Abstract
Clinical respiratory illness was compared in five homozygous chicken lines, originating from homozygous B2, B8, B12 and B19, and heterozygous B2/B12 birds after infection with either of two strains of the infectious bronchitis virus (IBV). All chickens used in these studies originated from White Leghorn and Ancona linages. IBV Gray strain infection of MHC homozygous B12 and B19 haplotype chicks resulted in severe respiratory disease compared to chicks with B2/B2 and B5/B5 haplotypes. Demonstrating a dominant B2 phenotype, B2/B12 birds were also more resistant to IBV. Respiratory clinical illness in B8/B8 chicks was severe early after infection, while illness resolved similar to the B5 and B2 homozygous birds. Following M41 strain infection, birds with B2/B2 and B8/B8 haplotypes were again more resistant to clinical illness than B19/B19 birds. Real time RT-PCR indicated that infection was cleared more efficiently in trachea, lungs and kidneys of B2/B2 and B8/B8 birds compared with B19/B19 birds. Furthermore, M41 infected B2/B2 and B8/B8 chicks performed better in terms of body weight gain than B19/B19 chicks. These studies suggest that genetics of B defined haplotypes might be exploited to produce chicks resistant to respiratory pathogens or with more effective immune responses.
Abbreviations: IBV, infectious bronchitis virus; MHC, major histocompatibility complex; M41, Massachusetts 41; RT-PCR, reverse transcription polymerase chain reaction; SARS, severe acute respiratory syndrome; RSV, Rous sarcoma virus; MDV, Marek’s disease virus; SPF, specific pathogen free; PI, post infection; EID50/ml, embryo infectious dose 50 per ml; NIU, Northern Illinois University; PBS, phosphate buffer saline; RNA, ribonucleic acid; 5′ UTR, 5′ untranslated region; bp, base pairs; ANOVA, analysis of variance; ARK, Arkansas; CTL, cytotoxic T lymphocyte; AIV, avian influenza virus; IFNγ, interferon gamma; poly I:C, polyinosinic polycytidylic acid; USDA, United States Department of Agriculture; NIFA, National Institute of Food and Agriculture
Keywords: Infectious bronchitis virus, Chicken MHC B haplotype, Clinical illness, Infection of trachea, Lungs and kidneys, Resistant
1. Introduction
Infectious respiratory diseases are responsible for considerable economic losses in commercial poultry operations. The respiratory avian coronavirus, infectious bronchitis virus (IBV), has consistently been a major agricultural problem in chickens for many years (Cavanagh, 2007, Collisson et al., 1992). IBV was the first coronavirus described (Schalk and Hawn, 1931) and the first for which the entire genome was sequenced (Binns et al., 1985, Boursnell et al., 1987, Collisson et al., 1992). As a non-zoonotic virus, IBV is ideal for characterizing avian host responses to respiratory viruses. Furthermore, it provides a model for the highly contagious severe acute respiratory syndrome (SARS) in humans, which resembles IBV in transmission, pathogenesis and genome structure (Jackwood, 2006).
In chickens, IBV causes a highly contagious acute respiratory disease especially in commercial flocks and is transmitted by aerosol and fecal contamination. While respiratory signs of illness are coughing, sneezing, nasal discharge, gasping and tracheal rales, IBV successfully replicates in various tissues including respiratory, intestinal and reproductive tracts, as well as kidneys and infections have been linked to nephrogenic, enteric and reproductive diseases (Cavanagh and Naqi, 2003, Lucio and Fabricant, 1990, Raj and Jones, 1997). Chickens of all ages can be infected, but morbidity and mortality are generally more severe in very young chicks. Economic losses are mainly attributed to decreased weight gain, feed efficiency, egg production and quality, mortality and condemnation (Cavanagh and Naqi, 2003).
IBV infection induces humoral and cellular immune responses in chickens, which result in viral clearance and protection against challenge infection (Cavanagh, 2007, Collisson et al., 2000, Ignjatovic and Galli, 1994, Pei et al., 2001, Raj and Jones, 1997, Seo et al., 2000). The first IBV vaccine strains, which have undergone many in vivo and in vitro passages, were derived from the classical IBV Massachusetts 41 (M41) and the closely related Beaudette strains (Cavanagh and Naqi, 2003, Hodgson et al., 2004). M41, a prototype strain of IBV, was first isolated in 1941 by Van Roekel at the University of Massachusetts (Fabricant, 1998, Jungherr et al., 1956). During the following decades, numerous serologically and genetically distinct strains were identified throughout the world. Molecular studies suggest that continuing evolution of the viral spike protein, the target protein of neutralization, can result in vaccine breaks and IBV outbreaks (Bochkov et al., 2006, Cavanagh and Naqi, 2003, Ignjatovic and Sapats, 2000, Ignjatovic and Galli, 1994). Variations are a result of the common occurrences of point mutations and recombination events (Cavanagh et al., 2005, Jackwood et al., 2012, Wang et al., 1994, Wang et al., 1993).
The severity of infection with IBV may depend, in part, on the genetics of the infected chickens, including differences in immune responses (Bacon et al., 2004, Cook et al., 1990, Nakamura et al., 1991, Otsuki et al., 1990, Zekarias et al., 2002). Successful selection and breeding of chickens for disease resistance is a rational approach to reducing disease-related losses. Inbred White Leghorn line 15I birds were shown to be more susceptible to IBV challenge than line C, which exhibited more severe clinical symptoms and a greater amount of virus was recovered from the respiratory tract and kidneys (Cook et al., 1990, Nakamura et al., 1991, Otsuki et al., 1990). A retrospective study implicated the MHC B region in resistance to IBV where the vaccinated chicks with B13 and B21 haplotypes experienced higher mortalities than those with the B15 haplotype (Bacon et al., 2004). The B15 haplotype has been associated with Rous sarcoma virus (RSV) progression of tumors and sensitivity to Marek’s disease virus (MDV), and the B2 haplotype was associated with RSV regression and the B21 with moderate regression of RSV induced tumors (Bacon et al., 2000).
The chicken B complex, which includes the B–F or MHC class I genes, B–L or MHC class II genes and B–G genes, unique to birds (Miller et al., 1988, Plachy et al., 1992), consists of at least 242 genes, mostly related to innate and adaptive immune responses (Briles and Briles, 1987, Shiina et al., 2007). There are over 50 genetically defined chicken MHC or B haplotypes (Fulton et al., 2006). Although the MHC B region has been implicated in resistance, a clinically controlled study examining the extent of susceptibility and resistance to IBV induced disease in a spectrum of B haplotypes has not been reported.
