Abstract
DC-SIGN, a human C-type lectin, is involved in the transmission of many enveloped viruses. Here we report the cloning and characterization of the cDNA and gene encoding porcine DC-SIGN (pDC-SIGN). The full-length pDC-SIGN cDNA encodes a type II transmembrane protein of 240 amino acids. Phylogenetic analysis revealed that pDC-SIGN, together with bovine, canis and equine DC-SIGN, are more closely related to mouse SIGNR7 and SIGNR8 than to human DC-SIGN. pDC-SIGN has the same gene structure as bovine, canis DC-SIGN and mouse SIGNR8 with eight exons. pDC-SIGN mRNA expression was detected in pig spleen, thymus, lymph node, lung, bone marrow and muscles. pDC-SIGN protein was found to express on the surface of monocyte-derived macrophages and dendritic cells, alveolar macrophages, lymph node sinusoidal macrophage-like, dendritic-like and endothelial cells but not of monocytes, peripheral blood lymphocytes or lymph node lymphocytes. A BHK cell line stably expressing pDC-SIGN binds to human ICAM-3 and ICAM-2 immunoadhesins in a calcium-dependent manner, and enhances the transmission of porcine reproductive and respiratory syndrome virus (PRRSV) to target cells in trans. The results will help better understand the biological role(s) of DC-SIGN family in innate immunity during the evolutionary process.
Keywords: C-type lectins, DC-SIGN, Pig, Innate immunity, Cell adhesion, Dendritic cells, Mammals, Gene
1. Introduction
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) located throughout the peripheral immune system. Invading foreign antigens trigger the migration of immature DCs from the blood into tissues where they detect and capture the antigens [1]. Subsequently, activated DCs process captured proteins into immunogenic peptides through MHC molecules and further present to T cells. Recognition of invading pathogens by DCs is mediated by pattern-recognition receptors (PRRs) including Toll-like receptors (TLRs) and lectins [2], [3], [4]. The lectins expressed on the surface of DCs are members of the calcium-dependent C-type lectin receptor (CLRs) family and play a key role in the antigen capture and internalization of DCs [4]. CLRs are also expressed on other APCs including macrophages.
Dendritic cells-specific intercellular-adhesion-molecule-3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209), a CLR, was initially identified as an ICAM-3 binding protein mediating DCs and T cell interaction [5] and a HIV-1 gp120 receptor mediating transmission of HIV-1 to susceptible cells in trans [6]. DC-SIGN was also found to interact with ICAM-2, regulating chemokine-induced trafficking of DCs across both resting and activated endothelium [7]. A second human DC-SIGN (hDC-SIGN) homologue, L-SIGN (CD209L), was subsequently identified and shown to have similar function to DC-SIGN [8]. DC-SIGN is expressed mainly on monocyte-derived human DCs in vitro and on immature and mature DCs in the normal human lymph node, dermis, mucosa and spleen and on macrophages in alveoli of the lung in vivo [5], [6], [9], [10], whereas L-SIGN is highly expressed in sinusoidal endothelial cells of the liver and lymph node [8]. Recently, a third DC-SIGN-related lectin, liver and lymph node sinusoidal endothelial cell (LSECs) C-type lectin (LSECtin) which is coexpressed with L-SIGN on LSECs, was identified with similar property of pathogen recognition and antigen capture [11]. DC-SIGN, L-SIGN and LSECtin form a tight gene cluster at human chromosome 19p13.3 and have analogous genomic structures [12].
Since their identifications, DC-SIGN and L-SIGN have generated considerable interests for their ability to bind and uptake pathogens including viruses, bacteria (Mycobacterium), fungi and parasites in vitro [13]. A broad spectrum of enveloped viruses including Retroviridae (human immunodeficiency virus, simian immunodeficiency virus and feline immunodeficiency virus), Flaviviridae (Dengue virus, West Nile virus and hepatitis C virus), Filoviridae (Ebola and Marburg virus), Coronaviridae (SARS-CoV), Togaviridae (Sindbis virus) and Herpesviridae (human cytomegalovirus), has been reported to use DC-SIGN and/or L-SIGN as recognition and adhesion receptor for enhanced infection in vitro [14]. DC-SIGN/L-SIGN contain C-type-lectin-specific carbohydrate recognition domain (CRD) that tightly binds asparagines-linked high mannose glycans in viral enveloped glycoproteins in a calcium (Ca2+)-dependent manner [15].
The searching for DC-SIGN-related homologues in nonhuman primates initially identified rhesus macaque and pigtailed macaque DC-SIGN [16]. A more detailed survey showed that Old World monkeys (OWM) such as rhesus macaque and apes (like chimpanzee) have orthologues of hDC-SIGN whereas L-SIGN is missing in OWM but presented in apes. Another DC-SIGN member, CD209L2 was identified in OWM and apes but not in humans [17]. The study indicated that the DC-SIGN gene family in primates has undergone duplications and deletions during recent evolutionary processes [17]. For non-primate species, five mouse homologues of hDC-SIGN, designated as SIGN1 to SIGN4 and mouse DC-SIGN (also called SIGNR5) were initially identified based on similarity searches in database and partially characterized [18], [19], [20]. SIGNR1 was found to activate the classical complement pathway on the surface of marginal-zone macrophage, leading to resistance to pneumococcal infection, which plays a different physiological role in mouse compared to its human counterpart [21]. Later, a detailed screening of the mouse genome revealed a pseudogene SIGN6 as well as two expressed proteins SIGN7 and SIGNR8, indicating widely divergent biochemical and probably physiological properties of mouse DC-SIGN related proteins [22]. More recently, DC-SIGN homologues from domestic animal species such as dog, cattle and horse have also been predicted from the genome databases.
Due to similarities in organ size and physiology with humans, pig is considered to be the preferred source animal for xenotransplantation [23]. Understanding the compatibilities across the human-pig species barrier of the molecular interactions is very critical for the clinical application of pig-to-human xenotransplantation. Interactions of the receptors on porcine hematopoietic cells with ligands on human endothelial cells play a crucial role in the event that porcine hematopoietic cells are used to induce tolerance in the human recipient [24]. In addition, T-cell-mediated xenograft rejection, a phenomenon probably caused by induction of stronger human T cell responses against pig antigen than that against alloantigens, also involved potential interactions of adhesion molecules between porcine APCs such as DCs and human T cells [25]. Considering that DC-SIGN has been shown as the endogenous adhesion receptor for ICAM-2 and ICAM-3 [5], [7], [26], it will be interesting to see whether the DC-SIGN homologue in pig potentially participates in those events by cross-interacting with human ICAM-2 and ICAM-3.
Porcine reproductive and respiratory syndrome virus (PRRSV), an economically important swine pathogen worldwide, is a member of the family Arteriviridae in the order of the Nidovirales. PRRSV isolates identified thus far worldwide are divided into two distinct genotypes, European (type 1) and North American (type 2) genotypes, which cause the same disease symptoms but are antigenically different. Like other enveloped virus such as HIV and HCV, the entry of PRRSV into the host cells, porcine alveolar macrophages, is a complex multistep process that involves the presence of several entry factors including sialoadhesin, CD163 and heparan sulphate [27]. However, the potential interaction between PRRSV and porcine PRRs on APCs has not been reported. Since human L-SIGN was shown to be associated with SARS-coronavirus entry in lung, we hypothesize that the porcine DC-SIGN/L-SIGN homologue may play a similar role during PRRSV infection in pig lung since PRRSV and coronavirus both belong to the Nidovirales order.
In this study, we report for the first time the cloning and characterization of DC-SIGN homologue in pigs (Sus scrofa). Unlike the computer-based screening of DC-SIGN homologues in genome databases of mouse and other species, DC-SIGN-related porcine gene sequences have not been available in pig genome database thus far. Therefore, by using degenerate RT-PCR primers based upon the human, nonhuman primates and mouse DC-SIGN genes, we first amplified a short fragment with sequence homologous to hDC-SIGN from in vitro cultured porcine monocyte-derived dendritic cells. Based upon the initial resulting sequence, both of the complete cDNA and the gene of porcine DC-SIGN (pDC-SIGN) were determined. Subsequently, a pDC-SIGN-specific antibody was generated and a stable cell line expressing pDC-SIGN was developed. The gene structure, tissue and cellular distributions and in vitro binding property of pDC-SIGN to human ICAM-3 and ICAM-2 immunoadhesins as well as the potential interaction between pDC-SIGN and PRRSV were characterized.
2. Materials and methods
2.1. Isolation and culture of porcine alveolar macrophages, porcine peripheral blood lymphocytes, monocytes, monocyte-derived dendritic cells and monocyte-derived macrophages
Healthy crossbred conventional pigs of 3–7 weeks of age were used for the collection of venous blood samples and porcine alveolar macrophages (PAM). Pigs were maintained in an isolated room under experimental conditions.
PAM were collected by lung lavage using cold PBS and resuspended in DMEM supplemented with 10% fetal bovine serum (FBS). Fresh or 3-day in vitro cultured PAM cultures were used for staining and subsequent analyses.
Porcine heparinized blood was diluted 1:2 with phosphate-buffered saline (PBS) and centrifuged over Ficoll-Paque PREMIUM (GE Healthcare, Sweden) at 1000 × g for 40 min at room temperature. The buffy coat layer containing peripheral blood mononuclear cells (PBMC) was isolated and washed three times with PBS at 250 × g for 10 min at 4 °C. CD14-positive monocytes on the surface of PBMC were sorted by immunomagnetic labeling MACS system of cells using anti-CD14 mAb (clone M-M9, VMRD Inc., WA, USA) and goat anti-mouse IgG1-magnetic microbeads (Miltenyi Biotec, Germany). CD14-negative cells, based on the cell morphology determined by flow cytometry analysis, were recognized as porcine peripheral blood lymphocytes (PBL). Purified monocytes were resuspended at 1 × 105 cells/ml in DMEM supplemented with 10% FBS, 55 μmol/l of β-mercaptoethanol and antibiotics. Monocytes were then cultured in six-well plates at 37 °C in the presence of 25 ng/ml of recombinant porcine granulocyte-macrophage colony stimulating factor (rpGM-CSF, R&D systems) and 25 ng/ml recombinant porcine interleukin-4 (rpIL-4, Endogen). Half of the culture medium was replaced by fresh medium every 3 days. The cells were collected on the third or seventh day and used as monocyte-derived dendritic cells (MDDCs). Monocyte-derived macrophages (MDMΦs) were developed in a similar procedure, but cultured in the absence of the two cytokines. Cells were collected on the fifth days and used as MDMΦs.
2.2. Culture of continuous cell lines
A baby hamster kidney fibroblast cell line BHK-21, a monkey kidney cell line MARC-145 and a porcine kidney epithelial cell line PK15 were grown in MEM supplemented with 10% FBS and antibiotics while a porcine monocytic cell line 3D4/31 (ATCC CRL-2844) was grown in RPMI 1640 medium supplemented with 10% FBS and antibiotics at a 37 °C incubator. A mouse fibroblast NIH 3T3 cell line stably expressing hDC-SIGN was obtained through the NIH AIDS Research and Reference Reagent Program (Germantown, MD) and was renamed as 3T3-HDCS in this study. This cell line was cultured in DMEM supplemented with 10% FBS.
2.3. RNA extraction, reverse transcription (RT) and degenerate PCR and rapid amplification of cDNA ends (RACE)-PCR
In vitro cultured porcine MDDCs, derived from porcine monocytes in the presence of rpGM-CSF and rpIL-4, were collected between the seventh and tenth days. Total RNA was isolated from MDDCs using the RNeasy mini kit (Qiagen Inc.) followed by an RNase-free DNase I treatment. First-strand cDNA was synthesized from total RNA with SuperScript II reverse transcriptase (Invitrogen) using oligo-dT (Promega) as the reverse primer. Several pairs of degenerate primers complementary to conserved sequences in human and mouse DC-SIGN genes were designed based on the multiple sequence alignments of the available human and mouse DC-SIGN related genes. PCR with degenerate primers was performed in 50 μl reaction with an Advantage 2 PCR kit (Clontech, Palo Alto, CA) using the following PCR parameters: 94 °C for 2 min, 30 cycles of 94 °C for 15 s, 57.5 °C for 30 s and 72 °C for 1 min, and a final incubation at 72 °C for 3 min. A PCR fragment was amplified only when one set of primers (NF-05 and NR-05, Table 1 ) was used for amplification. The obtained PCR products were directly sequenced and compared with the GenBank sequences of the human and mouse DC-SIGN related genes. RT and RACE-PCR were performed with a SMART RACE cDNA amplification kit (Clontech) according to the manufacturer's manual. The gene-specific primers used for 5′-RACE or 3′-RACE were PDR-1 and PDF-1, respectively (Table 1), which were designed based on the sequence information obtained from degenerate PCR products. The RACE reaction products were cloned into a pCR2.1 vector (Invitrogen) by TA cloning strategy and sequenced.
