi: 10.1093/intimm/8.12.1897. [DOI] [PubMed] [Google Scholar]
- 23.Reynaud C.A., Imhof B.A., Anquez V., Weill J.C. Emergence of committed B lymphoid progenitors in the developing chicken embryo. EMBO J. 1992;11(12):4349–4358. doi: 10.1002/j.1460-2075.1992.tb05534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormack W.T., Tjoelker L.W., Thompson C.B. Avian B-cell development: generation of an immunoglobulin repertoire by gene conversion. Annu Rev Immunol. 1991;9(1):219–241. doi: 10.1146/annurev.iy.09.040191.001251. [DOI] [PubMed] [Google Scholar]
- 25.Sinkora J., Rehakova Z., Sinkora M., Cukrowska B., Tlaskalova-Hogenova H. Early development of immune system in pigs. Vet Immunol Immunopathol. 2002;87(3-4):301–306. doi: 10.1016/s0165-2427(02)00056-9. [DOI] [PubMed] [Google Scholar]
- 26.Nourrit F., Doyen N., Kourilsky P., Rougeon F., Cumano A. Extensive junctional diversity of Ig heavy chain rearrangements generated in the progeny of single fetal multipotent hematopoietic cells in the absence of selection. J Immunol. 1998;160(9):4254–4261. [PubMed] [Google Scholar]
- 27.Knight K.L., Crane M.A. Generating the antibody repertoire in rabbit. Adv Immunol. 1994;56(1):179–218. doi: 10.1016/s0065-2776(08)60452-6. [DOI] [PubMed] [Google Scholar]
- 28.Butler J.E., Weber P., Wertz N. Antibody repertoire development in fetal and neonatal piglets. XIII. Hybrid VH genes and the preimmune repertoire revisited. J Immunol. 2006;177(8):5459–5470. doi: 10.4049/jimmunol.177.8.5459. [DOI] [PubMed] [Google Scholar]
- 29.Butler J.E., Weber P., Sinkora M., Sun J., Ford S.J., Christenson R. Antibody repertoire development in fetal and neonatal piglets. II. Characterization of heavy chain CDR3 diversity in the developing fetus. J Immunol. 2000;165(12):6999–7011. doi: 10.4049/jimmunol.165.12.6999. [DOI] [PubMed] [Google Scholar]
- 30.Butler J.E., Wertz N., Wang H., Sun J., Chardon P., Piumi F. Antibody repertoire development in fetal and neonatal pigs. VII. Characterization of the pre-immune kappa light chain repertoire. J Immunol. 2004;173(11):6794–6805. doi: 10.4049/jimmunol.173.11.6794. [DOI] [PubMed] [Google Scholar]
- 31.Jones M., Cordell J.L., Beyers A.D., Tse A.G.D., Mason D.Y. Detection of T and B cells in many animal species using cross-reactive anti-peptide antibodies. J Immunol. 1993;150(12):5429–5435. [PubMed] [Google Scholar]
- 32.Sinkora J., Rehakova Z., Sinkora M., Cukrowska B., Tlaskalova-Hogenova H., Bianchi A.T. Expression of CD2 on porcine B lymphocytes. Immunology. 1998;95(3):443–449. doi: 10.1046/j.1365-2567.1998.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinkora M., Sinkorova J., Cimburek Z., Holtmeier W. Two groups of porcine TCRγδ+ thymocytes behave and diverge differently. J Immunol. 2007;178(2):711–719. doi: 10.4049/jimmunol.178.2.711. [DOI] [PubMed] [Google Scholar]
- 34.Sinkora M., Butler J.E., Holtmeier W., Sinkorova J. Lymphocyte development in fetal piglets: facts and surprises. Vet Immunol Immunopathol. 2005;108(1–2):177–184. doi: 10.1016/j.vetimm.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Faldyna M., Samankova P., Leva L., Cerny J., Oujezdska J., Rehakova Z. Cross-reactive anti-human monoclonal antibodies as a tool for B-cell identification in dogs and pigs. Vet Immunol Immunopathol. 2007;119(1–2):56–62. doi: 10.1016/j.vetimm.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Wilson S.M., Wilkie B.N. B-1 and B-2 B-cells in the pig cannot be differentiated by expression of CD5. Vet Immunol Immunopathol. 2007;115(1–2):10–16. doi: 10.1016/j.vetimm.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 37.McAleer J., Weber P., Sun J., Butler J.E. Antibody repertoire development in fetal and neonatal piglets. XI. The thymic B cell repertoire develops independently from that in blood and mesenteric lymph nodes. Immunology. 2005;114(2):171–183. doi: 10.1111/j.1365-2567.2004.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y., Pan-Hammarstrom Q., Kacskovics I., Hammarstrom L. The porcine Igδ gene: Unique chimeric splicing of the first constant region domain in its heavy chain transcripts. J Immunol. 2003;171(3):1312–1318. doi: 10.4049/jimmunol.171.3.1312. [DOI] [PubMed] [Google Scholar]
