Abstract
Introduction
Bariatric surgery-induced weight loss may reduce resting energy expenditure (REE) and fat-free mass (FFM) disproportionately thereby predisposing patients to weight regain and sarcopenia.
Methods
We compared REE and body composition of African-American and Caucasian Roux-en-Y gastric bypass (RYGB) patients after surgery with a group of non-operated controls (CON). REE by indirect calorimetry; skeletal muscle (SM), trunk organs, and brain volumes by MRI; and FFM by DXA were measured at post-surgery visits and compared with CON (N = 84) using linear regression models that adjusted for relevant covariates. Ns in RYGB were 50,42, and 30 for anthropometry and 39, 27,17 for MRI body composition at years 1,2, and 5 after surgery, respectively.
Results
Regression models adjusted for age, weight, height, ethnicity, and sex showed REE differences (RYGB minus CON; mean ± s.e.): year 1 (43.2 ± 34 kcal/day, p = 0.20); year 2 (− 27.9 ± 37.3 kcal/day, p = 0.46); year 5 (114.6 ± 42.3 kcal/day, p = 0.008). Analysis of FFM components showed that RYGB had greater trunk organ mass (~ 0.4 kg) and less SM (~ 1.34 kg) than CON at each visit. REE models adjusted for FFM, SM, trunk organs, and brain mass showed no between-group differences in REE (− 15.9 ± 54.8 kcal/day, p = 0.8; − 46.9 ± 64.9 kcal/day, p = 0.47; 47.7 ± 83.0 kcal/day, p = 0.57, at years 1, 2, and 5, respectively).
Conclusions
Post bariatric surgery patients maintain a larger mass of high–metabolic rate trunk organs than non-operated controls of similar anthropometries. Interpreting REE changes after weight loss requires an accurate understanding of fat-free mass composition at both the organ and tissue levels.
Clinical Trial Registration
Long-term Effects of Bariatric Surgery (LABS-2) NCT00465829
Keywords: Bariatric surgery, Roux-en-Y gastric bypass, Resting energy expenditure, Body composition, Fat-free mass, Skeletal muscle, Organs, Brain
Introduction
Bariatric surgery is widely used in combatting obesity and its co-morbidities. Weight loss magnitude and maintenance following surgical procedures clearly surpass those of conventional methods such as low-calorie diets, exercise, and behavior modification. While the benefits of weight loss surgery are numerous and compelling [1,2], questions about changes in body composition and resting energy expenditure (REE) remain. The influence of weight loss on REE in the long term remains controversial, with REE adjusted for fat-free mass (FFM; body weight minus fat mass) reported to be lower [3–5], not different [6–8], or higher [9] after weight loss. Low REE at reduced body weight may contribute to weight regain [10].
Of concern are the loss of FFM and its components. Excessive loss of skeletal muscle (SM), a major constituent of FFM (~ 45%), has implications for sarcopenia, frailty, and loss of mobility, crucial for independent functioning [11–13]. SM is a significant contributor to REE (~ 20%) and REE makes up ~ 70% of total energy expenditure and, thus, energy balance. Except for one report on SM [14], changes in the organ-tissue composition of FFM after surgery (e.g., SM, liver, kidney, heart, brain) and their potential effect on REE have not been studied.
The lack of homogeneity in heat-producing tissues that constitute FFM has long been recognized [15–18] with the existence of large between-organ differences in the rates of energy flux. Brain and visceral organs have high rates of heat production in the post-absorptive state whereas adipose tissue and skeletal muscle have relatively low rates. Specifically, the brain, liver, heart, and kidneys account for 58% of total calculated REE yet comprise 5.7% of mean body weight and 6.9% of FFM [17].
