Abstract
Numerous preclinical studies have demonstrated the efficacy of viral gene delivery vectors, and recent clinical trials have shown promising results. However, the tight control of transgene expression is likely to be required for therapeutic applications and in some instances, for safety reasons. For this purpose, several ligand-dependent transcription regulatory systems have been developed. Among these, the tetracycline-regulatable system is by far the most frequently used and the most advanced towards gene therapy trials. This review will focus on this system and will describe the most recent progress in the regulation of transgene expression in various organs, including the muscle, the retina and the brain. Since the development of an immune response to the transactivator was observed following gene transfer in the muscle of nonhuman primate, focus will be therefore, given on the immune response to transgene products of the tetracycline inducible promoter.
Keywords: Gene regulation, Tetracycline, Doxycycline, Retina, Brain, Rat, Mouse, Nonhuman primate
1. Introduction
The clinical efficacy and safety as well as the application range of gene therapy will be broadened by developing systems capable of finely modulating the expression of therapeutic genes. A regulatable system that can turn “on” and “off” therapeutic gene expression will not only be crucial for maintaining appropriate levels of a gene product within the therapeutic range, thus preventing toxicity, but also allow the turning “off” of therapeutic gene expression to avoid harmful side effects. The development of ligand-dependent transcription regulatory systems is thus of great importance.
To be used in human gene therapy, a pharmacologically regulated system should fulfil several criteria as follows: (1) The ligand should activate (ON-switch) rather than silence transcription (OFF-switch). Off-systems suffer from 2 drawbacks: a prolonged exposure to the drug is required to silence the system and the induction kinetics is mainly determined by the rate of drug clearance; (2) the inducer drug should be orally bioavailable and penetrate the target tissue; (3) the system should not interfere with endogenous metabolic pathways and the inducer compound should have a drug metabolism profile compatible with prolonged therapeutic uses; (4) transcription should be fully and rapidly reversible to enable prompt changing of the dosing regimen; (5) the system should be inactive in the absence of the inducer drug but strongly stimulated by the drug administration; (6) a precise correlation must exist between drug dosage and target gene expression level; and (7) the transcriptional activator should exhibit a low potential of eliciting an immune response in humans.
Four drug-dependent regulatable systems, which meet most of the criteria listed above, were developed for the in vivo control of transgene expression: the tetracycline (tet) [1], [2] and rapamycin [3] regulatable systems, the mammalian steroid receptor (mifepristone and tamoxifen) [4], [5], and the insect steroid receptor (ecdysteroid)-based systems [6], [7]. The molecular mechanism underlying the regulatory effect and the pharmacologic characteristics of the inducer drug are distinct in these different regulatory systems, but transgene regulation has been documented in vitro and in rodent models (for review [8], [9]). Only the tetracycline and the rapamycin regulatable systems have been tested in large animal models so far [10], [11], [12], [13], [14], [15], [16], [17], [18].
This review will focus on the tetracycline-regulatable systems. Besides, the molecular mechanisms of different variants of the system and the pharmacologic characteristics of the inducer drug, the regulation of transgene expression in various organs including muscle, retina, and brain, will be explored in this review. Only the preclinical studies that are potentially most relevant clinically for future gene therapy clinical trials, will be discussed in detail.
2. Molecular mechanism involved in tetracycline-regulatable systems
Tet-regulatable systems are based on the E. coli Tn10 Tet resistance operon, which consists of the Tet repressor protein (TetR) and the Tet operator DNA sequence (TetO DNA) [19]. In the absence of Tet or its derivate doxycycline (Dox), the TetR protein gets attached to the TetO DNA sequence, while in the presence of the drug, TetR changes its conformation to detaching from the DNA [20].
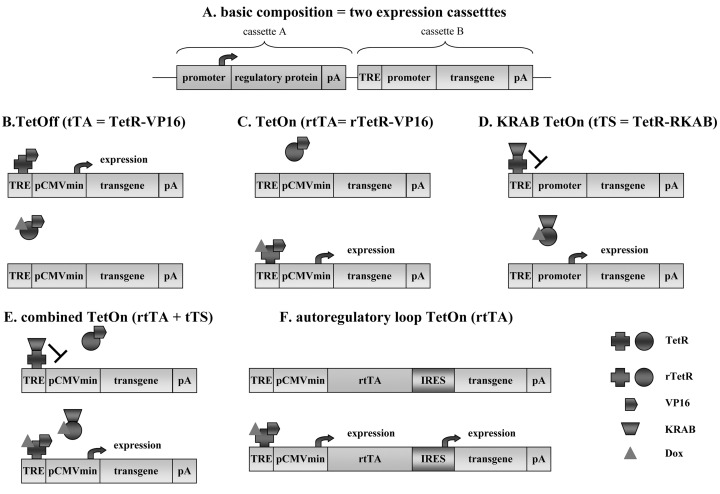
Five different Tet-responsive regulatory systems have been developed, based on this bacterial operon system, that are normally cloned in two different expression cassettes (Fig. 1 A). The first two systems contain the TetR protein fused to the transactivator VP16 of Herpes simplex virus (HSV) [1], [2]. If transgene expression is allowed only in the absence of Dox, the system is called TetOff (Fig. 1B), whereas if transgene expression is allowed only in the presence of Dox, the system is called TetOn (Fig. 1C). The third system contains the TetR protein fused to a transinhibitor protein, the Krüppel-associated box (KRAB) of Zink finger proteins [21]. The TetR-KRAB system is designed to allow transgene expression only in the presence of Dox (TetOn, Fig. 1D). In an attempt to minimize background levels of transgene expression, two alternative systems have been developed. The first one being a combination of the rTetR-VP16 and the TetR-KRAB systems (combined TetOn, Fig. 1E), and a second system based on an autoregulatory loop, in which the transactivator is not constitutively expressed but under control of the inducible promoter (autoregulatory loop, Fig. 1F), thus making it possible to have only one expression cassette with an internal ribosomal entry site (IRES) or a bidirectional promoter.
Fig. 1.
Schematic representations of the five different Tet-regulatable systems. (A) In general, Tet-regulatable systems are composed of two distinct expression cassettes, one containing the regulatory protein (tTA, rtTA, tTS) under the control of a ubiquitous cell type specific promoter (cassette A) and the second cassette containing the Tetracycline Responsive Element (TRE) fused to a promoter, regulating the expression of the reporter or therapeutic transgene (cassette B). (B) The chimeric protein tTA is composed of the TetR fused to the viral transactivator VP16. In the absence of Dox, the tTA protein binds to the TRE within the compound promoter PCMVmin to allow the expression of the transgene. In the presence of Dox, the tTA changes its conformation, gets detached from the TRE and no transgene expression is observed. (C) The rtTA transactivator is also a chimeric protein composed of the TetR and the VP16 domains. However, due to the presence of four mutations in the TetR (now called rTetR) domain, this protein fixes the TRE in the presence of Dox, thus allowing transgene expression. In the absence of Dox, rtTA gets detached from the TRE and no transgene expression is observed. (D) The chimeric protein tTS is composed of the TetR fused to the KRAB box of Zink finger proteins. In the absence of Dox, tTS binds to the TRE and inhibits transgene expression driven by a normal promoter (i.e. CMV). In the presence of Dox, tTS gets detached from the TRE, thus allowing transgene expression. (E) The combined TetOn system contains both the rtTA and the tTS protein, simultaneously expressed from cassette A. In the absence of Dox, the tTS protein binds to the minimal compound promoter and inhibits even the low background expression, while the rtTA protein stays detached from the TRE. In the presence of Dox, rtTA binds to the TRE and activates expression of the transgene, while tTS stays detached. (F) In the autoregulatory loop system, both rtTA (or tTA) and the transgene of interest are driven by the compound minimal promoter separated by an internal ribosomal entry site (IRES). In the absence of Dox, no transgene is expressed, while in the presence of Dox, both transgenes, transactivator and gene of interest, are expressed. Onset of expression takes longer, because initially, sufficient amounts of rtTA need to be produced to completely activate the system.
In this section, each system is going to be described on the molecular basis for the better understanding of the different in vivo applications.
2.1. TetOff (tTA, TetR-VP16)
In this system, the chimeric protein, which is the product of the DNA binding domain of the TetR and the transactivator domain of VP16, is called tTA (for Tet-controlled transcriptional activator). Seven repeats of the TetO DNA sequence, also referred to as TRE (for Tet-responsive elements), are fused upstream of a minimal CMV promoter (PminCMV), forming the compound promoter PCMV-1 [1]. In the absence of Dox, tTA binds to the TREs thus activating PCMV-1, which results in the expression of the adjacent gene (Fig. 1B). If Dox is present, tTA changes its conformation, detaches from the TREs and gene expression is silenced. Furth and colleagues have shown for the first time the functionality of the system in vivo in bigenic mice harbouring the tTA gene and a reporter gene [22]. To minimize possible interactions of the VP16 transactivator with other cellular promoters and to avoid the onset of immune reactions, this protein was reduced to three minimal repeats of 12 amino acids [23].
The TetOff system is useful only if gene expression is to be maintained to a switch-on state for a long time. To turn the system off, continued administration of Dox would be required and the activation of transgene expression depends mainly on the clearance kinetics of Dox. These reasons limit the usefulness of the system for potential human applications due to which the use of this system was restricted to early experiments in vivo.
2.2. TetOn (rtTA, rTetR-VP16)
Four mutations in the TetR domain of the transactivator tTA were shown to reverse the behaviour of the protein in interacting with the TRE in response to the presence or absence of Dox [2]. Rather than attaching to the TRE in the absence of Dox, this mutant reverse Tet repressor protein (rTetR) stays soluble and binds the TREs in the presence of Dox. When rTetR is fused to the VP16 domain, the resulting transactivator protein is called rtTA and the system changes to a TetOn system: only the presence of the inducer drug allows expression of a transgene (Fig. 1C).
Activation of transgene expression was found to be more rapid compared to the TetOff system [2], [24]. However, to induce transgene expression using the TetOn system, high concentrations of Dox were required, raising the question of whether these concentrations were possible to achieve in tissues such as in the brain [25]. Urlinger and colleagues studied the performance of several mutated rtTA proteins, which were developed by random mutagenesis and codon optimisation [26]. They found new rtTA transactivators, in particular rtTA2s-M2 and rtTAS-S2, which showed an improved sensitivity to the inducer drug and a reduced background activity in the absence of Dox [26], [27].
This system confers with most of the requirements to be used in human patients (see Introduction) and has therefore been used in numerous in vivo studies in rodent models and has already advanced to preclinical studies in nonhuman primates [10], [12], [13], [17], [18], [28].
2.3. KRAB TetOn (tTS, TetR-KRAB)
Due to the fact that a potential immune response against rtTA is primarily triggered against the viral part of the fusion protein, attempts were made to replace this part of rtTA by a less immunogenic protein. Deuschle and colleagues developed a system, in which the original TetR protein was fused to the transrepressing domain KRAB of the human Zink finger protein Kox1, forming a transinhibitor called TetR-KRAB, or tTS [21], [29], [30]. In other studies, the KRAB domain has been retrieved from the rodent Zink finger protein Kid-1 and the transrepressor is called tTSKid [31], [32]. Zink finger proteins are regulatory proteins and their KRAB domain is known to inhibit all RNA polymerases II or III within a range of 3 kB of its DNA attachment site [21], [33]. The second part of the TetR-KRAB system, the compound promoter (PTetO-CMVlg) used for the expression of the transgene, consists of TRE fused upstream to a normal CMV promoter that does not need activation. In the absence of Dox, TetR-KRAB gets attached to the TRE and inhibits the activity of the CMV promoter, while in the presence of Dox, TetR-KRAB gets detached from the TRE, thus allowing transgene expression (Fig. 1D).
The mechanism by which KRAB inhibits expression of genes is not entirely known. Three mechanisms seem to be involved: local change in chromatin structure (triggering heterochromatin), local histone deacetylation, and the indirect influence of the arrangement of the basal transcription machinery [34], [35], [36], [37], [38], [39], [40]. It has been proposed that only integrated promoters can be inhibited by the TetR-KRAB system [41]. However, different studies demonstrated efficient regulation of transgene expression in vivo using non-integrating adenoviral vectors [42], [43].
The system has been used in transformed cell lines [44] in vitro and in vivo in different organs in mice using lentiviral vectors, which integrate their viral DNA [41], [45].
2.4. Combined TetOn (rtTA and tTS)
To minimize background expression of the TetOn system, KRAB-mediated inhibition was incorporated in addition to the rtTA system [46]. For this purpose, the tTS gene is cloned into a bicistronic expression cassette downstream of the transactivator, separated by the use of an internal ribosomal entry site (IRES). In the absence of Dox, the rtTA would stay detached from the TRE while the tTS would fix and inhibit background expression of the transgene. In the presence of Dox, the two chimeric proteins would change their conformation and reverse their activity, thus allowing transgene expression (Fig. 1E).
This combined system has been used in several in vivo studies where low background activities were described, while the maximum levels of transgene expression in the presence of Dox were similar to those in pure TetOn systems. However, one group observed progressive loss of transgene expression in CHO cells transduced with lentiviral vectors containing this combined system with long-term culture and repeated inductions [47].
