Abstract
Demineralized bone matrix (DBM) is an osteoconductive and osteoinductive commercial biomaterial and approved medical device used in bone defects with a long track record of clinical use in diverse forms. True to its name and as an acid-extracted organic matrix from human bone sources, DBM retains much of the proteinaceous components native to bone, with small amounts of calcium-based solids, inorganic phosphates and some trace cell debris. Many of DBM's proteinaceous components (e.g., growth factors) are known to be potent osteogenic agents. Commercially sourced as putty, paste, sheets and flexible pieces, DBM provides a degradable matrix facilitating endogenous release of these compounds to the bone wound sites where it is surgically placed to fill bone defects, inducing new bone formation and accelerating healing. Given DBM's long clinical track record and commercial accessibility in standard forms and sources, opportunities to further develop and validate DBM as a versatile bone biomaterial in orthopedic repair and regenerative medicine contexts are attractive.
Keywords: Demineralized bone matrix, DBM, Bone graft, Growth factors, Bone regeneration, Osteoinductive
Graphical abstract
1. Introduction
Demineralized bone matrix (DBM) is an important therapeutic option for the appendicular, axial and craniofacial skeletons. In these skeletal locales, DBM has osteoconductive and osteoinductive properties that prompt bone regeneration. Consequently, about 20% (or about 108,000 procedures per year) of the $1 billion per year bone grafting market [1] focuses on using DBM products in bone repair and regenerative strategies.
United States Food and Drug Agency (FDA) approvals of recombinant human bone morphogenetic protein-2 (rhBMP-2) (InFuseTM, Medtronic/Sofamor-Danek) and recombinant human platelet-derived growth factor-BB (rhPDGF-BB) (Gem21STM, BioMimetic Therapeutics, Inc.) as purified therapeutic agents for bone repair have not significantly decreased either the clinical and corporate enthusiasm or the clinical need for DBM products. This clinical enthusiasm in DBM is underscored by the quantity of research and clinical publications regarding its use. A search of the PubMed database for publications mentioning ‘demineralized bone’ in the title or abstract over the past 40 years revealed a sustained and continuous number of DBM publications (Fig. 1 ).
Fig. 1.
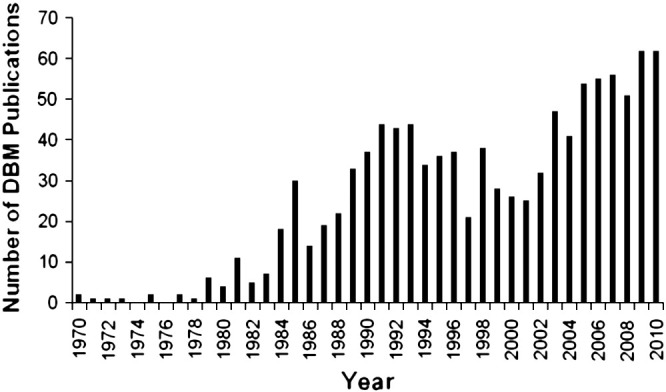
DBM publications by year based on PubMed search (through 2010).
With a robust clinical demand for DBM in human patients, tissue banks and companies continue to commit remarkable resources to produce a diverse array of DBM-containing products—to the point where clinicians are often confused by the DBM product diversity. Moreover, some uncertainty exists clinically about the validity of various claims made by commercial vendors about DBM-containing products. Additionally, combinations of DBMs with other biomaterials in several composite forms increase the versatility of this clinical human tissue-derived product to expand its capabilities for bone repair.
Several factors regarding DBM as a human-derived tissue product are important to understand in guiding use as a bone repair matrix and vehicle for delivering bioactive agents; for example, bone procurement techniques from human donors; donor age and gender, and DBM composition and properties [2], [3], [4], [5]. Differences in preparation and processing methods for bone can impact properties and clinical performance. Biological testing of processed bone and the outcome measures to validate biological activity (i.e., the so-called osteoinductive index, OI, described herein) are not uniform among tissue banks, and as a result DBM products have variable composition and properties. This variability has obvious significance for clinicians and patients, but also issues for understanding performance in research studies. In addition, DBM products consist of bone-derived particle sizes and particle size ranges, even protein fibers. DBM sterilization protocols are also variable. Finally, the composition of various carriers combined with the DBM, and DBM combined with various bioactive substances will influence clinical and research outcomes. Best practices and consensus for use are difficult to ascertain from the literature. The diverse types of DBM products as well as commercial claims regarding the performance of those products leads to many questions about clinical effectiveness, future opportunities and understanding how it is best used. Given DBM's substantial history that weaves biology together and clinical applications, an initial review of its history, contemporary and factual clinical basis for DBM properties and clinical utility in bone repair is useful to understand current usage trends and future opportunities. Importantly, in this context, DBM was the original source for discovery of several key growth factors that were subsequently cloned and recombinantly expressed to become FDA-approved clinical therapeutic proteins BMP-2 and BMP-7 in bone repair (rhBMP-7 is no longer FDA-approved but has a highly restrictive human device exemption label).
2. Historical perspective on DBM
Early publications by Harakas [6] and Glowacki and Mulliken [7] provide key historical information for first identifying and recognizing important pioneering work on DBM. Context for DBM use was prompted by clinical recognition of the value of bone grafting that alone has substantial history. A 1632 report regarding a Russian soldier successfully treated with dog bone graft (the first reported bone xenograft) elicited vociferous condemnation from the Christian church [8]. The treating surgeon, Meek'ren, fearing excommunication, removed the xenograft. Autografting was also reported by Macewen in 1881 [9] who speculated that transplanted osteoblasts carried in the autograft were responsible for success. Senn in 1889 used decalcified bone to treat osseous defects [10]. He soaked tibiae from oxen in hydrochloric acid (HCl), using logic that HCl had antiseptic effects on the xenograft and would benefit patients with osteomyelitis. Deaver [11], Curtis [12] and Mackie [13] validated Senn's work. During World War I (1914–1917), combat-incurred injuries requiring bone grafting prompted resurgence in autografting. Notable clinician-scientists Phemister [14] and Groves [15] systematically studied autografting using pre-clinical models. Phemister underscored the need for ‘mechanical stress’ for successful structural bone grafting. While the concept of load may not have been specifically stated, the implications were that limb function following grafting is an important, positive bone-promoting stimulus. Phemister then introduced the ‘creeping substitution’ concept where host bone grows into bulk allograft. Barth (1893) [16] and Axhausen [17] previously described a process where host bone grows into other grafted bone. Contemporary nomenclature for ‘creeping substitution’ is ‘osteoconduction’, the term coined by Urist (reviewed in [18]). Groves emphasized the importance of ‘secure fixation’ of the graft to the recipient bed as well as noting viable bone produced better outcome than non-viable bone [15].
Improved surgical skills and patient care produced a commensurate decrease in morbidity and mortality following bone autografting. However, despite these benefits, a compelling concern with autografts was the invasive ‘donor’ procedure to recover the graft. Moreover, inadequate donor bone improperly shaped for the recipient site, was an additional concern. Furthermore, time on the operating room table, infection risk and increased patient care costs were concerns that persist today as contemporary challenges. Noteworthy, the overall risk of infection from hospital internment remains substantial. Consequently, 20th century clinicians sought alternatives to autografting that minimize hospital exposure—a lingering quest remaining for 21st century clinicians as well.
An initial alternative to autografting was offered by Huggins [19], [20]. He described a propitious finding where transitional epithelium from the urogenital system promoted ectopic osteogenesis: the formation of bone in a connective tissue site. These reports were similar to Neuhof's observation [21], where fascia used to augment bladder in dogs elicited an ectopic osteogenic outcome. Interestingly, in 1998 Urist and colleagues reported detection of messenger RNA for bone morphogenetic proteins (BMPs) 2, 4 and 5 in bladder tissue, producing an important causal connection [22].
Important to DBM development, these reports on ‘ectopic osteogenesis’ were followed by two provocative papers in 1934 and 1938 describing the response in muscle to alcohol extracts prepared from bone [23], [24]. The alcohol extracts injected into skeletal muscle produced osteogenesis. These studies were phenomenological, and it was not until 1945 and 1947 that a biological explanation was offered by Lacroix [25], [26] who thought that bone contained ‘substances’ that enabled osteogenesis. His 1947 paper in Nature stated that ‘osteogenin’, speculated to be in bone, initiated its growth [26]. Ray and Holloway [27] and then Urist in his landmark paper in Science [28] determined ectopic osteogenesis occurred when demineralized bone was implanted into a non-bony site. This elegantly detailed scientific explanation of ectopic osteogenesis coined the term ‘autoinduction’. Urist posited that the substratum (the non-mineralized matrix of bone) was key, providing morphogenetic signals that prompted osteogenesis. It is highly noteworthy that in 1971, Urist and Strates introduced ‘bone morphogenetic protein’ (BMP) and ‘osteoinduction’ to the scientific and clinical communities [29]. Urist systematically and lucidly presented a visionary process—still highly relevant today—of bone morphogenesis promoted by the demineralized component of bone: the organic substratum (as Urist called it) [30].
Reddi [31], [32] further clarified the cellular and molecular biology of ‘ectopic osteogenesis’ caused by demineralized bone and dentin matrices. In 1976, Reddi and Anderson [33] refined and polished Urist's work on ‘induced osteogenesis’ and then provided the first credible and compelling explanation of the functional role of purified organic bone matrices (i.e., the bone's demineralized matrix) [34]. Sampath and Reddi stated ‘the functional collaboration between the soluble extract and insoluble collagenous substratum’ (i.e., the demineralized matrix) were necessary for ‘optimal osteogenic activity’. Reddi and his loyal colleagues identified factors in the ‘soluble extract’ crucial to ectopic ossification. Respecting the hallmark work of Lacroix along these lines, Reddi called one of these factors osteogenin [35], later also identified as BMP-3. Reddi's comprehensive and pioneering work on DBM enabled the cloning and expressing of recombinant human BMPs. Reddi acknowledged in 2000 [36], the ‘incisive work of Wozney and colleagues [37] who cloned bone morphogenetic proteins 2 and 4. This accomplishment was followed in 1990 by Özkaynak and co-workers who cloned BMPs 7 and 8 (also called osteogenic proteins 1 and 2, or OP-1, and ‐2, respectively) [38]. These BMP molecules are part of the ‘soluble extract’ Reddi first identified in his original demineralized bone matrix (DBM) (reviewed in [36]).
In summary then, the profound clinical ramifications from the soluble bone extract that contains BMPs and other influential bone growth promoting substances now known as DBM can be attributed to Reddi and his disciples for their clarity in taking clues from centuries of previous, largely phenomenological work and focusing research attention on the soluble component of bone's organic matrix. This demineralized component was proven to contain the biological drivers essential for osteogenesis. This history provides a conceptual foundation to develop several issues germane to the biological activity of DBM and its clinical utility, including procurement, donor profile (e.g., gender, age), processing, production issues, sterilization, and its combinations with carrier biomaterials.
3. DBM procurement
The origin for all DBM clinical products is the human donor. Donor bone, by contemporary jargon, is referred to as an allograft. However, the removal of bone from the donor, as well as the processing of that donor bone, renders the tissue void of viable cells. Therefore, by definition the DBM product is an alloimplant: it does not contain viable cells. By contrast the autograft contains viable cells. The procurement and processing of the donor bone tissue that will become DBM uses the term allograft despite the fact that the bone allograft is a cell-free matrix of bone containing the inorganic and organic matrices.
As a consequence of donor procurement to obtain the graft, the primary risk from the allograft bone products is transmission of infectious diseases from the donor to the recipient. Since the implementation of rigorous product testing began over 10 years ago, only rare, isolated instances of disease transmission are reported that may have resulted as a consequence of unscrupulous tissue banking procedures. Notably, these events occurred when irresponsible groups did not adhere to the tissue procurement guidelines advocated by the American Association of Tissue Banks (AATB).