Most studies have found decreases in FFM and REE following surgery but the critical issue is whether the degree of loss in FFM and REE is appropriate or disproportionate, and whether the declines are temporary or enduring. The matter has been studied in two ways: by comparing post-surgery measured vs predicted values based on regression equations (or ratios), derived either from own sample before surgery or from published equations; or by comparing post-surgery patients with matched non-surgery controls of similar anthropometries (BMI, weight, age, sex) who represent the normal range of FFM and REE. Many studies using the former method find evidence for excessive REE reductions [19–23] although there are exceptions [6,8,9]. In contrast, investigators using the second approach have not found evidence for excessive REE decline. Skogar et al. [24] compared 27 Roux-en-Y gastric bypass (RYGB) surgery and duodenal switch patients with 17 matched controls at 2 years post-surgery and reported no between-group differences in FFM (by BodPod) or REE (estimated by BodPod equations, not measured). Schiavo et al. [25] compared FFM and REE 3 years after sleeve gastrectomy with BMI-, age-, and sex-matched controls and reported no differences in REE or FFM. Mirahmadian et al. [26] compared FFM and REE of RYGB patients with age-, sex-, and weight-matched controls, 3 months post-surgery, and reported substantial decreases in FFM and REE, which did not differ between the surgery and controls. Strain et al. [27] reported greater FFM by bioelectrical impedance analysis (BIA) in patients 2 years post-surgery compared with controls.
Among the methodological problems of previous research are limitations of methods for body composition (e.g., known BIA measurement errors in severe obesity [28]); REE predictions from BIA or BodPod, or from anthropometric equations, rather than actual measurements; inappropriate use of ratios; small sample sizes; and imprecisely matched controls.
We present results from 3 post-surgery visits over 5 years on REE and organ-tissue body compartment sizes measured by DXA for FFM; MRI for SM, the liver, the kidney, the spleen, and the brain; and indirect calorimetry for REE, and compare these with measurements from an archived sample of non-operated, weight-stable, healthy controls.
Methods and Procedures
Surgery Participants
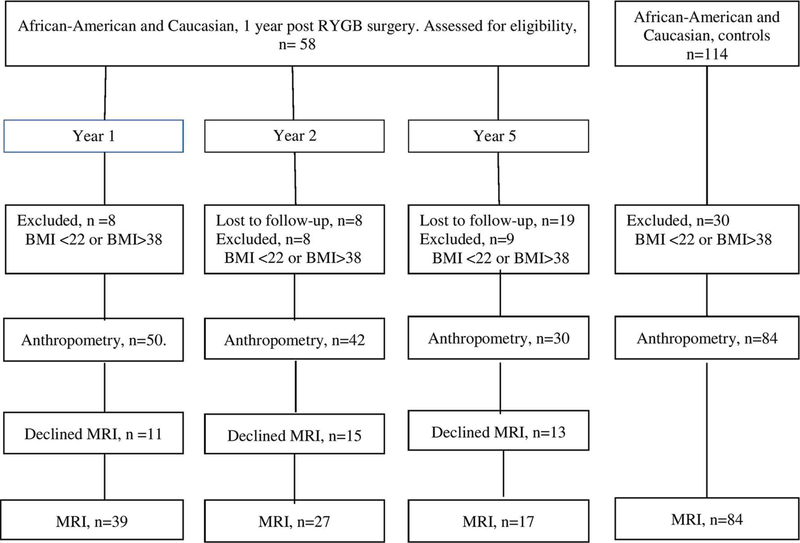
Between November 2006 and February 2009, 64 participants enrolled in the Longitudinal Assessment of Bariatric Surgery 2 (LABS-2) at the Weill Cornell Medical College and the University of Pittsburgh Medical Center sites were invited to participate in this ancillary study [29], An additional 41 non-LABS-2 participants were enrolled for a total of 105 participants. Ninety-two patients returned 1 year post-surgery. Of these, 77 were of the same ethnicity as our control group (AA or Caucasian). Fifty-eight had Roux-en-Y gastric bypass (RYGB) surgery, 8 had biliopancreatic diversion (BD), 8 had sleeve gastrectomy, and 3 had biliopancreatic diversion with duodenal switch. Since different procedures may have different effects on REE or body composition, and the sample of cases with other procedures is small, this analysis is confined to AA and Caucasian patients who had RYGB. The follow-up and disposition of cases during the study are shown in Fig. 1.
Fig. 1.