- 39.Butler J.E., Sinkora M. The isolator piglet: a model for studying the development of adaptive immunity. Immunol Res. 2007;39(1–3):33–51. doi: 10.1007/s12026-007-0062-7. [DOI] [PubMed] [Google Scholar]
- 40.Cukrowska B., Sinkora J., Mandel L., Splichal I., Bianchi A.T.J., Kovaru F. Thymic B cells of pig fetuses and germ free pigs spontaneously produce IgM. IgG and IgA: detection of ELISPOT method. Immunology. 1996;87(3):487–492. doi: 10.1046/j.1365-2567.1996.499573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jotereau F., Heuze F., Salomon-Vie V., Gascan H. Cell kinetics in the fetal mouse thymus: precursor cell input, proliferation, and emigration. J Immunol. 1987;138(4):1026–1030. [PubMed] [Google Scholar]
- 42.Coltey M., Jotereau F.V., Le Douarin N.M. Evidence for a cyclic renewal of lymphocyte precursor cells in the embryonic chick thymus. Cell Differ. 1987;22(1):71–82. doi: 10.1016/0045-6039(87)90414-3. [DOI] [PubMed] [Google Scholar]
- 43.Sinkora M., Sinkorova J., Holtmeier W. Development of γδ thymocyte subsets during prenatal and postnatal ontogeny. Immunology. 2005;115(4):544–555. doi: 10.1111/j.1365-2567.2005.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Offner F., Van Beneden K., Debacker V., Vanhecke D., Vandekerckhove B., Plum J. Phenotypic and functional maturation of TCRγδ cells in the human thymus. J Immunol. 1997;158(10):4634–4641. [PubMed] [Google Scholar]
- 45.Itohara S., Nakanishi N., Kanagawa O., Kubo R., Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor γδ: analysis of γδ T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci USA. 1989;86(13):5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher A.G., Ceredig R. γδ T cells expressing CD8 or CD4low appear early in murine foetal thymus development. Int Immunol. 1991;3(12):1323–1328. doi: 10.1093/intimm/3.12.1323. [DOI] [PubMed] [Google Scholar]
- 47.Bucy R.P., Chen C.H., Cooper M.D. Ontogeny of T cell receptors in the chicken thymus. J Immunol. 1990;144(4):1161–1168. [PubMed] [Google Scholar]
- 48.Aparicio P., Alonso J.M., Toribio M.L., Marcos M.A., Pezzi L., Martínez A.C. Isolation and characterization of γδ CD4+ T cell clones derived from human fetal liver cells. J Exp Med. 1989;170(3):1009–1014. doi: 10.1084/jem.170.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wucherpfennig K.W., Liao Y.J., Prendergast M., Prendergast J., Hafler D.A., Strominger J.L. Human fetal liver γ/δ T cells predominantly use unusual rearrangements of the T cell receptor δ and γ loci expressed on both CD4+CD8− and CD4−CD8− γ/δ T cells. J Exp Med. 1993;177(2):425–432. doi: 10.1084/jem.177.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saalmuller A., Hirt W., Reddehase M.J. Porcine γδ T lymphocyte subsets differing in their propensity to home to lymphoid tissue. Eur J Immunol. 1990;20(10):2343–2346. doi: 10.1002/eji.1830201026. [DOI] [PubMed] [Google Scholar]
- 51.Yang H., Parkhouse R.M. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology. 1996;89(1):76–83. doi: 10.1046/j.1365-2567.1996.d01-705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reddehase M.J., Saalmuller A., Hirt W. γδ T-lymphocyte subsets in swine. Curr Top Microbiol Immunol. 1991;173(1):113–117. doi: 10.1007/978-3-642-76492-9_16. [DOI] [PubMed] [Google Scholar]
