Abstract
The pathogenicity of feline infectious peritonitis virus (FIPV) is known to depend on macrophage tropism, and this macrophage infection is enhanced by mediation via anti-S antibody (antibody-dependent enhancement, ADE). In this study, we found that TNF-alpha production was increased with viral replication in macrophages inoculated with a mixture of FIPV and anti-S antibody, and demonstrated that this culture supernatant had feline PBMC apoptosis-inducing activity. We also demonstrated that the expression level of the FIPV virus receptor, feline aminopeptidase N (fAPN), was increased in macrophages of FIP cats. For upregulation of TNF-alpha and fAPN in macrophages, viral replication in macrophages is necessary, and their expressions were increased by ADE of FIPV infection. It was demonstrated that a heat-resistant fAPN-inducing factor was present in the culture supernatant of FIPV-infected macrophages, and this factor was TNF-alpha: fAPN expression was upregulated in recombinant feline TNF-alpha-treated macrophages, and FIPV infectivity was increased in these macrophages. These findings suggested that FIPV replication in macrophages increases TNF-alpha production in macrophages, and the produced TNF-alpha acts and upregulates fAPN expression, increasing FIPV sensitivity.
Keywords: FIP, ADE, TNF-alpha, fAPN
Introduction
Feline coronavirus (FCoV) is a coronavirus belonging to Group 1 of the family Coronaviridae. FCoV is mainly composed of nucleocapsid (N) protein, transmembrane (M) protein, and peplomer spike (S) protein (Olsen, 1993). FCoV is classified into types I and II according to the amino acid sequence of its spike protein (Hohdatsu et al., 1991a, Motokawa et al., 1995, Motokawa et al., 1996). Each of these types consists of two viruses: FIP-causing FIP virus (FIPV) and non-FIP-causing feline enteric coronavirus (FECV). FIPV and FECV of the same type cannot be distinguished by their antigenicity or at the gene level, and differ only in their pathogenicity for cats. Thus, there are types I and II FECV and FIPV in FCoV. FECV is asymptomatic in cats, but FIPV infection induces feline infectious peritonitis (FIP). FIP is a fatal disease mainly developing immune complex vasculitis accompanied by necrosis and pyogenic granulomatous inflammation. The difference in pathogenicity between FECV and FIPV is considered to be associated with macrophage tropism of each virus (Rottier et al., 2005).
When anti-FCoV antibody-positive cats are inoculated with FIPV, the onset time of FIP is earlier than that in antibody-negative cats, and symptoms are severer (Pedersen and Boyle, 1980). These phenomena are known as antibody-dependent enhancement (ADE) of infection. There have been several reports concerning ADE of other virus infections (dengue virus, HIV and respiratory syncytial virus, etc.; Suhrbier and La Linn, 2003, Sullivan, 2001, Tirado and Yoon, 2003), and ADE of SARS-CoV infection, belonging to coronavirus as FIPV, has recently been reported (Weingartl et al., 2004, Yang et al., 2005). ADE of FIPV infection has been reported to be induced by antibodies against FIPV spike (S) protein (Hohdatsu et al., 1991b, Corapi et al., 1992, Olsen et al., 1992). FIPV infects macrophages via the virus-bound anti-FIPV-S antibody Fc region and Fc receptor on the macrophage surface, inducing ADE. ADE-induced aggravation of the pathology in FIP cats is considered to be due to an increased viral infection rate in macrophages.
Various studies concerning the FIP pathology have been performed, including studies involving cytokine measurement in FIP cats. Increases in the IL-1, IL-6, IL-10, and TNF-alpha levels in FIP cats have been confirmed (Goitsuka et al., 1990, Goitsuka et al., 1991, Dean et al., 2003, Takano et al., 2007). Of these cytokines, TNF-alpha is a feline lymphocyte apoptosis-inducing factor, and its involvement in lymphopenia in FIP cats has been suggested (Dean et al., 2003, Takano et al., 2007). Increased TNF-alpha expression in monocytes and macrophages in FIP cats has been noted (Dean et al., 2003, Kipar et al., 2005, Takano et al., 2007). Kiss et al. (2004) reported that the expression level of TNF-alpha mRNA in FIP cat-derived PBMC was increased, whereas that of IFN-gamma mRNA was reduced. However, many points remain unclear, such as whether this increase in TNF-alpha is directly related to FIPV replication in macrophages, and whether induction of ADE of FIPV infection in macrophages affects TNF-alpha production.
Coronavirus belonging to Group 1 of the family Coronaviridae utilizes aminopeptidase N (APN) or angiotensin-converting enzyme 2 (ACE2) as its virus receptor (Delmas et al., 1992, Hohmann et al., 2005, Yeager et al., 1992, Tresnan et al., 1996). APN is widely distributed in respiratory and digestive epithelial cells and blood cells. In FCoV, type II FECV and FIPV are known to utilize feline APN (fAPN) as their virus receptors (Hohdatsu et al., 1998).
In this study, we discovered that the expression levels of TNF-alpha mRNA as well as fAPN mRNA were increased in alveolar macrophages collected form FIP cats compared to those from specific pathogen-free (SPF) cats. Thus, we investigated how these increases in TNF-alpha and fAPN expressions in macrophages were related to FIPV infection in vitro using macrophages derived from SPF cats. We also investigated whether these increases in TNF-alpha and fAPN expressions were affected by ADE on FIPV infection in macrophages.
