Highlights
► Molecular characterisation of RIG-I-like helicases in the fruit bat Pteropus alecto. ► Bat RLHs are structurally conserved with those in other vertebrates. ► Bat RLHs are ubiquitously expressed and enriched in immune tissues. ► Treatment of bat kidney cells with poly I:C produces an fibroblast-like IFN response. ► Poly I:C induces upregulation of bat RLHs, indicating they are also ISGs.
Keywords: Innate immunity, Viral infection, Fruit bat, Pteropus alecto, RIG-I, mda5, LGP2
Abstract
The RIG-I like helicases, RIG-I, mda5 and LGP2 are an evolutionarily conserved family of cytosolic pattern recognition receptors important in the recognition of viral RNA, and responsible for the innate induction of interferons and proinflammatory cytokines upon viral infection. Bats are natural reservoir hosts to a variety of RNA viruses that cause significant morbidity and mortality in other species; however the mechanisms responsible for the control of viral replication in bats are not understood. This report describes the molecular cloning and expression analysis of RIG-I, mda5 and LGP2 genes in the fruit bat Pteropus alecto, and is the first description of RIG-I like helicases from any species of bat. Our results demonstrate that P. alecto RIG-I, mda5 and LGP2 have similar primary structures and tissue expression patterns to their counterparts in humans and other mammals. Stimulation of bat kidney cells with synthetic dsRNA (poly I:C) induced high levels of interferon β and rapid upregulation of all three helicases. These findings reveal that the cytoplasmic virus sensing machinery is present and intact in P. alecto. This study provides the foundation for further investigations into the interactions between bat RIG-I-like helicases and viruses to elucidate the mechanisms responsible for the asymptomatic nature of viral infections in bats.
1. Introduction
Bats are poorly understood, yet have attracted increasing attention since being recognized as the source of numerous high-profile emerging viral diseases (Calisher et al., 2006). Fruit bats are known reservoir hosts of Hendra virus (Murray et al., 1995, Halpin et al., 2000), Nipah virus (Chua et al., 2000), Ebola virus (Leroy et al., 2005), Marburg virus (Towner et al., 2009), Melaka virus (Chua et al., 2007), and Australian bat lyssavirus (van der Poel et al., 2006, Speare et al., 1997), whereas microbats are known reservoir hosts of Rabies, and likely reservoir hosts of Severe acute respiratory syndrome coronavirus (SARS CoV) (Lau et al., 2005, Li et al., 2005). While these viruses are highly pathogenic in other mammals, experimental studies and field observations have shown that bats rarely display clinical signs upon viral infection (Williamson et al., 1998, Calisher et al., 2006, Middleton et al., 2007, Towner et al., 2009, van den Hurk et al., 2009). One hypothesis under consideration is that bats possess qualitative differences in their innate immune system. In order to address this hypothesis, we are seeking to identify and characterise elements of the bat innate immune system.
In higher organisms, cellular recognition of infection begins with the engagement of pattern recognition receptors (PRRs) which function by recognising conserved pathogen-associated molecular patterns (PAMPs), thereby initiating signalling cascades that lead to inflammatory responses. The two major classes of virus-sensing PRRs are the transmembrane Toll-like receptors (TLRs) expressed on the cell surface or within endosomes, and the retinoic acid-inducible gene I (RIG-I)-like RNA helicases (RLHs) which are expressed cytoplasmically. The RLH gene family is evolutionarily ancient and consists of three genes; RIG-I (also known as DDX58), melanoma differentiation associated protein 5 (mda5/IFIH1), and laboratory of genetics and physiology 2 (LGP2/DHX58). We previously showed that fruit bats transcribe intact TLRs 1–10 and a TLR13-like pseudogene, and thus possess all of the TLRs believed to be involved in antiviral immunity in other mammals (Cowled et al., 2011); however this is the first study to determine whether bats also possess an intact set of RLHs.
RIG-I, mda5 and LGP2 each contain an ATP-binding DExD/H-box helicase domain and a C-terminal regulatory domain, both of which are required for ligand recognition (Fuller-Pace, 2006, Lu et al., 2011). RIG-I and mda5 (but not LGP2) each contain two N-terminal caspase activation and recruitment domains (CARDs) required for downstream signalling (Johnson and Gale, 2006). RIG-I and mda5 recognise short and long dsRNAs respectively and RIG-I detects dsRNA with uncapped 5′-triphosphate RNA (Hornung et al., 2006, Kato et al., 2008). Ligand binding produces a conformational change in RIG-I and mda5 that exposes the CARD domains, thereby permitting interaction with mitochondria-located adaptor molecule (MAVS/IPS1). This event initiates a signalling pathway that culminates in activation of transcription factors including IRFs and NF-κB, which translocate to the nucleus to induce transcription of type I IFNs, proinflammatory cytokines and chemokines (Yoneyama and Fujita, 2010).
Knockout studies in mice have demonstrated critical roles for the RLHs in initiating antiviral immune responses in vivo, and have shown that rather than being redundant to each other, the different RLHs provide protection from specific classes of viruses. RIG-I is implicated in the response to negative sense RNA viruses including flaviviruses, orthomyxoviruses, paramyxoviruses and rhabdoviruses, whereas mda5 is important in the response to positive sense RNA viruses such as picornaviruses, and is also a potent cytoplasmic receptor for poly I:C (Kato et al., 2006, Gitlin et al., 2006). When initially identified, overexpression of LGP2 in vitro resulted in apparent negative regulation of RIG-I and mda5 (Yoneyama et al., 2005; Rothenfusser et al. 2005), however knockout studies have since shown that LGP2 can positively regulate both RIG-I and mda5 signalling in some instances (Venkataraman et al., 2007, Satoh et al., 2010). The exact role of LGP2 requires further clarification.
In the absence of commercial reagents or prototype sequence data, there is a strong need to identify and define components of the bat immune system. RLHs are important components of the innate antiviral immune system in other species but to date have not been described in bats. To address the hypothesis that bats may have atypical RLH genes, we performed molecular cloning and transcriptional analysis of the RLH gene family in the fruit bat, Pteropus alecto.
2. Materials and methods
2.1. Tissue collection and cell culture
P. alecto bats were trapped in Southern Queensland, Australia, and transported alive by air to the Australian Animal Health Laboratory (AAHL) in Victoria, where they were euthanised for dissection using methods approved by the AAHL animal ethics committee. Tissues were stored at −80 °C in RNAlater (Ambion). PaKiT03 cells are an immortalised kidney cell line derived from P. alecto primary tissue as described in Crameri et al. (2009). They were maintained in DMEM supplemented with 10% FCS and antibiotic/antifungal mixture.
