Summary
Ferrets (Mustela putorius furo) develop symptoms upon influenza infection that resemble those of humans, including sneezing, body temperature variation and weight loss. Highly pathogenic strains of influenza A, such as H5N1, have the capacity to cause severe illness or death in ferrets. The use of ferrets as a model of influenza infection is currently limited by a lack of species-specific immunological reagents. Interferon gamma (IFN-γ) plays a key role in the development of innate and adaptive immunity and the regulation of Th1-type immune responses. Here we describe the cloning of the full-length cDNA for ferret IFN-γ. Multiple sequence alignment of the predicted amino acid sequence with those of other species indicates that the predicted ferret protein shares the highest identity with Eurasian badger IFN-γ. We raised two hybridoma clones expressing monoclonal antibodies against recombinant ferret IFN-γ capable of detecting IFN-γ protein derived from mitogen-stimulated ferret PBMCs by immunoblotting, ELISA and ELISPOT assay. Finally, an ELISA utilizing the ferret-specific antibodies detected elevated levels of IFN-γ in serum samples from H3N2 influenza A-infected ferrets.
Keywords: Ferret, Influenza, Cytokine, IFN-γ ferret, Interferon gamma, Animal model, Influenza, Monoclonal antibody, Comparative study, Immunoassay, Cytokines
Introduction
Influenza A viruses infect millions of people world-wide resulting in high morbidity and mortality rates, particularly in the very young and elderly [1], [2], [3], [4]. Certain highly pathogenic avian influenza A viruses, such as the H5N1 strain, are a significant threat to global health. H5N1 influenza has a high mortality rate, even in healthy young adults, and while human-to-human transmission has been limited, mutation or genetic reassortment of H5N1 influenza A virus may create a strain readily capable of human-to-human transmission and with pandemic potential.
Cytokines are important mediators of the immune response and have been shown to play a pivotal role in host immune defense to microbial infection [5]. Interferon gamma (IFN-γ) has been recognized as a multipotent cytokine due to its broad range of regulatory functions during virus-induced host immune responses [6], [7]. IFN-γ can directly influence the differentiation of T cells and promote Th1-type immune responses in CD4+ and CD8+ T cells [5], [8]. Virtually all immune cell types express receptors for IFN-γ and are influenced by this cytokine. Signaling by IFN-γ leads to up-regulation of class II major histocompatibility complex (MHC) expression, which in turn increases the antigen presentation to CD4+ T cells by macrophages, dendritic cells, and B cells [9], [10]. IFN-γ also increases the expression of class I MHC, resulting in enhanced stimulation of antigen-specific CD8+ T cells [11], [12]. Upon stimulation of these cells by IFN-γ, CD8+ T cells are important in the eradication of intracellular virus during recovery from viral infections [13], [14], [15]. Moreover, high levels of IFN-γ bias the polarity of CD4+ T helper cells towards a Th1 phenotype, characterized by production of IL-2 and IFN-γ [16], [17]. A Th1-type immune response is fundamental to the development of host immunity against many pathogens, including influenza A virus [18], [19], [20]. IFN-γ is also necessary for T cell-mediated viral clearance and limitation of latent viral infections [21]. Moreover, IFN-γ production is considered to be critical in determining vaccine efficacy [22], [23]. Therefore, measurement of IFN-γ production in both natural and experimental influenza A virus infections will improve our understanding of the antiviral immune response, which may assist the development of more effective vaccines.
Several animal models have been developed for testing the efficacy of influenza vaccines and antiviral therapies, including mice, rats, guinea pigs, nonhuman primates and ferrets [24], [25], [26], [27]. Ferrets (Mustela putorius furo) have been used by researchers to identify novel viruses, determine strain toxicity and evaluate vaccines [28]. Ferrets are naturally susceptible to human strains of influenza virus and display many of the same symptoms that appear in humans, including sneezing, nasal discharge, weight loss and an increased body temperature [29]. Indeed, the Spanish flu virus (H1N1) was first identified in ferrets [28]. Ferrets therefore represent a fundamentally important model for studying the transmission and pathogenesis of influenza. A current limitation to the ferret influenza model, however, is the lack of species-specific immunological reagents required to assess host responses in vaccinated and infected animals. In the current study, we report the full-length cloning of the ferret IFN-γ cDNA, expression of the recombinant cytokine, and the generation of monoclonal antibodies suitable for ELISA and ELISPOT detection of natural ferret IFN-γ.
Materials and methods
Experimental animals
Six-month-old male ferrets (M. putorius furo) were purchased from Triple F Farms Inc. (Sayre, PA, USA) and housed at the Southern Research Institute (SRI) BSL-2 animal facility (Birmingham, AB). Approval by IACUC and IBC committees at SRI were obtained for all procedures. After arrival, ferrets were quarantined and monitored for 1 week prior to tissue and blood collection. Animal diets were based on a low fat, high protein regimen.
Female Balb/c mice (4–6 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME, USA).
