Abstract
Porcine epidemic diarrhea virus (PEDV) causes lethal diarrhea in piglets that leads to great economic losses in East Asia. It was reported that aminopeptidase N (APN) is the receptor for transmissible gastroenteritis virus (TGEV), human coronavirus 229E (HCoV-229E) and feline coronavirus (FeCoV) which all belong to group I coronavirus including as well as PEDV. It was also confirmed previously that porcine aminopeptidase N (pAPN) can bind to PEDV, and anti-pAPN antibodies may inhibit the combination. To investigate whether pAPN is a receptor for PEDV, we transfected MDCK cells with porcine aminopeptidase (pAPN) cDNA and this enabled non-susceptible cells to support PEDV replication and serial viral propagation. Moreover, the infection was blocked by antibodies against pAPN, implies the critical role of pAPN during virus entry. In addition, immunofluorescence assays for detection of pAPN and PEDV antigens, together with neutralization assays using antibodies against pAPN, further confirmed the correlation between pAPN expression and viral replication in pAPN-transfected MDCK cells. These results indicate that pAPN is a functional receptor for PEDV.
Keywords: Receptor, Porcine aminopeptidase, Porcine epidemic diarrhea virus, Coronavirus
Introduction
Porcine epidemic diarrhea virus (PEDV) is a member of the Coronaviridae, causing severe entero-pathogenic diarrhea especially in piglets. PEDV belongs to group I coronavirus which consists of porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV), human coronavirus 229E (HCoV-229E), feline coronavirus (FeCoV), canine coronavirus (CCV) and human coronavirus NL63 (NL or New Haven) (Van der Hoek et al., 2004). Among them, TGEV, HCoV-229E and FeCoV all use APN as their cellular receptor (Delmas et al., 1992, Yeager et al., 1992, Tresnan et al., 1996, Tresnan and Holmes, 1998, Kolb et al., 1998). It binds to spike proteins of coronaviruses to mediate infection (Godet et al., 1994, Bosch et al., 2003).
PEDV and TGEV induce rather similar clinical symptoms which are hardly differentiated. Both of them replicate in the differentiated enterocytes of the small intestine and cause severe diarrhea (Sanchez et al., 1992). TGEV could be propagated in many continuous cell lines, such as swine testicle (ST) cells and porcine kidney (PK-15) cells. However, the propagation of PEDV had been unsuccessful until serial passage of the virus in Vero cells (Hofmann and Wyler, 1988, Song et al., 2003). It is known that porcine aminopeptidase N (pAPN) is a cellular receptor for TGEV (Delmas et al., 1992, Delmas et al., 1993, Hansen et al., 1998, Schultze et al., 1995), pAPN is a 150-kDa glycosylated transmembrane protein that is highly expressed in small intestinal mucosa, where aminopeptidase represents about 8% of the protein content of the apical membrane of the differentiated enterocytes and catalyzes the removal of single amino acids from the amino terminus of peptides for the final steps of digestion (Delmas et al., 1992). It is a type II glycoprotein, trypsin can cleave it into 2 subunits, N-terminal part (95 kDa) and C-terminal part (50 kDa). The region between residues 717 and 813 which contains the epitopes for three neutralizing antibodies is essential for TGEV infection (Delmas et al., 1994, Posthumus et al., 1990).
It was also demonstrated PEDV could bind to pAPN specifically and this combination can be inhibited by anti-pAPN antibodies (Oh et al., 2003). And soluble pAPN can facilitate the production of PEDV in Vero cells, just as soluble CD4 may enhance simian immunodeficiency virus infection (Werner et al., 1990, Schenten et al., 1999).
In this study, we transfected non-susceptible cell lines Madin–Darby canine kidney (MDCK) cells for the transient expression of pAPN and found this confers susceptibility to PEDV and lead to serial passage. Then we further investigated the ability of pAPN to mediate viral replication through immunofluorescence and neutralization assays. The results confirmed the correlation between pAPN expression and PEDV production. All the data indicate that pAPN serves as a receptor for PEDV that mediates virus entry and determines tissue tropism.
Results
pAPN was expressed in MDCK cells through transfection
Proteins of transfected MDCK cells and native pAPN (Sigma) were separated by SDS–gel electrophoresis and electrotransferred onto a PVDF membrane for Western blotting. Fig. 1 shows that anti-pAPN antibodies recognized wild-type pAPN at 150 kDa, 95 kDa and 55 kDa, corresponding to uncleaved pAPN (150 kDa), amino subunits (95 kDa) and carboxy subunits (50 kDa) which were cleaved by trypsin. The anti-pAPN antibodies also reacted with the protein of MDCK cells transfected with pcDNA3.1–pAPN at 150 kDa, but not with the cells transfected with pcDNA3.1, indicating that only pcDNA3.1–pAPN conferred the expression of pAPN in MDCK cells.
Fig. 1.