The question of whether the expression of three foreign proteins in one cell will have negative effects on the immunological situation in vivo has to be addressed. In addition, due to size restrictions, this system cannot be incorporated in a single vector system using all available viral vectors (i.e. AAV vectors), which may cause problems for observing certain protocols. This system has been used in vivo in several organs, including muscle, retina and brain, which will be reviewed in Section 4.
2.5. Autoregulatory loop TetOn–TetOff
A second strategy to minimize background expression is to place both transgenes, the transactivator and the gene of interest, under the compound Tet-inducible promoter PCMV-1 (Fig. 1F) [48]. Using such a system, no rtTA is expressed in the absence of the inducer drug, which is in contrast to the original TetOn system, where rtTA expression is placed under a constitutive promoter. Therefore, the inducible PCMV-1 promoter cannot interact with constitutively expressed rtTA in a Dox-independent, non-specific manner. Dox-mediated induction of expression of the gene of interest may take longer, as the rtTA has to be expressed first, but the immunogenicity and potential toxicity of the transactivator is restricted to the time of induction. Evidently, the autoregulatory loop can also be used as a TetOff mechanism, placing the tTA rather than the rtTA transactivator under control of the minimal CMV promoter [49], [50].
Autoregulatory loop systems have been used as naked plasmids or cloned into lentiviral vectors in vitro [51], [52] and in several studies in vivo, which will be discussed in Section 4.
3. Pharmacology of Doxycyline
Dox, an analogue of Tet, is a well-documented antibiotic drug because it has been used in the clinics for more than 30 years. It is available for oral or intravenous application. The bioavailability of Dox after oral administration compared to intravenous administration is almost 100% and the serum half-life has been calculated to be 14–22h. The tissue penetration is excellent and includes the brain. Concentrations are the highest in liver, kidney and digestive tract, as it is eliminated primarily via urine and faeces (for review [53]).
Dox is currently considered as one treatment option for infectious diseases such as Lyme disease [54], malaria [55] or multi-resistant Staphylococcus aureus (MRSA) infections [56]. Interestingly, Dox has been approved by the FDA in 2006 for usage in the treatment of rosacea (acne) by sub-antimicrobial doses, so-called “anti-inflammatory-dose Dox” (40 mg/day per adult person, corresponding to less than 1 mg/kg body weight) [57], [58], [59].
Side effects of prolonged administration of Dox include renal toxicity and dose-dependent photosensitivity. Dox may accumulate in the bones and in young patients in the teeth, leading to teeth decolouration and decreased bone growth rate [57].
However, the main concern while using an antibiotic-driven regulatory switch remains to be the possibility of raising resistance to the antibiotic itself, even though Dox at doses of 40 mg/day (“anti-inflammatory-dose Dox”) or less are not considered to lead to the development of antibiotic-resistant organisms [57].
This problem of potentially raising antibiotic resistance to Dox may also be overcome by the use of 4-epidoxycycline, a hepatic metabolite of Dox lacking antibiotic activity in mice [60]. In conditional knockout mice, 4-epidoxycycline was as effective as Dox in switching transgene expression “on” or “off”.
4. Regulation of transgene expression in vivo
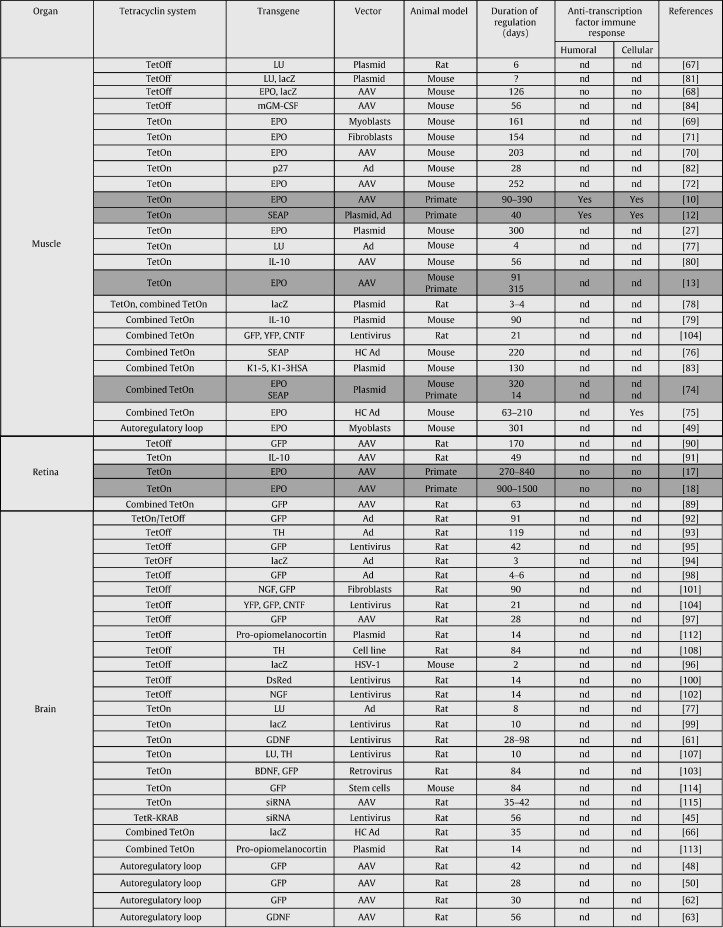
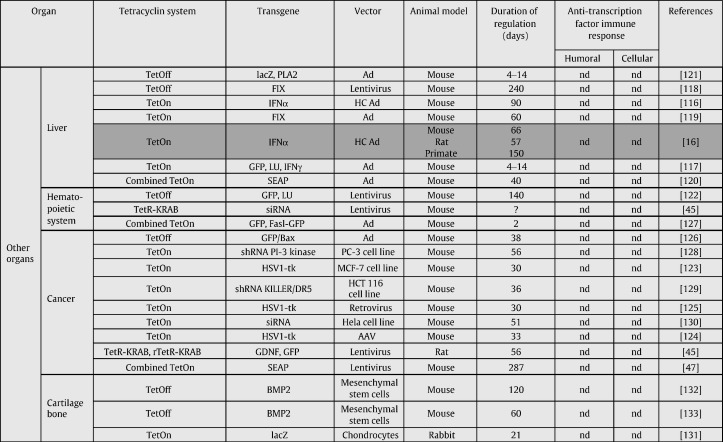
In this section, we aim to review the use of the Tet-regulatable system to regulate transgene expression in vivo (Table 1 ). Regarding each organ, focus will be given on the most advanced strategies in terms of their potential clinical application.
Table 1.
Regulation of transgene expression by tetracycline dependent systems in vivo (in gray: studies involving nonhuman primates).
Some strategies have already reached the level of nonhuman primates, which is the last stage before the clinical use in human patients (Table 1, highlighted in gray). These strategies have been developed to regulate transgene expression employing the TetOn system after AAV-mediated gene transfer either in the muscle (chapter 4.1) [10], [13], in the retina (chapter 4.2) [17], [18], or, following High Capacity (HC) adenoviral vector-mediated gene transfer, in the liver (chapter 4.4.1) [16]. In contrast, regulated transgene expression in the brain was studied predominantly in rat, often in rat models with CNS diseases. The focus here will be given on three different strategies: (i) the TetOn system following lentiviral vector-mediated gene transfer [61], (ii) the autoregulatory loop following AAV-mediated gene transfer [62], [63], [64], and (iii) the combined TetOn system following HC-Ad vector-mediated gene transfer [65], [66]. In other organs or in cancer models, the published studies were limited to early experiments performed in mice, and therefore will not be mentioned in detail in the following section.
4.1. Regulation of transgene expression in the muscle
The skeletal muscle is one of the largest organ systems in the mammalian organism and easily accessible. Therefore, attempts at expressing therapeutic proteins from skeletal muscle were one of the earliest applications for regulated gene therapy. Due to the facile quantification of erythropoietin (EPO) in the serum and the obvious biological effect in increasing the hematocrit levels in the induced animals, this hormone has been used in many published works as a reporter gene [13], [27], [49], [68], [69], [70], [71], [72], [73], [74], [75]. Besides EPO, other reporter genes have been used to study regulated transgene expression from the muscle, i.e. secretable alkaline phosphatase (SEAP) [12], [74], [76], luciferase (LU) [67], [77] or ß-galactosidase [78]. In addition, several studies employed potentially therapeutic proteins, for example IL-10 [79], [80].
Proof of principle experiments on regulated transgene expression using the TetOff system in the skeletal [81] or the heart muscle [67] of mice following direct plasmid injection precluded muscle directed gene transfer of a TetOff regulatory system encapsidated in AAV vectors [68]. Soon afterwards, the TetOn system has also been used to control EPO expression in the skeletal muscle following cell based or AAV-mediated gene transfer [69], [71].
Bohl and colleagues constructed an AAV vector, in which the original rtTA transactivator was placed under the control of a strong CMV promoter, and the murine EPO expression cassette was constructed in a way that both cassettes were laid directly opposite to each other [70]. Within one week of Dox administration via the drinking water, EPO concentrations in the serum raised by 10-fold and decreased to baseline levels within 14 days of Dox withdrawal. Following induction of EPO expression, hematocrit levels increased accordingly, demonstrating the biological activity of transgenic EPO. No immune response was observed in mice either to the rtTA transactivator, or to the EPO protein. Similar results leading to tight and long-term control of EPO expression were observed in a murine model of ß-thalassemia [72].
These encouraging results in mice led to the transfer of this system into the nonhuman primate muscle. Favre and colleagues injected AAV2 vectors carrying the TetOn (rtTA transactivator) into the skeletal muscle of 8 primates [28]. Initially, all primates showed tight regulation of transgene expression with rapid induction and de-induction kinetics [10]. Following intravenous Dox administration, serum EPO levels increased to a maximum within 3–4 days, which was followed by an increase of the reticulocyte counts in the serum, demonstrating once again the biological activity of the transgenic EPO. The animals were regularly bled to avoid high hematocrit discomfort following the inductions.
However, the inducibility of EPO expression following intravenous injection of Dox vanished and was completely lost following two to five Dox pulses [10]. This was due to the appearance of a humoral and cellular response against the transactivator protein, resulting in destruction of rtTA-expressing cells within the skeletal muscle (for further details, see chapter 5 of this review).
To obtain a more stringent regulation of transgene expression, optimised versions of the transactivator, such as rtTA-S2, rtTA-M2, and rtTA-M2nls, were cloned in different orientations into expression cassettes that were incorporated into AAV2 vectors and transferred into the muscle of mice and subsequently of nonhuman primates [13]. In addition, the woodchuck hepatitis post-transcriptional regulatory element (WPRE) was introduced following the EPO cDNA. Based on the evaluation of different designs of the expression cassette in mice, it was observed that when both promoters driving the expression of the transgene and the rtTA-M2 were positioned apart from each other, but oriented in the same direction (forward), inducibility was optimal and background expression remained minimal. This cassette (forward) was then studied in the muscle of nonhuman primates using the AAV1 and AAV2 serotypes. It was shown that using the AAV1 serotype for the transduction of primate skeletal muscle, almost 10 times higher serum EPO concentration could be observed compared to animals injected with serotype AAV2. In addition, the inducibility of the system seemed to be more effective in nonhuman primates than in mice, since a 50–200-fold increase of serum EPO could be observed with almost no baseline activity, while in mice the difference was around 20-fold. However, these values have to be studied with caution, as baseline expression of EPO could be quite undetectable in primates due to the large volume of blood in the circulatory system that would dilute the serum concentration of EPO [13].
The TetOn system was also used to regulate transgene expression following gene transfer by adenoviral vectors of antiangiogenic factor p27Kip1 in mice [82] or SEAP in primates [12]. EPO expression was also studied under the control of the TetOn system following naked DNA transfection in mice [27], [78]. Importantly, in the study performed by Latta-Mahieu and colleagues in primates, a strong cellular and humoral immune response to the transactivator led to the loss of transgene expression, probably by the destruction of transgene expressing cells, which is in agreement with the results obtained in the study performed by Favre et al. [10] (for further details, see chapter 5 of this review).
Interleukin 10 (IL-10) is a strong anti-inflammatory cytokine that may offer a potential treatment option for rheumatoid arthritis (RA). Construction and intramuscular injection of two AAV vectors containing the IL-10 gene under the control of a TetOn regulatory system resulted in profound expression of IL-10 from the transduced muscle in a mouse model of chronic inflammatory arthritis over the duration of 8 weeks [80]. A similar strategy, proposed by the same group, used a plasmid transfection strategy, in which the IL-10 expression was controlled by a combined TetOn system (rtTA-M2 and tTS) for the duration of 3 months [79].
The combined TetOn system was also employed in several studies to regulate EPO expression in the muscle, either following AAV-mediated gene transfer [73], adenoviral vector-mediated gene transfer [76], [77] or using plasmid transfection [74], [78]. One study examined the controlled expression of different domains of the antiangiogenic factor plasminogen (K1-5, K1-3HSA) following plasmid gene transfer [83]. All studies that were performed in mice demonstrated successful regulation of transgene expression. In addition, Lamartina and colleagues studied the regulated expression of SEAP in the skeletal muscle of primates following direct plasmid injection. Even though the control of EPO expression in the mouse muscle could be demonstrated for up to 1 year, the study performed in the primate muscle lasted only for 14 days, which was not sufficient for long-term analysis of Tet-mediated regulation [74].