2.2. RNA extraction and cDNA synthesis
Total RNA was extracted from frozen P. alecto tissues using a Precellys 24 tissue homogeniser (Bertin technologies) and an RNeasy mini kit (Qiagen) with on-column DNase-I treatment (Qiagen) to remove traces of genomic DNA. Total RNA was extracted from peripheral blood mononuclear cells (PBMC) harvested from P. alecto blood by density centrifugation using lymphoprep (Axis-Shield) and QIAshredders (Qiagen) for homogenisation, followed by RNA extraction using an RNeasy mini kit with on-column DNase-I treatment. RNA was quantified by absorption spectrometry, and then reverse transcribed into cDNA using Omniscript reverse transcriptase (Qiagen) according to the manufacturer’s instructions.
2.3. Sequence identification and molecular cloning
Sequences were identified in the genome of the Malaysian flying fox Pteropus vampyrus located in the Ensembl database (assembly pteVam1, 2.63x coverage, July 2008) and the corresponding GenBank trace file archive. Primers for Rapid Amplification of cDNA ends (RACE) and PCR were designed using Clone Manager 9.0 (Sci-Ed Software). Full-length amplification was performed using LongAmp Taq DNA polymerase (New England BioLabs Inc). 5′ and 3′ RACE were performed using a GeneRACER kit (Invitrogen). PCR and RACE products were cloned into the pCR4-TOPO vector using the TOPO TA Cloning Kit for sequencing (Invitrogen). M13 forward and M13 reverse universal sequencing primers were employed for DNA sequencing using a BigDye Terminator Cycle Sequencing Kit v3.1 (Applied Biosystems) and an Applied Biosystems 3130 XL Genetic Analyser.
2.4. Sequence and phylogenetic analysis
Sequence data were assembled with Seqman PRO (Lasergene) and analysed with Clone Manager 9.0 (Sci-Ed Software). Intron–exon maps were drawn using Fancy Gene v1.4 (Rambaldi and Ciccarelli, 2009). Protein domains were identified using the NCBI Conserved Domains search tool (Marchler-Bauer et al., 2011). Sequence alignments and phylogenetic trees were generated in MEGA 5 (Tamura et al., 2011) using the neighbour joining method with 1000 bootstrap replicates. Evolutionary distances were computed using Poisson correction.
2.5. Quantitative reverse transcription PCR (qRT-PCR)
qRT-PCR was performed on cDNA derived from tissues collected from three apparently healthy wild-caught P. alecto bats. Total RNA was prepared from peripheral blood mononuclear cells (PBMC), lymph node, spleen, liver, lung, heart, kidney, small intestine, brain and salivary glands following the procedure described above, including on-column DNaseI treatment to control for residual genomic DNA (gDNA) contamination. Quantitect reverse transcriptase for RT-PCR (Qiagen) was then used to perform a second round of gDNA digestion followed by cDNA synthesis. For each sample, 0.5–1 μg total RNA was used as template in a 20 μl cDNA synthesis reaction. qRT-PCR primers were designed using Primer Express 3.0 (Applied Biosystems) with default settings (listed in Table 2 ). Reactions were carried out using EXPRESS SYBR® GreenER™ qPCR Supermix Universal (Invitrogen) and an Applied Biosystems 7500 Fast Real-Time qPCR instrument. Final qPCR reaction volumes were 10 μl and carried out in 96 well plates. Each reaction contained a final concentration of 200 nmol each primer and 2 μl of 1:5 diluted cDNA. Thermocycling consisted of 50 °C/2 min, 95 °C/2 min, 40× cycles of 90 °C/15s followed by 60 °C/1 min, and was followed by melt curve analysis. Copy numbers of target sequences were calculated using standard curves and normalised to 18s ribosomal RNA (for baseline tissue expression) or analysed without normalisation (poly I:C stimulation experiments).
Table 1.
Accession numbers of protein sequences used in phylogenetic analysis.
| Species | RIG-I | mda5 | LGP2 |
|---|---|---|---|
| Homo sapiens (human) | NP_055129.2 | NP_071451.2 | NP_077024.2 |
| Mus musculus (mouse) | NP_766277.3 | NP_082111.2 | NP_084426.2 |
| Oryctolagus cuniculus (rabbit) | XP_002708086.1 | XP_002712267.1 | XP_002719437.1 |
| Rattus norvegicus (rat) | NP_001100115.1 | NP_001102669.1 | NP_001092258.1 |
| Ailuropoda melanoleuca (panda) | EFB16979.1 | EFB18884.1 | EFB20114.1 |
| Bos taurus (cattle) | XP_580928.2 | XP_615590.2 | NP_001015545.1 |
| Canis lupus familiaris (dog) | XP_545493.2 | XP_850033.1 | |
| Equus caballus (horse) | XP_001497895.2 | XP_001494380.1 | XP_001495262.2 |
| Sus scrofa (pig) | NP_998969.1 | NP_001093664.1 | NP_001186061.1 |
| Danio rerio (zebrafish) | XP_694124.2 | ||
| Salmo salar (salmon) | NP_001157171.1 | NP_001133649.1 |
Table 2.
qRT-PCR primers used in this study.
| Gene | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| RIG-I | CTCAGGTCGTTGGGCTGACT | CCAAGGCTTCACCTGTGCTT |
| mda5 | TCGCTATGGTCTCGTCACTAATG | CAGGACGTAGGTGCTCTCATCA |
| LGP2 | CCAGCTGCAGGAGCACAAC | CGCCTGTGGCAAAGATCATA |
| IFN-α | GAGACTCCCCTGCTGGATGA | ATAGAGGGTGATTCTCTGGAAGTATTTC |
| IFN-β | CTCTAGCACTGGCTGGAATGAA | TGCCCACCGAGTGTCTCA |
| IFN-λ (IL-28) | CATGTCCCAGTTCAGGTCTCTGT | CATCCTTGGCCCTCTTGAAA |
| 18s | CACGGCGACTACCATCGAA | CGGCGACGACCCATTC |
2.6. In vitro stimulation with polyinosinic-polycytidylic acid
PaKiT03 cells (an immortal cell line derived from P. alecto primary kidney cells) were challenged in vitro with 0.001–10 μg/ml of the dsRNA homologue polyinosinic-polycytidylic acid (poly I:C, Sigma) either by direct addition to the culture media or in complex with Lipofectamine 2000 (Invitrogen) to mimic viral infection. Three hours post challenge cells were collected into buffer RLT (Qiagen) for total RNA extraction and qRT-PCR as described above.
