Highlights
► Bat IFNγ appears to be conserved with IFNγ from other mammals. ► IFNγ is produced by activated bat splenocytes. ► Bat IFNγ displays antiviral activity in bat cells infected with Semliki forest virus and the bat borne virus, Hendra virus.
Keywords: Bats, Interferon gamma, Antiviral
Abstract
Bats are natural reservoir hosts to a variety of viruses, many of which cause morbidity and mortality in other mammals. Currently there is a paucity of information regarding the nature of the immune response to viral infections in bats, partly due to a lack of appropriate bat specific reagents. IFNγ plays a key role in controlling viral replication and coordinating a response for long term control of viral infection. Here we describe the cloning and expression of IFNγ from the Australian flying fox, Pteropus alecto and the generation of mouse monoclonal and chicken egg yolk antibodies specific to bat IFNγ. Our results demonstrate that P. alecto IFNγ is conserved with IFNγ from other species and is induced in bat splenocytes following stimulation with T cell mitogens. P. alecto IFNγ has antiviral activity on Semliki forest virus in cell lines from P. alecto and the microbat, Tadarida brasiliensis. Additionally recombinant bat IFNγ was able to mitigate Hendra virus infection in P. alecto cells. These results provide the first evidence for an antiviral role for bat IFNγ in vitro in addition to the application of important immunological reagents for further studies of bat antiviral immunity.
1. Introduction
Interferons (IFNs) provide the first line of defence against viral infection and play a role in shaping the adaptive immune response. Three classes of IFNs have been identified, designated types I, II and III, each of which can be distinguished according to their amino acid sequence and their receptor complex. Type I and type III IFNs are produced by virus infected cells and play an important role in the innate immune response. Type II IFN is represented by a single IFNγ in all mammals examined, is mainly produced by activated T cells and natural killer (NK) cells and acts on macrophages, T cells and NK cells. Constitutive production of IFNγ by NK cells reflects a role for this cytokine in the early innate immune response. However, IFNγ production by activated T cells plays an important role in shaping the adaptive immune response to viral infection and establishing longer term control of viral infection (Schroder et al., 2004). The importance of IFNγ has been demonstrated in type II IFN receptor deficient mice which are susceptible to low doses of virus often leading to lifelong viral persistence (van den Broek et al., 1995). Among its activities, IFNγ is responsible for the stimulation of bactericidal activity of phagocytes, stimulation of antigen presentation through class I and class II major histocompatibility complex (MHC) molecules, orchestration of leukocyte–endothelium interactions and effects on cell proliferation and apoptosis. IFNγ also acts through the stimulation and repression of a variety of genes including the upregulation of a number of host antiviral proteins including 2,5-oligoadenylate synthetase, dsRNA-dependent protein kinase PKR, guanylate binding protein and adenosine deaminase (Boehm et al., 1997).
Bats belong to the order Chiroptera which is divided into two suborders: Megachiroptera (megabats), and Microchiroptera (microbats). Bats are the second most species rich group after rodents, making up approximately 20% of mammalian diversity and they possess a variety of unique characteristics that distinguish them from other mammals. These include their capability for powered flight, relatively long lifespans relative to body size and their role as natural reservoir hosts for a variety of viruses, many of which have the potential to cause significant morbidity and mortality in other mammals, including humans but rarely causing any signs of disease in bats (Calisher, 2006, Wong et al., 2007). These include rabies virus, severe acute respiratory syndrome-like coronaviruses (SARS-like CoV), henipaviruses (Hendra and Nipah viruses) and Ebola virus among others (Leroy et al., 2005, Leroy et al., 2009, Swanepoel et al., 1996, Williamson et al., 1998, Williamson et al., 2000). Despite their array of unique characteristics bats are among the least studied of all mammalian taxa and there is little information on antiviral immunity in any species of bat and few bat specific reagents exist to study bat immunology. The few functional studies that have been performed on the adaptive immune responses of bats have revealed some interesting differences between bats and other mammalian species. Antibody mediated immune responses in several species of bats have been examined in response to immunisation with commonly used antigens and have demonstrated that antibody responses in bats are both qualitatively and quantitatively lower compared with conventional laboratory animals (Chakraborty and Chakravarty, 1984, Hatten et al., 1968, Wellehan et al., 2009). In vitro experiments have also revealed evidence for delayed cell mediated responses to T cell mitogens such as phytohaemagglutinin (PHA) and concanavalin A (ConA) and the B cell mitogen lipopolysaccharide (LPS) (Chakravarty and Paul, 1987, McMurray and Thomas, 1979, Paul and Chakravarty, 1986). However, as these studies were crude relative to comparable studies in humans and mice, further studies are required to determine whether differences in the adaptive immune response play a role in the asymptomatic nature of viral infections in bats.
In an effort to understand whether differences in the antiviral response of bats are responsible for their ability to remain asymptomatic to persistent viral infections, we have begun to develop the Australian flying fox Pteropus alecto as a model species for studying host virus relationships. Recently we described a number of immune genes in P. alecto providing important information on molecules associated with adaptive and innate immunity in pteropid bats (Baker et al., 2010, Cowled et al., 2011, Cowled et al., in press, Zhou et al., 2011a, Zhou et al., 2011b). P. alecto harbours a number of viruses including Hendra virus (HeV) from the genus Henipavirus. HeV is capable of spillover from bats to horses and subsequently from horses to humans. Although HeV causes no apparent signs of disease in bats, it is highly pathogenic in horses and humans (Field et al., 2007, Murray et al., 1995). Paramyxoviruses including HeV are capable of evading the host’s immune response and HeV has been demonstrated to antagonise the type I IFN production pathway in human cell lines (Gotoh et al., 2002, Rodriguez and Horvath, 2004, Virtue et al., 2011b). In bat cells, HeV has a similar effect on type I IFN production but is also capable of antagonising IFN signalling pathways (Virtue et al., 2011a). The role of IFNγ in antiviral immunity and in the control of long term viral infections in bats remains to be determined. Evidence for the presence of IFNγ in bats has been reported in an earlier investigation describing the in silico identification of Type I and II IFNs in the low coverage genome sequences of P. vampyrus and Myotis lucifugus (Kepler et al., 2010). Here we report the characterisation of P. alecto IFNγ and the development of bat specific antibodies critical for future investigations of the IFNγ response of bats to viral infections. Our data also demonstrate the antiviral activity of recombinant bat IFNγ on Semliki Forest Virus (SFV) and HeV, providing the first evidence for in vitro antiviral activity of bat IFNγ.
2. Materials and methods
2.1. Animals and RNA extraction
The P. alecto bats used in this study were caught in Queensland and transported to the Australian Animal Health Laboratory (AAHL) in Victoria Australia in accordance with the procedures prescribed by the AAHL Animal Ethics Committee (Protocol No. 1222). For the preparation of cDNA, the mesenteric lymph nodes from a bat that was experimentally infected with SARS-CoV and euthanised at 35 days following inoculation were collected in RNAlater (Ambion, Austin, TX). Total RNA was extracted as described previously (Baker et al., 2010).
2.2. PCR amplification of IFNγ
IFNγ was identified in the whole genome sequences of the Malaysian flying fox P. vampyrus and the little brown bat, M. lucifugus available in the Ensembl database (assembly pteVam1, 2.63× coverage, July 2008; myoLuc1, 1.7× coverage, March 2006) using the BLAT algorithm. Based on alignments of the P. vampyrus and M. lucifugus IFNγ sequences, degenerate oligonucleotide primers were designed (IFNγ7F: 5′-GCTATTMGAAGAGAAAGATCAGC-3′ and IFNγ8R: 5′-TGGCCCCTGAGATAAAGCCTT-3). P. alecto IFNγ was amplified from lymph node cDNA using Qiagen HotStar Hi Fidelity Polymerase kit with Q solution and 1 μM each of primers IFNγ7F and 8R. The DNA amplification conditions included an initial denaturation step of 95 °C for 5 min; denaturation at 94 °C for 40 s, annealing at 57 °C for 1 min and extension at 72 °C for 1 min for 45 cycles and a final extension at 72 °C for 10 min.