Table 1.
Oligonucleotide primers used for degenerate RT-PCR, 5′-RACE and 3′-RACE PCR, genomic PCR, gene sequencing, subcloning and PCR detection in pig tissues of pDC-SIGN.
| Primer ID | Sequence (5′–3′)a | Positionb |
|---|---|---|
| NF-05 | ATCAAAASTGMTGAGGAGCAGA | 473–494 |
| NR-05 | CATTTGTCRTCRTTCCAGCC | 671–690 |
| NF-06 | AACCGCTTCACCTGGATGGG | 524–543 |
| 5′-RACE PDR-1 | CAGAAGCTGAGTTGGAGGGGGCTG | 589–612 |
| 3′-RACE PDF-1 | GCCACCTGGATTGGCCTCAGTGATG | 530–554 |
| PCI-XHO | agtctcgagcgccaccATGGCAGAGATATG | 26–39 |
| DCS3 | tatctagaTCAGAGCATGGGGCAGGGAGA | 728–748 |
| 1F | GATGGCAGAGATATGTGACCCCAAGGA | 25–54 |
| 4R | CGGAGGGGCTGCTGAGACCATC | 966–987 |
| 2F | TCGTCTCATTGGGTTTCTTCATGCTCC | 168–194 |
| 3F | CTGCAGAGAGAGAGAGAGACCAGCAGGA | 236–263 |
| 4F | TGCCCCTGGCATTGGGAATTCTT | 359–381 |
| Nco-DCS-5 | ataccATGGCAGAGATATG | 26–39 |
| Xho-DCS-3 | agtctcgagTCAGAGCATGGGGCAGGGAGA | 728–748 |
| PDCS-E56F | GAATGCCACCCTGGCTGGCCT | 328–348 |
| PDCS-E78R | GGGTTCTCCTTCTTTCCAGAAGCTGAGTT | 600–628 |
The mixed bases (S = C + G, M = A + C, and R = A + G) designed for degenerate primers (NF-05 and NR-05) are shown in bold and underlined. It is noted that the sequences of primers NF-05, NF-06 and NR-05 are not fully identical to that of the final cDNA sequence of pDC-SIGN. For primers PCI-XHO, DCS3, Nco-DCS-5 and Xho-DCS-3, lowercase letters indicate the non-porcine-DC-SIGN sequences; underlined nucleotides represent restriction sites (XhoI, XbaI or NcoI) used for subcloning and italic nucleotides indicate the optimal Kozak sequence before start codon ATG.
Position is corresponding to the full-length cDNA of pDC-SIGN (Fig. 2a).
2.4. Genomic PCR and gene sequencing
The primers used for one-step genomic PCR were based on the determined sequence of pDC-SIGN cDNA in this study. The forward primer 1F contains the start codon ATG while the reverse primer 4R is complementary to the sequence within the 3′-noncoding region of the cDNA (Table 1). Genomic PCR was performed with a Platinum PCR HiFi Supermix kit (Invitrogen) using 150 ng of the pig genomic DNA (Novagen) in a total volume of 50 μl. The PCR condition was 35 cycles of 94 °C for 30 s, 68 °C for 5 min with an initial denaturing of the template DNA at 94 °C for 2 min. The resulting fragment was cloned into a pCR2.1 vector by TA cloning strategy. The M13 forward and reverse primers together with three gene-specific primers, 2F, 3F and 4F (Table 1), were used for sequencing. Assembly of the full-length gene was done with the SeqMan program from Lasergene package (DNASTAR Inc., Madison, WI).
2.5. Sequence and phylogenetic analyses
Analyses and alignment of DNA and amino acid sequences were performed using Lasergene package. The DC-SIGN-related cDNA and genes from various vertebrate species and their corresponding GenBank accession numbers used for the alignment and comparison are: human DC-SIGN cDNA (NM_021155), human L-SIGN cDNA (NM_014257), chimpamzee DC-SIGN cDNA (NM_001009064), chimpanzee L-SIGN1 cDNA (XM_512333), chimpanzee CD209L2 (renamed as L-SIGN2 in this study) cDNA(XM_001146279), rhesus monkey DC-SIGN1 (NM_001032870), rhesus monkey DC-SIGN2 (NM_001033089), rhesus L-SIGN2 cDNA (NM_001032951), mouse SIGNR1 (CD209b) cDNA (NM_026972), mouse SIGNR2 (CD209c) cDNA (NM_130903), mouse SIGNR3 (CD209d) cDNA (NM_130904), mouse SIGNR4 (CD209e) cDNA (NM_130905), mouse SIGNR5 (mouse DC-SIGN/CD209a) cDNA (NM_133238), mouse SIGNR7 (CD209g) cDNA (XM_284376), mouse SIGNR8 (CD209f) cDNA and gene (XM_284386 and NC_000074), bovine DC-SIGN cDNA and gene (XM_590928 and NC_007305), canis DC-SIGN cDNA and gene (XM_542118 and NC_006602), equine DC-SIGN cDNA and gene (XM_001496929 and NC_009150), opossum SIGNR8 cDNA1 (XM_001377290) and opossum SIGNR8 cDNA2 (XM_001377303). Phylogenetic tree was constructed by the neighbor-joining method in the PAUP 4.0 program (David Swofford, Smithsonian Institute, Washington, DC, distributed by Sinauer Associate Inc.) based upon the complete amino acid coding sequences of DC-SIGN family proteins using porcine LSECtin (Huang et al., unpublished data) as an outgroup. Prediction of the mRNA splicing of pDC-SIGN gene was performed with an online program ASPic (Alternative Splicing Prediction, http://t.caspur.it/ASPIC/home.php). Comparison of the gene sequences of porcine, bovine and canis DC-SIGN and mouse SIGNR8 was accomplished with the mVISTA program (http://genome.lbl.gov/vista/mvista/submit.shtml).
2.6. Generation of an anti-peptide polyclonal antibody specific to pDC-SIGN
To generate a pDC-SIGN-specific anti-peptide polyclonal antibody to detect the expression of pDC-SIGN protein, two peptides corresponding to regions predicted to be exposed within the CRD of pDC-SIGN (acetyl-VDNSPLQLSFWKEGEPNNHGC-amide, and Acetyl-AEQKFLKSWYRYNKAC-amide) were commercially synthesized (21st Century Biochemicals Corp., Marlboro, MA). The peptides were subsequently purified and used together to immunize two New Zealand white rabbits as a custom antibody production service at 21st Century Biochemicals Corp. pDC-SIGN-specific anti-peptide polyclonal antibody was produced from serum of immunized rabbits by affinity purification at the concentration of 0.73 mg/ml.
2.7. Construction of a recombinant vector expressing pDC-SIGN and in vitro expression
The complete coding region of pDC-SIGN was amplified by PCR using primers PCI-XHO and DCS3 (Table 1) and subsequently cloned into a pCI-neo vector (Promega) downstream of the CMV immediate-early enhancer/promoter using XhoI and XbaI restriction sites. The construct was sequenced to verify the identity and designated as pCI-PDCS. For transfection, BHK-21 cells were seeded at 4 × 104 cells/well onto 8-well Lab-Tek chamber slides (Nalge Nunc), and grown without antibiotics for 24 h. Plasmids pCI-DCS and pCI-neo were transiently transfected into BHK-21 cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol with minor modifications. Briefly, 0.4 μg of plasmid DNA was mixed with 1.5 μl Lipofectamine 2000 and 150 μl of Opti-MEM (Invitrogen) at room temperature for 20 min and subsequently added to the cells. Fresh growth medium was replaced after 6 h. Cells were cultured for 24–48 h, and were then applied to an immunofluorescence assay or western blot to detect the expression of pDC-SIGN protein.
2.8. Immunofluorescence assay (IFA) and western blot
Transfected cells were washed two times with PBS, fixed with 4% paraformaldehyde in PBS for 20 min and then permeabilized with 0.5% Triton X-100 for 10 min. One hundred microliters of the anti-peptide antibody, specific to pDC-SIGN at 1:100 dilution in PBS, was added over the cells and incubated for 1 h at 37 °C. Cells were washed three times with PBS and 100 μl FITC-labeled goat anti-rabbit IgG (KPL, Inc., Gaithersburg, MD, USA) at 1:100 dilution was then added. After 30 min incubation at 37 °C, the cells were washed three times with PBS and were visualized under a fluorescence microscope.
For western blot analysis, pCI-PDCS or pCI-neo transfected cells were lysed in 125 μl CelLytic M lysis buffer (Sigma–Aldrich Corp.) per 106 cells. Protein extracts were collected, aliquated and frozen at −20 °C. Samples and protein marker (Precision Plus Protein Kaleidoscope Standards, Bio-Rad Laboratories, Inc.) were resolved on SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membrane that was subsequently blocked with Tris-buffered saline (TBS) containing 3% bovine serum albumin (BSA) overnight at 4 °C. pDC-SIGN protein was detected using pDC-SIGN-specific antibody at a 1:200 dilution in TBS for 90 min at room temperature, followed by incubation with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (KPL, Inc.) for 90 min at room temperature. The membrane was then developed with chloronaphthol.
2.9. Tissue distribution of pDC-SIGN detected by RT-PCR
Total RNA was isolated from homogenized pig tissues, selected cell populations and cell lines using the RNeasy mini kit (Qiagen) followed by an RNase-free DNase I treatment, and cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen) using oligo-dT (Promega) as the reverse primer. For pig tissues that were difficult to isolate such as thymus and bone marrow, their tissue cDNA's were purchased from Zyagen Laboratories (San Diego, CA, USA). PCR was performed in 50 μl reactions with Clontech's Advantage 2 PCR kit using primer PDCS-E56F spanning the boundary between exon 5 and exon 6 and primer PDCS-E78R spanning the boundary of exons 7 and 8 of pDC-SIGN gene (Table 1). The PCR parameters include 30 cycles of 95 °C for 20 s, 68 °C for 1 min with an initial denaturing of the template DNA for 2 min. The house keeping gene, porcine glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was also amplified using primers GAPDH5 (5′-GCTGAGTATGTCGTGGAGTC-3′) and GAPDH3 (5′-CTTCTGGGTGGCAGTGAT-3′) by PCR (95 °C for 1 min, 30 cycles of 95 °C for 20 s, 55 °C for 20 s and 68 °C for 40 s, and 72 °C for 3 min). The expected size of the PCR products was 301 bp for pDC-SIGN and 285 bp for porcine GAPDH, respectively.
2.10. Flow cytometry analyses
BHK, 3D4/31, PK15 and 3T3-HDCS cells used for surface staining were collected by trypsin treatment, counted and adjusted to 1 × 106 cells/ml in chilled washing buffer (PBS buffer containing 0.1% sodium azide and 0.2% BSA). The cell concentration of porcine PBL, PAM, MDDCs and MDMΦs were each adjusted to (2–5) × 105 cells/ml. After microcentrifugation and removal of the buffer, approximately 2–10 × 105 cells were incubated with 10 μl of the pDC-SIGN-specific anti-peptide antibody at a 1:100 dilution in PBS for 30–60 min. The cells were washed to remove unbound antibody and stained with 10 μl of FITC-labeled goat anti-rabbit IgG (KPL, Gaithersburg, MD) at 1:100 dilution in PBS for 30 min. The two staining procedures were performed at 4 °C. For detection of human DC-SIGN expressed on 3T3-HDCS, a mouse anti-hDC-SIGN mAb (clone 120507, NIH AIDS Research and Reference Reagent Program) and a FITC-labeled goat anti-mouse IgG (KPL) were used for staining. Fluorescence was monitored using FACSAria (BD Biosciences, San Jose, CA) and the results were analyzed using FlowJo software (Tree Star, Ashland, OR).
2.11. Immunohistochemistry (IHC)
Paraffin sections of pig lymph nodes and livers (Zyagen Laboratories, San Diego, CA) were immunostained with avidin–biotin complex (ABC) method as previously described [28]. Briefly, to block endogenous peroxidase activity and nonspecific immunostaining, sections were immersed in 3% H2O2 for 10 min before treatment with 10% normal goat serum (NGS) in PBS (pH 7.4) for 30 min at room temperature. The primary antibody, pDC-SIGN-specific anti-peptide polyclonal antibody, and the secondary antibody, biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA), were both diluted in 2% NGS in PBS buffer. Primary and secondary antibodies were incubated overnight at 4 °C and 30 min at room temperature, respectively. The ABC reagent was prepared and used according to the manufacturer's instructions from Vectastain Elite ABC kits (Vector Lab) followed by applying DAB/Ni substrate (Vector Lab) for 5 min. Controls included omission of primary or secondary antibodies, replacement of primary antibody with rabbit IgG, normal rabbit serum, or antigen–antibody complex (pre-antibody absorption). Sections were counterstained with hematoxylin and sealed with Permount solution. IHC data were acquired with Nikon DS-Fi1 digital camera and NIS-Elements software.