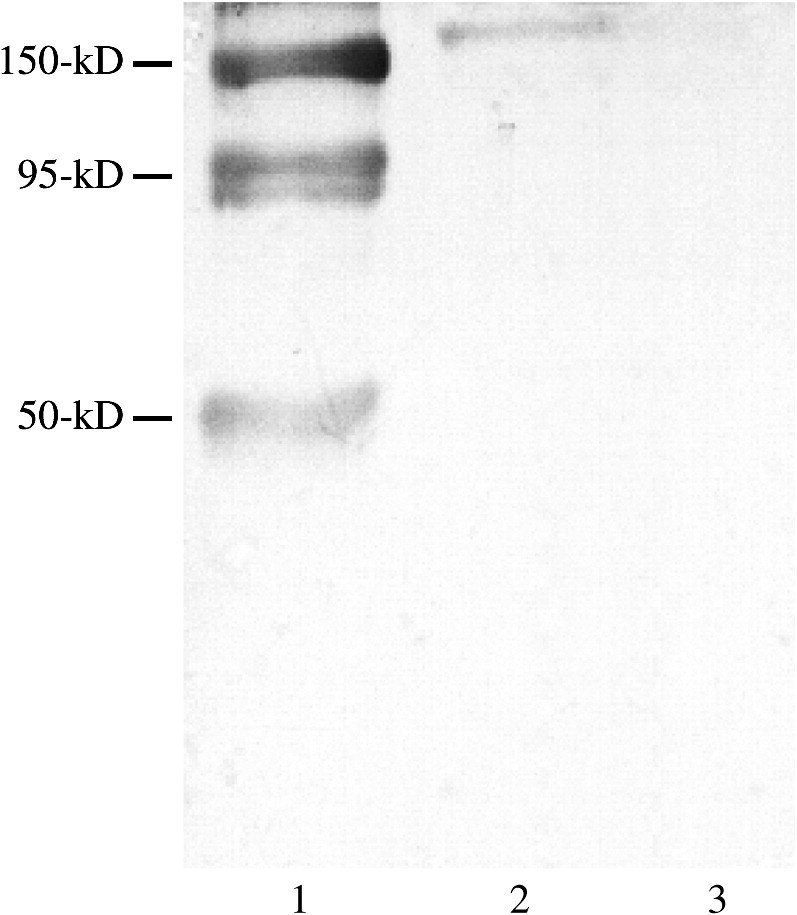
Western blotting of the pAPN expressed in MDCK cells after transfection. Lane 1, Native form of pAPN (Sigma) purified from porcine kidney. Lane 2, MDCK cells transfected with pcDNA3.1–pAPN. Lane 3, MDCK cells transfected with pcDNA3.1.
pAPN expressed at a high level in MDCK cells
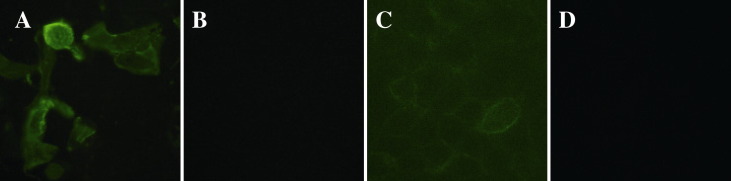
To determine the expression level of pAPN on the plasma membrane of MDCK cells compared with other cell lines, expression of pAPN in MDCK cells was confirmed by immunofluorescence by using polyclonal antibodies against pAPN. Fig. 2 shows that pAPN expressed at a high level in pAPN-transfected MDCK cells, strong fluorescence was observed on cell membrane. While the mock-transfected MDCK cells and Vero cells showed none green fluorescence, indicates that pAPN is not present. As for ST cells, weaker fluorescence was due to lower expression level of native pAPN compared with pAPN-transfected MDCK cells.
Fig. 2.
pAPN protein expressed on plasma membrane were detected by indirect immunofluorescence using pAPN-specific antibodies followed by FITC-conjugated secondary antibody. (A) pAPN-transfected MDCK cells. (B) MDCK cells. (C) ST cells. (D) Vero cells.
Expression of pAPN in MDCK cells renders susceptibility to PEDV
Based on the previous reports, we assumed that pAPN is a cellular receptor for PEDV. To test this hypothesis, we isolated total RNA from newborn piglet intestinal brush border membrane which expresses pAPN at a high level, and amplified pAPN gene. Then a recombinant plasmid expressing pAPN with the eukaryotic expression vector pcDNA3.1 was constructed (named pcDNA3.1–pAPN). A newborn piglet was orally infected in order to adapt the virus to grow to a high titer, especially for the virus variants which potentially have stronger replication ability in the transfected cells, and the virus was purified subsequently. To determine PEDV replication, MDCK cells which are not susceptible to PEDV were transfected for transiently expression of pAPN on the plasma membrane. The confluent monolayers were infected with the purified virus and PEDV was passaged up to 5 times through transiently transfected MDCK cells.