2.3. Sequencing and analysis
PCR products from two independent reactions were cloned into pCR-blunt (Invitrogen) for sequencing. M13 forward and reverse primers were employed for sequencing using BigDye Terminator Cycle Sequencing Kit v3.1 (Applied Biosystems, Foster City, CA) as described previously (Baker et al., 2010). Chromatograms were edited manually using DNASTAR Lasergene SeqMan Pro version 8 (Madison, WI) and CloneManager Professional version 9 (Scientific & Educational Software, Cary, NC) and were compared with sequences in the GenBank database using the BLAST algorithm (Altschul, 1990). Potential transcription factor binding sites were identified using the MatInspector program (http://www.genomatix.de). All sequences were aligned using the ClustalX program (Thompson et al., 1994). Nucleotide sequences were aligned and gapped manually using Bioedit software version 7.0.9 (Tom Hall, Ibis Biosciences, Carlsbad, CA), based on the protein alignment to retain codon positions. Based on the nucleotide alignments, phylogenetic trees were constructed by the neighbour joining method of Saitou and Nei (1987), maximum parsimony and minimum evolution using the MEGA4 program (Kumar et al., 2004). The GenBank accession numbers for sequences used in the phylogenetic analysis are as follows: horse, NP_001075418; dog, NM_001003174; cat, NM_001009873; camel, HM051108; cow, NM_174086; human, NM_000619; pig, NM_213948; rabbit, NM_001081991; mouse, NM_008337; ferret, EF492064; chicken, NM_205149.
2.4. Cells and cell lines
The P. alecto cells and cell lines used in this study included one cloned and immortalised kidney cell line, PaKiT02 generated in our laboratory and described previously (Crameri et al., 2009). A lung epithelial cell line Tb1-Lu derived from the free-tailed bat, Tadarida brasiliensis (ATCC #CCL 88) was used for comparative purposes.
2.5. Mitogen stimulation of bat splenocytes
Single cell suspensions of splenic lymphocytes were obtained as described previously (Janardhana et al., 2007). Viable lymphocytes were counted using a haemocytometer by trypan blue exclusion and resuspended at 107/ml in DMEM supplemented with 10% FCS, 15 mM HEPES, 15 mM l-glutamine, 100 U/ml penicillin (CSL, Parkville) and 100 μg/ml streptomycin (Sigma). For the induction of IFNγ, cells were cultured with either ConA (10 μg/ml, Sigma #C2010) or PHA (10 μg/ml, Sigma) for 36 h. Supernatants were collected for ELISAs and stimulated cells were harvested in RNAlater for RNA extraction for qRT-PCR analysis.
2.6. Quantitative reverse transcription PCR (qRT-PCR)
qRT-PCR was performed on total RNA extracted from cultured splenocytes as described previously (Cowled et al., 2011). The IFNγ expression level was calculated using the standard curve method. All data were normalised relative to 18S rRNA.
2.7. Expression and purification of bat IFNγ in Escherichia coli (rbIFNγ)
The full length IFNγ mature peptide excluding the signal peptide was PCR amplified using primers IFNγ9F (5′-ACTGGATCCCAGGCTACATTTTTAAAAGAAAT-3′) and IFNγ10R (5′-ACTAAGCTTCTATGCTTTCCAACCACGAAAC-3′) and cloned into the BamHI/HindIII sites of the pGEM-T vector (Promega). The DNA sequence coding for the mature IFNγ was confirmed by capillary sequencing. The mature IFNγ gene was cloned as a N-terminal hexahistidine (His)6 gene fusion in the BamHI/HindIII sites of the pQE30 expression vector (Qiagen) and transformed in E. coli strain JM109. Clones were analysed by restriction digestion to confirm correct orientation of the IFNγ gene.
Solubilisation and purification were performed using a modification of the protocol described previously (Andrew et al., 2007). Modifications included the use of Luria broth (LB, Oxoid Australia Ltd.) for growth of the bacterial cultures, 50 mM Tris, pH 8.0, 500 mM NaCl and 10 mM imidazole (Sigma) was substituted for resuspension of the cell pellet and subsequent protein purification steps; lysozyme for cell lysis was omitted. P. alecto IFNγ N-terminal (His)6 tagged protein was purified from the E. coli lysate using Ni-IDA agarose (Scientifix Pty Ltd., Melbourne, Australia). Protein was allowed to bind to the Ni-IDA agarose on a rotating wheel for 1 h at room temperature; unbound protein was removed and the agarose was washed once with resuspension buffer containing 0.1% v/v Triton X-100 followed by three washes with resuspension buffer containing 0.1% w/v zwittergent (Calbiochem). The recombinant protein was eluted from the column in a total of 2.5 ml of buffer containing 50 mM Tris, pH 8.0, 500 mM NaCl, 250 mM imidazole and 0.1% w/v zwittergent.
Bacterial endotoxin was removed from the purified IFNγ preparation using the Pierce Detoxi-Gel Endotoxin Removing Gel (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Protein concentration of the purified rbIFNγ was determined with a bicinchoninic acid Protein Assay kit (Pierce – Thermo Fisher Scientific) using bovine serum albumin as the protein standard. Purified rbIFNγ was stored at 4 °C in the presence of 0.01% w/v thimerosal (Sigma).
2.8. Expression of recombinant bat IFNγ in Chinese hamster ovary (CHO) cells (rbIFNγCHO)
A 715 bp EcoRI/PsiI fragment of P. alecto IFNγ from the original IFNγ clone (cloned into pCRblunt) was cloned into the EcoRI/SmaI sites of the mammalian expression vector pCI (Promega, Madison, WI, USA) then transformed into E. coli JM109 cells. The identity of the clone and orientation of the gene with respect to the CMV promoter was confirmed using restriction enzyme analysis. Endotoxin free plasmid DNA for the production of IFNγ specific monoclonal Abs (mAbs) and expression in CHO cells was purified using the Promega PureYield™ Plasmid Maxiprep System according to the manufacturer’s protocol. Purified plasmid DNA was concentrated by ethanol precipitation and resuspended at a concentration of 1 mg/ml in sterile PBS.
CHO (ATCC) cells grown to log phase in a 75 cm2 tissue culture flask were seeded into a 6 well tissue culture plate at 5 × 105 cells per well in OPTI-MEM (GIBCO) with no antibiotics. The following day the cells were transfected with the eukaryotic expression vector pCI containing bat IFNγ using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. The mock control consisted of cells transfected with pCI plasmid vector. The cells were incubated at 37 °C in a humidified CO2 incubator. Culture supernatants from pCI IFNγ (rbIFNγCHO) and pCI vector (CHO mock) transfected cells were collected after 48 or 72 h and tested for the expression of biologically active IFNγ in a virus inhibition assay described below.
2.9. Production of monoclonal antibodies (mAb) to bat IFNγ
Six week old mice were primed with an intramuscular inoculation of pCI plasmid expressing bat IFNγ followed by three intraperitoneal inoculations (four weeks apart) of E. coli expressed protein emulsified with a triple adjuvant cocktail containing QuilA, DEAE and Montanide (Than and Edgar, 1998). Mice were bled 7 days following each inoculation for serum collection and monitored by ELISA for IFNγ specific antibodies. When the serum antibody titres peaked, mice received a final intravenous boost of rbIFNγ protein in PBS followed by euthanasia 3 days later.