The current studies compare the clinical respiratory illness associated with the pneumotropic and nephrotropic IBV Gray strain infection of chickens from five homozygous lines and one heterozygous line defined by their MHC B haplotypes. Birds with B haplotypes selected on the basis of their Gray strain associated respiratory disease were infected with a second IBV strain, the pneumotropic M41, for scoring of clinical illness, viral load and performance.
2. Materials and methods
2.1. Viral stocks
The pneumopathogenic M41 and the moderately pneumopathogenic and moderately nephropathogenic Gray strains of IBV were separately propagated in 10-day-old specific pathogen free (SPF) chicken embryos (Sneed et al., 1989). The morbidity (any respiratory illness during any of the days examined) resulting from infection with either strain was >90% in these studies. The IBV M41 strain was further passaged twice in 4–5-day-old SPF chicks. The virus was collected from the pooled supernatants of trachea and lungs when respiratory signs were observed in chicks at 6 and 8 days post infection (PI) for passage 1 and at days 7, 9, 11 and 13 days PI for passage 2. The viral titers of the IBV strains were determined in embryonated chicken eggs by the Reed and Muench method (1938) and expressed as embryo infectious dose 50 per ml (EID50/ml). The viral stocks were stored at −80 °C.
2.2. Experimental animals
All chicks used in these experiments were hatched and housed in an SPF environment. Eggs with embryos, that were homozygous for B19, B2, B8, B5 and B12 haplotypes and the heterozygous B2/B12 haplotype, were obtained from Northern Illinois University (NIU), DeKalb, IL (Briles and Briles, 1982). The NIU chicken lines, with simultaneous segregation of nine alloantigen genes and particular MHC genes, were derived from unvaccinated chickens in flocks negative for IBV and avian influenza and have been maintained as closed flocks at NIU since 1970. Two chicken stocks of diverse origins (White Leghorn and Wisconsin inbred line 3 Ancona breeds) have been selected for crosses to yield full and half-sib families (Briles, 2004). All matings were designed to utilize parents from multiple families of the desired B haplotype with minimal inbreeding, where full and half sib matings were avoided. All NIU eggs were obtained from birds, whose MHC B haplotypes had been identified and were distinguishable by serology and microsatellite typing. All the homozygous B haplotype eggs were collected from several matings, in which all parents were homozygous for the 19, 12, 2, 5 or 8 B type allele (Briles and Briles, 1982, Fulton et al., 2006). The heterozygous B2/B12 chicks were obtained from several matings of B2/B2 dams and B12/B12 sires. The SPF eggs used for the titration of virus were obtained from Avian Vaccine Services (Charles River Laboratories, Inc., North Franklin, CT). All birds were humanely euthanized by isoflurane overdose. Animal experiments were approved by the institutional animal care and use committees of Texas A&M University and Western University of Health Sciences.
2.3. Experimental design
Each experimental group was housed separately in an SPF environment with free access to feed and water. Chicks were inoculated by the oculo-nasal route at 5–6 days of age with either the Gray or M41 strain of IBV or with buffered phosphate (PBS) as controls (Pei et al., 2001, Seo and Collisson, 1997). In the initial IBV Gray experiment, 24 birds in each experimental group (B19/B19, B12/B12, B2/B2, B5/B5 and B2/B12) were infected with 106 EID50 of Gray strain per bird, while the infected B8/B8 group had 36 birds and all uninfected control groups had 12 birds per group. In a separate second experiment, using B haplotype defined birds infected with 2 × 101.3 EID50 IBV M41, 13, 17 and 21 birds were used in the uninfected control B2/B2, B19/B19 and B8/B8 groups, respectively, while 16, 20 and 21 chicks were used in the infected B2/B2, B19/B19 and B8/B8 groups, respectively. In a separate experiment quantifying M41 in vivo, seven 6-day-old SPF chicks were used for inoculation of each 10-fold (10−1 and 10−5 in PBS) dilution of M41 (103.3 EID50/ml), while 12 age-matched uninfected control chicks were inoculated with an equivalent volume of PBS. The chicks given M41 were inoculated with 0.2 ml and those given Gray were inoculated with 0.1 ml total volume of virus per chick in PBS. The IBV Gray study was conducted in the Lab Animal Research Resources facility at Texas A&M University, College Station, TX; while the IBV M41 studies were performed in the Animal Resources facility at Western University of Health Sciences, Pomona, CA.
2.4. Clinical illness and chicken performance
All chicks were examined daily for signs of clinical illness. Clinical illness of control and IBV Gray-infected chicks was scored daily from 0 to 3+ according to the following criteria: 0 = no illness; 1+ = sneezing/coughing or nasal discharge; 2+ = sneezing/coughing or nasal discharge and tracheal rales and 3+ = sneezing/coughing or nasal discharge, tracheal rales and labored breathing or depression (Pei et al., 2001). More stringent criteria were instituted for scoring of clinical illness for IBV M41-infected chicks. All control and IBV M41-infected birds were examined daily for the following clinical signs: (1) depression, (2) ruffled feathers, (3) swollen sinuses or ocular discharges, (4) nasal discharge, (5) coughing/sneezing, (6) gasping and (7) tracheal rales. These seven clinical signs were scored according to the following criteria: 0 = no signs; 1+ = mild signs; 2+ = moderate signs and 3+ = severe signs with a range of 0 to a maximum of 21+. The average percentage of maximum possible observed clinical illness for each group in each study was calculated as: (A/B) × 100, where A was the average total clinical score observed for all chicks in a group and B was the maximum possible total clinical score for the total number of chicks in a group (Pei et al., 2001). Chicken performance was assessed by measuring the daily body weight in grams (g) of individual chicks and calculated as the average weight in grams per group per day.
2.5. Real-time RT-PCR for identifying IBV infection in harvested tissues
Four chicks from each IBV M41-infected group and three to four birds from each control group were randomly selected from each B haplotype (B19/B19, B2/B2 and B8/B8) at 4, 8, 12 and 16 days PI for collection of trachea, lungs and kidneys. Tissues were collected over ice and stored at −80 °C within the next 2 h. Viral RNA (ribonucleic acid) was extracted and used as templates for the amplification of the 5′ non-coding region of the viral genome. Forward and reverse primers for the IBV 5′ UTR (untranslated region) generated a 143 bp (base pairs) fragment that was used with a Taqman dual-labeled probe. Adjusted IBV genome copy number (#) was quantified from known weights of trachea, lung and kidney tissues using real-time Taqman RT-PCR (reverse transcription polymerase chain reaction). As few as 100 genome or template copies per 1 ml of sample could be quantified using this protocol (Callison et al., 2006). The percentages of birds with positive IBV genome copy numbers (containing more than 100 viral genome copies per 1 ml sample) were determined for each tissue at varying days PI.
