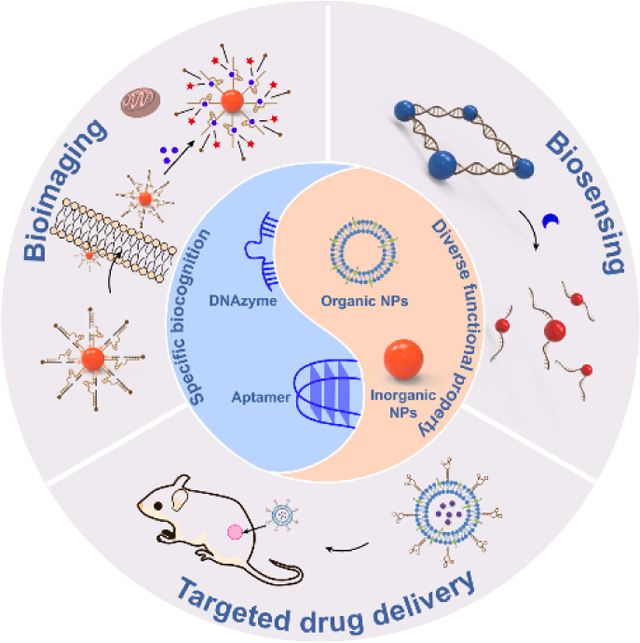
CONSPECTUS:
Nanoparticles (NPs) have enormous potential to improve disease diagnosis and treatment due to their intrinsic electronic, optical, magnetic, mechanical, and physiological properties. To realize their full potentials for nanomedicine, NPs must be biocompatible and targetable toward specific biomolecules to ensure selective sensing, imaging and drug delivery in complex environments such as living cells, tissues, animals and human bodies. In this Account, we summarize our efforts to impart specific biorecognition functionality to NPs by developing strategies to integrate inorganic and organic NPs with functional DNA (FDNA), including aptamers, DNAzymes, and aptazymes to create FDNA-NPs. These hybrid NPA take advantage of FDNA s capability to either bind targets or catalyze reactions in the presence of targets selectively and utilize their unique physicochemical properties including small size, low immunogenicity, and ease of synthesis and chemical modification in comparison with other molecules such as antibodies. By integrating inorganic NPs such as gold NPs, quantum dots, and iron oxide nanoparticles with FDNA, we designed stimuli-responsive FDNA-NPs that exhibit target induced assembly and disassembly of NPs, resulting in a variety of colorimetric, fluorescent, and magnetic resonance imaging-based sensors for diagnostic of a broad range of analytes. To impart both biocompatibility and selectivity on inorganic NPs for targeted bioimaging, we have demonstrated DNA-mediated surface functionalization, shape-controlled synthesis, and coordinative assembly of such NPs as specific bioprobes. A highlight is provided on the construction of FDNA-based nanoprobes with light-activatable sensing and imaging functions, which provides precise control of recognition property of FDNA with high spatiotemporal resolution. To explore the potential of organic NPs for biosensing applications, we have developed an enzyme-responsive FDNA-liposome as a universal sensing platform compatible with diverse biological targets as well as different detection methods including by fluorescence, MRI, or temperature, making it possible for point-of-care diagnostics. To expand the application regime of organic NPs, we collaborated with the Zimmerman group to prepare single-chain block copolymer-based NPs and incorporated it with a variety of functions, including monovalent DNA for assembly, tunable surface chemistry for cellular imaging, and coordinative Cu(II) sites for catalyzing intracellular click reactions, demonstrating the potential of using organic NPs to create promising FDNA-NP systems with programmable functionalities. Furthermore, we survey our recent endeavor in integration of cell-specific aptamers with different NPs for targeted drug delivery, showing that introducing stimuli-responsive properties into NPs that target tumor microenvironments would enable safer and more effective therapy for cancers. Finally, current challenges and future perspectives in FDNA-mediated engineering of NPs for biomedical applications are discussed.