The autoregulatory loop, constructed as a TetOff system, was also used to study the regulated expression of EPO from skeletal muscle in mice [49]. Encapsulated myoblasts that were transfected with plasmids containing the autoregulatory loop TetOff system and EPO as reporter gene were implanted and EPO expression was controlled over a period of 40 weeks (duration of the experiment).
The optimisation of the TetOff expression cassette was reported by Agha-Mohammadi et al. [84]. In their study, the number of Tet operator elements that are fused to the compound minimal promoter was increased to 8 and the promoter sequence shortened. These new components of the Tet-regulatable systems were incorporated into an AAV vector, and expression of mouse granulocyte–macrophage colony stimulating factor (mGM-GSF) was controlled over a period of 4 weeks following intramuscular injection.
The most recent experiment on transgene regulation in the skeletal muscle was performed by Lena and colleagues, who studied the capacity of the combined TetOn (rtTA-M2 and tTS-kid) system to regulate EPO expression in mice following HC-Ad-mediated gene transfer [75]. They observed the development of a cellular immune response leading to loss of transgene regulation within 8 weeks after the gene transfer. This observation is reminiscent of what has been observed in primates [10], [12]. However, the use of lower doses of adenoviral vector and the employment of a muscle-specific promoter was sufficient to avoid immune reactions in these mice and led to sustained regulation of transgene expression for 30 weeks [75].
In conclusion, stringent regulation of transgene expression in the muscle is possible using Tet-regulatable systems. However, the appearance of a strong immune response to the transactivators, regardless of original or optimised version, in nonhuman primates makes it difficult to further advance with this system [85]. This issue will be discussed in Section 5 of this review.
4.2. Regulation of transgene expression in the retina
The expression of several neurotrophic factors, such as ciliary neurotrophic factor (CNTF) or brain derived neurotrophic factor (BDNF), in the retina has shown to delay the onset of retinal degeneration in a variety of animal models of Retinitis pigmentosa. However, a long-term exposure of retinal cells to CNTF decreases the electroretinogram (ERG) responses due to the downregulation of several transcription factors in the photoreceptors [86]. Therefore, a regulated expression of neurotrophic proteins in the retina would avoid the onset of negative side effects while conserving the neuroprotective activity.
A similar strategy is currently being developed for neovascular disorders, the treatment of which consists currently in the injection of anti-VEGF molecules repeatedly into the vitreous to inhibit VEGF-mediated neovascularisation in age-related macula degeneration or diabetic retinopathy. The long-term blockage of VEGF activity by expressing such anti-VEGF molecules in the retina may be deleterious, as VEGF holds also some neuroprotective activities for neurons [87], and is responsible for normal development of vasculature [88]. Therefore, the regulation of transgene expression would be an important step towards a clinical application.
The first ever to evaluate Tet-regulatable control of transgene expression in the retina were McGee Sanftner et al. [89] by using a dual vector system: two AAV2/2 vectors containing either the combined TetOn (rtTA-M2 and tTS-Kid) cassette or the enhanced GFP gene under the control of the inducible minimal CMV promoter. A mix of both vectors was injected subretinally into rats, thus targeting photoreceptors and retinal pigment epithelium (RPE). The results demonstrated a successful induction and de-induction of GFP expression within 2 weeks of Dox administration or withdrawal respectively. No apparent side effects were observed. However, GFP expression was relatively low when compared to CMV driven expression, which was due to the ratio of both vectors injected (GFP containing vector and combined TetOn containing vector at 1:8) as well as the necessity of both vectors to transduce the same cell. Oral administration of Dox at very low levels (2 μM) allowed for the onset of GFP expression, which increased with the increasing dose of Dox administered.
Folliot and colleagues used a single AAV vector carrying a TetOff system to control transgene expression in the retina [90]. A recombinant AAV2/2 vector carrying the destabilised d2GFP gene under the control of the TetOff system was injected intravitreally into rats to warrant transduction of retinal ganglion cells. Regulation of d2GFP expression was very tight, with 95% of retinal GFP signal being gone within 48 h following oral administration of Dox via the drinking water. Upon Dox withdrawal, re-expression of d2GFP was slower, returning to full signal within 21 days, although it was readily detectable at 48 h.
The TetOn system was used to regulate interleukin 10 (IL-10) expression after intravitreal administration of rAAV2/2 into the rat eye [91]. IL-10 mRNA could be detected by RT-PCR in Dox-induced retinas two weeks after drug administration, while control non-induced retinas or mock-injected retinas remained undetected by RT-PCR. Upon induction of experimental autoimmune uveitis, animals that had been injected with AAV2/2.TetOn.vIL-10 and induced by Dox showed partial protection of the photoreceptors from degeneration due to the presence of transgenic IL-10, while non-induced animals or mock-injected animals developed severe uveitis with near complete loss of photoreceptors.
Our group developed a regulatory system based on the TetOn technique (rtTA-M2) that controls the expression of EPO in the eye of nonhuman primates [17], [18]. The use of EPO as reporter protein allows easy and objective quantification of transgene expression in the anterior chamber fluid, which represents an advantage over the subjective evaluation of GFP expression.
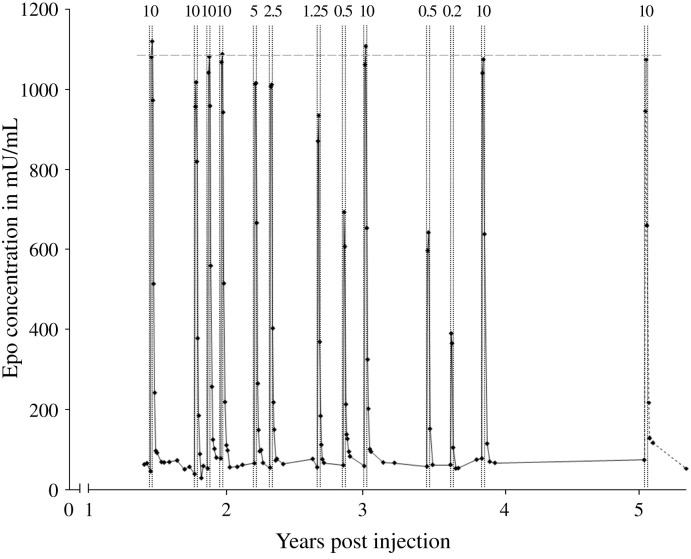
Three different vectors, AAV2/5.CMV.TetOn.epo, AAV2/4.CMV.TetOn.epo and AAV2/4.pRPE65.TetOn.epo, were injected into a total of seven primates. Induction of EPO expression was performed initially by a 3-day Dox pulse given intravenously (10 mg/kg/day). After 2–4 days post-Dox administration, EPO concentrations reached a peak and declined to background levels within 10–14 days of withdrawal of the inducer drug. Maximum EPO concentrations in the a.c.f. were 1000 mU/ml, 100 mU/ml and 10 mU/ml for animals injected with AAV2/5.CMV.TetOn.epo, AAV2/4.CMV.TetOn.epo, and AAV2/4.pRPE65.TetOn.epo vectors respectively. This shows a range of two orders of magnitude of possible concentrations for any given therapeutic protein, depending on the serotype and the amount of vector injected, and the promoter driving the expression of the rtTA-M2 protein. Regulated transgene expression is still being observed, to date that is for more than 5 years following administration of the AAV TetOn vector (Fig. 2 ). No immune response was observed either to transgene products, rtTA and EPO, or to the AAV vectors used.
Fig. 2.
Administration of Dox allows dose-dependent expression of EPO in the retina over 5 years after subretinal delivery of AAV2/5.CAG.TetOn.epo in macaque. Intravenous administration of Dox was performed by a 3-day Dox pulse (Dox-ratiopharm SF, Ratiopharm) and oral administration consisted of the daily uptake (3 days) of Dox hidden in dry fruits. Numbers above the peaks indicate the dose of Dox administered in mg/kg. Continuous graphs indicate intravenous administration of Dox and dotted graphs indicate oral administration of Dox. The horizontal dashed grey line indicates average peak EPO levels of the first 4 inductions by 10 mg/kg.
Since the possibility of oral administration of Dox is a key to considering advancing to clinical trials, we also evaluated the influence of oral administration on the capacity to control transgene expression [18]. In this study, we not only demonstrated the equally efficient and tight control of induction and de-induction of EPO expression following oral administration of Dox, but we were also able to show that the system is capable of continuously inducing transgene expression for a time period of 6 months, without eliciting any negative side effects.
Therefore, following these extensive preclinical experiments in the nonhuman primate model, we consider this regulatory system as a useful tool in the development of neurotrophic or antiangiogenic gene therapy strategies in the retina.
4.3. Regulation of transgene expression in the brain
The expression of therapeutic genes in the brain following viral vector-mediated gene transfer is a powerful option for the potential treatment of various neurological disorders, including Parkinson's disease, Huntington's disease (HD), Alzheimer's disease and brain cancer. Recent advances to identify key obstacles for successful gene therapy applications in the brain, i.e. the mode of delivery and potential immune responses to vector or transgene, raise the hope for clinical trials to be realized in the near future. However, the need for regulated transgene expression remains to be an issue in the treatment of brain disorders.
Over the last decade, a variety of strategies for the regulated expression of transgenes in the brain have been developed employing different versions of the Tet system, different reporter genes and different viral vectors. The model organism for brain directed gene transfer using such regulatory systems is until now predominantly the rat.
4.3.1. Reporter genes and proof of principle
Proof of principle of in vivo Tet regulated expression in the brain was performed using AAV vectors for the transfer of GFP under the control of an autoregulatory loop or the TetOn system, respectively [48], [50]. Adenoviral vectors were used for the transfer of GFP, LU and tyrosine hydroxylase (TH), comparing the TetOn (rtTA) and TetOff (tTA) systems side by side [92], or using the TetOn (rtTA) system [77], [93] or TetOff system alone [94]. In addition, the possibility to use lentiviral vectors for the transfer was demonstrated using the TetOff system to control GFP expression [95]. More recently, even the HSV-1 vector system has been used for the transfer of a regulatory system into the brain, in which the reporter gene lacZ was driven under the control of the TetOff system [96]. Jiang and colleagues studied the effect of the orientation of the two expression cassettes in a TetOff system within the same AAV vector following intracerebral gene transfer [97]. They observed best control of transgene expression and minimal basal leakiness with a construction, in which both promoters driving the tTA and the GFP expression were positioned at both ends of the vector in opposite directions.
In order to target specific cell populations in the brain following adenoviral vector-mediated gene transfer, the transactivator gene was placed under the control of cell specific promoters, such as synapsin I or glial fibrillary acidic protein (GFAP) for targeting neurons or glial cells, respectively [98].
Following the development of the next generation transactivator rtTA-M2, the new TetOn system was used to regulate ß-galactosidase expression following lentiviral vector-mediated gene transfer [99]. Using RT-PCR and immunostaining, the authors were able to demonstrate a tight regulation of ß-gal expression in transduced areas of the brain.
Very recently, a regulated transgene expression strategy was developed, in which the expression of the tTA transactivator in a TetOff system was placed under the control of a second enhancement system based on the Gal4-NF-κB p65 transcriptional activation system, in order to potentiate the expression of the tTA protein [100]. The authors reported high levels of transactivator production and an increased expression of red fluorescent protein (DsRed) in the rat brain.
4.3.2. Alzheimer's disease
Blesch and colleagues were among the first ever who demonstrated the regulated expression of neurotrophic factors in a brain disease model using the TetOff system to control the expression of nerve growth factor (NGF) or GFP in a rat model of Alzheimer's disease [101]. The transfer of the regulated expression cassette was performed using primary fibroblasts transduced by a retrovirus, and regulated expression was observed for 3 months. This initial study was followed by a modified experiment, in which the transfer of the regulated expression cassette was done by use of a lentivirus vector [102]. Production of NGF in the activated state and the therapeutic effect in cell rescue of cholinergic neurons was similar to animals treated with a vector that continuously expressed NGF. More recently, the same group studied the effect of regulated BDNF expression on axonal growth in a rat model of spinal cord injury [103]. Primary fibroblasts that were transduced by a retrovirus carrying a TetOn system to control BDNF or GFP expression were implanted in these rats immediately following the injury of the spinal cord. Regulation of transgene expression was observed for up to 3 months following gene transfer and production of BDNF resulted in strong axonal growth into the lesion site.
4.3.3. Huntington's disease
A rat model of Huntington's disease (HD), in which the striatal injection of quinoloic acid provoked pathologic changes reminiscent to HD pathology, especially the degeneration of striatal neurons, was used by Regulier and colleagues to study the regulated neurotrophic factor expression [104]. Following lentiviral vector-mediated gene transfer of CNTF under the control of the TetOff system, CNTF expression could be turned on and off by administering Dox via the drinking water, and striatal neurons were protected from degeneration by quinoloic acid.
4.3.4. Parkinson's disease
Different strategies have been developed for the treatment of Parkinson's disease by the use of regulated transgene expression. One possibility is the restoration of regulated dopamine production (which is inhibited in Parkinson's disease) in the striatum through temporal overexpression of tyrosine hydroxylase (TH), the rate limiting enzyme in dopamine biosynthesis. A second strategy would be the expression of neurotrophic factors, which protect dopaminergic neurons in the striatum from degeneration during the disease. For example, the neurotrophic factor GDNF has been shown to improve the phenotype of Parkinson's disease when administered over long time or following continuous expression in the brain. However, the continuous overexpression of GDNF in the brain leads to adverse effects, including the downregulation of tyrosine hydroxylase (TH) [105], [106].
