Abstract
Postweaning multisystemic wasting syndrome, which is primarily caused by porcine circovirus type 2 (PCV2), is an emerging and important swine disease. We have recently shown that PCV2 induces nuclear factor kappa B activation and its activation is required for active replication, but the other cellular factors involved in PCV2 replication are not well defined. The extracellular signal-regulated kinase (ERK) which served as an important component of cellular signal transduction pathways has been shown to regulate many viral infections. In this report, we show that PCV2 activates ERK1/2 in PCV2-infected PK15 cells dependent on viral replication. The PCV2-induced ERK1/2 leads to phosphorylation of the ternary complex factor Elk-1, which kinetically paralleled ERK1/2 activation. Inhibition of ERK activation with U0126, a specific MEK1/2 inhibitor, significantly reduced viral progeny release. Investigations into the mechanism of ERK1/2 regulation revealed that inhibition of ERK activation leads to decreased viral transcription and lower virus protein expression. These data indicate that the ERK signaling pathway is involved in PCV2 infection and beneficial to PCV2 replication in the cultured cells.
Keywords: PCV2, ERK1/2, Elk-1, Signal transduction, Viral replication, PK15 cells
Introduction
Porcine circovirus (PCV) is classified in the genus Circovirus of the family Circoviridae (Todd et al., 2005). Two genotypes of PCV have been identified. PCV type 1 (PCV1), which was first recognized in 1974 as a contaminant of a continuous porcine kidney cell line (PK15) (Tischer et al., 1982), is known to be non-pathogenic to pigs (Allan et al., 1995). Infection with PCV type 2 (PCV2) has been associated with postweaning multisystemic wasting syndrome (PMWS) in young weaned pigs, which was first recognized in Canada in 1991 (Clark, 1997). Nowadays, this disease and related PCV2-associated diseases are occurring in all swine-producing areas of the world and have become increasingly serious threats to global pig production (Allan et al., 1998, Allan and Ellis, 2000, Choi et al., 2000, Edwards and Sands, 1994, Fenaux et al., 2000, Mankertz et al., 2000, Onuki et al., 1999, Segalés and Domingo, 2002). Usually PMWS appears in pigs aged 5 to 18 weeks, and affected pigs show fever, wasting or unthriftiness, respiratory distress, enlarged lymph nodes and, occasionally, jaundice and diarrhea (Darwich et al., 2004, Harding, 1996, Segalés and Domingo, 2002). Mortality rates may vary from 1 to 2% up to 30% in complicated cases when co-infections with porcine reproductive and respiratory syndrome virus, porcine parvovirus, or Mycoplasma hyopneumoniae. Other risk factors described in the Madec principles have been developed to correlate with PMWS (Rose et al., 2003). Microscopic lesions are characterized by lymphocyte depletion of follicular and interfollicular areas together with macrophage infiltration of lymphoid tissues in PMWS-affected pigs. Several lines of field and experimental evidence have suggested that severely PMWS affected pigs may develop immunosuppression (Segalés et al., 2004).
PCV genome is a closed circular, single-stranded DNA molecule of about 1.7 kb. Two major open reading frames (ORFs) have been recognized for PCV, ORF1, called rep gene, which encodes a protein of 35.7 kDa involved in virus replication (Mankertz et al., 1998), and ORF2, called cap gene, which encodes the major immunogenic capsid protein of 27.8 kDa (Cheung, 2003, Nawagitgul et al., 2000). In addition, a third open reading frame (ORF3) coding for an apoptosis-associated protein has been reported for PCV2 and it is involved in viral pathogenesis in vitro and in vivo (Liu et al., 2005, Liu et al., 2006). A recent report has shown that PCV2 induces nuclear factor kappa B (NF-κB) activation in cultured cells, and further revealed the role of NF-κB activation in viral replication and PCV2-mediated apoptotic change (Wei et al., 2008). However, whether the other signaling pathways may also contribute to PCV2 infection in the cultured cells is still unclear.
The extracellular signal-regulated kinase (ERK) signaling pathway is one of the three mitogen-activated protein kinase (MAPK) cascades that play important roles in the regulation of cell proliferation and differentiation, cytokine production and apoptosis (Garrington and Johnson, 1999, Roux and Blenis, 2004). The ERK activation is initiated by receptor tyrosine kinases that signal through the small GTP-binding protein Ras. Activation of Ras leads to phosphorylation of Raf kinase and in turn phosphorylates MEK1/2 followed by activating ERK1/2 via phosphorylation on tyrosine and threonine residues (Rubinfeld and Seger, 2005). The activated ERK1/2 translocates into the nucleus and phosphorylates numerous downstream substrates such as transcription factors c-myc, Ets, Elk-1, and Egr-1, which ultimately regulate gene expression (Luttrell, 2003). Therefore, the ERK signaling pathway involves in a wide range of cellular functions including cell proliferation, transformatiom, differentiation, and cell survival and death (Sebolt-Leopold et al., 1999, Luttrell, 2003). Research data have shown that many viruses such as human cytomegalovirus (Johnson et al., 2001), human immunodeficiency virus type 1 (Yang and Gabuzda, 1999), influenza virus (Pleschka et al., 2001), herpes virus (Perkins et al., 2002), coxsackievirus B3 (Luo et al., 2002), vaccinia virus (Andrade et al., 2004, de Magalhães et al., 2001), respiratory syncytial virus (Kong et al., 2004), Borna disease virus (Planz et al., 2001), and coronavirus (Cai et al., 2007) manipulate the ERK signaling pathway to regulate viral replication and gene expression. However, the role of the ERK signaling pathway during PCV2 replication is not clear.