3. Results and discussion
3.1. Cloning and sequence analysis of P. alecto RLH’s
Although no whole genome sequence is currently available for P. alecto, a low coverage genome sequence is publicly available in Ensembl for the closely related pteropid bat, P. vampyrus. Three RIG-I like helicases were identified by keyword searching of the P. vampyrus genome using the search terms DDX58 (RIG-I), IFIH1 (mda5) and DHX58 (LGP2). Blast queries of the public databases using these sequences confirmed their homology to corresponding genes from other species. BLAT searches of the P. vampyrus genome using these sequences as well as human and mouse homologues of these genes did not identify any additional homologues in P. vampyrus, nor did BLAST searches of the trace file archive from which the genome was constructed.
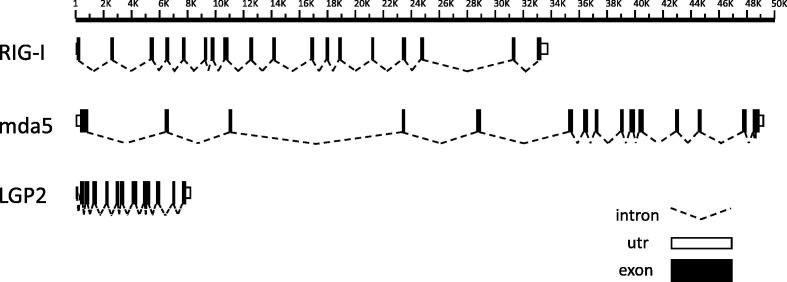
To determine the complete gene structure of RIG-I, mda5 and LGP2 in P. alecto, RACE PCR was performed using primers based on sequences identified in the P. vampyrus genome sequence using spleen cDNA as a template. Inwards facing PCR primers were then designed from the RACE products to amplify the entire coding region of each gene as a single large PCR product, which was then cloned for sequencing. The longest transcript obtained for each gene was designated the prototype and full-length mRNA sequences were deposited into GenBank under the accession numbers JN031514-JN031516. Each gene contained a single open reading frame corresponding in size to homologues in other mammalian species. P. alecto RLH mRNA sequences were compared to the corresponding P. vampyrus genomic DNA sequences to determine the intron–exon structure (Fig. 1 ). Mda5 was found to be the longest of the RLHs, consisting of 16 exons encoding a 3075 bp open reading frame (ORF). RIG-I consisted of 18 exons encoding a 2790 bp ORF, whereas LGP2 consisted of 12 coding exons plus one non-coding 5’ exon encoding a 2037 bp ORF. The bat RLHs are of typical length and organisation to corresponding genes in other mammals, however bat RIG-I and LGP2 occupy considerably less genomic DNA than their human counterparts (RIG-I: 34 vs. 71 kb, LGP2: 8 vs. 11 kb) due to reduced intron sizes, whereas mda5 is similar in bat and human (50 vs. 52 kb). This is not an unexpected finding, since bat genomes are known to be more constrained than in other vertebrates, a feature shared with birds and hypothesised to be a consequence of adaptation to flight (Smith and Gregory, 2009). Kozak consensus sequences for strong eukaryotic translation consist of either adenine or guanine in the −3 position and guanine in the +4 position relative to the start codon (Kozak, 1997). LGP2 contained both Kozak elements (−3: adenine,+4: guanine), whereas RIG-I and mda5 each contained only one Kozak element (−3: adenine). Polyadenylation signals (AATAAA) were present in the 3′ UTRs of all three transcripts in close proximity to the Poly A + tail.
Fig. 1.
Predicted intron–exon organisation of P. alecto RLHs. The intron–exon arrangements of pteropid bat RIG-I, mda5 and LGP2 were predicted by nucleotide alignment of P. alecto mRNA and P. vampyrus genomic DNA. Predicted coding and non-coding exons are drawn as light and dark rectangles respectively, while putative introns are indicated by dotted lines. The figure is drawn to scale and distances are given in bp, however some intron sizes may be underestimated due to gaps in the P. vampyrus genome assembly. mda5 exon 8 is absent from the P. vampyrus genome assembly due to a sequencing gap, however it is expected to be a contiguous exon in the pteropid bats as it is highly conserved and present as a single exon in other species examined. In all other cases, intron–exon boundaries were clearly identified by homology with other species and by the presence of splice site consensus sequences.
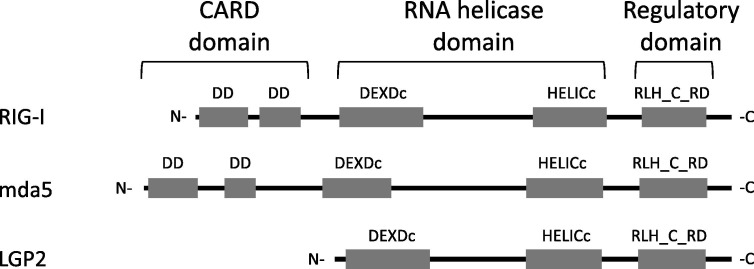
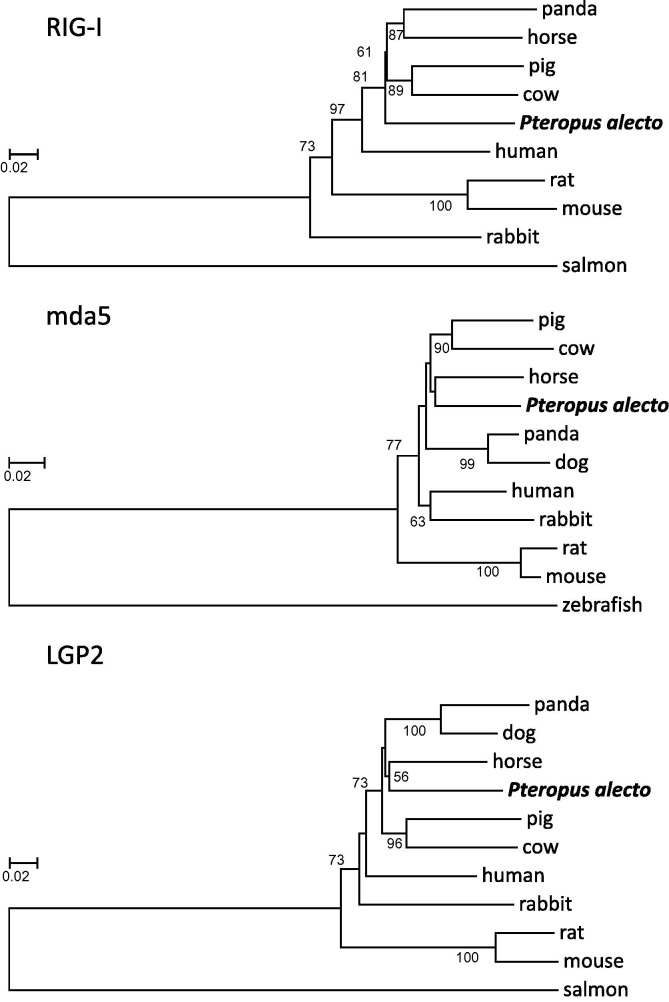