2.6. Statistical analysis
The average percentages of total possible illness of control and IBV Gray-infected chicks were statistically analyzed for each haplotype and day PI by ANOVA (analysis of variance) at a significance of p < 0.05. The average percentages of maximum possible observed clinical illness, based on accumulative scores for each clinical sign as described above in the “Clinical illness and chicken performance” Section 2.4, were calculated for each day for each bird. The average percentages of maximum possible observed clinical illness and the average weights of control and IBV M41-infected birds data were tested for normality and analyzed for significant differences (p < 0.05) among the various B haplotype/treatment groups using two-way analysis of variance (2-way ANOVA). All statistical analyses were conducted using GraphPad Prism 5 for Windows version 5.04 software 1992–2010 (GraphPad software, Inc., La Jolla, CA).
3. Results
3.1. Severity of clinical illness of birds infected with IBV Gray was associated with defined MHC B haplotypes
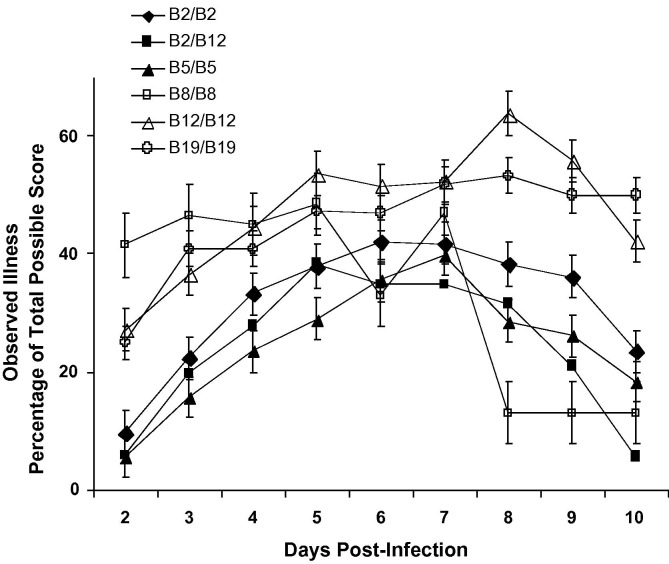
In the initial experiment, comparison of the clinical illness following infection with the moderately pneumopathogenic Gray IBV was evaluated in 6 lines of chickens defined by their unique MHC B region. Chicks in 5 lines were homozygous for the B loci (B2, B5, B8, B12 and B19) and chicks in the sixth line were heterozygous for B12 and B2. In this initial infectivity study, chicks were infected with the moderately pneumopathogenic/nephropathogenic IBV Gray strain. Following IBV Gray infection, the presentation of clinical illness (on the basis of 0 to 3+) of the homozygous B19 and B12 birds was greater than that of the homozygous B5 and B2 and the heterozygous B2/B12 birds. Differences were significant (p < 0.05) at 2, 5, 8 and 10 days PI (Fig. 1 ). While the B2/B2, B5/B5 and B2/B12 chicks showed a more rapid decline in clinical illness after the peak of severity at day 7 PI, the B12/B12 and B19/B19 chicks recovered only slightly by day 8 PI and still displayed greater clinical illness at 10 days PI. Although B8/B8 birds demonstrated a similar degree of clinical illness as that of the B12/B12 and B19/B19 birds until day 7 PI, there was definitely a more rapid decline in signs of respiratory illness by day 8 PI compared with those of the B2 and B5 homozygous birds. No signs of illness were observed in the PBS inoculated controls (data not shown).
Fig. 1.
Observed clinical illness of IBV Gray-infected chickens differed with the MHC B haplotype. Groups of 12–36 chickens homozygous for B2, B5, B8, B12 and B19 haplotypes and the heterozygous B2/B12 were examined daily for clinical respiratory signs. Indicators of illness included nasal discharge or sneezing, tracheal rales and depression, which were scored in a range from 0 to 3+. Each data point represents the average percentage of total possible clinical illness score ± SEM.
3.2. In vivo quantification of IBV M41 stock or Infectious
A second experiment was designed to examine the pathogenesis of a more virulent pneumopathogenic IBV M41 strain passaged in vivo in lungs and trachea. Stock IBV M41 was collected from the homogenates of lungs and tracheas from IBV M41-infected SPF chicks. The infectious dose was determined in chicks in vivo following oculo-nasal inoculation using illness criteria, ranging from 0 to 21+ per chick. Although illness was observed in chicks inoculated with all five dilutions of virus from 6 to 22 days PI, maximum illness was observed following infection with the 10−1–10−3 dilutions of the IBV M41 stock (Fig. 2 ). The second highest concentration of the virus (10−2) was chosen because it was the lowest concentration of the virus stock that caused a significant reproducible maximum clinical illness in SPF birds from 5 to 17 days PI with the shortest lag in appearance of respiratory signs (p < 0.05).
Fig. 2.
Clinical illness of specific pathogen free (SPF) birds post infection with 5 serial 10-fold dilutions of IBV M41 stock or phosphate buffer saline (PBS). Groups of 7–12 control and infected birds were examined daily. Indicators of illness included depression, ruffled feathers, swollen sinuses, nasal discharge, coughing/sneezing, tracheal rales and gasping, which were scored on the basis of severity of each sign (no signs = 0, mild = 1+, moderate = 2+ and severe = 3+) and scores collectively ranged from 0 to 21+. Each data point represents the average percentage (%) of maximum possible clinical illness ± SEM.
3.3. Clinical illness following infection with IBV M41 was associated with the chicken B haplotype
As indicated in the studies using the Gray strain of IBV, the homozygous B2, B8 and B19 chicks were selected for infection with the IBV M41 strain. The more comprehensive criteria used in quantifying the M41 stock were again used to compare clinical illness in birds with these selected representative haplotypes in a second experiment. Clinical illness was detected as early as 2 and 3 days PI in chicks of all three haplotypes (Fig. 3 ). The chicks with the B2/B2 and B8/B8 haplotypes consistently experienced less clinical disease than B19/B19 chicks on most days PI. The clinical illness of B8 homozygous birds reached a maximum at 5 days PI and began declining 2 days earlier than B2 and B19 homozygous birds. Although the clinical disease reached a maximum at 7 days PI for both B2/B2 and B19/B19 chicks, the degree of clinical illness observed for B2/B2 and B8/B8 chicks was less than that observed for B19/B19 chicks. Significant differences in the severity of respiratory signs between B19/B19 and B2/B2 chickens were observed from 5 to 11 days PI and between B19/B19 and B8/B8 chickens from 7 to 11 and 13 days PI. No significant differences were noted in the degree of clinical illness between the B2 and B8 homozygous chickens at any day PI. No signs of illness were observed in any of the control birds receiving only PBS throughout the studies.