Here, we reported that the ERK signaling pathway was activated in the cultured cells by PCV2 infection and found that activation of the host cellular pathway is essential for efficient PCV2 infection. Further experiments revealed that inhibition of this pathway reduces viral transcription, protein synthesis, and PCV2 viral progeny release. These results illustrate a mechanism by which PCV2 manipulates the ERK signaling pathway to facilitate its infection and replication during viral infection.
Results
Activation of ERK during PCV2 replication
It was reported that ERK is phosphorylated during varieties of virus replication. In order to assess whether PCV2 infection activated ERK1/2, the phosphorylation status of ERK1/2 was monitored on PK15 cells at different time points after infection by Western blot analysis. The PK15 cells were infected with PCV2 strain BJW at a MOI of 1 TCID50, and whole cell lysates were prepared at the indicated times after virus infection. Inoculation of PBS into PK15 cells served as mock-infected controls. As shown in Fig. 1 A, infection with PCV2 led to progressive accumulation of p-ERK1/2 signals over time, and the maximal induction of ERK1/2 was seen at 72 h postinfection. The increased levels of ERK1/2 phosphorylation were concurrent with expression of viral capsid protein ORF2 in the infected cells, but not due to the enhanced production of these molecules or the difference in protein extracts loaded, as the protein levels of total amounts of ERK2, as well as β-actin, in each sample were comparable.
Fig. 1.
PCV2 infection activates ERK1/2 signaling pathway in cultured cells. (A) Whole cell lysates from PK15 cells after infection with PCV2 strain BJW at a MOI of 1 TCID50. PCV2-infected cells 24, 48, 72, and 96 h were harvested, whole cell lysates were prepared and resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted by using antibody specific for ERK2, phosphorylated ERK1/2, as well as PCV2 viral capsid protein ORF2. The amounts of β-actin were also assessed to monitor the equal loadings of protein extracts. (B) ERK1/2 activation induced by PCV2 infection was quantitatively determined using FACS assay. PK15 cells were fixed at the indicated time points with 4% formaldehyde and incubated with normal or anti-phospho-specific antibodies directed against ERK1/2 followed by HRP-conjugated IgG antibodies. Total and phosphorylated ERK were each assayed in triplicate. Cell numbers were normalized using crystal violet. These results are representative of three independent experiments. Values are shown as the mean ± SD from triplicate wells. p-, phospho-.
To further determine activated ERK1/2 quantitatively in the infected cells, we used a Fast Activated Cell-Based ELISA (FACE) assay to investigate the levels of ability of ERK1/2 phosphorylation at different time points after PCV2 infection. Consistent with the results shown in Fig. 1A, there was a time-dependent increase in the ERK1/2 phosphorylation in the PCV2-infected cells at 72 h postinfection which decreased thereafter (Fig. 1B). At 72 h after infection, the activation of phosphorylated ERK1/2 showed approximately 3.1-fold higher than that in the mock-infected cells. In the mock-infected cells, the level of ERK1/2 phosphorylation fell to its basal level. In addition, the levels of total ERK1/2 remain unchanged in the PCV2-infected cells at various time points after infection when compared to that in the mock-infected cells. Thus, these data indicate that PCV2 infection induces the activation of the cellular signaling pathway mediated by ERK1/2.
PCV2 replication is required for ERK1/2 phosphorylation
To determine whether PCV2 replication was required for ERK1/2 phosphorylation, we used the FACE assay to examine ERK1/2 phosphorylation in the cultured cells when infected with a UV light-irradiated virus sample. PK15 cells were infected an unirradiated or irradiated virus samples for 72 h postinfection. As shown in Fig. 2 , no significant increase in activation of phosphorylated ERK1/2 was observed in the UV-irradiated PCV2-infected cells as compared to that in the mock-infected cells. In contrast, the levels of ERK1/2 phosphorylation increased by 3.5-fold when infected with the unirradiated PCV2 at 72 h postinfection. The levels of total ERK1/2 remain unchanged in the PCV2-infected cells when compared to that in the mock-infected cells. The result demonstrated that PCV2 replication was required for ERK1/2 phosphorylation.
Fig. 2.
PCV2 replication is required for ERK phosphorylation. Monolayer PK15 cells were infected with PCV2 strain BJW at a MOI of 1 TCID50. At 72 h postinfection, PCV2-infected cells and UV-irradiated PCV2-infected cells were fixed with 4% formaldehyde. The FACE assay was performed using normal or anti-phospho-specific antibodies directed against ERK followed by HRP-conjugated IgG antibodies. Total and phosphorylated ERK were each assayed in triplicate. Cell numbers were normalized using crystal violet. These results are representative of three independent experiments. Values are shown as the mean ± SD from triplicate wells.
ERK activation leads to Elk-1 phosphorylation
To confirm that phosphorylation of ERK1/2 following PCV2 infection truly activated its downstream substrate Elk-1, the phosphorylation of the transcription factor was measured in the infected cells by Western blotting. No activation of Elk-1 was detected in the mock-infected PK15 cells. Sustained ERK1/2 activation after PCV2 infection leads to prolonged phosphorylation of Elk-1 (Fig. 3 A), with kinetics that paralleled those observed for ERK1/2. Cells exposed to the UV-irradiated virus sample failed to phosphorylate Elk-1 (Fig. 3B). In addition, Elk-1 phosphorylation was specifically impaired by preincubation with MEK1/2 inhibitor U0126 at 20 μM (Fig. 3B). The result demonstrated that activation of ERK1/2 induced by PCV2 infection is possibly through the activation of its downstream components such as Elk-1.