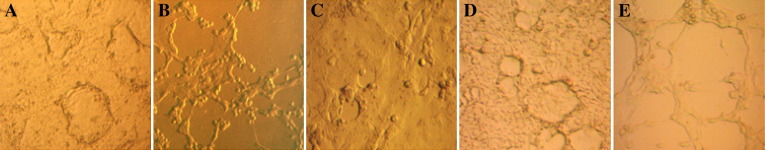
CPEs could be observed after about 36 h and the cell cultures would completely lysed 48 h after inoculation. Figs. 3A, B and C show that PEDV caused obvious CPEs in pAPN-expressing MDCK cells at 24 and 36 h after infection, but not in MDCK cells transfected with pcDNA3.1 alone at 36 h after inoculation.
Fig. 3.
MDCK cells were transiently transfected with pcDNA3.1–pAPN or empty vector and then infected with PEDV, the cytopathic effects (CPEs) was observed. (A) pAPN-transfected MDCK cells inoculated with PEDV were observed at 24 h after inoculation. (B) pcDNA3.1–pAPN was used to transfect MDCK cells followed by PEDV inoculation, CPEs was examined at 36 h after infection. (C) MDCK cells were transfected with pcDNA3.1 and then infected with PEDV. The cells were observed at 36 h after infection. pAPN-transfected MDCK cells were also infected with PEDV in the presence of anti-pAPN antiserum or a control antiserum. CPEs in cells were examined at 36 h after inoculation. (D) Anti-pAPN antiserum was added to the maintenance medium. (E) A control antiserum was incubated with cells throughout the infection.
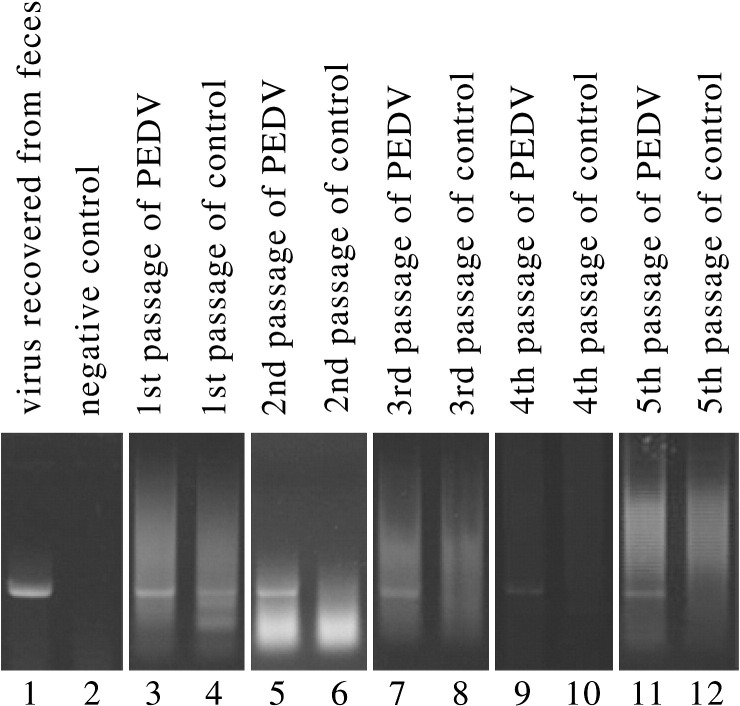
At every passage, total cellular RNA was isolated and subjected to nested RT–PCR for the detection of PEDV. As shown in Fig. 4 , PEDV was recovered after 5 successive passages from pAPN-expressing cells. In contrast, viral replication was not observed in the control cultures transfected with pcDNA3.1, even after serial infections with frozen–thawed cell monolayers, PEDV was not detectable by RT–PCR after the first passage. Likewise, no CPEs was observed.
Fig. 4.
Detection of PEDV RNA in virus suspension recovered from feces and all virus cultures from passage 1 to 5 by nested RT–PCR assays. 412-bp PCR fragments (sequence confirmed) could be observed. Lanes 1 and 2, PEDV of virus suspension purified from feces and negative control. Lanes 3 to 12, 5 serial passaged PEDV in pAPN-expressing MDCK cells and pAPN-deficient MDCK cells as control groups.
Detection of PEDV antigens by immunofluorescence assay
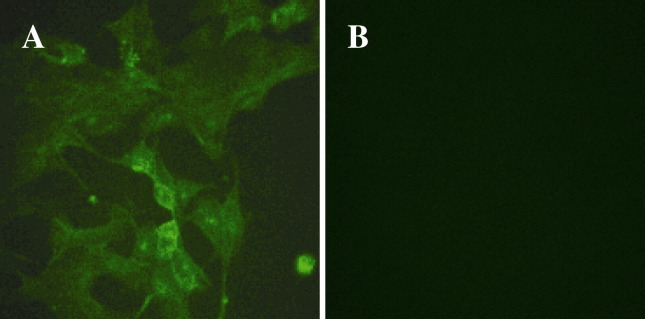
To confirm the proliferation of PEDV in pAPN-transfected MDCK cells. After inoculation, the presence of PEDV antigens in cells was examined by indirect immunofluorescence. Fig. 5 shows the expression of PEDV antigens in a pAPN-expressing MDCK cells as detected by immunofluorescence with polyclonal antibodies. Proteins of PEDV were detectable in pAPN-transfected cells. In contrast, extraordinary low level of fluorescence was observed in pAPN-deficient MDCK cells.