2.12. Generation of a stable cell line expressing pDC-SIGN
The complete coding region of pDC-SIGN was amplified by PCR using primers Nco-DCS-5 and Xho-DCS-3 (Table 1) and subsequently cloned into a bicistronic expression vector pTriEx-1.1 Neo (Novagen) using NcoI and XhoI restriction sites. The construct, designated as pTriEx-PDCS, was sequenced to verify the identity and subsequently used to generate a stable cell line expressing pDC-SIGN. BHK-21 cells were seeded at 2 × 105 cells/well onto a 6-well plate and grown until 80–90% confluency before transfection. Transfection with plasmid pTriEx-PDCS was performed as described above. The transfected cells were incubated for approximately 36 h to allow expression of the pDC-SIGN gene without the growth medium and then replaced with complete growth medium plus geneticin selective antibiotic (Invitrogen) at a concentration of 1 mg/ml. The geneticin-containing medium was changed every 2 days to remove dead or dying cells.
After 12 days, the surviving cells were treated with trypsin and plated in 60-mm dishes at a dilution such that single cells would give rise to well-separated, individual colonies. The cells were grown for approximately 2 weeks until individual colonies of several hundred cells were present and isolated by cloning rings technique. The cells were then transferred into an individual well of a 24-well plate. When the transferred cells had grown to sufficient density, they were re-plated in T-25 flasks, grown until 100% confluency and recognized as an engineered cell line. The representative cell line expressing pDC-SIGN on the surface that is confirmed by flow cytometry analysis was designated as BHK-PDCS. To obtain high level of pDC-SIGN expression, the cell line BHK-PDCS was further sorted using the pDC-SIGN antibody by fluorescence-activated cell sorting (FACS).
2.13. Human ICAM-3 and ICAM-2 binding assay
Adhesion of human ICAM-3 or ICAM-2 to pDC-SIGN proteins was assessed with BHK-PDCS and BHK-21 cells by measuring detectable cells that bound the soluble immunoadhesins through FACS analysis. 3T3-HDCS cells were used as the positive control. Cells ((1–3) × 105 per sample) were resuspended in 100 μl PBS containing 2% FBS and incubated for 60 min at 4 °C with 1 μg of recombinant human IgG1 Fc (hFc), human ICAM-3-Fc (hICAM3-Fc) chimera or hICAM2-Fc chimera (R&D systems) in the presence or absence of mannan (100 μg/ml) or ethylene glycol tetraacetic acid (EGTA, 10 mM). Cells were then washed twice and incubated for another 45 min at 4 °C with 0.5 μg of FITC-labeled anti-human IgG Fc antibody (KPL) in 100 μl PBS containing 2% FBS. Fluorescence was monitored using FACSAria.
2.14. Generation of PRRSV virus stocks
We used two PRRSV strains from different genotypes in this study. The virus stocks of genotype 1 PRRSV expressing green fluorescent protein (GFP), designated as PGXG in this study, was generated by transfection of MARC-145 cells capable of supporting PRRSV infection with a PRRSV infectious cDNA clone (a gift of Dr. Ying Fang, South Dakota State University), and this infectious clone had been modified to be DNA-launched with much higher efficiency by our lab followed by two serial passages on MARC-145 cells (Huang et al., unpublished data). The virus stocks were PRRSV-containing supernatants without cell debris, which was removed by centrifugation. The virus titers of PGXG, and a genotype 2 North American PRRSV strain VR2385 stored in our lab, were determined by limiting dilution on MARC-145 cells through IFA and quantified as fluorescent focus-forming unit (FFU) per ml, respectively.
2.15. PRRSV binding assay
BHK-PDCS and BHK-21 cell monolayers were dispersed by incubation with cell dissociation buffer (enzyme free PBS-based buffer, Invitrogen) and washed twice with PBS containing 2% FBS. A total of 5 × 105 cells in suspension were inoculated with a PRRSV strain VR2385 at a multiplicity of infection (M.O.I.) of 10 FFU/cell. After virus adsorption for 60 min at 4 °C and washing twice, cells were incubated with a PRRSV mAb SDOW17-A (Rural Technologies, Inc., Brookings, SD) at a 1:1000 dilution for 30 min at 4 °C. Cells were subsequently washed twice to remove free antibody and then incubated with a FITC-labeled goat anti-mouse IgG (KPL) at a 1:50 dilution to determine the binding of PRRSV to the cells by FACS analysis. For the PRRSV-blocking ICAM-3 binding assay, BHK-PDCS cells were incubated with either PGXG or VR2385 (M.O.I. = 10 FFU/cell) for 60 min at 4 °C before hICAM-3-Fc addition.
2.16. PRRSV capture and in trans transmission assay
BHK-PDCS, BHK-21 or MARC-145 donor cells (2.5 × 105 cells for each) were incubated with either PGXG or PRRSV VR2385 virus at a M.O.I. of 0.5 FFU/cell in a volume of 500 μl for 3 h to allow adsorption of the virus. Cells were then washed with PBS, mixed with MARC-145 target cells (1.0 × 105) in 1 ml MEM supplemented with 2% FBS and seeded onto individual wells of 12-well plates. Three days post-infection, cells were scraped and the PRRSV viruses were recovered by three cycles of freeze–thaw. Virus titers were determined as described above.
3. Results
3.1. Molecular cloning of a full-length porcine cDNA homologue to hDC-SIGN from in vitro cultured porcine MDDCs
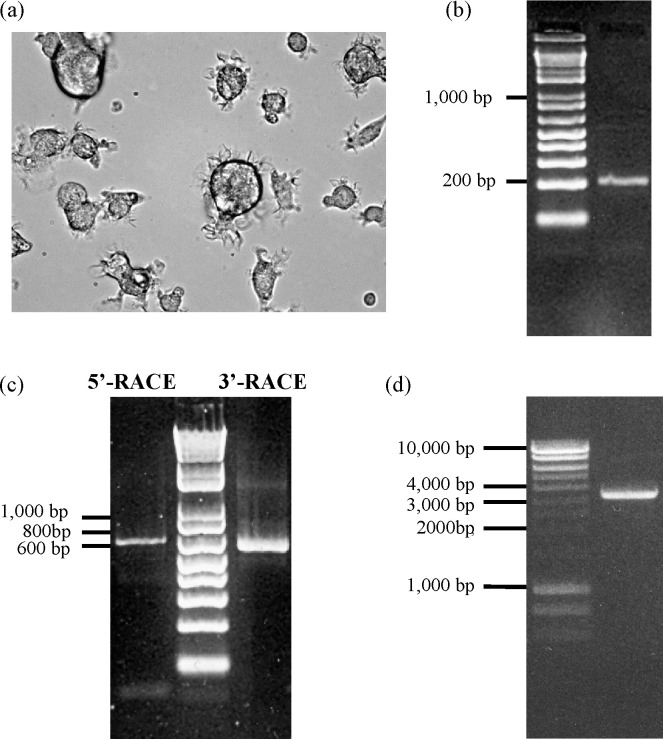
We initially hypothesized that the DC-SIGN homologue of the pig has similar expression and distribution patterns to hDC-SIGN, and thus may be mainly expressed at a high level on the surface of porcine MDDCs which can be used as the source for the cloning of the unknown pDC-SIGN cDNA. Generation of porcine MDDCs has been reported by several groups [29], [30], [31]. Using similar procedure, we observed single and aggregated veiled-shaped cells after three days of culture of adherent porcine CD14 positive monocytes in the presence of rpGM-CSF and rpIL-4. The characteristic dendritic morphology of the cells that had almost transformed from monocytes in the cultured dish was more significant after seven day (Fig. 1a). Phenotyping of the cells resulted in MHC II+CD1+CD11b/c+CD80/86+ which was consistent with other reports (data not shown) and thus recognized as MDDCs [29], [30], [31]. A sequence similarity search from the database of the Swine Genome Sequencing Project (SGSP, http://www.ncbi.nlm.nih.gov/sites/entrez?Db=genomeprj&cmd=ShowDetailView&TermToSearch=13421) in NCBI did not yield any sequences of DC-SIGN homologues. In addition, other predicted DC-SIGN homologues from domestic animal species had not been released from the genome databases when we started this project. Therefore, to identify a novel pDC-SIGN gene, we first designed a series of degenerate primers based on the conserved sequences from multiple alignments of the known human, non-human primates and mouse DC-SIGN related cDNAs [5], [16], [17], [19]. An approximately 210-bp product was first amplified by RT-PCR from the total RNA of MDDCs with the primers NF-05 and NR-05 (Fig. 1b). A nested-PCR using the gel-purified fragment as the template with the same forward primer NF-05 and a new reverse primer NR-06 upstream to primer NR-05 also amplified a fragment with smaller but expected size (data not shown), indicating the specificity of the PCR. Sequence analysis showed that the sequence of this initial PCR fragment shares 62.6%, 61.2%, and 57.6% sequence identity, respectively, to the corresponding region of hDC-SIGN, L-SIGN and mouse DC-SIGN (SIGNR5) cDNA sequences, which represents a region in the CRD of DC-SIGN.
Fig. 1.
Amplification of pDC-SIGN cDNA from in vitro cultured porcine monocyte-derived dendritic cells (MDDCs) by RT-PCR, RACE-PCR and amplification of pDC-SIGN gene from pig genomic DNA by genomic PCR. (a) Morphologic development of porcine MDDCs after 7 day in vitro culture of CD14 monocytes in the presence of rpGM-CSF and rpIL-4. Magnification = 400×. (b) Detection of a ∼210-bp product with expected size by RT-PCR with degenerate primers. (c) 5′-RACE and 3′-RACE PCR. (d) One-step genomic PCR.
Based upon this initial sequence we were able to design two gene-specific primers to amplify the 5′- and 3′-proximal regions of the cDNA by 5′-RACE and 3′-RACE PCR, respectively. Since the reverse primer PDR-1 for 5′-RACE PCR is located downstream of the 3′-RACE PCR primer PDF-1, the amplified 5′-RACE and 3′-RACE PCR products were expected to have a 82-nt overlapping region, thus covering the full-length sequence of the cDNA. The resulting two PCR products, each with approximately 600 bp from the respective RACE PCR (Fig. 1c), were assembled into a full-length cDNA sequence. A BLAST search with this cDNA did not yield any homologues sequence in Sus scrofa, indicting it's a novel porcine equivalent of the hDC-SIGN. We hence designated it as porcine DC-SIGN (pDC-SIGN).
3.2. Characterization of pDC-SIGN cDNA and its deduced protein product
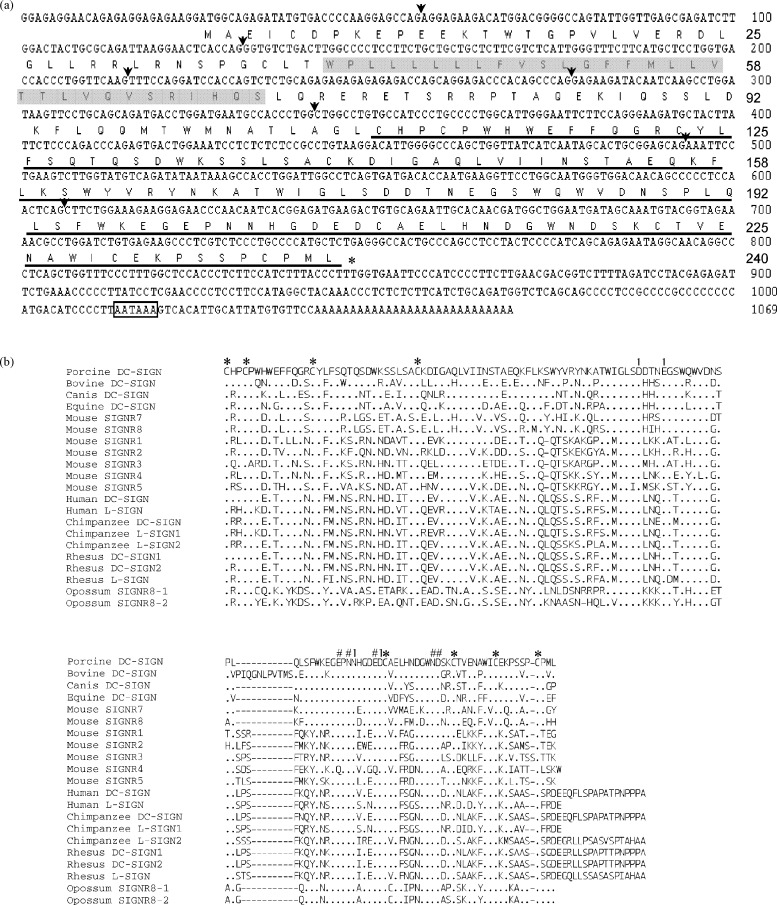
The 1069-bp pDC-SIGN cDNA encompassed an open reading frame (ORF) of 723 nucleotides from position 26–728 encoding a protein of 240 amino acids (Fig. 2a). Like other C-type lectins, the deduced pDC-SIGN protein product is predicted to be a type II transmembrane protein beginning from a putative 39-aa cytoplasmic tail (CT) followed by a putative 31-aa transmembrane domain (TMD). The extracellular domain consisted of a 38-aa neck region followed by a 132-aa CRD (Fig. 2a). An internalization motif, dileucine-based motif at aa position 27–28, was found within the CT. Since internalization motifs in the CT of the transmembrane receptors are important for the internalization of the ligand–receptor complex, it seems that the pDC-SIGN is likely able to mediate endocytosis and transfer the potential bound pathogen into the cytoplasm of the DCs. Human DC-SIGN, L-SIGN, nonhuman primate DC-SIGN and mouse SIGNR1 contains variable repeated sequence within the neck region whereas the remaining mouse SIGNR members, except SIGNR2 and SIGNR6, do not have repeated sequence [5], [8], [16], [19], [22], [32]. The sequence in the neck region of pDC-SIGN was non-repeated and the length was closer to SIGNRs 3–5 but was highly related to mouse SIGNR7 and SIGNR8 (data not shown). When the pDC-SIGN sequence was being analyzed, other computer-predicted DC-SIGN homologues from cattle, dog, equine and opossum were recently released. The neck region sequences of these DC-SIGN members are also non-repeated (data not shown).