Splenocytes from the immunised mice were fused with SP2 myeloma cells (ATCC). Cell fusion, selection, and cloning of hybridomas were performed using the semisolid clonacell-hybridoma cloning kit (Stem Cell Technologies) following the manufacturer’s instructions. Anti-bat IFNγ producing hybridomas were selected by an ELISA against bat IFNγ. One positive clone (2G6) was further expanded in 6 well tissue culture plates, supernatants collected and stored at 4 °C. Isotype of the mAb was determined by ELISA using a clonotyping system from SouthernBiotech (#5300-05).
2.10. Production of IgY specific antibodies to bat IFNγ
Adult egg laying hens were primed with pCI IFNγCHO and boosted with at least three doses of rbIFNγ protein emulsified in QuilA triple adjuvant through intra-abdominal and intramuscular routes. Seroconversion was monitored 7 days post inoculation by ELISA and eggs collected after the serum IFNγ antibody titres peaked. Egg yolk antibodies were purified using the yolk IgY purification kit (Gallus Immunotech Inc.) following the manufacturer’s instructions. The purified antibody was resuspended in PBS. The titre of IgY was determined in an indirect ELISA where Nunc MaxiSorp plates were coated with 2 μg/ml of rbIFNγ to which serial dilutions of purified yolk anti-bat IFNγ was added. The bound IgY was determined with 1:3000 dilution of rabbit anti-chicken HRP (ICN #612141) and the H2O2/TMB substrate and the absorbance measured at 450 nm using an ELISA plate reader.
2.11. Sandwich ELISA
A sandwich ELISA was developed to detect recombinant and native bat IFNγ in culture supernatants. In brief, 96 well microtitre plates (Nunc) were coated with a 1:1000 dilution of yolk anti-bat IFNγ in carbonate buffer for 2 h at room temperature then at 4 °C overnight. The following day the ELISA was carried out at room temperature with 1 h incubation between the addition of reagents. The plates were washed once with PBS + 0.05% Tween 20 (PBST), blocked with 1% power block (Biogenex), followed by the addition of serial dilutions of rbIFNγ or culture supernatant in a diluent containing 1% BSA in PBS. The captured IFNγ was detected using a 1:10 dilution of the supernatant from 2G6 hybridoma culture, followed by goat anti-mouse HRP (Zymed). At the end of incubation the plates were washed five times with distilled water. Addition of substrate and recording of absorbance was carried out as described above.
2.12. Western blot with IFNγ antibodies
Purified rbIFNγ protein was run on a 12% SDS–PAGE gel (Laemmli, 1970). Protein was then transferred to a Hybond C-extra membrane for 70 min (GE Healthcare Australia Pty Ltd., Rydalmere, NSW) using a Bio-Rad Western blot apparatus. The membrane was blocked for 1 h with 10% skim milk powder in PBS, incubated for 1 h with anti-recombinant bat IFNγ mAb or IgY antibody diluted in 10% skim milk powder in PBS then washed three times with PBST. rbIFNγ protein was detected by incubating the membrane for 1 h with a secondary antibody diluted in 10% skim milk in PBS, washing the membrane three times with PBST and once with PBS, and once with Supersignal West Pico reagent (Thermo Scientific, Rockford, IL, USA).
2.13. Antiviral activity of rbIFNγCHO against Semliki forest virus
Semliki Forest Virus (SFV) was a kind gift from Dr. J.W. Lowenthal (Livestock Industries, CSIRO). Inhibition of SFV replication in bat cells by rbIFNγCHO was assessed by a colorimetric assay (Finter, 1969). P. alecto kidney or T. brasiliensis Tb1-Lu cells were seeded at 4 × 104 cells per well in a 96 well tissue culture plate and incubated at 37 °C in a humidified CO2 atmosphere for 24 h. The culture medium was removed from the monolayer and three fold serial dilutions of rbIFNγCHO or CHO mock supernatant were added. Cultures were incubated overnight followed by the addition of SFV suspension containing 106 TCID50 particles per well or culture medium to uninfected control wells. After 48 h, medium was discarded, cells were washed once with PBS followed by the addition of 0.25% neutral red in PBS and incubated for a further 2 h. The cells were then washed three times in PBS and lysed with isopropanol containing 0.04 M HCl to release the dye. The absorbance was read at 540 nm using an ELISA plate reader.
2.14. Antiviral activity of rbIFNγCHO against Hendra virus
The antiviral effect of rbIFNγCHO on HeV infection was assessed using bat kidney cells cultured and treated with IFNγ as above for SFV inhibition. Cells were infected with a pre-optimised titre of HeV at 105 TCID50/well and immunofluorescent labelling for HeV antigens was carried out as described previously (Aljofan et al., 2008, Aljofan et al., 2009). Following immunofluorescent labelling, full field images of all wells were captured under 200× magnification using an inverted microscope (EVOS Fl, AMG). Enumeration of HeV positive cells in the captured images was carried out using analysis software (AnalySIS, Soft Imaging System, GmbH). All work with live HeV was carried out under biosafety level 4 (BSL-4) conditions at CSIRO, AAHL, Geelong, Australia.
2.15. Statistical analysis
To assess the effect of treatment with or without IFNγ prior to HeV infection, antigen positive immunofluorescent cells in different doses of rbIFNγCHO and CHO mock treated cells were compared using a one tailed t test. A p value ⩽0.05 was deemed to be statistically significant.
The statistical analysis was performed using the software GraphPad Prism (version 3.00 for Windows, GraphPad Software, San Diego, California, USA). All data were presented as the mean ± SEM of five replicates for HeV positive cells.
3. Results
3.1. Cloning and sequencing of P. alecto IFNγ
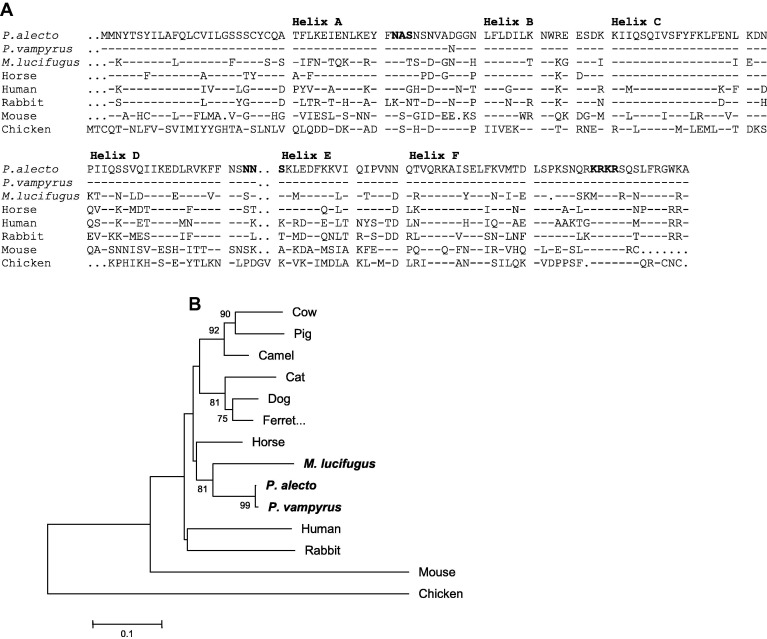
The IFNγ sequence was predicted from the whole genome sequences of P. vampyrus and M. lucifugus available in the Ensembl genome browser resulting in the identification of a single IFNγ locus in each species of bat. Degenerate oligonucleotide primers were used to amplify an 858 bp fragment of P. alecto IFNγ from the lymph node cDNA from a SARS-CoV infected bat. An open reading frame encoding the complete IFNγ sequence consisted of 495 bp, encoded by 164 amino acids (Fig. 1 a). This sequence has been submitted to Genbank (Accession No. JN656277). Alignment of the P. alecto cDNA with the corresponding genomic sequence identified in the whole genome of P. vampyrus revealed that bat IFNγ is encoded by four exons, similar to other species (data not shown; Savan et al., 2009). The 1200 bp sequence upstream of the start site of IFNγ in the P. vampyrus whole genome was also scanned for potential transcription factor binding sites. This analysis resulted in the identification of binding sites for transcription factors shown to be involved in the regulation of IFNγ in other species (Savan et al., 2009). These included GATA, BRAC, NFAT, STAT, OCT1, ISRE, IRF and NF-κB among others. Given the close relationship of P. alecto and P. vampyrus, it is likely that these sites are also conserved in P. alecto. These results are consistent with the regulation of bat IFNγ being similar to other mammals.