Fig. 5.
MDCK cells transfected with pcDNA3.1–pAPN and MDCK cells were infected with PEDV. 24 h post infection, immunofluorescence assays were performed for the detection of PEDV antigens with anti-PEDV polyclonal antibodies and FITC-conjugated secondary antibody. (A) pAPN-expressing MDCK cells infected with PEDV. (B) MDCK cells infected with PEDV.
Infection of Vero cells with serially passaged PEDV
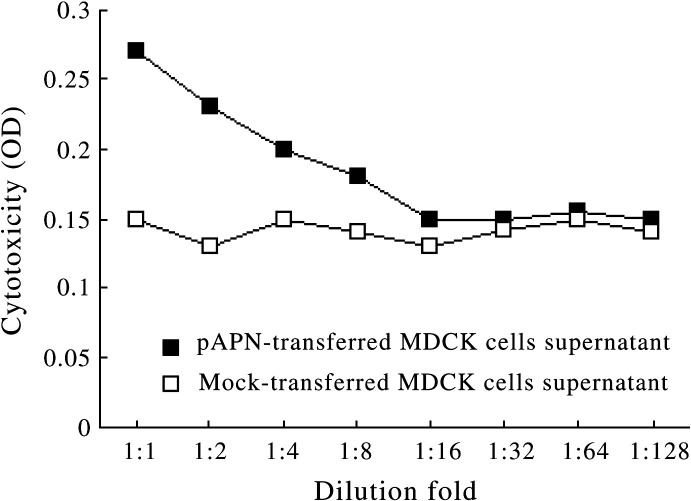
In order to confirm the critical role of pAPN expression during the serial passage of virus, we investigate whether PEDV virion could be recovered after 5 successive passages from pAPN-expressing cells or empty vector-transfected cells, supernatants at passage 5 were serially diluted and cytotoxicity were determined on Vero cells as described. As shown in Fig. 6 , it was found that supernatant of pAPN-transfected MDCK cells induced a significant loss of viability in Vero cell lines and the cytotoxicity was reduced with the dilution of the supernatant. Therefore, this confirmed the presence of virus. However, for the Vero cells infected with supernatant collected from pcDNA3.1-transfected MDCK cells, the loss of variability was minimal and did not correlated with the dilution. Combined with the results of electrophoresis, it was inferred that PEDV could not proliferate because of the absence of pAPN.
Fig. 6.
Vero cells were infected with serially diluted supernatants collected from PEDV-infected mock- and pAPN-transfected MDCK cells at serial passage 5. Inoculation with the serially diluted supernatant of pAPN-transfected cells corresponds to the decrease of cytotoxicity. However, infection with the serially diluted supernatant of pcDNA3.1-transfected cells merely resulted in minimal loss of viability without gradient change.
Antibodies against pAPN inhibit PEDV infection
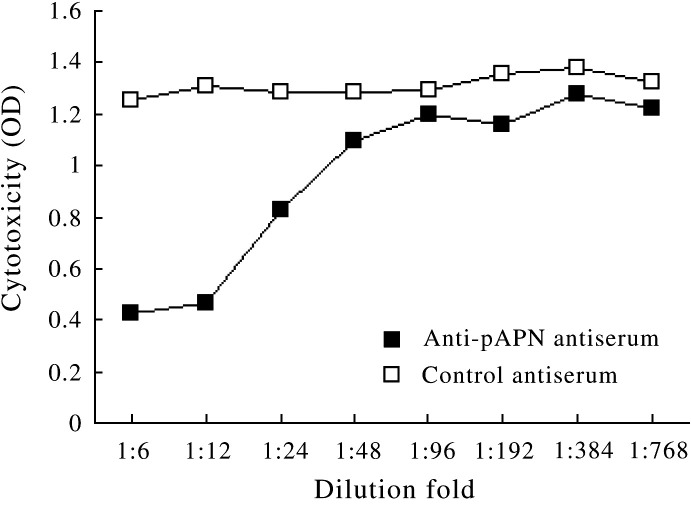
The ability of anti-pAPN antibodies to inhibit viral replication on pAPN-transfected MDCK cells was then investigated. Serial dilutions of anti-pAPN antiserum or a control antiserum was preincubated with pAPN-expressing MDCK cells for 1 h before infection, and the antibodies' presence in the medium throughout the infection. Loss of cell viability was determined by CytoTox 96. As shown in Fig. 7 , dose-dependent inhibition of cytopathic effect was observed in the MDCK cells transfected with pcDNA3.1–pAPN in response to the incubation with anti-pAPN polyclonal antibodies. With the addition of anti-pAPN antiserum, cell monolayers developed relatively weak CPEs even at a dilution of 1:24. At the same time, the control antibody showed no inhibition of the infection of PEDV.