Results
FIPV infection and TNF-alpha production in feline alveolar macrophages
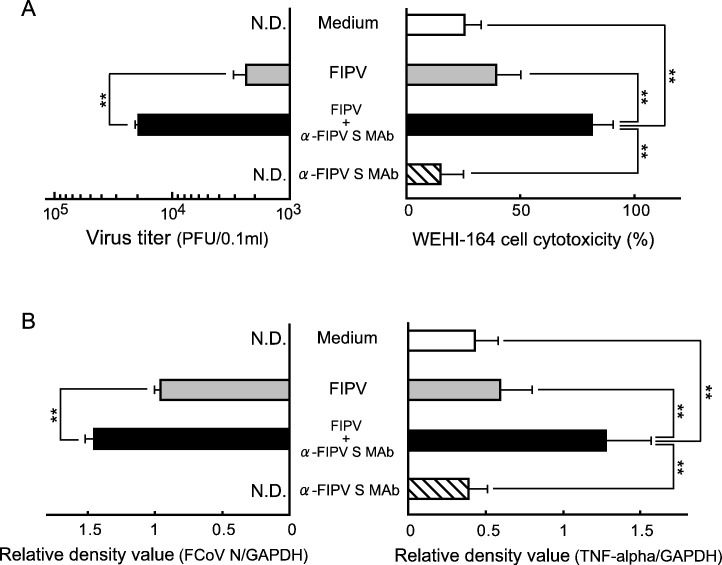
To investigate the relationship between FIPV infection and TNF-alpha production in feline alveolar macrophages, SPF cat-derived alveolar macrophages were inoculated with FIPV alone and a mixture of FIPV and monoclonal antibody (MAb) 6-4-2. After 3 days, the culture supernatant and cells were collected, and FIPV replication and TNF-alpha production were measured. The virus titer in the macrophage culture supernatant was measured by plaque assay. TNF-alpha was measured using cytotoxicity against WEHI-164 cells as an index. The virus titer and TNF-alpha production were significantly higher in the culture supernatant of macrophages inoculated with a mixture of FIPV and MAb 6-4-2, than in that of macrophages cultured with medium alone, virus alone, and MAb 6-4-2 alone (Fig. 1A). The intracellular FCoV gene and TNF-alpha mRNA expression levels were significantly increased in macrophages inoculated with a mixture of FIPV and MAb 6-4-2 (Fig. 1B), showing that TNF-alpha production was increased with an increase in virus production in macrophages in which ADE was induced.
Fig. 1.
Relationship between FIP infection and TNF-alpha production in feline macrophages. SPF cat-derived macrophages (2 × 106 cells) were cultured with medium alone, FIPV, FIPV + α-FIPV S MAb, and α-FIPV MAb alone for 3 days, and the cells and culture supernatant were collected. The virus titer in the culture supernatant was measured by plaque assay (A, left), and TNF-alpha production was measured using cytotoxicity against WEHI-164 cells as an index (A, right). In addition, the FCoV N gene (B, left) and TNF-alpha mRNA (B, right) in the cells were detected by RT–PCR. The FCoV N gene and TNF-alpha mRNA were quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH. n = 5, **p < 0.01. N.D.: not detected.
Relationship between TNF-alpha production and virus replication in FIPV-infected macrophages
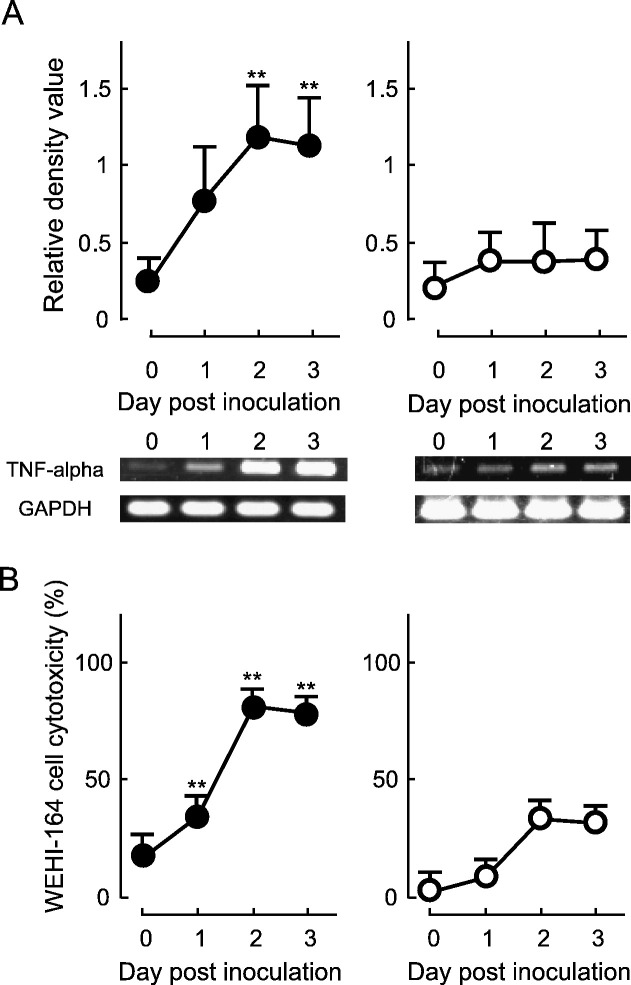
To investigate whether TNF-alpha in FIPV-infected macrophages is related to viral replication, the following experiment was performed. Feline alveolar macrophages were inoculated with a mixture of FIPV inactivated at 56 °C for 30 min and MAb 6-4-2, and TNF-alpha production was investigated with time. When macrophages were inoculated with a mixture of non-heat-inactivated (live) FIPV and MAb 6-4-2, the intracellular TNF-alpha mRNA expression level (Fig. 2A) and the culture supernatant TNF-alpha level (Fig. 2B) increased with time. However, in macrophages inoculated with a mixture of heat-inactivated FIPV and MAb 6-4-2, both levels were significantly lower than those in cells inoculated with a mixture of live FIPV and MAb on days 2 and 3 of culture (Figs. 2A and B).
Fig. 2.
TNF-alpha production in FIPV-infected macrophages depends on viral replication. SPF cat-derived macrophages (2 × 106 cells) were inoculated with a mixture of heat-inactivated FIPV and α-FIPV S MAb (white circle) or a mixture of live FIPV and α-FIPV S MAb (black circle), and the cells and culture supernatant were collected daily for 3 days. (A) TNF-alpha mRNA in the cells was detected by RT–PCR. TNF-alpha mRNA was quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH. (B) TNF-alpha production in the culture supernatant was measured using cytotoxicity against WEHI-164 cells as an index. n = 5, **p < 0.01.
