Abstract
Infectious Bronchitis Virus, a member of the Coronaviridae, is a respiratory pathogen in poultry. We found that in vitro stimulation with IBV resulted in ChIFN-γ production in splenocytes of both infected birds and uninfected birds. The non-specific stimulation did not occur when other avian viruses or other coronaviruses were used or when mammalian cells were stimulated with IBV. Inactivation of IBV reduced ChIFN-γ production, but ChIFN-γ remained elevated compared to unstimulated cells. An increase in ChIFN-γ mRNA was detected in splenocytes from IBV-infected and uninfected chickens as early as 1 h after stimulation with IBV. These results indicate that IBV acts as a polyclonal stimulus, inducing a rapid production of IFN-γ even without previous exposure to the virus.
Keywords: Infectious Bronchitis Virus, ELISPOT, Chicken, Polyclonal stimulation, Interferon-gamma, Superantigen
Introduction
Infectious Bronchitis Virus is a coronavirus of limited structural complexity that causes an acute, highly contagious respiratory disease in poultry. As well as being an economically relevant pathogen in poultry, IBV also bears close resemblance to the human pathogen severe acute respiratory syndrome coronavirus (SARS-CoV; Cavanagh, 2003). In order to study the immune responses to IBV in poultry in more detail, techniques are required that provide information about the immune cells participating to the response on a single-cell level. It has been shown that CD8+T cells play an important role in controlling IBV infection (Collisson et al., 2000). Furthermore, it is known that interferons are actively involved in the immune response against IBV (Hackney et al., 2003, Otsuki et al., 1988). Most of the techniques that are available to study T-cell responses in poultry do not provide any information about the response on single-cell level. We developed an ELISPOT assay and intracellular cytokine staining for the detection of ChIFN-γ production, which is often used as an indicator of T-cell activity (Ariaans et al., 2008b).
This ELISPOT assay allowed for the quantification of immune cell activity after mitogen stimulation and after vaccination with Newcastle Disease Virus (NDV), providing information about infection status of poultry and about the kinetics of the infection. An infection experiment was set up in which ChIFN-γ production by splenocytes from IBV-uninfected chickens was compared to that of IBV-infected chickens after in vitro restimulation with virulent IBV strain M41. Contrary to our expectations, the results indicated that ChIFN-γ production was not only significantly increased in splenocytes of IBV-infected chickens after in vitro restimulation with IBV, but also in splenocytes of IBV-uninfected chickens. Based on these findings, we investigated the possibility of polyclonal stimulation by IBV in more detail.
Results
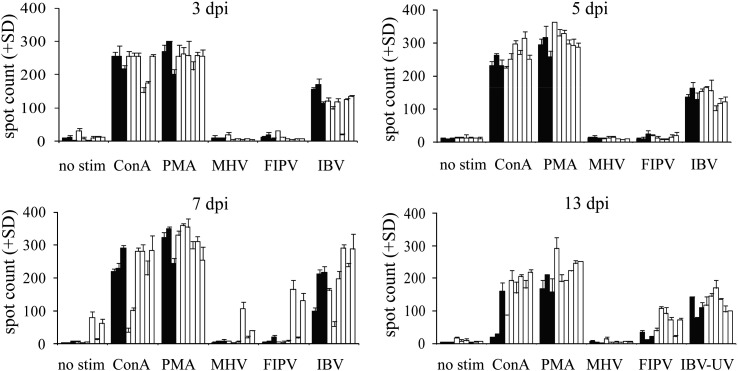
White Leghorn chickens were inoculated with virulent IBV strain M41 and at four different time points after inoculation spleens were harvested. Splenocytes of IBV-infected and uninfected chickens were incubated for 48 h with ConA, PMA and ionomycin, and different coronaviruses. ChIFN-γ production increased significantly after in vitro restimulation with IBV in both the IBV-infected and uninfected chickens (Fig. 1 ). Two mammalian coronaviruses did not cause an increase in ChIFN-γ production, indicating that the observed effect was specific for IBV and not for coronaviruses in general.
Fig. 1.
ELISPOT assay to measure ChIFN-γ production by splenocytes of IBV-uninfected (black bars; n = 3) versus IBV M41-infected (white bars; n = 6) chickens at 3, 5, 7, or 13 days post-infection. Splenocytes were added to ChIFN-γ-coated wells and stimulated for 48 h with medium, ConA (10 μg/ml), PMA (100 ng/ml) and ionomycin (500 ng/ml), MHV (106 pfu), FIPV (106 pfu), IBV M41 (105 EID50), or UV-inactivated IBV M41 (IBV-UV, 13 dpi; 105 EID50). Spot counts represent the number of ChIFN-γ secreting cells. Each bar represents the average of three samples per chicken, plus standard deviation.