Fig. 7.
pAPN-transfected MDCK cells were infected with PEDV in the presence of serial dilutions of anti-pAPN antiserum or a control antiserum. Anti-pAPN antibodies inhibited cytopathicity in a dose-dependent manner. In contrast, the control antibody could not inhibit virus-induced cytopathicity.
To further check the function of pAPN for infection, anti-pAPN antiserum or a control antiserum at a dilution of 1:20 was incubated with pAPN-expressing MDCK cells throughout the infection. As shown in Figs. 3D and E, with the addition of anti-pAPN antiserum, MDCK cells transfected with pcDNA3.1–pAPN were shown to develop relatively weak CPEs at 36 h after infection. At the same time, PEDV induced much stronger CPEs to the cells incubated with control antibodies.
Discussion
To examine whether pAPN is a relevant receptor for PEDV, MDCK cells transfected with pcDNA3.1–pAPN were exposed to virus. Control groups were transfected with pcDNA3.1 alone. PEDV can be experimentally adapted to pcDNA3.1–pAPN-transfected cells. Viral RNA could be detected up to 5 serial passages, and PEDV at serial passage 5 could infect Vero cells as judged by the dose-dependent infection effect. As for the control groups, the absence of pAPN results in the failure of virus replication in MDCK cell culture, and PEDV's presence in the cell culture. It is inferred that the expression of pAPN in MDCK cells enabled serial propagation of PEDV.
To further confirm that virus entry into pAPN-transfected MDCK cells via pAPN, western blot and immunofluorescence assays were carried out to detect pAPN expression on the plasma membrane of MDCK cells. pAPN expressed in MDCK cells was a bit larger than native protein, it may be due to glycolisation in MDCK cells. MDCK cell monolayers transfected with pcDNA3.1–pAPN expressed pAPN at a significantly high level compared with mock-transfected MDCK cells, ST cells and Vero cells (Delmas et al., 1992). This indicates that there is a direct correlation between the efficiency of viral replication and pAPN expression. In addition, the presence of PEDV antigens in pAPN-expressing MDCK cells was proved by indirect immunofluorescence. However, attempts to infect pAPN-deficient MDCK cells with PEDV resulted in a rather low level of antigen proteins as determined by immunofluorescence assays. Moreover, anti-pAPN antiserum could inhibit CPEs significantly with a dose-dependent effect. From these results, we deduce that high-level expression of pAPN correlates with high-efficiency replication of PEDV, and the susceptibility to PEDV was conferred by pAPN expression in MDCK cells.
Generally, PEDV is adapted to serial propagation in Vero cell cultures by adding trypsin but could not be propagated in ST cell lines which are commonly used for TGEV propagation (Hofmann and Wyler, 1988). The cellular receptor for TGEV in ST cells is pAPN which however can not render PEDV infection (Delmas et al., 1992). And what mechanism causes such discrimination?
One explanation may be that pAPN density plays a critical role in PEDV infection. It is known that cellular receptor density plays a critical role for the entry of some virus, such as oncolytic measles virus and human immunodeficiency virus type 1 (Reynes et al., 2000, Anderson et al., 2004). As for natural PEDV infection, porcine intestinal tissues are rather sensitive and produce high-titer virus. In porcine intestinal brush border membrane, pAPN is abundantly expressed and constitutes of about 8% of the total membrane proteins. However, in ST cells, as shown in Fig. 2, the expression level of pAPN is relatively low. The expression level of pAPN may be an important factor influencing PEDV entry. From our data, it can also be inferred that the density of pAPN and PEDV infectivity might be correlated, since high pAPN amount and enhanced replication of PEDV originate from the same source. It is possible that in the presence of trypsin, MDCK cells with high level of pAPN expression are quite similar to intestinal villus membrane. Thus we speculate that compared with TGEV, the infectivity of PEDV maybe much more restricted by pAPN density.
Another possible explanation for the restriction of PEDV host range may be summarized as follows. This restriction could be due to a defect in entry or a post-entry event. Other unknown mechanisms might provide some supporting factors required for virus entry and replication capacity. It remains to be determined whether pAPN is sufficient for viral entry in some cell lines or a secondary or earlier event is responsible for promoting entry. In another word, it is likely that multiple receptor molecules mediate PEDV infection or the replication is restricted at a post-entry step, and it is also possible that the modification of PEDV is needed. However, PEDV could infect Vero cells without pAPN. It may be due to other receptors or receptor-independent infection (Taguchi and Matsuyama, 2002).
From the results of our research and the previous reports, we deduced that pAPN serves as a cellular receptor for PEDV. However, the entire mechanism of PEDV entry is remained to be unrevealed and further research is needed. Since MDCK cells transiently expressing pAPN could be used for the propagation of PEDV, it pointed out an alternative way to construct an optimized cell line for the cultivation of PEDV and providing adequate quantity of virus to meet the vaccine requirements. Moreover, identifying the cellular receptor for PEDV can also provide data for viral pathogenesis and antiviral drug design.