Feline peripheral blood mononuclear cells (PBMC) apoptosis-inducing activity of FIPV-infected macrophage culture supernatant
Whether the culture supernatant of FIPV-infected macrophages induces apoptosis of SPF cat-derived PBMC was investigated. SPF cat-derived macrophages (2 × 106 cells) were culture with medium alone, FIPV alone, a mixture of FIPV and MAb 6-4-2, and MAb 6-4-2 alone, and the culture supernatants were collected after 3 days. The PBMC of SPF cats (2 × 106 cells) were cultured at 37 °C for 4 h in the presence of each culture supernatant, and apoptotic cells were detected by TUNEL. As shown in Fig. 3 , the feline PBMC apoptosis induction rate was significantly higher in the presence of the culture supernatant of macrophages inoculated with a mixture of FIPV and MAb 6-4-2 than in the other 3 groups.
Fig. 3.
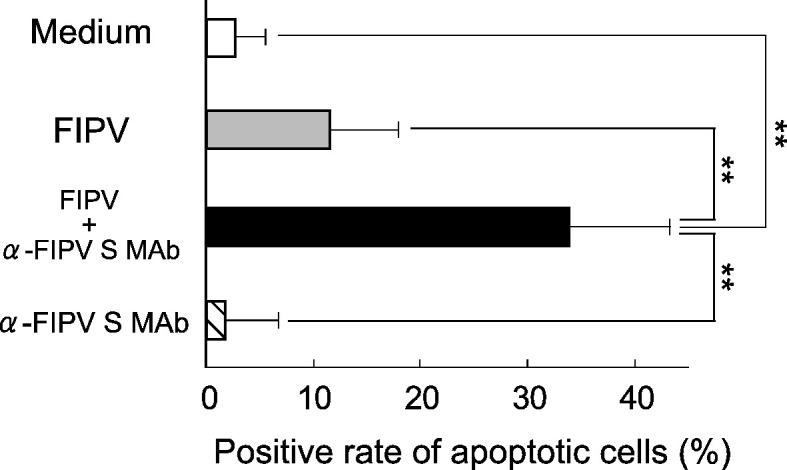
PBMC apoptosis-inducing activity of FIV-infected macrophage culture supernatant. SPF cat-derived macrophages (2 × 106 cells) were cultured with medium alone, FIPV, FIPV + α-FIPV S MAb, and α-FIPV MAb alone, and the culture supernatants were collected after 3 days. The PBMC of SPF cats (2 × 106) were cultured at 37 °C for 4 h in the presence of each culture supernatant, and apoptotic cells were detected by TUNEL. n = 6, **p < 0.01.
FCoV N gene and TNF-alpha and fAPN mRNA expression levels in macrophages of FIP and SPF cats
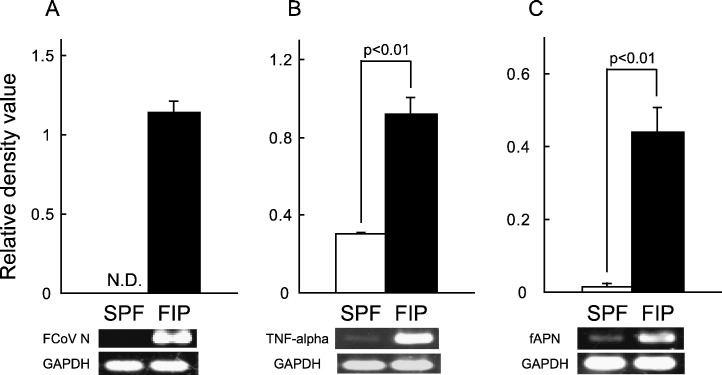
The FCoV N gene and TNF-alpha mRNA expression levels were compared between alveolar macrophages derived from FIP and SPF cats. The FCoV N gene and TNF-alpha mRNA expression levels were increased in alveolar macrophages derived from FIP cats (Figs. 4A and B). Interestingly, the mRNA expression level of FCoV type II virus receptor, fAPN, was also increased in FIP cat-derived alveolar macrophages (Fig. 4C).
Fig. 4.
FCoV N gene, TNF-alpha, and fAPN mRNA expression levels in macrophages of FIP and SPF cats. Alveolar macrophages (2 × 106 cells) were collected from FIP and SPF cats, and the FCoV N gene (A), TNF-alpha mRNA (B), and fAPN mRNA (C) were detected by RT–PCR. The FCoV N gene, TNF-alpha mRNA, and fAPN mRNA were quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH. n = 7, N.D.: not detected.
Relationship between fAPN mRNA expression level and FIPV infection in cultured feline macrophages
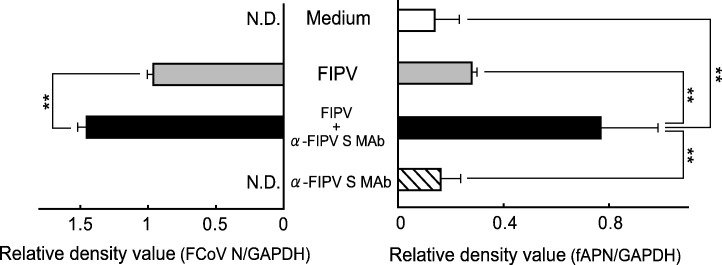
In FIP cat-derived alveolar macrophages, the TNF-alpha and fAPN mRNA expression levels increased with an increase in the FCoV N gene expression level. Thus, changes in the FCoV N gene and fAPN mRNA expression levels in FIPV-infected alveolar macrophages were compared. When SPF cat-derived alveolar macrophages were inoculated with FIPV and a mixture of FIPV and MAb 6-4-2, as in Fig. 1, Fig. 3, the intracellular FCoV N gene expression level increased, with which the fAPN mRNA level also increased (Fig. 5 ).
Fig. 5.
Relationship between fAPN mRNA expression and FIPV infection in cultured feline macrophages. SPF cat-derived macrophages (2 × 106 cells) were cultured with medium alone, FIPV, FIPV + α-FIPV S MAb, and α-FIPV MAb alone. The cells were collected after 3 days, and the FCoV N gene and fAPN mRNA were detected by RT–PCR. FCoV N gene and fAPN mRNA were quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH. n = 5, **p < 0.01. N.D.: not detected.