Fig. 3.
Phosphorylated ERK activates downstream target Elk-1 signal. (A) PK15 cells were mock-infected or infected with PCV2 strain BJW at a MOI of 1 TCID50. At 24, 48, 72, and 96 h postinfection, whole cell lysates were harvested and resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti-phospho-Elk-1 antibody. (B) PK15 cells were infected in the absence or presence of U0126 (at 20 μM). Cell lysates were harvested to examine Elk-1 phosphorylation. β-actin was probed as the loading control. p-, phospho-.
ERK regulates PCV2 replication
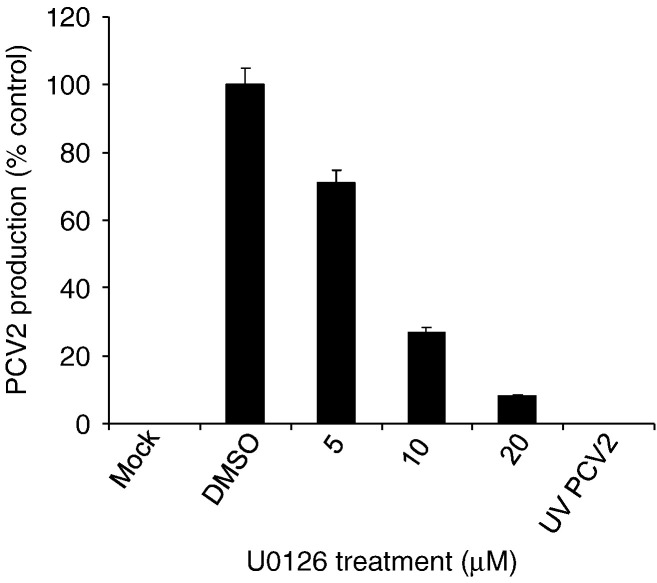
To determine whether activated ERK plays any role in the replication of PCV2, we examined the effect of the kinase on progeny virus production in the PCV2-infected PK15 cells by blocking ERK activation. We infected PK15 cells with PCV2 in the presence of the inhibitor U0126 (5–20 μM) or control (DMSO), and determined the viral concentration in the cell culture supernatant 72 h after infection. The kinase inhibitor was also present during infection and in subsequent incubation periods. The selected concentrations of the kinase inhibitor were tested to show their effectiveness in inhibiting virus-induced ERK1/2 activity by the FACS assay, which demonstrated that activation of ERK1/2 reduced dose-dependently (data not shown). Seventy-two hours postinfection supernatants were collected and viral production was determined by the IFA method. The inhibitor U0126 at 5 μM inhibited PCV2 production in the cultured cells by 29%, compared with controls (Fig. 4 ). Treatment with the inhibitor U0126 at 10 μM and 20 μM reduced PCV2 growth by 73% and 92%, respectively. As expected, the UV-irradiated PCV2 failed to grow comparable to those seen in the mock-infected cells (Fig. 4). The result suggested that ERK signal regulates the replication of PCV2 in PK15 cells.
Fig. 4.
Inhibition of ERK1/2 phosphorylation blocks PCV2 replication. Supernatants of PCV2-infected PK15 cells 72 h after treatment with various concentrations of the inhibitor U0126 as well as UV-irradiated PCV2-infected cell supernatants 72 h were inoculated on monolayers of PK15 cells. Virus productions were assayed by the IFA method under a fluorescence microscopy. The values represented are the means of the results for three independent experiments; error bars show the standard deviations.
Inhibition of ERK activation blocks PCV2 replication at the early stage of infection
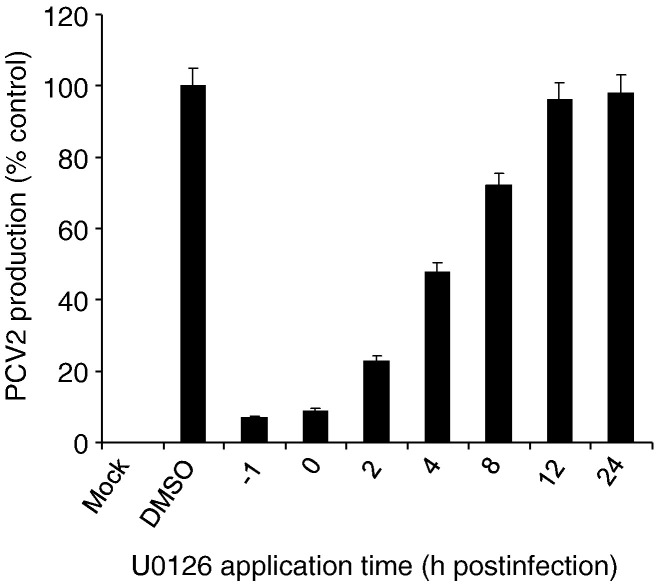
To more specifically identify the stage of PCV2 infection that was targeted by the inhibition of ERK activation, we quantified PCV2 production after applying the inhibitor U0126 at different time points postinfection. The cells were incubated until 72 h postinfection, and the supernatants were collected for virus production assay. As shown in Fig. 5 , addition of U0126 at the time of infection led to a 91% inhibition in virus production as compared to the DMSO-treated control. Treatment between 2 and 8 h postinfection resulted in significant reduction of virus production (73% to 28% reduction compared to the DMSO-treated control). In contrast, no significant differences in viral production were observed when the inhibitor was added at 12 or 24 h postinfection. The result indicates that inhibition of PCV2 infectivity by the inhibitor indeed occurs at the early stage in PCV2 infection.