Fig. 2.
(a) Complete nucleotide sequence of pDC-SIGN cDNA and its deduced amino acid sequence. The 1069-nucleotide sequence contains an open reading frame encoding a 240-aa protein beginning at nt position 26. The predicted transmembrane domain (TMD) is indicated by a grey background and the carbohydrate recognition domain (CRD) is underlined. The polyadenylation signal is boxed. Arrows show the boundary of exons. (b) Alignment of amino acid sequences of the CRD of pDC-SIGN and other DC-SIGN homologues among various vertebrate species. Amino acid residues that form Ca2+-binding site 1 are marked by “1”, residues that form Ca2+-binding site 2 and the primary sugar-binding site are indicated by “#” and conserved cysteine residues involved in disulfide bond formations are noted by “*”.
The CRD of pDC-SIGN had a similar size with all the other DC-SIGN homologue proteins, although their overall sizes were quite different due to the variation of the neck region. The CRD was also the most conserved region shared by porcine and all the other DC-SIGN homologue proteins, encompassing the key residues that form Ca2+- and carbohydrate-binding sites (Fig. 2b). The CRD of hDC-SIGN has been shown to bind two calcium ions using two close but distinct sites [15]. The Ca2+ site 1 contains amino acid residues Asp176, Glu180, Asn203 and Asp208 that are essential for the interaction of DC-SIGN with its ligands. All four of these residues were conserved in pDC-SIGN. pDC-SIGN also had the common Glu-Pro-Asn sequence (EPN sequences, aa position 200–202) and Glu207 as well as Asn218 involved in the Ca2+ site 2 that are critical for binding mannose-, fucose- or galactose-containing oligosaccharides. In addition, eight conserved cysteines predicted to form disulfide bonds were found in the CRD (Fig. 2b). It is noted that all the other DC-SIGN members except mouse SIGNR4 have these conserved residues, suggesting that the CRDs of DC-SIGN family share significant structural conservation (Fig. 2b).
3.3. DC-SIGN members of domestic animal species along with mouse SIGNRs 7 and 8 form a divergent evolution pathway distinct from primates and mouse SIGNRs 1–5
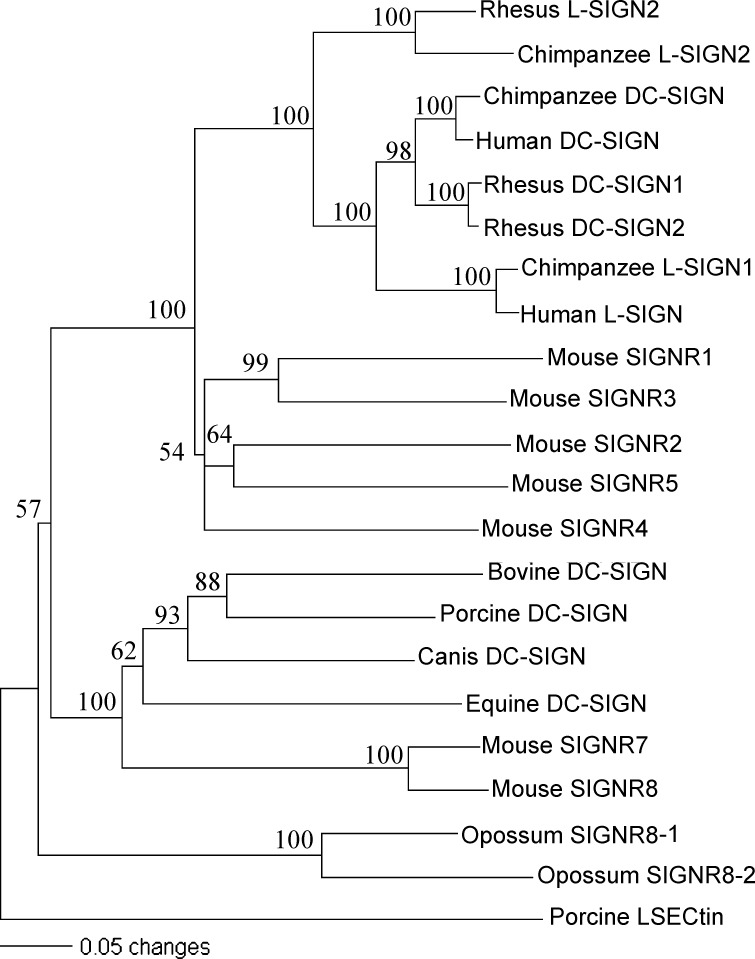
After cloning and sequencing the pDC-SIGN, sequence comparison of the DC-SIGN members in various mammalian species was conducted to determine the divergence level and evolution relationship among orthologous and paralogous genes. Since the computer-predicted bovine, canis, equine and opossum DC-SIGNs had been recently released in the respective genome database, we included their putative complete amino acid sequences, together with those from primates and mouse DC-SIGN related proteins, in the phylogenetic analysis. In addition, we used porcine LSECtin (Huang et al., unpublished data), a C-type lectin closely related to but distinct from DC-SIGN as shown by recent studies of human LSECtin [11], [12], as an outgroup to construct a neighbor-joining phylogenetic tree. The result showed that the porcine and bovine proteins are most closely related to each other than to others (Fig. 3 ). A surprising finding was that mouse SIGNR7, SIGNR8, canis and equine DC-SIGNs were clustered together with porcine and bovine proteins, forming an individual clade different from the clade containing other mouse and primates homologues (Fig. 3). Both clades were supported by bootstrap values of 100%. Opossum SIGNR8-1 and 8-2 formed another separate clade with bootstrap value of 57%. Considering opossum (Monodelphis domestica) is the common ancestor of other mammalian species selected for comparison here, the phylogenetic data indicated that opossum SIGNR8-1 and 8-2 are probably the ancestral mammalian gene in the DC-SIGN family. The tree also suggested that the later evolution of mammalian DC-SIGN family was likely originated from the mouse homologues, leading to two divergent pathways. The first pathway radiated mouse SIGNRs 1–5 and generated the lineage of primate proteins that underwent duplications (from DC-SIGN to L-SIGN2 to L-SIGN1) and deletions (L-SIGN2) during recent evolutionary processes [17], whereas the second pathway included the ancestor of SIGNRs 6–8 (SIGN6 is a pseudogene and thus was not shown here) and the homologues from the other species, including pDC-SIGN. There is no detectable orthologous relationship between the genes in these two clades. Phylogenetic analysis of the CRD sequences had the similar results with two separate clades observed in Fig. 3, except that the primate DC-SIGN is more closely related to L-SIGN2 than either is to L-SIGN1 (data not shown). Pairwise sequence comparison of the complete pDC-SIGN protein with the DC-SIGN homologues from other species revealed that pDC-SIGN was more homologous to bovine, canis and equine proteins as well as to SIGNR7 and SIGNR8 (over 50%) than to other DC-SIGN homologues (less than 50%), which was consistent with the phylogenetic analysis (data not shown).
Fig. 3.
Phylogenetic tree constructed by the neighbor-joining method based upon the amino acid sequences of DC-SIGN family proteins using porcine LSECtin as an outgroup. Bootstrap values are indicated for the nodes as a percentage of the data obtained from 1000 re-sampling.
3.4. Porcine DC-SIGN encodes a type II transmembrane protein
In order to determine if the pDC-SIGN is effectively translated and, if so, whether the translated product has the putative transmembrane property, we conducted a transfection experiment using BHK-21 cells. The full-length coding region of pDC-SIGN with 720 bp was amplified by PCR from RNA extracts of porcine MDDCs, and was subsequently subcloned into a eukaryotic expression vector pCI-neo, to obtain plasmid pCI-PDCS. BHK-21 cells were transfected with this construct or vector alone. The expression of pDC-SIGN protein was detected by IFA using a pDC-SIGN-specific anti-peptide antibody raised against two peptides in the CRD. The IFA results showed that most cells expressing pDC-SIGN had a spreading cytoplasmic and membrane staining (Fig. 4a). Some cells showed the fluorescent signals only localizing on the cell membrane (Fig. 4b). In contrast, cells transfected with pCI-neo vector did not have any positive IFA signals (Fig. 4c). We concluded from these results that the cDNA encoding pDC-SIGN is effectively translated in vitro and that the resulting product is indeed a type II transmembrane protein. The anti-pDC-SIGN antibody also detected a specific band of ∼48 kDa in the lysate of cells transfected with pCI-PDCS but not in cells transfected with the empty vector control (Fig. 4d). The molecular size was larger than that predicted from the deduced amino acids sequence (28 kDa) probably due to glycosylation, as pDC-SIGN contains a putative N-linked glycosylation site (aa 102) in the neck region. This glycosylation site is conserved in the bovine, canis and equine DC-SIGNs as well as mouse SIGNR7 and SIGNR8. A recent study showed that the expression of bovine DC-SIGN also resulted in a product of similar size in the range of 46–48 kDa [33].
Fig. 4.
Expression of pDC-SIGN (construct pCI-PDCS) in transfected BHK-21 cells. (a) Immunofluorescence assay (IFA) results at 48 h post-transfection with a pDC-SIGN-specific anti-peptide polyclonal antibody (magnification = 200×). Most cells had a spreading cytoplasmic and membrane staining. (b). A few cells only had cell membrane staining. Inner panels indicate the magnification (400×) of the stained cells. (c) Transfection of cells with the vector pCI-neo as a negative control (200×). (d) Western blot analysis using cell lysates of BHK-21 cells transfected with plasmids pCI-PDCS or pCI-neo.
3.5. Analysis and comparison of the gene structure of pDC-SIGN with bovine, canis DC-SIGN and mouse SIGNR8
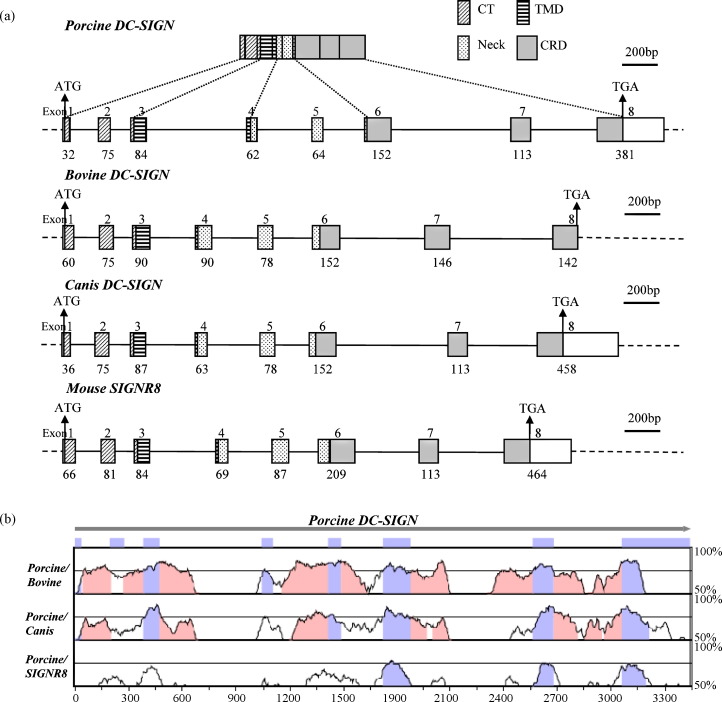
After cloning and sequencing the cDNA of the pDC-SIGN, we next sought to obtain the gene sequence of pDC-SIGN. By using one-step genomic PCR, a unique band of approximately 3.5 kb was amplified only when the annealing and extension steps of the PCR cycle were combined together at 68 °C (Fig. 1d). The PCR product was cloned into the TA vector and sequenced. The consensus sequence of the pDC-SIGN gene with 3438 bp in length was obtained by comparison of the sequences among three different independent clones. Sequence analysis and pairwise alignment with the cDNA sequence revealed that the pDC-SIGN gene was encoded by eight exons spanning the complete coding region of the gene in which exons 1 and 8 had undetermined sizes (Fig. 5a). Although extra nucleotide sequences at both termini in the noncoding region of the determined porcine cDNA were not included in the gene, the sequence of all the eight exons was fully identical to that of the coding region and partial 3′ end noncoding region of the cDNA, indicating the authenticity of the gene.