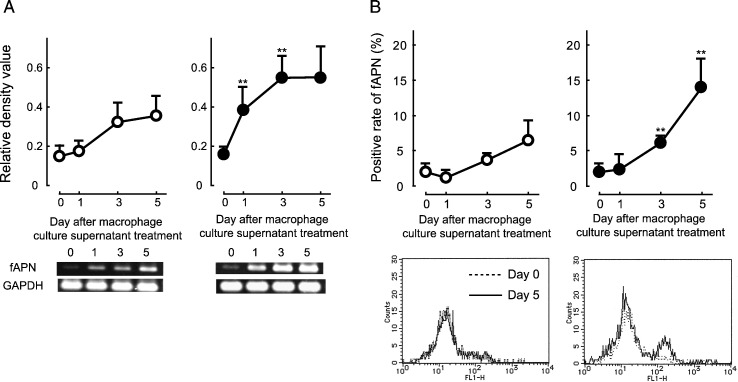
The presence of fAPN-inducing factor in FIPV-infected macrophage culture supernatant
The presence of a fAPN-inducing factor in the FIPV-infected macrophage culture supernatant was investigated. The culture supernatant of macrophages inoculated with a mixture of FIPV and MAb 6-4-2 for 3 days was heated at 56 °C for 30 min to inactivate viruses. The supernatant was then added to medium at 10%, and used for culturing fresh alveolar macrophages. The intracellular fAPN mRNA and cell surface fAPN expression levels were measured over time. As the control, alveolar macrophages were cultured with heat-inactivated culture supernatant of uninfected macrophages. Both the intracellular fAPN mRNA and cell surface fAPN expression levels were significantly increased in cells inoculated with the mixture of FIPV and MAb 6-4-2 compared to those in cells cultured with the control supernatant (Figs. 6A and B).
Fig. 6.
fAPN expression in feline macrophages treated with FIPV-infected macrophage culture supernatant. SPF cat-derived macrophages (2 × 106 cells) were inoculated with a mixture of FIPV and α-FIPV S MAb. The culture supernatant was collected after 3 days, and heated at 56 °C for 30 min to inactivate viruses. SPF cat-derived macrophages (2 × 106 cells) were cultured with medium containing 10% inactivated culture supernatant. The cells were collected on days 0, 1, 3, and 5 of culture, and the intracellular fAPN mRNA (A) and cell surface fAPN (B) expression levels were measured. fAPN mRNA was quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH. White circle: Treatment with control macrophage culture supernatant, black circle: treatment with culture supernatant of macrophages inoculated with a mixture of FIPV and α-FIPV S MAb. n = 5, **p < 0.01 vs. treatment with culture supernatant of intact (control) macrophages.
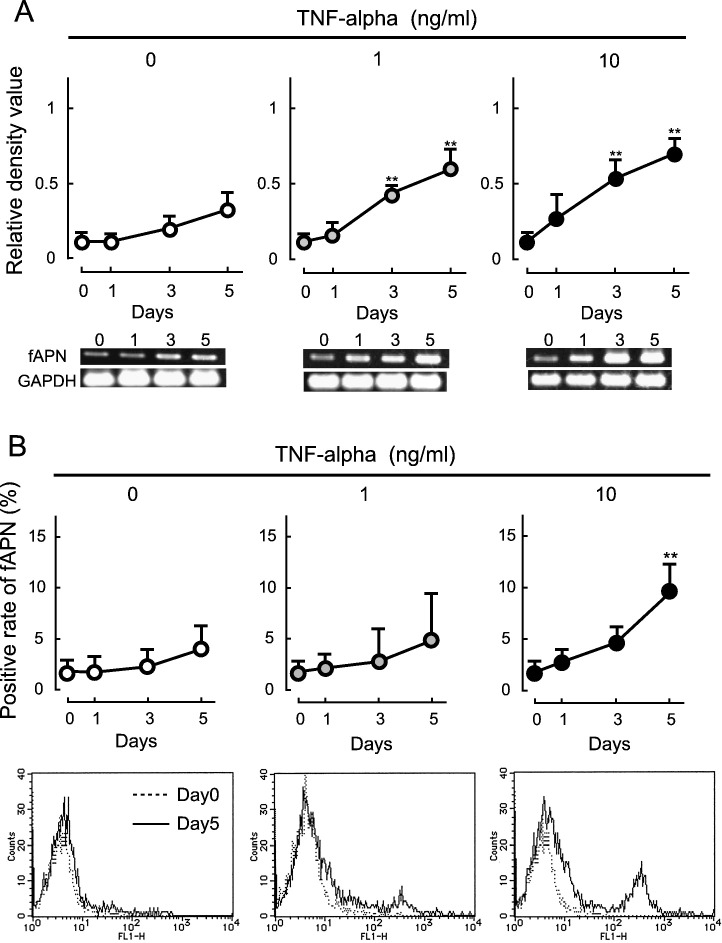
Changes in the fAPN expression level in macrophages treated with recombinant feline TNF-alpha
The fAPN expression level increased with an increase in TNF-alpha production in alveolar macrophages derived from FIP cats and macrophages inoculated with FIPV in vitro. To investigate the relationship between TNF-alpha and fAPN expression, cultured macrophages were treated with recombinant feline TNF-alpha, and changes in the fAPN expression level were investigated. Alveolar macrophages were cultured with medium containing recombinant feline TNF-alpha for 5 days, and the intracellular fAPN mRNA and cell surface fAPN expression levels were measured over time. When TNF-alpha was added to the culture at 1 and 10 ng/ml, the fAPN mRNA expression level was significantly increased on days 3 and 5 in both treatment groups compared to the control group (TNF-alpha: 0 ng/ml) (Fig. 7A). The surface fAPN expression level was also significantly increased on day 5 in cells cultured with 10 ng/ml TNF-alpha (Fig. 7B).
