Abstract
Infectious bronchitis virus (IBV), the first coronavirus described, has been a continuing problem in poultry for more than 70 years. IBV, causing a highly contagious respiratory disease in chickens, resembles the recently described severe acute respiratory syndrome virus in pathogenesis and genome organization. While previous studies demonstrated that effector and memory CD8+ T lymphocytes are critical in controlling acute IBV infection and disease in chickens, here chicken anti-IBV antibody (IgG) secreting cells (ASC) in both peripheral blood mononuclear cells (PBMC) and spleens collected following IBV Gray infection were evaluated using an ELISPOT assay. The ASC in peripheral blood and spleens can be detected from 3 to 7 days post-infection (p.i.), which is 3–7 days earlier than anti-IBV IgG detected in the serum. The ASC frequency reached a maximum at 7–10 days p.i., and decreased more than 90% in the spleen and 70% in PBMC by 14 days p.i. The ASC levels in the PBMC then decreased gradually to 0.5 ASC/106 over the next 8 weeks. The higher concentration of about 20 ASC/106 cells in spleens may, at least partially, account for the presence of antibody in the serum although bone marrow ASC were not determined. In vitro stimulation of PBMC and splenocytes with IBV antigen demonstrated that memory B cells can be activated to secrete antibody by 3 weeks p.i. ELISPOT detection of primary B cells could be useful in the early detection of infection following infection with respiratory coronaviruses.
Keywords: Coronavirus, Chicken, Antibody secreting cell, ELISPOT, Memory B cell
1. Introduction
It has been recently shown that the highly contagious severe acute respiratory syndrome (SARS) in humans is caused by a coronavirus (CoV) that resembles infectious bronchitis in transmission, pathogenesis and genome structure [10], [19], [26]. Infectious bronchitis virus (IBV) infection causes a highly contagious respiratory disease in chickens, especially in young chicks [3], [5]. The disease was first described in 1931, and has since remained a major problem in the poultry industry worldwide [3], [13]. Vaccines are available, but they are not effective long-term in controlling IBV infection, especially for variant strains. Genetic variations are common in new strains because of both point mutations and recombinants [14], [15], [40], [41]. The many years of experience dealing with IBV should provide a valuable model for understanding SARS CoV infection in humans.
Recent studies have shown that effector CD8+ T cells are critical in controlling acute IBV infection [6], [9], [30]. Adoptive transfer of T cells collected at 10 days post-infection (p.i.) protected syngenic chicks from clinical illness [30]. IBV specific memory T cells can be generated at 3 weeks p.i., and adoptive transfer of the memory T cells passed protection to the recipient chicks [22]. Innate immunity may also be instrumental in controlling IBV infection. Chicken interferon type I (ChIFN-I) inhibits IBV replication in vitro and in vivo [23]. Local administration of ChIFN-I inhibited IBV associated respiratory illness [23]. The importance of humoral immunity was indicated by Cook et al. (1991), who demonstrated that after IBV infection, bursectomised chicks suffered more severe and longer illness than intact chicks. The viral titers in tissues were also higher and lasted longer in bursectomised chicks than in normal chicks [7].
Individual antibody secreting cells (ASC) and activated T cells can be detected using ELISPOT assays, powerful tools for quantifying individual cell responses [1], [27], [28], [35], [42]. In the current experiments, IBV specific IgG secreting cells were detected in peripheral blood and spleens using an ELISPOT assay, while memory B cells were detected after antigen stimulation.
2. Materials and methods
2.1. Animals and virus
SPAFAS specific, pathogen-free (SPF) chickens were hatched in our laboratory and housed in an SPF environment at the Laboratory Animal Resources and Research Facility (Texas A&M University, College Station, TX). Immune chickens were generated by inoculating 1-week-old chickens with 107 EID50 of the IBV Gray strain by the eye–nasal routes. The virus, propagated by inoculating the allantoic sac of 11-day-old chicken embryos with the Gray strain of IBV and harvesting allantoic fluid 36 h p.i., was used for in vivo inoculation [34]. The IBV antigen used in the ELISA and ELISPOT assay was purified by polyethylene glycol (PEG) 8000 precipitation. Briefly, allantoic fluid collected at 36 h p.i. was centrifuged at 10,000 rpm for 25 min to remove any cells and cell debris. Sodium chloride (2.33%) and PEG 8000 (7%) were added to the supernatant and incubated overnight at 4 °C. The virus was collected by centrifuging at 12,000 rpm for 40 min and resuspended in PBS (pH 7.4).