Fig. 5.
Organization of the pDC-SIGN gene (GenBank accession no. EU684955) from this study and comparison with bovine DC-SIGN (GenBank accession no. NC_007305), canis DC-SIGN (GenBank accession no. NC_006602) and mouse SIGNR8 (GenBank accession no. NC_000074) genes. (a) Gene structures of porcine, bovine, canis DC-SIGN and mouse SIGNR8 genes. The top row represents the domain structure of the putative pDC-SIGN coding region. CT: cytoplasmic tail; TMD: transmembrane domain; CRD: carbohydrate recognition domain. The bottom row displayed the exon allocation of domains. Un-translated regions in exons 1 and 8 are shown as open boxes. The numbers below the exons indicate the length of the base pairs. (b) Comparison of the gene sequences of pDC-SIGN obtained from this study with bovine DC-SIGN, canis DC-SIGN and mouse SIGNR8 generated by the mVISTA program. Conserved regions between pairs of sequences (pDC-SIGN/bovine DC-SIGN, pDC-SIGN/canis DC-SIGN and pDC-SIGN/mouse SIGNR8) are displayed as peaks of similarity (Y axe) relative to the positions of the gene sequence of pDC-SIGN (X axe). The blue–violet boxes above the plots represent the eight exons of the pDC-SIGN gene. The peaks in the same color indicate conserved regions within exons while the peaks in pink color denote conserved regions within introns. The cutoff value of percent identity is set to 70%.
The intron sizes vary from 113 to 689 bp and all acceptor and donor sequences on the introns conform to the GT-AG rule. Additional alternatively spliced mRNA isoforms were not predicted by the computer software program ASPic, suggesting that the identified cDNA is likely the only existing isoform of the pDC-SIGN expression, which is consistent with the RACE-PCR result described previously. The translation start site begins in exon 1. The 3′ end of exon 1, the entire exon 2 and the 5′ end of exon 3 encode the CT. The remaining part of exon 3 and the 5′ end of exon 4 encode the TMD. The neck region follows the TMD sequence in exon 4, spans the entire exon 5 and the first 8 nucleotides of exon 6. The rest of exon 6, the entire exon 7 and the 5′ end of exon 8 encode the CRD (Fig. 2, Fig. 5).
The pDC-SIGN gene shares a similar structure and size of eight exons with the predicted bovine, canis DC-SIGN gene and the identified mouse SIGNR8 gene (Fig. 5a), including the localization of the four domains to the corresponding exons. This is consistent with the phylogenetic analysis of DC-SIGN proteins (Fig. 3). For the other two DC-SIGN orthologues in the same clade, mouse SIGNR7 has nine exons whereas equine DC-SIGN has seven exons (data not shown). In addition to the exon number, the size of the first intron of equine DC-SIGN gene is significantly larger than the others, with 36,282 bp in length (data not shown). Pairwise comparison of the genomic sequences of pDC-SIGN with bovine DC-SIGN, canis DC-SIGN or mouse SIGNR8 (Fig. 5b) revealed that the last three exons encoding the CRD have the highest sequence identity (70–85%). Overall identity of the pDC-SIGN genomic sequences with other species (bovine DC-SIGN > canis DC-SIGN > mouse SIGNR8) was also consistent with the result from the phylogenetic analysis of DC-SIGN proteins (Fig. 3). Although limited sequence identity in the overall intron sequences was shown in the four genes, some of the intron regions adjacent to the exons were conserved, especially between the porcine and bovine DC-SIGN genes and the porcine and canis DC-SIGN genes (Fig. 5b). These conserved sequences may contain the common elements regulating the gene expression.
Human DC-SIGN gene is localized on chromosome 19p13.3 according to the NCBI map viewer build 36.2. Mapping of pDC-SIGN (CD209) including Ssc UniGene and Ssc RNA on pig genome has not been released (data not shown). Based on the correspondence between human and pig chromosomal segments, the pDC-SIGN gene is predicted to assign on pig chromosome 2 between SSC 2q1.1 to q2.1.
3.6. Tissue and cellular distribution of pDC-SIGN
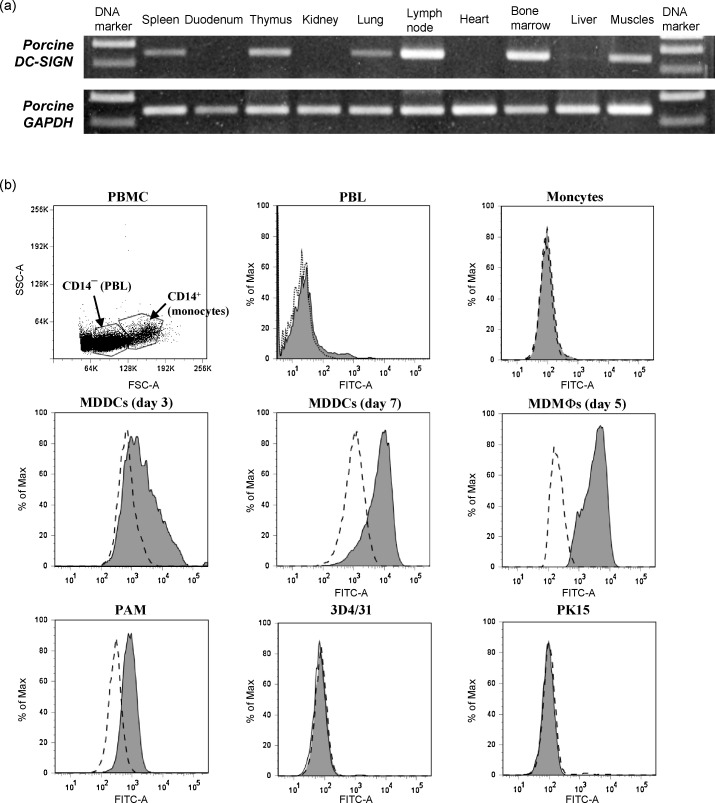
Expression of pDC-SIGN mRNA was detected in both of the primary (thymus and bone marrow) and the secondary lymphoid organs (lymph node and spleen) as well as lung and skeletal muscles but not in duodenum, kidney, heart or liver of pig by RT-PCR (Fig. 6a). The expression level in lymph node and bone marrow was the highest. The detection of DC-SIGN expressed in muscles was intriguing but not unexpected since mouse SIGNRs 7 and 8 were also found to express in skeletal muscle [22].
Fig. 6.
Detection of pDC-SIGN expression in selected pig tissues and cell populations by RT-PCR and flow cytometry, respectively. (a) RT-PCR expression profile showing the mRNA expression of pDC-SIGN. Pig tissue cDNA were used as templates in PCR reactions with primers PDCS-E56F/PDCS-E78R or porcine GAPDH-specific primers. (b) Detection of pDC-SIGN expression on defined porcine cell populations and cell lines. Porcine PBMC were isolated by centrifugation on Ficoll and assessed for forward and side scatter properties (topleft panel). The peripheral blood lymphocytes (PBL, CD14− cells) and monocytes (CD14+ cells) were separated by immunomagnetic labeling MACS system using anti-CD14 monoclonal antibody. In the other panels, the expression of pDC-SIGN on PBL, monocytes, monocyte-derived dendritic cells (MDDCs), monocyte-derived macrophages (MDMΦs), porcine alveolar macrophages (PAM), porcine monocytic cell line 3D4/31 and porcine kidney epithelial cell line PK15 were assessed by staining with anti-pDC-SIGN antibody (grey histograms). Dashed open histograms indicate background controls. Data are representative of three independent experiments.
Taking pDC-SIGN expression in various lymphoid organs into account, we speculate that pDC-SIGN may be also expressed by specific hematopoietic cell populations in addition to MDDCs. We hence performed flow cytometry analysis to detect the surface expression of pDC-SIGN protein on PBL, monocytes, MDDCs, MDMΦs and PAM (Fig. 6b). Scatter profile of porcine PBMC clearly indicated two cell populations, PBL and monocytes according to their morphology. Since CD14 molecule is the surface marker for porcine monocytes [34], these two cell populations could be separated by immunomagnetic labeling MACS system using anti-porcine CD14 monoclonal antibody (data not shown). CD14+ monocytes were further used to develop MDDCs with the addition of rpGM-CSF and rpIL-4 or MDMΦs in the absence of the cytokines, respectively. PBL and the monocytes did not show any pDC-SIGN expression, which was expected since hDC-SIGN or L-SIGN is not expressed on lymphocytes or monocytes. Accordingly, there was no detectable pDC-SIGN expression on a porcine monocytic cell line 3D4/31 (Fig. 6b, bottom-middle panel). Upon differentiation of the monocytes into MDDCs in culture, the pDC-SIGN expression was up-regulated with an approximately eight-fold increase of median fluorescence intensity from days 3 to 7. pDC-SIGN expression was also found on MDMΦs. Compared to MDDCs, the majority of MDMΦs gave a pDC-SIGN phenotype. PAM's were also dominated by a pDC-SIGN phenotype, but the expression level was lower than that on MDMΦs. In accordance with undetectable expression of pDC-SIGN mRNA in pig kidney, the protein was not expressed in an epithelial cell line PK15 derived from pig kidney (Fig. 6b).
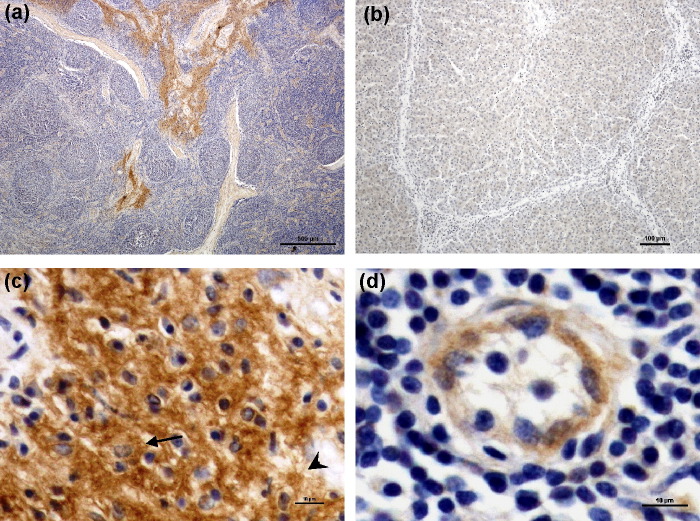
To further confirm whether pDC-SIGN protein was indeed expressed in particular cell populations of lymphoid tissues, IHC analysis on paraffin sections of pig lymph node and liver tissues was performed. We found that pDC-SIGN protein showed a predominant sinusoidal pattern of expression in lymph nodes (Fig. 7a). However, there was no detectable expression in pig livers (Fig. 7b), which was consistent with the RT-PCR results (Fig. 6a). Most of the cells immunostained with pDC-SIGN-specific anti-peptide antibody in sinuses of lymph nodes were macrophage-like and dendritic-like cells (Fig. 7c). Endothelial cells in lymphatic vessel of parenchyma were also immunostained with pDC-SIGN-specific anti-peptide antibody (Fig. 7d). The expression pattern of pDC-SIGN protein in pig lymph nodes is analogous to that of hDC-SIGN in human lymph nodes where hDC-SIGN protein was identified not only on sinusoidal macrophages but also on endothelial cells by IHC [35]. The absence of pDC-SIGN expression in pig livers further supported that the cloned pDC-SIGN is not the L-SIGN homologue since the presumed porcine L-SIGN, if exists, should be strongly expressed on LSECs.
Fig. 7.