The group of Vogel and colleagues used a lentiviral vector for the transfer of an expression cassette containing TH or LU controlled by the TetOn (rtTA-M2) system [107]. The authors reported an inducibility of transgene expression by two orders of magnitude after 10 days of Dox administration via the drinking water at a dose of 3 mg/ml. Another group transplanted a neuronal cell line (PC12), which was stably transfected with a TetOff system for regulated production of TH, into the striatum of rats [108]. The production of dopamine was partially controlled over a period of 2 months in this study.
In order to attempt a regulated expression of neurotrophic factors, the expression of GDNF was placed under the control of the TetOn system employing the rtTA-M2 transactivator in a two vector system [61]. To prove the feasibility of this strategy, two vectors, one encoding the reporter gene GFP and the second encoding the TetOn system, were initially injected in a 1:2 ratio into the striatum. Dox was given via the drinking water (1 mg/ml) over 4 weeks, and GFP expressing cells were found only in induced animals. Injection of two vectors expressing GDNF under control of the TetOn system resulted in a 7-fold difference of GDNF expression between non-induced and induced animals. Background levels were elevated, indicating a rather strong leakage of the system. Diluting the vector stock by 10 resulted in lower GDNF levels in the induced state, but also almost undetectable GDNF expression in the off state. Upon withdrawal of Dox from the drinking water, GDNF protein levels were completely reduced within 2 weeks and TH protein expression returned to normal within 2–8 weeks, indicating that GDNF-induced downregulation of TH is a reversible event.
The use of the TetR-KRAB system for the regulation of GDNF expression following lentiviral vector-mediated gene transfer was studied by Szulc et al. [45]. Lasting over a period of 8 weeks, they were able to turn on and off GDNF expression with a 9-fold difference following Dox administration via the drinking water (0.2 mg/ml).
Another strategy to express GDNF was developed using the autoregulatory loop to control transgene expression following injection of a single AAV vector [62], [63], [64]. Initially, it was shown, that the expression of GFP could be induced in the rat brain through the administration of Dox supplemented food which displayed an 80-fold increase in the mean total fluorescence intensity [62]. After the introduction of the GDNF cDNA into the vector and injection into the striatum, animals were continuously induced with 0.6 mg/ml Dox via the drinking water. GDNF expression was increased 12-fold in induced animals and remained undistinguishable in non-induced animals. The introduction of the WPRE sequence behind the GDNF gene did not increase protein levels in the induced state whereas basal levels were 2.5-fold higher [63]. The biological activity of the transgenic GDNF was proven by the downregulation of TH in dopaminergic terminals of neurons in induced animals. Recently, the difference in the expression pattern between expression cassettes containing the TetOn system or the CMV promoter driving GFP expression following AAV-mediated gene transfer was assessed by the group and found to be different [64].
4.3.5. Brain cancer
A widely used strategy for the treatment of cancer constitutes in the expression of suicide genes that render cancer cells susceptible to specific antitumour drugs. The combination of HSV-1-thymindine kinase (HSV-1-TK) with gancyclovir (GCV) is the most widely used system in cancer gene therapy. It has been shown that an immunotherapeutic approach that combines the cytotoxic properties of HSV-1-TK, which kills glioma cells in the presence of GCV alone or in combination with the immunostimulatory effects of FMS-like tyrosine kinase 3 ligand (Flt3L), is very efficient in eliminating large, intracranial, malignant glioblastoma in rats [109]. However, the use of first generation adenoviral vectors and the detection of an immune response against HSV-1-TK in survivors of glioma cancer demonstrated the need for a regulated expression of the transgene, particularly the “turn off” in case of a successful therapeutic outcome.
In order to meet these challenges, a strategy was developed, in which the regulation of HSV-1-TK expression is mediated by the combined TetOn strategy. The vector to be transferred into the brain parenchyma is a High Capacity adenovirus, which is devoid of all wild type adenoviral sequences and, therefore, should be less immunogenic as compared to previous adenovirus based vector systems.
Initially, ß-gal was used as a reporter gene and expressed under the control of the combined TetOn system and rtTA-M2 and tTS-Kid expressions driven either by the human (hCMV) or the mouse (mCMV) cytomegalovirus early promoter element [66]. Within 6 days of Dox administration via the drinking water, transgene expression reached to a maximum and decreased to background levels within 10–14 days of the withdrawal of Dox. As observed in other studies, the expression of ß-gal in the induced state was dependent on the dose of Dox administered. Inducibility of transgene expression was the best and background levels the lowest with the mCMV-TetOn system [66], [110]. Importantly, the regulated transgene expression was possible even in the presence of a strong systemic immune reaction against adenoviruses, highlighting the possibility to use this system in patients with prior exposure to adenovirus, a common human infection. Therefore, the authors continued to work with the murine promoter to construct an expression cassette that would control the expression of the immunostimulatory protein, Flt3L, using the HC-Ad vector system [110]. A second vector encoded the HSV-1-TK protein, which is toxic only in the presence of GCV, driven by a continuous promoter. Cytotoxic activity would be initiated by the application of GCV. After having shown the tight control of transgene expression in human glioma cells [110], the system was studied in canine glioma cells [111]. The spontaneous development of glioblastoma multiforme in a variety of dog breeds makes these animals a precious large animal model for the development of gene therapy strategies for this disease. Both HC-Ads successfully transduced dog glioma cells and exerted anti-tumoural effects in these cells in vitro. Therefore, this strategy may be a possible treatment option for glioblastoma multiforme in dogs, and subsequently in human clinical trials.
An interesting observation made by this group was that immunisation of mice against the reporter gene but not against the transactivators would elicit an immune response and subsequent loss of expression from transduced cells in the brain of mice [65].
4.3.6. Chronic pain
Chronic constriction injury (CCI) of the sciatic nerve in rats is a model for the development of antinociceptive therapies for neuropathic pain diseases, in which patients suffer from intractable pain. The overproduction of β-endorphin in the brain can reduce pain sensitivity to CCI and therefore might represent a potential therapy for patients suffering from neuropathic pain. A two-plasmid gene therapy strategy using the TetOff system to regulate expression of pro-opiomelanocortin, which is the precursor gene of β-endorphin, has been developed and tested in the rat model of CCI [112]. Expression of β-endorphin was regulated over a duration of 14 days by intraperitoneal administration of Dox; nociceptive sensitivity was reduced in rats that expressed this protein. In an attempt to develop the strategy into a more clinically relevant setting, the authors produced a three-plasmid strategy coding for the pro-opiomelanocortin gene, the rtTA transactivator and the tTS silencer [113]. The three plasmids were coelectroporated into the spinal cord of rats and the analgesic potential was measured by heat and mechanical sensitivity tests. Expression of β-endorphin was robust upon intraperitoneal Dox administration for a period of 14 days (duration of the experiment).
4.3.7. Stem cells and siRNA regulation
Another strategy for regulated expression of transgenes in the brain is the use of stably transfected embryonic stem cells, whose progeny cells express the transgene following transfer into certain organs [114]. GFP expressing stem cells, in which the expression is controlled by a TetOn system, were implanted into the brain of mice and expression was efficiently controlled by Dox administration over a period of 84 days.
Very recently, the TetOn system was used for the regulated expression of siRNA to block the expression of integrin-linked kinase (ILK) following rAAV-mediated gene transfer into the nucleus accumbens of rats [115]. This conditional knock-down of ILK expression was constructed to study the function of this kinase in normal brain structures. The use of a neuron specific promoter, synapsin 1 gene promoter, to drive rtTA-M2 expression assured highly specific neuronal transgene expression. Within two weeks of Dox administration (3 mg/kg), the expression of ILK was markedly reduced, returning to normal values within 8 days of Dox withdrawal. This study shows that conditional gene silencing can be established in distinct brain regions using rAAV delivered and TetOn-regulated siRNA expression. Such a system could potentially be useful to down-regulate the production of toxic proteins in the brain in certain CNS disorders.
4.4. Regulation of transgene expression in other organs
The possibility of regulated transgene expression was studied by several groups in the liver of rodents and primates, and in rodent models of human hematopoiesis, cancer, and cartilage tissue.
4.4.1. Liver
One strategy to treat patients with Hepatitis C is the systemic administration of the antiviral molecule interferon α (IFN-α). The treatment of this viral infection is not very effective, as the relatively short half-life of the protein in the circulation makes it difficult to obtain sufficient protein levels in the liver over a sustained period. However, expression of IFN-α in the liver following viral vector-mediated gene transfer may constitute an effective treatment option for patients with Hepatitis C. As mentioned above, an inducible gene expression system would be most appropriate in order to avoid the occurrence of potential negative side effects of the expression of such a protein over time.
The TetOn system (rtTA-S2) has been employed to control the expression of IFN-α (HD-Tet-IFN) in the liver of mice following adenoviral vector-mediated gene transfer [116]. After 3 days of Dox administration (200 μg/ml) via the drinking water, IFN-α concentrations of 4000 U/ml were observed in the serum of HD-Tet-IFN injected mice. Withdrawal of Dox from the drinking water resulted in a rapid decrease of serum IFN-α concentration to undetectable levels within 3 days. The level of IFN-α in the serum of induced mice was dependent on the dose of Dox administered. Finally, the authors tested the effect of regulated IFN-α expression on the survival rate of mice challenged with a lethal dose of liver toxic coronavirus. IFN-α expression resulted in the prolonged survival of the animals and reduced liver damage due to the activation of the expression of antiviral genes.
Following these encouraging results, the authors aimed to extrapolate their system from the mouse to nonhuman primates [16]. Monkeys of the genus Sanguinus, commonly called tamarins, have been proposed to be a surrogate model to study virus induced hepatitis after infection with a flavivirus, GB virus B (GBV-B). To study the effect of IFN-α expression following gene transfer to the liver in this model, an adenoviral vector was developed that controlled the expression of tamarin IFN-α by use of the TetOn (rtTA-S2) system. Following successful demonstration of the functionality of the system in mice and rats, the vector was injected intravenously into 4 monkeys and initially tested for the regulation of IFN-α expression in the liver. At 14 days post-injection, transgene expression was induced for 3 days by oral administration of Dox (10 mg/kg). Two of the monkeys exhibited an 8-to-16 fold increase of IFN-α serum levels, while the other two monkeys showed lower increases of IFN-α in the serum. Within 3–5 days of Dox withdrawal, serum IFN-α levels decreased to baseline levels. The inductions were repeated three times and demonstrated the reinducibility of the system. At day 42 post-injection of the adenoviral vector, IFN-α expression was induced by Dox administration and the animals were challenged with a dose of GBV-B virus. From this moment on, Dox was given continuously until day 150, the end of the experiment, to ensure local liver expression of IFN-α. Even though all animals became viraemic, the expression of IFN-α delayed significantly the onset of the disease. More importantly, all except one animal exhibited low but measurable levels of IFN-α secretion despite inflammation in the expressing organ. The authors discussed various reasons for the lack of complete protection from viral hepatitis compared to the rodent study, including the difference of the maximum level of IFN-α in the serum of induced mice (4000 fold) compared to primates (maximal 32-fold). This is an often observed discrepancy that needs to be taken into account when developing a gene therapy strategy from the mouse to large animal models.
In a different mouse model of chronic hepatitis B virus infection, the regulated expression of IFN-γ was controlled by a TetOn system and delivered systemically into mice by two adenoviral vectors [117]. Liver-specific transgene expression was assured by use of the liver-activated protein promoter (p-LAP). The authors reported low basal expression and tight regulation of IFN-γ expression upon Dox administration via the drinking water.
Hemophilia is a coagulation disorder caused to defective blood clotting factor (FIX) production. In order to treat patients with this disease, the regulated expression of the missing factor in the liver would provide a potential alternative to repeated administration of the protein or prolonged expression without regulatory system.
Vigna and colleagues reported on the regulated expression of FIX in the liver of SCID mice following systemic administration of a single lentiviral vector containing the TetOff system for regulation [118]. In this study, mice were injected with different amounts of the lentiviral vector, and regulation of FIX expression upon Dox administration via the drinking water (50 μg/ml) was observed over a period of 240 days. Another study employed an adenoviral vector for delivery and the TetOn system for control of FIX expression [119]. Upon delivery of the vector into SCID mice, the expression of FIX could be regulated by oral administration of Dox (200 μg/ml) via the drinking water for at least 2 months. The group of Mizuguchi and colleagues studied the regulated expression of an adenoviral vector containing the SEAP gene under the control of the combined TetOn system in mice [120]. In addition, the regulated expression of lacZ and phospholipase A2 (PLA2) was studied in mice following adenoviral vector injection into the lung (lung-targeted gene transfer) or intravenous administration (liver-targeted gene transfer), respectively [121].
4.4.2. Hematopoietic cells
The expression of transgenes from hematopoietic precursor cells, which can be efficiently transduced ex vivo, requires the integration of the expression cassette into the genome. Such systems could potentially be applied to regulate the expression of certain genes in defined lineages of precursor cells during hematopoiesis.