Methods to prepare DBM from donor bone generally sterilize the product and eliminate potential infectious agents. Nevertheless, the first line of defense against disease transmission is donor screening. Donor screening begins at the procurement level. To avoid transmission of infectious diseases, potential donors are screened for specific exclusionary risk factors. The procurement process is highly regulated by the FDA and specific requirements are found in Title 21 Code of Federal Regulations (CFR 21) Part 1270. Compliance with these tissue processing guidelines requires the cooperation of independent organizations, including hospital-based medical staff, recovery and screening organizations, tissue processing facilities and sales and distribution organizations. The starting point is an assessment of the suitability of a donor conducted by a recovery or screening organization based on general criteria followed by an initial medical screening. The family of the deceased donor is consulted to obtain an informed consent for tissue donation. If the triage and consent steps are successful, the tissue processing facility is contacted and takes responsibility for final screening.
A number of guidance documents, continuously updated to account for newly identified risk factors, are available from the FDA with recommendations for screening potential donors for exclusionary risk factors. For example, the current FDA guidance document includes potential exposure to newly identified zoonotic diseases such as West Nile Virus (WNV) and Severe Acute Respiratory Syndrome (SARS) as exclusionary risk factors. FDA mandates that a detailed medical and social history is obtained to uncover social and behavioral risk factors (see Supplementary Table 1). The information is obtained through interviews with family and other close contacts of the donors. In some instances identification of a behavioral risk factor disqualifies a donor and in other cases it triggers further investigation. The next assessment is a physical examination that may reveal evidence of risk factors or signs of pathologies not otherwise detected through serological testing and could lead to donor exclusion (Supplementary Table 2). Finally, a series of clinical tests are completed to rule out Human Immunodeficiency Virus (HIV) types 1 and 2, Hepatitis B virus (HBV), Hepatitis C virus (HCV), and treponema pallidum (syphilis). The human donor qualification process for DBM sourcing is rigorous and stringent. The intent is to ensure procurement of disease-free tissue and DBM biomaterials with requisite safely and efficacy for patient use.
4. DBM processing
As a direct product of donor allograft bone, DBM is a composite of collagens (mostly type I with some types IV and X), non-collagenous proteins and growth factors, a variable percent of residual calcium phosphate mineral (1–6%) and some small percent cellular debris. In general, the DBM preparation protocol includes donor bone debridement of adherent soft tissues and removal of blood and lipids. At this point, often an antibiotic soak is used to initiate the sterilization process. Subsequently, cleansed donor bone is morsellized to defined particles or fibers and subjected to acid demineralization followed by one or more rounds of freeze-drying. The mineral phase is extracted from the particulate whole donor bone with 0.5–0.6 N HCl, leaving the organic matrix intact. Upon freeze-drying, the resulting demineralized bone powder (synonymous with DBM) can be formulated into putties, pastes and more recently, flexible, pre-formed strips for implant use. The freeze-drying as one step in the processing has led to the alternative DBM term: Demineralized Freeze-Dried Bone Allograft (DFDBA).
The biological and clinical justification to demineralize donor bone to yield DBM (or DFDBA) was provided above: DBM contains abundant bone morphogenetic proteins known to be essential for bone growth and regeneration [28], [29]. The biological and subsequent clinical impact of bone demineralization on DBM is that residual calcium may influence its osteoinductivity [39]. Honsaek and co-workers suggested that BMPs in DBM maybe more readily extracted as calcium content in the DBM decreases [40]. However, BMP content of the DBM is also suggested to likely be less important than its extractability and release into the host implant site.
If the extent of bone demineralization is constant, then DBM particle size remaining after morselization in the powder, or DBM fiber geometry produced by processing the bone-derived collagen proteins then defines DBM surface area as a clinical variable. Different surface geometries may impact host cellular interactions as well as diffusion rates of DBM-resident biological molecules and endogenous agents such as BMPs or growth factors in and out of DBM. Consequently, some discussion regarding the optimal size and size range of particle for DBM preparations suggests that particles less than 250 μm are not as osteoinductive as larger-sized (420–840 μm) particles [5], [41], [42]. Generally, preclinical data are inconsistent regarding DBM compositions as particles and their size ranges, as well as for fibers, and DBM formulations in sheets and gels. Inconsistency is due to different animal models and outcome measures, many of which are highly subjective and are unique to one laboratory and not universally accepted. Moreover, human data on DBM are weak due in part to the emphasis on DBM as a bone graft extender rather than as a stand-alone therapy. Consequently, the efficacy for different formulations for DBM has not been clearly elucidated. Formulation design features are largely empirically ascertained in arbitrary test beds.
An additional concern regards possible effects that bisphosphonate use may have on DBM donors who have taken this drug to treat osteoporosis. Recent work indicates that DBM processed from human donors with a known history of bisphosphonate use can be purified free of alendronate, eliminating concerns about inadvertent DBM contamination from donors using this bone-binding drug [43]. To address the related concern about the possible paucity of bioactive components in alendronate-treated bone and resulting DBM, the Boyan group found no detectable changes in the ability of DBM from human donors treated with bisphosphonates to induce bone formation compared to other DBMs. However, they did not determine if the new bone quality was affected [44].
5. DBM carriers
The end product of the bone demineralization process is a DBM powder that may be difficult to manage clinically. Consequently, several carriers have been used to incorporate high mass fractions of DBM powder, facilitate handling, formulation and reliable delivery of DBM products clinically. Diverse types of commercial DBM-carrier products are known and available, listed in Table 1 .
Table 1.
Commercial demineralized bone matrix preparations.
| Company | Commercially available product | Composition | Commercially available forms | Claimed mechanisms of action | Burden of proof | FDA status |
|---|---|---|---|---|---|---|
| AlloSource | AlloFuseTM | Heat sensitive copolymer with DBM | Injectable gel and putty | • Osteoconduction • Bioresorbable • Osteoinduction |
• Case reports • Animal studies • Cell culture |
510(k) cleared • Bone graft extender • Bone void filler |
| Biomet Osteobiologics | InterGro® | DBM in a lecithin carrier | Paste, putty and mix with HA/CC composite granules | • Osteoconduction • Bioresorbable • Osteoinduction |
• Case reports • Animal studies • Every lot tested for osteoinduction |
510(k) cleared • Bone graft extender • Bone void filler |
| Exactech | Optecure® | DBM suspended in a hydrogel carrier | Dry mix kit delivered with buffered saline | • Osteoconduction • Bioresorbable • Osteoinduction • Osteogenesis when mixed with autogenous bone graft |
• Human studies • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft extender • Bone void filler |
| Optecure® + CCC | DBM and CCC suspended in a hydrogel carrier | Dry mix kit delivered with buffered saline | • Osteoconduction • Bioresorbable • Osteoinduction • Osteogenesis when mixed with autogenous bone graft |
• Human studies • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft extender • Bone void filler |
|
| Optefil® | DBM suspended in gelatin carrier | Injectable bone paste-dry powder ready to be hydrated | • Osteoconduction • Bioresorbable • Osteoinduction • Osteogenesis when mixed with autogenous bone graft |
• Human studies • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone void filler |
|
| Opteform® | DBM and cortical cancellous chips suspended in gelatin carrier | Formable putty or dry powder ready to be hydrated | • Osteoconduction • Bioresorbable • Osteoinduction • Osteogenesis when mixed with autogenous bone graft |
• Human studies • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone void filler |
|
| Integra Orthobiologics/(IsoTis OrthoBiologic) | Accell Connexus® | DBM, Accell Bone Matrix, Reverse Phase Medium | Injectable putty | • Osteoconduction • Bioresorbable • Osteoinduction |
• Human studies • Case reports • Animal studies • Every DBM lot tested for osteoinduction |
510(k) cleared extremities, pelvis • Bone void filler extremities, pelvis, spine • Bone graft extender |
| Integra Orthobiologics/(IsoTis OrthoBiologic | Accell Evo3TM | DBM, Accell Bone Matrix, Reverse Phase Medium | Injectable putty | • Osteoconduction • Bioresorbable • Osteoinduction |
• Animal studies • Every DBM lot tested for osteoinduction |
510(k) cleared extremities, pelvis • Bone void filler extremities, pelvis, spine • Bone graft extender |
| Accell TBM® | DBM, Accell Bone Matrix | Various sized strips | • Osteoconduction • Bioresorbable • Osteoinduction |
• Human studies • Case reports • Animal studies • Every DBM lot tested for osteoinduction |
510(k) cleared extremities, pelvis • Bone void filler extremities, pelvis, spine • Bone graft extender |
|
| DynaGraft II | DBM, Reverse Phase Medium | Injectable putty | • Osteoconduction • Bioresorbable • Osteoinduction |
• Human studies • Case reports • Animal studies • Every DBM lot tested for osteoinduction |
510(k) cleared | |
| OrthoBlast II | DBM, cancellous bone, Reverse Phase Medium | Injectable putty | • Osteoconduction • Bioresorbable • Osteoinduction |
• Human studies • Case reports • Animal studies • Every DBM lot tested for osteoinduction |
510(k) cleared extremities, pelvis • Bone void filler extremities, pelvis, spine • Bone graft extender |
|
| LifeNet Health | IC Graft Chamber® | DBM particles and cancellous chips | Lyophilized and packaged in various sizes within a delivery chamber | • Osteoconduction • Bioresorbable • Osteoinduction • Designed to be used with blood, PRP or bone marrow to enhance DBM activity |
• Animal studies • Case reports |
• Regulated under CFR 1270 and 1271 as a human tissue |
| Optium DBM® | DBM combined with glycerol carrier | Formable putty (bone fibers) and injectable gel (bone particles) | • Osteoconduction • Bioresorbable • Osteoinduction |
• Human studies • Case reports • Animal studies |
510(k) cleared • Bone void filler |
|
| Medtronic Spinal & Biologics | Osteofil® DBM | DBM in porcine gelatin | Injectable paste and moldable strips | • Osteoconduction • Bioresorbable • Osteoinduction |
• Animal studies • Case reports |
510(k) cleared • Bone void filler |
| ProgenixTM Plus | DBM in Type-1 bovine collagen and sodium alginate | Putty with demineralized cortical bone chips | • Osteoconduction • Bioresorbable • Osteoinduction |
• Animal studies • Case reports |
510(k) cleared • Bone graft substitute • Bone graft extender • Bone void filler |
|
| Progenix™ Putty | DBM in Type-1 bovine collagen and sodium alginate | Ready to use injectable putty | • Osteoconduction • Bioresorbable • Osteoinduction |
• Animal studies • Case reports |
510(k) cleared extremities, pelvis • Bone graft substitute • Bone void filler spine • Bone graft extender: must be used with autograft bone |
|
| MTF/Orthofix | Trinity EvolutionTM | Viable cellular bone matrix | Multiple volumes available | • Osteogenesis • Osteoinduction • Osteoconduction |
• Animal studies • Case reports |
• Regulated under CFR 1270 and 1271 as a human tissue |
| MTF/Synthes | DBX® | DBM in sodium hyaluronate carrier | Paste, putty mix and strip | • Osteoconduction • Bioresorbable • Osteoinduction |
• Human studies • Case reports • Animal studies |
510(k) cleared • Bone graft extender • Bone void filler |
| Osteotech | GRAFTON® A-Flex® | DBM fiber technology | Round flexible sheet | • Osteoinduction • Osteoconduction • Incorporation/complete remodeling • Osteogenesis when mixed with bone marrow aspirate or autogenous bone graft |
• Peer-reviewed published human studies (incl. Levels I–II prospective studies) • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft substitute • Bone graft extender • Bone void filler |
| GRAFTON® Crunch® | DBM fibers with demineralized cortical cubes | Packable graft | • Osteoinduction • Osteoconduction • Incorporation/complete remodeling • Osteogenesis when mixed with bone marrow aspirate or autogenous bone graft |
• Peer-reviewed published human studies (incl. Levels I–II prospective studies) • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft substitute • Bone graft extender • Bone void filler |
|