P. alecto RIG-I, mda5 and LGP2 genes are predicted to encode proteins with molecular weights of 107, 116, and 77 kDa, respectively. The predicted amino acid sequences were analysed by conserved domain analysis to identify putative domains (Fig. 2 ). Both RIG-I and mda5 (but not LGP2) contained two N-terminal CARD domains, while all three proteins contained a DExD/H-box RNA helicase domain and a C-terminal regulatory domain. The protein domain organisation of bat RLHs is therefore highly conserved with other mammalian species and thus indicative of similar function. At the amino acid sequence level, P. alecto RIG-I and LGP2 were 82% identical to their human counterparts, while P. alecto mda5 was 85% identical to human mda5. Phylogenetic trees were constructed based on the amino acid sequences of RIG-I, mda5 and LGP2 from P. alecto and other mammals (Fig. 3 ). P. alecto mda5 and LGP2 were most closely related to horse genes, followed by other members of Laurasiatheria. P. alecto RIG-I was slightly more divergent but still clustered with Laurasiatheria. At both the nucleotide and amino acid level, interspecies differences appeared to be evenly distributed across the open reading frame with no obvious hypervariable regions. Although several of the predicted branch points were only weakly supported by bootstrap analysis, these findings support the idea that fruit bats are quite closely related to horses. This is supported by a recent phylogenetic study based on retrotransposon insertion analysis (Nishihara et al., 2006), as well as findings from previous studies of bat immune genes (Fujii et al., 2010, Iha et al., 2009, Iha et al., 2010, Cowled et al., 2011) and our own additional observations, and might be considered significant in light of the fact that horses are the main spillover host for Hendra virus.
Fig. 2.
Predicted protein domain architecture of P. alecto RLHs. Conserved domain architecture analysis was performed for P. alecto RIG-I, mda5 and LGP2 proteins. Abbreviations: DEATH domain superfamily (DD); DEAD-like helicases superfamily (DEXDc), helicase superfamily c-terminal domain (HELICc), RIG-I like helicase superfamily c-terminal regulatory domain (RLH_C_RD).
Fig. 3.
Phylogenetic analysis of RLHs in P. alecto and other vertebrates. Phylogenetic trees were generated from amino acid sequence alignments of RIG-I, mda5 and LGP2 from P. alecto and other vertebrates (Accession numbers are listed in Table 1). Phylogenetic relationships were inferred using the Neighbor-Joining method with 1000 bootstrap replicates. Bootstrap values above 50% are shown next to the branches. The scale represents the number of amino acid substitutions per site.
3.2. Transcriptional analysis of P. alecto RLHs
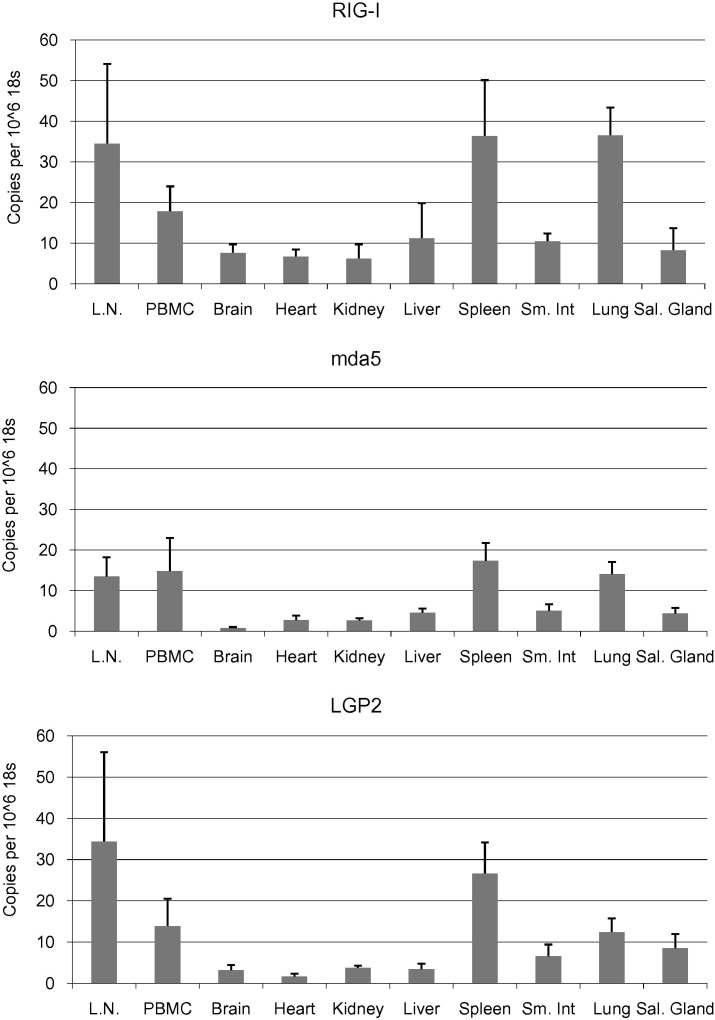
The transcriptional levels of RIG-I, mda5 and LGP2 were determined by qRT-PCR in 10 tissues collected from P. alecto (Fig. 4 ). All bats appeared healthy and returned negative test results for Hendra virus antibodies (data not shown); however we cannot exclude the possibility that one or more animals carried a naturally acquired infection at the time of capture. The fact that all three individual bats had very similar transcription profiles, however, suggests reliable baseline data for this species. All three RLHs were expressed at moderate levels in all tissues examined, however they were most highly expressed in tissues rich in immune cell types: PBMC, spleen, lymph node and lung. Previously we described the distribution of TLRs in P. alecto tissues (Cowled et al., 2011), demonstrating that the viral recognition receptors TLRs 7, 8 and 9 were also expressed at high levels in immune-rich tissues, at copy numbers substantially higher than observed here for the RLHs. In contrast, all three RLHs were expressed at higher copy numbers than TLR3 in all tissues examined, with the exception of liver in which TLR3 mRNA is elevated. Baseline expression of mda5 mRNA was minimal in P. alecto brain tissue, as is the case in both humans and mice (Kang et al., 2002, Furr et al., 2008).