Fig. 1.
Pteropus alecto IFNγ is closely related to other mammalian IFNγ genes. (A) Alignment of the deduced amino acid sequence of bat IFNγ genes with IFNγ genes from other species. The six α helixes corresponding to the conserved secondary structure of IFNγ are indicated. Potential N-linked glycosylation sites and the conserved NLS (KRKR) are shown in bold. Dashes indicated similarity and dots indicate gaps. (B) Phylogenetic analysis based on amino acid alignments of bat IFNγ with representative vertebrate species. Branch support is indicated as the percentage out of 1000 bootstrap replicates and is shown where support is greater than 60%. The P. vampyrus and M. lucifugus sequences were obtained from the publicly available whole genome sequences available in Ensembl. The accession numbers of all other IFNγ sequences can be found in Section 2.
The deduced protein sequence encoded by IFNγ shown in Fig. 1a contained many features conserved with IFNγ genes from other species. 3D modelling of the structure of bat IFNγ revealed six putative α-helices (A–F), the locations of which are illustrated in Fig. 1a (Arnold et al., 2006). The bat IFNγ gene also contains two N-linked glycosylation sites and a KRKR motif which acts as a nuclear localisation signal (NLS) in IFNγ molecules from other species and appears to be crucial for the biologic activity of the molecule (Slodowski et al., 1991, Subramaniam et al., 1999).
The P. alecto IFNγ sequence shared 60–99% nucleotide and 44–99% amino acid identity to IFNγ genes from other mammalian species, sharing highest similarity to the two bat genes and lowest similarity to mouse IFNγ (Table 1 ). Phylogenetic analysis based on nucleotide alignments of the coding region of P. alecto IFNγ with sequences from a variety of other mammals and non-mammals is shown in Fig. 1b. Consistent with the pairwise analysis, the bat IFNγ gene was closely related to other mammalian IFNγ genes and clustered with the two bat IFNγ sequences. The tree shown in Fig. 1b was reconstructed using the neighbour joining method; however, identical results were found when maximum parsimony and minimum evolution were used (data not shown).
Table 1.
Percentage nucleotide and amino acid identity between bat, human and mouse IFNγ.
| P. alecto | P. vampyrus | M. lucifugus | Human | Mouse | |
|---|---|---|---|---|---|
| P. alecto | 99 | 70 | 64 | 44 | |
| P. vampyrus | 99 | 70 | 64 | 44 | |
| M. lucifugus | 83 | 83 | 56 | 39 | |
| Human | 78 | 78 | 74 | 40 | |
| Mouse | 60 | 61 | 59 | 60 |
Bold numbers indicate amino acid identity; non-bold numbers represent nucleotide identity.
3.2. Production of recombinant bat IFNγ protein and generation of antibody reagents
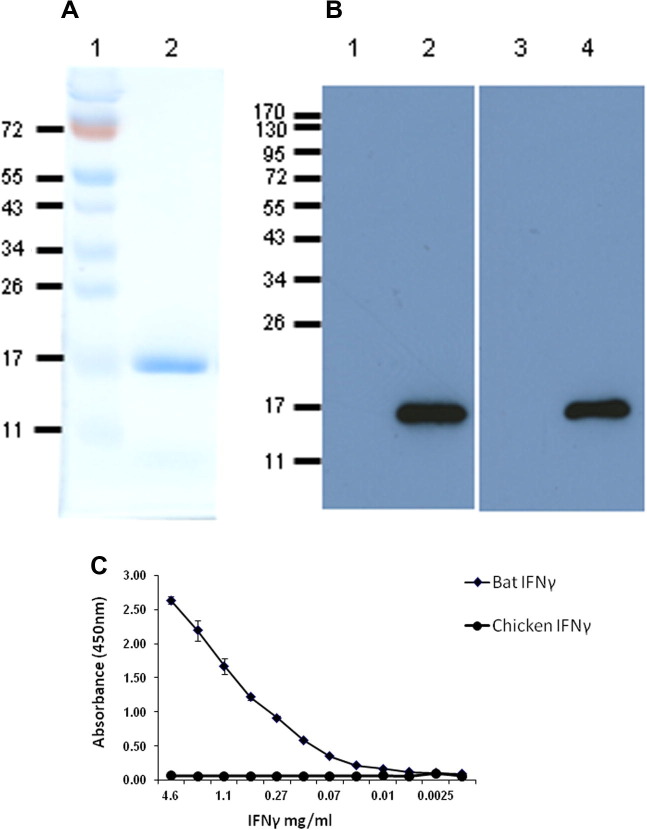
Recombinant bat IFNγ was expressed as an N-terminal 6 histidine tagged protein in the E. coli strain JM109, purified by affinity chromatography and run on a denaturing gel revealing the presence of a single band at 17 kDa (Fig. 2 a). This protein was used as an antigen to immunise mice and chickens to produce mAb and IgY specific antibodies, respectively. One of the hybridomas generated, clone 2G6, produced a large quantity of highly specific bat IFNγ that did not cross react with other cytokines including chicken IFNγ and bat IL1β (data not shown). This mAb was further cloned and isotyped, demonstrating that it was an IgG1 with a κ light chain. Similarly, IgY antibody was purified from chicken egg yolks from chickens immunised with recombinant bat IFNγ and tested for IFNγ specificity. As shown in Fig. 2b, Western blot analysis confirmed that both the mAb and IgY antibodies bind to rbIFNγ. An indirect sandwich ELISA developed using anti-bat IFNγ IgY for coating and the bat specific IFNγ mAb for detection was capable of detecting rbIFNγ down to 10 ng/ml (Fig. 2c).
Fig. 2.
Detection of bat IFNγ in Western blot and ELISA using mouse monoclonal and IgY antibodies to recombinant bat IFNγ. (A) SDS–PAGE and coomassie blue staining of the purified rbIFNγ (lane 2) indicates its approximate molecular weight as 17 kDa. (B) Western blot of rbIFNγ was probed with anti-bat mAb 2G6 (lane 2) and yolk derived polyclonal anti-bat IgY (lane 4) to rbIFNγ and were visualised with anti-mouse HRP and anti-chicken HRP, respectively, in a chemiluminescence system. Negative controls run on lanes 1 and 3 was an unrelated E. coli expressed and purified recombinant bat protein of similar size to rbIFNγ. (C) Capture ELISA developed using the anti-bat IgY (for coating the plate), anti-bat mAb 2G6 (for detection) and anti-mouse-HRP (secondary Ab) has the sensitivity to detect 10 ng/ml of rbIFNγ. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Purified bat IFNγ plasmid DNA used for the generation of mAb and IgY antibodies was also used to generate a transient expression system in CHO cells. The production of recombinant bat IFNγ from a mammalian cell line was performed to provide a source of IFNγ protein with a higher likelihood of corresponding to the native protein and of retaining biological activity on storage for assessing the biological activity of IFNγ. The IFNγ protein produced by CHO cells is referred to throughout the text as rbIFNγCHO.