Follow-up and disposition of surgery cases and non-surgery controls
Non-surgery Participants
The control sample was 114 healthy, weight-stable (< 2 kg change within 6 months by self-report), AA and Caucasian men and women enrolled in a study between 1995 and 2006 with similar data collection methods [30, 31].
Measures
In the early morning after an 8-h fast, subjects were weighed in a hospital gown to the nearest 0.1 kg (Weigh-Tronix, New York, NY; and BWB 800 Tanita Corp., Pittsburgh) and their height was measured to the nearest 0.5 cm using a stadiometer (Holtain; Crosswell, Wales-New York; and Perspective Enterprises, Portage, MI-Pittsburgh).
Indirect Calorimetry
REE was measured by indirect calorimetry (TrueOne® 2400, ParvoMedics Inc, Salt Lake City, UT). Under thermoneutral and quiet conditions, subjects rested comfortably on a bed, and a plastic transparent ventilated hood placed over the head for 40–60 min sampled expiratory gases from which the rates of oxygen consumption and carbon dioxide production were measured by system analyzers. Gas exchange results were evaluated during the stable measurement phase (10–20 min) and converted to REE (kcal/day) using the formula of Weir [32].
Magnetic Resonance Imaging
Skeletal muscle mass was measured using a whole-body multislice MRI protocol, as previously described [33]. Participants were placed on a 1.5-T MRI scanner (GE, 6X Horizon, Milwaukee, WI) table and scanned with arms above their heads. Approximately 40 axial images with 10-mm thickness and a 40-mm interslice gap were acquired across the entire body. SliceOmatic image analysis software (Tomovision, Montreal, Canada) was used by a single image analyst to tag skeletal muscle (SM) on each image and SM volume was converted to mass using an assumed density of 1.04 kg/L [34], The coefficient of variation for SM obtained from a repeat blinded analysis of the same whole-body MRI by a single analyst in our lab is 2.4%.
The protocol for organs and brain volumes has been previously described [30] and included 40 contiguous abdominal axial MRI images for liver, kidney, and spleen volumes. Brain volumes were acquired by using 1 of 2 protocols: an axial orientation for images collected before 2001 (most CON) and a 3-dimensional coronal orientation for RYGB patients.
Dual-Energy X-ray Absorptiometry
Fat-free mass (calculated as body weight minus total body fat) was measured using a whole-body dual-energy X-ray absorptiometry (DXA) scanner (GE Lunar, DPX or DPXL for controls; GE iDXA for bariatric, Madison, WI). The between-measurement technical errors for TBF and FFM in the same subject are 3.4% and 1.2%, respectively [35].
Statistical Methods
Descriptive statistics (number, mean, standard deviation) were calculated for subject characteristics at each visit year [1, 2, 5] post-surgery (RYGB) and for CON. Linear regression models compared adjusted mean REE of the RYGB at each visit with CON covarying for anthropometries (age, sex, ethnicity, height, and weight). Between-group differences in body composition were investigated with similar models. A subsequent analysis evaluated adjusted group REE differences after covarying for body composition.
Regression models were checked for outliers and violations of modeling assumptions including normality and homoscedasticity of residuals. Ethnicity was retained in the models as it reduced error variability but no interaction effects of ethnicity on the RYGB vs CON differences were noted. A sensitivity analysis that included all bariatric and control cases regardless of BMI led to conclusions similar to the analyses reported here and are not reported separately. Interaction tests of group by FFM, SM, organs, and brain at each visit on REE tested whether the energy cost of tissue was different after weight loss due to metabolic adaptation.
Maximum likelihood regression models were fitted using the MIXED procedure in SAS v9.4 (SAS Institute, Cary, NC). Statistical significance was set at p< 0.05, two-tailed, except when adjustment for multiple comparisons was made as noted in the text or footnotes.
Results
For the comparison of post-surgery RYGB with CON, a BMI range of 22–38 kg/m2 was chosen. This range allowed for the inclusion of 84 of 114 CON, and 50 of 58 RYGB with same organ-tissue body composition data, yet ensured that RYGB were substantially below their pre-surgery baseline weight at the time of visit. Mean change from baseline to year 1 was as follows: BMI –15.2 kg/m2, range – 3.2 to – 28.5; weight –42 kg, range – 10 to – 76 (data not shown). Table 1 shows the anthropometric characteristics and body composition of RYGB at each visit and CON.