Fig. 4.
Quantitative baseline mRNA expression analysis of RLHs in P. alecto tissues. Tissue mRNA expression levels of RIG-I, mda5 and LGP2 were determined by qRT-PCR and normalised relative to 18s rRNA. N = 3 individual apparently healthy wild-caught bats. Error bars represent standard deviation. Abbreviations: Lymph node (L.N.), Small intestine (Sm. Int.), Salivary gland (Sal. Gland).
3.3. Induction of Type I interferon and RLH transcription in bat cells
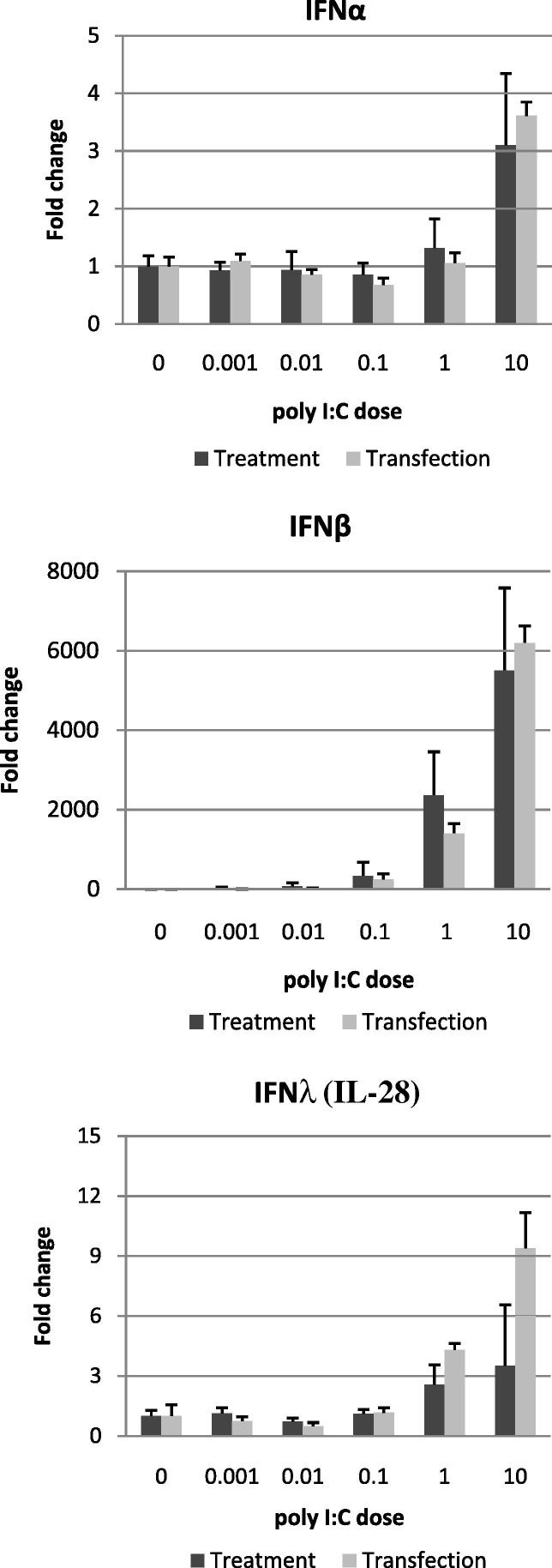
In other mammals, viral sensing via RIG-I and mda5 leads to production of type I interferon (IFN). mda5 (and to a lesser extent, RIG-I), recognise the synthetic ligand poly I:C in a response that mimics the response to viral dsRNA, including induction of high levels of Type I IFN (Kato et al., 2006, Yoneyama et al., 2005). In addition, poly I:C is also recognised by TLR3 (Matsumoto and Seya, 2008). Poly I:C can be delivered to cells either by direct addition to the media (treatment), or in combination with Lipofectamine 2000 (transfection) to mimic viral infection. We previously found that treatment or transfection with high doses of poly I:C (10–33 μg/ml) in a variety of bat cell types induced potent induction of Type I and Type III IFN (Zhou et al., 2011). To expand upon this, we challenged an immortalised P. alecto kidney fibroblast-like cell line (PaKiT03) by transfection or treatment with a wide dose range of poly I:C for 3 h, then used qRT-PCR to quantify IFN mRNA (Fig. 5 ).
Fig. 5.
Type I IFNs are upregulated in P. alecto kidney cells upon poly I:C challenge. PaKiT03 cells were challenged for 3 h with the indicated concentrations of poly I:C, either by direct addition to the supernatant (treatment), or in combination with Lipofectamine 2000 (transfection). Levels of IFN-α, IFN-β and IFNλ (IL-28) mRNA were measured by qRT-PCR using the standard curve method. Results are given as fold-change relative to mock treatment or mock transfection, respectively. N = 4 replicate cultures. Error bars represent standard deviation.
Poly I:C stimulation of PaKiT03 cells induced a potent IFN response dominated by IFNβ. At the highest dose of poly I:C, IFN-β showed >5000-fold upregulation, while neither IFN-α or IFNλ exceeded 10-fold upregulation. IFNβ was upregulated even at the lowest challenge dose of poly I:C (0.001 μg/ml), whereas IFNα and IFNλ were upregulated only in response to higher doses of poly I:C (⩾1 μg/ml). Very little difference was observed between treatment and transfection groups. Overall the IFN response to poly I:C was typical of a fibroblast-like cell line.
A low level of TLR3 mRNA was detected in PaKiT03 cells but was not upregulated at 3 h by either treatment or transfection with poly I:C (data not shown). While TLR3 is typically expressed in endosomes, it can also be expressed on the outer membrane in some cell types, and in any event, reagents and methods are unavailable to determine the subcellular localisation of either TLR3 or the RLHs in bat cells. Whether or not bat TLR3 is surface expressed, a recent study has shown that in both humans and mice, cell surface-expressed receptors called class A scavenger receptors (SR-As) bind and internalise dsRNA where it can be recognised by TLR3, RIG-I and mda5, thereby providing an explanation for how exogenous dsRNA can induce activation of intracellular dsRNA receptors (DeWitte-Orr et al., 2010). Therefore, while PaKiT03 cells exhibit a typical fibroblast pattern of type I IFN induction in response to poly I:C stimulation, further studies are required to determine the relative contributions of TLR3, RIG-I and mda5.