Fig. 5.
Inhibition of ERK1/2 pathway reduces PCV2 infectivity at the early stage of infection. U0126 (20 μM) was added to PCV2-infected PK15 cells at − 1, 0, 2, 4, 8, 12, and 24 h postinfection. At 72 h postinfection, the supernatants were collected and determined for virus production by the IFA method. Experiments were performed in triplicate; the data shown are mean values ± SD based on three independent experiments; error bars represent the standard deviations.
Inhibition of ERK activation reduces PCV2 viral protein synthesis and viral transcription
To help delineate the mechanism of ERK1/2 regulation of PCV2 infection, we examined the effect of ERK inhibition on viral protein expression. PK15 cells were infected with PCV2 (MOI of 1) in the presence or absence of U0126 (20 μM), and ORF2 protein expression was monitored by a fluorescence microscopy. The ORF2 protein expression was significantly reduced when cells were treated with U0126, as demonstrated by the increased number of PCV2-positive cells observed in DMSO-treated infected cells (Fig. 6 A). No significant differences were seen in the ORF2 protein expression between DMSO-treated infected cells and untreated infected cells (data not shown). No ORF2 protein expression was detected in the UV-irradiated PCV2-infected as well as mock-infected cells (Fig. 6A).
Fig. 6.
(A) Inhibition of ERK1/2 phosphorylation decreases protein synthesis in the PCV2-infected PK15 cells. The PCV2-Infected PK15 cells 72 h in the presence of U0126 (20 μM) as well as UV-irradiated PCV2-infected cells 72 h were assayed for the amount of PCV2 viral capsid expression by the IFA method. The amounts of PCV2 ORF2 protein expression are shown as percentages of the PCV2-positive signals in PCV2 alone-infected cells. (B) Inhibition of ERK1/2 phosphorylation decreases viral transcription in the PCV2-infected PK15 cells. Total RNAs (1 μg) isolated from PCV2-infected cells 48 h after treatment with the inhibitor U0126 at the indicated concentrations were subjected to a real-time RT-PCR analysis. The relative amount of ORF2 mRNA was normalized to that of β-actin mRNA and is expressed as multiples of the normalized value for PCV2-infected and treated cells or mock-infected cells (control) in the same sample. The data shown are mean values ± SD based on three independent experiments; error bars show the standard deviations.
We then examined the effect of ERK1/2 inhibition on PCV2 viral mRNA synthesis, the real-time RT-PCR analysis was performed with RNA extracted from PK15 cells 48 h after treatment with U0126 at 20 μM. The amount of each viral mRNA was normalized to that of β-actin mRNA in the same sample. The abundance of PCV2 mRNA was significantly decreased in the PCV2-infected cells with U0126 treatment (Fig. 6B). At the indicated time point, the amount of PCV2-specific mRNA in U0126-treated cells was approximately 2.6% that of untreated cells. In contrast, the mRNA was detected in the DMSO-treated PCV2-infected cells as seen in that in the PCV2-aloned-infected cells (data not shown). No viral mRNA accumulation was detected in the UV-irradiated PCV2-infected as well as mock-infected cells (Fig. 6B). The results indicate that inhibition of ERK1/2 activation did have an inhibitory effect on viral protein synthesis and the accumulation of viral-specific mRNA in the PCV2-infected cells.
Discussion
Infection with a variety of viruses lead to the perturbation of host cell signaling pathways including ERK MAPK cascade, which can affect cellular function and virus replication. In the present study, we show that ERK1/2 MAPK pathway was activated during the course of PCV2 infection in PK15 cells (Figs. 1A and B), and its activation was required for viral replication (Fig. 2). Also, we show that PCV2 infection induced the transcription factor Elk-1, which is a downstream substrate of ERK1/2, with kinetics that paralleled those observed for ERK1/2 (Fig. 3). PCV2 replication was inhibited with the MEK1/2 U0126 (Fig. 4). U0126 inhibited viral replication by 28% when added up to 8 h postinfection (Fig. 5), but subsequent addition had little effect, confirming that ERK is important at early steps of the viral cycle. In addition, PCV2 viral transcription (Fig. 6B) and virus protein synthesis (Fig. 6A) were reduced in the presence of U0126. Together, these data suggest that the ERK1/2 signaling pathway is manipulated by PCV2 and that the pathway plays a beneficial role in PCV2 replication.