2.2. Cell culture and antigen stimulation
Peripheral blood mononuclear cells (PBMC) and spleen cells were prepared at varying times p.i. [22]. Briefly, 0.5 ml of blood was collected from each of three chicks at each time point through a wing vein with a 1-ml syringe containing anticoagulant EDTA-K3 and mixed with an equal volume of PBS. PBMC were isolated by centrifuging the diluted blood for 20 min at 2000 rpm through Histopaque-1077 (Sigma). The cells collected from the interface were pooled for each group, washed three times and cultured in complete RPMI-1640 in six-well plates at a density of 107 cells/ml. Single splenocyte suspensions were prepared as described [22], [23]. To detect memory responses, the PBMC and splenocytes were stimulated with UV-inactivated IBV Gray strain (107.3 EID50/well). One hundred microliters of cell suspension were collected at 3, 6, 9, and 12 days after stimulation. The cells were washed three times with RPMI-1640 before using in an IgG ELISPOT assay. The supernatants were saved for antibody detection.
2.3. Detection of antibody by ELISA
One-week-old chicks were infected with the IBV Gray strain through the eyes and nose. Sera were collected from three chicks at various times p.i., and antibody responses were determined using ELISA [21]. Briefly, 96-well microtiter ELISA plates (Nunc Maxisorb) were coated with 100 μl/well of purified IBV antigen, diluted in bicarbonate/carbonate buffer (2.93 g NaHCO3, 1.59 g Na2CO3, 0.203 g MgCl2 in 1 l of distilled water, pH 9.6) at a concentration of 10 μg/ml. The plates were incubated overnight at 4 °C before blocking with 5% non-fat milk in PBS (pH 7.4) for 1 h at room temperature or overnight at 4 °C. After washing the plates three times with PBS (pH 7.4) and 0.1% Tween 20 (PBS-T), the chicken sera were diluted to 1:200 in PBS-T with 5% FBS and added to the ELISA plates. The plates were incubated at room temperature for 1 h and washed again with PBS-T. Secondary antibody (goat anti-chicken IgG horseradish peroxidase conjugate) diluted to 1:5000 in PBS-T with 5% FBS was added and the plates were incubated at room temperature for 1 h before thoroughly washing with PBS-T. The color was developed by adding 100 μl/well of ABTS one component microwell peroxidase substrate (Kirkegaard and Perry Laboratories, Inc.) and optical densities (OD) were read at 630 nm with an ELISA reader (Emax Precision Microplate Reader, Molecular Devices Corporation).
2.4. ELISPOT assay
Nitrocellulose bottomed 96-well multiscreen filtration plates (Millipore Corporation) were coated with 100 μl/well of purified IBV antigen (5 μg/ml) and incubated overnight at 4 °C [31]. Plates were then washed with PBS and blocked with 10% FCS in PBS for 1 h at room temperature. The cells were diluted in 100 μl complete RPMI-1640 were added to each well and incubated overnight at 37 °C, 5% CO2. The cells were removed by rinsing with PBS, and secondary antibody (goat anti-chicken IgG–horseradish peroxidase conjugate), diluted to 1:2000 in PBS-T with 5% FBS, was added before incubating at room temperature for 1 h. TMB membrane peroxidase substrate (Kirkegaard and Perry Laboratories, Inc.) was used to develop the spots representing ASC. The spots were counted under a dissecting microscope [31]. The ASC frequency (ASC/106 cells) is equal to ASC number from infected chickens minus the ASC number from uninfected chickens (background).