Total RNA purification and cDNA cloning of ferret IFN-γ
Ferret whole blood was diluted 1:1 with RPMI 1640 cell culture media (Invitrogen, Carlsbad, USA) and stimulated with mitogens LPS (1 μg/ml, Sigma Chemicals, St. Louise, MO, USA), PMA (50 ng/ml, Sigma), ionomycin (0.1 mM, Sigma) and poly I:C (25 μg/ml, Sigma) and incubated at 37 °C in 5% CO2 for 2, 4, 8, and 12 h prior to RNA purification. Paxgene RNA isolation method (Qiagen, Mississauga, Canada) was used according to manufacturer's protocols. cDNA was synthesized from purified total RNA by reverse transcriptase II (Invitrogen) according to supplier's protocol. A 907 bp cDNA fragment was amplified by PCR using consensus sequence primers based on nucleotide sequence alignment of IFN-γ from multiple species, including dog, cat, and pig. The PCR fragment was isolated, cloned and sequenced to confirm that it encompassed the full-length coding sequence for ferret IFN-γ. An expression vector for IFN-γ was generated by PCR-based subcloning using the following primers: 5′-CGATGAATTATACAAGCTATATCTTA-3′ (forward) and 5′-TTTCGATGCTCTG CGGCCGGGAAA-3′ (reverse). The resulting 501 bp fragment was ligated into pCR 3.1-TOPO vector (Invitrogen). Nucleotide sequences of positive clones were confirmed by dideoxy sequencing using ABI 3730XL DNA analyzers (Center for Applied Genomics, Toronto, Ontario). Gene identification was carried out by Basic Local Alignment Search Tool (BLAST) analyses against National Centre for Biotechnology Information (NCBI) databases at National Institutes of Health, USA. Multiple sequence alignment was performed using Clustal X included in the Lasergene Software package (DNAStar Inc., Madison, WI).
Gene transfection, expression in COS-7 cells, and protein purification
PCR was used to generate a Kozak sequence at the 5′ end of the ferret cDNA, and to remove 3′ end termination codons before subcloning into pcDNA3.1/His6.V5/TOPO expression vector (Invitrogen) to produce a His6/V5 tagged form of the protein. Expression constructs were sequenced to verify sequence and orientation.
COS-7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen), supplemented with 10% fetal bovine serum (Invitrogen) at 37 °C, 5% CO2.
COS-7 cells were transiently transfected with plasmid encoding the recombinant ferret IFN-γ using Effectene (Qiagen) according to recommended protocols of the manufacturer. After 24–48 h, the cell culture supernatant from the IFN-γ-transfected cells was run through Ni-NTA metal immobilized affinity column (Novagen, EMS Chemicals, San Diego, CA, USA) to bind HIS-tagged recombinant ferret IFN-γ protein. The purified protein was washed and eluted according to manufacturer's suggested protocol. Fractions collected from the washing and elution steps were screened by immunoblotting using an antibody recognizing the V5 epitope (Invitrogen). Fractions containing the IFN-γ protein were pooled and dialyzed against phosphate buffered saline (PBS) at 4 °C and concentrated by spin column (Nanosep 10k OMEGA, Pall Life Science, East Hills, NY, USA). The protein concentration was determined by protein assay kit (Pierce, Rockford, IL, USA).
Western blot analysis
10–15% SDS-poylacrylamide gel electrophoresis (SDS-PAGE) was performed with pre-cast gels (Bio-Rad, USA), or 10% NuPAGE pre-cast gels (Invitrogen) according to standard protocols. Protein was transferred to nitrocellulose and the membranes were blocked with 5% fat-free milk, 0.01% Tween-20 in PBS (TPBS) for 1 h at room temperature. Membranes were incubated at 4 °C for 4–16 h with monoclonal anti-V5Ab (1:1000) (Invitrogen) or hybridoma culture supernatant (1:50). Hybridized membranes were washed with TPBS and incubated with goat–anti-mouse-HRP (1:5000) (Santa Cruz, CA, USA) for 1 h at room temperature. Protein blots were visualized using enhanced chemiluminescent (ECL) reagents according to supplier's protocol (GE Healthcare, Canada).
Isolation of peripheral blood mononuclear cells (PBMC)
PBMCs were isolated from ferret blood gradient centrifugation through Histopaque solution (Sigma) according to manufacturer's protocol. Briefly, whole blood was diluted 1:1 with PBS (10 ml), layered on to 5 ml Histopaque solution, and centrifuged 400g for 20 min. The enriched mononuclear cell layer at the Histopaque–plasma interface was harvested and used as the source of PBMCs.
Mouse B cell hybridoma preparation
Recombinant ferret IFN-γ (50 μg) and 2 mg of keyhole limpet hemocyaine (KLH) (Calbiochem, San Diego, CA, USA) were diluted in 0.5 ml PBS. Five microliters of glutaraldehyde was added and the mixture was allowed to incubate at room temperature for 1 h. Due to the appearance of aggregates, the whole mixture was washed on a spin column (Nanosep 10 k OMEGA, Pall Life Science) and concentrated to 0.1 ml volume. Following centrifugation, 0.5 ml PBS was added and the mixture was centrifuged again. After two rounds of PBS addition and centrifugation, the mixture was made up to 0.5 ml in PBS and used as the source for the priming antigen. Mice were immunized with 25 μl antigen suspension in emulsified Complete Freund's Adjuvant. Mice were injected an additional two times at bi-weekly intervals with 5 μg of recombinant ferret IFN-γ. Three days after the third injection, spleen cells were removed and isolated for fusion with Sp2/0-Ag14 using polyethylene glycol (Roche, Mannheim, Germany) and hypoxanthine aminopterin thymidine (HAT) resistant hybridomas were selected. Hybridoma cells were screened for the reactivity against IFN-γ by ELISA using Nunc MaxiSorp 96 well plates coated with ferret IFN-γ (100 μl, 0.1 μg/ml).