Materials and methods
Cells and medium
The MDCK-2 and Vero cells were cultured in growth medium containing alpha minimum essential medium (Gibco) supplemented with 8% FCS (Hyclone). Maintenance medium consisted of alpha minimal essential medium supplemented with 0.3% tryptose phosphate broth (Difco) and 0.02% yeast extract (Oxoid) and 10 mg/ml trypsin (Sigma).
Virus
One local strain LJB/03 of PEDV was used for the experiments (Jinghui and Yijing, 2005, Junwei et al., 2006). A newborn piglet orally infected with PEDV fecal specimens was sacrificed (Hofmann and Wyler, 1988). The content of the small intestine were diluted 1:10 in PBS and homogenized (Dounce homogenizer). The homogenate was centrifuged at low-speed centrifugation (1000×g for 15 min at 4 °C), and debris were pelleted by centrifugation (10, 000×g for 15 min at 4 °C). The supernatants were concentrated by ultracentrifugation (150 ,000×g for 1 h at 4 °C). The pellet was resuspended up in 1 ml maintenance medium and clarified by centrifugation (10,000×g for 10 min at 4 °C). Then 1 ml 50% (w/v) filter-sterilized sucrose was injected directly to the bottom of the tube carefully without disturbing the pellet. Bacteria was then removed through centrifugation (10 000×g for 20 min at 4 °C) by using of swinging bucket rotors. The resulting suspension containing PEDV was aliquoted and stored at − 80 °C. The presence of PEDV was confirmed by RT–PCR with primer sets used for diagnosis reported previously (Ishikawa et al., 1997, Kim et al., 2000, Kocherhans et al., 2001). The PCR fragment was sequenced subsequently.
Antibodies
Polyclonal antibodies recognizing pAPN were prepared from a BALB/c mice immunized with pAPN (Sigma) purified from porcine kidney. Antiserum against PEDV was prepared from a rabbit immunized with PEDV which was purified from feces as described above.
Isolation of the porcine aminopeptidase cDNA
Total RNA was extracted from intestinal brush border membrane of a newborn piglet using TRIzol Reagent (Invitrogen) according to the manual. The porcine aminopeptidase coding sequence was amplified by RT–PCR (Delmas et al., 1992, Olsen et al., 1989). Primers used for the PCR were 5′ CTCCCTTCTCACCCTCACC 3′ and 5′ CCACAGGCTTTGTCCCTA 3′. The PCR product was subcloned into pMD18-T vector (TaKaRa) followed by sequencing. The resulting plasmid was named pMD-18T-pAPN. The full-length pAPN cDNA with BamHI and HindIII restriction sites was PCR-amplified from pMD-18T-pAPN, primers used for this step were 5′ TGGAAGCTTCTCCCTTCTCACCCT 3′ and 5′ CTGGGATCCCCACAGGCTTTGTC 3′, sequences underlined are sites for restriction enzymes. The PCR product was subsequently subcloned into the corresponding restriction sites of pcDNA3.1 and sequenced to identify the orientation and integrity. The constructed vector was named pcDNA3.1–pAPN. Plasmid DNA was purified with a plasmid DNA purification kit (Promega) for subsequent transfection.
Western blot analysis of the expression of pAPN
To determine the expression of pAPN in MDCK cells, western blot analysis was performed. Freshly thawed MDCK cells were seeded into a 24-well plate and cultured in growth medium. Then the cells were grown overnight to 80–90% confluency. In the presence of 8% FCS, transient transfection was performed with 1.6 μg of pcDNA3.1–pAPN or pcDNA3.1 and 4 μl of Lipofectamine 2000 (Invitrogen) each well. 5 h later, the transfection medium was replaced with fresh growth medium.
36 h after transfection, cell monolayers were scrapped and solubilized by boiling in SDS sample buffer. With pAPN protein (Sigma) purified from porcine kidney as a positive control group, the proteins were fractionated by SDS–polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride (PVDF) membrane, PVDF membrane was blocked for 2 h using 5% non-fat milk in PBS and incubated for 1 h with a 1:100 dilution of the mouse anti-pAPN antibodies. And 1:2000 dilution of horseradish peroxidase-labeled goat anti-mouse IgGs were used as a secondary antibody. Each of the incubations was performed at 37 °C followed by 3 times wash with phosphate-buffered saline with 0.1% Tween 20 (PBST). Finally, the antibodies were revealed with a 4-chloro-1-naphthol-based color development.
Immunofluorescence analysis of the expression of pAPN
Next we analyzed the expression level of pAPN in MDCK cells. With ST and Vero cells as control groups, 24 h after transfection of MDCK cells with plasmid-expressing pAPN or pcDNA3.1 alone, the monolayers were washed with PBS and fixed in 3% PFA at 4 °C for 15 min followed by 3 times wash with PBS. Cells were then blocked with PBS containing 1% BSA and incubated with a 1:50 dilution of mouse polyclonal anti-pAPN antiserum. After 3 times wash with PBS–BSA, the monolayer were stained by FITC-labeled goat anti-mouse IgGs. The results were observed with a Zeiss fluorescence microscope.