3. Results
3.1. Anti-IBV IgG responses detected in serum
It has been shown that cell-mediated immunity can control acute IBV infection in chickens [6], [9], [22], [29]. However, the role of humoral immune responses in resolving IBV infection is not clearly defined [7], [8], [11], [20], [24]. To detect B cell antibody responses, 50 one-week-old chicks were divided into two groups. Twenty-five chicks in Group 1 were infected with the IBV Gray strain and 25 uninfected chicks in Group 2 were used as controls. Sera were collected randomly from three of the 25 chicks from each group at varying times p.i., and anti-IBV IgG was determined by ELISA (Fig. 1 ). Low levels of serum anti-IBV IgG were detected at 7 days p.i. Relatively low levels of antibody specific for IBV were detected in all infected chicks by 10 days p.i. However, the antibody titers reached maximum levels at 2 weeks p.i. and remained positive until at least week 10 p.i., the last week examined (Fig. 1).
Fig. 1.
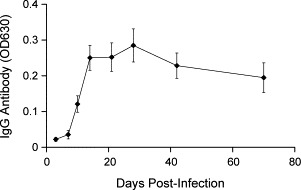
Serum anti-IBV IgG. Sera were collected from three infected chicks at indicated times p.i. and anti-IBV IgG levels were detected by ELISA. The values represent the means±SD (n=3) of OD630.
3.2. ASC detected in peripheral blood and spleens
To determine the time course of specific ASC in peripheral blood and spleen, PBMC and splenocytes were prepared from three infected and uninfected chicks at various times p.i. Concentrations of 107 and 106 cells/ml were prepared with complete RPMI-1640. One hundred microliters of the cell suspensions were added to IBV antigen coated plates, and the ASC were detected using an antibody ELISPOT assay (Fig. 2 ). Low numbers of specific ASC were identified in spleens and PBMC by 3 and 7 days p.i., respectively (Fig. 3 ), 3–7 days earlier than specific IgG was detected in sera (Fig. 1). The ASC frequencies were maximum (592.5±364.2 ASC/106 cells) in spleens at 7 days p.i., then sharply decreased more than 20-fold to 25±9.9 ASC/106 cells by 14 days p.i. This level of ASC was maintained in spleens until week 10 p.i., the last week examined (Fig. 3). The numbers of ASC in PBMC followed the same kinetics as in the spleens, but at much lower frequencies, reaching a maximum at 10 days p.i. with only 69±4.2 detectable ASC/106 PBMC. The levels of IBV specific IgG producing cells declined to 15±7.1 ASC/106 cells at 14 days p.i. and continued to gradually decrease to 0.5 ASC/106 cells at 10 weeks p.i. (Fig. 3).
Fig. 2.
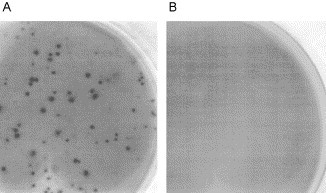
ELISPOT assay detects anti-IBV IgG secreting cells. (A) Anti-IBV secreting cells detected from infected chicken PBMC. (B) Control PBMC from uninfected chickens.
Fig. 3.
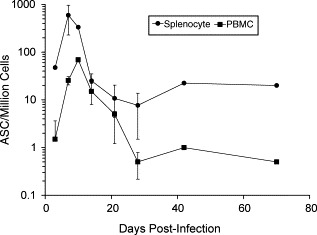
Antibody secreting cells (ASC) detected in PBMC and spleens. PBMC and spleen cells were collected and pooled from three chickens at indicated times after IBV infection. The ASC were detected by an ELISPOT assay. The results equal the means±SD of two separate experiments.
3.3. Memory B cells can be detected at 3 weeks p.i.
Memory B cells are identified on the basis of their ability to proliferate and differentiate, and their dependence on stimulation by antigen for recall of antibody production [31]. To detect memory B cells in IBV infected chickens, PBMC were collected from the chickens at 3 and 10 weeks p.i., and stimulated in vitro with the IBV Gray whole virus antigen. The antibody titers in the cell supernatants were detected with ELISA at 3, 6, 9, and 12 days post-stimulation (p.s.) (Fig. 4 A). To determine the frequency of the ASC in the cell cultures, 106 cells in 100 μl of media were collected and washed with RPMI-1640 after stimulating in vitro for 3, 6, 9, and 12 days (Fig. 4B).