Ferret IFN-γ-specific ELISA
A 96-well ELISA plate (MaxiSorb, Nunc) was coated with 100 μl/well monoclonal anti-IFN-γ (2 μg/ml) overnight at 4 °C. The wells were blocked with 150 μl 1% BSA in PBS for 1 h at 37 °C. Supernatants from mitogen-stimulated PBMC cultures or serum from influenza A virus-infected ferrets were loaded into each well at dilutions described in the text and incubated for 1 h at 37 °C. Wells were washed with PBS/0.5% Tween-20 and then incubated for 1 h at room temperature with biotin-conjugated anti-IFN-γ antibody (1 μg/ml in 0.5% Tween-20/1%BSA). The wells were washed three times with PBS/0.5% Tween-20 before incubation with HRP-Avidin for 30 min. The substrate (o-phenylenediamine, Sigma) was applied for 15 min at room temperature. Colorimetric changes were quantitated using an automated ELISA reader (μQuant, BIO-TEK Instruments, Winooski, VT, USA).
Ferret IFN-γ-specific ELISPOT assay
PVDF plates (Millipore, MAIPS4510) or MaxiSorp plates (Nunc) were coated with a monoclonal anti-ferret IFN-γ antibody as the capture antibody, and blocked with 1% BSA-PBS. Ferret PBMCs were cultured in the presence of stimulating reagents as described in the text for 18 h. Wells were washed with water to remove cells and then captured IFN-γ was detected by a biotin-conjugated detection antibody coupled to HRP-avidin (Sigma). The ELISPOT was developed using DAB (Vector Laboratories, Burlingame, CA, USA).
Infection of ferrets with influenza A virus
Male ferrets (castrated, descented) weighing approximately 800–1000 g were infected intranasally with 106 EID50 influenza virus (H3N2 strain: A/Panama/2007/99) in 1 ml PBS. An additional three animals were mock-infected using PBS diluent alone. Animals were euthanized humanely 6 days post-infection, and serum was obtained using 10 ml SST vacutainer tubes according to manufacturer's recommended procedure.
Results
Cloning of ferret IFN-γ cDNA and the expression of His6-,V5-tagged recombinant IFN-γ in COS-7 transfectant cells
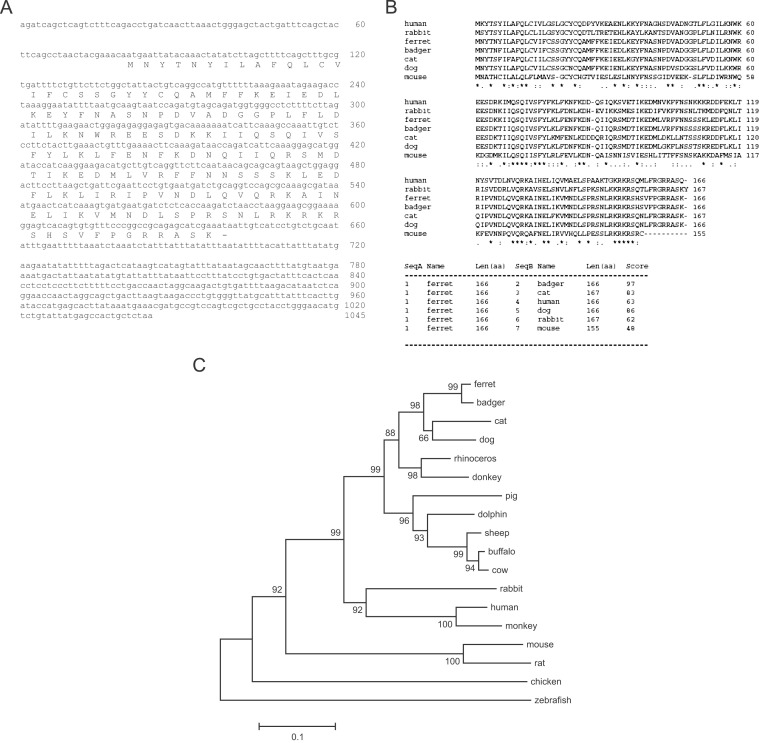
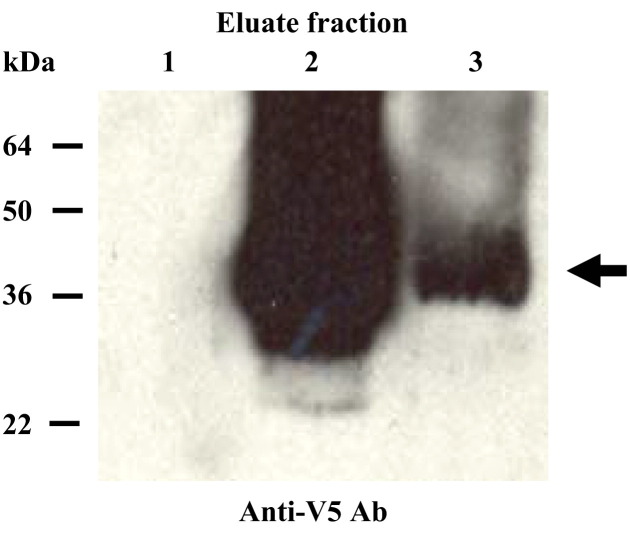
A cDNA encoding ferret IFN-γ was cloned from isolated total RNA derived from mitogen-stimulated ferret PBMCs as described in the materials and methods. The cDNA sequence and predicted amino acid sequence for ferret IFN-γ are depicted in Figure 1A . Amino acid sequence homology to the predicted ferret IFN-γ was highest in the Eurasian badger (Meles meles) (97%), followed by the canine (86%) and feline (83%) sequences (Figure 1B). The homology of ferret IFN-γ to human and mouse IFN-γ was 63% and 48%, respectively. Phylogenetic analysis using BLAST (see Figure 1C) indicates that closest orthologue of ferret IFN-γ is that of the badger. The ferret IFN-γ cDNA was subcloned into the His6-,V5-fusion protein expression vector and subsequently transfected into COS-7 cells to produce recombinant IFN-γ protein. Ferret IFN-γ protein was secreted from the COS-7 transfectants (fIFNγ-COS-7) and migrated as a 35–45 kDa band when subjected to immunoblotting experiments using a V5-specific antibody (Figure 2 ). Since the estimated molecular weight of ferret IFN-γ is ∼17 kDa as the monomer, the band detected in Coomassie blue staining and immunoblotting is consistent with the dimerized form of IFN-γ.