Fig. 3.
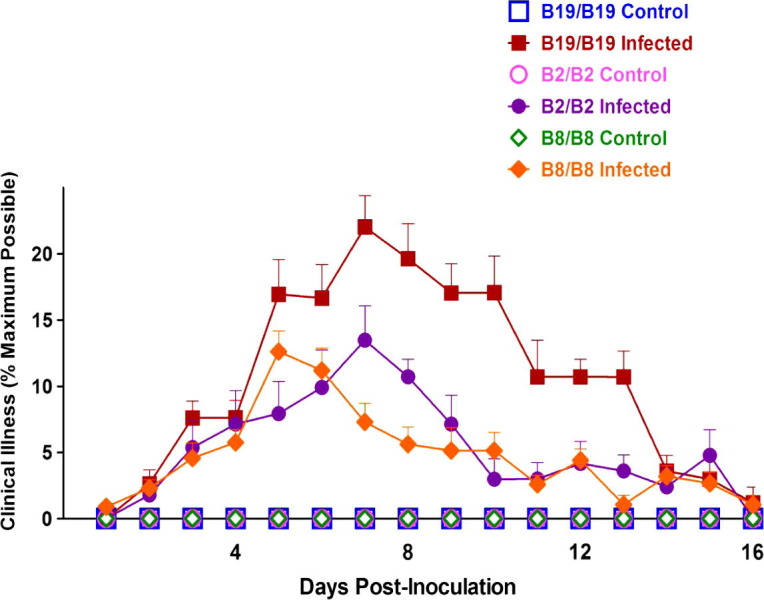
Observed clinical illnesses of B2 and B8 homozygous chickens were significantly less severe than the B19/B19 birds following infection with the IBV M41 strain. Groups of 13–21 control and infected B19/B19, B2/B2 and B8/B8 birds were examined daily. Indicators of illness included depression, ruffled feathers, swollen sinuses, nasal discharge, coughing/sneezing, tracheal rales and gasping, which were scored on the basis of severity of each sign (no signs = 0, mild = 1+, moderate = 2+ and severe = 3+) and collectively ranged from 0 to 21+. Each data point represents the average percentage (%) of maximum possible clinical illness ± SEM.
3.4. Chicken performance after infection with the M41 strain of IBV
The only significant differences in the average weights among the control birds were observed between the B19 homozygous birds and B8 homozygous birds from 11 to 16 days following inoculation with PBS (Fig. 4 ). While all birds in the IBV M41-infected groups had consistently lower average weights after 13 days PI than those of the corresponding control bird groups, the differences in the average weights of only B8/B8 control and infected chicks were significant from 7 to 12 and 14 to 15 days PI. Furthermore, the average weights of the infected B19/B19 birds were significantly lower than those of the infected B2/B2 from 14 to 16 days PI and significantly lower than those of the infected B8/B8 birds from 15 to 16 days PI.
Fig. 4.
The average weights, as a measure of performance, of control (PBS-inoculated) and IBV M41-infected birds with distinct B haplotypes. Each data point represents the average body weight in grams (g) ± SEM.
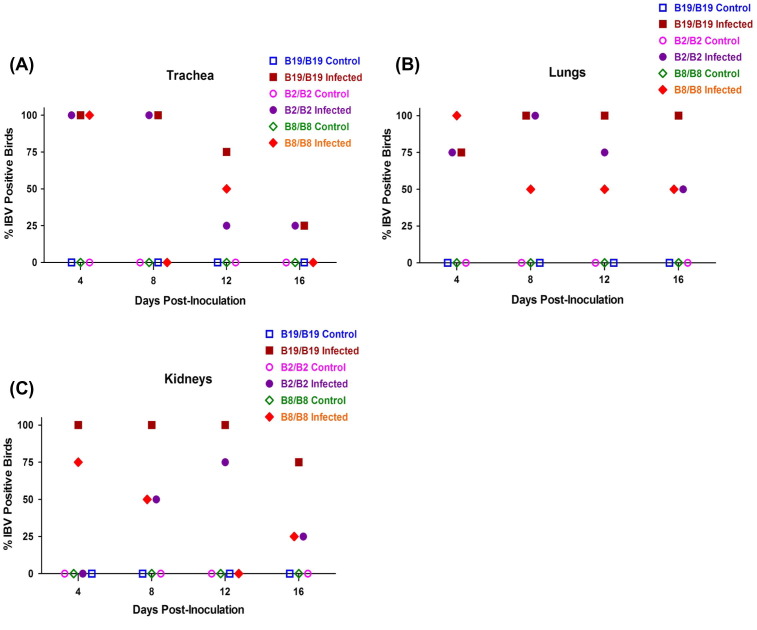
3.5. A greater percentage of B2/B2 and B8/B8 chickens cleared IBV M41 more readily than B19/B19 chickens
The percentages of birds positive for IBV were determined for the trachea, lungs and kidneys at 4, 8, 12 and 16 days PI using real-time RT-PCR. The tracheas of all B2, B8 and B19 homozygous birds were positive for IBV on day 4 PI (Fig. 5 a). From days 8 to 16 PI, the tracheas of fewer B8/B8 chicks were positive compared with trachea tissues from B19/B19 birds, whereas the tracheas of fewer B2/B2 birds had detectable IBV RNA after day 8 PI than B19/B19 birds. Overall, virus could be detected in 75–100% of the tissues from lungs and kidneys of B19 homozygous birds. In contrast, the percentages of B2 and B8 homozygous chicks positive for IBV in the lungs and kidneys were lower than B19/B19 after day 8 PI (Fig. 5b and c). Viral RNA was not detected in any tissue of the control chicks inoculated with PBS.
Fig. 5.