3.3. IFNγ is produced by bat splenocytes following stimulation with T cell mitogens
IFNγ is a Th1 cytokine that is mainly produced by activated T or NK cells following viral infection or stimulation with mitogens such as PHA (de Ley et al., 1980). To determine whether bat splenocytes were capable of an IFNγ response similar to that of other mammals, splenocytes were stimulated with T cell mitogens, ConA and PHA for 36 h. As shown in Fig. 3 a, qRT-PCR on mRNA extracted from stimulated cells from two individual bats demonstrated a strong IFNγ response in PHA stimulated bat splenocytes and weaker responses in ConA stimulated cells.
Fig. 3.
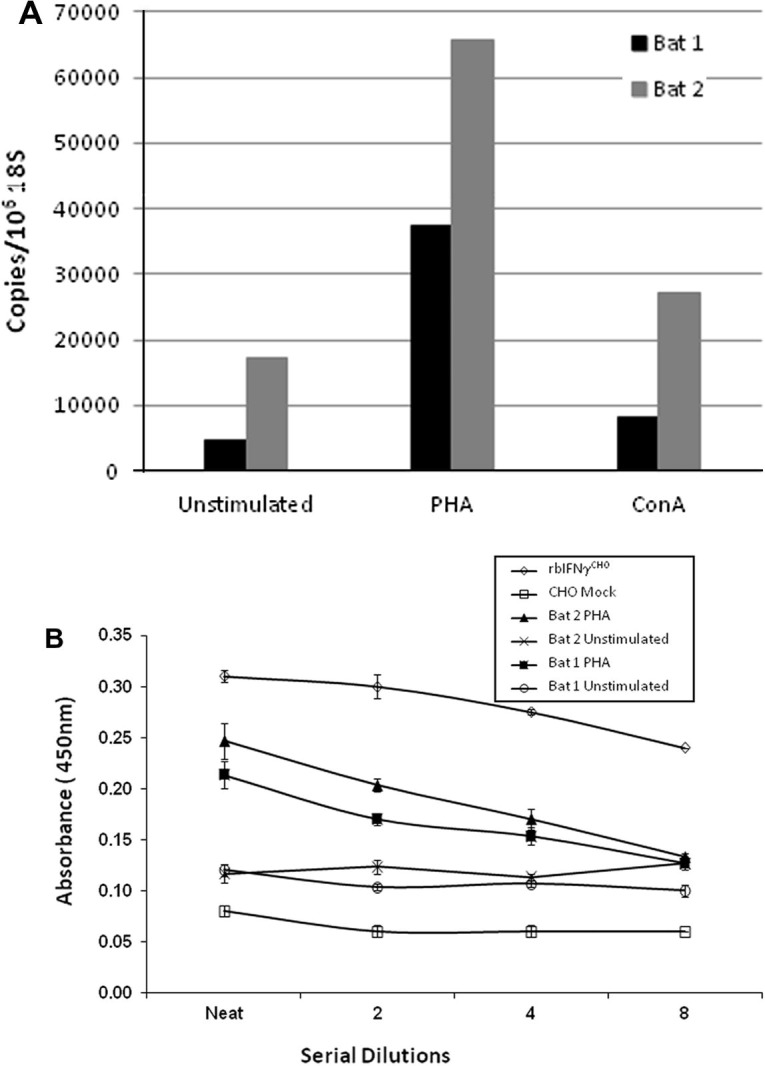
Pteropus alecto spenocytes produce IFNγ in response to mitogen stimulation. (A) IFNγ mRNA was measured in RNA from cultured splenocytes from two individual P. alecto by qRT-PCR and normalised against 18s rRNA. (B) Native IFNγ protein was measured in supernatant from cultured cells by capture ELISA using IFNγ specific anti-bat IgY as the coating antibody and IFNγ specific anti-bat mAb as the detection antibody. Data are mean values of triplicates, and the bars represent ± standard error of the means.
To further examine IFNγ production by the stimulated bat cells we used the capture ELISA described above to detect IFNγ protein in supernatant from mitogen stimulated bat splenocytes using our bat specific IFNγ IgY and mAb reagents. This ELISA was capable of detecting rbIFNγCHO and native IFNγ produced by PHA stimulated bat splenocytes (Fig. 3b). The detection of IFNγ by ELISA in supernatant collected from PHA stimulated splenocytes from both bats is consistent with the results obtained by qRT-PCR. However, no native IFNγ was detected in supernatants from cells that were stimulated with ConA from either bat despite the presence of mRNA in the stimulated cells (Fig. 3a and data not shown). The lower level of transcript produced by ConA stimulated cells correlates with the production of a low quantity of protein that is below the detectable limit of our ELISA.
3.4. Bat IFNγ inhibits replication of Semliki forest virus in cell lines from two bat species
To assess whether pre-treatment with bat IFNγ could protect cells from virus infection, the antiviral activity of E. coli and CHO produced recombinant IFNγ on SFV infected cells was examined. Although rbIFNγ displayed antiviral activity against SFV, this protein demonstrated a gradual loss of activity during storage (data not shown), and therefore all subsequent functional assays were performed using the more stable rbIFNγCHO.
The antiviral activity of rbIFNγCHO was tested not only on P. alecto cloned kidney cells but also on T. brasiliensis Tb1-Lu lung cells (ATCC) to assess its ability to cross react with cells from other bat species. Serial dilutions of rbIFNγCHO supernatant were used to treat P. alecto kidney and T. brasiliensis lung cell lines. As shown in Fig. 4 a and b, rbIFNγCHO inhibited SFV growth in both cell lines in a dose dependent manner, conferring self and cross species protection against SFV. Pretreatment of cells with rbIFNγCHO inhibited SFV infection of P. alecto kidney cells in a dose dependent manner (Fig. 4a). Maximum antiviral activity was conferred at a 1:4 dilution, decreasing with subsequent dilutions and this effect had almost disappeared by a dilution of 1:128. In the Tb1-Lu cell line, maximum antiviral activity was observed at a dilution of 1:64 and this effect had disappeared by a dilution of 1:256 (Fig. 4b).
Fig. 4.
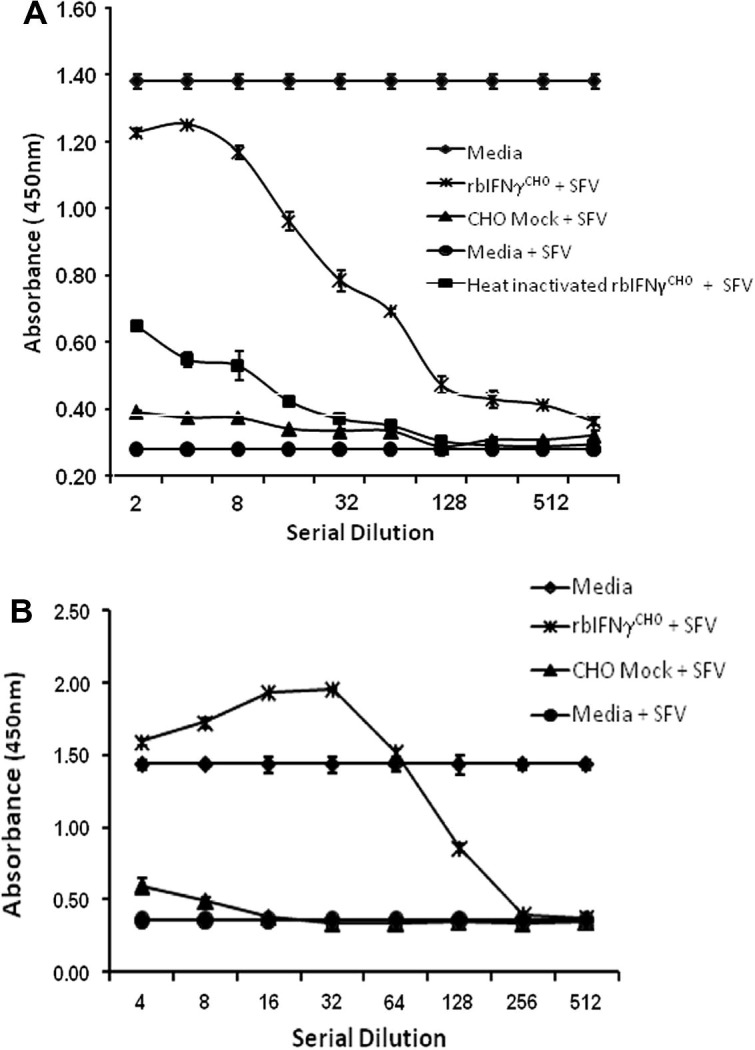
Recombinant bat IFNγ displays antiviral activity against Semliki forest virus in P. alecto and T. brasiliensis cell lines. Cells were pre-treated overnight with serial dilutions of supernatants from CHO cells transfected with pCI plasmid containing bat IFNγ (rbIFNγCHO) or pCI vector alone (CHO mock). Cells were then infected with SFV for 48 h. Assay controls included replicates pre-treated with media alone and cultured for a further 48 h either infected (media + SFV) or uninfected (media). Cell death due to viral infection was determined by a colorimetric assay using the viral dye neutral red. Pre-treatment of (A) P. alecto PaKiT02 cells or (B) T. brasiliensis Tb1-Lu cells with rbIFNγCHO protects from SFV infection in a dose dependent manner. Data are mean values of triplicates, and the error bars represent SEs.