| GRAFTON® Flex® | DBM fiber technology | Various sizes of flexible sheets | • Osteoinduction • Osteoconduction • Incorporation/complete remodeling • Osteogenesis when mixed with bone marrow aspirate or autogenous bone graft |
• Peer-reviewed published human studies (incl. Levels I–II prospective studies) • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft substitute • Bone graft extender • Bone void filler |
|
| GRAFTON® Gel | DBM in a syringe | MIS and Percutaneous injectable graft | • Osteoinduction • Osteoconduction • Incorporation/complete remodeling • Osteogenesis when mixed with bone marrow aspirate or autogenous bone graft |
• Peer-reviewed published human studies (incl. Levels I–II prospective studies) • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft substitute • Bone graft extender |
|
| GRAFTON® Matrix PLF | DBM fiber technology | Single and double troughs | • Osteoinduction Osteoconduction Incorporation/complete remodeling • Osteogenesis when mixed with bone marrow aspirate or autogenous bone graft |
• Peer-reviewed published human studies (incl. Levels I–II prospective studies) • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft substitute • Bone graft extender |
|
| Osteotech | GRAFTON® Matrix Scoliosis Strips | DBM fiber technology | Various sizes of strips | • Osteoinduction • Osteoconduction • Incorporation/complete remodeling • Osteogenesis when mixed with bone marrow aspirate or autogenous bone graft |
• Peer-reviewed published human studies (incl. Levels I–II prospective studies) • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft substitute • Bone graft extender • Bone void filler |
| GRAFTON® Orthoblend Large Defect | DBM fibers with crushed cancellous chips | Packable graft | • Osteoinduction • Osteoconduction • Incorporation/complete remodeling • Osteogenesis when mixed with bone marrow aspirate or autogenous bone graft |
• Peer-reviewed published human studies (incl. Levels I–II prospective studies) • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft substitute • Bone graft extender • Bone void filler |
|
| GRAFTON® Orthoblend Small Defect | DBM fibers with larger cancellous chips | Packable moldable graft | • Osteoinduction • Osteoconduction • Incorporation/complete remodeling • Osteogenesis when mixed with bone marrow aspirate or autogenous bone graft |
• Peer-reviewed published human studies (incl. Levels I–II prospective studies) • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft substitute • Bone graft extender • Bone void filler |
|
| GRAFTON Plus® Paste | DBM in a syringe | Injectable MIS graft, resists irrigation | • Osteoinduction • Osteoconduction • Incorporation/ complete remodeling • Osteogenesis when mixed with bone marrow aspirate or autogenous bone graft |
• Peer-reviewed published human studies (incl. Levels I–II prospective studies) • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft substitute • Bone graft extender • Bone void filler |
|
| GRAFTON® Putty | DBM fiber technology | Packable moldable graft | • Osteoinduction • Osteoconduction • Incorporation/complete remodeling • Osteogenesis when mixed with bone marrow aspirate or autogenous bone graft |
• Peer-reviewed published human studies (incl. Levels I–II prospective studies) • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone graft substitute • Bone graft extender • Bone void filler |
|
| RegenerationTechnologies | BioSetTM | DBM combined with natural gelatin carrier | Injectable paste, injectable putty, strips and blocks with cortical cancellous chips | • Osteoconduction • Bioresorbable • Osteoinduction |
• Human studies • Case reports • Animal studies • Every lot tested in vivo for osteoinduction |
510(k) cleared • Bone void filler |
| Smith &Nephew | VIAGRAF | DBM combined with glycerol | Putty, paste, gel, crunch and flex | • Osteoconduction • Bioresorbable • Osteoinduction |
• Animal studies | 510(k) cleared • Bone void filler |
| Wright Medical Technology | ALLOMATRIX® | DBM with/without CBM in surgical grade calcium sulfate powder | Various volumes of injectable/formable putty | • Osteoconduction • Bioresorbable • Osteoinduction |
• Human studies • Case reports • Animal studies • Cell culture |
510(k) cleared • Bone void filler |
| ALLOMATRIX® RCS | DBM with CACIPLEXTM technology in surgical grade calcium sulfate powder | Various volumes of formable putty | • Osteoconduction • Bioresorbable • Osteoinduction |
• Animal studies | 510(k) cleared • Bone void filler |
|
| IGNITE® | DBM in surgical grade calcium sulfate powder to be mixed with bone marrow aspirate | Percutaneous graft for problem fractures | • Osteoconduction • Bioresorbable • Osteoinduction |
• Human studies • Case reports • Animal studies • Cell culture |
510(k) cleared • Bone void filler |
|
| PRO-STIM™ Injectable Inductive Graft | 50% calcium sulfate, 10% calcium phosphate, and 40% DBM by weight | Procedure kits, various volumes of injectable paste/formable putty | • Osteoconduction • Bioresorbable • Osteoinduction |
• Case reports • Animal studies |
510(k) cleared • Resorbable calcium salt bone void filler device |
|
| Zimmer | Puros® DBM | Allograft DBM putty (putty with chips includes allograft chips from the same donor) | Putty and putty with chips | • Osteoconduction • Bioresorbable • Osteoinduction |
• Every lot tested in an in vivo rat assay for osteoinductive potential demonstrating bone formation in an ectopic model | • 100% derived from allograft tissue • Regulated under 21 CFR Parts 1270 and 1271 as a human tissue |
The current most popular clinical DBM format is a moldable putty that can be packed into bone defects and resists dispersion from irrigation and blood during surgery. Conversion of DBM powder to putty involves formulation with a biocompatible viscous carrier that provides a stable suspension of DBM powder particles. The viscous carriers can be classified as water-soluble polymers such as sodium hyaluronate or carboxymethylcellulose, or anhydrous water-miscible solvents such as glycerol. In some cases, the carrier selection has implications for other processing steps, compatibilities, applications, and even sterilization. Further, DBM can be mixed with these carriers to produce flexible sheets that may contain both DBM and cortical bone chips as a composite biomaterial. Additionally, the polymer carrier Pluronic (BASF product, synonymous with poloxamer), is a temperature-sensitive biomedical copolymer carrier used with DBM. The composition becomes firmer as it warms to body temperature. Another carrier is a thermoplastic, porcine collagen‐based hydrogel that is non-water‐soluble. The DBM-porcine collagen can be extruded through a syringe after it is heated to 46–50 °C. In situ at body temperature the composition becomes firm.
Studies designed to discriminate the effectiveness of various carriers on DBM (pre)clinical efficacy are limited. Wang implanted athymic rats with commercially available DBMs Dynagraft putty, Grafton putty, or Osteofil allograft bone paste [45] for spinal fusion. Most of the segments implanted with Grafton and Osteofil fused and none of the segments implanted with Dynagraft fused. Sassard and colleagues reported on a retrospective review of patients who had undergone instrumented posterolateral lumbar spinal fusion with an autogenous bone graft and Grafton gel [46]. Age-, gender-, and procedure-matched group of patients were involved. There were no differences between treatment groups (autograft vs autograft plus Grafton) based on radiographs up to 24 months after surgery. The fusion rates in the autograft with Grafton group and the autograft-only groups were only 60% and 56%, respectively. The most important predictor of 24-month bone mineralization was a correlation between the type of instrumentation and fusion success. Bostrom and co-workers implanted Grafton subcutaneously into athymic rats and reported acute tubular necrosis [47]. It was speculated the glycerol component in Grafton led to the nephrotxoicity. However, the dose was 10 times the human clinical dose. Nevertheless, glycerol-containing products should be used with caution in pediatric patients and in those at risk of renal disease [48].
Acarturk and Hollinger determined in a pre-clinical model in an orthopic critical-sized defect site that treatment with either Grafton or DBX promoted significantly more bone regeneration than other DBM-carrier products [49] They concluded that differences in osteogenic activity among commercial DBM products may be related to differences in the carrier, the amount of DBM in the carrier and ability of the carrier to localize the DBM particulate to the bone defect site for a sufficient period to promote bone regeneration.
An important variable inherent to these products is that the DBM content among different commercial composites (i.e., DBM plus carrier) is non-standardized and inconsistent. Therefore, different DBM doses will be delivered to tissue sites by different products. This could explain the variability in responses reported by Acarturk and Hollinger [49]. Moreover, individual ‘DBM lots’ processed by the same tissue bank may possess different osteoinductive capacities, and these capacities may vary among different donors. While biological activity is measured by osteoinduction and is reported as the osteoinductive index, OI, no DBM “potency” or “compositional” regulatory standards are enacted across DBM products to understand or control these differences. It is also noteworthy to emphasize the significance of shelf life and carrier stability for DBM composite products. If precise control of conditions is not maintained, then endogenous osteogenic proteins in the DBM, most importantly, BMPs, might be susceptible to chemical and physical degradation [50]. Variations in the shelf life of a specific carrier may or may not affect the overall activity of the product. This is an important clinical and manufacturing issue that has not been sufficiently described or controlled.
6. DBM sterilization
DBM is considered by the USA FDA not to be a medical device and it is categorized under the heading of human cells, tissues, and cellular and tissue-based products (HCT/PS). When DBM is combined with a ‘synthetic carrier’, it becomes a device. With all implants, there is a finite increased risk of infection with DBM implantation. Medical devices must be provided to the surgeon in a certified sterile condition. The FDA regulates the sterility requirements for devices and the most recent guidance document for sterility of 510(K) medical devices such as DBM was provided in August 2002 (Updated 510(K) Sterility Review Guidance K90-1; Guidance for Industry and FDA). The degree of sterility must be quantified to provide an achievable benchmark or set point for industry. Currently the sterility assurance level (SAL) required for DBM and all other implants is set at SAL 10− 6. This SAL nomenclature signifies that no more than one unit out of one million devices sterilized would fail sterility testing. Implant sterilization methods are categorized by the FDA as either “Traditional” or Non-Traditional” methods. Traditional methods include dry heat sterilization, moist heat sterilization, ethylene oxide (ETO) in a fixed ETO chamber, gamma and electron beam radiation and liquid chemical sterilants. Non-traditional methods are defined as ETO not using fixed chambers, high intensity light, chlorine dioxide, ultraviolet light, combined vapor and gas plasma, vapor systems (peroxide or peracetic acid) and filtration. Manufacturers of DBM can use either category provided that the SAL 10− 6 threshold is met. Table 1 lists methods applied to specific products.
While there is evidence that demineralization processes can inactivate certain viruses [51], tissue banks and industries that process and prepare DBM products rely on additional steps to ensure that the DBM is free of bacterial and viral contamination. Sterility is a challenge for producers of DBM-based products. The sterilization protocol may inactivate or attenuate the BMPs in DBM that confer its clinically important biological activity. Terminal sterilization of DBM was reported 30 years ago [52]. Sterilization methods and the resulting impact on OI have been recently reviewed [53], [54]. Munting and co-workers prepared rat DBM preparation and sterilized it using different protocols: gamma irradiation, merthiolate, glutaraldehyde, formaldehyde and ethylene oxide (ETO) [55]. Glutaraldehyde, formaldehyde and ETO abolished OI while merthiolate and gamma radiation were less damaging to the OI. Other reports document the impact of sterilizing BMPs derived from DBM [56], [57]. However, it is unclear whether the BMP within the DBM will be affected differently than BMPs extracted from the DBM and sterilized alone.
The lack of predictive tools for assessing the impact of sterilization protocols on OI underscores the need to test sterilization methods for particular DBM preparations and compare different methods on different DBM preparations. Traditionally, certain chemical sterilization methods such as alcohol treatment and combinations of solvents and detergents yielded positive results and these methods are still in use. Suitable conditions were also identified for radiation-based methods. Radiation doses of approximately 3–5 Mrad are considered sufficient to sterilize medical implants and these doses are within a range shown not to degrade DBM [58], [59], [60] and also capable of inactivating certain viruses [61].
