Abstract
Mesenchymal chondrosarcoma (MCS) is a rare high-grade sarcoma of bone and soft tissue with highly aggressive behavior and a peak incidence in the second and third decades. We report a case of primary orbital MCS in a 30 year-old female, with radiological and clinicopathological features. Orbital MCS is an entity that should be considered in the differential diagnosis of calcified orbital lesions.
Keywords: chondrosarcoma, mesenchymal, orbit
INTRODUCTION
Mesenchymal chondrosarcoma (MCS) is a rare high-grade sarcoma of bone and soft tissue firstly described by Lightenstein and Bernstein in 1959 [1].
MCS has a highly aggressive behavior and accounts for approximately 3% of all chondrosarcoma, with a peak incidence in the second and third decades [2].
MCS is composed of undifferentiated neoplastic cells associated with areas of mature cartilage and usually a hemangiopericytoma-like pattern of vascularization. Up to one-third of cases of MSC arise primarily in the soft tissue, with approximately 30 cases of orbital MSC described in the literature. Here, we report a case with primary orbital MCS in a 30 year-old patient, an entity that should be considered in the differential diagnosis of calcified orbital lesions.
CASE REPORT
A 30-year-old Caucasian female without any past medical illness presented with an endorbital mass of the right eye, without proptosis. Physical examination revealed a decreased right visual acuity. No abnormality of the left eye was detected. Computed tomography (CT) and magnetic resonance imaging (MRI) scans revealed a well-defined right intrasonic mass, in the upper-external quadrant with optical nerve displacement without involvement of it. The endorbital mass was adherent to the superior rectus muscle with a cleavage plane. CT scans demonstrated an ovoidal soft tissue mass with central calcification. T1 and T2 weighted images showed an oval tumor with iso-hyperintense signal with a calcified central component. Enhanced T2 weighted images demonstrated an omogenous tumoral enhancement except for the central calcified part (Fig. 1). These findings have led to a radiological-suggested differential diagnosis between vascular malformation, cavernous hemangioma and solitary fibrous tumor.
Figure 1.
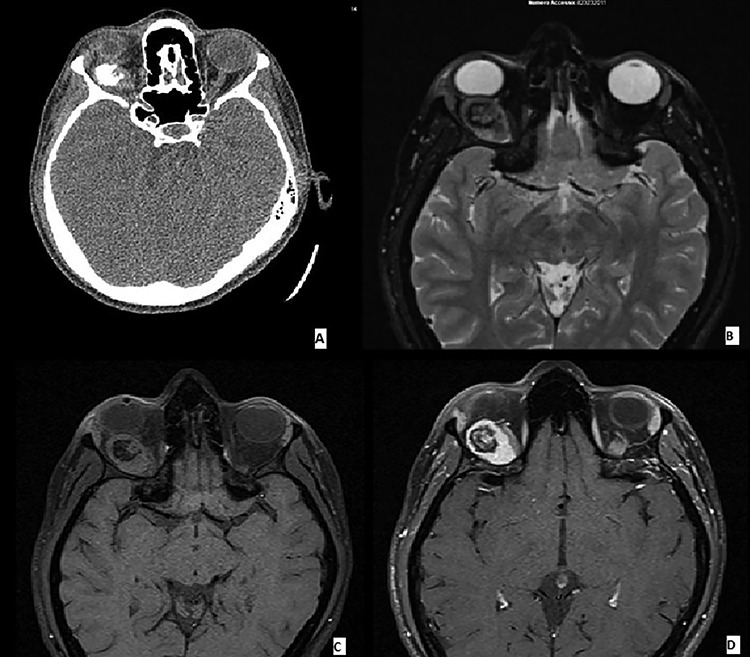
(A–D) Imaging features of MCS: CT scans demonstrated an ovoidal soft tissue mass with central calcification (A), T2 (B) and T1 (C) weighted images showing an oval tumor with iso-hyperintense signal with a calcified central component. Enhanced T1 weighted image demonstrating an omogenous tumoral enhancement except for the central calcified part (D).
An excisional biopsy of the orbital mass was performed. We found a firm, whitish nodule that measured 2.8 × 1.9 × 1.7 cm. Microscopically, the neoplasm had a typical biphasic pattern, with a hypercellular small round cell component intermixed with islands of well-differentiated cartilage showing metaplastic bone ossification. There was an abrupt transition between the two different components of the neoplasm, and a hemangiopericytoma-like vascular pattern was noted (Figs 2–4). Immunohistochemically, the mesenchymal small cell component showed focal cytoplasmic positivity for CD99, while the cartilaginous component was S100 protein-positive (Figs 5 and 6). The neoplastic cells showed negativity for cytokeratin AE1/AE3, STAT6 (signal transducer and activator of transcription-6), CD31 and CD34. The findings were consistent with MCS. This case was sent for a second opinion to Prof. Angelo Paolo Dei Tos (Department of Pathology, Azienda ULSS 2 Marca Trevigiana, Treviso, Italy) who confirmed the diagnosis of MCS, also reporting nuclear positivity for SOX9, a master regulator of the differentiation of mesenchymal cells into chondrocytes.
Figure 2.