Table 1.
Characteristics of the bariatric and control groups
| Variable | Years after RYGB surgery | Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 5 | ||||||
| African-American/Caucasian, N | 8/42 | 8/34 | 7/23 | 40/44 | ||||
| Female/male, N | 40/10 | 35/7 | 23/7 | 52/32 | ||||
| N | Mean | N | Mean | N | Mean | N | Mean | |
| Age, years | 50 | 45.7 ±11.4 | 42 | 45.8 ±11.6 | 30 | 50.6 ±11.1 | 84 | 47.4 ± 20.7 |
| BMI, kg/m2 | 50 | 28.7 ± 3.7 | 42 | 30.1 ±4.4 | 30 | 29.3 ±3.3 | 84 | 27.0 ± 3.7 |
| Weight kg | 50 | 81.3 ± 15.0 | 42 | 84.3 ± 15.7 | 30 | 83.7 ± 12.7 | 84 | 76.3 ± 12.2 |
| FFM, kg | 48 | 53.2 ± 10.7 | 41 | 52.9 ±10.0 | 30 | 52.4 ± 10.5 | 81 | 53.3 ±11.4 |
| SM, kg | 35 | 22.2 ± 5.6 | 26 | 21.9 ±3.4 | 19 | 22.5 ± 6.0 | 84 | 25.3 ± 7.0 |
| Organs, kg | 39 | 2.63 ± 0.45 | 27 | 2.63 ± 0.41 | 17 | 2.53 ± 0.47 | 83 | 2.05 ± 0.39 |
| Brain, kg | 22 | 1.17 ±0.13 | 8 | 1.19 ± 0.11 | 10 | 1.23 ±0.14 | 84 | 1.15 ±0.16 |
| REE, keal/day | 43 | 1534 ±206 | 39 | 1501 ±240 | 25 | 1623 ± 302 | 84 | 1448 ± 260 |
| Adjusted REE, keal/day, (s.e.)* | 1568 (37) | 1521 (40) | 1640 (46) | |||||
Values are mean ± SD.
Least squares means adjusted for age, sex, ethnicity, height, and weight s.e., standard error. P for year = 0.024
REE During Weight Maintenance (Year 1-Year 5 Post-RYGB)
To describe the trajectory of REE changes from years 1 to 5 in RYGB, a repeated measures regression model adjusting for age, ethnicity, sex, height, and weight (final row of Table 1, p for year = 0.024) was fitted. REE increased by ~ 118 keal/day from years 2 to 5 (p = 0.022, Tukey-Kramer adjusted).
REE and Body Composition of RYGB Compared with Controls
REE of RYGB and CON were compared using multiple regression models adjusting for age, ethnicity, sex, height, and weight (Table 2). Younger age, male sex, Caucasian ethnicity, and greater body weight were independently associated with higher REE.
Table 2.
Multiple linear regression comparing resting energy expenditure in surgery and non-surgery controls
| Years after RYGB surgery |
|||
|---|---|---|---|
| Year 1 | Year 2 | Year 5 | |
| Intercept | 447 (378) | 234 (373) | 383 (411) |
| Age, years | − 4.25 (0.78)*** | − 4.27 (0.80)*** | − 4.16 (0.84)*** |
| Ethnicity | 86 (33)* | 128 (34)*** | 105 (36)** |
| Sex | 193 (45)*** | 128 (45)** | 160 (49)** |
| Height, cm | 198 (253) | 387 (244) | 208 (270) |
| Weight, kg | 9.8 (1.4)*** | 8.5 (1.4)*** | 10.4 (1.6)*** |
| REE difference (RYGB minus CON), kcal/day | 43.2 (34.0), p = 0.20 (1528 vs 1485) | − 27.9 (37.3), p = 0.46 (1456 vs 1483) | 114.6 (42.3), p = 0.008 (1596 vs 1482) |
Values are coefficients and standard errors (β (s.e.)) adjusted for age, ethnicity, sex, height, and weight Ethnicity coded as Caucasian 1, African-American 0; sex coded as male 1, female 0; group coded as RYGB 1, CON 0.