Fig. 7.
Changes in fAPN expression level in macrophages treated with recombinant feline TNF-alpha. SPF cat-derived macrophages (2 × 106 cells) were cultured with medium containing 1 and 10 ng/ml recombinant feline TNF-alpha. The cells were collected on days 0, 1, 3, and 5 of culture, and the intracellular fAPN mRNA (A) and cell surface fAPN (B) expression levels were measured. fAPN mRNA was quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH. n = 5, **p < 0.01 vs. TNF-alpha 0 ng/ml.
FIPV replication in recombinant feline TNF-alpha-treated macrophages
To investigate the influence of macrophages cultured in the presence of recombinant feline TNF-alpha on FIPV infection, whether an increase in fAPN expression in macrophages increased the FIPV infectivity of macrophages was investigated. Macrophages cultured with medium containing TNF-alpha for 5 days were inoculated with FIPV strain 79-1146, and the virus titer in the culture supernatant was measured over time. FIPV production was significantly increased in macrophages cultured with TNF-alpha and inoculated with FIPV, compared to cells without TNF-alpha (Fig. 8 ), and the FIPV titer increased in a manner dependent on the TNF-alpha concentration in the culture medium.
Fig. 8.
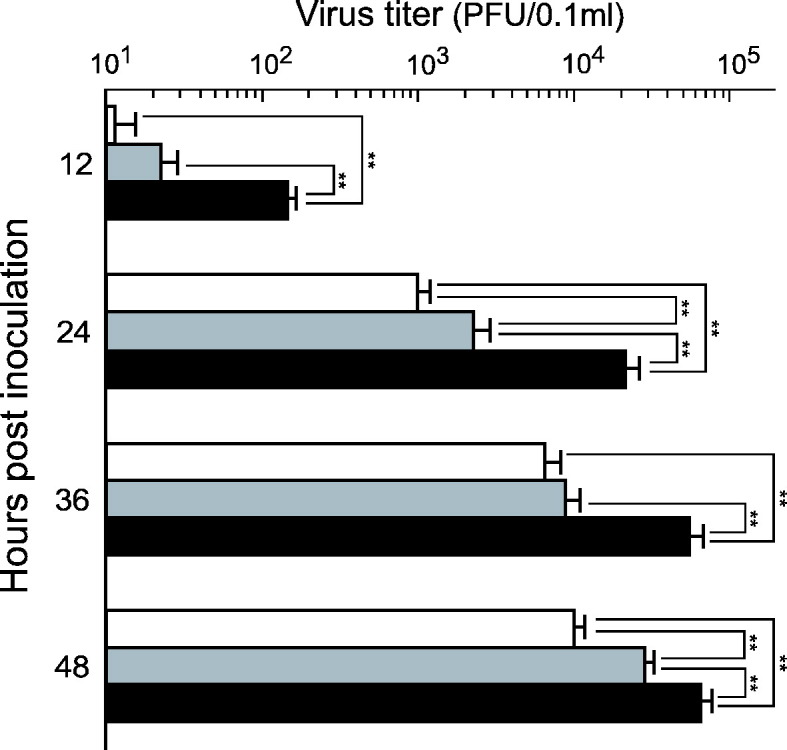
FIPV replication in macrophages treated with recombinant feline TNF-alpha. SPF cat-derived macrophages (2 × 106 cells) were cultured with medium containing 1 and 10 ng/ml recombinant feline TNF-alpha for 5 days. The cells were then inoculated with FIPV, and the culture supernatants were collected every 12 h for 2 days. The virus titers in the supernatants were measured by plaque assay. White bar: without TNF-alpha, gray bar: 1 ng/ml TNF-alpha was added, black bar: 10 ng/ml TNF-alpha was added. n = 4, **p < 0.01.
Discussion
When anti-FCoV antibody-positive cats are inoculated with FIPV, FIP develops earlier and symptoms are severer compared to antibody-negative cats (Pedersen and Boyle, 1980). This ADE-induced aggravation of the pathology in FIP cats has been interpreted that binding of FIPV with antibodies elevates the macrophage infection efficiency, increasing virus production. We demonstrated that TNF-alpha production was elevated with an increase in virus production in macrophages inoculated with a mixture of antibody and FIPV (ADE-induced FIPV-infected macrophages). When macrophages were inoculated with heat-inactivated FIPV, TNF-alpha production was not increased, suggesting that FIPV replication in macrophages is necessary to induce TNF-alpha. TNF-alpha is a cytokine involved in the regulation of immune function, cell proliferation, and cell differentiation. However, overproduction of TNF-alpha is known to have a negative influence on the body. For example, aggravation of the pathology by overproduction of TNF-alpha in inflammatory diseases, such as rheumatoid arthritis and Crohn's disease, and viral infections, such as dengue and Ebola virus infections, has been reported (Atrasheuskaya et al., 2003, Schreiber et al., 1999, Shanahan and St Clair, 2001, Stroher et al., 2001).
We detected an increased feline PBMC apoptosis-inducing activity in the culture supernatant of ADE-induced FIPV-infected macrophages. We have recently reported that the culture supernatant of peritoneal exudate cells (adherent cells with macrophage morphology) from FIP cats had a feline PBMC apoptosis-inducing activity (Takano et al., 2007). We also reported the possibility that this apoptosis induction was due to TNF-alpha contained in the culture supernatant. In this study, the TNF-alpha content in the culture supernatant of ADE-induced FIPV-infected macrophages was higher than that in the culture supernatant of macrophages inoculated with FIPV alone, supporting that TNF-alpha is important as an apoptosis-inducing factor, as we previously reported.