Additional sterilization methods such as e-beam [62], [63] and hydrogen peroxide gas plasma [64] have been successfully used. In contrast, satisfactory methods for ETO sterilization remained more challenging. The advantages of ETO sterilization are that, next to gamma irradiation, it is an industry standard. In the context of allografts there is evidence ETO can deactivate viruses [65]. However, studies suggested an ETO dose-dependent decline of OI [66], [67] while others show little loss of activity [5], [68], [69], [70].
The sterilization controversy may hinge on interactions between physical properties of the DBM preparation and process variables associated with its sterilization cycle. For example, a study that evaluated the pro-inflammatory responses to ETO-sterilized DBM in vitro suggested small particle sizes are more pro-inflammation than larger particles [71]. In another study the humidity during the ETO exposure process modulated the effects of ETO on OI [72]. As a reactive gas, ETO penetrates and sorbs into soft, porous biomaterials like DBM so that de-gassing of ETO commonly used for porous implants might be poorly controlled in these comparative studies and remains partially unaddressed. Empirical studies suggest that procedures for ETO sterilization of DBM appear to be a combination of physical processing steps, including adequate removal of lipids, time and temperature for ethylene oxide exposure as well as the thoroughness of the process to remove residual ETO and reactant products ethylene glycol and ethylene chlorohydrin [7].
Given the potential for traditional and non-traditional sterilization methods to adversely impact DBM OI, many manufacturers have resorted to the more expensive option of aseptic processing. Sterility is achieved by aseptic processing through a combination of manufacturing controls and standard operating procedures. The FDA provided a guidance document in September 2004 entitled “Guidance for Industry, Sterile Drug Products Produced by Aseptic Processing-Current Good Manufacturing Practice to help manufacturers achieve sterility through aseptic processing. The document provides information on the engineering and human controls that can be used to ensure sterility. Compliance with the guidelines to ensure a sterile product depends on adequate manufacturing spaces called “clean rooms” with sufficient air quality controls. Clean rooms must have High-Efficiency Particulate Air (HEPA) filtration and allow entry and exit of staff and materials without the introduction of infectious agents. Chemical and physical conditions typical of other sterilization methods that could degrade or alter biologically derived substances are avoided in the aseptic processing of DBM. Several DBM manufacturers have endorsed the cost effectiveness of aseptic processing in preserving the DBM OI.
6.1. DBM storage conditions and shelf life
DBM producers are motivated to develop DBM with the longest shelf life and broadest stability under various storage parameters so that storage and distribution systems are streamlined and cost effective. Room temperature storage is the most inexpensive mode and is often considered a product feature promoted in marketing biomaterials. Storage conditions and product expiration dates must be explicitly stated in the DBM product insert. Most producers of DBM test transient product exposures to elevated temperatures to simulate potential temperature extremes that can occur during shipping. If temperature limits are identified, then manufacturers place temperature limit indicators on product packaging and clinicians are directed to not use the product if the indicator has been triggered. Unfortunately, there is a limited understanding of how storage conditions impact DBM shelf-life and OI. Consequently, different DBM producers may indicate on their respective packet inserts either storage at room temperature or freezing the DBM product. A recent report sheds light on a potential mechanistic approach to storage and stability issues for DBM [73]. The authors used an in vitro alkaline phosphatase assay and an ectopic bone formation assay in nude mice to determine the OI of either hydrous or anhydrous DBM preparations after prolonged exposure to temperatures as high as 65 °C. Anhydrous DBM was substantially more resistant to temperature related effects on OI than hydrous versions of DBM.
6.2. Distinguishing DBM products for clinical applications
The decision to select one type of DBM-carrier formulation over another may be determined by the desired clinical target. For example, in some clinical situations, such as a bone void, an injectable formulation would be favored whereas in other clinical situations that may include a spine fusion procedure, a flexible putty may be better advocated. However, the clinical target alone may be insufficient to distinguish one product formulation from another given the redundancy among several manufacturers and their DBM product lines. Consequently, it is necessary to compare similar DBM products in preclinical models to determine outcome efficacy and clinical handling advantages. Table 2 provides such a comparison.
Table 2.
Comparative testing of commercially available DBM products.
| Reference | Animal model | Materials tested | Comments |
|---|---|---|---|
| Schwartz et al. [74] | Ectopic bone formation in nude mice | 14 lots of DBM from six different processors | Samples from three banks formed bone after 1 month and samples from two banks formed bone after 2 months. Samples from one bank failed to from new bone. |
| Bostrom et al. [47] | Ectopic bone formation in athymic rats | Grafton Dynagraft Osteofil, Opteform |
Toxicity associated with the glycerol carrier used in Grafton confounded the comparative analysis |
| Takikawa et al. [75] | Ectopic bone formation in athymic rats | Grafton Osteofil Rat DBM |
No significant differences in bone formation |
| Oakes et al. [76] | Femoral defects in athymic rats | Grafton DBX |
No significant differences in radiographic scores Endochondral ossification more prominent in DBX |
| Bomback et al [77] | Single level interprocess spinal fusions: athymic rats | Grafton OP-1 Autograft |
OP-1 promoted 100% fusion rates at 3 and 6 weeks while Grafton promoted 13% and 33% respectively. |
| Peterson et al. [78] | Spinal fusion in athymic rats | Grafton DBX Allomatrix |
Manual palpation after 8 weeks: 6 of 6 fusions for Grafton 3 of 6 fusions for DBX |
| Acarturk and Hollinger [79] | Critical sized defects in athymic rats | Allomatrix, DBM Grafton Dynagraft DBX Regenafil |
Statistically significant differences observed between DBM preparations; less new bone was observed with Allomatrix; Meshplate: Dynagraft; and Regenafil. |
Differences in outcome measures, methods and animal models make direct comparisons among these study reports difficult. However, a few trends may be determined. Notably, differences among the OI across DBM preparations from different sources exist as seen in Table 2. As mentioned above these differences may be caused by DBM processing and donor variables. Processing can include sterilization protocols versus aseptic procurement, as well as the quantity and OI potency per unit volume of DBM found within the carrier. Consequently, since processing and sterilization methods may change over time, it is useful to collect contemporary comparative data in standardized models, especially in light of the recent 510(K) clearance requirements that mandate controlled and validated manufacturing processes. Unfortunately, peer-reviewed reports are not routinely available from non-biased sources and companies often provide their own data to indicate superiority of their DBM product line without detailed scientific validation.
7. Regulatory issues
Given its unique sourcing from human bone tissue, the regulation of DBM is fairly unique and interesting. Three major aspects of the DBM medical device supply chain are regulated:
-
1.
donation of the tissue and its qualification for processing;
-
2.
processing methods and product quality;
-
3.
approval to distribute the finished product for specific clinical indications.
In the first instance, the Uniform Anatomical Gift Act enacted in 1968 and revised in 1987 was the first legislation passed into law that defined how human tissue is legally donated. The act defines the steps for donation of tissue and also establishes the principle that donated tissue may not be sold or purchased. The foregoing does not exclude “…reasonable payment for the removal, processing, disposal, preservation, quality control, storage, transportation, or implantation…” This provision of the law is particularly important for DBM because it provides the basic framework that enables processing facilities to invest in the development cost and capital equipment necessary to process bone into DBM and provide it as a clinically useful product.
Additional government regulations are promulgated by the Food and Drug Administration (FDA). As described above, the DBM source procurement process is a regulated activity with the goal of limiting the risk of disease transmission. The resulting products were regulated for many years as human cell, tissue or cellular and tissue based products (HCT/P) regulated under section 361 of the Public Health Service (PHS) Act and in 21 CFR Part 1271.
Under the HCT/P regulation, processing facilities were originally permitted to distribute DBM without the FDA premarket approval process. However, in early 2001 the FDA further clarified the products that can be solely regulated as HCT/P such that the regulations apply only if:
-
1.
HCT/P is minimally manipulated;
-
2.
HCT/P is intended for homologous use only;
-
3.
manufacture of the HCT/P does not involve the combination of the cell or tissue component with a drug or device, except for a sterilizing, preserving, or storage agent, if the addition of the agent does not raise new clinical safety concerns with respect to the HCT/P; and
-
4.Either:
-
A.HCT/P does not have a systemic effect and is not dependent upon the metabolic activity of living cells for its primary function; or
-
B.HCT/P has a systemic effect or is dependent upon the metabolic activity of living cells for its primary function, and is for:
-
1)autologous use
-
2)allogeneic use in a first or second degree relative; or
-
3)reproductive use.
-
1)
-
A.
Importantly, the FDA concluded that DBM carriers for improved use and handling do not satisfy the provision of “minimal manipulation”. By issuing the new rule, DBM preparations became regulated under the FDA's 510(k) approval process. The 510(k) approval process required medical device manufacturers to disclose to the FDA their manufacturing process, quality control methods and animal safety testing data for review and approval prior to marketing the DBM-based device. In the 510(k) approval process, the FDA also determines the specific label claims and indications that distributors can communicate to clinicians.
In the years following that ruling, the FDA sent a number of letters to processors and distributors of DBM identifying deadlines for submission of 510(k) applications. Submitted 510(k) applications for DBM product approval were received by the FDA in 2005. Non-compliant producers of DBM and DBM-carriers had their products removed from the market.
A final comment on regulatory issues for DBM is that the tissue banking industry has relied on peer group organizations to maintain standards such as those produced by the American Association of Tissue Banks (AATB) and the American Society for Testing and Materials (ASTM). The AATB was founded in 1976 as a nonprofit organization to facilitate the quality and availability of allograft cells and tissues. The ASTM is a century-old organization that provides standardized testing methods for many industries at an international level. The AATB and ASTM provide a resource to standardize procurement and processing methods. These organizations have been highly beneficial in improving the quality of products such as DBM and DBM-carrier combinations.
8. DBM biological considerations
8.1. Osteoconductivity
The original Urist definition of osteoconduction is [18]:
‘The three-dimensional process of ingrowth of sprouting capillaries, perivascular tissue, and osteoprogenitor cells from the recipient bed into the structure of an implant or bone graft.’
The concept was further detailed by Glowacki and Mulliken [7]:
‘Osteoconduction occurs as the dead bone acts as a scaffold for the ingrowth of vessels, followed by resorption of the implant and deposition of new bone derived from the edges of the defect. This process is very slow and may require years to unite a large segmental defect.’
8.2. Osteoinductivity
The origin of the term “osteoinduction” is also Urist's, and was as accurate in 1971 as it is today [29]:
‘The process of differentiation of pluripotential mesenchymal cells into osteoprogenitor cells and ultimately into osteoblasts that form bone as a consequence of a stimulating agent, that is, bone morphogenetic protein.’
Urist [80] emphasized that:
‘Bone matrix is … a morphogenetic substratum (and) … the solid structure supports morphogenesis … . (Further) … the (bone matrix) substratum play a … permissive (supportive) role in morphogenesis.’
Reddi further underscored not only the role of bone matrix but also the anatomic field from which it was derived [81]:
‘On allogeneic transplantation demineralized bone matrix of rat transforms responding fibroblasts to chondroblasts and osteoblasts. Transformation … is critically dependent on the geometry and surface charge (authors' emphasis) of the transformant and on the site where the ossicle is created. The transforming potency varies widely in matrices of different bones of the rat; the demineralized residues of tubular bones are more active than matrices of flat bones.’
The teachings of this bone induction principle as it related to demineralized bone matrix were an epochal event coming from Reddi's laboratory at the USA's NIDR lab [34]. In 1981, Sampath and Reddi tested the hypothesis that ‘a portion dissociatively extracted from the DBM was the inductive factor’. Significantly, the matrix itself was not inductive as Reddi succinctly restated almost 20 years later [82]:
‘Thus, it would appear that for optimal osteogenic activity there was a collaboration (authors' emphasis added) between soluble extract and the insoluble collagenous substratum.’