Graphical Abstract
1. INTRODUCTION
Development and application of nanoparticles (NPs) in the diagnosis and treatment of human disease is a major branch of nanomedicine.1,2 NPs intended for medical use can be divided into two broad classes: inorganic and organic NPs. The intrinsic electronic, optical, magnetic, and mechanical properties of inorganic NPs can be harnessed for the development of nanoprobes for a range of applications in sensing, imaging, and diagnostic medicine.3,4 Alongside, organic NPs include those made of synthetic polymers and lipids are ideal for encapsulation and delivery of imaging probes and therapeutic agents due to their biocompatibility.5,6 In addition, because of their versatility of chemical compositions and surface functional groups, cargo release can be controlled through appropriate biodegradation or stimulus activation mechanisms.5 The specific interactions between these NPs and their targets in biological systems are a main driver of nanomedicine. However, NPs often lack specificity toward targets of interests. How to impart designer biorecognition and targeting functionality to NPs is a major focus in the fields of precision and personalized nanomedicine.
Functional DNA (FDNA) molecules include aptamers (which selectively bind targets), DNAzymes (which catalyze reactions like enzymes), and aptazymes (which are a combination of the two) and can be obtained by in vitro selection or Systematic Evolution of Ligands by Exponential Enrichment (SELEX).7 Moreover, FDNA exhibits many favorable features for biological applications, such as small size, low immunogenicity, ease of synthesis and chemical modification, and facile surface immobilization. Integration of FDNA into NPs imparts specific recognition capability to NPs for a wide variety of targets, ranging from small molecules to biomacromolecules, and even viruses or cells. Many groups have explored the development of new methods to construct FDNA-engineered NPs and pursue their diagnostic and therapeutic applications in nanomedicine.7–12 In the last two decades, our laboratories have focused on selection of new FDNA, characterization of its structural properties, as well as engineering it onto different inorganic and organic NPs for diagnostic and therapeutic applications. This Account summarizes our recent efforts to develop FDNA-engineered NPs that enable target-specific biosensing, bioimaging, and drug delivery. Novel design strategies for combination of FDNA with different organic and inorganic NPs to enable their applications in nanomedicine are a special focus of this Account.
2. FDNA ENGINEERED INORGANIC NPs AND THEIR APPLICATIONS IN BIOSENSING, IMAGING AND TARGETED DRUG DELIVERY
2.1. FDNA engineered inorganic NPs and their applications for in vitro diagnostics
FDNA does not normally possess functional groups that can generate detectable signals directly. To transform FDNA into sensing probes, we have integrated FDNA with other NPs by taking advantage of their unique optical and magnetic properties.7,13,14 Mirkin and co-workers have used DNA-functionalized gold nanoparticles (AuNPs) for highly sensitive colorimetric detection of nucleic acids.15 By using FDNA as the biorecognition element, we designed FDNA-AuNPs-based colorimetric sensors for detection of a wide range of analytes beyond nucleic acids.16–19 Both labeled and label-free methods have been employed for the construction of such colorimetric sensors. The labeled approach is based on conjugating FDNA to AuNPs first and specific binding of the FDNA with analytes triggering either the assembly or disassembly of AuNPs, resulting in a distinct color change in solution due to the plasmon resonance peak shift of AuNPs (Figure 1A). Using this strategy, FDNAs-based colorimetric sensors for metal ions16,17 and biomolecules18,19 have been developed. For example, a limit of detection (LOD) of 100 nM Pb2+ in paint with tunable dynamic range was achieved using DNAzyme-AuNP,16 and this LOD can meet the demands of on-site and real-time detection of lead in paint, as US Environmental Protection Agency (EPA) has defined the threshold for leaded paint that needs remediation as 1.0 mg/cm2. In contrast to the labeled method that requires the covalent conjugation of FDNA on AuNPs, the label-free method relies on different adsorption behaviors of single stranded and double stranded DNA molecules on AuNPs, with analyte-triggered aggregation of AuNPs in high salt solution.17 The label-free method enables a low LOD of 1 nM for UO22+ and 3 nM for Pb2+ using DNAzyme-AuNPs.17 Recently, Guo et al. introduced a silver stain enhancement strategy into the DNAzyme-AuNP system and achieved a LOD of 2 nM for Pb2+ detection.20 Since US EPA has defined maximum contamination level for Pb2+ in drinking water as 72 nM and in children’s blood as 5 μg/dL, the LODs of these methods can meet the demand of biomedical applications.