Figure 1.
Ferret IFN-γ cDNA. (A) Full-length ferret IFN-γ cDNA sequence including 80 base pairs in the 5′ untranslated region (UTR), 501 base pairs of coding sequence with predicted amino acid sequence, and 404 base pairs in the 3′UTR. (B) Alignment of the amino acid sequences of ferret, Eurasian badger, rabbit, cat, dog, mouse, and human IFN-γ precursor proteins (accession numbers Y11647, P30123, P46402, P42161, P01580, and P01579, respectively) is shown. Asterisks indicate positions displaying identical amino acid residues in all sequences in the alignment, and periods indicate positions displaying semiconserved substitutions. Scores of amino acid homology between ferret IFN-γ and IFN-γ from different species are shown in the lower panel. (C) Phylogenetic tree showing the relationship between ferret and other known vertebrate IFN-γ sequences. This tree was constructed using CLUSTAL W and MEGA 3.1 packages and bootstrapped 10,000 times. †Bootstrapping confidence values are between 66 and 100. The Gene peptide accession numbers for IFN-γ are: badger, CAA72346; dog, AAD314233; panda, ABE02189; cat, BAA06309; rhinoceros, ABC18310; donkey, AAC42595; pig, ABG56234; dolphin, BAA82042; sheep, ABD64367; buffalo, BAE75855; cow, NP_776511; armadillo, AAZ57195; woodchuck, AAC31963; rabbit, BAA24439; human, P01579; monkey, AAM21477; mouse, P01580; rat, NP_620235; chicken, CAA69227; zebrafish, BAD06253.
Figure 2.
Secretion of ferret IFN-γ by fIFNγ-COS-7. Detection of recombinant ferret IFN-γ by V5-tag-specific immunoblotting. Secreted recombinant ferret IFN-γ was enriched using a His-affinity column. The first three eluate fractions, shown in lanes 1, 2, and 3, respectively, were analyzed by V5-specific immunoblotting. The arrow indicates the dominant protein band observed in fractions 2 and 3.
Generation of monoclonal Abs specific for ferret IFN-γ
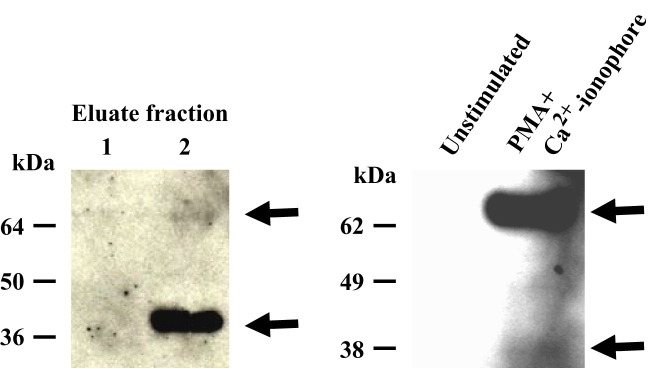
In the early attempts to monitor IFN-γ in ferret cell cultures, we tried commercially available cytometric bead arrays, which employ human or mouse IFN-γ-specific antibodies. Neither human nor mouse-specific arrays resulted in the positive detection as antibodies did not cross react with ferret IFN-γ. We are therefore prompted to generate monoclonal antibodies specific to ferret IFN-γ. To produce ferret IFN-γ specific antibodies, recombinant IFN-γ was conjugated to a carrier protein, KLH (keyhole limpet hemocyanin) using glutaraldehyde. The resulting KLH-IFN-γ complex was injected i.p. into Balb/c mice. Following fusion of splenocytes isolated from IFN-γ-immunized mice with hybridoma parent cells, IFN-γ-reactive B cell hybridomas were established. Hybridoma clones, selected by ELISA reactivity, were tested for specificity by immunoblotting against recombinant ferret IFN-γ and supernatants from mitogen-stimulated ferret PBMCs (Figure 3 ). Clone 3E7 recognized a 36 kDa band in samples of recombinant ferret IFN-γ, demonstrating that the ferret IFN-γ reactive monoclonal antibody can also detect the dimerized form of IFN-γ (Figure 3). Additionally, a weak band at ∼65 kDa was consistently observed indicating a possible detection of another oligomeric form of ferret IFN-γ (Figure 3). Interestingly, the study of culture supernatants derived from mitogen-stimulated PBMCs showed prominent bands at 60–70 kDa and the band at 36 kDa was only weakly detectable (Figure 3). The data indicates that the native ferret IFN-γ exists as an oligomeric form (likely a dimer of the dimer), while the recombinant IFN-γ exists predominantly as a single dimer.