The percentage of B2, B8 and B19 homozygous birds positive for the virus in (A) Trachea, (B) Lungs and (C) Kidneys following IBV M41 infection indicated B2 and B8 homozygous birds more effectively cleared virus. Tissues were collected from 3 control and 4 infected birds at 4, 8, 12 and 16 days post inoculation with PBS or IBV M41. Viral RNA was extracted and adjusted IBV genome copy number was quantified using real-time Taqman RT-PCR. Each data point represents the average percentage (%) of chickens containing more than 100 genome copies per 1 ml of RNA sample.
4. Discussion
Infectious bronchitis virus causes a non-zoonotic, highly contagious respiratory disease of birds resulting in invasive infection with virus not only replicating in the trachea and lungs, but also the kidneys, and reproductive and gastrointestinal tracts (Cavanagh and Naqi, 2003, Jackwood, 2006). In the current studies, birds with the homozygous B2, B5 and B8 haplotypes were identified as having only mild clinical disease to IBV while those with the homozygous B12 and B19 haplotypes presented with severe clinical disease. Interestingly, birds heterozygous for the less susceptible B2 and the more susceptible B12 haplotypes were at least as resistant as B2/B2 birds, suggesting a dominance of the resistant B2 phenotype. Similarly, Joiner et al. (2007) reported that the severity and duration of illness following Ark (Arkansas) IBV strain challenge was not significantly different in IBV-vaccinated B2/B15 and B2/B21 birds, possibly reflecting the dominant B2 phenotype. In contrast, the kinetics of clinical illness differed for the B8 homozygous birds. The clinical illness of the B8/B8 birds resolved earlier following infection with the Gray strain than the more susceptible B12/B12 and B19/B19 birds or than the B19/B19 birds following infection with the M41 strain. However, the initial respiratory signs of the B8/B8 birds were more severe early PI with the Gray strain similar to that observed for the B19 and B12 homozygous chicks although they recovered faster than even the B2/B2, B5/B5 and B2/B12 birds. The early differences observed following infection of the B8/B8 birds could be due to inherent differences in the B8/B8 line of chicks.
It is noteworthy that our classifications for resistance and susceptibility based on clinical illness correlated with clades or branches I (B2, B5 and B8) and III (B12 and B19) of sequence clusters described by Fulton et al. (2006) based on cluster analysis and sequence homology. Furthermore, the sequences in the B complex encompassing 14 gene loci from BG1 to BF2 have been shown to be identical at nine loci between the B12 and B19 haplotypes and at seven loci between the B5/B5 and B8/B8 (Hosomichi et al., 2008). However, sequences of the B2/B2 haplotype were not found to be relatively close to either the B12 and B19, nor the B5 and B8 haplotypes. Overall, the striking differences in disease resistance following IBV infection may be, at least in part, a direct consequence of differences in MHC B region. This is consistent with the importance of cellular and humoral immunity on resolution of IBV associated disease (Collisson et al., 2000, Ignjatovic and Galli, 1994, Ignjatovic and Sapats, 2000, Seo and Collisson, 1997, Seo et al., 1997).
Congenic lines have been used to associate B haplotype with immune protection to MD vaccines (Bacon and Witter, 1993), while non-MHC genes have also been implicated in MDV vaccine efficacy (Bacon et al., 1996, Chang et al., 2010). Infection of congenic lines of birds with a highly pathogenic strain of avian influenza virus (AIV) suggested that resistance could also be dependent on genes outside of the B locus (Hunt et al., 2010). However, the strain of AIV used was lethal even in many of the more resistant birds. Thus, the pathogenesis is very different from IBV strains that cause respiratory illness with low mortality, although the resistance and susceptibility in our studies were similar regardless of the IBV strain used. It is possible that genes outside that B region do influence, if not determine, resistance to viral infection. Future studies, determining the pathogenesis of IBV in inbred congenic chicken lines homozygous and heterozygous for these haplotypes, would more precisely define the impact of the B region and/or non-B regions on viral resistance. Similar studies were also suggested in the review by Bacon et al. (2000).
The negative impact of IBV M41 infection on body weight of chickens with the selected B2/B2, B8/B8 and B19/B19 haplotypes, determined as an indication of performance, correlated with the greater susceptibility of B19 homozygous birds, which had significantly lower body weights than the more resistant B2/B2 and B8/B8 birds. The decrease in body weight due to infection is an expected finding. Although not defined by their MHC, similar results were reported by Otsuki et al. (1990), where there was an indication that the effect on body weight was more marked in the more susceptible line 151 compared with the more resistant line C by 21 days PI following IBV infection.
In the current studies with IBV M41, the more clinically resistant B2/B2 and B8/B8 birds cleared the virus from all tissues (trachea, lungs and kidneys) examined more readily than the B19/B19 birds. Similarly, considerably more IBV was recovered from the respiratory tract organs and kidneys of the more susceptible line 151 chicks than the more resistant line C chicks (Otsuki et al., 1990). In contrast, Ignjatovic et al. (2003) reported that IBV distribution was similar in tissues (trachea, lungs and kidneys) of the susceptible and the resistant chicks throughout the acute phase (between 3 and 7 days) of infection. Moreover, IBV persisted longer in all tissues examined especially the lungs of B19 homozygous chicks than in the tissues of the B2 and B8 homozygous birds.
The greater susceptibility to clinical illness observed in B19/B19 birds could be a consequence of a more permissive environment for viral replication or due to a less effective immune response to infection, or to a combination of both. A more favorable environment for infection could explain why clinical illness was more severe and lasted longer in B19/B19 than in B2/B2 and B8/B8 birds.
Since variations in pathogenesis of IBV in various chicken haplotypes were observed early, the distinct differences in susceptibility and resistance to IBV may be impacted by innate immunity. The clearance of IBV from the tissues of the more resistance birds, especially early after infection could be attributed directly or indirectly to more responsive innate immune responses (Dawes & Drechsler, unpublished data). In companion studies, the B2/B2 macrophages differentiated more readily and were dramatically more responsive to both IFNγ (interferon gamma) and poly I:C (polyinosinic polycytidylic acid) than B19/B19 macrophages (Dawes & Drechsler, unpublished data). The latter stimulant simulates infection with an RNA virus, such as IBV, and the IFNγ response was intended to reflect the interphase between innate and adaptive immunity. Moreover, lymphocytes that infiltrate trachea, lungs and kidneys following infection with IBV have been shown to be T cells (Janse et al., 1994). MHC-restricted, IBV specific cytotoxic T cell responses have been shown to correlate with initial decreases in viral load and clinical signs (Collisson et al., 2000, Pei et al., 2003, Seo et al., 1997). Elucidating the interphase between innate and adaptive immune responses to IBV needs to be further examined in resistant and susceptible birds of defined B haplotypes.