Activation of the ERK pathway seems to be an essential requirement to regulate signals associated with biological functions during virus infections. Many viruses utilize the ERK pathway for maximal viral replication. Activated ERK1/2 was reported to enhance the infectivity of human immunodeficiency virus (HIV), whereas treatment of cells with the MEK1/2 inhibitor significantly inhibited HIV infectivity (Yang and Gabuzda, 1998, Yang and Gabuzda, 1999). ERK MAPK is required for Visna virus replication and virus-induced neuropathology (Barber et al., 2002). Treating cells with the MEK1/2 inhibitor also significantly inhibited the propagation of influenza A virus (Pleschka et al., 2001), Borna disease virus (Planz et al., 2001), Coxsackievirus B3 (Luo et al., 2002), human cytomegalovirus (Johnson et al., 2001), vaccinia virus (Andrade et al., 2004), coronavirus (Cai et al., 2007), and astrovirus (Moser and Schultz-Cherry, 2008). In the present study, we determined whether PCV2-induced ERK1/2 activation is involved in viral replication by examining the effect of the inhibitor U0126 on PCV2 production and found that inhibition of ERK1/2 activation significantly reduced the production of progeny virus (Fig. 4). This shows that optimal PCV2 replication requires the ERK1/2 activity, and raises the possibility that PCV2 has acquired the ability to activate the kinase to aid its replication. Therefore, our findings indicate that PCV2-induced activation of ERK is truly involved in the replication of PCV2 in PK15 cells and add a new member to the growing list of viruses whose replication is modulated by the ERK signaling pathway.
ERK1/2 regulation of viral replication can act at specific steps of the replication cycle, such as attachment, entry, gene transcription, protein expression, and assembly. Borna disease virus requires active ERK for entry (Planz et al., 2001). HIV-1 depends on the activation of MEK ERK pathway to deliver its genomes into the cultured cells (Liu et al., 2002). Inhibition of ERK during coronavirus infection specifically decreases genomic and sgRNA production but has no effect on protein synthesis (Cai et al., 2007). ERK activates adenovirus gene transactivators, modulating protein expression (Schümann and Dobbelstein, 2006, Whalen et al., 1997), with little effect on RNA levels (Schümann and Dobbelstein, 2006). Inhibition of MEK/ERK pathway reduces Kaposi's sarcoma-associated herpesvirus infectivity but has no effect on virus binding to the cell surface receptor (Naranatt et al., 2003). Vaccinia virus-induced ERK activation was significantly blocked by inhibition of ERK, which was simultaneously paralleled by both delayed viral early-gene expression and a decrease in viral DNA synthesis (Andrade et al., 2004). Inhibition of ERK activation mediated by astrovirus reduces all steps of the viral life cycle, including early and late protein expression as well as subgenomic and genomic RNA transcription (Moser and Schultz-Cherry, 2008). The function of activated ERK during PCV2 infection was investigated in the present study. Our data showed that treatment of cells with U0126 suppressed virus propagation at the step of viral RNA transcription as well as viral protein synthesis. PCV replicates via rolling circle replication involving an intermediate double-stranded replicative form of DNA, and the rep proteins nick and join the nucleotide segments at the initiation and termination of the replication cycle (Cheung, 2006, Steinfeldt et al., 2006). Thus, it was not surprising that treatment of PCV2-infected cells with inhibition of ERK reduced viral transcripts as well as protein expression, because viral transcription and protein synthesis are closely related in the replication of PCV2. However, a detailed mechanistic understanding of the inhibition of ERK activation on PCV2 replication requires the identification of particular cellular factors which are the components of the ERK signaling pathway.
In conclusion, we have shown that PCV2 infection induces the activation of ERK and its involvement in Elk-1 activation in the cultured cells and demonstrate that the activation of ERK is required for efficient PCV2 replication. Inhibition of ERK activation significantly reduces viral protein expression and viral RNA transcription. The role of ERK activation in PCV2 replication will contribute important information about the molecular mechanism of PCV2 infection.
Materials and methods
Virus and cells
The permanent PK15 cell line, which was free of PCV, was maintained in minimal essential medium (MEM) supplemented with 5% heat-inactivated fetal bovine serum (FBS), 5% l-glutamine, 100 U of penicillin G/ml, and 100 μl of streptomycin/ml at 37 °C in a humidified 5% CO2 incubator. The PCV2 virus used in the study was originally isolated from a kidney tissue sample of a pig with naturally occurring PMWS (strain BJW) (Liu et al., 2005).
For PCV2 infection, PK15 cells seeded the day before were infected with PCV2 strain BJW at a multiplicity of infection (MOI) of 1 TCID50. Cells were additionally treated with 300 mM d-glucosamine at 24 h after infection as described previously (Tischer et al., 1987).
Reagents and antibodies
U0126 was purchased from Calbiochem (La Jolla, Calif.). PK15 cells were treated with either DMSO which is the solvent for U0126 or various concentrations (5–20 μM) for 1 h prior to infection. After 1 h of virus adsorption, the virus inoculum was removed and fresh basal medium containing fresh inhibitor was added to the culture. The cytotoxicity of the inhibitors on PK15 cells was determined by trypan blue exclusion dye staining. It was noted that throughout all doses of the inhibitor used in this study, cell viability assay showed no detectable cell death in PK15 cells.
Rabbit antibodies against ERK2, β-actin, as well as phosphorylated ERK1/2 (p-ERK1/2) were purchased from Santa Cruz Biotechnology (Hercules, CA). Antibody specific for phosphorylated forms of Elk-1 (p-Elk-1) was obtained from Cell Signaling Technology. Horseradish peroxidise (HRP)-linked secondary antibodies were purchased from Sigma.