Vigna and colleagues developed a lentivirus vector based system for the regulated expression of GFP and LU in human cord blood CD34+ cells that where engrafted into sublethally irradiated NOD/SCID mice [122]. The Tet-system employed was the TetOff system and kinetics of tTA-mediated control of transgene expression were similar to what had been observed in other studies. To switch the system “off”, Dox was administered via the drinking water (200 μg/ml) and, within 8 days, transgene expression was not detectable. The “on” switch, however, took 4 weeks to completely restore transgene expression in these animals, as the slow clearance of the inducer drug defines the kinetics in this system. A second group developed a lentivirus vector, in which SEAP expression was controlled by different versions of the pure TetOn and combined TetOn system [47]. Based on their observations, the authors proposed the simple TetOn system as the most appropriate regulatory system for the control of transgene expression in the hematopoietic system.
4.4.3. Cancer
As it has been mentioned above (chapter 4.3), a widely used strategy to treat cancer is to introduce a suicide gene, such as HSV-1-TK into cancer cells and, thus, render them susceptible to antitumour drugs, such as GCV.
A strategy based on the HSV-1-TK/GCV system has been developed for the treatment of a human breast cancer model in mice [123], [124], [125]. Initially, two plasmids, the first containing the HSV-1-TK gene under control of an inducible promoter and the second containing the rtTA Transactivator, were generated in retroviral vector plasmids [123]. These two vector plasmids were co-transduced into human breast cancer cells (MCF-7) and 7 days following Dox induction, these cells were implanted into mice with severe combined immunodeficiency (SCID) bearing breast cancer xenografts. Following GCV administration, tumour cells bearing the HSV-1-TK produced toxic molecules and were subsequently eradicated from the tissue. More importantly, due to a bystander effect, which is a known effect of HSV-1-TK, adjacent cells from the implanted tumour xenograft were also arrested in their growth and tumour size remarkably decreased. In the following step, the experiments were performed using retroviral vectors based on the above-mentioned plasmids for the transfer of the regulatory system directly into tumour xenografts transplanted in nude mice [125]. Transduced cells were sensitive to GCV beginning at 14 days following retrovirus vector administration. HSV-1-TK mRNA increased significantly in tumour tissue and within 6 weeks, the tumour size decreased remarkably and the treated mice achieved long-term survival. To further adjust the treatment strategy for subsequent use in the clinics, the two-retroviral vector system was replaced by a one vector strategy using rAAV2 virus [124]. Again, direct injection of rAAV2 vectors resulted in GCV sensitivity within the tumour 14 days following vector and GCV administration. Similar results concerning tumour size and HSV-1-TK mRNA levels were observed compared to the retroviral vector study. The authors conclude that the regulated expression of HSV-1-TK together with the administration of GCV may be a useful method for the treatment of breast cancer and other solid tumours.
Another anticancer strategy employs the tumour specific expression of toxic proteins in cancer cells. Gu and colleagues designed an expression cassette, where the tTA transactivator is placed under the control of the human telomere reverse transcriptase (hTERT) promoter and controls the expression of cytotoxic protein Bax [126]. The expression cassette was encapsidated into adenoviral vectors and injected into subcutaneous xenograft tumours in mice, where tumour growth was efficiently suppressed for the period of 38 days (duration of the experiment). Adenoviral vectors carrying the TetOff or the combined TetOn regulatory systems to drive FasL-GFP expression in tumour cells have been developed by Rubinchik et al. [127].
The generation of stable cell lines, which express siRNA against certain important tumour-associated proteins under the control of the TetOn system, is a third anticancer strategy, where Tet regulated expression systems have been studied in vivo. Czauderna and colleagues developed stable human prostate cancer cells containing siRNA directed against subunits of the phosphatidylinositol 3 kinase under a modified TetOn system [128]. Another group developed a stable human colon cancer cell line in which the expression of a receptor for a pro-oncogenic protein, KILLER/DR5, is controlled by the TetOn system [129]. A third group developed HeLa cells, which contained a stably integrated expression cassette composed of the TetOn system driving siRNA expression against human polo-like kinase 1 [130].
4.4.4. Cartilage and bone tissue
Cartilage damage following acute injury or degenerative disorders of the joint are frequent and strategies aimed to regenerating damaged cartilage and bone tissue have been investigated. The expression of growth factors that would stimulate the regeneration of cartilage and bone tissue is a potential treatment method.
In order to control the expression of transgenes in the joint, Ueblacker and colleagues developed an expression cassette, in which the reporter gene, lacZ, was under the control of the TetOn system and transfected it into chondrocytes [131]. These cells were implanted into osteochondral defects in the knees of New Zealand rabbits. Following three weeks of Dox-mediated gene expression, lacZ protein could be detected in well integrated implants.
A different strategy involved the use of mesenchymal stem cells, which have been transfected with an expression cassette containing the TetOn system regulating the expression of bone morphogenic protein-2 (BMP-2), a highly potent osteoinductive protein. Upon transplantation of these cells into the radii of osteopenic mice, expression of BMP-2 could be well detected in induced animals and bone formation and regeneration was observed over a duration of up to 4 months [132]. Another group reported that even the short-term induction of BMP-2 expression following intra-articular injection of stem cells, which contained a TetOff controlled expression cassette for BMP-2, resulted in the induction of bone formation in knee tissue [133].
5. Immune response to the TetOn transactivator is observed following gene transfer in the muscle, but not following gene transfer in the retina of nonhuman primates
The Tet-regulatable system was studied by several groups in the last preclinical model, the nonhuman primate, following gene transfer to the muscle [10], [12], [13] to the retina [17], [18], or to the liver [16]. In contrast to what has been observed in the retina, where regulated transgene expression persists for more than 5 years without any apparent cellular or humoral immune response, muscle directed gene transfer exhibited a more differentiated image.
When rtTA was used to regulate the expression of macaque EPO or of SEAP from skeletal muscle, following rAAV or adenovirus-mediated gene transfer, a large majority of individuals developed an immune response to the rtTA protein [10], [12], [13] (Moullier et al. unpublished data). This immune response was responsible for the rapid loss of rtTA-mediated control of transgene expression due to the destruction of the genetically modified cells and included both reactions, humoral and cellular. Such immune responses occurred even upon using the muscle-specific desmin promoter instead of a ubiquitous promoter to drive rtTA expression (Moullier unpublished data). After the generation of novel rtTA variants, which showed an improved sensitivity to Dox and reduced background activity, it was hoped that these variants would be less immunogenic in muscle directed gene transfer.
However, when using these variants to regulate transgene expression from the muscle, an immune response to the transactivator was still detectable (Moullier et al., unpublished data). It has been demonstrated that CD8+-specific epitopes, which were identified on rtTA, resulted in vitro in lysis of rtTA-expressing cells by cytotoxic T lymphocytes. Mutation analysis of the dominant epitope led to the appearance of subdominant epitopes, suggesting that an anti-rtTA immune response could not be prevented by selective engineering of the transactivator [134].
In intramuscularly rAAV-injected nonhuman primates, lymphomonocytic infiltrates associated with myofibre destruction were documented. Phenotypic analysis of the infiltrating cells showed the presence of CD4+, CD8+ and CD86+ cells. Presence of these infiltrates was concomitant with the detection of circulating antibodies against the rtTA protein, and a quantitative reduction of the rAAV copy number at the injection sites. In addition, a weak IFN-γ secretion, specific for the rtTA protein, was detected in AAV vector injected animals using in vitro activated lymph node mononuclear cells (LNMC) [10] and in Ad vector injected animals ex vivo in fresh peripheral blood mononuclear cells (PBMC) stimulated with the rtTA polypeptide [12]. In vitro stimulated PBMC of the injected animals could also specifically lyse target cells expressing the rtTA peptides.
The reason why not all of the injected animals did mount an immune response to the rtTA transactivator remains unknown [10]. The fact that one of the animals injected did not have an immune response to rtTA and developed neutralising antibodies to the rAAV capsid (Moullier et al., unpublished data) indicated that this individual was immunocompetent for other non-self peptides and may have possibly developed a spontaneous tolerance for this particular peptide.
Factors involved in transgene-specific immune response are likely numerous, but depend mainly on the vector system used and the route of administration. For example, when naked plasmids are injected, the chemical formulation of the plasmid DNA, the physical method for injecting the DNA and the dose injected can affect the immune response profile [12]. Regarding the route of rAAV and rAd administration, the poor diffusion of the vectors following intramuscular injection is likely one of the major reasons for the development of transgene-specific immune responses [85], [135].
On the contrary, ability of the TetOn system to establish long-term transgene expression regulation was demonstrated in the retina of nonhuman primates following subretinal injection of rAAV vectors. The fact that no antibody directed against the transactivator rtTA could be detected in the eye or in the serum of these nonhuman primates, and that all animals displayed persistent regulation of EPO secretion for up to 5 years (Fig. 2) [17], [18] is consistent with the fact that, unlike skeletal muscle, the retina is likely an immune-privilege site [136].
6. Conclusion
The Tet-regulatable system has now been used in a variety of versions embedded in plasmids, rAAV, adenovirus, lentivirus or HSV vectors to explore the potential of such a system in different organs.
Most applications were experimental studies to demonstrate the feasibility of such an approach but some advanced from the small animal (rodent) model into the large animal model, i.e. the nonhuman primate. This has been in particular the case in muscle, retina or liver directed gene transfer using rAAV or HC-Ad as the transfer vector.
Important information have been derived from these regulated transgene expression studies in large animals. First, the difference between the level of transgene expression in the induced and the non-induced state is smaller in nonhuman primate compared to what is observed in rodents or even in vitro. For example in the muscle, a difference of 100–1000 fold is observed in mice while in nonhuman primate, the difference is 20-to-50 fold.
Second, immune reactions against the TetOn transactivator was detected in nonhuman primate following gene transfer in the muscle. Initial studies in mice did not reveal such immune reactions.
To advance further with these Tet-regulatable systems, several milestones need to be achieved. Concerning the muscle directed gene transfer approach, the problem of the immune reaction against the transactivator needs to be overcome. The imminent step in brain directed gene therapy studies is to extrapolate the results from the rat model to large animal models in order to evaluate, in a clinically relevant setting, the immune status and efficiency of the Tet-regulatable systems. This may soon be the case for the brain cancer study, where a canine model for glioblastoma multiforme exists. Regarding gene transfer to the retina, clinically relevant disease models should now be evaluated, using vectors in which the Tet-regulatable system controls the expression of the therapeutic transgene.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Gene Regulation for Effective Gene Therapy”.