Figure 3.
Detection of ferret IFN-γ by a monoclonal anti-ferret IFN-γAb. Western blot analysis of recombinant ferret IFN-γ using monoclonal Abs. (LEFT PANEL) Lysates from fIFNγ-COS-7 samples were analyzed by Western blot using a monoclonal antibody established from mouse immunized by recombinant ferret IFN-γ and shown in the left panel. (RIGHT PANEL) Supernatants derived from ferret PBMC cultures stimulated with PMA plus ionomycin, were analyzed by Western blot using anti-ferret IFN-γ monoclonal antibody. Arrows at the right of each panel indicate the dimers for lower molecular weight protein bands and putative tetramer as higher molecular weight species.
Utilization of monoclonal antibodies for the detection of ferret IFN-γ by ELISA and ELISPOT immunoassays
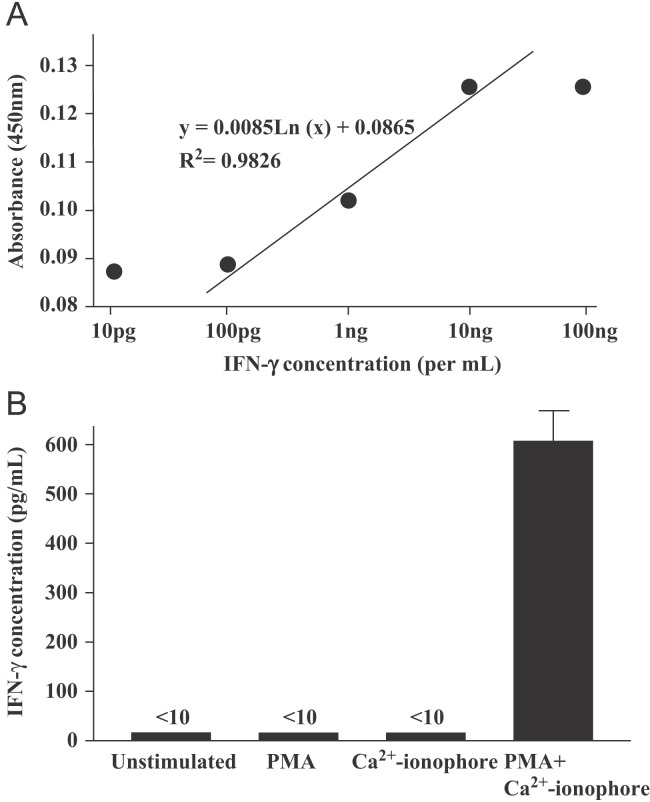
An important application for monoclonal anti-ferret IFN-γ antibodies is the detection and quantitation of IFN-γ protein in biological samples. To this end, we tested our monoclonal antibodies for utility in a ferret IFN-γ-specific ELISA assay. To select the antibody pair for IFN-γ recognition in ELISA assays, we tested monoclonal antibodies derived from different clones. The clone 3E7 was conjugated to biotin and used as the detection antibody against ferret IFN-γ and antibodies from five other clones were used to coat the assay wells. Of the five monoclonal anti-ferret IFN-γ antibodies that we screened in this manner, the clone 1E3 consistently showed the presence of Ferret IFN-γ in mitogen-stimulated ferret spleen cell supernatants. Therefore, we decided to continue using 1E3 as the ELISA capture antibody paired with 3E7 as the detection antibody. As shown in Figure 4A , an ELISA was carried out using the paired capture and biotin-conjugated detection antibodies, and demonstrated an increase in optical density that correlated directly with the concentration of purified recombinant IFN-γ. The increase in optical density exhibited a smooth logarithmic correlation between 10 pg/ml and 10 ng/ml of recombinant IFN-γ protein (R 2=0.9826). Using this standard curve, we measured the concentration of IFN-γ in in vitro-stimulated PBMC supernatants. IFN-γ was present only in samples that were treated with PMA in conjunction with a Ca2+ ionophore (Figure 4B). The results indicate that the monoclonal antibodies selected are applicable for ferret IFN-γ-specific ELISA.
Figure 4.
Measurement of IFN-γ in the mitogen-stimulated ferret PBMC culture supernatants by ELISA. (A) Standard curve for ferret IFN-γ ELISA. ELISA plate well was coated with a monoclonal anti-ferret antibody generated in our laboratory. Recombinant ferret IFN-γ was sequentially diluted and loaded to the antibody-coated wells. Captured ferret IFN-γ was detected by a second monoclonal anti-ferret IFN-γ antibody generated in our laboratory conjugated to biotin, using the avidin-HRP detection method. Logarithmic dilution was used to derive a standard curve for downstream applications of the ELISA. (B) IFN-γ in mitogen stimulated ferret PBMC supernatants. ELISA utilizing the monoclonal ferret IFN-γ antibody as a capture antibody was performed on ferret PBMC cells treated with PMA, ionomycin or both. Results represent the mean values of triplicate samples.