Disease resistance of poultry to a number of avian viruses, such as Marek’s disease virus (Bacon et al., 2001, Hepkema et al., 1993), Rous sarcoma virus (Aeed et al., 1993, Collins et al., 1977, Taylor, 2004), avian leukosis virus (Yoo and Sheldon, 1992), infectious bursal disease virus (Fadly and Bacon, 1992), AIV (Hunt et al., 2010) and Newcastle disease virus (Dunnington et al., 1992), has been associated with the MHC B region. The DNA sequencing of the MHC I (B–F) and MHC II (B–L) genes indicated that, although promoters of the avian are very similar, enhancer A sequences vary for the B2, B4, B8 and B21 haplotypes and are missing entirely in the B12 and B19 haplotypes (Kaufman et al., 1999, Miller et al., 2004). Genetic resistance linked to the chicken B complex could be explained by variations in expressed MHC molecules. Although the IBV epitopes recognized by the MHC in these lines have not been defined, the nucleocapsid proteins of both IBV and AIV induced vigorous MHC matched virus specific CD8+ T lymphocyte responses (Seo et al., 1997, Singh et al., 2010a, Singh et al., 2010b).
5. Conclusions
Resistance to IBV disease has not been previously examined in birds with these haplotypes. The results in this manuscript indicate that these lines may be useful in defining B haplotype-linked influences on resistance to respiratory viruses, as well as differences in vaccines responses. These studies associated chicken MHC or B complex with IBV respiratory illness regardless of the IBV strain used. They also suggest that one might determine the sequences within the B2, B5 and B8 loci for eventual selection of chickens with resistance to IBV strains of distinct pathotypes. The chickens with the resistant haplotypes showed less severe clinical illness, cleared the virus infection sooner and performed better in terms of body weight than the susceptible chickens. An implication of these studies is the potential to breed birds with resistance genes from B2, B5 or B8 haplotypes in order to increase resistance to IBV and perhaps to other respiratory viral pathogens. While ongoing studies (Dawes and Drechsler, unpublished data) have identified functional differences in macrophages that would likely impact the sequelae of viral infection, future studies should investigate differences in adaptive immunity that would be further correlated with viral resistance.
Acknowledgements
This work was funded by grants from the U.S. Poultry and Egg Association (297), USDA (U.S. Department of Agriculture) Formula Animal Health Funds (1433), USDA Cooperative State Research Service (97035204-5069), USDA NIFA, National Institute of Food and Agriculture (2008-00875) and the USDA Agricultural Initiative Research Program with California State Polytechnic University, Pomona. The authors would like to thank Dr. Yvonne Drechsler, Dr. Maisie Dawes and Jianwu Pei for useful discussions and Ms. Lisa Griggs, Dr. Shailbala Singh and Dr. Ibrahim El-Sabagh for invaluable technical support.
Contributor Information
Ghida R. Banat, Email: drgb09@gmail.com.
Suzana Tkalcic, Email: stkalcic@westernu.edu.
Jennifer A. Dzielawa, Email: dzielawa@gmail.com.
Mark W. Jackwood, Email: mjackwoo@uga.edu.
Miguel D. Saggese, Email: msaggese@westernu.edu.
W.E. Briles, Email: ebriles@niu.edu.
Ellen W. Collisson, Email: ecollisson@westernu.edu.
References
- Aeed P.A., Briles W.E., Zsigray R.M., Collins W.M. Influence of different B-complex recombinants on the outcome of Rous sarcomas in chickens. Anim. Genet. 1993;24:177–181. doi: 10.1111/j.1365-2052.1993.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Bacon L.D., Witter R.L. Influence of B-haplotype on the relative efficacy of Marek’s disease vaccines of different serotypes. Avian Dis. 1993;37:53–59. [PubMed] [Google Scholar]
- Bacon L.D., Hunter D.B., Zhang H.M., Brand K., Etches R. Retrospective evidence that the MHC (B haplotype) of chickens influences genetic resistance to attenuated infectious bronchitis vaccine strains in chickens. Avian Pathol. 2004;33:605–609. doi: 10.1080/03079450400013147. [DOI] [PubMed] [Google Scholar]
- Bacon L.D., Hunt H.D., Cheng H.H. Genetic resistance to Marek’s disease. Curr. Top. Microbiol. Immunol. 2001;255:121–141. doi: 10.1007/978-3-642-56863-3_5. [DOI] [PubMed] [Google Scholar]
- Bacon L.D., Hunt H.D., Cheng H.H. A review of the development of chicken lines to resolve genes determining resistance to diseases. Poult. Sci. 2000;79:1082–1093. doi: 10.1093/ps/79.8.1082. [DOI] [PubMed] [Google Scholar]
- Bacon L.D., Motta J., Cheng H., Vallejo R., Witter R. Use of recombinant congenic chicken strains to define non-HMC genes influencing Marek’s disease susceptibility. In: Silva R.F., Cheng H.H., Coussens P.M., Lee L.F., Velicer L.F., editors. Current Research on Marek’s Disease. American Association of Avian Pathologists; Kennett, PA: 1996. pp. 63–68. [Google Scholar]
- Binns M.M., Boursnell M.E.G., Cavanagh D., Pappin D.J.C., Brown T.D.K. Cloning and sequencing of the gene encoding the spike protein of the coronavirus IBV. J. Gen. Virol. 1985;66:719–726. doi: 10.1099/0022-1317-66-4-719. [DOI] [PubMed] [Google Scholar]
- Bochkov Y.A., Batchenko G.V., Shcherbakova L.O., Borisov A.V., Drygin V.V. Molecular epizootiology of avian infectious bronchitis virus in Russia. Avian Pathol. 2006;35:379–393. doi: 10.1080/03079450600921008. [DOI] [PubMed] [Google Scholar]
- Boursnell M.E.G., Brown T.D.K., Foulds I.J., Green P.F., Tomley F.M., Binns M.M. Completion of the sequence of the genome of the coronavirus avian infectious bronchitis virus. J. Gen. Virol. 1987;68:57–77. doi: 10.1099/0022-1317-68-1-57. [DOI] [PubMed] [Google Scholar]
- Briles W.E. Non-major histocompatibility complex alloantigen genes affecting immunity. Poult. Sci. 2004;83:606–610. doi: 10.1093/ps/83.4.606. [DOI] [PubMed] [Google Scholar]
- Briles W.E., Briles R.W. Genetics and classification of the major histocompatibility complex antigens of the chicken. Poult. Sci. 1987;66:776–781. doi: 10.3382/ps.0660776. [DOI] [PubMed] [Google Scholar]