We also initially discovered that the mRNA expression level of the FIPV virus receptor, fAPN, was increased in FIP cat-derived macrophages. Furthermore, we revealed that fAPN expression was increased in macrophages infected with FIPV in vitro, particularly in ADE-induced FIPV-infected macrophages, and confirmed the presence of a factor inducing fAPN expression in the culture supernatant of these macrophages. Heating this culture supernatant at 56 °C for 30 min did not inactivate the fAPN expression-inducing activity, suggesting that this factor is heat-resistant. Since the TNF-alpha level was increased in the FIPV-infected macrophage culture supernatant, as described above, and TNF-alpha is heat-resistant, we predicted that this fAPN-inducing factor is TNF-alpha. To verify this hypothesis, we treated macrophages with recombinant feline TNF-alpha, and investigated its influence on fAPN expression. As we expected, the fAPN expression level was increased in the macrophages. We speculated that TNF-alpha may have been produced as FIPV replicated, and autocrinally or paracrinally acted on macrophages and promoted fAPN expression. To verify this concept, it is necessary to examine whether anti-feline TNF-alpha antibody can inhibit the ability of the FIPV-infected macrophage culture supernatant to induce fAPN in macrophages. We also revealed that infection of recombinant feline TNF-alpha-treated macrophages with FIPV increased FIPV production. TNF-alpha is known to affect other viral infections. It has been reported that TNF-alpha makes vascular endothelial cell invasion by viruses easier in Ebola virus infection (Yonezawa et al., 2005). In rhinovirus infection, when cells were infected after treatment with TNF-alpha, the infection efficiency was increased compared to that in untreated cells (Subauste et al., 1995), and this was considered to be due to an increase in the expression level of the rhinovirus receptor, ICAM-1 (Shafren et al., 1999). In FIPV infection, TNF-alpha may similarly act on macrophages and increase the fAPN expression level, increasing FIPV sensitivity.
We initially demonstrated that TNF-alpha production increased with viral replication in FIPV-infected macrophages, and this TNF-alpha acted on macrophages and promoted fAPN expression. We also confirmed that FIPV infectivity was elevated as fAPN expression increased in macrophages. TNF-alpha production was significantly increased in ADE-induced FIPV-infected macrophages compared to macrophages infected with FIPV alone. It was suggested that when humoral immunity is induced in FIPV-infected cats, ADE induces viral replication in macrophages and overproduction of TNF-alpha, resulting in: 1) lymphocyte apoptosis induction, and 2) advanced FIP onset due to the virus sensitivity being upregulated by the increased virus receptor (fAPN) expression in macrophages. Based on the fact that weakly pathogenic FECV is less likely to infect macrophages than fatal FIPV, Rottier et al. (2005) suggested that the macrophage tropism of the virus was involved in the development of FIP due to infection with FCoV (FIPV). Investigation of a balance with induction of cellular immunity is expected.
Materials and methods
Experimental animals
Seven 6- to 8-month-old anti-FCoV antibody-negative SPF cats were inoculated with 104 TCID50/ml of FIPV strain 79-1146. These seven cats developed FIP symptoms, such as fever, weight loss, peritoneal or pleural effusion, dyspnea, ocular lesions, and neural symptoms, and were used as FIP cats. Seven 6- to 8-month-old SPF cats as controls were also used in this study.
Cell cultures
Felis catus whole fetus-4 (fcwf-4) cells were grown in Eagle's minimum essential medium containing 50% L-15 medium, 5% fetal calf serum (FCS), and antibiotics.
Feline PBMC, alveolar macrophages, and WEHI-164 murine sarcoma cells were maintained in RPMI 1640 growth medium supplemented with 10% FCS, antibiotics, 50 μM 2-mercaptoethanol, and 2 μg/ml of polybrene. WEHI-164 murine sarcoma cells (ATCC CRL1751) were obtained from the American Type Culture Collection.
Monoclonal antibodies (MAbs)
MAb 6-4-2 (IgG2a) used in the present study recognizes S protein of the virus, as demonstrated by immunoblotting. It has been reported that MAb 6-4-2 exhibits a neutralizing activity in fcwf-4 and CrFK cells, but exhibits an enhancing activity in feline macrophages depending on the reaction conditions (Hohdatsu et al., 1993). For MAb recognizing fAPN, R-G-4 (IgG1) prepared by our laboratory was used (Hohdatsu et al., 1998).
Recovery of alveolar macrophages
Feline alveolar macrophages were obtained by broncho-alveolar lavage with HBSS from SPF cats and FIP cats, as previously described by Hohdatsu et al. (1991b).
Inoculation of feline alveolar macrophages with FIPV
Viral suspension (FIPV strain 79-1146, 2 × 103 TCID50/0.1 ml) and MAb 6-4-2 solution were mixed at an equivalent volume ratio and reacted at 4 °C for 1 h, and 0.1 ml of this reaction solution was used to inoculate feline alveolar macrophages (2 × 106 cells) cultured in each well of 24-well multi-plates. As the control, medium alone, virus suspension alone, and MAb 6-4-2 solution alone were added to feline alveolar macrophages. After virus adsorption at 37 °C for 1 h, the cells were washed with HBSS and 1 ml of growth medium. The cells and culture supernatant were collected every 24 h thereafter. The cells were used for measurement of the FCoV N gene, TNF-alpha mRNA, and fAPN mRNA, and the culture supernatant was used for the quantitative analysis of the virus titer and cytotoxic activity against TNF-alpha using WEHI-164 cells.
Plaque assay
Confluent fcwf-4 cell monolayers in 24-well multi-plates were inoculated with 100 μl of the sample dilutions. After virus adsorption at 37 °C the cells were washed with HBSS and 1 ml of growth medium containing 1.5% carboxymethyl cellulose was added to each well. The cultures were incubated at 37 °C for 2 days, fixed in 10% buffered formalin, and stained with 1% crystal violet.
RNA isolation and cDNA preparation
RNA isolation and cDNA preparation were performed by the method of Takano et al. (2007).
Determination of levels of feline GAPDH, TNF-alpha, fAPN mRNA, and FCoV N gene expression
cDNA was amplified by PCR using specific primers for feline GAPDH, TNF-alpha, fAPN, and FCoV N genes. The primer sequences are shown in Table 1 .
Table 1.