Glowacki and Mulliken in 1985 [7] further refined the definition of osteoinduction:
‘(It) is the phenotypic conversion of connective tissue into bone by an appropriate stimulus. This concept implies that formation of bone can be demonstrated in nonskeletal sites. Demineralized bone and dentin are such osteoinducers.’
8.3. Osteoinduction: donor gender and age
Banked donor bone can have diverse biological properties as a consequence of donor age and gender. The effects of donor age and gender on DBM osteoinductivity are the most accessible variables to explore. However, there is limited data to date to support exclusionary criterion based upon either one. A potential age-related factor may include metabolic competence and relevancy to the balance between bone resorption and deposition. Investigations of correlative properties in vitro suggest age-related effects. For example, Groessner-Schreiber et al. reported osteoclast recruitment assessed in a chick chorioallentoic membrane assay was decreased in human DBM prepared from donors of increasing age [2]. However, their report used DBM from a cohort of only eight donors ranging in age from 18 to 72. Consequently, the study may have been insufficiently powered. Aaboe and colleagues found that ectopic bone formation by DBM from differently aged rats seemed to indicate a modest age-related increase in osteoinductivity from younger vs. older rat donors [3]. Moreover, an evaluation of human DBM preparations from age and sex stratified donors indicated a correlation between donor age and the amount extractable BMP-2 suggesting with an increase in age there was a decrease in BMP content (reviewed in [83]). A similar profile was observed for BMP-4 [84]. In contrast to these reports, no significant correlation was suggested between donor age and BMP-2, -4 and ‐7 [83]. Given that BMP-2 levels are in the nanogram per gram level in cortical bone, it is unclear that age related effects of either BMP-2 or TGF- are clinically meaningful [83].
Aside from BMPs in DBM, there may be additional endogenous growth factor considerations. The quantity of insulin-like growth factor I (IGF-I) and transforming growth factor beta (TGF- in DBM may have co-regulatory affects on BMP [85]. It is noteworthy that IGF-I and TGF-remain relatively consistent during aging [86]. However, Bae and co-workers have reported variability in growth factor content across commercial DBM preparations [87]. It is noteworthy however, that differential growth factor extraction efficiencies both during DBM processing and during growth factor content assays might have contributed to suggested DBM product content differences.
Donor-recipient gender and age have been mentioned as variables that could affect the activity of DBM. However, as of the preparation of this review, data neither validate the speculation that donor age and gender affect DBM activity nor does recipient age influence response to DBM. Work by Schwartz and colleagues found no evidence for gender related differences in DBM OI [4]. Moreover, Zhang's group reported OI of DBM that suggested males between 41 and 50 years of age and females between 51 and 60 years of age had a better OI than DBM prepared from young donors [5].
In contrast, Lohmann and coworkers reported that increasing donor age decreased OI [88]. They prepared DBM from donors with an average age of 32.8 years and 75.6 years and found the 32.8 year-old average-aged cohort had a higher OI than the older group. Additional work from the Lohman group suggested a decrease in OI with an increase in age of the donor. Data from this group has not been validated by others. For example, Traianedes and colleagues determined the OI of human DBM prepared from 133 male and 115 female donors [89]. Data indicated DBM from donors as old as 85 had an OI comparable to younger donors.
In the absence of convincing data to the contrary and until the work from the Lohman group can be duplicated that suggests age affects on DBM OI, definitive guidance for tissue processors of DBM use an age cut-off of 70 for donor acceptability and there is no gender differentiation.
8.4. In vitro biological assays
The Glowacki–Mulliken definition of osteoinduction (vida supra) clearly raised the bar for osteoinduction validation for all products. Their accepted definition derived from Urist and Reddi underscores that an in vivo environment will be required to test osteoinduction. It is a challenge to duplicate complex biological in vivo environment with in vitro assays. This is, however, a challenge that needs to be addressed by the commercial sector selling DBM products. There is a profound and compelling fiduciary stimulus to develop an in vitro correlate to the in vivo environment that reliably validates DBM biological activity (that is, accurately verify osteoinduction). The simple rationale for the corporate sector is that in vitro assays are quicker and less expensive than in vivo assays!
A number of skillful workers have tried to develop in vitro paradigms with selected bone cell lines and identify marquis bone markers that may be used to verify biological activity of DBM [90], [91]. Carnes and co-workers [90] reported that the 2 T9 cell line is not effective to verify osteoinductivity and concluded that:
‘This study underscores the frustration inherent in developing a reliable and rapid assay of bone induction ability, either in cell culture or in vivo.’
In contrast, Adkisson and colleagues reported that Saos human osteosarcoma cells proliferate in response to DBM [91]. While these transformed cancer cell culture proliferation results are not directly supportive, this response can be correlated to in vivo osteoinduction. Nonetheless, the bold conclusion by Glowacki as a basic bone biology expert is [53]:
‘Evaluation of the current state of knowledge leads to the fact that we cannot conclude that performance of different lots of demineralized bone allograft in in vivo or in vitro test systems can be used as a measure of clinical performance. It may be possible to adopt an osteoinductivity standard for release-to-market, but it should be followed by clinical monitoring and further research.’
8.5. In vivo biological assays
Several fundamental criteria must be fulfilled by a successful biological osteoinductive assay. The assay must be standardized and reproducible within the same laboratory and among different laboratories. The outcome must be accurate and quantifiable within the same ‘batch’ of DBM and across ‘batches’ within each laboratory and among laboratories. Moreover, if the biological assay will be used as a clinical predictor of performance, it must correlate accurately and precisely to clinical outcome.
The standard in vivo biological assay for bone induction is the mouse ‘hamstring’ implant described by Urist in 1965 [28]. Numerous variations have followed that include both subcutaneous and pectoralis major implantations.
The procedure to assay DBM for OI biological activity involves implanting a known weight (e.g., 25 mg) of DBM into the rodent muscle. While loading is critical to stimulate new bone, DBM implantation is performed in the absence of controlled mechanical loading. After approximately 28 days, the rodent is euthanized and the explanted muscle mass is radiographed and tissues are processed for histological assessment. The radiograph is examined for radiopacity. The histological tissue assessment includes a subjective grading of bone formation among a number of random histological sections at a certain magnification using a stain of choice for the laboratory (e.g., Goldner trichrome). An arbitrary ‘value’ (i.e., some numerical ‘grade’) is given by the examiner to the histology slide based on the bone observed without any clear standard or endpoint for bone architecture defined for the field. Because of this, there may be little relevance for numerical scores among different bone banking laboratories and sometimes there are differences in ‘scores’ within the same laboratory. Therefore, a standardized procedure needs to be adopted for bone banks for ‘grading’ biological (e.g., osteoinductive) outcome from the DBM in vivo assay. Standardization in ‘grading’ is necessary to ensure suitable quality control both within the same bone bank laboratory and among different bone bank laboratories.
9. Clinical targets
Clinical trends have witnessed an increasing passion for biological research to stimulate the innate capacity of bone to regenerate itself in a more rapid and predictable manner, irrespective of the nature of the bone malformation, defect or fracture. It has been logical to use DBM with its inherent BMPs and other growth factors to modulate bone repair. However, to date the most impressive implant results still come from DBM in conjunction with autogenous bone grafts, as this combination provides a scaffold, viable cells, and proper signals to effectively recapitulate tissue regeneration. As mentioned above, demineralized bone products exist in many forms (powders, putties, flexible sheets, and gels, Table 1), but their desired clinical outcome is what dictates the form used.
9.1. Composite grafts
Burwell first suggested that the osteoinductive potential of alloimplants could be maximized if they were combined with autogenous marrow [92]. Pike and Boyne later used surface-decalcified allogeneic bone as bone graft trays to retain autogenous cancellous marrow [93]. Combining autogenous bone and marrow with demineralized products is known to provide greater osteogenic potential than either bone marrow or demineralized matrix alone [94], [95].
9.2. Dental applications
Demineralized bone products are used in dentistry primarily in periodontal regeneration and in reconstruction of the jaws for dental implant placement. Periodontal regeneration is challenging because grafting is performed in an infected site, often complicated by patients who smoke or are diabetic. Furthermore, the grafting attempts to reconstruct not only bone, but also cementum, and periodontal ligament. The classical approach to periodontal regeneration has been to use bone grafts. However, demineralized products began to attract attention for periodontal regeneration in the early 1980s [96], [97]. Bowers and colleagues showed that the average new attachment formation was only 1.21 mm compared to no attachment in periodontal defects that were only debrided [98], [99].
Currently, three questions dominate the dental field for DBM:
-
1)
Are demineralized bone products osteoinductive?
-
2)
Are demineralized products significantly better for periodontal regeneration than other products (e.g., mineralized xenografts, hydroxyapatite, polymers, bioactive ceramics, growth factors, such as rhPDGF-GEM21S: BioMimetic Therapeutics, Inc.)?
-
3)
What is the long-term fate of periodontal defects grafted with demineralized bone?
Recent data from several clinical studies now validate superiority of the rhPDGF-containing product GEM21S that is FDA-approved for periodontal regeneration (BioMimetic Therapeutics, Inc., USA) over either DBM or DFDBA [100], [101], [102].
9.3. Cranial and facial surgery applications
Craniofacial applications of demineralized bone products are limited by the current product designs. Contemporary demineralized products are designed as bone fillers for bone defects that have ‘walls’, that is, the defect is bounded by existing, intact bone. Craniofacial applications are challenging, requiring structural support and protection of the dura as essential elements of a successful bone substitute material. In the early 1980s, Mulliken et al. [103] reported on demineralized bone powder for cranial reconstruction. Since then, several groups have used demineralized bone preparations for cranial reconstruction and in the reconstruction of nasomaxillary cleft defects [104], [105]. Ousterhout placed demineralized implants in facial defects and LeFort I osteotomies [106]. He found that demineralized products to augment facial contours resulted in considerable resorption, while demineralized bone in defects generally resulted in satisfactory osteogenesis.
9.4. Applications in hand and foot surgery
Applications of demineralized bone products in hand surgery have been reported with limited frequency. Demineralized bone products have been successfully used in the reconstruction of phalangeal and metacarpal defects following enchondroma and congenital hand surgery [107], [108]. Similarly, demineralized bone products in foot surgery have been infrequently reported [109], [110]. Weinraub and Cheung used allogeneic bone either with or without demineralized bone in several procedures including arthrodesis, segmental lengthening, and treatment of nonunion. Michelson and Curl evaluated demineralized and autogenous bone for subtalar fusions and triple arthrodesis in 55 patients over a 5 year period [111]. They concluded that demineralized bone grafts aid arthrodesis at least as well as iliac crest bone graft and without the increased blood loss, cost, and postoperative pain associated with iliac crest bone harvest.
9.5. Orthopaedic applications
Autogenous bone is the grafting material of choice in orthopaedic science. However, demineralized bone has garnered attention in the repair of cysts [112], fractures, segmental bone defects, and non-unions. Tiedman and his group used demineralized bone, alone, and as a composite graft with autogenous bone marrow in orthopaedic applications [113]. They concluded that DBM and marrow composite grafting is a suitable alternative to autogenous iliac crest bone graft for clinical situations such as bone defects in children, comminuted fractures with associated bone loss, non-united fractures, or for augmentation of arthodesis [113]. In one of the few prospective studies using demineralized bone products in orthopaedics, Geesink et al. [114] compared Osteogenic Protein-1 (OP-1: Stryker Biomedical, Inc.; also known as rhBMP-7) to Grafton Gel in fibular defects. At early time points, OP-1 had superior union, however, at 12 months, there was no difference in bone mineral density scores between OP-1 and DBM Grafton Gel. Although controversial, controlled studies and anecdotal reports suggest that bone graft substitutes, including demineralized bone matrix, may result in improved treatment outcomes for patients with fractures of the distal radius [115].