Fig. 4.
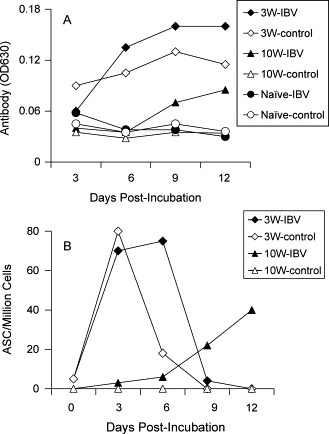
Memory B cells can be detected at 3 weeks p.i. PBMC were collected and pooled from three IBV infected chickens at 3 weeks (3W) and 10 weeks (10W) p.i. The cells were stimulated in vitro with IBV Gray strain antigen (IBV) or without antigen stimulation (Control) for 12 days. (A) The antibody titers in the culture supernatants were detected by ELISA at indicated times. (B) The ASC frequencies in the cultured cells were detected by an ELISPOT assay. The results are representative of two separate experiments.
PBMC collected from chickens at 3 weeks p.i. secreted IBV specific antibody even without in vitro antigen stimulation, suggesting that plasma cells were present in the peripheral blood at this time (Fig. 4A). However, higher levels of antibody were detected after antigen stimulation (Fig. 4A), indicating that memory B cells were generated at 3 weeks p.i. The antibody levels in the stimulated cultures reached a plateau at 9 days p.s. that lasted for at least 3 days. The ELISPOT assay indicated that the plasma cells replicated after in vitro incubation (without antigen stimulation), but died within 3 days of incubation (Fig. 4B). After in vitro antigen stimulation, ASC were detected at 3–9 days, but were not detected by 12 days p.s. The frequency of the ASC in IBV antigen stimulated cultures was similar to that in unstimulated cultures; however, the ASC survived longer in the presence of in vitro antigen stimulation (Fig. 4B). Although the numbers of ASC were detectable prior to day 9 of stimulation, the anti-IBV antibody could be detected in low levels from cell cultures at 3 days p.s. and reached a maximum after 9 days of antigen stimulation. Therefore, most of the detected antibody was likely produced and accumulated before 9 days p.s. (Fig. 4).
In contrast, antibody was not detected in the supernatants of PBMC collected at 10 weeks p.i. in the absence of IBV antigen stimulation (Fig. 4A). As expected, antibody was detected in the supernatants of cells collected from chicks at 10 weeks p.i., following in vitro stimulation with IBV antigen (Fig. 4A). The ASC frequency detected by ELISPOT assay correlated with antibody production in the supernatants with in vitro stimulation (Fig. 4A, B).
3.4. Memory B cells detected in spleens
Previous studies showed that memory T cells can be detected in peripheral blood and spleens [22]. In the present study, low frequencies of IBV specific plasma cells were detected in the spleens after the infection was resolved (Fig. 3), and the memory B cells were detected even at 10 weeks p.i. (Fig. 4). To detect memory B cells in spleens, splenocytes were collected at 10 weeks p.i. and stimulated in vitro with the IBV Gray strain antigen. The results indicated that the memory spleen cells secreted antibody only after antigen stimulation (Fig. 5 A). Antibody could neither be detected from splenocytes without antigen stimulation nor from splenocytes of uninfected chickens (Fig. 5A). Furthermore, ELISPOT assays demonstrated that ASC could be detected only in antigen stimulated cultures (Fig. 5B), not in unstimulated cultures (data not shown).
Fig. 5.