Sequences of PCR primers for feline GAPDH, TNF-alpha, fAPN, and FCoV N
| Orientation | Nucleotide sequence | Location | Length (bp) | Reference | |
|---|---|---|---|---|---|
| GAPDH | Forward | 5′-AATTCCACGGCACAGTCAAGG-3′ | 158–178 | 97 | Avery and Hoover (2004) |
| Reverse | 5′-CATTTGATGTTGGCGGGATC-3′ | 235–254 | |||
| TNF-alpha | Forward | 5′-TGGCCTGCAACTAATCAACC-3′ | 195–214 | 251 | Avery and Hoover (2004) |
| Reverse | 5′-GTGTGGAAGGACATCCTTGG-3′ | 426–445 | |||
| fAPN | Forward | 5′-AGGCTACAGATGCTCGGAAA-3′ | 614–633 | 249 | GenBank Accession No. U96104 |
| Reverse | 5′-ACTGGGAGCTCTTGTCTCCA-3′ | 863–882 | |||
| FCoV N | Forward | 5′-CAACTGGGGAGATGAACCTT-3′ | 876–895 | 788 | GenBank Accession No. X56496 |
| Reverse | 5′-GGTAGCATTTGGCAGCGTTA-3′ | 1644–1663 |
PCR was performed by the method of Takano et al. (2007).
Band density was quantified under appropriate UV exposure by video densitometry using Scion Image software (Scion Corporation, USA).
To quantify TNF-alpha, fAPN mRNA and the FCoV N gene were quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH.
Cytotoxic activity against TNF-alpha using WEHI-164 cells
Cytotoxic activity against TNF-alpha were detected by the method of Takano et al. (2007) using WEHI-164 cells.
Separation of PBMC
Heparinized blood (10 ml) was 2-fold diluted with phosphate-buffered saline (PBS), and subjected to Ficoll–Hypaque density gradient centrifugation at 1700 rpm for 20 min. The PBMC layer was collected, washed twice with PBS, and resuspended with growth medium at 2 × 106 cells/ml.
Apoptosis-inducing activities of culture supernatant of alveolar macrophage on PBMC
PBMC (2 × 106 cells) were cultured for 4 h in the presence of culture supernatant of alveolar macrophage (final concentration of 1:2), and were examined for their apoptosis-inducing activities. For alveolar macrophage culture supernatants, macrophages (2 × 106 cells) were inoculated with FIPV alone, anti-FIPV (α-FIPV) S MAb alone, and a mixture of FIPV + α-FIPV S MAb, and the supernatants were collected after 72 h.
Apoptosis induction was detected by the TUNEL method using flow cytometric analysis.
Detection of apoptosis by TUNEL
The apoptotic cells was detected by TUNEL according to the method of Takano et al. (2007).
Measurement of fAPN expression level in feline alveolar macrophages cultured with recombinant feline TNF-alpha
Feline alveolar macrophages (2 × 106 cells) were cultured in medium containing recombinant feline TNF-alpha (R&D Systems, USA) at 1 and 10 ng/ml. The cells were collected after culturing for 1, 3, and 5 days, and fAPN mRNA expression level and macrophage surface fAPN expression level were measured.
Measurement of macrophage surface fAPN expression level by flow cytometric analysis
A total of 2 × 106 cells were incubated with α-fAPN MAb at 4 °C for 50 min. The cells were washed twice in PBS containing 0.1% sodium azide and incubated with FITC-conjugated Fab of goat anti-mouse IgG antibody (MP Biomedicals, USA). The cells were again washed twice, and the fluorescence intensity was measured using a FACS 440 cell sorter. The value calculated by subtracting the positive rate on reaction with control MAb, belonging to the same subclass as α-fAPN MAb, from the positive rate on reaction with α-fAPN MAb was regarded as the positive rate of fAPN.
FIPV replication in feline alveolar macrophages cultured with recombinant feline TNF-alpha
Feline alveolar macrophages (2 × 106 cells) were cultured in medium containing recombinant feline TNF-alpha (R&D Systems, USA) at 1 and 10 ng/ml for 5 days. The cells were then inoculated with FIPV strain 79-1146 (103 TCID50/0.1 ml). The culture supernatant was collected every 12 h, and the virus infection titer was measured by plaque assay.
Statistical analysis
Data were analyzed by Student's t test. P values < 0.05 were considered to indicate a significant difference between compared groups.
Acknowledgment
This work was supported by Ministry of Health, Labor, and Welfare Grant H16-Shinkoh-9.