Figure 1.
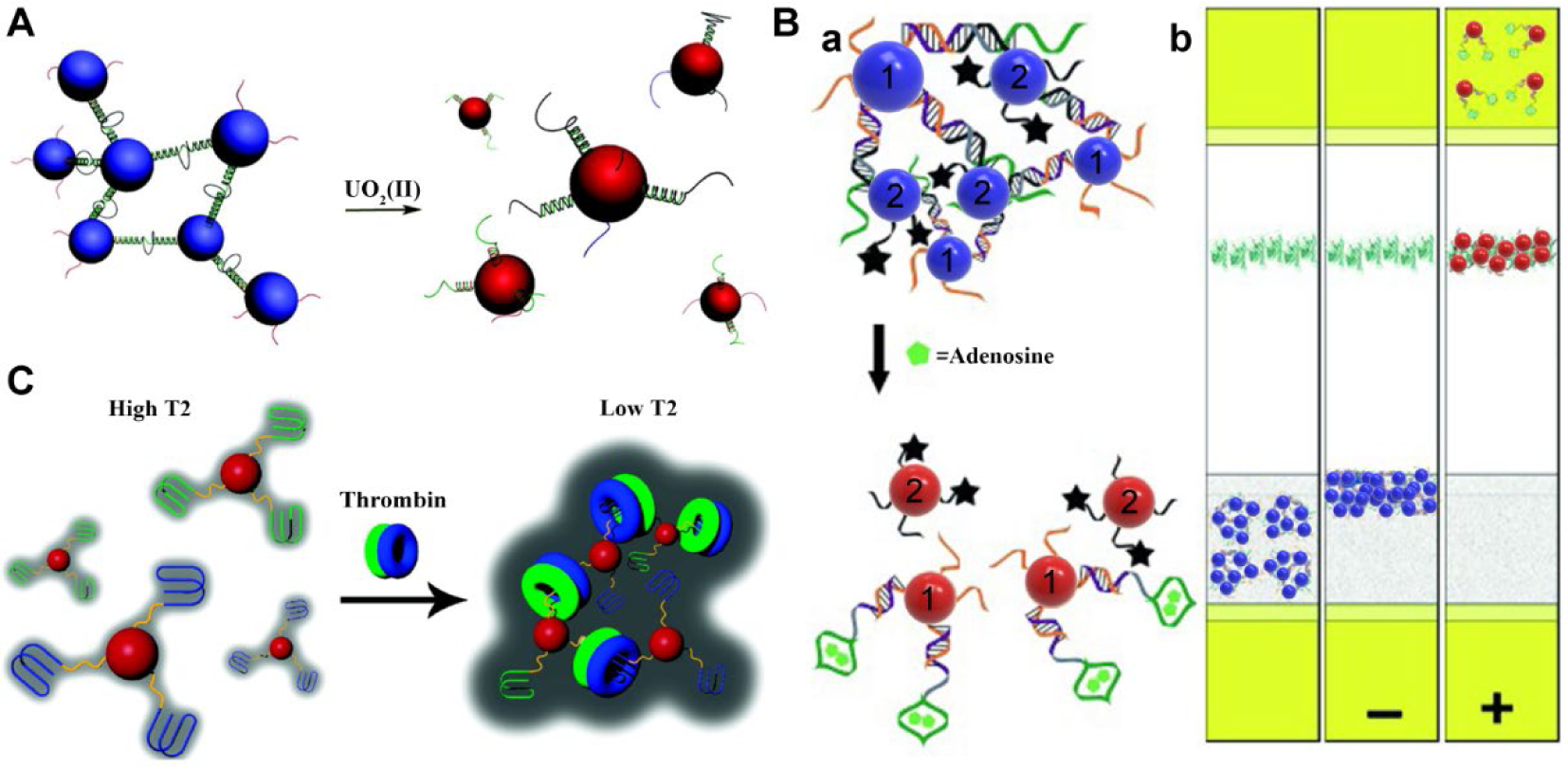
(A) Colorimetric sensors based on the disassembly of AuNPs linked by uranyl specific DNAzyme. Reproduced from refs 17. Copyright 2008 American Chemical Society. (B) Aptamer/AuNP-based lateral flow device. (a) Adenosine-induced disassembly of aptamer-linked AuNPs. (b) Schematic illustration of lateral flow devices loaded with assembled AuNPs before use (left strip), in a negative (middle strip), or a positive (right strip) test. Reproduced with permission from refs 22. Copyright 2006 Wiley. (C) Design of MRI contrast agent based on thrombin-induced assembly of the aptamer-functionalized SPIOs. Reproduced from refs 24. Copyright 2008 American Chemical Society.