In mammals, type II IFN is distinguished from type I IFN by its heat lability and rapidly loses its antiviral activity following exposure to heat (Farrar and Schreiber, 1993). To examine the degree of heat lability of bat IFNγ, rbIFNγCHO was subjected to heat treatment at 60 °C for 30 min. As shown in Fig. 4a, heat treated rbIFNγCHO was no longer effective in protecting cells from SFV, providing further evidence that the bat IFNγ protein is functionally similar to IFNγ from other mammalian species.
3.5. Bat IFNγ inhibits replication of Hendra virus in P. alecto cells
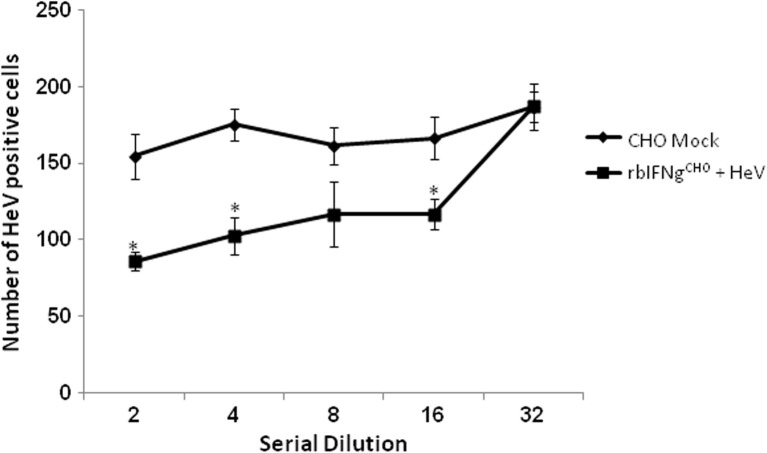
To investigate the antiviral activity of bat IFNγ on a virus naturally harboured by P. alecto, we investigated the antiviral activity of rbIFNγCHO on HeV. The colorimetric assay that was used for SFV inhibition was found to be unsuitable for testing inhibition of HeV infection by IFNγ. Therefore we adapted an immunofluorescence assay previously standardised in our laboratory for studies involving HeV infections (Aljofan et al., 2008, Aljofan et al., 2009). Treatment of cells with rbIFNγCHO prior to HeV infection resulted in a significant reduction in the number of HeV glycoprotein G positive cells relative to CHO mock treated cells (p < 0.05) and this effect occurred in a dose dependent manner (Fig. 5 ). Pre-treatment of bone marrow derived cells from P. alecto with rbIFNγCHO or CHO mock followed by HeV infection yielded similar results (data not shown). These results provide evidence that rbIFNγCHO is capable of mitigating HeV infection in bat cells in vitro.
Fig. 5.
Recombinant bat IFNγ displays antiviral activity against Hendra virus. P. alecto PaKiT02 cells were pre-treated overnight with serial dilutions of supernatant from CHO cells transfected with pCI plasmid containing bat IFNγ (rbIFNγCHO) or pCI vector alone (CHO mock) and then infected with HeV for a further 48 h. HeV positive cells were enumerated by immunofluorescent labelling with anti HeV glycoprotein G antibody. Data are mean values of five replicates, and the error bars represent SEs. Asterisks indicate statistical significance with p ⩽ 0.05.
4. Discussion
Upon activation, CD4+ T cells can differentiate into T helper 1 (Th1) cells which potentiate pro-inflammatory responses or Th2 cells which participate in the humoral immune response. IFNγ is regarded as one of the hallmarks of the Th1 response and plays an important role in cell mediated immunity. Although previous studies have provided limited evidence for reduced cell mediated immune responses in bats, the lack of immunological reagents has limited the ability to define the kinetics of these responses in detail (Chakravarty and Paul, 1987, McMurray and Thomas, 1979, Paul and Chakravarty, 1986). As an initial step towards understanding the cell mediated antiviral response in bats, we report the first characterisation of IFNγ from a Chiropteran with the identification and functional characterisation of IFNγ from P. alecto. This study demonstrates that P. alecto IFNγ is conserved and functionally similar to IFNγ from other mammalian species. Additionally we have generated bat specific IFNγ reagents for further characterisation of IFNγ and the Th1 mediated immune responses in bats.
Two low coverage bat genome sequences are publicly available in the Ensembl database, one from the megabat, P. vampyrus, a member of the Pteropidae family and a second from the microbat, M. lucifugus, a member of the Vespertilionidae family. Both P. vampyrus and M. lucifugus appear to have a single IFNγ locus in their genomes similar to other mammalian species. Using tissue obtained from an individual P. alecto that had been experimentally infected with SARS-CoV, we identified a single transcribed IFNγ gene in our model pteropid species. Compared to other mammalian IFNγ genes, the P. alecto sequence shared greatest amino acid identity with the two bat IFNγ sequences from P. vampyrus (99%) and M. lucifugus (70%) and lowest similarity with mouse (44%). A number of conserved features were identified in the bat IFNγ sequence that are consistent with it being functionally equivalent to IFNγ proteins identified in other species. These include the six α helical structure of the translated protein, the NLS and the KRKR in the C-terminal region that has been shown to be essential for IFNγ function. The NLS has been demonstrated to be conserved across evolution from fish and amphibians through to mammals and has been implicated in binding to an acidic stretch on the IFNγ receptor (Griggs et al., 1992, Savan et al., 2009). In common with IFNγ genes from other species, the P. alecto IFNγ sequence also appeared to be highly hydrophobic and contained a number of potential N-linked glycosylation sites.
IFNγ is induced predominantly as a result of the activation of NK and T cells (Schroder et al., 2004). To examine the ability of activated T cells to produce IFNγ, we examined IFNγ production by mitogen activated bat splenocytes, a rich source of T cells. IFNγ was transcribed in P. alecto splenocytes stimulated with PHA and to a lesser extent in ConA stimulated splenocytes. As ConA and PHA typically stimulate T cells in other mammals, these results are consistent with the possibility that the IFNγ producing cells in P. alecto are also activated T cells. Using P. alecto recombinant bat IFNγ as an immunogen we successfully generated a bat specific IFNγ mAb and a polyclonal chicken IgY antibody against the E. coli expressed bat IFNγ protein. A sandwich ELISA developed using these two bat specific IFNγ reagents resulted in the detection of IFNγ in supernatants from PHA stimulated bat splenocytes, thus demonstrating the production of IFNγ protein. Overall, these results demonstrate the production of IFNγ by stimulated bat splenocytes and the use of important bat specific immunological reagents. The production of IFNγ has been used as an indicator of cell mediated immunity in a number of species and as a diagnostic marker for disease. For example, IFNγ has been used for the diagnosis of tuberculosis in cattle (Wood and Jones, 2001) and a relationship between IFNγ production and disease protection has been reported in pigs infected with classical swine fever virus (Suradhat et al., 2001). The availability of anti-bat IFNγ reagents will now allow us to characterise the timing and production of IFNγ following viral infection in bats, providing information on the role of cell mediated immunity in viral infections.