References
- Atrasheuskaya A., Petzelbauer P., Fredeking T.M., Ignatyev G. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol. Med. Microbiol. 2003;35:33–42. doi: 10.1111/j.1574-695X.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Avery P.R., Hoover E.A. Gamma interferon/interleukin 10 balance in tissue lymphocytes correlates with down modulation of mucosal feline immunodeficiency virus infection. J. Virol. 2004;78:4011–4019. doi: 10.1128/JVI.78.8.4011-4019.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corapi W.V., Olsen C.W., Scott F.W. Monoclonal antibody analysis of neutralization and antibody-dependent enhancement of feline infectious peritonitis virus. J. Virol. 1992;66:6695–6705. doi: 10.1128/jvi.66.11.6695-6705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean G.A., Olivry T., Stanton C., Pedersen N.C. In vivo cytokine response to experimental feline infectious peritonitis virus infection. Vet. Microbiol. 2003;97:1–12. doi: 10.1016/j.vetmic.2003.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L'Haridon R., Vogel L.K., Sjostrom H., Noren O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goitsuka R., Ohashi T., Ono K., Yasukawa K., Koishibara Y., Fukui H., Ohsugi Y., Hasegawa A. IL-6 activity in feline infectious peritonitis. J. Immunol. 1990;144:2599–2603. [PubMed] [Google Scholar]
- Goitsuka R., Furusawa S., Mizoguchi M., Hasegawa A. Detection of interleukin 1 in ascites from cats with feline infectious peritonitis. J. Vet. Med. Sci. 1991;53:487–489. doi: 10.1292/jvms.53.487. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T., Okada S., Koyama H. Characterization of monoclonal antibodies against feline infectious peritonitis virus type II and antigenic relationship between feline, porcine, and canine coronaviruses. Arch. Virol. 1991;117:85–95. doi: 10.1007/BF01310494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Nakamura M., Ishizuka Y., Yamada H., Koyama H. A study on the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection in feline macrophages by monoclonal antibodies. Arch. Virol. 1991;120:207–217. doi: 10.1007/BF01310476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Yamada H., Ishizuka Y., Koyama H. Enhancement and neutralization of feline infectious peritonitis virus infection in feline macrophages by neutralizing monoclonal antibodies recognizing different epitopes. Microbiol. Immunol. 1993;37:499–504. doi: 10.1111/j.1348-0421.1993.tb03242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Izumiya Y., Yokoyama Y., Kida K., Koyama H. Differences in virus receptor for type I and type II feline infectious peritonitis virus. Arch. Virol. 1998;143:839–850. doi: 10.1007/s007050050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann H., Pyrc K., van der Hoek L., Geier M., Berkhout B., Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipar A., May H., Menger S., Weber M., Leukert W., Reinacher M. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet. Pathol. 2005;42:321–330. doi: 10.1354/vp.42-3-321. [DOI] [PubMed] [Google Scholar]
- Kiss I., Poland A.M., Pedersen N.C. Disease outcome and cytokine responses in cats immunized with an avirulent feline infectious peritonitis virus (FIPV) – UCD1 and challenge – exposed with virulent FIPV-UCD8. J. Feline Med. Surg. 2004;6:89–97. doi: 10.1016/j.jfms.2003.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa K., Hohdatsu T., Aizawa C., Koyama H., Hashimoto H. Molecular cloning and sequence determination of the peplomer protein gene of feline infectious peritonitis virus type I. Arch. Virol. 1995;140:469–480. doi: 10.1007/BF01718424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa K., Hohdatsu T., Hashimoto H., Koyama H. Comparison of the amino acid sequence and phylogenetic analysis of the peplomer, integral membrane and nucleocapsid proteins of feline, canine and porcine coronaviruses. Microbiol. Immunol. 1996;40:425–433. doi: 10.1111/j.1348-0421.1996.tb01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C.W. A review of feline infectious peritonitis virus: molecular biology, immunopathogenesis, clinical aspects, and vaccination. Vet. Microbiol. 1993;36:1–37. doi: 10.1016/0378-1135(93)90126-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C.W., Corapi W.V., Ngichabe C.K., Baines J.D., Scott F.W. Monoclonal antibodies to the spike protein of feline infectious peritonitis virus mediate antibody-dependent enhancement of infection of feline macrophages. J. Virol. 1992;66:956–965. doi: 10.1128/jvi.66.2.956-965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Boyle J.F. Immunologic phenomena in the effusive form of feline infectious peritonitis. Am. J. Vet. Res. 1980;41:868–876. [PubMed] [Google Scholar]
- Rottier P.J., Nakamura K., Schellen P., Volders H., Haijema B.J. Acquisition of macrophage tropism during the pathogenesis of feline infectious peritonitis is determined by mutations in the feline coronavirus spike protein. J. Virol. 2005;79:14122–14130. doi: 10.1128/JVI.79.22.14122-14130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Nikolaus S., Hampe J., Hamling J., Koop I., Groessner B., Lochs H., Raedler A. Tumor necrosis factor alpha and interleukin 1beta in relapse of Crohn's disease. Lancet. 1999;353:459–461. doi: 10.1016/S0140-6736(98)03339-X. [DOI] [PubMed] [Google Scholar]
- Shafren D.R., Gardner J., Mann V.H., Antalis T.S., Suhrbier A. Picornavirus receptor down-regulation by plasminogen activator inhibitor type 2. J. Virol. 1999;73:7193–7198. doi: 10.1128/jvi.73.9.7193-7198.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan J.C., St Clair W. Tumor necrosis factor-alpha blockade: a novel therapy for rheumatic disease. Clin. Immunol. 2001;103:231–242. doi: 10.1006/clim.2002.5191. [DOI] [PubMed] [Google Scholar]
- Stroher U., West E., Bugany H., Klenk H.D., Schnittler H.J., Feldmann H. Infection and activation of monocytes by Marburg and Ebola viruses. J. Virol. 2001;75:11025–11033. doi: 10.1128/JVI.75.22.11025-11033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subauste M.C., Jacoby D.B., Richards S.M., Proud D. Infection of a human respiratory epithelial cell line with rhinovirus. Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J. Clin. Invest. 1995;96:549–557. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhrbier A., La Linn M. Suppression of antiviral responses by antibody-dependent enhancement of macrophage infection. Trends Immunol. 2003;24:165–168. doi: 10.1016/s1471-4906(03)00065-6. [DOI] [PubMed] [Google Scholar]
- Sullivan N.J. Antibody-mediated enhancement of viral disease. Curr. Top. Microbiol. Immunol. 2001;260:145–169. doi: 10.1007/978-3-662-05783-4_8. [DOI] [PubMed] [Google Scholar]
- Takano T., Hohdatsu T., Hashida Y., Kaneko Y., Tanabe M., Koyama H. A “possible” involvement of TNF-alpha in apoptosis induction in peripheral blood lymphocytes of cats with feline infectious peritonitis. Vet. Microbiol. 2007;119:121–131. doi: 10.1016/j.vetmic.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado S.M., Yoon K.J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- Tresnan D.B., Levis R., Holmes K.V. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup: I. J. Virol. 1996;70:8669–8674. doi: 10.1128/jvi.70.12.8669-8674.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J., Smith G., Jones S., Proulx R., Deschambault Y., Grudeski E., Andonov A., He R., Li Y., Copps J., Grolla A., Dick D., Berry J., Ganske S., Manning L., Cao J. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 2004;78:12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Y., Werner H.C., Kong W.P., Leung K., Traggiai E., Nabel G.J. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc. Natl. Acad. Sci. U. S. A. 2005;102:797–801. doi: 10.1073/pnas.0409065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa A., Cavrois M., Greene W.C. Studies of Ebola virus glycoprotein-mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. J. Virol. 2005;79:918–926. doi: 10.1128/JVI.79.2.918-926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]