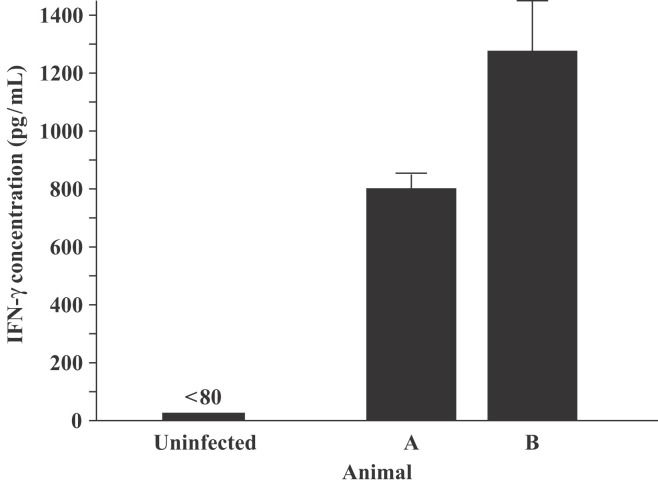
Our primary scope in developing the ferret IFN-γ-specific monoclonal antibodies was to develop a reagent with which to measure IFN-γ immune responses in tissues or cells derived from influenza A virus-infected ferrets. To this end, an ELISA using the capture-detection monoclonal antibody pair was used to assess the level of IFN-γ in sera obtained from influenza A-infected ferrets. The assay showed substantial levels of circulating IFN-γ on day 6 post-infection (Figure 5 ). The level of IFN-γ in serum from the non-infected control ferret was below the detection limit. These results show that the ELISA assay using our anti-ferret IFN-γ monoclonal antibodies will be invaluable in monitoring systemic IFN-γ responses during a host response against virus infection.
Figure 5.
Detection of increased levels of IFN-γ in ferret serum after infection with H3N2 influenza A virus. Serum from two ferrets (animals A and B) was taken 6 days post-infection. Sera from infected ferrets and one uninfected control were loaded on to ELISA plates coated with the ferret IFN-γ specific capture antibody-coated and the samples were analyzed as indicated in Figure 4. Results represent the means of triplicate samples.
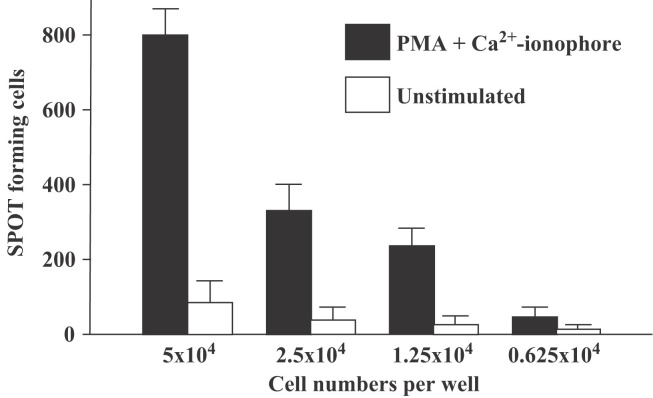
ELISPOT IFN-γ assays are also a key tool in the determination and quantitation of IFN-γ-secreting cells. To develop a ferret-specific IFN-γ ELISPOT assay we employed the same set of monoclonal ferret IFN-γ antibodies, clone 3E7 and 1E3. ELISPOT was performed on ferret PBMCs that had been stimulated with PMA plus ionomycin. As demonstrated in Figure 6 , increasing numbers of IFN-γ secreting cells were detected in direct proportion to the number of stimulated cells plated, while the number of IFN-γ positive cells did not increase above background when increasing numbers of unstimulated cells were plated. The results indicate that 3E7 and 1E3 anti-ferret IFN-γ monoclonal antibodies will be invaluable for performing ELISPOT analysis of IFN-γ levels in ferret tissue.
Figure 6.
ELISPOT assay for the IFN-γ producing cells in mitogen-stimulated ferret PBMCs. ELISPOT assay was performed in the same manner outlined for Figure 4 for capture and detection. Ferret PBMCs were plated in serial dilution and stimulated with PMA plus ionomycin for 18 h and IFN-γ secreting cells were detected by biotinylated capture antibody. The y-axis depicts the number of IFN-γ spot forming cells per well; total cells per well are indicated on the x-axis. Data shown are the average of triplicate samples.
Discussion
Experimental models that would allow the testing of effective treatments or vaccines for infection by novel influenza A viruses are currently in high demand. Ferrets have been used as an animal model of infection with influenza A viruses to test the severity of the disease and also to evaluate efficacy of potential vaccines [27]. IFN-γ synthesis in vivo is a central regulator in host immune responses against viral infection. In this study we describe the cloning of a full-length cDNA for IFN-γ and expression of the recombinant IFN-γ protein. Furthermore, we describe the generation of two monoclonal antibodies specific for ferret IFN-γ and the subsequent development of immunoassays for the detection of native IFN-γ. We anticipate that the IFN-γ immunoassays established in this study will be useful in gaining insight into ferret antiviral responses and in other immune processes in general. Previous studies on IFN-γ have shown that there is a strict species-specific activity of IFN-γ. IFN-γ genes isolated from diverse species such as guinea pig, turkey, rhino, and catfish have been previously described in the literature [30], [31], [32], [33], [34]. Since ferrets are susceptible to human viral diseases, including influenza A virus [35] and SARS-coronavirus [29], we expect that the availability of the ferret IFN-γ assay system described in this study will greatly expand the utility of the ferret infection model.