To determine if the IBV-induced ChIFN-γ production was caused by virus invasion and replication or an exclusively cell-mediated response to IBV, the virus was inactivated by exposure to ultraviolet light (Fig. 1, 13 dpi). ChIFN-γ production after exposure to the inactivated IBV did decrease in the IBV-uninfected birds when compared to IBV-infected birds, but it was still higher than in unstimulated cells.
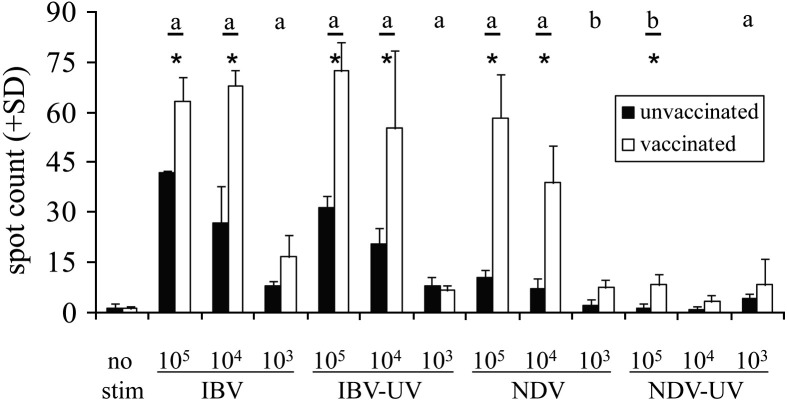
Although a decrease in ChIFN-γ production was detected after UV-inactivation of IBV, it cannot be excluded that the immune response in the IBV-infected chickens had already passed its peak at this time point. The experiment was repeated with IBV and with another chicken virus, NDV, both live and UV-inactivated. Splenocytes of an unvaccinated chicken and a chicken that had been vaccinated for both IBV and NDV 4 months prior to the experiment were restimulated in vitro with different concentrations of either virulent or UV-inactivated IBV M41 or NDV (Fig. 2 ).
Fig. 2.
ELISPOT assay to measure ChIFN-γ. Splenocytes of an unvaccinated (black bars) and an IBV- and NDV-vaccinated White Leghorn chicken (white bars) were stimulated in triplicate with three different doses of untreated or UV-inactivated IBV M41 or NDV. Bars marked with ⁎ show a significant difference (p < 0.05) between vaccinated and unvaccinated birds. Bars marked with ‘a’ show a significant difference between the vaccinated and unvaccinated birds on the one hand and the unstimulated controls on the other. Bars marked with ‘b’ show a significant difference between vaccinated birds and unstimulated controls.
Only the splenocytes from the NDV-vaccinated chicken produced ChIFN-γ after NDV restimulation, and hardly any response to the UV-inactivated virus was detected. In contrast, both the IBV-vaccinated and the unvaccinated chicken showed a dose-dependent response to restimulation with IBV, with no discernable difference between treated and untreated virus in the vaccinated chicken. Stimulation with UV-inactivated IBV resulted in decreased ChIFN-γ production in the unvaccinated chicken compared to the vaccinated chicken, but the response was still elevated compared to the unstimulated control.
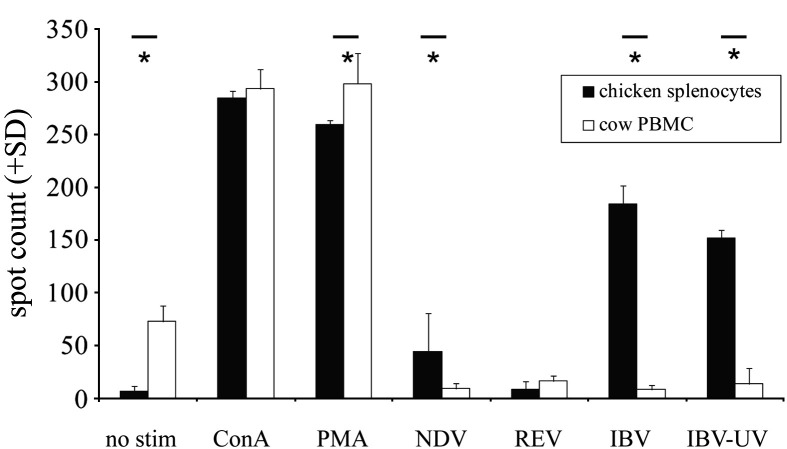
To test if the observed effect is a specific interaction between the virus and its natural host, or if IBV can activate leukocytes regardless of the species from which the cells were obtained, we stimulated chicken and cow leukocytes with mitogens and different avian viruses (Fig. 3 ). Splenocytes of an IBV- and NDV-vaccinated chicken produced ChIFN-γ after incubation with IBV and UV-inactivated IBV, whereas cow PBMC did not show any response. A third poultry virus, REV, did not induce IFN-γ. These results demonstrate that the immune activation by IBV is species-specific, and that two other poultry viruses that we have tested (NDV and REV) do not cause a similar rapid immune activation upon first exposure.