p< 0.05
p<0.01
p<0.001. The REE in the RYGB group is significantly higher than that in the CON group at year 5, Bonferroni corrected for 3 comparisons against a single control (0.05/3 = 0.016)
Given the central role of body composition in determining REE, body composition of CON and RYGB was compared (Table 3). A pattern emerged of greater FFM and less SM in RYGB than in CON at each visit. Importantly, however, RYGB has greater abdominal organ mass throughout the follow-up years. Given the high energy cost of these organs (> 200 kcal/kg/day) [17], this difference in organ mass could account for an elevated REE despite the adjustment for anthropometric characteristics (Table 2, year 5) while mitigating or obscuring any decrease in REE that could otherwise follow RYGB at years 1 and 2.
Table 3.
Differences in body composition compartment size between surgery and non-surgery controls
| Years after RYGB surgery |
Controlsa | |||
|---|---|---|---|---|
| Year 1 | Year 2 | Year 5 | Mean, kg | |
| FFM, kg | 2.55 (0.75)*** | 2.14 (0.82)** | 1.07(0.88) | 55.2 (0.43) |
| SM, kg | − 1.37 (0.61)* | − 1.34 (0.70) | − 2.20 (0.77)** | 26.5 (0.30) |
| Trunk organs, kg | 0.43 (0.07)*** | 0.47 (0.08)*** | 0.36 (0.09)*** | 2.08 (0.03) |
| Brain, kg | 0.008 (0.031) | 0.054 (0.04) | 0.086 (0.042)* | 1.16(0.013) |
Values are mean differences and standard errors (RYGB minus controls) adjusted for age, ethnicity, sex, height, and weight
Adjusted means and standard errors for controls varied by ~ 1–2% If om year to year. Median values are shown.
p < 0.05
p < 0.01,
p < 0.001
REE Multiple Regression Models Adjusting for Tissue-Organ Mass
To investigate whether the between-group REE differences observed in the REE-anthropometric analysis would be sustained after adjusting for body composition differences, the anthropometric variables in the regression model were replaced with FFM, SM, trunk organs, brain, and weight (Table 4). The regression coefficients give an indication of the energy cost per unit of each tissue-organ. The between-group difference in REE (RYGB minus CON) was not statistically significant at any year.
Table 4.
Multiple linear regression comparing resting energy expenditure in surgery and non-surgery controls
| Years after RYGB surgery |
|||
|---|---|---|---|
| Year 1 | Year 2 | Year 5 | |
| Intercept | 29 (154) | − 17.6 (164) | −2(172) |
| Weight, kg | 5.00 (1.69)** | 5.14 (1.71)** | 5.42 (1.79)** |
| FFM, kg | − 4.41 (4.94) | −4.14(5.14) | − 6.02 (5.50) |
| SM, kg | 17.37 (7.85)* | 15.11 (8.21) | 17.70 (8.78)* |
| Trunk organs, kg | 201.3 (51.2)*** | 226.2 (57.0)*** | 211.1 (55.7)*** |
| Brain, kg | 363.8 (119.7)** | 387.8 (125.0)** | 412.4 (127.1)** |
| REE difference (RYGB minus CON), kcal/day | − 15.9 (54.8),p = 0.8 (1448 vs 1464) | − 46.9 (64.9), p = 0.47 (1415 vs 1462) | 47.7 (83.0), p = 0.57 (1513 vs 1465) |
Values are coefficients and standard errors (β (s.e.)) adjusted for weight, FFM, SM, organs, and brain. Group coded as RYGB 1, CON 0.
p < 0.05
p < 0.01
p < 0.001
Additional Analyses
Adding FFM to the regression of REE on anthropometric variables to account for year 5 between-group REE difference showed the difference decreased by ~ 15 kcal to 99.0 kcal/day but remained significant (p = 0.02).
We tested for body compartment by group (RYGB vs CON) interactions at each year, an effect that might signal a difference in energy cost per unit of mass (adaptive thermogenesis) in RYGB who have undergone substantial weight loss compared with CON. No significant interactions were found.