Serial passage of PEDV in pAPN-deficient and pAPN-expressing MDCK cells
Viral inoculation of the cells was carried out 24 h after transfection. The growth medium of the confluent cells was removed and the monolayers were washed three times with maintenance medium. The cell cultures were subsequently inoculated with 0.3 ml per well of the clarified virus suspension recovered from feces described above. After 1 h of adsorption at 37 °C, without removing the viral inoculum, 1 ml of maintenance medium was transferred into each well. Subsequently, the cells were incubated at 37 °C. Since trypsin is thermolabile, 5 μl trypsin concentration (1 mg/ml) was added to each well 24 h after inoculation. Infected cells were harvested when the cells reached 70–80% CPEs. Then the cultures were subjected to one freeze–thaw cycle and the suspension was used for the next inoculation of pAPN-expressing or control transfected MDCK cells. Serial passages of PEDV were continued by level 5 according to the method described above. PEDV was identified by nested RT–PCR for each passage using the former primers, the PCR product of the fifth passage of virus was sequenced.
Detection of viral antigens by immunofluorescence
For investigating the ability of pAPN-transfected MDCK cells to support virus replication. Mock-transfected and pAPN-transfected MDCK cells were inoculated with PEDV according to the protocol described above. At 24 h post infection, the monolayer were fixed by 3% PFA followed by permeabilization using 0.2% Triton X-100. After blocking with PBS–BSA and incubation with rabbit polyclonal anti-PEDV antibodies at a 1:100 dilution, they were then treated with FITC-labeled goat anti-rabbit IgGs before examination under a fluorescence microscope.
Infection of Vero cells with serially passaged PEDV
To identify the presence of virus after serial passage of PEDV in pAPN-transfected MDCK cells. CytoTox 96 nonradioactive cytotoxicity assay (Promega) was performed according to the manual of the manufacturer. Vero cells were seeded into 96-well plates and grown for 24 h to achieve a confluency of 80–90%, supernatants collected from PEDV infected mock- and pAPN-transfected MDCK cells at serial passage 5 were used to inoculate Vero cell at 1:1 dilution, 1:2 dilution, 1:4 dilution, 1:8 dilution, 1:16 dilution, 1:32 dilution, 1:64 dilution and 1:128 dilution in triplicate. Cytopathic effect was then monitored for each well 36 h after infection.
Neutralization analysis using anti-pAPN antibodies
To ensure the critical role of pAPN during virus entry, we examined whether anti-pAPN antiserum could inhibit PEDV infection. Confluent monolayers of MDCK cells transiently expressing pAPN in 96 plates were pretreated with 1:6 dilution, 1:12 dilution, 1:24 dilution, 1:48 dilution, 1:96 dilution, 1:192 dilution, 1:384 dilution and 1:768 dilution of polyclonal anti-pAPN antibodies or a control antibody in medium for 1 h. And cells were then infected with PEDV with antibodies' presence in the medium. After 36 h, virus-induced cytotoxicity was measured at 490 nm using CytoTox 96 nonradioactive cytotoxicity assay (Promega) according to the instruction of the manufacturer. The experiment was performed in 3 replicate wells for each antibody concentration.
Cytopathic effect was also observed to confirm the neutralization effect of anti-pAPN antibodies. MDCK cells transiently expressing pAPN were incubated with 20-fold diluted polyclonal antibodies against pAPN or a control antiserum 1 h prior to virus inoculation, and the antiserum was present in the medium throughout the infection process. CPEs were subsequently examined under a microscope.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 30671574).
References
- Anderson B.D., Nakamura T., Russell S.J., Peng K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64(14):4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- Bosch B.J., Van Der Zee R., De Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77(16):8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L'Haridon R., Vogel L.K., Sjostrom H., Noren O., Laude H. Aminopeptidase N is a major receptor for the enteropathogenic coronavirus TGEV. Nature. 1992;357(6377):417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., Sjostrom H., Noren O., Laude H. Further characterization of aminopeptidase-N as a receptor for coronaviruses. Adv. Exp. Med. Biol. 1993;342:293–298. doi: 10.1007/978-1-4615-2996-5_45. [DOI] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., Kut E., Sjostrom H., Noren O., Laude H. Determinants essential for the transmissible gastroenteritis virus–receptor interaction reside within a domain of aminopeptidase-N that is distinct from the enzymatic site. J. Virol. 1994;68(8):5216–5224. doi: 10.1128/jvi.68.8.5216-5224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet M., Grosclaude J., Delmas B., Laude H. Major receptor-binding and neutralization determinants are located within the same domain of the transmissible gastroenteritis virus (coronavirus) spike protein. J. Virol. 1994;68(12):8008–8016. doi: 10.1128/jvi.68.12.8008-8016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen G.H., Delmas B., Besnardeau L., Vogel L.K., Laude H., Sjostrom H., Noren O. The coronavirus transmissible gastroenteritis virus causes infection after receptor-mediated endocytosis and acid-dependent fusion with an intracellular compartment. J. Virol. 1998;72(1):527–534. doi: 10.1128/jvi.72.1.527-534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988;26(11):2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Sekiguchi H., Ogino T., Suzuki S. Direct and rapid detection of porcine epidemic diarrhea virus by RT–PCR. J. Virol. Methods. 1997;69(1–2):191–195. doi: 10.1016/s0166-0934(97)00157-2. [DOI] [PubMed] [Google Scholar]