Fig. 3.
ELISPOT assay to measure the production of IFN-γ by splenocytes (black bars) of an IBV- and NDV-vaccinated White Leghorn chicken and by cow PBMC (white bars) in triplicate after in vitro stimulation with ConA (10 μg/ml), PMA (100 ng/ml) and ionomycin (500 ng/ml), NDV (105 EID50), REV (105 EID50), IBV M41 (105 EID50), or UV-inactivated IBV M41 (105 EID50). Bars marked with ⁎ are significantly different (p < 0.05).
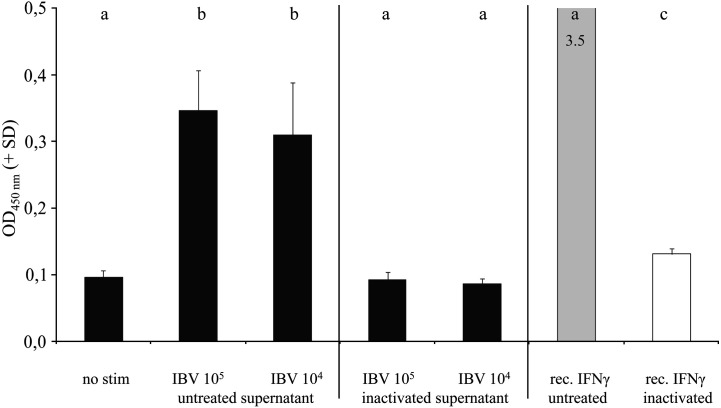
IBV is known to induce rapid production of type-I IFNs by chicken leukocytes in both naïve and IBV-infected chickens (Pei et al., 2001). Type-I IFNs are heat-stable, whereas IFN-γ is heat-sensitive. To exclude that the anti-IFN-γ antibodies used in our ELISPOT assay are cross-reacting with type-I IFNs or other heat-stable antigens produced by IBV-infected cells, we tested the effect of heat inactivation. Chicken splenocytes were cultured for 48 h in the presence of different stimuli, the supernatants were collected and part of the samples heat-inactivated at 65 °C for 30 min. We tested all the samples using a commercial ELISA for chIFN-γ detection (Fig. 4 ).
Fig. 4.
Chicken splenocytes were incubated in triplicate for 48 h with medium or IBV M41 (105 and 104 EID50). After 48 h, culture supernatants were collected and half of the samples were incubated at 65 °C for 30 min. Supernatants were tested using a commercial ELISA for ChIFN-γ. Recombinant ChIFN-γ protein (10 ng/ml) with or without heat treatment was included as a control. Groups with different letters are significantly different (p < 0.05).
After heat-inactivation, ChIFN-γ levels in the supernatants decreased significantly when compared to untreated supernatants, confirming that the observed signal was indeed specific for ChIFN-γ and not due to cross-reactivity with type-I IFNs or other heat-stable proteins. Based on ChIFN-γ standard curves, before heat inactivation the ChIFN-γ levels were around 200–250 pg/ml, whereas after inactivation these levels dropped below the detection limit.
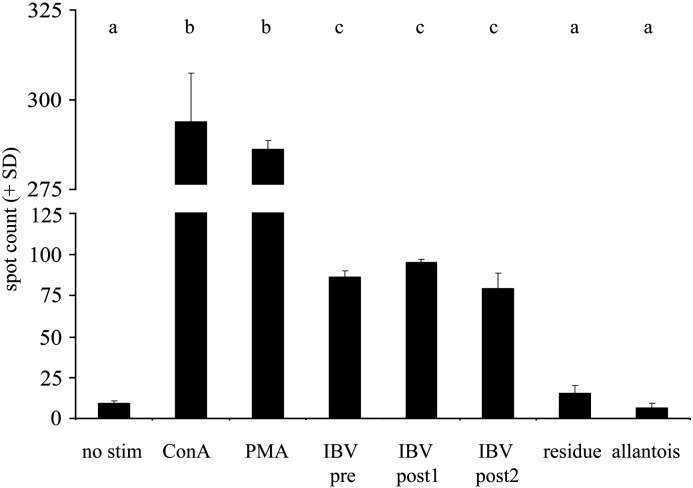
To ensure that the induction of ChIFN-γ was not due to contaminating components derived from the allantois fluid in which the virus was grown or from the production process, the virus was purified with a sucrose gradient and tested in an ELISPOT assay. Splenocytes from four IBV-uninfected chickens were stimulated with purified and unpurified IBV (Fig. 5 ). A fraction of the sucrose gradient obtained from outside the IBV band and allantois fluid from an IBV-uninfected egg (10 × diluted in PBS) were also included. All splenocyte cultures showed a significant increase in ChIFN-γ production after stimulation with IBV M41 before and after sucrose-purification, with no significant differences between them. Incubation with a fraction of the sucrose gradient obtained from outside the IBV band (‘residue’) and with IBV-free allantois fluid did show any effect. We therefore conclude that the increase in ChIFN-γ production is caused by the IBV itself and not by other components.