References
- 1.Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U. S. A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gossen M., Freundlieb S., Bender G., Muller G., Hillen W., Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 3.Rivera V.M., Clackson T., Natesan S., Pollock R., Amara J.F., Keenan T., Magari S.R., Phillips T., Courage N.L., Cerasoli F., Jr., Holt D.A., Gilman M. A humanized system for pharmacologic control of gene expression. Nat. Med. 1996;2:1028–1032. doi: 10.1038/nm0996-1028. [DOI] [PubMed] [Google Scholar]
- 4.Vegeto E., Allan G.F., Schrader W.T., Tsai M.J., McDonnell D.P., O'Malley B.W. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992;69:703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- 5.Roscilli G., Rinaudo C.D., Cimino M., Sporeno E., Lamartina S., Ciliberto G., Toniatti C. Long-term and tight control of gene expression in mouse skeletal muscle by a new hybrid human transcription factor. Mol. Ther. 2002;6:653–663. [PubMed] [Google Scholar]
- 6.Karns L.R., Kisielewski A., Gulding K.M., Seraj J.M., Theodorescu D. Manipulation of gene expression by an ecdysone-inducible gene switch in tumor xenografts. BMC Biotechnol. 2001;1:11. doi: 10.1186/1472-6750-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palli S.R., Kapitskaya M.Z., Kumar M.B., Cress D.E. Improved ecdysone receptor-based inducible gene regulation system. Eur. J. Biochem. 2003;270:1308–1315. doi: 10.1046/j.1432-1033.2003.03501.x. [DOI] [PubMed] [Google Scholar]
- 8.Toniatti C., Bujard H., Cortese R., Ciliberto G. Gene therapy progress and prospects: transcription regulatory systems. Gene Ther. 2004;11:649–657. doi: 10.1038/sj.gt.3302251. [DOI] [PubMed] [Google Scholar]
- 9.Vilaboa N., Voellmy R. Regulatable gene expression systems for gene therapy. Curr. Gene Ther. 2006;6:421–438. doi: 10.2174/156652306777934829. [DOI] [PubMed] [Google Scholar]
- 10.Favre D., Blouin V., Provost N., Spisek R., Porrot F., Bohl D., Marme F., Cherel Y., Salvetti A., Hurtrel B., Heard J.M., Riviere Y., Moullier P. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J. Virol. 2002;76:11605–11611. doi: 10.1128/JVI.76.22.11605-11611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auricchio A., Rivera V.M., Clackson T., O'Connor E.E., Maguire A.M., Tolentino M.J., Bennett J., Wilson J.M. Pharmacological regulation of protein expression from adeno-associated viral vectors in the eye. Mol. Ther. 2002;6:238–242. doi: 10.1006/mthe.2002.0660. [DOI] [PubMed] [Google Scholar]
- 12.Latta-Mahieu M., Rolland M., Caillet C., Wang M., Kennel P., Mahfouz I., Loquet I., Dedieu J.F., Mahfoudi A., Trannoy E., Thuillier V. Gene transfer of a chimeric trans-activator is immunogenic and results in short-lived transgene expression. Hum. Gene Ther. 2002;13:1611–1620. doi: 10.1089/10430340260201707. [DOI] [PubMed] [Google Scholar]
- 13.Chenuaud P., Larcher T., Rabinowitz J.E., Provost N., Joussemet B., Bujard H., Samulski R.J., Favre D., Moullier P. Optimal design of a single recombinant adeno-associated virus derived from serotypes 1 and 2 to achieve more tightly regulated transgene expression from nonhuman primate muscle. Mol. Ther. 2004;9:410–418. doi: 10.1016/j.ymthe.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Lebherz C., Auricchio A., Maguire A.M., Rivera V.M., Tang W., Grant R.L., Clackson T., Bennett J., Wilson J.M. Long-term inducible gene expression in the eye via adeno-associated virus gene transfer in nonhuman primates. Hum. Gene Ther. 2005;16:178–186. doi: 10.1089/hum.2005.16.178. [DOI] [PubMed] [Google Scholar]
- 15.Rivera V.M., Gao G.P., Grant R.L., Schnell M.A., Zoltick P.W., Rozamus L.W., Clackson T., Wilson J.M. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- 16.Aurisicchio L., De Tomassi A., La Monica N., Ciliberto G., Traboni C., Palombo F. Regulated and liver-specific tamarin alpha interferon gene delivery by a helper-dependent adenoviral vector. J. Virol. 2005;79:6772–6780. doi: 10.1128/JVI.79.11.6772-6780.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stieger K., Le Meur G., Lasne F., Weber M., Deschamps J.Y., Nivard D., Mendes-Madeira A., Provost N., Martin L., Moullier P., Rolling F. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol. Ther. 2006;13:967–975. doi: 10.1016/j.ymthe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Stieger K., Mendes-Madeira A., Meur G.L., Weber M., Deschamps J.Y., Nivard D., Provost N., Moullier P., Rolling F. Oral administration of doxycycline allows tight control of transgene expression: a key step towards gene therapy of retinal diseases. Gene Ther. 2007;14:1668–1673. doi: 10.1038/sj.gt.3303034. [DOI] [PubMed] [Google Scholar]
- 19.Wissmann A., Meier I., Wray L.V., Jr., Geissendorfer M., Hillen W. Tn10 tet operator mutations affecting Tet repressor recognition. Nucleic Acids Res. 1986;14:4253–4266. doi: 10.1093/nar/14.10.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orth P., Cordes F., Schnappinger D., Hillen W., Saenger W., Hinrichs W. Conformational changes of the Tet repressor induced by tetracycline trapping. J. Mol. Biol. 1998;279:439–447. doi: 10.1006/jmbi.1998.1775. [DOI] [PubMed] [Google Scholar]
- 21.Deuschle U., Meyer W.K., Thiesen H.J. Tetracycline-reversible silencing of eukaryotic promoters. Mol. Cell. Biol. 1995;15:1907–1914. doi: 10.1128/mcb.15.4.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furth P.A., St Onge L., Boger H., Gruss P., Gossen M., Kistner A., Bujard H., Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc. Natl. Acad. Sci. U. S. A. 1994;91:9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron U., Gossen M., Bujard H. Tetracycline-controlled transcription in eukaryotes: novel transactivators with graded transactivation potential. Nucleic Acids Res. 1997;25:2723–2729. doi: 10.1093/nar/25.14.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kistner A., Gossen M., Zimmermann F., Jerecic J., Ullmer C., Lubbert H., Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baron U., Bujard H. Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods. Enzymol. 2000;327:401–421. doi: 10.1016/s0076-6879(00)27292-3. [DOI] [PubMed] [Google Scholar]
- 26.Urlinger S., Baron U., Thellmann M., Hasan M.T., Bujard H., Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamartina S., Roscilli G., Rinaudo C.D., Sporeno E., Silvi L., Hillen W., Bujard H., Cortese R., Ciliberto G., Toniatti C. Stringent control of gene expression in vivo by using novel doxycycline-dependent trans-activators. Hum. Gene Ther. 2002;13:199–210. doi: 10.1089/10430340252769734. [DOI] [PubMed] [Google Scholar]
- 28.Favre D., Provost N., Blouin V., Blancho G., Cherel Y., Salvetti A., Moullier P. Immediate and long-term safety of recombinant adeno-associated virus injection into the nonhuman primate muscle. Mol. Ther. 2001;4:559–566. doi: 10.1006/mthe.2001.0494. [DOI] [PubMed] [Google Scholar]
- 29.Bellefroid E.J., Poncelet D.A., Lecocq P.J., Revelant O., Martial J.A. The evolutionarily conserved Kruppel-associated box domain defines a subfamily of eukaryotic multifingered proteins. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3608–3612. doi: 10.1073/pnas.88.9.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margolin J.F., Friedman J.R., Meyer W.K., Vissing H., Thiesen H.J., Rauscher F.J., III Kruppel-associated boxes are potent transcriptional repression domains. Proc. Natl. Acad. Sci. U. S. A. 1994;91:4509–4513. doi: 10.1073/pnas.91.10.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witzgall R., O'Leary E., Leaf A., Onaldi D., Bonventre J.V. The Kruppel-associated box-A (KRAB-A) domain of zinc finger proteins mediates transcriptional repression. Proc. Natl. Acad. Sci. U. S. A. 1994;91:4514–4518. doi: 10.1073/pnas.91.10.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witzgall R., Volk R., Yeung R.S., Bonventre J.V. Genomic structure and chromosomal location of the rat gene encoding the zinc finger transcription factor Kid-1. Genomics. 1994;20:203–209. doi: 10.1006/geno.1994.1154. [DOI] [PubMed] [Google Scholar]
- 33.Senatore B., Cafieri A., Di Marino I., Rosati M., Di Nocera P.P., Grimaldi G. A variety of RNA polymerases II and III-dependent promoter classes is repressed by factors containing the Krüppel-associated/finger preceding box of zinc finger proteins. Gene. 1999;234:381–394. doi: 10.1016/s0378-1119(99)00182-1. [DOI] [PubMed] [Google Scholar]
- 34.Ryan R.F., Schultz D.C., Ayyanathan K., Singh P.B., Friedman J.R., Fredericks W.J., Rauscher F.J., III KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: a potential role for Krüppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol. Cell. Biol. 1999;19:4366–4378. doi: 10.1128/mcb.19.6.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pengue G., Lania L. Krüppel-associated box-mediated repression of RNA polymerase II promoters is influenced by the arrangement of basal promoter elements. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1015–1020. doi: 10.1073/pnas.93.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuda E., Agata Y., Sugai M., Katakai T., Gonda H., Shimizu A. Targeting of Krüppel-associated box-containing zinc finger proteins to centromeric heterochromatin. Implication for the gene silencing mechanisms. J. Biol. Chem. 2001;276:14222–14229. doi: 10.1074/jbc.M010663200. [DOI] [PubMed] [Google Scholar]
- 37.Urrutia R. KRAB-containing zinc-finger repressor proteins. Genome Biol. 2003;4:231. doi: 10.1186/gb-2003-4-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sripathy S.P., Stevens J., Schultz D.C. The KAP1 corepressor functions to coordinate the assembly of de novo HP1-demarcated microenvironments of heterochromatin required for KRAB zinc finger protein-mediated transcriptional repression. Mol. Cell. Biol. 2006;26:8623–8638. doi: 10.1128/MCB.00487-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz D.C., Ayyanathan K., Negorev D., Maul G.G., Rauscher F.J., III SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 2002;16:919–932. doi: 10.1101/gad.973302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz D.C., Friedman J.R., Rauscher F.J., III Targeting histone deacetylase complexes via KRAB-zinc finger proteins: the PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2alpha subunit of NuRD. Genes Dev. 2001;15:428–443. doi: 10.1101/gad.869501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiznerowicz M., Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 2003;77:8957–8961. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rittner K., Schultz H., Pavirani A., Mehtali M. Conditional repression of the E2 transcription unit in E1–E3-deleted adenoviral vectors is correlated with a strong reduction in viral DNA replication and late gene expression in vitro. J. Virol. 1997;71:3307–3311. doi: 10.1128/jvi.71.4.3307-3311.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H., Cao H., Wohlfahrt M., Kiem H.P., Lieber A. Tightly regulated gene expression in human Haematopoietic stem cells after transduction with helper-dependent Ad5/35 vectors. Exp. Hematol. 2008;36:823–831. doi: 10.1016/j.exphem.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi C., Gibellini D., Barbanti-Brodano G., Betti M., Boarini C., Pengue G., Lania L., Caputo A. Transiently transfected and stably integrated HIV-1 LTR responds differentially to the silencing activity of the Krüppel-associated box (KRAB) transcriptional repressor domain. J. Med. Virol. 1999;58:264–272. doi: 10.1002/(sici)1096-9071(199907)58:3<264::aid-jmv12>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 45.Szulc J., Wiznerowicz M., Sauvain M.O., Trono D., Aebischer P. A versatile tool for conditional gene expression and knockdown. Nat. Methods. 2006;3:109–116. doi: 10.1038/nmeth846. [DOI] [PubMed] [Google Scholar]
- 46.Freundlieb S., Schirra-Muller C., Bujard H. A tetracycline controlled activation/repression system with increased potential for gene transfer into mammalian cells. J. Gene Med. 1999;1:4–12. doi: 10.1002/(SICI)1521-2254(199901/02)1:1<4::AID-JGM4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 47.Barde I., Zanta-Boussif M.A., Paisant S., Leboeuf M., Rameau P., Delenda C., Danos O. Efficient control of gene expression in the Haematopoietic system using a single Tet-on inducible lentiviral vector. Mol. Ther. 2006;13:382–390. doi: 10.1016/j.ymthe.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Haberman R.P., McCown T.J., Samulski R.J. Inducible long-term gene expression in brain with adeno-associated virus gene transfer. Gene Ther. 1998;5:1604–1611. doi: 10.1038/sj.gt.3300782. [DOI] [PubMed] [Google Scholar]
- 49.Sommer B., Rinsch C., Payen E., Dalle B., Schneider B., Deglon N., Henri A., Beuzard Y., Aebischer P. Long-term doxycycline-regulated secretion of erythropoietin by encapsulated myoblasts. Mol. Ther. 2002;6:155–161. doi: 10.1006/mthe.2002.0646. [DOI] [PubMed] [Google Scholar]