Fast activated cell-based ELISA (FACE)
FACE kit to monitor the levels of ERK1/2 MAPK activation was obtained from Active Motif. Procedure was performed strictly according to the manufacturer's instructions. Briefly, PK15 cells were seeded in 96-well plates 1 day prior to infection. After treatment and/or infection, cells were fixed with 4% formaldehyde in PBS. After washing and blocking, cells were reacted overnight with an anti-ERK1/2 or anti-phospho-ERK1/2 antibody. Following incubation with a HRP-conjugated secondary antibody, colorimetric analysis was performed. A450 was determined using a plate spectrophotometer.
Quantitative real-time RT-PCR
Total cell RNAs were prepared from PCV2-infected PK15 cell 48 h after being treated with various concentrations of the inhibitor U0126 by using Trizol RNA extract reagent (Invitrogen). The following primers were used: ORF2 (sense) (5′-ATCAAGCGAACCACAG-3′) and ORF2 (antisense) (5′-GGTCATAGGTGAGGGGC-3′) for PCV2 ORF2 and sense (5′-CACGCCATCCTGCGTCTGGA-3′) and antisense (5′-AGCACCGTGTTGGCGTAGAG) for β-actin. The RNA samples were incubated with DNase I for 60 min at 37 °C to remove any contaminating viral DNA. cDNAs were reverse transcribed from total RNAs by the use of antisense primers and the First-Strand synthesis system (Avian Myeloblastosis Virus Reverse Transcriptase kit; Roche). Quantitative real-time PCR was performed on a LightCycler (Roche) instrument according to the instructions of the LightCycler Fast Start DNA Masterplus SYBR Green I kit (Roche). The PCR parameters consisted of an initial denaturation at 94 °C for 5 min, followed by 40 cycles of 94 °C for 10 s, 52 °C for 5 s and 72 °C for 10 s. Subsequent melting curve analysis and C T value determination were performed using Roche LightCycler software version 3.5. Each sample was run in triplicate. The relative amount of target viral mRNA was normalized to that of β-actin mRNA in the same sample.
Indirect immunofluorescence assay (IFA)
PK15 monolayer cells seeded in 24-well culture plates were infected with PCV2 strain BJW. At 72 h, the cells were washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde (PFA). After three washes, the cells were incubated with mouse anti-ORF2 antibody diluted in 3% bovine serum albumin (BSA)-PBS at room temperature (RT) for 1 h. After three further washes, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (Sigma) at RT for 1 h and washed with PBS three times. The cells were examined under a fluorescence microscopy and cells positive for PCV2 viral antigens were counted in six fields of view.
Whole cell lysates
Whole cell lysate extracts from PK15 cells after infection at various time points were prepared with the Nuclear Extract kit (Active Motif) according to the manufacturer's protocol. Protein concentration was determined by the Bio-Rad protein assay (Bio-Rad, Hercules, CA) with BSA as a standard.
Western blotting
The whole cell lysate extracts prepared as described above were diluted in 2× sample buffer and boiled for 5 min. Twenty micrograms of each extract was resolved on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose (NC) membranes (Stratagene) with a semidry transfer cell (Bio-Rad Trans-Blot SD). The membranes were blocked for 2 h at RT in blocking buffer TBST (20 mM Tris–HCl [pH 7.4], 150 mM NaCl, 0.1% Tween-20) containing 5% skim milk powder to prevent nonspecific binding, and then incubated with specific primary antibodies raised against ORF2, ERK2, phosphorylated (p)-ERK1/2, and (p)-ElK-1, as well as β-actin at RT for 2 h. The membranes were washed three times with TBST buffer, and incubated for 2 h at RT with HRP-conjugated secondary antibodies diluted in blocking buffer (1:2 000). Immunoreactive bands were visualized by enhanced chemiluminescence system (Amersham Biosciences).
Statistical analysis
Results are presented as averages ± the standard deviations or standard errors of the means, as indicated. Statistical comparisons are made by using Student's t test, and differences between groups were considered significant if the P value was < 0.05.
Acknowledgments
This work was supported by grants from National Natural Science Foundation (30871866) and Beijing Municipal Science and Technology Contract Project (Z07010501780701), the People's Republic of China.