Fig. 5.
ELISPOT for detection of ChIFN-γ produced by splenocytes of 4 chickens after 24 h incubation with the following stimuli: culture medium, ConA (10 μg/ml), PMA (100 ng/ml) and ionomycin (500 ng/ml), 105 EID50 IBV M41 before (IBV pre) sucrose purification, IBV M41 pooled from the 3 IBV-richest sucrose fractions (IBV post1, ∼ 105 EID50) and pooled from 3 surrounding fractions (IBV post2, ∼ 105 EID50), a pool of 3 sucrose fractions outside the IBV band (residue, same volume as ‘post2’ sucrose fraction), and allantois from an IBV-uninfected egg. Groups with different letters are significantly different (p < 0.05).
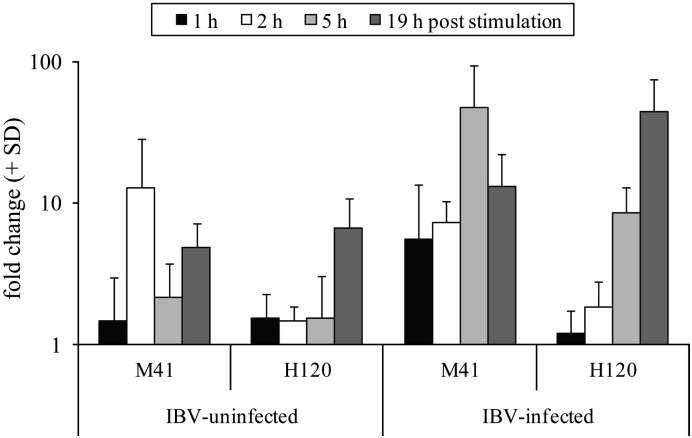
To study the kinetics with which IFN-γ was induced, we performed quantitative RT-PCR (qPCR) for detection of ChIFN-γ mRNA in commercial broilers (Fig. 6 ) and layer chickens (Fig. 7 ). Both layers and broilers show increased ChIFN-γ mRNA expression after IBV stimulation. An increase in ChIFN-γ mRNA expression was detected as early as 2 h after stimulation with virulent IBV M41 and with IBV vaccine strain H120 in splenocytes of layer birds.
Fig. 6.
Real-time quantification of ChIFN-γ mRNA expression by splenocytes collected 7 days after IBV infection from four IBV-infected and four IBV-uninfected 5 wk old commercial broiler chickens, 6 h after stimulation with PMA (100 ng/ml) and ionomycin (500 ng/ml), or IBV M41 (105 EID50). Bars represent the mean fold increase (plus standard deviation) in ChIFN-γ mRNA production compared to unstimulated splenocytes. All groups are significantly different from the unstimulated splenocytes (⁎p < 0.05; ⁎⁎p < 0.01).
Fig. 7.
Real-time quantification of ChIFN-γ mRNA expression by splenocytes from four IBV-infected layer chickens, at four different time points after stimulation with IBV M41 (105 EID50) or IBV H120 (105 EID50). Each bar represents the mean fold increase (plus standard deviation) in ChIFN-γ mRNA production compared to unstimulated splenocytes from the same birds.