- Briles W.E., Briles R.W. Identification of haplotypes of the chicken major histocompatibility complex (B) Immunogenetics. 1982;15:449–459. doi: 10.1007/BF00345904. [DOI] [PubMed] [Google Scholar]
- Callison S.A., Hilt D.A., Boynton T.O., Sample B.F., Robison R., Swayne D.E., Jackwood M.W. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J. Virol. Methods. 2006;138:60–65. doi: 10.1016/j.jviromet.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Naqi S.A. Infectious bronchitis. In: Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougald L.R., Swayne D.E., editors. Diseases of Poultry. eleventh ed. Iowa State University Press; Ames, Iowa: 2003. pp. 101–119. [Google Scholar]
- Cavanagh D., Picault J.P., Gough R., Hess M., Mawditt K., Britton P. Variation in the spike protein of the 793/B type of infectious bronchitis virus in the field and during alternate passage in chickens and embryonated eggs. Avian Pathol. 2005;34:20–25. doi: 10.1080/03079450400025414. [DOI] [PubMed] [Google Scholar]
- Chang S., Dunn J.R., Heidari M., Lee L.F., Song J., Ernest C.W., Ding Z., Bacon L.D., Zhang H. Genetics and vaccine efficacy: host genetic variation affecting Marek’s disease vaccine efficacy in white leghorn chickens. Poult. Sci. 2010;89:2083–2091. doi: 10.3382/ps.2010-00740. [DOI] [PubMed] [Google Scholar]
- Collins W.M., Briles W.E., Zsigray R.M., Dunlop W.R., Corbett A.C., Clark K.K., Marks J.L., McGrail T.P. The B locus (MHC) in the chicken: association with the fate of RSV-induced tumors. Immunogenetics. 1977;5:333–343. [Google Scholar]
- Collisson E.W., Pei J., Dzielawa J., Seo S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev. Comp. Immunol. 2000;24:187–200. doi: 10.1016/s0145-305x(99)00072-5. [DOI] [PubMed] [Google Scholar]
- Collisson E.W., Williams A., Parr R., Wang L. An overview of the molecular characteristics of avian infectious bronchitis virus. Poult. Sci. Rev. 1992;4:41–55. [Google Scholar]
- Cook J., Otsuki K., Huggins M., Bumstead N. Investigations into resistance of chicken lines to infection with infectious bronchitis virus. Adv. Exp. Med. Biol. 1990;276:491–496. doi: 10.1007/978-1-4684-5823-7_68. [DOI] [PubMed] [Google Scholar]
- Dunnington E.A., Larsen C.T., Gross W.B., Siegel P.B. Antibody responses to combinations of antigens in White Leghorn chickens of different background genomes and major histocompatibility complex genotypes. Poult. Sci. 1992;71:1801–1806. doi: 10.3382/ps.0711801. [DOI] [PubMed] [Google Scholar]
- Fabricant J. The early history of infectious bronchitis. Avian Dis. 1998;42:648–650. [PubMed] [Google Scholar]
- Fadly A.M., Bacon L.D. Response of B congenic chickens to infection with infectious bursal disease virus. Avian Dis. 1992;36:871–880. [PubMed] [Google Scholar]
- Fulton J.E., Juul-Madsen H.R., Ashwell C.M., McCarron A.M., Arthur J.A., O’Sullivan N.P., Taylor R.L., Jr. Molecular genotype identification of the Gallus gallus major histocompatibility complex. Immunogenetics. 2006;58:407–421. doi: 10.1007/s00251-006-0119-0. [DOI] [PubMed] [Google Scholar]
- Hepkema B.G., Blankert J.J., Albers G.A.A., Tilanus M.G.J., Egberts M.E., van der Zijpp A.J., Hensen E.J. Mapping of susceptibility to Marek’s disease within the major histocompatibility (B) complex by refined typing of White Leghorn chickens. Anim. Genet. 1993;24:283–287. doi: 10.1111/j.1365-2052.1993.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Hodgson T., Casais R., Dove B., Britton P., Cavanagh D. Recombinant infectious bronchitis coronavirus Beaudette with the spike protein gene of the pathogenic M41 strain remains attenuated but induces protective immunity. J. Virol. 2004;78:13804–13811. doi: 10.1128/JVI.78.24.13804-13811.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosomichi K., Miller M.M., Goto R.M., Wang Y., Suzuki S., Kulski J.K., Nishibori M., Inoko H., Hanzawa K., Shiina T. Contribution of mutation, recombination, and gene conversion to chicken MHC-B haplotype diversity. J. Immunol. 2008;181:3393–3399. doi: 10.4049/jimmunol.181.5.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt H.D., Jadhao S., Swayne D.E. Major histocompatibility complex and background genes in chickens influence susceptibility to high pathogenicity avian influenza virus. Avian Dis. 2010;54:572–575. doi: 10.1637/8888-042409-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Ignjatovic J., Galli L. The S1 glycoprotein but not the N or M proteins of avian infectious bronchitis virus induce protection in vaccinated chickens. Arch. Virol. 1994;138:117–134. doi: 10.1007/BF01310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatovic J., Sapats S. Avian infectious bronchitis virus. Rev. Sci. Tech. 2000;19:493–508. doi: 10.20506/rst.19.2.1228. [DOI] [PubMed] [Google Scholar]
- Ignjatovic J., Reece R., Ashton F. Susceptibility of three genetic lines of chicks to infection with a nephropathogenic T strain of avian infectious bronchitis virus. J. Comp. Pathol. 2003;128:92–98. doi: 10.1053/jcpa.2002.0609. [DOI] [PubMed] [Google Scholar]
- Jackwood M.W. The relationship of severe acute respiratory syndrome coronavirus with avian and other coronaviruses. Avian Dis. 2006;50:315–320. doi: 10.1637/7612-042006R.1. [DOI] [PubMed] [Google Scholar]
- Jackwood M.W., Hall D., Handel A. Molecular evolution and emergence of avian gammcoronaviruses. Infect. Genet. Evol. 2012;12:1305–1311. doi: 10.1016/j.meegid.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse E.M., van Roozelaar D., Koch G. Leukocyte subpopulations in kidney and trachea of chickens infected with infectious bronchitis virus. Avian Pathol. 1994;23:513–523. doi: 10.1080/03079459408419021. [DOI] [PubMed] [Google Scholar]
- Joiner K.S., Hoerr F.J., Ewald S.J., van Santen V.L., Wright J.C., van Ginkel F.W., Toro H. Pathogenesis of infectious bronchitis virus in vaccinated chickens of two different major histocompatibility B complex genotypes. Avian Dis. 2007;51:758–763. doi: 10.1637/0005-2086(2007)51[758:POIBVI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Jungherr, E.I., Chomiak, T.W., Luginbuhl, R.E., 1956. Immunologic differences in strains of infectious bronchitis virus. In: Proc. 60th Annual Meeting US Livestock Sanitary Association, pp. 203–209.