Detection of pDC-SIGN protein expression by immunohistochemistry (IHC) in pig lymph node tissues but not in pig liver tissues. Localization of pDC-SIGN protein expression was examined by IHC using ABC method on paraffin sections of pig lymph nodes (a, c and d) and pig livers (b). (a) pDC-SIGN protein preferentially expressed in lymph node sinuses, supcapsular sinuses. (b) pDC-SIGN protein was not expressed in pig liver. (c) Most of the cells immunostained with pDC-SIGN-specific anti-peptide antibody in sinuses were morphologically macrophage-like (arrow) and dendritic-like cells (arrowhead). (d) Lymphatic vessel endothelial cells in parenchyma were also immunostained with pDC-SIGN-specific antibody.
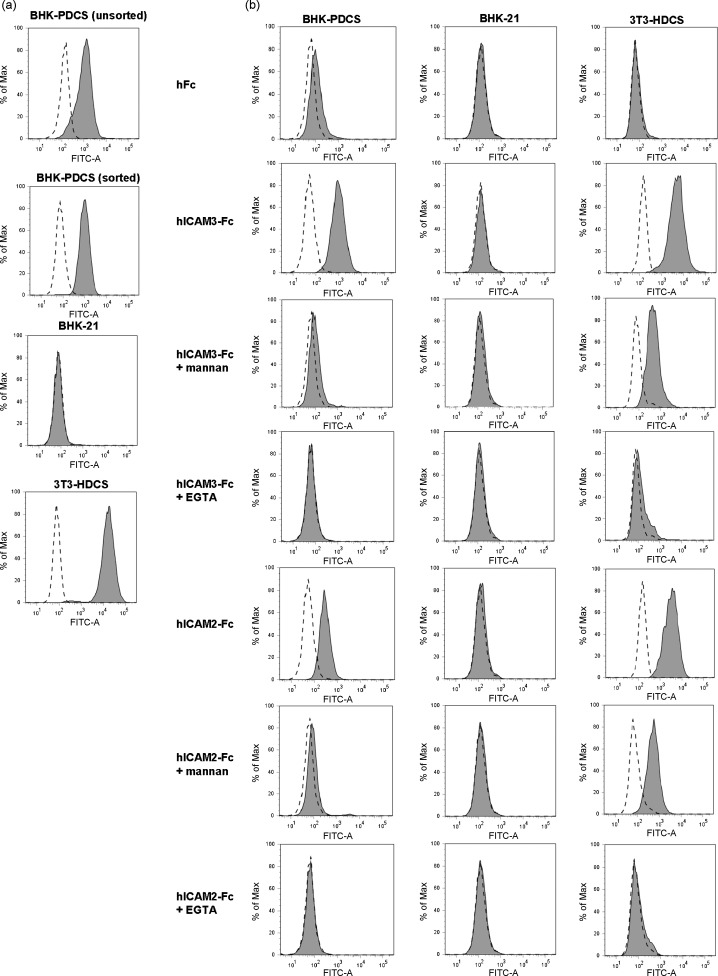
3.7. Binding of human ICAM-3 and ICAM-2 immunoadhesins to BHK cells stably expressing pDC-SIGN
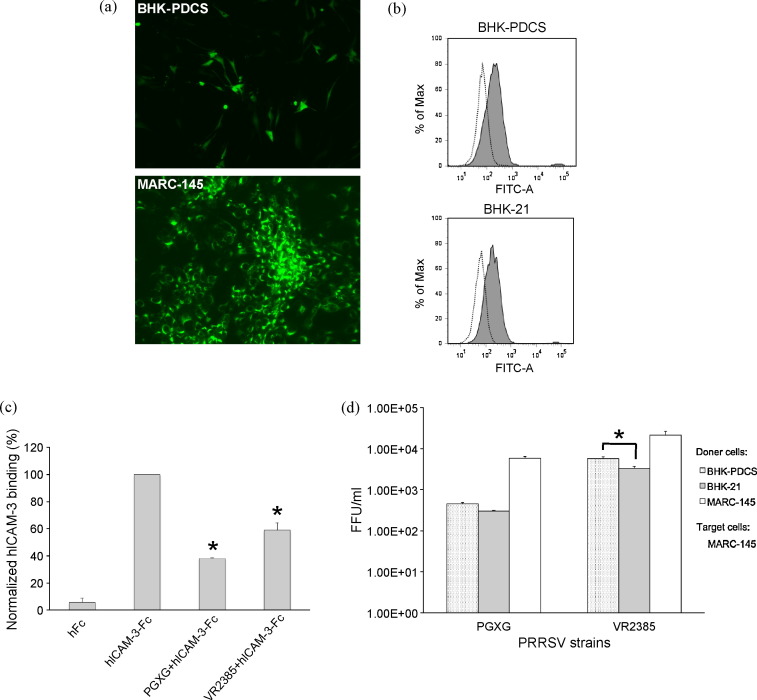
A representative BHK-21 cell colony transfected with the pDC-SIGN expression plasmid pTriEx-PDCS was developed into a cell line under the selection of geneticin antibiotic, and this cell line was designated as BHK-PDCS. To determine whether pDC-SIGN can be expressed on the cell surface, cell lines BHK-PDCS and BHK-21 were stained with polyclonal pDC-SIGN antibody for flow cytometry analysis. As shown in Fig. 8a, no detectable staining with the antibodies was displayed on BHK-21 cells whereas the surface expression of pDC-SIGN protein on BHK-PDCS cells was detected, indicating that BHK-PDCS cell line was able to synthesize pDC-SIGN proteins. The result also confirmed that pDC-SIGN belongs to a type II integral membrane protein family. BHK-PDCS was further enriched and sorted, to obtain a purer cell population with pDC-SIGN expression, by FACS and used for the subsequent binding experiments. In addition, 3T3-HDCS cell line stably expressing hDC-SIGN [36], verified by staining with a hDC-SIGN specific mAb (Fig. 8a, bottom panel), was used for a positive control.
Fig. 8.
Binding of human ICAM-3 and ICAM-2 immunoadhesins to BHK cells stably expressing pDC-SIGN. (a) Detection of surface expression of pDC-SIGN protein on stable BHK cell lines. BHK-21, and unsorted or sorted BHK-PDCS cell lines were stained with anti-pDC-SIGN antibody and FITC-labeled goat anti-rabbit IgG, respectively, and analyzed by flow cytometry. Dashed open histograms represented the background staining. The expression of pDC-SIGN was indicated by the solid grey histograms. Expression of hDC-SIGN on the surface of 3T3-HDCS cell line was also verified by staining with a hDC-SIGN monoclonal antibody (the lowest panel). (b) Calcium-dependent binding of human ICAM-3 and ICAM-2 immunoadhesins to BHK-PDCS and 3T3-HDCS cells. Dashed open histograms represent cells staining only with a FITC labeled anti-human IgG Fc antibody. Results are representative of three independent experiments. Data are expressed as histogram analysis of 10,000 cells.
Binding of soluble hFc, hICAM-3-Fc and hICAM-2-Fc in the presence or absence of either mannan or EGTA to pDC-SIGN-negative BHK-21 cells was not observed (Fig. 8b, middle column). However, we observed a binding of either hICAM-3-Fc or hICAM-2-Fc to both of the DC-SIGN-positive cells. Binding of hICAM-3-Fc to BHK-PDCS cells had a higher affinity than binding of hICAM-2-Fc to BHK-PDCS cells. The binding was specific since binding of hFc alone to BHK-PDCS or 3T3-HDCS was negative. Furthermore, the addition of mannan blocked the binding of both hICAM-3-Fc and hICAM-2-Fc, so did the presence of EGTA. Inhibition by EGTA was more efficient than that by mannan in both of the DC-SIGN-positive cells (Fig. 8b). The results indicated that pDC-SIGN is able to cross-react with human ICAM-3 or ICAM-2 and the interaction is dependent on Ca2+ and is mediated by the CRD of pDC-SIGN.
3.8. Porcine DC-SIGN expressed on the surface of BHK cells is not involved PRRSV virus entry but enhances PRRSV transmission to target MARC-145 cells in trans
BHK-21 was shown to support enveloped PRRSV replication inside the cell but not allow cell-to-cell spread of the virus [37]. Since pDC-SIGN was expressed on PAM, the susceptible host cells for PRRSV which contains highly glycosylated envelope viral proteins, it was important to see if pDC-SIGN expressed on the cell surface was involved in PRRSV attachment and entry. Transfection of BHK-PDCS cells with a genotype 1 PRRSV infectious cDNA clone could recover the virus with GFP expression that subsequently propagated in target MARC-145 cells (Fig. 9a). However, the virus (PGXG) released into the cell culture medium was unable to infect the untransfected BHK-PDCS cells (data not shown), demonstrating that pDC-SIGN is not involved in PRRSV entry. Since BHK-21 cell line was also known to be susceptible for PRRSV binding [38], we subsequently performed a PRRSV specific binding assay to compare the virus attachment on the cell surface between BHK-PDCS and BHK-21 cells. We found that PRRSV indeed bound to both cell lines, although it was difficult to quantify the difference (Fig. 9b). To determine whether the attachment of PRRSV on BHK-PDCS cells could interfere with the pDC-SIGN-hICAM-3 interaction, cells were pretreated with either genotype 1 PRSV strain PGXG or genotype 2 PRRSV strain VR2385 before the hICAM-3-Fc binding. The results showed that both PRRSV strains blocked the hICAM-3 binding, suggesting a correlation between PRRSV attachment and the expression of pDC-SIGN on the BHK cell surface (Fig. 9c).
Fig. 9.
(a) Porcine reproductive and respiratory syndrome virus (PRRSV) is replication-competent in BHK-PDCS cells by transfection with a modified PRRSV infectious cDNA clone expressing GFP (upper panel) and the released virus is able to infect target MARC-145 cells (lower panel). GFP signal was directly monitored at 48 h post-transfection in BHK-PDCS cells or at 72 h post infection in MARC-145 cells (magnification = 100×). (b) Comparison of PRRSV binding on BHK-PDCS and BHK-21 cell lines. Dotted open histograms represent control cells incubated without PRRSV inoculation but stained with anti-PRRSV mAb SDOW17-A and FITC-labeled goat anti-mouse IgG. Cells inoculated with the virus and incubated with the two antibodies are indicated by solid grey histograms. (c) Both PRRSV strain PGXG and strain VR2385 blocked hICAM-3 binding to the BHK-PDCS cell line. BHK-PDCS cells were incubated with either PGXG or VR2385 (M.O.I. = 10 FFU/cell) for 60 min at 4 °C before the addition of hICAM-3-Fc. The stained cells were analyzed using FACS as described for Fig. 8b. Data are presented as the mean fluorescence intensity normalized to the untreated control (addition of a hICAM-3-Fc and a FITC labeled anti-human IgG Fc antibody only) ± S.D. Asterisks indicated statistical difference compared with the untreated control (p < 0.05). (d) PRRSV transmission mediated by BHK cells was enhanced by pDC-SIGN. Transmission of either PRRSV PGXG or PRRSV VR2385 using BHK-PDCS, BHK-21 and MARC-145 cells as donor cells and MARC-145 cells as target cells and titration of PRRSV were performed as described in materials and methods section. Donor cells co-cultured with MARC-145 target cells not exposed to PRRSV were used as mock-transmission control and the results were not shown in the figure (no virus detected). Asterisk indicated statistical difference for PRRSV VR2385 strain between BHK-PDCS and BHK-21 cells (p < 0.05).
Furthermore, since hDC-SIGN has been shown to efficiently transmit viruses to target cells, it will be important to see whether pDC-SIGN has the analogous ability to facilitate PRRSV transmission through donor cell-to-target cell contacts in trans. BHK-PDCS and BHK-21 cells were used as donor cells whereas the susceptible MARC-145 cells were used as the target cells (or donor cells in the control) in the PRRSV capture and transmission assay. The donor cells were incubated with culture medium (as a mock-incubation control), PRRSV PGXG strain and PRRSV VR2385 strain, respectively. Compared to the virus titers obtained from direct infection of MARC-145 cells with PRRSV at the same M.O.I. of 0.5 FFU/cell that could reach up to 1 × 107 FFU/ml (data not shown), the virus titers of PRRSV grown in MARC-145 cells transmitted by three types of donor cells were much lower, ranging from 2.9 × 102 to 2.5 × 104 FFU/ml (Fig. 9d), indicating that the transmission of PRRSV could be quantified in spite of the low efficiency. PRRSV transmitted by MARC-145 cells was more efficient than that by the two BHK cells due to the presence of more cells (donor cells were also used as target cells). PRRSV transmission by BHK-PDCS was enhance by 52% (p = 0.07) for PRRSV PGXG strain and by 72% (p = 0.02) for PRRSV VR2385 strain compared to that by BHK-21 cells, respectively (Fig. 9d), suggesting that pDC-SIGN is probably associated with PRRSV transmission in trans under these conditions.