IFNγ was first recognised on the basis of its anti-viral activity and only later shown to act primarily as an immunomodulator (Schroder et al., 2004, Wheelock, 1965). Only one report of IFN antiviral activity has been reported in bats with the demonstration that recombinant P. alecto type III IFN is capable of inhibiting the replication of the bat orthoreovirus, Pulau virus (Zhou et al., 2011b). To determine whether IFNγ in bats also exhibits antiviral activity similar to IFNs from other species, we examined the ability of rbIFNγCHO to inhibit the replication of SFV. Although SFV has not been identified in bats, it replicates to a high level in cells from a variety of species, including our bat cell lines. P. alecto IFNγ displayed antiviral activity in SFV infected kidney cells from P. alecto and in the lung cell line derived from the microbat, T. brasiliensis. Both cell lines responded to a similar range of IFNγ dilutions, thus demonstrating that the recombinant P. alecto IFNγ protein has reactivity across both suborders of bats (megabats and microbats). Bovine IFNγ demonstrates cross reactivity with closely related species such as cattle, buffalo, sheep and goat but not between different orders of mammals such as pigs and horses (Rothel et al., 1990). The close similarity of the P. alecto IFNγ sequence with the two other bat IFNγ genes is consistent with the cross reactivity of recombinant IFNγ observed in our antiviral assays. However, the lower sequence similarity with other mammalian species indicates its activity is likely highly specific for the order Chiroptera.
To assess the ability of bat IFNγ to exert antiviral activity against a virus known to be carried by bats, we tested the antiviral activity of rbIFNγCHO against HeV which is naturally harboured by P. alecto. HeV has been demonstrated to antagonise type I IFN production in virus infected human cells and antagonise both type I IFN production and signalling pathways in virus infected bat cell lines (Virtue et al., 2011a, Virtue et al., 2011b). However, the role of type II IFN in HeV infection has not been investigated. Our results provide the first evidence that bat IFNγ is capable of mitigating HeV infection in our P. alecto cell line. This result provides preliminary information on the antiviral effect of IFNγ on HeV infection in bat cells and will form the basis of future experiments to examine the role of IFNγ during viral infection in bats, including its immunoregulatory roles.
As natural reservoirs for a variety of zoonotic viruses, bats have the potential to provide important insights into antiviral strategies that may result in the development of new therapeutics for other mammals. This study represents the first evidence, to our knowledge for a type II IFN response in any species of bat, demonstrating that bat IFNγ is produced by a similar subset of cells to other species and has antiviral activity. The availability of recombinant bat IFNγ will allow us to examine the immunoregulatory effects of IFNγ in bats on various cell types and virus infections. Reagents to detect native IFNγ will allow us to examine the kinetics of IFNγ production during viral infection and provide valuable insights into the cell mediated immune responses of bats. These tools are an important step in elucidating the mechanisms responsible for the asymptomatic nature of viral infections in bats.
Acknowledgements
The authors wish to acknowledge support from a CSIRO CEO Science Leaders award to L.-F.W. We thank Craig Smith, Hume Field, Jenn Barr, Deborah Middleton and Susanne Wilson for provision of bat tissues for this study, Matt Bruce for statistical analyses and John Lowenthal and Peng Zhou for critical reading of the manuscript.
References
- Aljofan M., Porotto M., Moscona A., Mungall B.A. Development and validation of a chemiluminescent immunodetection assay amenable to high throughput screening of antiviral drugs for Nipah and Hendra virus. Journal of Virological Methods. 2008;149:12–19. doi: 10.1016/j.jviromet.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljofan M., Saubern S., Meyer A.G., Marsh G., Meers J., Mungall B.A. Characteristics of Nipah virus and Hendra virus replication in different cell lines and their suitability for antiviral screening. Virus Research. 2009;142:92–99. doi: 10.1016/j.virusres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.F. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Andrew M., Morris K., Bruce M., O’Neil T., Jansen E., Coupar B., Strom D. Sustained biological effects of porcine interleukin 5 delivered to pigs as recombinant protein or via a DNA vector. Cytokine. 2007;40:193–200. doi: 10.1016/j.cyto.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Arnold K., Bordoli L., Kopp J., Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Baker M., Tachedjian M., Wang L.-F. Immunoglobulin heavy chain diversity in pteropid bats: evidence for a diverse and highly specific antigen binding repertoire. Immunogenetics. 2010;62:173–184. doi: 10.1007/s00251-010-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm U., Klamp T., Groot M., Howard J.C. Cellular responses to interferon-γ. Annual Review of Immunology. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Calisher C.H. Bats: important reservoir hosts of emerging viruses. Clinical Microbiology Reviews. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A.K., Chakravarty A.K. Antibody-mediated immune response in the bat, Pteropus giganteus. Developmental and Comparative Immunology. 1984;8:415–423. doi: 10.1016/0145-305x(84)90048-x. [DOI] [PubMed] [Google Scholar]
- Chakravarty A.K., Paul B.N. Analysis of suppressor factor in delayed immune responses of a bat, Pteropus giganteus. Developmental and Comparative Immunology. 1987;11:649–660. doi: 10.1016/0145-305x(87)90053-x. [DOI] [PubMed] [Google Scholar]
- Cowled C., Baker M., Tachedjian M., Zhou P., Bulach D., Wang L.-F. Molecular characterisation of Toll-like receptors in the black flying fox Pteropus alecto. Developmental and Comparative Immunology. 2011;35:7–18. doi: 10.1016/j.dci.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowled, C., Baker, M., Zhou, P., Tachedjian, M., Wang, L.-F., in press. Molecular characterisation of RIGI-like helicases in the Black flying fox, Pteropus alecto. Developmental and Comparative Immunology. doi:10.1016/j.dci.2011.11.008. [DOI] [PMC free article] [PubMed]
- Crameri G., Todd S., Grimley S., McEachern J.A., Marsh G.A., Smith C., Tachedjian M., De Jong C., Virtue E.R., Yu M., Bulach D., Liu J.-P., Michalski W.P., Middleton D., Field H.E., Wang L.-F. Establishment, immortalisation and characterisation of pteropid bat cell lines. PLoS ONE. 2009;4:e8266. doi: 10.1371/journal.pone.0008266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ley M., van Damme J., Claeys H., Weening H., Heine J.W., Billiau A., Vermylen C., de Somer P. Interferon induced in human leukocytes by mitogens: production, partial purification and characterization. European Journal of Immunology. 1980;10:877–883. doi: 10.1002/eji.1830101113. [DOI] [PubMed] [Google Scholar]
- Farrar M.A., Schreiber R.D. The molecular cell biology of interferon-gamma and its receptor. Annual Review of Immunology. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Field H.E., Breed A.C., Shield J., Hedlefs R.M., Pittard K., Pott B., Summers P.M. Epidemiological perspectives on Hendra virus infection in horses and flying foxes. Australian Veterinary Journal. 2007;85:268–270. doi: 10.1111/j.1751-0813.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- Finter N.B. Dye uptake methods for assessing viral cytopathogenicity and their application to interferon assays. Journal of General Virology. 1969;5:419–427. [Google Scholar]