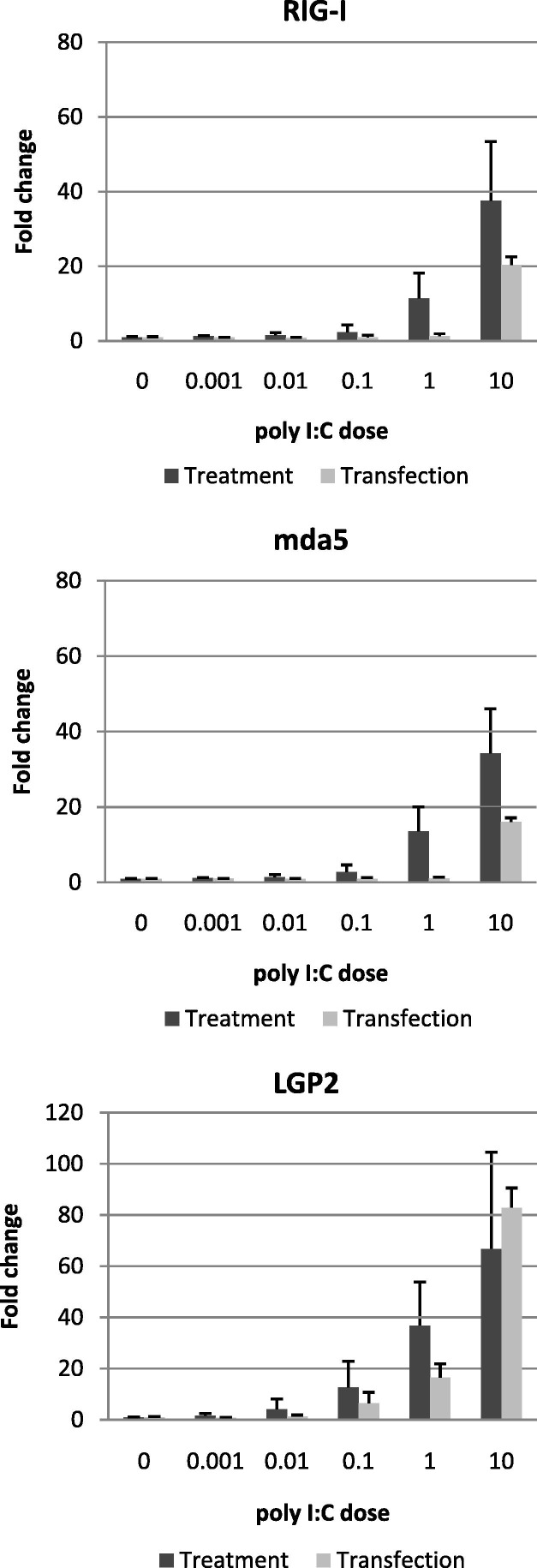
In other mammals, signalling through Type I and Type III IFN receptors results in upregulation of a large number of genes collectively known as IFN stimulated genes (ISGs). These include well-characterised antiviral genes such as Mx, PKR and OAS1, which help to establish the ‘antiviral state’ (Onoguchi et al., 2007). RLHs are important mediators of IFN production, but are also upregulated by IFN signalling in a positive feedback loop; hence they are also considered to be ISGs. Having confirmed that poly I:C stimulation induced rapid IFN upregulation in PaKiT03 cells, we tested to see whether this correlated with increased transcription of RIG-I, mda5 and LGP2 (Fig. 6 ). RIG-I, mda5 and LGP2 were all strongly upregulated at 3 h post poly I:C challenge, supporting the assertion that bat RLHs are also ISGs. This result is consistent with our previous results demonstrating upregulation of RIG-I following stimulation with recombinant IFN-λ in a bat foetal cell line (Zhou et al., 2011). At higher doses of poly I:C (⩾1 μg/ml), upregulation of all three RLHs was higher for treated cells than transfected cells. This may reflect minor differences in the kinetics of the response or differences in sensitivity between endogenous and exogenous dsRNA-sensing pathways, but does not directly correlate with the IFN response. It was noted that LGP2 was more strongly upregulated than either RIG-I or mda5 upon poly I:C stimulation.
Fig. 6.
RLHs are upregulated in P. alecto kidney cells upon poly I:C challenge. PaKiT03 cells were challenged for 3 h with the indicated concentrations of poly I:C, either by direct addition to the supernatant (treatment), or in combination with Lipofectamine 2000 (transfection). Levels of RIG-I, mda5 and LGP2 mRNA were measured by qRT-PCR using the standard curve method. Results are given as fold-change relative to mock treatment or mock transfection, respectively. N = 4 replicate cultures. Error bars represent standard deviation.
4. Conclusions
It has been proposed that bats’ role as reservoir hosts of zoonotic viruses combined with an apparent ability to withstand viral infections without showing signs of disease may reflect fundamental differences in the bat immune system. Despite the potential significance of this proposal and the increasing scientific and public interest in bats, major gaps exist in our basic understanding of bat immunity. Due to the critical role of cytoplasmic RNA sensors in innate immune responses to viral infection in other mammals, we sought to characterise the RIG-I-like helicase gene family in the fruit bat P. alecto. We hypothesised that bat RLHs may have atypical features that could imply altered immune response to viral infections; however our data lead us to conclude that they instead share a high degree of similarity with those in other mammalian species. Previous studies on pteropid bats to date have similarly shown high levels of sequence similarity between bat immune genes and their counterparts in other mammals, including descriptions of immunoglobulin heavy chains (Baker et al., 2010), CD4 (Omatsu et al., 2006), IFN-α/β (Omatsu et al., 2008, Kepler et al., 2010), IFN-λ (Zhou et al., 2011), STAT1(Fujii et al., 2010), IL-2, IL-4, IL-6, IL-10, IL-12p40, TNFα (Iha et al., 2009) and TLRs (Iha et al., 2010, Cowled et al., 2011).
It seems increasingly likely that if fundamental differences in the bat immune system are to be identified, new methods are required for functional studies in bat cell lines, such as overexpression of innate immune genes and siRNA-mediated gene silencing. It is also clear that new approaches are required to identify components that do not have a clear homologue in other mammals, or function only in immune cell types. It is clear that the publically available genome of the pteropid bat P. vampyrus contains many errors, as many genes are incomplete, miss-assembled, or missing entirely. Furthermore, as it has been assembled based on similarity to the human genome, it is possible that elements which may contribute to altered immune function are excluded from the assembly for the very reason that they do not have a human counterpart. Basic characterisation of clearly-defined prototype bat immune genes therefore remains a high priority that will lead to greater understanding of the bat immune system and enable the development of much-needed reagents. We thus conclude that the RLHs are intact and apparently functional in P. alecto, and therefore likely to be critical components of the bat innate antiviral immune system, as they are in other mammals.