Unexpectedly, the monomeric form of IFN-γ (17 kDa) was detectable at only low levels in recombinant ferret IFN-γ samples. Instead, a dominant band at ∼36 kDa was consistently observed during Western blotting whereas a weak band at 65–70 kDa was also observed in the same sample. Conversely, when native IFN-γ in mitogen-stimulated PBMC culture supernatants were investigated with monoclonal antibodies, dominant bands were identified at 65–70 kDa and the ∼36 kDa band was barely detectable. Oligomeric forms of IFN-γ were very stable aggregates, as treatment with reducing reagents, guanidine or urea did not disrupt the IFN-γ into its monomeric form (unpublished data). Since the IFN-γ dimer was also observed in supernatants from mitogen-stimulated ferret PBMC cultures, it is unlikely that the dimerization is caused by the epitope tags present on the recombinant protein. The recombinant IFN-γ dimer was found to have biological function consistent with known IFN-γ activity [36]. Treatment of ferret PBMC cultures with the dimerized recombinant IFN-γ increased the surface presentation of class II MHC (unpublished data), suggesting that the recombinant ferret IFN-γ is able to maintain biological function in the dimerized form. We observed, however, an IFN-γ-specific band in Western blotting of mitogen-stimulated PBMC culture supernatants at a molecular mass at 65 kDa. This suggests that the probable form of ferret IFN-γ in vivo exists in tetrameric form rather than a dimeric form. Our observation of recombinant ferret IFN-γ seems to contradict the recent report on guinea pig IFN-γ in which recombinant IFN-γ was shown to exist primarily as a monomer [30]. Recombinant guinea pig IFN-γ was expressed in bacteria, and the purified protein was capable of stimulating the class II MHC expression in guinea pig cells. It has been reported previously that human IFN-γ shows a dominant band at approximately 65 kDa in immunoblotting and human IFN-γ specific receptors are shown to be reactive with IFN-γ dimers [37]. Other groups have demonstrated that carbohydrate modification alters the molecular nature of human IFN-γ [38]. Taken together, these observations suggest that it is not unreasonable to find that ferret IFN-γ may exist in monomeric, dimeric and oligomeric forms. Subtle variation in amino acid sequences between two species may result in differences in post-translational modification by glycosylation, which could result in differential association of monomers into oligomers. Alternatively, the origin of cells secreting IFN-γ, such as CD8+ T cells or NK cells, may determine the differences observed in IFN-γ oligomerization. The interaction between ferret IFN-γ and its receptor under physiological conditions may be mediated by IFN-γ dimerization or oligomerization. Whether or not dimerization is the mechanism of IFN-γ activation in other species obviously requires further investigation.
Cloning and expression of biologically active ferret IFN-γ is critical in the development of immunoassays such as ELISPOT and ELISAs for detection of interferon production and interferon producing cells. These reagents will be invaluable in the assessment of vaccine efficacy against influenza A and other emerging infectious viruses.
Acknowledgments
We are grateful to Wei Sun and Feseha Abebe-Akele for technical assistance. This project was supported by the funding from NIAID through NIH/NIAID Contract no. N01-AI-30063 Task Order no. 03, and the Canadian Institutes of Health Research.
References
- 1.Mills C.E., Robins J.M., Bergstrom C.T., Lipsitch M. Pandemic influenza: risk of multiple introductions and the need to prepare for them. PLoS Med. 2006;3:e135. doi: 10.1371/journal.pmed.0030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morens D.M., Fauci A.S. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018–1028. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 3.Poland GA, Jacobson RM, Targonski PV. Avian and pandemic influenza: an overview. Vaccine 2007. [DOI] [PubMed]
- 4.Thomas J.K., Noppenberger J. Avian influenza: a review. Am J Health Syst Pharm. 2007;64:149–165. doi: 10.2146/ajhp060181. [DOI] [PubMed] [Google Scholar]
- 5.Muller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 6.Bouley D.M., Kanangat S., Wire W., Rouse B.T. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]
- 7.Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R. Aguet M Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 8.Whitmire J.K., Tan J.T., Whitton J.L. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steeg P.S., Moore R.N., Johnson H.M., Oppenheim J.J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982;156:1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadee A.A., Kuschke R.H., Dooms T.G. The inhibitory effects of Mycobacterium tuberculosis on MHC class II expression by monocytes activated with riminophenazines and phagocyte stimulants. Clin Exp Immunol. 1995;100:434–439. doi: 10.1111/j.1365-2249.1995.tb03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maudsley D.J., Morris A.G. Regulation of IFN-gamma-induced host cell MHC antigen expression by Kirsten MSV and MLV. II. Effects on class II antigen expression. Immunology. 1989;67:26–31. [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas H.E., Parker J.L., Schreiber R.D., Kay T.W. IFN-gamma action on pancreatic beta cells causes class I MHC upregulation but not diabetes. J Clin Invest. 1998;102:1249–1257. doi: 10.1172/JCI2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maraskovsky E., Chen W.F., Shortman K. IL-2 and IFN-gamma are two necessary lymphokines in the development of cytolytic T cells. J Immunol. 1989;143:1210–1214. [PubMed] [Google Scholar]