4. Discussion
In this study, we have, for the first time, cloned and characterized the complete cDNA as well as the gene of pDC-SIGN, characterized its tissue and cellular distribution, showed cross-interactions between pDC-SIGN and human ICAM-3 and between pDC-SIGN and human ICAM-2, and demonstrated the enhancement of PRRSV transmission to target cells in trans by pDC-SIGN. Human DC-SIGN and related homologues have become attractive targets for their important roles in various immune responses including mediating DCs adhesion, migration, inflammation, activating primary T cell, and for their interactions with various pathogens as well as involvement in immune escape of the pathogens [5], [6], [7], [13], [14], [26], [32]. To establish a small animal model for DC-SIGN research, mouse DC-SIGN homologues, from SIGNR1 to SIGNR8, were screened from the available mouse genome or expressed sequence tag (EST) databases and subsequently cloned by RT-PCR [19], [22]. Most recently, a bovine homologue to DC-SIGN was identified using the same strategy [33]. However, since there is no relevant sequence available in the SGSP database, we used an entire different strategy to clone the pDC-SIGN gene compared to that used for mouse SIGNR molecules. By using degenerate primers based upon human and mouse DC-SIGN sequences, we firstly amplified a 210-bp fragment with sequence homologous to the hDC-SIGN and mouse SIGNRs by RT-PCR. Based upon this initial sequence, the complete cDNA sequence of the pDC-SIGN was subsequently obtained in two overlapping fragments by 5′- and 3′-RACE-PCR, respectively. In addition, the complete pDC-SIGN gene was cloned based upon the cDNA sequence by one-step genomic PCR. The cloning strategy used in this study should be very useful for the identification of the DC-SIGN homologues in other animal species with no available sequence information.
A surprising finding from this study was that pDC-SIGN and the putative bovine, canis and equine DC-SIGNs as well as mouse SIGNR7 and SIGNR8 form a distinct evolutionary pathway from primates DC-SIGNs and others mouse SIGNR members according to the phylogenetic analysis (Fig. 3), which should shed a new light on the evolution of DC-SIGN gene family. The duplications, deletions and rearrangements of DC-SIGN-related genes and DC-SIGN family members during evolutionary processes have been shown by accumulating evidences, suggesting that DC-SIGN is the functional target for selective pressure. It was proposed that hDC-SIGN, L-SIGN, LSECtin and another type II C-type lectin, CD23, are derived from a common ancestor since they form a tight gene cluster at chromosome 19p13.3 and have overall protein domain structure, similar genomic organization and possible analogous function [12]. It was reported that the current L-SIGN gene, presented in apes and human but not in OWM, was newly duplicated from the ancestral DC-SIGN, whereas the older duplicator, L-SIGN2, was lost in human but still retained in OWM and apes [17]. The promoter polymorphisms of DC-SIGN and variable tandem-neck repeats of L-SIGN among multi-ethnic groups are also found [39]. For non-primate species, mouse DC-SIGN homologues were identified as eight members in which SIGNRs 7 and 8 form an individual lineage [22]. However, it was impossible to analyze the overall outline of evolution of the mammalian DC-SIGN species until DC-SIGN members from other species are identified. The experimental identification and characterization of pDC-SIGN in this study as well as the functional characterization of bovine DC-SIGN [33] are the necessary first step to towards understanding the evolution of the DC-SIGN family. Since no other DC-SIGN sequences were detected in the bovine, canis and equine genomes (data not shown), the pDC-SIGN is likely to exist as a single gene analogously, although the relevant porcine genomic region has not been filled. Interestingly, we found thirteen rat SIGNR genes related to mouse SIGNRs 1–8 on the rat chromosome 12p12 (data not shown), suggesting that DC-SIGN homologues are more widely presented in rodents than in other mammalian species.
The discovery of eight mouse DC-SIGN homologues indicated that they had widely divergent biochemical and physiological properties [22]. However, none of them was experimentally verified to be the functional orthologue to hDC-SIGN. This is partly due to the fact that mouse does not have the ICAM-3 homologue in its genome [40]. Moreover, mouse SIGNRs 1, 3 and 5 do not interact with mouse ICAM-2 and do not support ICAM-2 mediated transmigration of immature DCs across resting endothelium [41]. SIGNR5 plays no role in T cell-DCs interactions and did not bind to pathogens known to interact with hDC-SIGN [42]. SIGNR1 was able to function as an adhesion receptor and recently was found to activate the classical complement pathway on the surface of marginal-zone macrophage, leading to resistance to pneumococcal infection [21]. SIGNR 3 shared the ability with hDC-SIGN to bind both high mannose and fucose-containing glycans and also mediated endocytosis of glycoprotein ligands but the exact function is still unknown [22]. The physiological role of SIGNR7 and SIGNR8 in mouse has not been determined. While the mouse DC-SIGN proteins have not been found to share functions with the human proteins, bovine DC-SIGN was recently shown to express on bovine MDDCs, bind and internalize HIV-1 gp120 as well as Mycobacterium bovis bacillus Calmette-Guerin (BCG), suggesting that it is functionally related to hDC-SIGN [33], even though they are classified into two different evolutionary pathway. This conclusion is also supported by the evidence of tissue and cellular distribution and binding characteristics of pDC-SIGN with human ICAM ligands in this study.
It has been reported that hDC-SIGN is expressed in vivo not only on DCs, but also on macrophages [9], [10], [35], activated B cells [43], lymph node endothelial cells [35], dermis of the skin [5], the placenta [44], the intestinal and genital mucosae [45], and various lymphoid tissues [5]. Bovine DC-SIGN protein was found to express on bovine MDDCs but not on CD14+ monocytes, B cells or T cells [33]. In this study, we showed that pDC-SIGN mRNA expression is mainly distributed in various lymphoid organs and the protein expression is not detected on the surface of CD14+ monocytes or PBL. Porcine DC-SIGN is not only expressed on MDDCs but also on MDMΦs and PAM, suggesting that it is activated during the development of porcine DCs and macrophages. By using IHC analysis, we further confirmed that pDC-SIGN was indeed expressed on lymph node sinusoidal APCs including macrophage-like and dendritic-like cells but not on B or T lymphocytes (Fig. 7). pDC-SIGN expression was also detected on lymph node endothelial cells, which shares an analogous pattern with that of hDC-SIGN expression [35]. However, neither pDC-SIGN mRNA nor protein was detectable in pig liver tissues using RT-PCR and IHC analysis, respectively.
Based on these results, we conclude that the cloned porcine gene is the DC-SIGN homologue (instead of the L-SIGN homologue) although the amino acid sequence of pDC-SIGN does not show significant sequence identity with hDC-SIGN or hL-SIGN. The L-SIGN genes emerged from a duplication event in the common DC-SIGN ancestor of anthropoids and probably does not exist in non-primate mammalian species as shown on the bovine, canis and equine genomic regions where the C-type lectins arrange as a three gene cluster CD23/LSECtin/DC-SIGN instead of a four gene cluster CD23/LSECtin/DC-SIGN/L-SIGN on human chromosome 19p13.3. The evolutionary pathway of DC-SIGN homologues in these non-primate mammalian species is distinct from that in primates resulting in the existence of DC-SIGN as a single gene. Phylogenetic analysis and comparison of gene organization indicated that porcine DC-SIGN is highly related to these non-primate mammalian species and thus should share the same characteristics. Furthermore, the absence of pDC-SIGN expression in pig livers by IHC and RT-PCR also supports this conclusion, since, if the cloned pDC-SIGN is the porcine L-SIGN homologue, its RNA and protein expression should have been detected in liver tissues by RT-PCR and IHC, respectively.
The interactions of hDC-SIGN with ICAM-2, ICAM-3 and HIV-1 gp120 required Ca2+ and could be inhibited by mannan and EGTA [5], [6], [7], [46], [47], indicating that the CRD of hDC-SIGN and L-SIGN plays key roles in cellular adhesion and viral attachment. Crystal structures of hDC-SIGN and L-SIGN fragments, determined in complex with a variety of carbohydrate ligands, showed that the CRD has the characteristic C-type lectin fold and revealed the oligosaccharides recognized by them [48], [49], [50]. Both DC-SIGN and L-SIGN recognize high-mannose glycans. For the mouse SIGNR members, SIGNR3 shares the ability with hDC-SIGN to bind both high-mannose and fucose-containing glycans [22]. SIGNR2 binds almost exclusively to GlcNAc-terminated glycans, and SIGNR7 binds preferentially to the 6-sulfo-sialyl Lewisx glycan [22]. The structural basis for the specific recognition has not been determined in these SIGNR members. Although the sequence alignment results suggest that pDC-SIGN and bovine DC-SIGN may have distinct carbohydrate binding specificity from hDC-SIGN and L-SIGN because of the lower sequence homology compared to other DC-SIGN-related proteins, they do share the analogous ligand-binding capacity with hDC-SIGN. This is mainly because both of the porcine and bovine DC-SIGN proteins have all structural conserved residues facilitating in the proper folding of the CRD and involved in calcium-dependent carbohydrate binding (Fig. 2b). On the other hand, these interactions may involve protein–protein interaction in addition to protein–carbohydrate interaction, which has been implicated by the scanning-mutagenesis analysis of hDC-SIGN binding to hICAM-2 and hICAM-3 [47]. Furthermore, it was shown that hDC-SIGN has a distinct but overlapping binding fashion for gp120 and ICAM-3 [15], [47]. A single mutation from valine to glycine at aa position 351 in hDC-SIGN abrogated ICAM-3 binding but not HIV-1 gp120 interaction [15]. However, the binding to either ICAM-3 or ICAM-2 was unaffected when valine was mutated to alanine [47]. The pDC-SIGN protein has the histidine residue at this position that is uniquely shared by the bovine, canis and equine DC-SIGN proteins. The change from valine to histidine likely has minimal effect on pDC-SIGN-hICAM-3/hICAM-2 interaction.
The carbohydrate binding specificity of hDC-SIGN is also determined by its multimerization and clustering on the cell surface [32]. DC-SIGN is a functional receptor as a tetramer on DCs formed by the neck region repeats [46], [51]. It has been shown that recognition of small carbohydrate compounds by individual CRD alone is not sufficient to achieve the high-affinity interactions of DC-SIGN and L-SIGN with pathogens like HIV-1 gp120. Biochemical studies with repeat domain deletion mutants in the neck region also showed that a minimum of three repeats are required to form tetramers, and that additional repeats would stabilize the tetramer [50]. Neither mouse SIGNR1 nor SIGNR3 bound to the polysaccharide dextran, whereas cellular-expressed SIGNR1 interacted with dextran [52], further indicating the importance of the multivalent nature of mouse SIGNR1. The pDC-SIGN along with the bovine, canis and equine DC-SIGN proteins did not have repeat sequences in the neck region, suggesting that they may be unable to form a tetramer. However, this is not directly associated with the binding capacity of these proteins since we showed that pDC-SIGN is capable of effectively interacting with the potential ligands like hICAM-3 and hICAM-2, capture and transmit PRRSV to the target cells. Similarly, bovine DC-SIGN without repeat sequences in the neck region also has the ability to bind and internalize HIV-1 gp120 as well as Mycobacterium bovis BCG [33]. Another example is the hDC-SIGN-related lectin LSECtin, which is devoid of repeat sequences in the neck region, and yet it is still able to mediate antigen capture and pathogen binding by human myeloid cells [11]. Although pDC-SIGN is not involved in PRRSV entry, we showed that it can enhance the virus transmission from the engineered BHK donor cells to target MARC-145 cells. Since these two cell lines are not of pig origin, further experiments using porcine primary cells, which is beyond the scope of this study, are warranted to determine whether this in trans transmission occurs in pigs with enhanced PRRSV infectivity. It will also be interesting to see whether pDC-SIGN has the ability to capture and internalize other swine pathogens similar to bovine DC-SIGN.
We have demonstrated the binding of pDC-SIGN expressed on the cell surface to soluble human ICAM ligands. It will be interesting to see in the future whether the interactions can extend to in vivo cell–cell adhesion, which may have important implications for clinical applications of pig-to-human xenotransplantation. Recipient endothelial cells play a significant role in tolerance induction whereas recipient T cells mainly mediate xenograft rejection [23]. Improving the binding of hICAM-2 to pDC-SIGN or blocking the binding of hICAM-3 to pDC-SIGN may have therapeutic value. Furthermore, it will also be interesting to assess the actual physiological role of pDC-SIGN in pigs. The tissue and cellular location and the property of pDC-SIGN and its cross-binding to human natural ligands strongly implicate analogous physiologic roles for this lectin in cell adhesion.
Note added in proof
After the submission of our manuscript, a sequence with GenBank accession number NM_001129972.1 (EU442799), identical to the coding region of the full-length pDC-SIGN cDNA sequence (EU684956) reported in this manuscript, was released in the NCBI GenBank database.