Discussion
IFN-α and IFN-β, both type-I interferons, are important in the innate immune defence against virus infection and are quickly upregulated after infection with viruses (Kawai and Akira, 2006). IFN-γ, a type-II interferon, is associated with adaptive immune responses and as such would be expected to be upregulated later in the immune response (Takaoka and Yanai, 2006). In contrast, we observed a rapid increase in ChIFN-γ production within 24 h after stimulation of chicken splenocytes with coronavirus IBV strain M41, and an increased expression of ChIFN-γ mRNA as early as 1 h after stimulation. This increase was detected not only in in vitro stimulated splenocytes of chickens that had previously been exposed to IBV, but also in splenocytes of IBV-naïve chickens. Furthermore, two chicken viruses that are not members of the Coronaviridae family, the avian paramyxovirus NDV and the retrovirus REV, did not induce ChIFN-γ production in splenocytes of uninfected chickens. IBV did not induce production of IFN-γ by leukocytes of mammalian origin, cow PBMC. This indicates that the observed IFN-γ production appears to be a specific interaction between IBV and its natural host, and not an effect generally observed after exposure of non-avian leukocytes to IBV, or of chicken leukocytes to non-avian coronaviruses or to other avian viruses.
We also observed an increase in IFN-γ production after FIPV stimulation at 13 dpi in IBV-infected chickens, which might be due to cross-presentation of certain conserved epitopes shared between the IBV and FIPV. It has been shown that coronaviruses are not necessarily restricted to one host species, as SARS-CoV can replicate in humans, mice, hamsters and ferrets (Roberts et al., 2008), and bovine coronavirus was shown to replicate in turkeys and cause enteritis (Ismail et al., 2001).
To ensure that the observed response was indeed due to ChIFN-γ detection and not to type-I interferons, samples were heated to 65 °C after incubation. This inactivates IFN-γ but not type-I interferons (Lowenthal et al., 2001). After heat-treatment, the concentration of ChIFN-γ protein in the IBV-stimulated samples dropped to background levels, indicating that the observed response is indeed ChIFN-γ-specific and not due to type-I interferons.
In virus taxonomy, coronaviruses are currently assigned into four different groups, with groups 1 and 2 consisting of various mammalian coronaviruses, group 3 formed exclusively by avian coronaviruses such as IBV, Turkey coronavirus (TCoV) and Pheasant coronavirus, and group 4 currently only containing the recently discovered human coronavirus SARS-CoV (Cavanagh, 2007). Chicken splenocytes were in vitro stimulated with members of the other coronavirus groups that have mammalian hosts, the group 1 coronavirus FIPV and group 2 coronavirus MHV. Both viruses did not induce ChIFN-γ production by chicken splenocytes. For MHV several studies have shown a clear difference in IFN-γ production between infected and uninfected mice by ELISA and qPCR (Hooks et al., 2003, Lucchiari et al., 1991). Elevated levels of IFN-γ mRNA were detected in a pilot study with a FIPV-infected cat when compared to uninfected cats (Kiss et al., 2004).
Castilletti et al. (2005) showed that the group 4 human SARS-CoV, which is thought to be closely related to IBV, induced both IFN-α and IFN-γ mRNA and protein after stimulation of PBMC from healthy donors in a dose-dependent manner within 24 h, without the need for viral replication. They suggest that exposure of PBMC to high virus titers in vivo at the site of infection is biologically likely and as such concomitant activation of IFN-α and IFN-γ after first-time exposure to relatively high levels of coronavirus might be relevant to the pathogenesis of the disease. A comparison between PBMC of healthy donors and SARS patients was not made, and it therefore remains unknown whether the kinetics of IFN-γ production after exposure to SARS-CoV is similar in SARS patients and healthy donors.
Both IBV and SARS-CoV infect and replicate in the respiratory tract, and have been reported to spread to other enteric tissues such as the gastrointestinal tract (Cavanagh, 2007, Navas-Martin and Weiss, 2004). Infection with these viruses leads to leukocyte influx and damage of respiratory tract epithelium. Matthijs et al. (in press) found very high numbers of macrophages and CD4+ T cells in trachea and lung of IBV H120 and M41 infected birds. The local immune deregulation might in turn give rise to enhanced susceptibility to secondary bacterial infections, such as colibacillosis in poultry after IBV infection, or to organ destruction and pneumonia-like symptoms in the case of SARS. Interestingly, highly elevated levels of IFN-γ and related cytokines and chemokines, a so-called “cytokine storm”, were found already at the day of fever onset in SARS patients (Huang et al., 2005), and improperly modulated IFN type I and II responses are thought to be closely linked to the clinical course of SARS (Cameron et al., 2007). These elevated cytokine and chemokine levels could be one of the causes of the observed leukocyte influx in the lungs and this in turn could result in the organ destruction and immune system exhaustion that are a hallmark of SARS. Unfortunately, the cytokine kinetics in the SARS study were examined at a later time interval than in our study and can therefore not support or disprove the possibility of SARS-CoV acting as a polyclonal stimulus.