- Gotoh B., Komatsu T., Takeuchi K., Yokoo J. Paramyxovirus strategies for evading the interferon response. Reviews in Medical Virology. 2002;12:337–357. doi: 10.1002/rmv.357. [DOI] [PubMed] [Google Scholar]
- Griggs N., Jarpe M., Pace J., Russell S., Johnson H. The N-terminus and C-terminus of IFN-gamma are binding domains for cloned soluble IFN-gamma receptor. The Journal of Immunology. 1992;149:517–520. [PubMed] [Google Scholar]
- Hatten B.A., Allen R., Sulkin S.E. Immune response in chiroptera to bacteriophage øX174. The Journal of Immunology. 1968;101:141–150. [PubMed] [Google Scholar]
- Janardhana V., Ford M.E., Bruce M.P., Broadway M.M., Neil T.E., Karpala A.J., Asif M., Browning G.F., Tivendale K.A., Noormohammadi A.H., Lowenthal J.W., Bean A.G.D. IFN-gamma enhances immune responses to E. coli infection in the chicken. Journal of Interferon and Cytokine Research. 2007;27:937–946. doi: 10.1089/jir.2007.0020. [DOI] [PubMed] [Google Scholar]
- Kepler T., Sample C., Hudak K., Roach J., Haines A., Walsh A., Ramsburg E. Chiropteran types I and II interferon genes inferred from genome sequencing traces by a statistical gene-family assembler. BMC Genomics. 2010;11:444. doi: 10.1186/1471-2164-11-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Tamura K., Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Briefings in Bioinformatics. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leroy E.M., Epelboin A., Mondonge V., Pourrut X., Gonzalez J.-P., Muyembe-Tamfum J.-J., Formenty P. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Diseases. 2009;9:723–728. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P., Délicat A., Paweska J.T., Gonzalez J.-P., Swanepoel R. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- McMurray D.N., Thomas M.E. Cell-mediated immunity in two species of bats. Journal of Mammalogy. 1979;60:576–581. [Google Scholar]
- Murray K., Selleck P., Hooper P., Hyatt A., Gould A., Gleeson L., Westbury H., Hiley L., Selvey L., Rodwell B. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- Paul B.N., Chakravarty A.K. In vitro analysis of delayed immune response in a bat, Pteropus giganteus: process of con-A mediated activation. Developmental and Comparative Immunology. 1986;10:55–67. doi: 10.1016/0145-305x(86)90044-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez J.J., Horvath C.M. Host evasion by emerging paramyxoviruses: Hendra virus and Nipah virus v proteins inhibit interferon signaling. Viral Immunology. 2004;17:210–219. doi: 10.1089/0882824041310568. [DOI] [PubMed] [Google Scholar]
- Rothel J.S., Jones S.L., Corner L.A., Cox J.C., Wood P.R. A sandwich enzyme immunoassay for bovine interferon-γ and its use for the detection of tuberculosis in cattle. Australian Veterinary Journal. 1990;67:134–137. doi: 10.1111/j.1751-0813.1990.tb07730.x. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Savan R., Ravichandran S., Collins J.R., Sakai M., Young H.A. Structural conservation of interferon gamma among vertebrates. Cytokine and Growth Factor Reviews. 2009;20:115–124. doi: 10.1016/j.cytogfr.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K., Hertzog P.J., Ravasi T., Hume D.A. Interferon-γ: an overview of signals, mechanisms and functions. Journal of Leukocyte Biology. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Slodowski O., BÖHm J., SchÖNe B., Otto B. Carboxy-terminal truncated rhuIFN-γ with a substitution of Gln133 or Ser132 to leucine leads to higher biological activity than in the wild type. European Journal of Biochemistry. 1991;202:1133–1140. doi: 10.1111/j.1432-1033.1991.tb16481.x. [DOI] [PubMed] [Google Scholar]
- Subramaniam P.S., Mujtaba M.G., Paddy M.R., Johnson H.M. The carboxyl terminus of interferon-γ contains a functional polybasic nuclear localization sequence. Journal of Biological Chemistry. 1999;274:403–407. doi: 10.1074/jbc.274.1.403. [DOI] [PubMed] [Google Scholar]
- Suradhat S., Intrakamhaeng M., Damrongwatanapokin S. The correlation of virus-specific interferon-gamma production and protection against classical swine fever virus infection. Veterinary Immunology and Immunopathology. 2001;83:177–189. doi: 10.1016/s0165-2427(01)00389-0. [DOI] [PubMed] [Google Scholar]
- Swanepoel R., Leman P.A., Burt F.J., Zachariades N.A., Braack L.E., Ksiazek T.G., Rollin P.E., Zaki S.R., Peters C.J. Experimental inoculation of plants and animals with Ebola virus. Emerging Infectious Diseases. 1996;2:321–325. doi: 10.3201/eid0204.960407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than, K.A., Edgar, J.A., 1998. Saponin adjuvant composition. In: CSIRO (Ed.), Australia.
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek M.F., Muller U., Huang S., Aguet M., Zinkernagel R.M. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. Journal of Virology. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue E.R., Marsh G.A., Baker M.L., Wang L.-F. Interferon production and signaling pathways are antagonized during henipavirus infection of fruit bat cell lines. PLoS ONE. 2011;6:e22488. doi: 10.1371/journal.pone.0022488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue E.R., Marsh G.A., Wang L.-F. Interferon signaling remains functional during henipavirus infection of human cell lines. Journal of Virology. 2011;85:4031–4034. doi: 10.1128/JVI.02412-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellehan J.F.X., Jr., Green L.G., Duke D.G., Bootorabi S., Heard D.J., Klein P.A., Jacobson E.R. Detection of specific antibody responses to vaccination in variable flying foxes (Pteropus hypomelanus) Comparative Immunology, Microbiology and Infectious Diseases. 2009;32:379–394. doi: 10.1016/j.cimid.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock E.F. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science. 1965;149:310–311. [PubMed] [Google Scholar]
- Williamson M.M., Hooper P.T., Selleck P.W., Gleeson L.J., Daniels P.W., Westbury H.A., Murray P.K. Transmission studies of Hendra virus (equine morbilli-virus) in fruit bats, horses and cats. Australian Veterinary Journal. 1998;76:813–818. doi: 10.1111/j.1751-0813.1998.tb12335.x. [DOI] [PubMed] [Google Scholar]
- Williamson M.M., Hooper P.T., Selleck P.W., Westbury H.A., Slocombe R.F. Experimental Hendra virus infectionin pregnant guinea-pigs and fruit bats (Pteropus poliocephalus) Journal of Comparative Pathology. 2000;122:201–207. doi: 10.1053/jcpa.1999.0364. [DOI] [PubMed] [Google Scholar]
- Wong S., Lau S., Woo P., Yuen K.-Y. Bats as a continuing source of emerging infections in humans. Reviews in Medical Virology. 2007;17:67–91. doi: 10.1002/rmv.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P.R., Jones S.L. BOVIGAMTM: an in vitro cellular diagnostic test for bovine tuberculosis. Tuberculosis (Edinburgh, Scotland) 2001;81:147–155. doi: 10.1054/tube.2000.0272. [DOI] [PubMed] [Google Scholar]
- Zhou P., Cowled C., Marsh G.A., Shi Z., Wang L.-F., Baker M.L. Type III IFN receptor expression and functional characterisation in the pteropid bat, Pteropus alecto. PLoS ONE. 2011;6:e25385. doi: 10.1371/journal.pone.0025385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Cowled C., Todd S., Crameri G., Virtue E.R., Marsh G.A., Klein R., Shi Z., Wang L.F., Baker M.L. Type III IFNs in pteropid bats: differential expression patterns provide evidence for distinct roles in antiviral immunity. Journal of Immunology. 2011;186:3138–3147. doi: 10.4049/jimmunol.1003115. [DOI] [PMC free article] [PubMed] [Google Scholar]