- 50.Fitzsimons H.L., McKenzie J.M., During M.J. Insulators coupled to a minimal bidirectional tet cassette for tight regulation of rAAV-mediated gene transfer in the mammalian brain. Gene Ther. 2001;8:1675–1681. doi: 10.1038/sj.gt.3301582. [DOI] [PubMed] [Google Scholar]
- 51.Gould D.J., Berenstein M., Dreja H., Ledda F., Podhajcer O.L., Chernajovsky Y. A novel doxycycline inducible autoregulatory plasmid which displays “on”/“off” regulation suited to gene therapy applications. Gene Ther. 2000;7:2061–2070. doi: 10.1038/sj.gt.3301354. [DOI] [PubMed] [Google Scholar]
- 52.Markusic D., Oude-Elferink R., Das A.T., Berkhout B., Seppen J. Comparison of single regulated lentiviral vectors with rtTA expression driven by an autoregulatory loop or a constitutive promoter. Nucleic Acids Res. 2005;33:e63. doi: 10.1093/nar/gni062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agwuh K.N., MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006;58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- 54.Ljostad U., Skogvoll E., Eikeland R., Midgard R., Skarpaas T., Berg A., Mygland A. Oral doxycycline versus intravenous ceftriaxone for European Lyme neuroborreliosis: a multicentre, non-inferiority, double-blind, randomised trial. Lancet Neurol. 2008;7:690–695. doi: 10.1016/S1474-4422(08)70119-4. [DOI] [PubMed] [Google Scholar]
- 55.Nosten F., McGready R., d'Alessandro U., Bonell A., Verhoeff F., Menendez C., Mutabingwa T., Brabin B. Antimalarial drugs in pregnancy: a review. Curr. Drug Saf. 2006;1:1–15. doi: 10.2174/157488606775252584. [DOI] [PubMed] [Google Scholar]
- 56.Powell J.P., Wenzel R.P. Antibiotic options for treating community-acquired MRSA. Expert Rev. Anti Infect. Ther. 2008;6:299–307. doi: 10.1586/14787210.6.3.299. [DOI] [PubMed] [Google Scholar]
- 57.Berman B., Perez O.A., Zell D. Update on rosacea and anti-inflammatory-dose doxycycline. Drugs Today (Barc.) 2007;43:27–34. doi: 10.1358/dot.2007.43.1.1025697. [DOI] [PubMed] [Google Scholar]
- 58.Berman B., Zell D. Subantimicrobial dose doxycycline: a unique treatment for rosacea. Cutis. 2005;75:19–24. [PubMed] [Google Scholar]
- 59.Sloan B., Scheinfeld N. The use and safety of doxycycline hyclate and other second-generation tetracyclines. Expert Opin. Drug Saf. 2008;7:571–577. doi: 10.1517/14740338.7.5.571. [DOI] [PubMed] [Google Scholar]
- 60.Eger K., Hermes M., Uhlemann K., Rodewald S., Ortwein J., Brulport M., Bauer A.W., Schormann W., Lupatsch F., Schiffer I.B., Heimerdinger C.K., Gebhard S., Spangenberg C., Prawitt D., Trost T., Zabel B., Sauer C., Tanner B., Kolbl H., Krugel U., Franke H., Illes P., Madaj-Sterba P., Bockamp E.O., Beckers T., Hengstler J.G. 4-Epidoxycycline: an alternative to doxycycline to control gene expression in conditional mouse models. Biochem. Biophys. Res. Commun. 2004;323:979–986. doi: 10.1016/j.bbrc.2004.08.187. [DOI] [PubMed] [Google Scholar]
- 61.Georgievska B., Jakobsson J., Persson E., Ericson C., Kirik D., Lundberg C. Regulated delivery of glial cell line-derived neurotrophic factor into rat striatum, using a tetracycline-dependent lentiviral vector. Hum. Gene Ther. 2004;15:934–944. doi: 10.1089/hum.2004.15.934. [DOI] [PubMed] [Google Scholar]
- 62.Chtarto A., Bender H.U., Hanemann C.O., Kemp T., Lehtonen E., Levivier M., Brotchi J., Velu T., Tenenbaum L. Tetracycline-inducible transgene expression mediated by a single AAV vector. Gene Ther. 2003;10:84–94. doi: 10.1038/sj.gt.3301838. [DOI] [PubMed] [Google Scholar]
- 63.Chtarto A., Yang X., Bockstael O., Melas C., Blum D., Lehtonen E., Abeloos L., Jaspar J.M., Levivier M., Brotchi J., Velu T., Tenenbaum L. Controlled delivery of glial cell line-derived neurotrophic factor by a single tetracycline-inducible AAV vector. Exp. Neurol. 2007;204:387–399. doi: 10.1016/j.expneurol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 64.Bockstael O., Chtarto A., Wakkinen J., Yang X., Melas C., Levivier M., Brotchi J., Tenenbaum L. Differential transgene expression profiles from rAAV2/1 vectors using the TetOn and CMV promoters in the rat brain. Hum. Gene Ther. 2008;2:2. doi: 10.1089/hum.2008.099. [DOI] [PubMed] [Google Scholar]
- 65.Xiong W., Candolfi M., Kroeger K.M., Puntel M., Mondkar S., Larocque D., Liu C., Curtin J.F., Palmer D., Ng P., Lowenstein P.R., Castro M.G. Immunization against the transgene but not the TetOn switch reduces expression from gutless adenoviral vectors in the brain. Mol. Ther. 2008;16:343–351. doi: 10.1038/sj.mt.6300375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiong W., Goverdhana S., Sciascia S.A., Candolfi M., Zirger J.M., Barcia C., Curtin J.F., King G.D., Jaita G., Liu C., Kroeger K., Agadjanian H., Medina-Kauwe L., Palmer D., Ng P., Lowenstein P.R., Castro M.G. Regulatable gutless adenoviral vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J. Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fishman G.I., Kaplan M.L., Buttrick P.M. Tetracycline-regulated cardiac gene expression in vivo. J. Clin. Invest. 1994;93:1864–1868. doi: 10.1172/JCI117174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rendahl K.G., Leff S.E., Otten G.R., Spratt S.K., Bohl D., Van Roey M., Donahue B.A., Cohen L.K., Mandel R.J., Danos O., Snyder R.O. Regulation of gene expression in vivo following transduction by two separate rAAV vectors. Nat. Biotechnol. 1998;16:757–761. doi: 10.1038/nbt0898-757. [DOI] [PubMed] [Google Scholar]
- 69.Bohl D., Naffakh N., Heard J.M. Long-term control of erythropoietin secretion by doxycycline in mice transplanted with engineered primary myoblasts. Nat. Med. 1997;3:299–305. doi: 10.1038/nm0397-299. [DOI] [PubMed] [Google Scholar]
- 70.Bohl D., Salvetti A., Moullier P., Heard J.M. Control of erythropoietin delivery by doxycycline in mice after intramuscular injection of adeno-associated vector. Blood. 1998;92:1512–1517. [PubMed] [Google Scholar]
- 71.Serguera C., Bohl D., Rolland E., Prevost P., Heard J.M. Control of erythropoietin secretion by doxycycline or mifepristone in mice bearing polymer-encapsulated engineered cells. Hum. Gene Ther. 1999;10:375–383. doi: 10.1089/10430349950018823. [DOI] [PubMed] [Google Scholar]
- 72.Samakoglu S., Bohl D., Heard J.M. Mechanisms leading to sustained reversion of beta-thalassemia in mice by doxycycline-controlled EPO delivery from muscles. Mol. Ther. 2002;6:793–803. doi: 10.1006/mthe.2002.0810. [DOI] [PubMed] [Google Scholar]
- 73.Rendahl K.G., Quiroz D., Ladner M., Coyne M., Seltzer J., Manning W.C., Escobedo J.A. Tightly regulated long-term erythropoietin expression in vivo using tet-inducible recombinant adeno-associated viral vectors. Hum. Gene Ther. 2002;13:335–342. doi: 10.1089/10430340252769842. [DOI] [PubMed] [Google Scholar]
- 74.Lamartina S., Silvi L., Roscilli G., Casimiro D., Simon A.J., Davies M.E., Shiver J.W., Rinaudo C.D., Zampaglione I., Fattori E., Colloca S., Gonzalez Paz O., Laufer R., Bujard H., Cortese R., Ciliberto G., Toniatti C. Construction of an rtTA2(s)-m2/tts(kid)-based transcription regulatory switch that displays no basal activity, good inducibility, and high responsiveness to doxycycline in mice and non-human primates. Mol. Ther. 2003;7:271–280. doi: 10.1016/s1525-0016(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 75.Lena A.M., Giannetti P., Sporeno E., Ciliberto G., Savino R. Immune responses against tetracycline-dependent transactivators affect long-term expression of mouse erythropoietin delivered by a helper-dependent adenoviral vector. J. Gene Med. 2005;7:1086–1096. doi: 10.1002/jgm.758. [DOI] [PubMed] [Google Scholar]
- 76.Salucci V., Scarito A., Aurisicchio L., Lamartina S., Nicolaus G., Giampaoli S., Gonzalez-Paz O., Toniatti C., Bujard H., Hillen W., Ciliberto G., Palombo F. Tight control of gene expression by a helper-dependent adenoviral vector carrying the rtTA2(s)-M2 tetracycline transactivator and repressor system. Gene Ther. 2002;9:1415–1421. doi: 10.1038/sj.gt.3301813. [DOI] [PubMed] [Google Scholar]
- 77.Fender P., Jeanson L., Ivanov M.A., Colin P., Mallet J., Dedieu J.F., Latta-Mahieu M. Controlled transgene expression by E1–E4-defective adenoviral vectors harbouring a “Tet-on” switch system. J. Gene Med. 2002;4:668–675. doi: 10.1002/jgm.315. [DOI] [PubMed] [Google Scholar]
- 78.Sander A., Guth A., Brenner H.R., Witzemann V. Gene transfer into individual muscle fibres and conditional gene expression in living animals. Cell Tissue Res. 2000;301:397–403. doi: 10.1007/s004410000247. [DOI] [PubMed] [Google Scholar]
- 79.Perez N., Plence P., Millet V., Greuet D., Minot C., Noel D., Danos O., Jorgensen C., Apparailly F. Tetracycline transcriptional silencer tightly controls transgene expression after in vivo intramuscular electrotransfer: application to interleukin 10 therapy in experimental arthritis. Hum. Gene Ther. 2002;13:2161–2172. doi: 10.1089/104303402320987851. [DOI] [PubMed] [Google Scholar]
- 80.Apparailly F., Millet V., Noel D., Jacquet C., Sany J., Jorgensen C. Tetracycline-inducible interleukin-10 gene transfer mediated by an adeno-associated virus: application to experimental arthritis. Hum. Gene Ther. 2002;13:1179–1188. doi: 10.1089/104303402320138961. [DOI] [PubMed] [Google Scholar]
- 81.Dhawan J., Rando T.A., Elson S.L., Bujard H., Blau H.M. Tetracycline-regulated gene expression following direct gene transfer into mouse skeletal muscle. Somat. Cell Mol. Genet. 1995;21:233–240. doi: 10.1007/BF02255778. [DOI] [PubMed] [Google Scholar]
- 82.Goukassian D., Diez-Juan A., Asahara T., Schratzberger P., Silver M., Murayama T., Isner J.M., Andres V. Overexpression of p27(Kip1) by doxycycline-regulated adenoviral vectors inhibits endothelial cell proliferation and migration and impairs angiogenesis. FASEB J. 2001;15:1877–1885. doi: 10.1096/fj.01-0065com. [DOI] [PubMed] [Google Scholar]
- 83.Martel-Renoir D., Trochon-Joseph V., Galaup A., Bouquet C., Griscelli F., Opolon P., Opolon D., Connault E., Mir L., Perricaudet M. Coelectrotransfer to skeletal muscle of three plasmids coding for antiangiogenic factors and regulatory factors of the tetracycline-inducible system: tightly regulated expression, inhibition of transplanted tumor growth, and antimetastatic effect. Mol. Ther. 2003;8:425–433. doi: 10.1016/s1525-0016(03)00201-6. [DOI] [PubMed] [Google Scholar]
- 84.Agha-Mohammadi S., O'Malley M., Etemad A., Wang Z., Xiao X., Lotze M.T. Second-generation tetracycline-regulatable promoter: repositioned tet operator elements optimize transactivator synergy while shorter minimal promoter offers tight basal leakiness. J. Gene Med. 2004;6:817–828. doi: 10.1002/jgm.566. [DOI] [PubMed] [Google Scholar]
- 85.Le Guiner C., Stieger K., Snyder R.O., Rolling F., Moullier P. Immune responses to gene product of inducible promoters. Curr. Gene Ther. 2007;7:334–346. doi: 10.2174/156652307782151461. [DOI] [PubMed] [Google Scholar]
- 86.Wen R., Song Y., Kjellstrom S., Tanikawa A., Liu Y., Li Y., Zhao L., Bush R.A., Laties A.M., Sieving P.A. Regulation of rod phototransduction machinery by ciliary neurotrophic factor. J. Neurosci. 2006;26:13523–13530. doi: 10.1523/JNEUROSCI.4021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oosthuyse B., Moons L., Storkebaum E., Beck H., Nuyens D., Brusselmans K., Van Dorpe J., Hellings P., Gorselink M., Heymans S., Theilmeier G., Dewerchin M., Laudenbach V., Vermylen P., Raat H., Acker T., Vleminckx V., Van Den Bosch L., Cashman N., Fujisawa H., Drost M.R., Sciot R., Bruyninckx F., Hicklin D.J., Ince C., Gressens P., Lupu F., Plate K.H., Robberecht W., Herbert J.M., Collen D., Carmeliet P. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 88.Campochiaro P.A. Molecular targets for retinal vascular diseases. J. Cell. Physiol. 2007;210:575–581. doi: 10.1002/jcp.20893. [DOI] [PubMed] [Google Scholar]
- 89.McGee Sanftner L.H., Rendahl K.G., Quiroz D., Coyne M., Ladner M., Manning W.C., Flannery J.G. Recombinant AAV-mediated delivery of a Tet-inducible reporter gene to the rat retina. Mol. Ther. 2001;3:688–696. doi: 10.1006/mthe.2001.0308. [DOI] [PubMed] [Google Scholar]
- 90.Folliot S., Briot D., Conrath H., Provost N., Cherel Y., Moullier P., Rolling F. Sustained tetracycline-regulated transgene expression in vivo in rat retinal ganglion cells using a single type 2 adeno-associated viral vector. J. Gene Med. 2003;5:493–501. doi: 10.1002/jgm.367. [DOI] [PubMed] [Google Scholar]
- 91.Smith J.R., Verwaerde C., Rolling F., Naud M.C., Delanoye A., Thillaye-Goldenberg B., Apparailly F., De Kozak Y. Tetracycline-inducible viral interleukin-10 intraocular gene transfer, using adeno-associated virus in experimental autoimmune uveoretinitis. Hum. Gene Ther. 2005;16:1037–1046. doi: 10.1089/hum.2005.16.1037. [DOI] [PubMed] [Google Scholar]
- 92.Harding T.C., Geddes B.J., Murphy D., Knight D., Uney J.B. Switching transgene expression in the brain using an adenoviral tetracycline-regulatable system. Nat. Biotechnol. 1998;16:553–555. doi: 10.1038/nbt0698-553. [DOI] [PubMed] [Google Scholar]