- Kaufman J., Jacob J., Shaw I., Walker B., Milne S., Beck S., Salomonsen J. Gene organization determines evolution of function in the chicken MHC. Immunol. Rev. 1999;167:101–117. doi: 10.1111/j.1600-065x.1999.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Lucio B., Fabricant J. Tissue tropism of three cloacal isolates and Massachusetts strain of infectious bronchitis virus. Avian Dis. 1990;34:865–870. [PubMed] [Google Scholar]
- Miller M.M., Bacon L.D., Hala K., Hunt H.D., Ewald S.J., Kaufman J., Zoorob R., Briles W.E. Nomenclature for the chicken major histocompatibility (B and Y) complex. Immunogenetics. 2004;56:261–279. doi: 10.1007/s00251-004-0682-1. [DOI] [PubMed] [Google Scholar]
- Miller M.M., Goto R., Briles W.E. Biochemical confirmation of recombination within the B–G subregion of the chicken major histocompatibility complex. Immunogenetics. 1988;27:127–132. doi: 10.1007/BF00351086. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Cook J.K., Otsuki K., Huggins M.B., Frazier J.A. Comparative study of respiratory lesions in two chicken lines of different susceptibility infected with infectious bronchitis virus: histology, ultrastructure and immunohistochemistry. Avian Pathol. 1991;20:241–257. doi: 10.1080/03079459108418761. [DOI] [PubMed] [Google Scholar]
- Otsuki K., Huggins M.B., Cook J.K.A. Comparison of the susceptibility to infectious bronchitis virus infection of two inbred lines of White Leghorn chickens. Avian Pathol. 1990;19:467–475. doi: 10.1080/03079459008418700. [DOI] [PubMed] [Google Scholar]
- Pei J., Briles W.E., Collisson E.W. Memory T cells protect chicks from acute infectious bronchitis virus infection. Virology. 2003;306:376–384. doi: 10.1016/s0042-6822(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Pei J., Sekellick M.J., Marcus P., Collisson E.W. Chicken interferon type I inhibits infectious bronchitis virus replication and associated respiratory illness. J. Interferon Cytokine Res. 2001;21:1071–1077. doi: 10.1089/107999001317205204. [DOI] [PubMed] [Google Scholar]
- Plachy J., Pink J.R.L., Hala K. Biology of the chicken MHC (B complex) Crit. Rev. Immunol. 1992;12:47–79. [PubMed] [Google Scholar]
- Raj G.D., Jones R.C. Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathol. 1997;26:677–706. doi: 10.1080/03079459708419246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method for estimation fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Schalk A.F., Hawn M.C. An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 1931;78:413–422. [Google Scholar]
- Seo S.H., Collisson E.W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J. Virol. 1997;71:5173–5177. doi: 10.1128/jvi.71.7.5173-5177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Pei J., Briles W.E., Dzielawa J., Collisson E.W. Adoptive transfer of infectious bronchitis virus primed alpha beta T cells bearing CD8 antigen protects chicks from acute infection. Virology. 2000;269:183–189. doi: 10.1006/viro.2000.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Wang L., Smith R., Collisson E.W. The carboxyl terminal 120-residue polypeptide of infectious bronchitis virus nucleocapsid induces cytotoxic T lymphocytes and protects chickens from acute infection. J. Virol. 1997;71:7889–7894. doi: 10.1128/jvi.71.10.7889-7894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina T., Briles W.E., Goto R.M., Hosomichi K., Yanagiya K., Shimizu S., Inoko H., Miller M.M. Extended gene map reveals tripartite motif, C-type lectin, and Ig superfamily type genes within a subregion of the chicken MHC-B affecting infectious disease. J. Immunol. 2007;178:7162–7172. doi: 10.4049/jimmunol.178.11.7162. [DOI] [PubMed] [Google Scholar]
- Singh S., Briles W.E., Lupiani B., Collisson E.W. Avian influenza viral nucleocapsid and hemagglutinin proteins induce chicken CD8+ memory T lymphocytes. Virology. 2010;399:231–238. doi: 10.1016/j.virol.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Toro H., Tang D.C., Briles W.E., Yates L.M., Kopulos R.T., Collisson E.W. Non-replicating adenovirus vectors expressing avian influenza virus hemagglutinin and nucleocapsid proteins induce chicken specific effector, memory and effector memory CD8+ T lymphocytes. Virology. 2010;405:62–69. doi: 10.1016/j.virol.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed L., Butcher G., Wang L., Parr R., Collisson E.W. Comparisons of the structural proteins of avian bronchitis virus as determined by Western blot analyses. Viral Immunol. 1989;2:221–227. doi: 10.1089/vim.1989.2.221. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., Jr. Major histocompatibility (B) complex control of response against Rous sarcomas. Poult. Sci. 2004;83:636–649. doi: 10.1093/ps/83.4.638. [DOI] [PubMed] [Google Scholar]
- Wang L., Junker D., Hock L., Ebiary E., Collisson E.W. Evolutionary implications of genetic variations in the S1 gene of infectious bronchitis virus. Virus Res. 1994;34:327–338. doi: 10.1016/0168-1702(94)90132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Junker D., Collisson E.W. Evidence of natural recombination within the S1 gene of infectious bronchitis virus. Virology. 1993;192:710–716. doi: 10.1006/viro.1993.1093. [DOI] [PubMed] [Google Scholar]
- Yoo B.H., Sheldon B.L. Association of the major histocompatibility complex with avian leukosis virus infection in chickens. Br. Poult. Sci. 1992;33:613–620. doi: 10.1080/00071669208417500. [DOI] [PubMed] [Google Scholar]
- Zekarias B., Ter Huurne A.A., Landman W.J., Rebel J.M., Pol J.M., Gruys E. Immunological basis of differences in disease resistance in the chicken. Vet. Res. 2002;33:109–125. doi: 10.1051/vetres:2002001. [DOI] [PubMed] [Google Scholar]