Discussion
This study provides evidence that RYGB patients are not dis-advantaged by a disproportionately low post-surgery REE or loss of FFM compared with a non-operated, weight-stable, CON group at 1, 2, or 5 years post-RYGB surgery. Rather, RYGB body composition favored higher REE compared with anthropometrically similar controls. The metabolic contribution of ~ 1.3–2.2 kg less SM (~ 16–25 kcal/day) is more than offset by larger abdominal organs (0.36–0.47 kg; ~ 90–115 kcal/day). In view of these results, the role of a reduced REE in weight regain after RYGB surgery is questionable.
The longitudinal analysis of REE over the RYGB follow-up period indicated an increase in REE from years 2 to 5. The means appear to suggest a decrease from years 1 to 2 and an increase thereafter; but only the increase from years 2 to 5 is significant. The non-significant REE differences between RYGB and CON in years 1 and 2 after adjustment for body composition suggest that surgery may lead to a transient decrease in REE during the initial 2 years. However, this decrease is not noticed in comparisons with anthropometrically similar controls due to body composition differences.
We note that adding FFM, a composite of the lean tissues in the body, to the anthropometric regressions did not explain the between-group REE differences observed in year 5; a 99-kcal difference remained. Thus, controlling for FFM with weight and other anthropometries was inadequate to explain body composition effects on REE and is in contrast to reports from longitudinal studies that changes in REE could be explained by changes in FM and FFM [6, 8].
It is difficult to reconcile the numerous longitudinal studies that report metabolic adaptations with the many longitudinal and cross-sectional studies that do not. One obvious variation is the post-surgery follow-up time; reports of adaptation soon after surgery (≤ 6 months) are sometimes no longer observed at a later follow-up [36, 37] but this is not a consistent difference. Another variation is whether ratios (e.g., REE/FFM, REE/BW) or regressions are used in the analysis. While convenient and easily calculated, ratios often lead to erroneous conclusions [38]. Some longitudinal investigations are weakened by body composition measurements on severely obese patients using BIA which is known to be affected by hydration. However, the critical vulnerability of all two-compartment body composition longitudinal studies is that they require the questionable assumption that the components of FFM (SM, liver, kidney, digestive organs, skin, connective tissue, etc.), which have vastly different energy requirements, remain in the same proportion to all other lean tissues after weight loss as before. This is not the case for SM and FFM [14], Research on other organ-tissue changes after conventional weight loss has yielded conflicting results [39, 40]. Absent this stability in proportions, metabolic adaptation (a measured REE lower than predicted REE) may be a consequence of changes in the composition of FFM.
This study has several strengths compared with previous work. One is the use of high-quality measures of body composition including SM, trunk organs, and brain, and a long follow-up period. Another is the availability of a large archived control group that had undergone similar high-quality REE and body composition measurements. Additionally, the use of multiple regression analyses allowed us to compare groups precisely matched for covariates without the requirement of initial matching on BMI, sex, age, and ethnicity.
Limitations include subject attrition effects on statistical power. Organ mass calculated from organ volume used a constant density that may be questionable in severe obesity where ectopic fat infiltration is common. The unmeasured organs not included in our models could help clarify the role of shifts in the organ-tissue composition of FFM on REE after weight loss. Levels of thyroid hormones were not measured in the surgery group at follow-up and hormone differences could have contributed to the observed REE differences with controls. Statistically non-significant differences in this study do not necessarily mean clinically insignificant—small differences may be of clinical significance if sustained over long durations. More definitive conclusions await further, more powerful, studies.
Conclusion
REE of RYGB patients was not different at years 1 and 2 but was higher at year 5 than that of non-surgery controls of similar anthropometries. RYGB patients maintained a larger mass of trunk organs (liver and kidney) which, if included in regression models, account for a higher REE at year 5. Unmeasured changes in organ-tissue composition of FFM following bariatric weight loss surgery may be confounding our understanding of REE changes.