- 93.Corti O., Sanchez-Capelo A., Colin P., Hanoun N., Hamon M., Mallet J. Long-term doxycycline-controlled expression of human tyrosine hydroxylase after direct adenovirus-mediated gene transfer to a rat model of Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12120–12125. doi: 10.1073/pnas.96.21.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith-Arica J.R., Morelli A.E., Larregina A.T., Smith J., Lowenstein P.R., Castro M.G. Cell-type-specific and regulatable transgenesis in the adult brain: adenovirus-encoded combined transcriptional targeting and inducible transgene expression. Mol. Ther. 2000;2:579–587. doi: 10.1006/mthe.2000.0215. [DOI] [PubMed] [Google Scholar]
- 95.Kafri T., van Praag H., Gage F.H., Verma I.M. Lentiviral vectors: regulated gene expression. Mol. Ther. 2000;1:516–521. doi: 10.1006/mthe.2000.0083. [DOI] [PubMed] [Google Scholar]
- 96.Yao F., Theopold C., Hoeller D., Bleiziffer O., Lu Z. Highly efficient regulation of gene expression by tetracycline in a replication-defective herpes simplex viral vector. Mol. Ther. 2006;13:1133–1141. doi: 10.1016/j.ymthe.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 97.Jiang L., Rampalli S., George D., Press C., Bremer E.G., O'Gorman M.R., Bohn M.C. Tight regulation from a single Tet-off rAAV vector as demonstrated by flow cytometry and quantitative, real-time PCR. Gene Ther. 2004;11:1057–1067. doi: 10.1038/sj.gt.3302245. [DOI] [PubMed] [Google Scholar]
- 98.Ralph G.S., Bienemann A., Harding T.C., Hopton M., Henley J., Uney J.B. Targeting of tetracycline-regulatable transgene expression specifically to neuronal and glial cell populations using adenoviral vectors. NeuroReport. 2000;11:2051–2055. doi: 10.1097/00001756-200006260-00048. [DOI] [PubMed] [Google Scholar]
- 99.Koponen J.K., Kankkonen H., Kannasto J., Wirth T., Hillen W., Bujard H., Yla-Herttuala S. Doxycycline-regulated lentiviral vector system with a novel reverse transactivator rtTA2S-M2 shows a tight control of gene expression in vitro and in vivo. Gene Ther. 2003;10:459–466. doi: 10.1038/sj.gt.3301889. [DOI] [PubMed] [Google Scholar]
- 100.Liu B., Wang S., Brenner M., Paton J.F., Kasparov S. Enhancement of cell-specific transgene expression from a Tet-Off regulatory system using a transcriptional amplification strategy in the rat brain. J. Gene Med. 2008;10:583–592. doi: 10.1002/jgm.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blesch A., Conner J.M., Tuszynski M.H. Modulation of neuronal survival and axonal growth in vivo by tetracycline-regulated neurotrophin expression. Gene Ther. 2001;8:954–960. doi: 10.1038/sj.gt.3301480. [DOI] [PubMed] [Google Scholar]
- 102.Blesch A., Conner J., Pfeifer A., Gasmi M., Ramirez A., Britton W., Alfa R., Verma I., Tuszynski M.H. Regulated lentiviral NGF gene transfer controls rescue of medial septal cholinergic neurons. Mol. Ther. 2005;11:916–925. doi: 10.1016/j.ymthe.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 103.Blesch A., Tuszynski M.H. Transient growth factor delivery sustains regenerated axons after spinal cord injury. J. Neurosci. 2007;27:10535–10545. doi: 10.1523/JNEUROSCI.1903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Regulier E., Pereira de Almeida L., Sommer B., Aebischer P., Deglon N. Dose-dependent neuroprotective effect of ciliary neurotrophic factor delivered via tetracycline-regulated lentiviral vectors in the quinolinic acid rat model of Huntington's disease. Hum. Gene Ther. 2002;13:1981–1990. doi: 10.1089/10430340260355383. [DOI] [PubMed] [Google Scholar]
- 105.Georgievska B., Kirik D., Bjorklund A. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J. Neurosci. 2004;24:6437–6445. doi: 10.1523/JNEUROSCI.1122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Georgievska B., Kirik D., Rosenblad C., Lundberg C., Bjorklund A. Neuroprotection in the rat Parkinson model by intrastriatal GDNF gene transfer using a lentiviral vector. NeuroReport. 2002;13:75–82. doi: 10.1097/00001756-200201210-00019. [DOI] [PubMed] [Google Scholar]
- 107.Vogel R., Amar L., Thi A.D., Saillour P., Mallet J. A single lentivirus vector mediates doxycycline-regulated expression of transgenes in the brain. Hum. Gene Ther. 2004;15:157–165. doi: 10.1089/104303404772679968. [DOI] [PubMed] [Google Scholar]
- 108.Kobayashi K., Yasuhara T., Agari T., Muraoka K., Kameda M., Ji Yuan W., Hayase H., Matsui T., Miyoshi Y., Shingo T., Date I. Control of dopamine-secretion by Tet-Off system in an in vivo model of parkinsonian rat. Brain Res. 2006;1102:1–11. doi: 10.1016/j.brainres.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 109.Dewey R.A., Morrissey G., Cowsill C.M., Stone D., Bolognani F., Dodd N.J., Southgate T.D., Klatzmann D., Lassmann H., Castro M.G., Lowenstein P.R. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat. Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 110.Candolfi M., Curtin J.F., Xiong W.D., Kroeger K.M., Liu C., Rentsendorj A., Agadjanian H., Medina-Kauwe L., Palmer D., Ng P., Lowenstein P.R., Castro M.G. Effective high-capacity gutless adenoviral vectors mediate transgene expression in human glioma cells. Mol. Ther. 2006;14:371–381. doi: 10.1016/j.ymthe.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Candolfi M., Pluhar G.E., Kroeger K., Puntel M., Curtin J., Barcia C., Muhammad A.K., Xiong W., Liu C., Mondkar S., Kuoy W., Kang T., McNeil E.A., Freese A.B., Ohlfest J.R., Moore P., Palmer D., Ng P., Young J.D., Lowenstein P.R., Castro M.G. Optimization of adenoviral vector-mediated transgene expression in the canine brain in vivo, and in canine glioma cells in vitro. Neuro Oncol. 2007;9:245–258. doi: 10.1215/15228517-2007-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu C.M., Lin M.W., Cheng J.T., Wang Y.M., Huang Y.W., Sun W.Z., Lin C.R. Regulated, electroporation-mediated delivery of pro-opiomelanocortin gene suppresses chronic constriction injury-induced neuropathic pain in rats. Gene Ther. 2004;11:933–940. doi: 10.1038/sj.gt.3302244. [DOI] [PubMed] [Google Scholar]
- 113.Chen K.H., Wu C.H., Tseng C.C., Shiau J.M., Lee C.T., Lin C.R. Intrathecal coelectrotransfer of a tetracycline-inducible, three-plasmid-based system to achieve tightly regulated antinociceptive gene therapy for mononeuropathic rats. J. Gene Med. 2008;10:208–216. doi: 10.1002/jgm.1132. [DOI] [PubMed] [Google Scholar]
- 114.Xia X., Ayala M., Thiede B.R., Zhang S.C. In vitro- and in vivo-induced transgene expression in human embryonic stem cells and derivatives. Stem Cells. 2008;26:525–533. doi: 10.1634/stemcells.2007-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Q., Xiong X., Lee T.H., Liu Y., Sun Q.A., Wetsel W., Zhang X. Adeno-associated virus-mediated ILK gene silencing in the rat NAc core. J. Neurosci. Methods. 2008;173:208–214. doi: 10.1016/j.jneumeth.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 116.Aurisicchio L., Bujard H., Hillen W., Cortese R., Ciliberto G., La Monica N., Palombo F. Regulated and prolonged expression of mIFN(alpha) in immunocompetent mice mediated by a helper-dependent adenoviral vector. Gene Ther. 2001;8:1817–1825. doi: 10.1038/sj.gt.3301596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dumortier J., Schonig K., Oberwinkler H., Low R., Giese T., Bujard H., Schirmacher P., Protzer U. Liver-specific expression of interferon gamma following adenoviral gene transfer controls hepatitis B virus replication in mice. Gene Ther. 2005;12:668–677. doi: 10.1038/sj.gt.3302449. [DOI] [PubMed] [Google Scholar]
- 118.Vigna E., Amendola M., Benedicenti F., Simmons A.D., Follenzi A., Naldini L. Efficient Tet-dependent expression of human factor IX in vivo by a new self-regulating lentiviral vector. Mol. Ther. 2005;11:763–775. doi: 10.1016/j.ymthe.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 119.Srour M.A., Fechner H., Wang X., Siemetzki U., Albert T., Oldenburg J., Hanfland P., Poller W., Brackmann H.H., Schwaab R. Regulation of human factor IX expression using doxycycline-inducible gene expression system. Thromb. Haemost. 2003;90:398–405. doi: 10.1160/TH03-01-0010. [DOI] [PubMed] [Google Scholar]
- 120.Mizuguchi H., Xu Z.L., Sakurai F., Mayumi T., Hayakawa T. Tight positive regulation of transgene expression by a single adenoviral vector containing the rtTA and tTS expression cassettes in separate genome regions. Hum. Gene Ther. 2003;14:1265–1277. doi: 10.1089/104303403767740803. [DOI] [PubMed] [Google Scholar]
- 121.Tietge U.J., Kozarsky K.F., Donahee M.H., Rader D.J. A tetracycline-regulated adenoviral expression system for in vivo delivery of transgenes to lung and liver. J. Gene Med. 2003;5:567–575. doi: 10.1002/jgm.384. [DOI] [PubMed] [Google Scholar]
- 122.Vigna E., Cavalieri S., Ailles L., Geuna M., Loew R., Bujard H., Naldini L. Robust and efficient regulation of transgene expression in vivo by improved tetracycline-dependent lentiviral vectors. Mol. Ther. 2002;5:252–261. doi: 10.1006/mthe.2002.0542. [DOI] [PubMed] [Google Scholar]
- 123.Hu W.X., Zeng Z.J., Luo S.Q., Chen Q. Suicide gene therapy of human breast cancer in SCID mice model by the regulation of Tet-On. Chin. Med. J. (Engl.) 2004;117:434–439. [PubMed] [Google Scholar]
- 124.Li Z.B., Zeng Z.J., Chen Q., Luo S.Q., Hu W.X. Recombinant AAV-mediated HSVtk gene transfer with direct intratumoral injections and Tet-On regulation for implanted human breast cancer. BMC Cancer. 2006;6:66. doi: 10.1186/1471-2407-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zeng Z.J., Li Z.B., Luo S.Q., Hu W.X. Retrovirus-mediated tk gene therapy of implanted human breast cancer in nude mice under the regulation of Tet-On. Cancer Gene Ther. 2006;13:290–297. doi: 10.1038/sj.cgt.7700889. [DOI] [PubMed] [Google Scholar]
- 126.Gu J., Zhang L., Huang X., Lin T., Yin M., Xu K., Ji L., Roth J.A., Fang B. A novel single tetracycline-regulative adenoviral vector for tumor-specific Bax gene expression and cell killing in vitro and in vivo. Oncogene. 2002;21:4757–4764. doi: 10.1038/sj.onc.1205582. [DOI] [PubMed] [Google Scholar]
- 127.Rubinchik S., Woraratanadharm J., Yu H., Dong J.Y. New complex Ad vectors incorporating both rtTA and tTS deliver tightly regulated transgene expression both in vitro and in vivo. Gene Ther. 2005;12:504–511. doi: 10.1038/sj.gt.3302437. [DOI] [PubMed] [Google Scholar]
- 128.Czauderna F., Santel A., Hinz M., Fechtner M., Durieux B., Fisch G., Leenders F., Arnold W., Giese K., Klippel A., Kaufmann J. Inducible shRNA expression for application in a prostate cancer mouse model. Nucleic Acids Res. 2003;31:e127. doi: 10.1093/nar/gng127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang S., El-Deiry W.S. Inducible silencing of KILLER/DR5 in vivo promotes bioluminescent colon tumor xenograft growth and confers resistance to chemotherapeutic agent 5-fluorouracil. Cancer Res. 2004;64:6666–6672. doi: 10.1158/0008-5472.CAN-04-1734. [DOI] [PubMed] [Google Scholar]
- 130.Kappel S., Matthess Y., Zimmer B., Kaufmann M., Strebhardt K. Tumor inhibition by genomically integrated inducible RNAi-cassettes. Nucleic Acids Res. 2006;34:4527–4536. doi: 10.1093/nar/gkl628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ueblacker P., Wagner B., Kruger A., Vogt S., DeSantis G., Kennerknecht E., Brill T., Hillemanns M., Salzmann G.M., Imhoff A.B., Plank C., Gansbacher B., Martinek V. Inducible nonviral gene expression in the treatment of osteochondral defects. Osteoarthr. Cartil. 2004;12:711–719. doi: 10.1016/j.joca.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 132.Moutsatsos I.K., Turgeman G., Zhou S., Kurkalli B.G., Pelled G., Tzur L., Kelley P., Stumm N., Mi S., Muller R., Zilberman Y., Gazit D. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol. Ther. 2001;3:449–461. doi: 10.1006/mthe.2001.0291. [DOI] [PubMed] [Google Scholar]
- 133.Noel D., Gazit D., Bouquet C., Apparailly F., Bony C., Plence P., Millet V., Turgeman G., Perricaudet M., Sany J., Jorgensen C. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells. 2004;22:74–85. doi: 10.1634/stemcells.22-1-74. [DOI] [PubMed] [Google Scholar]
- 134.Ginhoux F., Turbant S., Gross D.A., Poupiot J., Marais T., Lone Y., Lemonnier F.A., Firat H., Perez N., Danos O., Davoust J. HLA-A⁎0201-restricted cytolytic responses to the rtTA transactivator dominant and cryptic epitopes compromise transgene expression induced by the tetracycline on system. Mol. Ther. 2004;10:279–289. doi: 10.1016/j.ymthe.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 135.Toromanoff A., Cherel Y., Guilbaud M., Penaud-Budloo M., Snyder R.O., Haskins M.E., Deschamps J.Y., Guigand L., Podevin G., Arruda V.R., High K.A., Stedman H.H., Rolling F., Anegon I., Moullier P., Le Guiner C. Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol. Ther. 2008;16:1291–1299. doi: 10.1038/mt.2008.87. [DOI] [PubMed] [Google Scholar]
- 136.Streilein J.W. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev., Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]