- 14.Refaeli Y., Van Parijs L., Alexander S.I., Abbas A.K. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith P.M., Wolcott R.M., Chervenak R., Jennings S.R. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma) Virology. 1994;202:76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- 16.Bradley L.M., Dalton D.K., Croft M. A direct role for IFN-gamma in regulation of Th1 cell development. J Immunol. 1996;157:1350–1358. [PubMed] [Google Scholar]
- 17.Constant S.L., Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 18.Halford W.P., Halford K.J., Pierce A.T. Mathematical analysis demonstrates that interferons-beta and -gamma interact in a multiplicative manner to disrupt herpes simplex virus replication. J Theor Biol. 2005;234:439–454. doi: 10.1016/j.jtbi.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Karupiah G., Fredrickson T.N., Holmes K.L., Khairallah L.H., Buller R.M. Importance of interferons in recovery from mousepox. J Virol. 1993;67:4214–4226. doi: 10.1128/jvi.67.7.4214-4226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katze M.G., He Y., Gale M., Jr Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 21.Steed A.L., Barton E.S., Tibbetts S.A., Popkin D.L., Lutzke M.L., Rochford R. Gamma interferon blocks gammaherpesvirus reactivation from latency. J Virol. 2006;80:192–200. doi: 10.1128/JVI.80.1.192-200.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asanuma H., Sharp M., Maecker H.T., Maino V.C., Arvin A.M. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J Infect Dis. 2000;181:859–866. doi: 10.1086/315347. [DOI] [PubMed] [Google Scholar]
- 23.Lee S., Gierynska M., Eo S.K., Kuklin N., Rouse B.T. Influence of DNA encoding cytokines on systemic and mucosal immunity following genetic vaccination against herpes simplex virus. Microbes Infect. 2003;5:571–578. doi: 10.1016/s1286-4579(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 24.Alarcon J.B., Hartley A.W., Harvey N.G., Mikszta J.A. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol. 2007;14:375–381. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben Yedidia T., Arnon R. Towards an epitope-based human vaccine for influenza. Hum Vaccin. 2005;1:95–101. doi: 10.4161/hv.1.3.1851. [DOI] [PubMed] [Google Scholar]
- 26.Giebink G.S. Otitis media: the chinchilla model. Microb Drug Resist. 1999;5:57–72. doi: 10.1089/mdr.1999.5.57. [DOI] [PubMed] [Google Scholar]
- 27.Suguitan A.L., Jr., McAuliffe J., Mills K.L., Jin H., Duke G., Lu B. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3:e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid A.H., Taubenberger J.K., Fanning T.G. The 1918 Spanish influenza: integrating history and biology. Microbes Infect. 2001;3:81–87. doi: 10.1016/s1286-4579(00)01351-4. [DOI] [PubMed] [Google Scholar]
- 29.Roberts A., Subbarao K. Animal models for SARS. Adv Exp Med Biol. 2006;581:463–471. doi: 10.1007/978-0-387-33012-9_83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeevan A., McFarland C.T., Yoshimura T., Skwor T., Cho H., Lasco T. Production and characterization of guinea pig recombinant gamma interferon and its effect on macrophage activation. Infect Immun. 2006;74:213–224. doi: 10.1128/IAI.74.1.213-224.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loa C.C., Hsieh M.K., Wu C.C., Lin T.L. Molecular identification and characterization of turkey IFN-gamma gene. Comp Biochem Physiol B Biochem Mol Biol. 2001;130:579–584. doi: 10.1016/s1096-4959(01)00469-9. [DOI] [PubMed] [Google Scholar]
- 32.Milev-Milovanovic I., Long S., Wilson M., Bengten E., Miller N.W. Chinchar VG Identification and expression analysis of interferon gamma genes in channel catfish. Immunogenetics. 2006;58:70–80. doi: 10.1007/s00251-006-0081-x. [DOI] [PubMed] [Google Scholar]
- 33.Morar D., Tijhaar E., Negrea A., Hendriks J., van Haarlem D., Godfroid J. Cloning, sequencing and expression of white rhinoceros (Ceratotherium simum) interferon-gamma (IFN-gamma) and the production of rhinoceros IFN-gamma specific antibodies. Vet Immunol Immunopathol. 2007;115:146–154. doi: 10.1016/j.vetimm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Svitek N., von M.V. Early cytokine mRNA expression profiles predict Morbillivirus disease outcome in ferrets. Virology. 2007;362:404–410. doi: 10.1016/j.virol.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maher J.A., DeStefano J. The ferret: an animal model to study influenza virus. Lab Anim (NY) 2004;33:50–53. doi: 10.1038/laban1004-50. [DOI] [PubMed] [Google Scholar]
- 36.Farrar M.A., Schreiber R.D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 37.Kudo S., Kawano K. Association and dissociation properties of natural human interferon gamma. J Chromatogr B Biomed Sci Appl. 1999;723:25–30. doi: 10.1016/s0378-4347(98)00540-4. [DOI] [PubMed] [Google Scholar]
- 38.Fukuta K., Abe R., Yokomatsu T., Kono N., Asanagi M., Omae F. Remodeling of sugar chain structures of human interferon-gamma. Glycobiology. 2000;10:421–430. doi: 10.1093/glycob/10.4.421. [DOI] [PubMed] [Google Scholar]