- Jinghui F., Yijing L. Cloning and sequence analysis of the M gene of porcine epidemic diarrhea virus LJB/03. Virus Genes. 2005;30(1):69–73. doi: 10.1007/s11262-004-4583-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junwei G., Baoxian L., Lijie T., Yijing L. Cloning and sequence analysis of the N gene of porcine epidemic diarrhea virus LJB/03. Virus Genes. 2006;33(2):215–219. doi: 10.1007/s11262-005-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim O., Choi C., Kim B., Chae C. Detection and differentiation of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus in clinical samples by multiplex RT–PCR. Vet. Rec. 2000;146(22):637–640. doi: 10.1136/vr.146.22.637. [DOI] [PubMed] [Google Scholar]
- Kocherhans R., Bridgen A., Ackermann M., Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23(2):137–144. doi: 10.1023/A:1011831902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb A.F., Hegyi A., Maile J., Heister A., Hagemann M., Siddell S.G. Molecular analysis of the coronavirus–receptor function of aminopeptidase N. Adv. Exp. Med. Biol. 1998;440:61–67. doi: 10.1007/978-1-4615-5331-1_8. [DOI] [PubMed] [Google Scholar]
- Oh J.S., Song D.S., Park B.K. Identification of a putative cellular receptor 150 kDa polypeptide for porcine epidemic diarrhea virus in porcine enterocytes. J. Vet. Sci. 2003;4(3):269–275. [PubMed] [Google Scholar]
- Olsen J., Sjostrom H., Noren O. Cloning of the pig aminopeptidase N gene. Identification of possible regulatory elements and the exon distribution in relation to the membrane-spanning region. FEBS Lett. 1989;251(1–2):275–281. doi: 10.1016/0014-5793(89)81470-x. [DOI] [PubMed] [Google Scholar]
- Posthumus W.P., Lenstra J.A., Schaaper W.M., van Nieuwstadt A.P., Enjuanes L., Meloen R.H. Analysis and simulation of a neutralizing epitope of transmissible gastroenteritis virus. J. Virol. 1990;64(7):3304–3309. doi: 10.1128/jvi.64.7.3304-3309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynes J., Portales P., Segondy M., Baillat V., Andre P., Reant B., Avinens O., Couderc G., Benkirane M., Clot J., Eliaou J.F., Corbeau P. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J. Infect. Dis. 2000;181(3):927–932. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- Sanchez C., Gebauer M.F., Sune C., Mendez A., Dopazo J., Enjuanes L. Genetic evolution and tropism of transmissible gastroenteritis coronaviruses. Virology. 1992;190(1):92–105. doi: 10.1016/0042-6822(92)91195-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenten D., Marcon L., Karlsson G.B., Parolin C., Kodama T., Gerard N., Sodroski J. Effects of soluble CD4 on simian immunodeficiency virus infection of CD4-positive and CD4-negative cells. J. Virol. 1999;73(7):5373–5380. doi: 10.1128/jvi.73.7.5373-5380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze B., Enjuanes L., Herrler G. Analysis of the sialic acid-binding activity of the transmissible gastroenteritis virus. Adv. Exp. Med. Biol. 1995;380:367–370. doi: 10.1007/978-1-4615-1899-0_59. [DOI] [PubMed] [Google Scholar]
- Song D.S., Yang J.S., Oh J.S., Han J.H., Park B.K. Differentiation of a Vero cell adapted porcine epidemic diarrhea virus from Korean field strains by restriction fragment length polymorphism analysis of ORF 3. Vaccine. 2003;21(17–18):1833–1842. doi: 10.1016/S0264-410X(03)00027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Matsuyama S. Soluble receptor potentiates receptor-independent infection by murine coronavirus. J. Virol. 2002;76(3):950–958. doi: 10.1128/JVI.76.3.950-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresnan D.B., Holmes K.V. Feline aminopeptidase N is a receptor for all group I coronaviruses. Adv. Exp. Med. Biol. 1998;440:69–75. doi: 10.1007/978-1-4615-5331-1_9. [DOI] [PubMed] [Google Scholar]
- Tresnan D.B., Levis R., Holmes K.V. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J. Virol. 1996;70(12):8669–8674. doi: 10.1128/jvi.70.12.8669-8674.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C., Wertheim-Van Dillen P.M., Kaandorp J., Spaargaren J., Berkhout B. Identification of a new human coronavirus. Nat. Med. 2004;10(4):368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Winskowsky G., Kurth R. Soluble CD4 enhances simian immunodeficiency virus SIVagm infection. J. Virol. 1990;64(12):6252–6256. doi: 10.1128/jvi.64.12.6252-6256.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357(6377):420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]