References
- Allan G.M., Ellis J. Porcine circoviruses: a review. J. Vet. Diagn. Invest. 2000;13:3–14. doi: 10.1177/104063870001200102. [DOI] [PubMed] [Google Scholar]
- Allan G.M., McNeilly F., Cassidy J.P., Reilly G.A., Adair B., Ellis W.A., McNulty M.S. Pathogenesis of porcine circovirus: experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet. Microbiol. 1995;44:49–64. doi: 10.1016/0378-1135(94)00136-k. [DOI] [PubMed] [Google Scholar]
- Allan G.M., McNceilly F., Kennedy S., Daft B., Clark E.G., Ellis J.A., Haines D.M., Meehan B.M., Adair B.M. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J. Vet. Diagn. Invest. 1998;10:3–10. doi: 10.1177/104063879801000102. [DOI] [PubMed] [Google Scholar]
- Andrade A.A., Silva P.N.G., Pereira A.C.T.C., de Sousa L.P., Ferreira P.C.P., Gazzinelli R.T., Kroon E.G., Ropert C., Bonjardim C.A. The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication. Biochem. J. 2004;381:437–446. doi: 10.1042/BJ20031375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber S.A., Bruett L., Douglass B.R., Herbst D.S., Zink M.C., Clements J.E. Visna virus-induced activation of MAPKs is required for virus replication and correlates with virus-induced neuropathology. J. Virol. 2002;76:817–828. doi: 10.1128/JVI.76.2.817-828.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Liu Y., Zhang X. Suppression of coronavirus replication by inhibition of the MEK signaling pathway. J. Virol. 2007;81:446–456. doi: 10.1128/JVI.01705-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A.K. Transcriptional analysis of porcine circovirus type 2. Virology. 2003;305:168–180. doi: 10.1006/viro.2002.1733. [DOI] [PubMed] [Google Scholar]
- Cheung A.K. Rolling-circle replication of an animal circovirus genome in a theta-replicating bacterial plasmid in Escherichia coli. J. Virol. 2006;80:8686–8694. doi: 10.1128/JVI.00655-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C., Chae C., Clark E.G. Porcine postweaning multisystemic wasting syndrome in Korean pig: detection of porcine circovirus 2 infection by immunohistochemistry and polymerase chain reaction. J. Vet. Diagn. Invest. 2000;12:151–153. doi: 10.1177/104063870001200209. [DOI] [PubMed] [Google Scholar]
- Clark E.G. Post-weaning multisystemic wasting syndrome. Proc. Am. Assoc. Swine Pract. 1997;28:490–501. [Google Scholar]
- Darwich L., Segales J., Mateu E. Pathogenesis of postweaning multisystemic wasting syndrome caused by porcine circovirus 2: an immune riddle. Arch. Virol. 2004;149:857–874. doi: 10.1007/s00705-003-0280-9. [DOI] [PubMed] [Google Scholar]
- de Magalhães J.C., Andrade A.A., Silva P.N., Sousa L.P., Ropert C., Ferreira P.C., Kroon E.G., Gazzinelli R.T., Bonjardim C.A. A mitogenic signal triggered at an early stage of vaccinia virus infection: implication of MEK/ERK and protein kinase A in virus multiplication. J. Biol. Chem. 2001;276:38353–38360. doi: 10.1074/jbc.M100183200. [DOI] [PubMed] [Google Scholar]
- Edwards S., Sands J.J. Evidence of circovirus infection in British pigs. Vet. Rec. 1994;134:680–681. doi: 10.1136/vr.134.26.680. [DOI] [PubMed] [Google Scholar]
- Fenaux M., Halbur P.G., Gill M., Toth T.E., Meng X.J. Genetic characterization of type 2 porcine circovirus (PCV-2) from pigs with postweaning multisystemic wasting syndrome in different geographic regions of North America and development of a differential PCR-restriction fragment length polymorphism assay to detect and differentiate between infections with PCV-1 and PCV-2. J. Clin. Microbiol. 2000;38:2494–2503. doi: 10.1128/jcm.38.7.2494-2503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrington T.P., Johnson G.L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- Harding J.C. Post-weaning multisystemic wasting syndrome (PMWS): preliminary epidemiology and clinical presentation. Proc. West Can. Assoc. Swine Prac. 1996;1996:21. [Google Scholar]
- Johnson R.A., Ma X.L., Yurochko A.D., Huang E.S. The role of MKK1/2 kinase activity in human cytomegalovirus infection. J. Gen. Virol. 2001;82:493–497. doi: 10.1099/0022-1317-82-3-493. [DOI] [PubMed] [Google Scholar]
- Kong X., San Juan H., Behera A., Peeples M.E., Wu J., Lockey R.F., Mohapatra S.S. ERK-1/2 activity is required for RSV efficient infection. FEBS Lett. 2004;559:33–38. doi: 10.1016/S0014-5793(04)00002-X. [DOI] [PubMed] [Google Scholar]
- Liu N.Q., Lossinsky A.S., Popik W., Li X., Gujuluva C., Kriederman B., Roberts J., Pushkarsky M., Bukrinsky M., Wite M., Weinard M., Fiala M. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and mitogen-activated protein kinase signaling pathway. J. Virol. 2002;76:6689–6700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chen I., Kwang J. Characterization of a previously unidentified viral protein of porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J. Virol. 2005;79:8262–8274. doi: 10.1128/JVI.79.13.8262-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chen I., Du Q., Chua H., Kwang J. The ORF3 protein of porcine circovirus type 2 is involved in viral pathogenesis in vivo. J. Virol. 2006;80:5065–5073. doi: 10.1128/JVI.80.10.5065-5073.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Yanagawa B., Zhang J., Luo Z., Zhang M., Esfandiarel M., Carthy C., Wilson J.E., Yang D., McManus B.M. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 2002;76:3365–3373. doi: 10.1128/JVI.76.7.3365-3373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L.M. “Location, location, location”: activation and targeting of MAP kinases by G protein-coupled receptors. J. Mol. Endocrinol. 2003;30:117–126. doi: 10.1677/jme.0.0300117. [DOI] [PubMed] [Google Scholar]