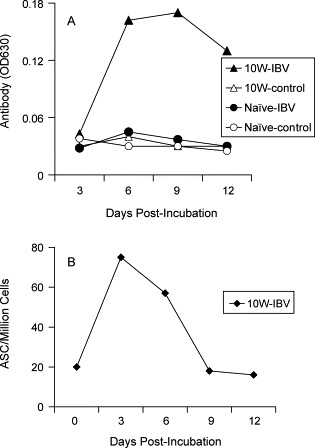
Memory B cells can be detected in spleens at 10 weeks p.i. Spleen cells were collected and pooled from three IBV infected chickens (10W) and uninfected chicken (Naive) at 10 weeks p.i., and stimulated in vitro with IBV antigen (IBV) or without antigen stimulation (Control) for 12 days. (A) The antibody titers in the culture supernatants were detected by ELISA at indicated time points. (B) The ASC frequencies in the cultured cells were detected by an ELISPOT assay. The results are representative of two separate experiments.
4. Discussion
Effector and memory CD8+ T cells play critical roles in controlling IBV acute infection [22]. However, the precise role of antibody in the control of IBV infection remains controversial. Some reports have shown that circulating antibody titer did not correlate with protection from IBV infection [11], [12], [24]. However, other studies demonstrated that humoral immunity plays an important role in disease recovery and virus clearance [7], [37], [38]. In our own studies, although the transfer of MHC compatible immune T cells eliminated initial illness and viral replication, mild illness was observed following the acute stage of infection [22], [30]. Therefore, total recovery involves other mechanisms in addition to T cells. The current study revealed that the ASC frequency in spleens and peripheral blood reached maximum levels between 7 and 10 days p.i., correlating with the maximum virus load in the lungs [29], suggesting that ASC are available early in chicks after IBV infection, although their role in antibody production at this time is not know.
Previous studies have evaluated antibody and not the B cells producing specific antibody. This is the first report enumerating the effector and memory ASC specific for a respiratory coronavirus. In this study, the frequency of anti-IBV ASC in PBMC and spleens, detected by an ELISPOT assay, implicated anti-IBV IgG secreting cells and subsequent accumulation of antibody in recovery. Memory B cells in peripheral blood and spleens could be detected by 3 weeks, and as late as 10 weeks, p.i. This is also the first report of direct detection of avian memory B cells, and the evaluation of the frequencies of avian antibody secreting B cells in peripheral blood. Wu et al. detected ASC in chicken spleens after infectious bursal disease virus (IBDV) infection using ELISPOT assay [42]. Although they did not determine the dynamics of the generation of ASC after virus infection, the IgG ASC frequency at 14 days p.i. (22.67±6.11 ASC/106 splenocytes) was similar to our results (25±9.9 ASC/106 splenocytes).
Because by definition, memory B cells do not secret antibody without recall antigen stimulation [31], the ASC we detected in the PBMC and spleen cells without IBV antigen stimulation would be plasma cells (Fig. 2). Similar to the T cell responses described in chickens [22], [29], three distinct stages can be observed in the chicken B cell response: expansion, decreases in numbers, and memory (Fig. 3). At the first stage, the IBV specific ASC expanded dramatically and reached a maximum between 7 and 10 days p.i. During this time, IBV in vivo infection increases to its maximum titer [6]. After 10 days infection, the virus was cleared by the host immune system. As in T cell responses, following antigen depletion, the IBV specific ASC response entered the second stage when the numbers decreased precipitously, perhaps as a result of apoptosis. In the third stage, a low level of residual memory B cells were generated and could be detected at 3 weeks p.i., similar to the maintenance of memory T cells [22].
It has been reported in mammals that long-lived plasma cells can be detected in bone marrow and spleen [18], [33], [36]. The antibody level in serum was maintained by these long-lived plasma cells [33] that survive independent of antigen stimulation [16], [17]. In this study, we also detected a low level (∼20 ASC/106) of plasma cells in chicken spleens up to at least 18 weeks p.i. (data not shown), and their survival was likely independent of antigen stimulation because IBV was cleared after 2 weeks p.i. [6]. Therefore, we presume that these cells were long-lived plasma cells as in mammals. These plasma cells could maintain the anti-IBV IgG in the serum. However, future studies should quantify the ASC, especially long-lived plasma cells, in chicken bone marrow [32].