9.6. Applications in spinal surgery
Spinal fusion using autogenous bone is a gold-standard treatment for many disorders of the spine. Sassard et al. [116] and Cammisa and co-workers [117] evaluated the mineralization efficiency and bone mass in patients have posterolateral lumbar spinal fusion with either autogenous bone alone or as a composite with Grafton DBM. Fusion rates and mineralization efficiency were similar for both groups. Simpson et al. [118] analyzed fusion results of an allograft-Grafton composite versus autograft in a prospective series of patients undergoing anterior cervical fusion surgery for cervical disc disease. They concluded that autografts demonstrated less graft collapse and pseudoarthrosis compared to the allograft-Grafton composite group. Overall differences in study outcomes from pre-clinical and clinical reports likely reflect differences among animal models (pre-clinical), DBM formulations and outcome measures [119].
9.7. Summary of DBM clinical utilities: lessons learned
The knowledge gained over decades from DBM product use has led to general guidelines concerning clinical application. First, using demineralized bone in conjunction with autogenous bone demonstrates a synergistic osteogenic response. Second, demineralized bone products in bone defects provide qualitative and quantitative bone healing results depending on the volumetric characteristics of the defects. Osseous defects with only two walls (e.g., segmental or continuity defects) would be expected to have less predictable osteogenesis than defects with only one wall. Third, demineralized bone products are not advised for augmenting facial and cranial contour deficits due to the tendency for resorption. Fourth, considerable variability in tissue banking processing of demineralized products, as well as lot-to-lot variability in composition and OI are confusing and concerning still to DBM use. Though DBM can be successful if used alongside allograft bone, its use alone in applications undergoing high compressive loads is contraindicated, leading to greater graft collapse in the inherent absence of structural rigidity.
10. Summary and outlook
The clinical demand for bone void fillers that obviate the need to harvest autograft has prompted the development of synthetic and biological autograft substitutes. Among the clinically most successful filler is DBM. DBM is osteoconductive, osteoinductive, and relatively easy to use clinically, especially in carrier formulations of diverse offerings. Moreover, DBM can extend the volume of an autograft. Maturation of DBM as a bone biology tool to understand BMP growth factor biology and pioneer bone regenerative recombinant therapeutics has led to tissue banking protocols that have standardized donor procurement, processing and sterilization. DBM initially was a non-FDA regulated tissue-derived product. However, as its clinical appeal expanded, innovative DBM formulations with carriers were designed and developed to increase clinical utility. Consequently, with this manipulation for clinical use, the FDA now regulates DBM as a 510(k) medical device. Therefore, DBM products must satisfy regulatory guidelines to ensure biocompatibility, manufacturing validation, shelf life and osteoinductive potential. It is the latter biological property that garners the most interest from clinicians in bone applications: DBM's intrinsic osteoinductive mechanism by releasing endogenous BMPs and growth factors by diffusion and matrix biodegradation.
Compelling questions regarding DBM's future utility in this regard remain to be answered:
-
1.
How to standardize DBM OI across the bone banking industry? Can improved processing and quality control assist such standardization?
-
2.
Is there a DBM threshold in a carrier formulation that must be fulfilled to ensure optimum OI?
An important concern in the DBM industry is the lack of reliable correlation metrics between OI potential assessed by in vitro and in vivo models and that significant to human clinical efficacy. While comparative preclinical performance has been pursued in animal models, it may be impractical to address this in the human clinical setting. Despite the lack of comparative clinical performance data, clinicians will benefit from the emerging competition among manufacturers to provide improved DBM formulations with rigorous testing and validation of the OI.
Other outstanding issues regarding DBM clinical use that lack current supporting studies to date include:
-
1.
What is the impact on DBM OI of a recipient patient who is osteoporotic? On bisphosphonate activity? On NSAIDs pharmacology? For patients who smoke or are diabetic?
-
2.
Are there unique regional physiological differences across the appendicular, axial and craniofacial skeletons that may require designer DBM compositions and formats with improved clinical outcomes?
-
3.
Can DBM compositions be designed that are load-bearing and calibrated to remodel with bone regeneration?
New work must be done to address these clinically relevant issues affecting DBM efficacy.
Overall, the bone graft substitutes market is growing at a rate of about 2.9% per year while the DBM segment expands at a more modest 4% (US Markets for Orthopedic Biomaterials 2011, Millenium Research Group, September 2010). Consequently, given commercial distractions by new biotechnologies that might one day supplant DBM, it may be up to academic institutions rather than within corporate and bone bank organizations to address important unanswered DBM scientific and therapeutic questions, concerns and issues relevant to future clinical opportunities. DBM OI modulation, standardization, and product validation seem like near-term needs. However, despite the modest product growth rates anticipated for DBM in the surgical marketplace, DBM is currently a highly valued therapeutic device for surgeons that should continue to remain important, especially in light of emerging markets and current health care systems that cannot bare substantial costs associated with recombinant growth factor delivery (see contributions in this theme issue on protein therapeutics for bone). Consequently, research efforts with DBM must be sustained to expand clinical applications, produce validated utility, demonstrate new options and opportunities to enhance clinical outcomes in bone repair.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Targeted delivery of therapeutics to bone and connective tissues”.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.addr.2012.06.008.
Appendix A. Supplementary data.
Supplementary materials.
References
- 1.Group M.R. 2005, 2004. US Markets for Orthopaedic Bioimaterials. [Google Scholar]
- 2.Groessner-Schreiber B., Krukowski M., Lyons C., Osdoby P. Osteoclast recruitment in response to human bone matrix is age related. Mech. Ageing Dev. 1992;62:143–154. doi: 10.1016/0047-6374(92)90051-e. [DOI] [PubMed] [Google Scholar]
- 3.Aaboe M., Pinholt E.M., Schou S., Hjorting-Hansen E. Incomplete bone regeneration of rabbit calvarial defects using different membranes. Clin. Oral Implants Res. 1998;9:313–320. doi: 10.1034/j.1600-0501.1998.090504.x. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz Z., Somers A., Mellonig J.T., Carnes D., Dean D., Cochran D., Boyan B. Ability of demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but not gender. J. Periodontol. 1998;69:470–478. doi: 10.1902/jop.1998.69.4.470. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M., Powers R.M., Jr., Wolfinbarger L., Jr. A quantitative assessment of osteoinductivity of human demineralized bone matrix. J. Periodontol. 1997;68:1076–1084. doi: 10.1902/jop.1997.68.11.1076. [DOI] [PubMed] [Google Scholar]
- 6.Harakas N.K. Demineralized bone matrix-induced osteogenesis. Clin. Orthop. Relat. Res. 1984;188:239–251. [PubMed] [Google Scholar]
- 7.Glowacki J., Mulliken J.B. Demineralized bone implants. Clin. Plast. Surg. 1985;12:233–241. [PubMed] [Google Scholar]
- 8.Meek'ren J.J.V. HT Bloom; Amsterdam: 1632. Observationes medico-chirurgicae, Observationes medico-chirurgicae; p. 6. [Google Scholar]
- 9.Macewen W. Observations concerning transplantation of bone. Proc. R. Soc. London. 1881;32:232–237. [Google Scholar]
- 10.Senn N. On the healing of aseptic cavities by implantation of antiseptic decalcified bone. Am. J. Med. Sci. 1889;98:219–243. doi: 10.1097/00000658-188907000-00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deaver J.B. Secondary bone implantation by a modification of Senn's method. Med. News. 1889;55:714–721. [Google Scholar]
- 12.Curtis B.F. Case of bone implantation and transplantation for cyst of tibia, osteomyelytic cavities and ununited fracture. Am. J. Med. Sci. 1890;106:30–34. [Google Scholar]
- 13.Mackie W. Clinical observations of the healing of aseptic bone cavities by Senn's method of implantation of antiseptic decalcified bone. Med. News. 1890;57:202–210. [Google Scholar]
- 14.Phemister D.B. The fate of transplanted bone and regenerative power of its various constituents. Surg. Gynecol. Obstet. 1914;19:303. [Google Scholar]
- 15.Groves E.W. Methods and results of transplantation of bone in the repair of defects. Br. J. Surg. 1917;5:185. [Google Scholar]
- 16.Barth F. Ueber histologische befunde nach knochen-implantation. Arch. Klin. Chir. 1893;46:409. [Google Scholar]
- 17.Axhausen G. Ueber histologische vorgang bei der transplanation von gelenkenden. Arch. Klin. Chir. 1912;99:1. [Google Scholar]
- 18.Urist M.R. J.B. Lippencott; Philadelphia: 1980. Fundamental and Clinical Bone Physiology. [Google Scholar]
- 19.Huggins C.B. Experimental osteogenesis—influence of urinary tract mucosa on the experimental formation of bone. Proc. Soc. Exp. Biol. Med. 1930;27:349–353. [Google Scholar]
- 20.Huggins C.B. The formation of bone under the influence of epithelium of the urinary tract. Arch. Surg. 1931;22:377. [Google Scholar]
- 21.Neuhof H. Fascia transplantation into visceral defects. Surg. Gynecol. Obstet. 1917;24:383–427. [Google Scholar]
- 22.Urist M.R., Maeda H., Shamie A.N., Teplica D. Endogenous bone morphogenetic protein expression in transplants of urinary bladder. Plast. Reconstr. Surg. 1998;101:408–415. doi: 10.1097/00006534-199802000-00023. (discussion 416–407) [DOI] [PubMed] [Google Scholar]
- 23.Levander G. On the formation of new bone in bone transplantation. Acta Chir. Scand. 1934;74:425–426. [Google Scholar]
- 24.Levander G. A study of bone regeneration. Surg. Gynecol. Obstet. 1938;67:705–714. [Google Scholar]
- 25.Lacroix P. Recent investigations on the growth of bone. Nature. 1945;156:576–586. [Google Scholar]
- 26.Lacroix P. Organizers and the growth of bone. J. Bone Joint Surg. 1947;29:292–296. [PubMed] [Google Scholar]
- 27.Ray R.D., Holloway J.A. Bone implants. Preliminary report of an experimental study. J. Bone Joint Surg. 1957;39A:1119. [PubMed] [Google Scholar]