- 53.Yang H., Parkhouse R.M. Differential expression of CD8 epitopes amongst porcine CD8-positive functional lymphocyte subsets. Immunology. 1997;92(1):45–52. doi: 10.1046/j.1365-2567.1997.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Bruin M.G., van Rooij E.M., Voermans J.J., de Visser Y.E., Bianchi A.T., Kimman T.G. Establishment and characterization of porcine cytolytic cell lines and clones. Vet Immunol Immunopathol. 1997;59(3-4):337–347. doi: 10.1016/s0165-2427(97)00085-8. [DOI] [PubMed] [Google Scholar]
- 55.MacDonald H.R., Schreyer M., Howe R.C., Bron C. Selective expression of CD8α (Ly-2) subunit on activated thymic γ/δ cells. Eur J Immunol. 1990;20(4):927–930. doi: 10.1002/eji.1830200431. [DOI] [PubMed] [Google Scholar]
- 56.Moebius U., Kober G., Griscelli A.L., Hercend T., Meuer S.C. Expression of different CD8 isoforms on distinct human lymphocyte subpopulations. Eur J Immunol. 1991;21(8):1793–1800. doi: 10.1002/eji.1830210803. [DOI] [PubMed] [Google Scholar]
- 57.Hori T., Paliard X., de Waal Malefijt R., Ranes M., Spits H. Comparative analysis of CD8 expressed on mature CD4+ CD8+ T cell clones cultured with IL-4 and that on CD8+ T cell clones: implication for functional significance of CD8β. Int Immunol. 1991;3(7):737–741. doi: 10.1093/intimm/3.7.737. [DOI] [PubMed] [Google Scholar]
- 58.Spetz A.L., Kourilsky P., Larsson-Sciard E.L. Induction of CD8 molecules on thymic γ/δ T cells in vitro is dependent upon α/β T cells. Eur J Immunol. 1991;21(11):2755–2759. doi: 10.1002/eji.1830211116. [DOI] [PubMed] [Google Scholar]
- 59.Saalmuller A., Werner T., Fachinger V. T-helper cells from naive to committed. Vet Immunol Immunopathol. 2002;87(3-4):137–145. doi: 10.1016/s0165-2427(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 60.Sinkora M., Sun J., Butler J.E. Antibody repertoire development in fetal and neonatal piglets. V. VDJ gene chimeras resembling gene conversion products are generated at high frequency by PCR in vitro. Mol Immunol. 2000;37(17):1025–1034. doi: 10.1016/s0161-5890(01)00022-0. [DOI] [PubMed] [Google Scholar]
- 61.Saalmuller A., Hirt W., Reddehase M.J. Phenotypic discrimination between thymic and extrathymic CD4−CD8− and CD4+CD8+ porcine T lymphocytes. Eur J Immunol. 1989;19(11):2011–2016. doi: 10.1002/eji.1830191107. [DOI] [PubMed] [Google Scholar]
- 62.Zuckermann F.A., Husmann R.J. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology. 1996;87(3):500–512. [PMC free article] [PubMed] [Google Scholar]
- 63.Pabst R., Rothkötter H.J. Postnatal development of lymphocyte subsets in different compartments of the small intestine of piglets. Vet Immunol Immunopathol. 1999;72(1-2):167–173. doi: 10.1016/s0165-2427(99)00129-4. [DOI] [PubMed] [Google Scholar]
- 64.Butler J.E., Sun J., Weber P., Navarro P., Francis D. Antibody repertoire development in fetal and newborn piglets, III. Colonization of the gastrointestinal tract selectively diversifies the preimmune repertoire in mucosal lymphoid tissues. Immunology. 2000;100(1):119–130. doi: 10.1046/j.1365-2567.2000.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butler J.E., Lemke C.D., Weber P., Sinkora M., Lager K.M. Antibody repertoire development in fetal and neonatal piglets. XIX. Undiversified B cells with hydrophobic HCDR3s preferentially proliferate in the porcine reproductive and respiratory syndrome. J Immunol. 2007;178(10):6320–6331. doi: 10.4049/jimmunol.178.10.6320. [DOI] [PubMed] [Google Scholar]
- 66.Butler J.E., Weber P., Sinkora M., Baker D., Schoenherr A., Mayer B. Antibody repertoire development in fetal and neonatal piglets. VIII. Colonization is required for newborn piglets to make serum antibodies to T-dependent and type 2 T-independent antigens. J Immunol. 2002;169(12):6822–6830. doi: 10.4049/jimmunol.169.12.6822. [DOI] [PubMed] [Google Scholar]