IBV infected chicks showed the greatest clinical illness between 5 and 10 days p.i., and the viral load in the lungs reached maximum levels by 7–10 days p.i. [29]. Interestingly, the ASC frequency reached a maximum at 7–10 days p.i., while the antibody in the sera was barely detectable at 7 days p.i. and became unquestionably positive only by 10 days p.i. or beyond. When the ASC frequencies were decreasing between 10 and 14 days, the serum antibody was increasing. Therefore, serum antibody did not correlate with clinical illness or viral titers. However, the ASC frequency in this study and effector T cells shown in previous studies did correlate [6]. Although the lower levels of antibody, detected in the sera at 7–10 days p.i., could be saturated by the presence of maximum levels of virus in vivo, plasma B cells may not have matured until later. The decrease in primary ASC may be a consequence of the decreasing levels of antigen stimulation as the virus is eliminated from the host by cellular mechanisms. It is possible that continued presence of initial B, as well as T, cell numbers depends on the presence of antigen or that activated B cells have migrated elsewhere. While B cell function certainly depends on the levels of secreted specific antibody. Local specific IgA and IgM in the early infection might also be involved in controlling IBV infection, and their role should be addressed in the future [25], [29], [37], [39]. Furthermore, the levels of neutralizing antibody were not examined.
The recent pandemic of SARS has demonstrated that human coronaviruses are potentially a major public health problem [10], [19], [26]. The SARS associated clinical illness, epidemiology, and viral genome structure resemble those that have been described for IBV in chicken for more than 70 years [2], [4], [19], [26]. IBV infection should be the most practical animal model for SARS. RT-PCR is a sensitive method for diagnosis of SARS CoV. Serological testing for specific antibody, including indirect immunofluorescence assays and ELISA, has been developed [4]. However, it typically takes 2–3 weeks for patients to produce detectable antibody. In the current study, we demonstrated that the ELISPOT assay detected ASC 3–7 days earlier than antibody. Therefore, this technique could be used in earlier diagnosis of specific infections including SARS CoV.
Acknowledgements
This work was supported by grants from the US Poultry and Egg Association (297), USDA Formula Animal Health Funds (1433), USDA Cooperative State Research Service (97-35204-5069), and a College of Veterinary Medicine Signature Grant (900004). We are also grateful to support of the Department of Veterinary Pathobiology, Texas A&M University.
References
- 1.Arvilommi H. ELISPOT for detecting antibody-secreting cells in response to infections and vaccination. APMIS. 1996;104:401–410. doi: 10.1111/j.1699-0463.1996.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 2.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P., Ephtimios I.E., Kitai I., Mederski B.D., Shadowitz S.B., Gold W.L., Hawryluck L.A., Rea E., Chenkin J.S., Cescon D.W., Poutanen S.M., Detsky S. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 3.Cavanagh D., Naqi S. Infectious bronchitis. In: Calnek B.W., editor. Diseases of Poultry. 10th ed. Iowa State University; Iowa: 1997. pp. 511–526. [Google Scholar]
- 4.CDC Severe acute respiratory syndrome (SARS) and coronavirus testing—United States, 2003. JAMA. 2003;289:2203–2206. doi: 10.1001/jama.289.17.2203. [DOI] [PubMed] [Google Scholar]
- 5.Collisson E.W., Parr R., Wang L., Williams A.K. An overview of the molecular characteristics of avian infectious bronchitis virus. Poult Sci. 1992:41–55. [Google Scholar]
- 6.Collisson E.W., Pei J., Dzielawa J., Seo S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev Comp Immunol. 2000;24:187–200. doi: 10.1016/s0145-305x(99)00072-5. [DOI] [PubMed] [Google Scholar]