To further facilitate practical applications, we applied the principle of target-induced assembly and disassembly of FDNAs-AuNPs into a dipstick format. In this system, non-cross-linked DNAzyme-AuNP conjugates21 or aptamer-linked AuNP aggregates (Figure 1B)22 were loaded onto a lateral flow device (LFD), enabling solution-based assays on LFDs for one-step colorimetric detection of a diverse range of targets. These LFDs significantly simplify the test operation and thus enable an even wider application of the FDNA-engineered NPs for point-of-care diagnostics at home or in low-resource settings.
Taking advantage of the highly tunable emission properties of quantum dots (QDs), we further incorporated QDs into the AuNPs aggregates for fluorescent detection of multiple targets in one pot.23 In addition to the advanced optical properties, some inorganic NPs, such as superparamagnetic iron oxide NPs (SPIOs), possess unique magnetic properties, and the assembly and disassembly of SPIOs could produce a distinct change of spin-spin relaxation time (T2) for adjacent water protons. Based on this principle, we have developed a general strategy to construct smart magnetic resonance imaging (MRI) contrast agents using FDNAs engineered SPIOs. In one case, a “turn-off” MRI sensor for thrombin was designed based on the thrombin-induced assembly of SPIOs, leading to a decrease in brightness of MR image (Figure 1C).24 In another case, a “turn-on” MRI sensor for adenosine was developed based on adenosine-induced disassembly of pre-formed SPIOs aggregates crosslinked by aptamer strands, leading to a brighter image and larger T2.25 “Turn-on” MRI sensors are preferred in T2-weighted MR imaging as on-signals are easier to visualize and quantify above noise. Based on this mechanism, Tan reported a nanosensor for the sensitive detection of cancer cells using aptamer-modified SPIOs.26 While most current MRI methods can detect tissue damage and tumor growth, which is often too late for preventive medicine, the methods presented here may allow detection of protein and metabolite biomarkers before tumor development, indicating the key role of nanomedicine in early detection and prevention.
2.2. FDNA engineered inorganic NPs and their bioimaging in living cells
2.2.1. Targeted bioimaging of cancer cells
Lanthanide ion doped upconversion nanoparticles (UCNPs) have become an exciting new class of inorganic nanophosphors because of their exceptional ability to upconvert near-infrared (NIR) radiation into shorter-wavelength luminescence.4,27–29 Advances in UCNP syntheses in the early 2000s made them more accessible for diverse biomedical applications.4 In particular, there was significant enthusiasm for developing UCNPs for bioimaging due to the advantage of anti-Stokes-shift luminescent properties, such as lack of background fluorescence, deep tissue penetration of the NIR light, resistance to photobleaching, and feasibility of multicolor labeling.28 Despite these advantages, the highest-quality UCNPs are generally synthesized in organic solvents with hydrophobic capping agents that render them insoluble in water and lack any functional groups for surface functionalization.4 As a result, it was challenging to use UCNPs for biomedical applications that require water solubility and surface functionalization in order to improve biorecognition ability. Although many strategies have been developed to attach DNA on the surface of UCNPs for bioimaging,4 these protocols often require three steps, transformation of UCNPs into water soluble forms, followed by surface-modification of function groups, and then conjugation of DNA. As a result, these multi-step syntheses often lead to low conjugation efficiency and reproducibility. To meet this challenge, we have developed straightforward approaches to prepare DNA-functionalized UCNPs for biological sensing and imaging.