Acknowledgments
The authors wish to acknowledge support from a CSIRO CEO Science Leaders award to L-F.W. We thank Craig Smith, Carol De Jong, Hume Field, Gary Crameri, Jenn Barr and Susanne Wilson for provision of bat tissues.
Contributor Information
Christopher Cowled, Email: chris.cowled@csiro.au.
Michelle L. Baker, Email: michelle.baker@csiro.au.
References
- Baker M.L., Tachedjian M., Wang L.F. Immunoglobulin heavy chain diversity in Pteropid bats: evidence for a diverse and highly specific antigen binding repertoire. Immunogenetics. 2010;62:173–184. doi: 10.1007/s00251-010-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua K.B., Bellini W.J., Rota P.A., Harcourt B.H., Tamin A., Lam S.K., Ksiazek T.G., Rollin P.E., Zaki S.R., Shieh W., Goldsmith C.S., Gubler D.J., Roehrig J.T., Eaton B., Gould A.R., Olson J., Field H., Daniels P., Ling A.E., Peters C.J., Anderson L.J., Mahy B.W. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- Chua K.B., Crameri G., Hyatt A., Yu M., Tompang M.R., Rosli J., McEachern J., Crameri S., Kumarasamy V., Eaton B.T., Wang L.F. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc. Natl. Acad. Sci. USA. 2007;104:11424–11429. doi: 10.1073/pnas.0701372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowled C., Baker M., Tachedjian M., Zhou P., Bulach D., Wang L.F. Molecular characterisation of Toll-like receptors in the black flying fox Pteropus alecto. Dev. Comp. Immunol. 2011;35:7–18. doi: 10.1016/j.dci.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri G., Todd S., Grimley S., McEachern J.A., Marsh G.A., Smith C., Tachedjian M., De Jong C., Virtue E.R., Yu M., Bulach D., Liu J.P., Michalski W.P., Middleton D., Field H.E., Wang L.F. Establishment, immortalisation and characterisation of pteropid bat cell lines. PLoS One. 2009;4:e8266. doi: 10.1371/journal.pone.0008266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitte-Orr S.J., Collins S.E., Bauer C.M., Bowdish D.M., Mossman K.L. An accessory to the ‘Trinity’: SR-As are essential pathogen sensors of extracellular dsRNA, mediating entry and leading to subsequent type I IFN responses. PLoS Pathog. 2010;6:e1000829. doi: 10.1371/journal.ppat.1000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Watanabe S., Yamane D., Ueda N., Iha K., Taniguchi S., Kato K., Tohya Y., Kyuwa S., Yoshikawa Y., Akashi H. Functional analysis of Rousettus aegyptiacus “signal transducer and activator of transcription 1” (STAT1) Dev. Comp. Immunol. 2010;34:598–602. doi: 10.1016/j.dci.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Pace F.V. DExD/H box RNA helicases: multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res. 2006;34:4206–4215. doi: 10.1093/nar/gkl460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr S.R., Chauhan V.S., Sterka D., Jr., Grdzelishvili V., Marriott I. Characterization of retinoic acid-inducible gene-I expression in primary murine glia following exposure to vesicular stomatitis virus. J. Neurovirol. 2008;14:503–513. doi: 10.1080/13550280802337217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L., Barchet W., Gilfillan S., Cella M., Beutler B., Flavell R.A., Diamond M.S., Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin K., Young P.L., Field H.E., Mackenzie J.S. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J. Gen. Virol. 2000;81:1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- Hornung V., Ellegast J., Kim S., Brzozka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K.K., Schlee M., Endres S., Hartmann G. 5’-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Iha K., Omatsu T., Watanabe S., Ueda N., Taniguchi S., Fujii H., Ishii Y., Kyuwa S., Akashi H., Yoshikawa Y. Molecular cloning and sequencing of the cDNAs encoding the bat interleukin (IL)-2, IL-4, IL-6, IL-10, IL-12p40, and tumor necrosis factor-alpha. J. Vet. Med. Sci. 2009;71:1691–1695. doi: 10.1292/jvms.001691. [DOI] [PubMed] [Google Scholar]
- Iha K., Omatsu T., Watanabe S., Ueda N., Taniguchi S., Fujii H., Ishii Y., Kyuwa S., Akashi H., Yoshikawa Y. Molecular cloning and expression analysis of bat toll-like receptors 3, 7 and 9. J. Vet. Med. Sci. 2010;72:217–220. doi: 10.1292/jvms.09-0050. [DOI] [PubMed] [Google Scholar]
- Johnson C.L., Gale M., Jr. CARD games between virus and host get a new player. Trends Immunol. 2006;27:1–4. doi: 10.1016/j.it.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Kang D.C., Gopalkrishnan R.V., Wu Q., Jankowsky E., Pyle A.M., Fisher P.B. Mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA. 2002;99:637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Takeuchi O., Mikamo-Satoh E., Hirai R., Kawai T., Matsushita K., Hiiragi A., Dermody T.S., Fujita T., Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K.J., Yamaguchi O., Otsu K., Tsujimura T., Koh C.S., Reis e Sousa C., Matsuura Y., Fujita T., Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kepler T.B., Sample C., Hudak K., Roach J., Haines A., Walsh A., Ramsburg E.A. Chiropteran types I and II interferon genes inferred from genome sequencing traces by a statistical gene-family assembler. BMC Genomics. 2010;11:444. doi: 10.1186/1471-2164-11-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Recognition of AUG and alternative initiator codons is augmented by G in position +4 but is not generally affected by the nucleotides in positions +5 and +6. EMBO J. 1997;16:2482–2492. doi: 10.1093/emboj/16.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Woo P.C., Li K.S., Huang Y., Tsoi H.W., Wong B.H., Wong S.S., Leung S.Y., Chan K.H., Yuen K.Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P., Delicat A., Paweska J.T., Gonzalez J.P., Swanepoel R. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H., Zhang J., McEachern J., Field H., Daszak P., Eaton B.T., Zhang S., Wang L.F. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Lu C., Ranjith-Kumar C.T., Hao L., Kao C.C., Li P. Crystal structure of RIG-I C-terminal domain bound to blunt-ended double-strand RNA without 5’ triphosphate. Nucleic Acids Res. 2011;39:1565–1575. doi: 10.1093/nar/gkq974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A., Lu S., Anderson J.B., Chitsaz F., Derbyshire M.K., DeWeese-Scott C., Fong J.H., Geer L.Y., Geer R.C., Gonzales N.R., Gwadz M., Hurwitz D.I., Jackson J.D., Ke Z., Lanczycki C.J., Lu F., Marchler G.H., Mullokandov M., Omelchenko M.V., Robertson C.L., Song J.S., Thanki N., Yamashita R.A., Zhang D., Zhang N., Zheng C., Bryant S.H. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225–D229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv. Drug Deliv. Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Middleton D.J., Morrissy C.J., van der Heide B.M., Russell G.M., Braun M.A., Westbury H.A., Halpin K., Daniels P.W. Experimental Nipah virus infection in pteropid bats (Pteropus poliocephalus) J. Comp. Pathol. 2007;136:266–272. doi: 10.1016/j.jcpa.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Murray K., Rogers R., Selvey L., Selleck P., Hyatt A., Gould A., Gleeson L., Hooper P., Westbury H. A novel morbillivirus pneumonia of horses and its transmission to humans. Emerg. Infect. Dis. 1995;1:31–33. doi: 10.3201/eid0101.950107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara H., Hasegawa M., Okada N. Pegasoferae, an unexpected mammalian clade revealed by tracking ancient retroposon insertions. Proc. Natl. Acad. Sci. USA. 2006;103:9929–9934. doi: 10.1073/pnas.0603797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu T., Bak E.J., Ishii Y., Kyuwa S., Tohya Y., Akashi H., Yoshikawa Y. Induction and sequencing of Rousette bat interferon alpha and beta genes. Vet. Immunol. Immunopathol. 2008;124:169–176. doi: 10.1016/j.vetimm.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu T., Nishimura Y., Bak E.J., Ishii Y., Tohya Y., Kyuwa S., Akashi H., Yoshikawa Y. Molecular cloning and sequencing of the cDNA encoding the bat CD4. Vet. Immunol. Immunopathol. 2006;111:309–313. doi: 10.1016/j.vetimm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Onoguchi K., Yoneyama M., Takemura A., Akira S., Taniguchi T., Namiki H., Fujita T. Viral infections activate types I and III interferon genes through a common mechanism. J. Biol. Chem. 2007;282:7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- Rambaldi D., Ciccarelli F.D. FancyGene: dynamic visualization of gene structures and protein domain architectures on genomic loci. Bioinformatics. 2009;25:2281–2282. doi: 10.1093/bioinformatics/btp381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenfusser S., Goutagny N., DiPerna G., Gong M., Monks B.G., Schoenemeyer A., Yamamoto M., Akira S., Fitzgerald K.A. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J. Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- Satoh T., Kato H., Kumagai Y., Yoneyama M., Sato S., Matsushita K., Tsujimura T., Fujita T., Akira S., Takeuchi O. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc. Natl. Acad. Sci. USA. 2010;107:1512–1517. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.D., Gregory T.R. The genome sizes of megabats (Chiroptera: Pteropodidae) are remarkably constrained. Biol. Lett. 2009;5:347–351. doi: 10.1098/rsbl.2009.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speare R., Skerratt L., Foster R., Berger L., Hooper P., Lunt R., Blair D., Hansman D., Goulet M., Cooper S. Australian bat lyssavirus infection in three fruit bats from north Queensland. Commun. Dis. Intell. 1997;21:117–120. doi: 10.33321/cdi.1997.21.25. [DOI] [PubMed] [Google Scholar]
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., Kumar, S., 2011. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- Towner J.S., Amman B.R., Sealy T.K., Carroll S.A., Comer J.A., Kemp A., Swanepoel R., Paddock C.D., Balinandi S., Khristova M.L., Formenty P.B., Albarino C.G., Miller D.M., Reed Z.D., Kayiwa J.T., Mills J.N., Cannon D.L., Greer P.W., Byaruhanga E., Farnon E.C., Atimnedi P., Okware S., Katongole-Mbidde E., Downing R., Tappero J.W., Zaki S.R., Ksiazek T.G., Nichol S.T., Rollin P.E. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009;5:e1000536. doi: 10.1371/journal.ppat.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk A.F., Smith C.S., Field H.E., Smith I.L., Northill J.A., Taylor C.T., Jansen C.C., Smith G.A., Mackenzie J.S. Transmission of Japanese Encephalitis virus from the black flying fox, Pteropus alecto, to Culex annulirostris mosquitoes, despite the absence of detectable viremia. Am. J. Trop. Med. Hyg. 2009;81:457–462. [PubMed] [Google Scholar]
- van der Poel W.H., Lina P.H., Kramps J.A. Public health awareness of emerging zoonotic viruses of bats: a European perspective. Vector Borne Zoonotic Dis. 2006;6:315–324. doi: 10.1089/vbz.2006.6.315. [DOI] [PubMed] [Google Scholar]
- Venkataraman T., Valdes M., Elsby R., Kakuta S., Caceres G., Saijo S., Iwakura Y., Barber G.N. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J. Immunol. 2007;178:6444–6455. doi: 10.4049/jimmunol.178.10.6444. [DOI] [PubMed] [Google Scholar]
- Williamson M.M., Hooper P.T., Selleck P.W., Gleeson L.J., Daniels P.W., Westbury H.A., Murray P.K. Transmission studies of Hendra virus (equine morbillivirus) in fruit bats, horses and cats. Aust. Vet. J. 1998;76:813–818. doi: 10.1111/j.1751-0813.1998.tb12335.x. [DOI] [PubMed] [Google Scholar]
- Yoneyama M., Fujita T. Recognition of viral nucleic acids in innate immunity. Rev. Med. Virol. 2010;20:4–22. doi: 10.1002/rmv.633. [DOI] [PubMed] [Google Scholar]
- Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y.M., Gale M., Jr., Akira S., Yonehara S., Kato A., Fujita T. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- Zhou P., Cowled C., Todd S., Crameri G., Virtue E.R., Marsh G.A., Klein R., Shi Z., Wang L.F., Baker M.L. Type III IFNs in pteropid bats: differential expression patterns provide evidence for distinct roles in antiviral immunity. J. Immunol. 2011;186:3138–3147. doi: 10.4049/jimmunol.1003115. [DOI] [PMC free article] [PubMed] [Google Scholar]