- 7.Cook J.K., Davison T.F., Huggins M.B., McLaughlan P. Effect of in ovo bursectomy on the course of an infectious bronchitis virus infection in line C White Leghorn chickens. Arch Virol. 1991;118:225–234. doi: 10.1007/BF01314032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darbyshire J.H., Peters R.W. Humoral antibody response and assessment of protection following primary vaccination of chicks with maternally derived antibody against avian infectious bronchitis virus. Res Vet Sci. 1985;38:14–21. [PubMed] [Google Scholar]
- 9.Dhinakar R.G., Jones R.C. Cross-reactive cellular immune responses in chickens vaccinated with live infectious bronchitis virus vaccine. Avian Pathol. 1997;26:641–649. doi: 10.1080/03079459708419240. [DOI] [PubMed] [Google Scholar]
- 10.Enserink M. Calling all coronavirologists. Science. 2003;300:413–414. doi: 10.1126/science.300.5618.413. [DOI] [PubMed] [Google Scholar]
- 11.Gelb J., Jr, Nix W.A., Gellman S.D. Infectious bronchitis virus antibodies in tears and their relationship to immunity. Avian Dis. 1998;42:364–374. [PubMed] [Google Scholar]
- 12.Gough R.E., Alexander D.J. Comparison of duration of immunity in chickens infected with a live infectious bronchitis vaccine by three different routes. Res Vet Sci. 1979;26:329–332. [PubMed] [Google Scholar]
- 13.Ignjatovic J., Sapats S. Avian infectious bronchitis virus. Rev Sci Tech. 2000;19:493–508. doi: 10.20506/rst.19.2.1228. [DOI] [PubMed] [Google Scholar]
- 14.Jia W., Karaca K., Parrish C.R., Naqi S.A. A novel variant of avian infectious bronchitis virus resulting from recombination among three different strains. Arch Virol. 1995;140:259–271. doi: 10.1007/BF01309861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusters J.G., Jager E.J., Niesters H.G., van der Zeijst B.A. Sequence evidence for RNA recombination in field isolates of avian coronavirus infectious bronchitis virus. Vaccine. 1990;8:605–608. doi: 10.1016/0264-410X(90)90018-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manz R.A., Cassese G., Thiel A., Radbruch A. Long-lived plasma cells survive independent of antigen. Curr Top Microbiol Immunol. 1999;246:71–74. doi: 10.1007/978-3-642-60162-0_9. discussion 74–5. [DOI] [PubMed] [Google Scholar]
- 17.Manz R.A., Lohning M., Cassese G., Thiel A., Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- 18.Manz R.A., Thiel A., Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 19.Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 20.Mockett A.P., Cavanagh D., Brown T.D. Monoclonal antibodies to the S1 spike and membrane proteins of avian infectious bronchitis coronavirus strain Massachusetts M41. J Gen Virol. 1984;65(Pt 12):2281–2286. doi: 10.1099/0022-1317-65-12-2281. [DOI] [PubMed] [Google Scholar]
- 21.Ndifuna A., Waters A.K., Zhou M., Collisson E.W. Recombinant nucleocapsid protein is potentially an inexpensive, effective serodiagnostic reagent for infectious bronchitis virus. J Virol Methods. 1998;70:37–44. doi: 10.1016/S0166-0934(97)00170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pei J., Briles W.E., Collisson E.W. Memory T cells protect chicks from acute infectious bronchitis virus infection. Virology. 2003;306:376–384. doi: 10.1016/s0042-6822(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 23.Pei J., Sekellick M.J., Marcus P.I., Choi I.S., Collisson E.W. Chicken interferon type I inhibits infectious bronchitis virus replication and associated respiratory illness. J Interferon Cytokine Res. 2001;21:1071–1077. doi: 10.1089/107999001317205204. [DOI] [PubMed] [Google Scholar]
- 24.Raggi L.G., Lee G.G. Lack of correlation between infectivity, serologic response and challenge results in immunization with an avian infectious bronchitis vaccine. J Immunol. 1965;94:538–543. [PubMed] [Google Scholar]
- 25.Raj G.D., Jones R.C. Local antibody production in the oviduct and gut of hens infected with a variant strain of infectious bronchitis virus. Vet Immunol Immunopathol. 1996;53:147–161. doi: 10.1016/0165-2427(95)05545-2. [DOI] [PubMed] [Google Scholar]