We first coated a UCNP with functionalizable phospholipids that would serve as the conjugation target of biomolecules (Figure 2A).30 The phospholipids, consisting of a poly(ethylene glycol) (PEG) segment, two fatty acid chains, and a functional group, which mimics the composition of the external cell membrane, was used for the surface engineering of UCNPs. The one-pot self-assembly process is driven by the hydrophobic van der Waals interactions between the oleate ligands on the UCNP surface and the hydrophobic tail of the phospholipids. Since the hydrophilic part of phospholipids points outward the aqueous environment after coating on UCNPs, the method facilitates modification of PEG on the surface of UCNPs, making the hydrophobic UNCPs water soluble. Moreover, functional groups (e.g. amine, carboxylic acid, maleimide) at the end of the phospholipids allows the UCNPs to be modified with diverse biomolecules. We accomplished this goal using sulfhydryl-maleimide coupling reaction to synthesize DNA-UCNP conjugates. Essentially, DNA molecules grafted onto Lipo-UCNPs retained their specific biorecognition properties.
Figure 2.
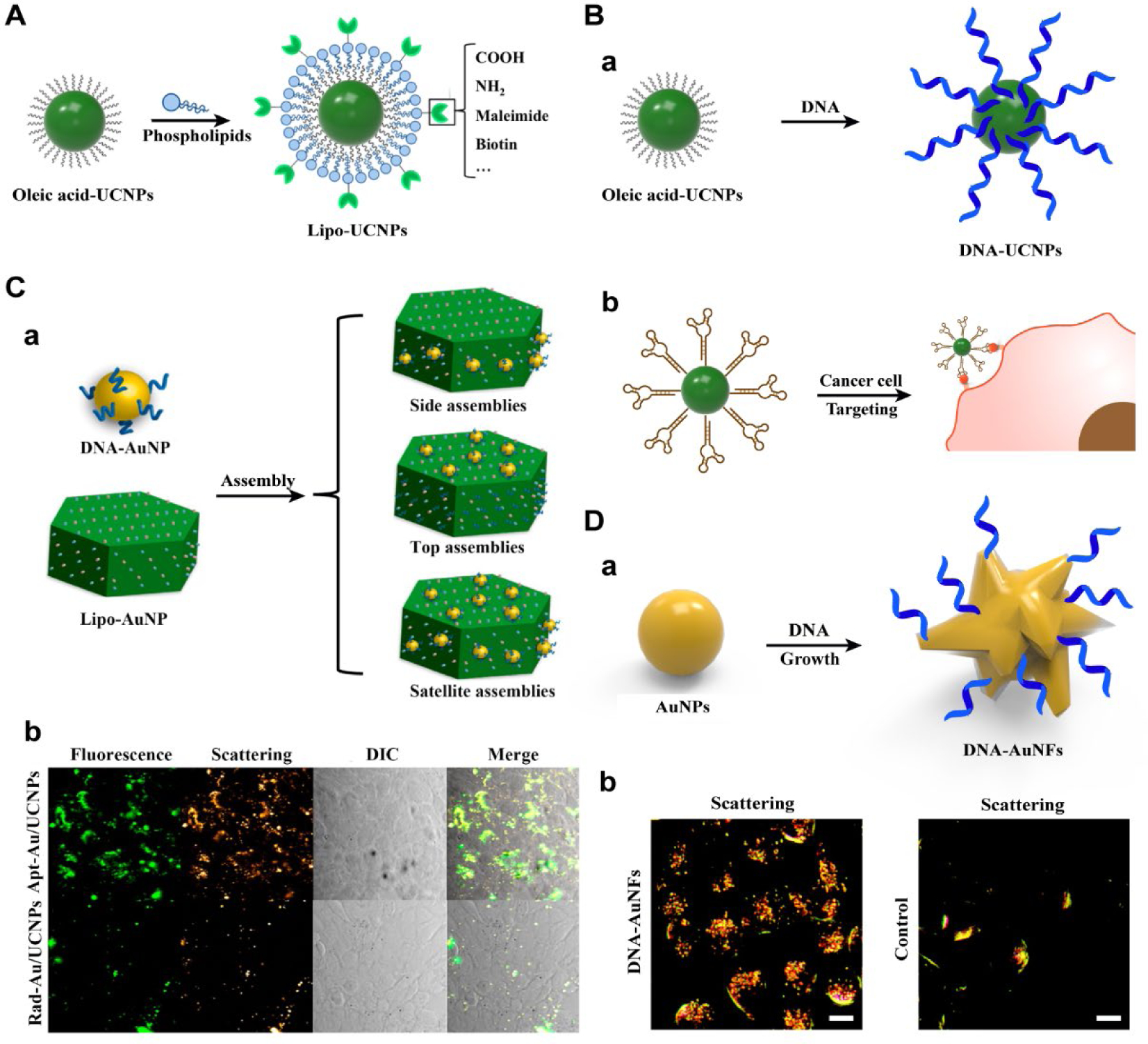
(A) Synthesis of water-dispersible and functionalizable UCNPs by the biomimetic surface engineering with phospholipids. Reproduced with permission from refs 30. Copyright 2012 Wiley. (B) (a) Schematics of synthesis of DNA-functionalized UCNPs through a one-step ligand exchange strategy. (b) Schematics of targeted imaging of cancer cells with aptamer-UCNP bioconjugates. Reproduced from refs 31. Copyright 2013 American Chemical Society. (C) Controlled hetero-assembly of DNA-Au/UCNP superstructures. (b) Confocal microscopy images of 4T1 cells with different treatment. Reproduced with permission from refs 32. Copyright 2015 American Chemical Society. (D) (a) Schematics of sequence-specific synthesis of DNA-AuNFs. (b) Dark field image of the CHO cells treated with DNA-AuNFs or without treatment. Reproduced from refs 33. Copyright 2010 American Chemical Society.