Low-power view of the lesion showing central ossification (H&E).
Figure 4.

Typical hemangiopericytoma-like vascular pattern (H&E, high power view).
Figure 5.

Immunohistochemical features of MCS: cytoplasmic positivity for CD99 in the small cell component (IHC stain).
Figure 6.

Nuclear positivity for S100 in the cartilaginous component (IHC stain).
Figure 3.

Abrupt transition from small cell component to well differentiated cartilaginous area (H&E, high power view).
DISCUSSION
Clinically, the patient did not present with proptosis, a very common finding in orbital chondrosarcoma patients [3]. Similar to previous reports [4–6], we observed ossification of the orbital mass, a characteristic TC finding of orbital chondrosarcoma [4, 5].
We also observed optic nerve displacement that is a common finding in intraorbital MCS. Optic nerve tissue involvement, which has rarely been reported in huge tumors associated with intracranial extension [7], was not seen.
In our case, nuclear positivity for Sox9 was reported. This immunohistochemical finding could be useful to distinguish this entity from other small round cell tumors [8]. In addition, molecular studies have demonstrated a recurrent HEY1-NCOA2 fusion in MSC [9], which could support the diagnosis of this tumor. In conclusion, although orbital MSC is a rare entity, it should be considered in calcified lesions affecting children and young adults.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1). Lightenstein L, Bernstein D. Unusual benign and malignant chondroid tumors of bone. A survey of some mesenchymal cartilage tumors and malignant chondroblastic tumors, including a few multicentric ones, as well as many atypical benign chondroblastomas and chondromyxoid fibromas. Cancer 1959;12:1142–57. [DOI] [PubMed] [Google Scholar]
- 2). Tos D, Paolo A. Soft Tissue Sarcoma: A Pattern Approach to Diagnosis. Cambridge University Press, 2019, 336–40ISBN 9781107040809 [Google Scholar]
- 3). Alkatan HM, Eberhart CG, Alshomar KM, Elkhamary SM, Maktabi AMY. Primary mesenchymal chondrosarcoma of the orbit: histopathological report of 3 pediatric cases. Saudi J Ophthalmol 2018;32:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Tsuchiya M, Masui T, Otsuki Y, Sakahara H. Mesenchymal chondrosarcoma of the orbit: imaging features of CT and MRI. Br J Radiol 2018;91:20170579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Yang BT, Wang YZ, Wang XY, Wang ZC. Mesenchymal chondrosarcoma of the orbit: CT and MRI findings. Clin Radiol 2012;67:346–51. [DOI] [PubMed] [Google Scholar]
- 6). Font RL, Ray R, Mazow ML, Del Valle M. Mesenchymal chondrosarcoma of the orbit: a unique radiologic-pathologic correlation. Ophthalmic Plast Reconstr Surg 2009;25:219–22. [DOI] [PubMed] [Google Scholar]
- 7). Bagheri A, Abbaszadeh M, Torbati P, Rezaei KM. Mesenchymal chondrosarcoma of the orbit attached to the optic nerve. J Craniofac Surg 2018;29:e591–4. [DOI] [PubMed] [Google Scholar]
- 8). Wehrli BM, Huang W, De Crombrugghe B, Ayala AG, Czerniak B. Sox9, a master regulator of chondrogenesis, distinguishes mesenchymal chondrosarcoma from other small blue round cell tumors. Hum Pathol 2003;34:263–9. [DOI] [PubMed] [Google Scholar]
- 9). Wang L, Motoi T, Khanin R, Olshen A, Mertens F, Bridge J, et al. Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer 2012;51:127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