We did not investigate which cells were involved in the observed rapid ChIFN-γ production. Apart from certain T cell sub-populations, NK cells are known to be major producers of IFN-γ (Yenan and Bryceson, 2006). However, as opposed to mammals, NK cells can only be found in very low numbers in the spleen or peripheral blood of birds (Göbel et al., 2001), and little is known about their activation kinetics. Identifying the cells involved in the rapid ChIFN-γ production after IBV stimulation will be an important focus for further research.
In conclusion, we have shown a rapid increase in ChIFN-γ production by chicken leukocytes after in vitro stimulation with IBV. This increase is independent of the infection status of the chicken and appears to be IBV-specific, as we did not observe this effect with other chicken viruses, other coronaviruses or components from allantoic fluid from which the virus was isolated. The observed effect suggests that IBV acts as a polyclonal stimulus on chicken immune cells.
Materials and methods
Animals
Commercial White Leghorn chickens and Mycoplasma-free broiler chickens (Cobb) were housed in groups and fed ad libitum on commercial feed. Chickens were housed, handled and treated following approval by the Animal Experimental Committee of the Veterinary Faculty of Utrecht University, The Netherlands, in accordance with the Dutch regulation on experimental animals.
Virus
The virulent Massachusetts IBV strain M41 was obtained from Intervet, Boxmeer, the Netherlands, as freeze-dried vials containing 108.3 EID50/1.2 ml/vial. The virus had been passaged twice in SPF embryonated eggs. The IB vaccine virus H120 was obtained as commercial freeze-dried 1000 doses vials which contained at least 103.0 EID50 (egg infective dose 50%) per dose (Nobilis® IB H120). Just prior to infection, all IBV inocula were prepared in distilled water and contained at least 103.0 EID50/ml of H120 virus and 104.6 EID50/ml of M41 virus. NDV was obtained as commercial freeze-dried vials which contained at least 106 EID50 per dose (Nobilis; ND clone 30). Feline Infectious Peritonitis Virus (FIPV) and Mouse Hepatitis Virus (MHV; both 108 pfu/ml) were a kind gift from the Virology Department of the Faculty of Veterinary Medicine, Utrecht University. Reticulo-Endotheliosis Virus (REV-C; 5 × 106 TCID/ml) was a kind gift from Dr. G. Koch (CIDC-Lelystad). All inocula were prepared in LPS-free PBS prior to in vitro stimulation at a concentration of 106 EID50 or pfu/ml.
IBV M41 was purified using a sucrose gradient (Cornelissen et al., 1997, Koopmans et al., 1986). The gradient consisted of layers of 20–50% sucrose (top to bottom) in TES-V (20 mM TRIS–HCl at pH 7.3, 1 mM EDTA, 100 mM NaCl). This gradient was autoclaved, left to linearise at 4 °C overnight, loaded with an IBV virus sample and centrifuged at 15,000 ×g for 3 h, 4 °C. Gradient fractions were collected from the bottom (50% sucrose solution) to the top (20%) with a fluid pump. For in vitro restimulation, inactivation of virus was achieved by direct exposure of the virus to UV illumination (∼ 1 mW/cm3) for 1 h (Ravindra et al., 2008, Stern and Sefton, 1982). Egg allantois fluid was a kind gift from Dr. J. de Wit (GD-Animal Health Service, Deventer).
ELISPOT assay
Preparation of splenocytes was described before in Ariaans et al. (2008b). Briefly, spleen tissue was squeezed through 70 μm mesh in RPMI1640 culture medium containing 2% FCS to prepare a single cell suspension. Splenocytes were isolated by density gradient centrifugation using FICOLL-Hypaque.
The ELISPOT assay has been described by Ariaans et al. (2008b). Briefly, MultiScreentm-IP 96-well plates were coated with mouse-anti-ChIFN-γ, blocked and splenocytes were seeded at 3 × 105 cells/well in triplicate in culture medium. Cells were incubated in the presence of either culture medium or medium supplemented with Concanavalin A (ConA; 10 μg/ml), phorbol 12-myristate 13-acetate (PMA; 100 ng/ml) and ionomycin (500 ng/ml), or virus and incubated for 24 or 48 h at 41 °C, 5% CO2. ChIFN-γ was detected by incubation with biotinylated mouse-anti-ChIFN-γ and streptavidin-alkaline phosphatase. The assay was developed using BCIP/NBT substrate, and analyzed using the A·EL·VIS machine and the Eli.Analyse software (Version 4.0) that allows for automated counting of the number of spots based on size and intensity. The cow PBMC ELISPOT was performed as described by Koets et al. (2006).