- 28.Urist M.R. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 29.Urist M.R., Strates B.S. Bone morphogenetic protein. J. Dent. Res. 1971;50:1392–1406. doi: 10.1177/00220345710500060601. [DOI] [PubMed] [Google Scholar]
- 30.Urist M.R. The substratum for bone morphogenesis. Symp. Soc. Dev. Biol. 1970;29:125–163. [PubMed] [Google Scholar]
- 31.Reddi A.H., Huggins C.B. Influence of geometry of transplanted tooth and bone on transformation of fibroblasts. Proc. Soc. Exp. Biol. Med. 1973;143:634–637. doi: 10.3181/00379727-143-37381. [DOI] [PubMed] [Google Scholar]
- 32.Reddi A.H., Huggins C.B. Formation of bone marrow in fibroblast-transformation ossicles. Proc. Natl. Acad. Sci. U. S. A. 1975;72:2212–2216. doi: 10.1073/pnas.72.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddi A.H., Anderson W.A. Collagenous bone matrix-induced endochondral ossification and hemopoiesis. J. Cell Biol. 1976;69:557–572. doi: 10.1083/jcb.69.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampath T.K., Reddi A.H. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc. Natl. Acad. Sci. U. S. A. 1981;78:7599–7603. doi: 10.1073/pnas.78.12.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthukumaran N., Ma S., Reddi A.H. Dose-dependence of and threshold for optimal bone induction by collagenous bone matrix and osteogenin-enriched fraction. Coll. Relat. Res. 1988;8:433–441. doi: 10.1016/s0174-173x(88)80016-5. [DOI] [PubMed] [Google Scholar]
- 36.Reddi A.H. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000;6:351–359. doi: 10.1089/107632700418074. [DOI] [PubMed] [Google Scholar]
- 37.Wozney J.M., Rosen V., Celeste A.J., Mitsock L.M., Whitters M.J., Kriz R.W., Hewick R.M., Wang E.A. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 38.Özkaynak E., Rueger D.C., Drier E.A., Corbett C., Ridge R.J., Sampath T.K., Oppermann H. OP-1 cDNA encodes an osteogenic protein in the TGF-ß family. EMBO J. 1990;9:2085–2093. doi: 10.1002/j.1460-2075.1990.tb07376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chesmel K.D., Branger J., Wertheim H., Scarborough N. Healing response to various forms of human demineralized bone matrix in athymic rat cranial defects. J. Oral Maxillofac. Surg. 1998;56:857–863. doi: 10.1016/s0278-2391(98)90015-5. (discussion 864–855) [DOI] [PubMed] [Google Scholar]
- 40.Honsawek S., Powers R.M., Wolfinbarger L. Extractable bone morphogenetic protein and correlation with induced new bone formation in an in vivo assay in the athymic mouse model. Cell Tissue Bank. 2005;6:13–23. doi: 10.1007/s10561-005-1445-4. [DOI] [PubMed] [Google Scholar]
- 41.Hollinger J.O., Mark D.E., Goco P., Quigley N., Desverreaux R.W., Bach D.E. A comparison of four particulate bone derivatives. Clin. Orthop. Relat. Res. 1991;267:255–263. [PubMed] [Google Scholar]
- 42.Schouten C.C., Hartman E.H., Spauwen P.H., Jansen J.A. DBM induced ectopic bone formation in the rat: the importance of surface area. J. Mater. Sci. Mater. Med. 2005;16:149–152. doi: 10.1007/s10856-005-6034-3. [DOI] [PubMed] [Google Scholar]
- 43.Hunter S.A., Orheim R., Sazon M., Newman H., Woll J.E., Bergevin M. Demineralization removes residual alendronate in allograft bone procured from donors with a history of bisphosphonate use. J. Periodontol. 2011;82:281–286. doi: 10.1902/jop.2010.100236. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz Z., Hyzy S.L., Moore M.A., Hunter S.A., Ronholdt C.J., Sunwoo M., Boyan B.D. Osteoinductivity of demineralized bone matrix is independent of donor bisphosphonate use. J. Bone Joint Surg. Am. 2011;93:2278–2286. doi: 10.2106/JBJS.J.01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J.C., Davies M., Kanin L.E. Prospective Comparison of Commercially Available Demineralized Bone Matrix for Spinal Fusion. North American Spine Society; New Orleans, LA: 2000. pp. 35–37. [Google Scholar]
- 46.Sassard W., Eidman D., Gray P.W. Augmenting local bone with Grafton demineralized bone matrix for posterior lumbar spine fusion: avoiding second site autologous bone harvest. Orthopaedics. 2000;23:1059–1065. doi: 10.3928/0147-7447-20001001-17. [DOI] [PubMed] [Google Scholar]
- 47.Bostrom M., Yang X., Keenan M., Andhu H., Dicarlo E., Lane J. An unexpected outcome during testing of commercially available demineralized bone graft materials: How safe are the nonallograft components. Spine. 2001;26:1425–1428. doi: 10.1097/00007632-200107010-00007. [DOI] [PubMed] [Google Scholar]
- 48.Safdar N., Khan M., Fraser J.R., Sandhu H., Cammisa F.P., Giradi F., Lane J. Use of osteopromotive growth factors. Spine. 2001;26:1425–1428. [Google Scholar]
- 49.O. Acarturk, J.O. Hollinger, Comparison of commercially available particulate demineralized bone products, J. Plastic. Recon., in press.
- 50.Carpener J., Pikal M., Chang B.H., Randolph T. Rational design of stable lyophilized protein formulations: some practical advice. Pharmacol. Res. 1997;14:969–975. doi: 10.1023/a:1012180707283. [DOI] [PubMed] [Google Scholar]
- 51.Scarborough N., White E., Hughes J.L. Autograft safety: Viral inactivation with bone demineralization. Contemp. Orthop. 1995;31:257–261. [PubMed] [Google Scholar]
- 52.Urist M.R., Dowell T.A., Hoy P.H., Strates B.S. Inductive substrates for bone formation. Clin. Orthop. 1968;59:59. [PubMed] [Google Scholar]
- 53.Glowacki J. A review of osteoinductive testing methods and sterilization processes for demineralized bone. Cell Tissue Bank. 2005;6:3–12. doi: 10.1007/s10561-005-4252-z. [DOI] [PubMed] [Google Scholar]
- 54.Russell J.L., Block J.E. Clinical utility of demineralized bone matrix for osseous defects, arthrodesis, and reconstruction: impact of processing techniques and study methodology. Orthopedics. 1999;22:524–531. (quiz 532–523) [PubMed] [Google Scholar]
- 55.Munting E.V., Wilmart J.-F., Wijen A., Hennebert P.I., Delloye C. Effect of Sterilization on osteoinduction: comparison of five methods in demineralized rat bone. Acta Orthop. Scand. 1988;59:34–38. doi: 10.3109/17453678809149340. [DOI] [PubMed] [Google Scholar]
- 56.Puolakkainen P., Ranchalis J., Strong D., Twardzik D. The effect of sterilization on transforming growth factor beta isolated from demineralized human bone. Transfusion. 1993;33:679–685. doi: 10.1046/j.1537-2995.1993.33893342752.x. [DOI] [PubMed] [Google Scholar]
- 57.Ijiri S., Yamauro T., Nakamura T., Kotani S., Notoya K. Effect of sterilization on bone morphogenetic protein. J. Orthop. Res. 1994;12:628–636. doi: 10.1002/jor.1100120505. [DOI] [PubMed] [Google Scholar]
- 58.Urist M.R., Hernandez A. Excitation transter in bone. Deleterious effects of cobalt 60 radiation–sterilization of bank bone. Arch. Surg. 1974;109:486–493. doi: 10.1001/archsurg.1974.01360040012004. [DOI] [PubMed] [Google Scholar]
- 59.Glowacki J., Altobeli J., Mulliken J.B. Fate of mineralized and demineralized ossedous implants in cranial defects. Calcif. Tissue Int. 1981;33:71–76. doi: 10.1007/BF02409414. [DOI] [PubMed] [Google Scholar]
- 60.Wientroub S., Reddi A.H. Influence of irradiation on the osteoinductive potential of demineralized bone matrix. Calcif. Tissue Int. 1988;42:255–260. doi: 10.1007/BF02553752. [DOI] [PubMed] [Google Scholar]
- 61.Pruss A., Kao M., Gohs U., Koscielny J., von Versen R., Pauli G. The effect of gamma irradiation on human cortical bone transplants contaminated with enveloped and non-enveloped viruses. Biologicals. 1993;30:125–133. doi: 10.1006/biol.2002.0326. [DOI] [PubMed] [Google Scholar]
- 62.Lekishvili M., Snetkov A., Vasiliev M., Il'ina V., Tarasov N., Gorbunova E., Pankratov A., Barakina O., Gavryushenko N., Batrakov S. Experimental and clinical study of the demineralized bone allografts manufactured in the tissue bank of CITO. Cell Tissue Bank. 2004;5:231–238. doi: 10.1007/s10561-004-1443-y. [DOI] [PubMed] [Google Scholar]
- 63.Dziedzic-Goclawska A., Kaminski A., Uhrynowska-Tyszkiewicz I., Stachowicz W. Irradiation as a safety procedure in tissue banking. Cell Tissue Bank. 2005;6:201–219. doi: 10.1007/s10561-005-0338-x. [DOI] [PubMed] [Google Scholar]
- 64.Ferreira S.D., Dernell W.S., Powers B.E., Schochet R.A., Kuntz C.A., Withrow S.J., Wilkins R.M. Effect of gas–plasma sterilization on the osteoinductive capacity of demineralized bone matrix. Clin. Orthop. Relat. Res. 2001:233–239. doi: 10.1097/00003086-200107000-00032. [DOI] [PubMed] [Google Scholar]
- 65.Moore T., Gendler E., Gendler E. Viruses adsorbed on musculoskeletal allografts are inactivated by terminal ethylene oxide disinfectant. J. Orthop. Res. 2004;22:1358–1361. doi: 10.1016/j.orthres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Aspenberg P., Lohmander L.S., Thorngren K.-G. Monkey bone matrix induces bone formation in the athymic rat, but not in adult monkeys. J. Orthop. Res. 1990;9:20–25. doi: 10.1002/jor.1100090104. [DOI] [PubMed] [Google Scholar]
- 67.Doherty M.M., Mollan R., Wilson D. The effect of ethylene oxide sterilization on human demineralized bone. Biomaterials. 1993;14:994–998. doi: 10.1016/0142-9612(93)90191-4. [DOI] [PubMed] [Google Scholar]
- 68.Solheim E., Pinholt E.M., Bang G., Sudmann E. Ethylene oxide gas sterilization does not reduce the osteoinductive potential of demineralized bone in rats. J. Craniofac. Surg. 1995;6:195–198. doi: 10.1097/00001665-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Zhang M., Powers R.M., Jr., Wolfinbarger L., Jr. Effect(s) of the demineralization process on the osteoinductivity of demineralized bone matrix. J. Periodontol. 1997;68:1085–1092. doi: 10.1902/jop.1997.68.11.1085. [DOI] [PubMed] [Google Scholar]
- 70.Dupoirieux L., Costes V., Jammet P., Souyris F. Experimental study on demineralized bone matrix (DBM) and coral as bone graft substitutes in maxillofacial surgery. Int. J. Oral Maxillofac. Surg. 1994;23:395–398. doi: 10.1016/s0901-5027(05)80028-1. [DOI] [PubMed] [Google Scholar]
- 71.Lomas R.J., Gillan H.L., Matthews J.B., Ingham E., Kearney J.N. An evaluation of the capacity of differently prepared demineralised bone matrices (DBM) and toxic residuals of ethylene oxide (EtOx) to provoke an inflammatory response in vitro. Biomaterials. 2001;22:913–921. doi: 10.1016/s0142-9612(00)00255-6. [DOI] [PubMed] [Google Scholar]
- 72.Aspenberg P., Lindqvist S.B. Ethene oxide and bone induction. Controversy remains. Acta Orthop. Scand. 1998;69:173–176. doi: 10.3109/17453679809117622. [DOI] [PubMed] [Google Scholar]
- 73.Han B., Yang Z., Nimni M. Effects of moisture and temperature on the osteoinductivity of demineralized bone matrix. J. Orthop. Res. 2005;23:855–861. doi: 10.1016/j.orthres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz Z., Mellonig J., Carnes D., Fontaine J., Cochran D., Dean D., Boyan B. Ability of commericial demineralized frezze-dried bone allograft to induce new bone formation. J. Periodontol. 1996;67:918–926. doi: 10.1902/jop.1996.67.9.918. [DOI] [PubMed] [Google Scholar]