- 26.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rassmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 27.Scheibenbogen C., Romero P., Rivoltini L., Herr W., Schmittel A., Cerottini J.C., Woelfel T., Eggermont A.M., Keilholz U. Quantitation of antigen-reactive T cells in peripheral blood by IFNgamma-ELISPOT assay and chromium-release assay: a four-centre comparative trial. J Immunol Methods. 2000;244:81–89. doi: 10.1016/s0022-1759(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 28.Schmittel A., Keilholz U., Bauer S., Kuhne U., Stevanovic S., Thiel E., Scheibenbogen C. Application of the IFN-gamma ELISPOT assay to quantify T cell responses against proteins. J Immunol Methods. 2001;247:17–24. doi: 10.1016/s0022-1759(00)00305-7. [DOI] [PubMed] [Google Scholar]
- 29.Seo S.H., Collisson E.W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J Virol. 1997;71:5173–5177. doi: 10.1128/jvi.71.7.5173-5177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo S.H., Pei J., Briles W.E., Dzielawa J., Collisson E.W. Adoptive transfer of infectious bronchitis virus primed alpha beta T cells bearing CD8 antigen protects chicks from acute infection. Virology. 2000;269:183–189. doi: 10.1006/viro.2000.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slifka M.K., Ahmed R. Limiting dilution analysis of virus-specific memory B cells by an ELISPOT assay. J Immunol Methods. 1996;199:37–46. doi: 10.1016/s0022-1759(96)00146-9. [DOI] [PubMed] [Google Scholar]
- 32.Slifka M.K., Ahmed R. Long-term antibody production is sustained by antibody-secreting cells in the bone marrow following acute viral infection. Ann NY Acad Sci. 1996;797:166–176. doi: 10.1111/j.1749-6632.1996.tb52958.x. [DOI] [PubMed] [Google Scholar]
- 33.Slifka M.K., Antia R., Whitmire J.K., Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 34.Sneed L.W., Butcher G.D., Parr R., Wang L., Collisson E.W. Comparisons of the structural proteins of avian infectious bronchitis virus as determined by western blot analysis. Viral Immunol. 1989;2:221–227. doi: 10.1089/vim.1989.2.221. [DOI] [PubMed] [Google Scholar]
- 35.Stott D.I. Immunoblotting, dot-blotting, and ELISPOT assays: methods and applications. J Immunoassay. 2000;21:273–296. doi: 10.1080/01971520009349537. [DOI] [PubMed] [Google Scholar]
- 36.Sze D.M., Toellner K.M., Garcia de Vinuesa C., Taylor D.R., MacLennan I.C. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med. 2000;192:813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson G., Mohammed H., Bauman B., Naqi S. Systemic and local antibody responses to infectious bronchitis virus in chickens inoculated with infectious bursal disease virus and control chickens. Avian Dis. 1997;41:519–527. [PubMed] [Google Scholar]
- 38.Toro H., Fernandez I. Avian infectious bronchitis: specific lachrymal IgA level and resistance against challenge. Zentralbl Veterinarmed B. 1994;41:467–472. doi: 10.1111/j.1439-0450.1994.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 39.Toro H., Reyes E., Redmann T., Kaleta E.F. Local and systemic specific antibody response of different chicken lines after ocular vaccination against infectious bronchitis. Zentralbl Veterinarmed B. 1996;43:449–454. doi: 10.1111/j.1439-0450.1996.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang L., Junker D., Collisson E.W. Evidence of natural recombination within the S1 gene of infectious bronchitis virus. Virology. 1993;192:710–716. doi: 10.1006/viro.1993.1093. [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Xu Y., Collisson E.W. Experimental confirmation of recombination upstream of the S1 hypervariable region of infectious bronchitis virus. Virus Res. 1997;49:139–145. doi: 10.1016/S0168-1702(97)01466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C.C., Thiagarajan D., Lin T.L. Research notes: ELISPOT assay for detection of antibody secreting cells to infectious bursal disease virus in chickens. Poult Sci. 1998;77:662–665. doi: 10.1093/ps/77.5.662. [DOI] [PubMed] [Google Scholar]