ELISA for ChIFN-γ detection
A total of 2 × 106 splenocytes per chicken were incubated for 48 h in a 24-wells plate in 500 μl of culture medium at 41 °C, 5% CO2, without stimulation or in the presence of ConA. Afterwards, supernatants were tested for ChIFN-γ using a commercial sandwich ELISA kit (Biosource International, California, USA) according to manufacturer's instructions.
Real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR)
RNA samples isolated from spleen were screened for mRNA encoding ChIFN-γ, as described in Ariaans et al. (2008a). Briefly, total RNA was isolated using the RNeasy Mini Kit (Qiagen) following the manufacturer's instructions. Reverse transcription was performed using iScript cDNA Synthesis Kit (Biorad). The forward primer (5′-GTGAAGAAGGTGAAAGATATCATGGA-3′) and reverse primer (5′-GCTTTGCGCTGGATTCTCA-3′) and the probe (5′-FAM-TGGCCAAGCTCCCGATGAACGA-TAMRA-3′) for ChIFN-γ were designed according to Kaiser et al. (Kaiser et al., 2003). Amplification and detection of specific products was achieved with the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad). Results were expressed as fold change between samples (Philbin et al., 2005).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 for Windows. Analysis of data was performed using a Mann-Whitney test. Results were considered statistically significant if p ≤ 0.05.
Acknowledgments
We thank Daphne van Haarlem for technical assistance. This work was supported by BSIK VIRGO consortium grant (G 03012), The Netherlands to P.M.H. and L.V.
References
- Ariaans M.P., Matthijs M.G.R., Van Haarlem D., Van de Haar P., Van Eck J.H.H., Hensen E.J., Vervelde L. The role of phagocytic cells in enhanced susceptibility of broilers to colibacillosis after Infectious Bronchitis Virus infection. Vet. Immunol. Immunopathol. 2008;123:240–250. doi: 10.1016/j.vetimm.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariaans M.P., Van de Haar P.M., Lowenthal J.W., Van Eden W., Hensen E.J., Vervelde L. ELISPOT and intracellular cytokine staining: novel assays for quantifying T cell responses in the chicken. Dev. Comp. Immunol. 2008;32:1398–1404. doi: 10.1016/j.dci.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Cameron M.J., Ran L., Xu L., Danesh A., Bermejo-Martin J.F., Cameron C.M., Muller M.P., Gold W.L., Richardson S.E., Poutanen S.M., Willey B.M., DeVries M.E., Fang Y., Seneviratne C., Bosinger S.E., Persad D., Wilkinson P., Greller L.D., Somogyi R., Humar A., Keshavjee S., Louie M., Loeb M.B., Brunton J., McGeer A.J., Kelvin D.J. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilletti C., Bordi L., Lalle E., Rozera G., Poccia F., Agrati C., Abbate I., Capobianchi M.R. Coordinate induction of IFN-alpha and -gamma by SARS-CoV also in the absence of virus replication. Virology. 2005;341:163–169. doi: 10.1016/j.virol.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian Infectious Bronchitis Virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Collisson E.W., Pei J., Dzielawa J., Seo S.H. Cytotoxic T lymphocytes are critical in the control of Infectious Bronchitis Virus in poultry. Dev Comp Immunol. 2000;24:187–200. doi: 10.1016/s0145-305x(99)00072-5. [DOI] [PubMed] [Google Scholar]
- Cornelissen L.A., Wierda C.M., van der Meer F.J., Herrewegh A.A., Horzinek M.C., Egberink H.F., de Groot R.J. Hemagglutinin-esterase, a novel structural protein of torovirus. J. Virol. 1997;71:5277–5286. doi: 10.1128/jvi.71.7.5277-5286.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel T.W.F., Kaspers B., Stangassinger M. NK and T cells constitute two major, functionally distinct intestinal epithelial lymphocyte subsets in the chicken. Int. Immunol. 2001;13:757–762. doi: 10.1093/intimm/13.6.757. [DOI] [PubMed] [Google Scholar]
- Hackney K., Cavanagh D., Kaiser P., Britton P. In vitro and in ovo expression of chicken gamma interferon by a defective RNA of avian coronavirus Infectious Bronchitis Virus. J. Virol. 2003;77:5694–5702. doi: 10.1128/JVI.77.10.5694-5702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks J.J., Wang Y., Detrick B. The critical role of IFN-gamma in experimental coronavirus retinopathy. Invest. Ophthalmol. Vis. Sci. 2003;44:3402–3408. doi: 10.1167/iovs.02-1106. [DOI] [PubMed] [Google Scholar]
- Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C., Lei H.Y. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail M., Cho K., Ward L., Saif L., Saif Y. Experimental bovine coronavirus in turkey poults and young chickens. Avian Dis. 2001;45:157–163. [PubMed] [Google Scholar]
- Kaiser P., Underwood G., Davison F. Differential cytokine responses following Marek's disease virus infection of chickens differing in resistance to Marek's disease. J. Virol. 2003;77:762–768. doi: 10.1128/JVI.77.1.762-768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- Kiss I., Poland A.M., Pedersen N.C. Disease outcome and cytokine responses in cats immunized with an avirulent feline infectious peritonitis virus (FIPV)-UCD1 and challenge-exposed with virulent FIPV-UCD8. J. Feline Med. Surg. 2004;6:89–97. doi: 10.1016/j.jfms.2003.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koets A., Hoek A., Langelaar M., Overdijk M., Santema W., Franken P., Eden W., Rutten V. Mycobacterial 70 kD heat-shock protein is an effective subunit vaccine against bovine paratuberculosis. Vaccine. 2006;24:2550–2559. doi: 10.1016/j.vaccine.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Koopmans M., Ederveen J., Woode G.N., Horzinek M.C. Surface proteins of Breda virus. Am. J. Vet. Res. 1986;47:1896–1900. [PubMed] [Google Scholar]
- Lowenthal J.W., Staeheli P., Schultz U., Sekellick M.J., Marcus P.I. Nomenclature of avian interferon proteins. J. Interf. Cytok. Res. 2001;21:547–549. doi: 10.1089/10799900152434439. [DOI] [PubMed] [Google Scholar]
- Lucchiari M.A., Martin J.P., Modolell M., Pereira C.A. Acquired immunity of A/J mice to mouse hepatitis virus 3 infection: dependence on interferon-gamma synthesis and macrophage sensitivity to interferon-gamma. J. Gen. Virol. 1991;72:1317–1322. doi: 10.1099/0022-1317-72-6-1317. [DOI] [PubMed] [Google Scholar]
- Matthijs, M.G.R., Ariaans, M.P., Dwars, R.M., van Eck, J.H.H., Bouma, A., Stegeman, A., Vervelde, L., in press. Course of infection and immune responses in the respiratory tract of Infectious Bronchitis Virus infected broilers after superinfection with E. coli. Vet. Imm. Immunopathol. doi:10.1016/j.vetimm.2008.09.016. [DOI] [PubMed]
- Navas-Martin S.R., Weiss S. Coronavirus replication and pathogenesis: implications for the recent outbreak of severe acute respiratory syndrome (SARS), and the challenge for vaccine development. J. Neurovirol. 2004;10:75–85. doi: 10.1080/13550280490280292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki K., Nakamura T., Kawaoka Y., Tsubokura M. Interferon induction by several strains of avian Infectious Bronchitis Virus, a coronavirus, in chickens. Acta Virol. 1988;32:55–59. [PubMed] [Google Scholar]
- Pei J., Sekellick M.J., Marcus P.I., Choi I.S., Collisson E.W. Chicken interferon type I inhibits Infectious Bronchitis Virus replication and associated respiratory illness. J. Interf. Cytok. Res. 2001;21:1071–1077. doi: 10.1089/107999001317205204. [DOI] [PubMed] [Google Scholar]
- Philbin V.J., Iqbal M., Boyd Y., Goodchild M.J., Beal R.K., Bumstead N., Young J., Smith A.L. Identification and characterization of a functional, alternatively spliced Toll-like receptor 7 (TLR7) and genomic disruption of TLR8 in chickens. Immunology. 2005;114:507–521. doi: 10.1111/j.1365-2567.2005.02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindra P.V., Tiwari A.K., Ratta B., Chaturvedi U., Palia S.K., Subudhi P.K., Kumar R., Sharma B., Rai A., Chauhan R.S. Induction of apoptosis in Vero cells by Newcastle disease virus requires viral replication, de-novo protein synthesis and caspase activation. Virus Res. 2008;133:285–290. doi: 10.1016/j.virusres.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Roberts A., Lamirande E.W., Vogel L., Jackson J.P., Paddock C.D., Guarner J., Zaki S.R., Sheahan T., Baric R., Subbarao K. Animal models and vaccines for SARS-CoV infection. Virus Res. 2008;133:20–32. doi: 10.1016/j.virusres.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D.F., Sefton B.M. Synthesis of coronavirus mRNAs: kinetics of inactivation of Infectious Bronchitis Virus RNA synthesis by UV light. J. Virol. 1982;42:755–759. doi: 10.1128/jvi.42.2.755-759.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A., Yanai H. Interferon signalling network in innate defence. Cell Microbiol. 2006;8:907–922. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- Yenan T., Bryceson M.E.M.H.G.L.E.O.L. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]