- 75.Takikawa S., Bauer W., Kambic H., Togawa D. Comparative evaluation of the osteoinductivity of two formulations of human demineralized bone matrix. J. Biomed. Mater. Res. 2003;65:37–42. doi: 10.1002/jbm.a.10345. [DOI] [PubMed] [Google Scholar]
- 76.Oakes D.A., Lee C., Liberman J. An evaluation of human demineralized bone matrices in a rat femoral defect model. Clin. Orthop. Relat. Res. 2003;413:281–290. doi: 10.1097/01.blo.0000073347.50837.16. [DOI] [PubMed] [Google Scholar]
- 77.Bomback D., Grauer J., Lugo R., Troiano N., Patel T., Friedlaender G. Comparison of posterolateral lumbar fusion rates of Grafton Putty and OP-1 in an athymic rat model. Spine. 2004;29:1612–1617. doi: 10.1097/01.brs.0000132512.53305.a1. [DOI] [PubMed] [Google Scholar]
- 78.Peterson B., Whang P., Iglesias R., Wang J., Lieberman J. Osteoinductivity of commercially available demineralized bone matrix: preparation in a spine fusion model. J. Bone Joint Surg. 2004;86-A:2243–2250. doi: 10.2106/00004623-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 79.Acarturk O., Hollinger J.O. Comparison of commercially available particulate demineralized bone products. J. Plast. Reconstr. 2006;118:862–873. doi: 10.1097/01.prs.0000232385.81219.87. [DOI] [PubMed] [Google Scholar]
- 80.Urist M.R. The substratum for bone morphogenesism. Dev. Biol. Suppl. 1970;4:125–163. [PubMed] [Google Scholar]
- 81.Reddi A.H. The matrix of rat calvarium as transformant of fibroblasts (39028) Proc. Soc. Exp. Biol. Med. 1975;150:324–326. doi: 10.3181/00379727-150-39028. [DOI] [PubMed] [Google Scholar]
- 82.Reddi A.H. Morphogenetic messages are in the extracellular matrix: biotechnology from bench to bedside. Biochem. Soc. Trans. 2000;28:345–349. [PubMed] [Google Scholar]
- 83.Pietrzak W.S., Woodell-May J., McDonald N. Assay of bone morphogenetic protein-2, -4 and ‐7 in human demineralized bone matrix. J. Craniofac. Surg. 2006;17:84–90. doi: 10.1097/01.scs.0000179745.91165.73. [DOI] [PubMed] [Google Scholar]
- 84.Honsawek S., Dhitiseith D. Content of bone morphogenetic protein-4 in human demineralized bone: relationship to donor age and ability to induce new bone formation. J. Med. Assoc. Thai. 2005;88:S260–S265. [PubMed] [Google Scholar]
- 85.Blum B., Moseley J., Miller L., Richelsoph K., Haggard W. Measurement of bone morphogenetic proteins and other growth factors in demineralized bone matrix. Orthopedics. 2004;27:s161–s165. doi: 10.3928/0147-7447-20040102-17. [DOI] [PubMed] [Google Scholar]
- 86.Pfeilschifter J., Diel I., Scheppach B., Bretz A., Krempien R., Erdmann J., Schmid G., Reske N., Bismar H., Seck T., Krempien B., Ziegler R. Concentration of transforming growth factor beta in human bone tissue: relationship to age, menopause, bone turnover, and bone volume. J. Bone Miner. Res. 1998;13:716–730. doi: 10.1359/jbmr.1998.13.4.716. [DOI] [PubMed] [Google Scholar]
- 87.Bae H., Zhao L., Kanin L., Wong P., Dawson E. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine. 2006;31:1299–1306. doi: 10.1097/01.brs.0000218581.92992.b7. [DOI] [PubMed] [Google Scholar]
- 88.Lohmann C.H., Andreacchio D., Koster G., Carnes D.L., Jr., Cochran D.L., Dean D.D., Boyan B.D., Schwartz Z. Tissue response and osteoinduction of human bone grafts in vivo. Arch. Orthop. Trauma Surg. 2001;121:583–590. doi: 10.1007/s004020100291. [DOI] [PubMed] [Google Scholar]
- 89.Traianedes K., Russell J., Edwards J., Stubbs H., Shanahan I., Knaack D. Donor age and gender effects on osteoinductivity of deminralized b one matrix. J. Biomed. Mater. Res. 2004;70:21–29. doi: 10.1002/jbm.b.30015. [DOI] [PubMed] [Google Scholar]
- 90.Carnes D.J., De La Fontaine J., Cochran D., Mellonig J.T., Keogh B., Harris S.E., Ghosh-Choudhury N., Dean D.D., Boyan B.D., Schwartz Z. DFDBA donor age effects in vitro and in vivo. J. Periodontal. 1999;70 doi: 10.1902/jop.1999.70.4.353. [DOI] [PubMed] [Google Scholar]
- 91.Adkisson H.A., Straus-Schoenberger J., Gillis M., Wilkins R., Jackson M.T., Hrushka K.A. Rapid quantitative bioassay for osteoinduction. J. Orthop. Res. 2005;18:503–511. doi: 10.1002/jor.1100180326. [DOI] [PubMed] [Google Scholar]
- 92.Burwell R.G. Studies in the transplantation of bone. VII. The fresh composite homograft-autografts of cancellous bone: an analysis of inductive mechanisms in bone transplantation. J. Bone Joint Surg. 1964;46-B:110–140. [PubMed] [Google Scholar]
- 93.Pike R.L., Boyne P.J. Composite autogenous marrow and surface-decalcified implants in mandibular defects. J. Oral Surg. 1973;31:905–912. [PubMed] [Google Scholar]
- 94.Lindholm T.S., Urist M.R. A quantitative analysis of new bone formation by induction in compositive grafts of bone marrow and bone matrix. Clin. Orthop. 1980;150:288–300. [PubMed] [Google Scholar]
- 95.Lindholm T.S., Nilsson O.S., Lindholm T.C. Extraskeletal and intraskeletal new bone formation induced by demineralized bone matrix combined with bone marrow cells. Clin. Orthop. 1982;171:251–255. [PubMed] [Google Scholar]
- 96.Pearson G.E., Rosen S., Deporter D.A. Preliminary observations on the usefulness of a decalcified, freeze-dried cancellous bone allograft material in periodontal surgery. J. Periodontol. 1981;52:55–59. doi: 10.1902/jop.1981.52.2.55. [DOI] [PubMed] [Google Scholar]
- 97.Sonis S.T., Kaban L.B., Glowacki J. Clinical trial of demineralized bone powder in the treatment of periodontal defects. J. Oral Med. 1983;38:117–122. [PubMed] [Google Scholar]
- 98.Bowers G.M., Chadroff M., Carnevale R., Mellonig J., Corio R., Emerson J., Stevens M., Romberg E. Histologic evaluation of new attachment apparatus formation in humans. Part III. J. Periodontol. 1989;60:683–693. doi: 10.1902/jop.1989.60.12.683. [DOI] [PubMed] [Google Scholar]
- 99.Bowers G.M., Chadroff M., Carnevale R., Mellonig J., Corio R., Emerson J., Stevens M., Romberg E. Histologic evaluation of new attachment apparatus formation in humans. Part II. J. Periodontol. 1989;60:675–682. doi: 10.1902/jop.1989.60.12.675. [DOI] [PubMed] [Google Scholar]
- 100.Nevins M.L., Camelo M., Nevins M., King C.J., Oringer R.J., Schenk R.K., Fiorellini J.P. Human histologic evaluation of bioactive ceramic in the treatment of periodontal osseous defects. Int. J. Periodontics Restorative Dent. 2000;20:458–467. [PubMed] [Google Scholar]
- 101.Nevins M., Camelo M., Nevins M.L., Schenck R., Lynch S. Periodontal regeneration in humans using recombinant human platelet-derived growth factor BB (rhPDGF-BB) and allogeneic bone. J. Periodontol. 2003;74:1282–1292. doi: 10.1902/jop.2003.74.9.1282. [DOI] [PubMed] [Google Scholar]
- 102.Nevins M., Giannobile W.V., McGuire M.H., Kao R., Mellonig J., Hinrichs J., McAllister B., Murphy K.G., McClain P., Nevins M.L., Paquette D.W., Han T., Reddy M., Lavin P.T., Genco R., Lynch S. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J. Periodontol. 2005;76:2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 103.Mulliken J.B., Glowacki J., Kaban L.B., Folkman J., Murray J.E. Use of demineralized allogenic bone implants for the correction of maxillocraniofacial deformities. Ann. Surg. 1981;194:366–372. doi: 10.1097/00000658-198109000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Habal M.B. Reconstruction of a large congenital cranioorbital defect with a species-specific demineralized bone implant. J. Craniofac. Surg. 1992;3:113–118. doi: 10.1097/00001665-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 105.Salyer K.E., Gendler E., Menendez J.L., Simon T.R., Kelly K.M., Bardach J. Demineralized perforated bone implants in craniofacial surgery. J. Craniofac. Surg. 1992;3:55–62. doi: 10.1097/00001665-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 106.Ousterhout D.K. Clinical experience in cranial and facial reconstruction using demineralized bone. Ann. Plast. Surg. 1985;15:367–373. doi: 10.1097/00000637-198511000-00001. [DOI] [PubMed] [Google Scholar]
- 107.Upton J., Glowacki J. Hand reconstruction with allograft demineralized bone: twenty-six implants in twelve patients. J. Hand Surg. 1992;17:704–713. doi: 10.1016/0363-5023(92)90321-f. [DOI] [PubMed] [Google Scholar]
- 108.Whiteman D., Gropper P.T., Wirtz P., Monk P. Demineralized bone powder. Clinical applications for bone defects of the hand. J. Hand Surg. Br. 1993;18:487–490. doi: 10.1016/0266-7681(93)90154-8. [DOI] [PubMed] [Google Scholar]
- 109.Kado K.E., Gambetta L.A., Perlman M.D. Uses of Grafton for reconstructive foot and ankle surgery. J. Foot Ankle Surg. 1996;35:59–66. doi: 10.1016/s1067-2516(96)80014-7. [DOI] [PubMed] [Google Scholar]
- 110.Weinraub G.M., Cheung C. Efficacy of allogenic bone implants in a series of consecutive elective foot procedures. J. Foot Ankle Surg. 2003;42:86–89. doi: 10.1016/s1067-2516(03)70006-4. [DOI] [PubMed] [Google Scholar]
- 111.Michelson J.J., Curl L.A. Use of demineralized bone in hindfoot arthrodesis. Clin. Orthop. 1996;325:203–208. doi: 10.1097/00003086-199604000-00024. [DOI] [PubMed] [Google Scholar]
- 112.Killian J.T., Wilkinson L., White S., Brassard M. Treatment of unicameral bone cyst with demineralized bone matrix. J. Pediatr. Orthop. 1998;18:621–624. doi: 10.1097/00004694-199809000-00013. [DOI] [PubMed] [Google Scholar]
- 113.Tiedman J.J., Garvin K.L., Kile T.A., Connolly J.F. The role of composite, demineralized bone marrow in the treatment of osseous defects. Orthopaedics. 1995;18:1153–1158. doi: 10.3928/0147-7447-19951201-05. [DOI] [PubMed] [Google Scholar]
- 114.Geesink R.G., Hoefnagels N.H., Bulstra S.K. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J. Bone Joint Surg. Br. 1999;81:710–718. doi: 10.1302/0301-620x.81b4.9311. [DOI] [PubMed] [Google Scholar]
- 115.Ladd A.L., Pliam N.B. Use of bone-graft substitutes in distal radius fractures. J. Am. Acad. Orthop. Surg. 1999;7:279–290. doi: 10.5435/00124635-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 116.Sassard W.R., Eidman D.K., Gray P.M., Block J.E., Russo R., Russell J.L., Taboada E.M. Augmenting local bone with Grafton demineralized bone matrix for posterolateral lumbar spine fusion: avoiding second site autologous bone harvest. Orthopaedics. 2000;23:1059–1064. doi: 10.3928/0147-7447-20001001-17. [DOI] [PubMed] [Google Scholar]
- 117.Cammisa F.P., Lowery G.L., Geisler F.H., McGuire R., Stubbs H., Block J.A. Two year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine. 2004;29:660–666. doi: 10.1097/01.brs.0000116588.17129.b9. [DOI] [PubMed] [Google Scholar]
- 118.An H.S., Simpson J.M., Glover J.M., Stephany J. Abstract, S.O. 15;20(20):2211–6., Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine. 1995;20:2211–2216. [PubMed] [Google Scholar]
- 119.Lee K., Roper J., Wang J. Demineralized bone matrix and spine arthrodesis. Spine. 2005;5:217S–223S. doi: 10.1016/j.spinee.2005.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.