Acknowledgements
This project is funded in part by a grant from Fort Dodge Animal Health Inc. We thank Dr Tonya LeRoith for the collection of porcine alveolar macrophages, Dr. Ying Fang for her contribution of the PRRSV infectious cDNA clone, Ms. Yanyan Ni for her help to develop PRRSV titration protocol using fluorescent focus-forming assay, Ms. Melissa Makris for her assistance in flow cytometry, and Dr. Stephen Wu for his support.
Footnotes
References
- 1.Palucka K., Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999;19:12–25. doi: 10.1023/a:1020558317162. [DOI] [PubMed] [Google Scholar]
- 2.Thoma-Uszynski S., Stenger S., Takeuchi O., Ochoa M.T., Engele M., Sieling P.A. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 3.Weis W.I., Taylor M.E., Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 4.Cambi A., Koopman M., Figdor C.G. How C-type lectins detect pathogens. Cell Microbiol. 2005;7:481–488. doi: 10.1111/j.1462-5822.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 5.Geijtenbeek T.B., Torensma R., van Vliet S.J., van Duijnhoven G.C., Adema G.J., van Kooyk Y. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 6.Geijtenbeek T.B., Kwon D.S., Torensma R., van Vliet S.J., van Duijnhoven G.C., Middel J. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 7.Geijtenbeek T.B., Krooshoop D.J., Bleijs D.A., van Duijnhoven G.C., Adema G.J., van Kooyk Y. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol. 2000;1:353–357. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- 8.Bashirova A.A., Geijtenbeek T.B., van Duijnhoven G.C., van Vliet S.J., Eilering J.B., Martin M.P. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J Exp Med. 2001;193:671–678. doi: 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tailleux L., Pham-Thi N., Bergeron-Lafaurie A., Herrmann J.L., Charles P., Schwartz O. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2005;2:e381. doi: 10.1371/journal.pmed.0020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soilleux E.J., Morris L.S., Leslie G., Chehimi J., Luo Q., Levroney E. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- 11.Dominguez-Soto A., Aragoneses-Fenoll L., Martin-Gayo E., Martinez-Prats L., Colmenares M., Naranjo-Gomez M. The DC-SIGN-related lectin LSECtin mediates antigen capture and pathogen binding by human myeloid cells. Blood. 2007;109:5337–5345. doi: 10.1182/blood-2006-09-048058. [DOI] [PubMed] [Google Scholar]
- 12.Liu W., Tang L., Zhang G., Wei H., Cui Y., Guo L. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J Biol Chem. 2004;279:18748–18758. doi: 10.1074/jbc.M311227200. [DOI] [PubMed] [Google Scholar]
- 13.van Kooyk Y., Geijtenbeek T.B. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 14.Lozach P.Y., Burleigh L., Staropoli I., Amara A. The C type lectins DC-SIGN and L-SIGN: receptors for viral glycoproteins. Methods Mol Biol. 2007;379:51–68. doi: 10.1007/978-1-59745-393-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geijtenbeek T.B., van Duijnhoven G.C., van Vliet S.J., Krieger E., Vriend G., Figdor C.G. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J Biol Chem. 2002;277:11314–11320. doi: 10.1074/jbc.M111532200. [DOI] [PubMed] [Google Scholar]
- 16.Baribaud F., Pohlmann S., Sparwasser T., Kimata M.T., Choi Y.K., Haggarty B.S. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J Virol. 2001;75:10281–10289. doi: 10.1128/JVI.75.21.10281-10289.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bashirova A.A., Wu L., Cheng J., Martin T.D., Martin M.P., Benveniste R.E. Novel member of the CD209 (DC-SIGN) gene family in primates. J Virol. 2003;77:217–227. doi: 10.1128/JVI.77.1.217-227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caminschi I., Lucas K.M., O’Keeffe M.A., Hochrein H., Laabi Y., Brodnicki T.C. Molecular cloning of a C-type lectin superfamily protein differentially expressed by CD8alpha(−) splenic dendritic cells. Mol Immunol. 2001;38:365–373. doi: 10.1016/s0161-5890(01)00067-0. [DOI] [PubMed] [Google Scholar]
- 19.Park C.G., Takahara K., Umemoto E., Yashima Y., Matsubara K., Matsuda Y. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int Immunol. 2001;13:1283–1290. doi: 10.1093/intimm/13.10.1283. [DOI] [PubMed] [Google Scholar]
- 20.Parent S.A., Zhang T., Chrebet G., Clemas J.A., Figueroa D.J., Ky B. Molecular characterization of the murine SIGNR1 gene encoding a C-type lectin homologous to human DC-SIGN and DC-SIGNR. Gene. 2002;293:33–46. doi: 10.1016/s0378-1119(02)00722-9. [DOI] [PubMed] [Google Scholar]
- 21.Kang Y.S., Do Y., Lee H.K., Park S.H., Cheong C., Lynch R.M. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. doi: 10.1016/j.cell.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Powlesland A.S., Ward E.M., Sadhu S.K., Guo Y., Taylor M.E., Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem. 2006;281:20440–20449. doi: 10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y.G., Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7:519–531. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]
- 24.Warrens A.N., Simon A.R., Theodore P.R., Sykes M. Human-porcine receptor–ligand compatibility within the immune system: relevance for xenotransplantation. Xenotransplantation. 1999;6:75–78. doi: 10.1034/j.1399-3089.1999.00020.x. [DOI] [PubMed] [Google Scholar]
- 25.Dorling A., Lombardi G., Binns R., Lechler R.I. Detection of primary direct and indirect human anti-porcine T cell responses using a porcine dendritic cell population. Eur J Immunol. 1996;26:1378–1387. doi: 10.1002/eji.1830260630. [DOI] [PubMed] [Google Scholar]
- 26.Bleijs D.A., Geijtenbeek T.B., Figdor C.G., van Kooyk Y. DC-SIGN and LFA-1: a battle for ligand. Trends Immunol. 2001;22:457–463. doi: 10.1016/s1471-4906(01)01974-3. [DOI] [PubMed] [Google Scholar]
- 27.Delputte P.L., Costers S., Nauwynck H.J. Analysis of porcine reproductive and respiratory syndrome virus attachment and internalization: distinctive roles for heparan sulphate and sialoadhesin. J Gen Virol. 2005;86:1441–1445. doi: 10.1099/vir.0.80675-0. [DOI] [PubMed] [Google Scholar]
- 28.Li W., Quigley L., Yao D.L., Hudson L.D., Brenner M., Zhang B.J. Chronic relapsing experimental autoimmune encephalomyelitis: effects of insulin-like growth factor-I treatment on clinical deficits, lesion severity, glial responses, and blood brain barrier defects. J Neuropath Exp Neurol. 1998;57:426–438. doi: 10.1097/00005072-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Carrasco C.P., Rigden R.C., Schaffner R., Gerber H., Neuhaus V., Inumaru S. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology. 2001;104:175–184. doi: 10.1046/j.0019-2805.2001.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paillot R., Laval F., Audonnet J.C., Andreoni C., Juillard V. Functional and phenotypic characterization of distinct porcine dendritic cells derived from peripheral blood monocytes. Immunology. 2001;102:396–404. doi: 10.1046/j.1365-2567.2001.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loving C.L., Brockmeier S.L., Sacco R.E. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology. 2007;120:217–229. doi: 10.1111/j.1365-2567.2006.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koppel E.A., van Gisbergen K.P., Geijtenbeek T.B., van Kooyk Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell Microbiol. 2005;7:157–165. doi: 10.1111/j.1462-5822.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 33.Yamakawa Y., Pennelegion C., Willcocks S., Stalker A., Machugh N., Burt D. Identification and functional characterization of bovine orthologue to DC-SIGN. J Leukoc Biol. 2008;83:1396–1403. doi: 10.1189/jlb.0807523. [DOI] [PubMed] [Google Scholar]
- 34.Ziegler-Heitbrock H.W., Appl B., Kafferlein E., Loffler T., Jahn-Henninger H., Gutensohn W. The antibody MY4 recognizes CD14 on porcine monocytes and macrophages. Scand J Immunol. 1994;40:509–514. doi: 10.1111/j.1365-3083.1994.tb03497.x. [DOI] [PubMed] [Google Scholar]
- 35.Martens J.H., Kzhyshkowska J., Falkowski-Hansen M., Schledzewski K., Gratchev A., Mansmann U. Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages in human lymph node sinuses, the primary sites of regional metastasis. J Pathol. 2006;208:574–589. doi: 10.1002/path.1921. [DOI] [PubMed] [Google Scholar]
- 36.Wu L., Martin T.D., Vazeux R., Unutmaz D., KewalRamani V.N. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J Virol. 2002;76:5905–5914. doi: 10.1128/JVI.76.12.5905-5914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meulenberg J.J., Bos-de Ruijter J.N., van de Graaf R., Wensvoort G., Moormann R.J. Infectious transcripts from cloned genome-length cDNA of porcine reproductive and respiratory syndrome virus. J Virol. 1998;72:380–387. doi: 10.1128/jvi.72.1.380-387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Therrien D., St-Pierre Y., Dea S. Preliminary characterization of protein binding factor for porcine reproductive and respiratory syndrome virus on the surface of permissive and non-permissive cells. Arch Virol. 2000;145:1099–1116. doi: 10.1007/s007050070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barreiro L.B., Patin E., Neyrolles O., Cann H.M., Gicquel B., Quintana-Murci L. The heritage of pathogen pressures and ancient demography in the human innate-immunity CD209/CD209L region. Am J Hum Genet. 2005;77:869–886. doi: 10.1086/497613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugino H. ICAM-3, a ligand for DC-SIGN, was duplicated from ICAM-1 in mammalian evolution, but was lost in the rodent genome. FEBS Lett. 2005;579:2901–2906. doi: 10.1016/j.febslet.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 41.Wethmar K., Helmus Y., Luhn K., Jones C., Laskowska A., Varga G. Migration of immature mouse DC across resting endothelium is mediated by ICAM-2 but independent of beta2-integrins and murine DC-SIGN homologues. Eur J Immunol. 2006;36:2781–2794. doi: 10.1002/eji.200526311. [DOI] [PubMed] [Google Scholar]
- 42.Caminschi I., Corbett A.J., Zahra C., Lahoud M., Lucas K.M., Sofi M. Functional comparison of mouse CIRE/mouse DC-SIGN and human DC-SIGN. Int Immunol. 2006;18:741–753. doi: 10.1093/intimm/dxl011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rappocciolo G., Piazza P., Fuller C.L., Reinhart T.A., Watkins S.C., Rowe D.T. DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS Pathog. 2006;2:e70. doi: 10.1371/journal.ppat.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soilleux E.J., Morris L.S., Lee B., Pohlmann S., Trowsdale J., Doms R.W. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J Pathol. 2001;195:586–592. doi: 10.1002/path.1026. [DOI] [PubMed] [Google Scholar]
- 45.Gurney K.B., Elliott J., Nassanian H., Song C., Soilleux E., McGowan I. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J Virol. 2005;79:5762–5773. doi: 10.1128/JVI.79.9.5762-5773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell D.A., Fadden A.J., Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 47.Su S.V., Hong P., Baik S., Negrete O.A., Gurney K.B., Lee B. DC-SIGN binds to HIV-1 glycoprotein 120 in a distinct but overlapping fashion compared with ICAM-2 and ICAM-3. J Biol Chem. 2004;279:19122–19132. doi: 10.1074/jbc.M400184200. [DOI] [PubMed] [Google Scholar]
- 48.Feinberg H., Mitchell D.A., Drickamer K., Weis W.I. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163–2166. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- 49.Guo Y., Feinberg H., Conroy E., Mitchell D.A., Alvarez R., Blixt O. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat Struct Mol Biol. 2004;11:591–598. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- 50.Snyder G.A., Colonna M., Sun P.D. The structure of DC-SIGNR with a portion of its repeat domain lends insights to modeling of the receptor tetramer. J Mol Biol. 2005;347:979–989. doi: 10.1016/j.jmb.2005.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernhard O.K., Lai J., Wilkinson J., Sheil M.M., Cunningham A.L. Proteomic analysis of DC-SIGN on dendritic cells detects tetramers required for ligand binding but no association with CD4. J Biol Chem. 2004;279:51828–51835. doi: 10.1074/jbc.M402741200. [DOI] [PubMed] [Google Scholar]
- 52.Galustian C., Park C.G., Chai W., Kiso M., Bruening S.A., Kang Y.S. High and low affinity carbohydrate ligands revealed for murine SIGN-R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN-R3 and langerin. Int Immunol. 2004;16:853–866. doi: 10.1093/intimm/dxh089. [DOI] [PubMed] [Google Scholar]