- Mankertz A., Mankertz J., Wolf K., Buhk H.J. Identification of a protein essential for replication of porcine circovirus. J. Gen. Virol. 1998;79:381–383. doi: 10.1099/0022-1317-79-2-381. [DOI] [PubMed] [Google Scholar]
- Mankertz A., Domingo M., Folch J.M., LeCann P., Jestin A., Segalés J., Chmielewicz B., Plana-Durán J., Soike D. Characterization of PCV2 isolates from Spain, Germany, and France. Virus Res. 2000;66:65–77. doi: 10.1016/s0168-1702(99)00122-7. [DOI] [PubMed] [Google Scholar]
- Moser L.A., Schultz-Cherry S. Suppression of astrovirus replication by an ERK1/2 inhibition. J. Virol. 2008;82:7475–7482. doi: 10.1128/JVI.02193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranatt P., Akula S., Zien C., Krishnan H., Chandran B. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-ζ-NEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 2003;77:1524–1539. doi: 10.1128/JVI.77.2.1524-1539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawagitgul P., Morozov I., Bolin S.R., Harms P.A., Sorden S.D., Paul P.S. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 2000;81:2281–2287. doi: 10.1099/0022-1317-81-9-2281. [DOI] [PubMed] [Google Scholar]
- Onuki A., Abe K., Togashi K., Kawashima K., Taneichi A., Tsunemitsu H. Detection of porcine circovirus from lesions of a pig with wasting disease in Japan. J. Vet. Med. Sci. 1999;61:1119–1123. doi: 10.1292/jvms.61.1119. [DOI] [PubMed] [Google Scholar]
- Perkins D., Pereira E.F.R., Gober M., Yarowsky P.J., Aurelian L. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 2002;76:1435–1449. doi: 10.1128/JVI.76.3.1435-1449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planz O., Pleschka S., Ludwig S. MEK-specific inhibitor U0126 blocks spread of Borna disease virus in cultured cells. J. Virol. 2001;75:4871–4877. doi: 10.1128/JVI.75.10.4871-4877.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleschka S., Wolff T., Ehrhardt C., Hobom G., Planz O., Rapp U.R., Ludwig S. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 2001;3:301–305. doi: 10.1038/35060098. [DOI] [PubMed] [Google Scholar]
- Rose N., Larour G., Le Diguerher G., Eveno E., Jolly J.P., Blanchard P., Oger A., Le Dimna M., Jestin A., Madec F. Risk factors for porcine post-weaning multisystemic wasting syndrome (PMWS) in 149 French farrow-to-finish herds. Prev. Vet. Med. 2003;61:209–225. doi: 10.1016/j.prevetmed.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Roux P.P., Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld H., Seger R. The ERK cascade: a prototype of MAPK signaling. Mol. Biotechnol. 2005;31:151–174. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- Schümann M., Dobbelstein M. Adenovirus-induced extracellular signal-regulated kinase phosphorylation during the late phase of infection enhances viral protein levels and virus progeny. Cancer Res. 2006;66:1282–1288. doi: 10.1158/0008-5472.CAN-05-1484. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold J.S., Dudley D.T., Herrera R., Van Becelaere K., Wiland A., Gowan R.C., Tecle H., Barrett S.D., Bridges A., Przybranowski S., Leopold W.R., Saltiel A.R. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat. Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- Segalés J., Domingo M. Postweaning multisystemic wasting syndrome (PMWS) in pigs: a review. Vet. Q. 2002;24:109–124. doi: 10.1080/01652176.2002.9695132. [DOI] [PubMed] [Google Scholar]
- Segalés J., Domingo M., Chianini F., Majó N., Domínguez J., Darwich L., Mateu E. Immunosuppression in postweaning multisystemic wasting syndrome affected pigs. Vet. Microbiol. 2004;98:151–158. doi: 10.1016/j.vetmic.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Steinfeldt T., Finsterbusch T., Mankertz A. Demonstration of nicking/joining activity at the origin of DNA replication associated with the rep and rep' proteins of porcine circovirus type 1. J. Virol. 2006;80:6225–6234. doi: 10.1128/JVI.02506-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer I., Glederblom H., Vettermann W., Koch M.A. A very small porcine virus with circular single-stranded DNA. Nature. 1982;295:64–66. doi: 10.1038/295064a0. [DOI] [PubMed] [Google Scholar]
- Tischer I., Peters D., Rasch R., Pociuli S. Replication of porcine circovirus: induction by glucosamine and cell cycle dependence. Arch. Virol. 1987;96:39–57. doi: 10.1007/BF01310989. [DOI] [PubMed] [Google Scholar]
- Todd D., Biagini P., Bendinelli M., Hino S., Mankertz A., Mishiro S., Niel C., Okamoto H., Raidal S., Ritchie B.W., Teo G.C. Circoviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy, VIIIth Report of the International Committee for the Taxonomy of Viruses. Elsevier/Academic Press; London: 2005. pp. 327–334. [Google Scholar]
- Wei L., Kwang J., Wang J., Shi L., Yang B., Li Y., Liu J. Porcine circovirus type 2 induces the activation of nuclear factor kappa B by IκBα degradation. Virology. 2008;378:177–184. doi: 10.1016/j.virol.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Whalen S.G., Marcellus R.C., Whalen A., Ahn N.G., Ricciardi R.P., Branton P.E. Phosphorylation within the transactivation domain of adenovirus E1A protein by mitogen-activated protein kinase regulates expression of early region 4. J. Virol. 1997;71:3545–3553. doi: 10.1128/jvi.71.5.3545-3553.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Gabuzda D. Mitogen-activated protein kinase phosphorylates and regulates the HIV-1 Vif protein. J. Biol. Chem. 1998;273:29879–29887. doi: 10.1074/jbc.273.45.29879. [DOI] [PubMed] [Google Scholar]
- Yang X., Gabuzda D. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J. Virol. 1999;73:3460–3466. doi: 10.1128/jvi.73.4.3460-3466.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]