Acknowledgments
Funding Information This study was supported by the National Institutes of Health grants RO1-DK-72507, P30-DK-26687, and T32-DK007559 (supported TL, EW, and LD).
Conflict of Interest Nerys M. Astbury declares having been supported by a research grant from the Cambridge Weight Plan UK Ltd to the University of Oxford. Also, the terms of Nerys M. Astbury’s support require that the following statement be included in the paper: “Nerys M. Astbury is supported by funding from the National Institute for Health Research (NIHR), Oxford Biomedical Research Centre (BRC). The views expressed are those of the authors) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.”
Footnotes
The remaining authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Procedures performed in studies involving human participants were approved by the Institutional Review Boards of the relevant institutions and were in accordance with the ethical standards of the institutions and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent Statement Written informed consent was obtained from all individual participants included in the study.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):Cd003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. [DOI] [PubMed] [Google Scholar]
- 3.Elliot DL, Goldberg L, Kuehl KS, et al. Sustained depression of the resting metabolic rate after massive weight loss. Am J Clin Nutr. 1989;49(1):93–6. [DOI] [PubMed] [Google Scholar]
- 4.Leibel RL, Hirsch J. Diminished energy requirements in reduced-obese patients. Metabolism. 1984;33(2):164–70. [DOI] [PubMed] [Google Scholar]
- 5.Weyer C, Pratley RE, Salbe AD, et al. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab. 2000;85(3): 1087–94. [DOI] [PubMed] [Google Scholar]
- 6.Das SK, Roberts SB, McCrary MA, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78(1):22–30. [DOI] [PubMed] [Google Scholar]
- 7.Weinsier RL, Nagy TR, Hunter GR, et al. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am J Clin Nutr. 2000;72(5):1088–94. [DOI] [PubMed] [Google Scholar]
- 8.Coupaye M, Bouillot JL, Coussieu C, et al. One-year changes in energy expenditure and serum leptin following adjustable gastric banding in obese women. Obes Surg. 2005;15(6):827–33. [DOI] [PubMed] [Google Scholar]
- 9.Wilms B, Ernst B, Thumheer M, et al. Resting energy expenditure after Roux-en Y gastric bypass surgery. Surg Obes Relat Dis. 2018;14(2):191–9. [DOI] [PubMed] [Google Scholar]
- 10.Johannsen DL, Knuth ND, Huizenga R, et al. Metabolic slowing with massive weight loss despite preservation of fet-free mass. J Clin Endocrinol Metab. 2012;97(7):2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta C, Hadley EC. The significance of sarcopenia in old age. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:l–4. [DOI] [PubMed] [Google Scholar]
- 12.Prado CM, Purcell SA, Alish C, et al. Implications of low muscle mass across the continuum of care: a narrative review. Ann Med. 2018;50(8):675–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shephard RJ, Bouhlel E, Vandewalle H, et al. Muscle mass as a factor limiting physical work. J Appl Physiol (1985). 1988;64(4): 1472–9. [DOI] [PubMed] [Google Scholar]
- 14.Davidson LE, Yu W, Goodpaster BH, et al. Fat-free mass and skeletal muscle mass five years after bariatric surgery. Obesity (Silver Spring). 2018;26(7): 1130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grande F Nutrition and energy balance in body composition studies In: Brozek J, Henschel A, editors. Techniques for measuring body composition. Washington, DC: National Academy of Sciences-National Research Council; 1959. p. 168–88. [Google Scholar]
- 16.Holliday MA, Potter D, Jarrah A, et al. The relation of metabolic rate to body weight and organ size. Pediatr Res. 1967;1:185–95. [DOI] [PubMed] [Google Scholar]
- 17.Elia M Organ and tissue contribution to metabolic rate I. In: Kinney JM, editor. Energy metabolism: tissue determinants and cellular corollaries. New York: Raven; 1992. p. 61–77. [Google Scholar]
- 18.Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Phys. 1998;275(2):249–58. [DOI] [PubMed] [Google Scholar]
- 19.van Gemert WG, Westerterp KR, Greve JW, et al. Reduction of sleeping metabolic rate after vertical banded gastroplasty. Int J Obes Relat Metab Disord. 1998;22(4):343–8. [DOI] [PubMed] [Google Scholar]
- 20.Carrasco F, Papapietro K, Csendes A, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17(5):608–16. [DOI] [PubMed] [Google Scholar]
- 21.Tam CS, Redman LM, Greenway F, et al. Energy metabolic adaptation and cardiometabolic improvements one year after gastric bypass, sleeve gastrectomy, and gastric band. J Clin Endocrinol Metab. 2016;101(10):3755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettini S, Bordigato E, Fabris R, et al. Modifications of resting energy expenditure after sleeve gastrectomy. Obes Surg. 2018;28(8):2481–6. [DOI] [PubMed] [Google Scholar]
- 23.Chu L, Steinberg A, Mehta M, et al. Resting energy expenditure and metabolic adaptation in adolescents at 12 months following bariatric surgery. J Clin Endocrinol Metab. 2019. [DOI] [PubMed] [Google Scholar]
- 24.Skogar M, Holmback U, Hedberg J, et al. Preserved fet-free mass after gastric bypass and duodenal switch. Obes Surg. 2017;27(7): 1735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiavo L, Scalera G, Pilone V, et al. Fat mass, fet-free mass, and resting metabolic rate in weight-stable sleeve gastrectomy patients compared with weight-stable nonoperated patients. Surg Obes Relat Dis. 2017;13(10):1692–9. [DOI] [PubMed] [Google Scholar]
- 26.Mirahmadian M, Hasani M, Taheri E, et al. Influence of gastric bypass surgery on resting energy expenditure, body composition, physical activity, and thyroid hormones in morbidly obese patients. Diabetes Metab Syndr Obes. 2018;11:667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strain GW, Ebel F, Honohan J, et al. Fat-free mass is not lower 24 months postbariatric surgery than nonoperated matched controls. Surg Obes Relat Dis. 2017;13(l):65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widen EM, Strain GW, King WC, et al. Validity of bioelectrical impedance analysis for measuring changes in body water and percent fat after bariatric surgery. Obes Surg. 2014;24(6):847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belle SH, Berk PD, Chapman WH, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013;9(6):926–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher D, Albu J, He Q, et al. Small organs with a high metabolic rate explain lower resting energy expenditure in African American thanin white adults. Am J Clin Nutr. 2006;83(5):1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Javed F, He Q, Davidson LE, et al. Brain and high metabolic rate organ mass: contributions to resting energy expenditure beyond fet- free mass. Am J Clin Nutr. 2010;91(4):907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol Lond. 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song MY, Ruts E, Kim J, et al. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79(5):874–80. [DOI] [PubMed] [Google Scholar]
- 34.Snyder WS, Cooke M, Mnassett ES, et al. Report of the Task Group on Reference Man. Oxford: Pergamon Press; 1975. [Google Scholar]
- 35.Gallagher D, Koveia AJ, Clay-Williams G, et al. Weight loss in postmenopausal obesity: no adverse alterations in body composition and protein metabolism. Am J Physiol Endocrinol Metab. 2000;279(1):E124–31. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe BM, Schoeller DA, McCrady-Spitzer SK. Resting metabolic rate, total daily energy expenditure, and metabolic adaptation 6 months and 24 months after bariatric surgery. Obesity (Silver Spring). 2018;26(5):862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knuth ND, Johannsen DL, Tamboli RA, et al. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity (Silver Spring). 2014;22(12):2563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Browning MG, Khoraki J, Campos GM. Regression-based approach is needed to compare predicted and measured resting metabolic rate after weight loss and body composition changes. Surg Obes Relat Dis. 2018;14(6):807–9. [DOI] [PubMed] [Google Scholar]
- 39.Bosy-Westphal A, Kossel E, Goele K, et al. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. Am J Clin Nutr. 2009;90(4):993–1001. [DOI] [PubMed] [Google Scholar]
- 40.Gallagher D, Kelley DE, Thornton J, et al. Changes in skeletal muscle and organ size after a weight-loss intervention in overweight and obese type 2 diabetic patients. Am J Clin Nutr. 2017;105(l):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]