To make the DNA conjugation process simpler and more accessible, we sought to avoid the extra step of synthesis of functionalizable UCNPs by direct preparation of DNA-UCNPs hybrids from as-prepared hydrophobic UCNPs (Figure 2B).31 The strategy relies on the replacement of original oleate ligands on UCNPs with DNA molecules through one-step ligand-exchange process. Importantly, DNA molecules on the resulting DNA-UCNPs retain their recognition capability, allowing programmable NP assembly. Despite being coated with negatively charged DNA, the DNA-UCNPs were able to enter cells without the need of transfection agents, and their application for long-term continuous imaging and DNA delivery were also demonstrated. Furthermore, the approach allows to endow UCNPs with specific recognition abilities for targeted imaging of cancer cells based on DNA aptamer-functionalized UCNPs. As a demonstration, AS1411, a DNA aptamer that can target nucleolin, was modified on UCNPs for targeting the cancer cell line MCF-7 (Figure 2B). When MCF-7 cells were incubated with AS1411-conjugated UCNPs, a strong upconversion luminescent image was observed, whereas a weak luminescent image was observed when cells were treated with UCNPs-modified with a control DNA of a randomized sequence. These results demonstrated that the surface engineered UCNPs entered preferentially into MCF-7 cells via aptamer-dependent nucleolin-receptor-mediated endocytosis.
Integration of AuNPs with fluorescent materials has been of great interest for multimodal bioimaging. We further developed a novel approach for regiospecific assembly of DNA-AuNPs onto UCNPs for targeted dual-modality bioimaging (Figure 2C).32 Based on selective binding of DNA toward specific crystal facets of UCNPs, the DNA-AuNPs could be specifically assembled onto the different faces of the hexagonal plate-like UCNPs with well-regulated stoichiometry, forming various addressable superstructures. Since minimal quenching of upconversion luminescence was observed after formation of the superstructures, the obtained DNA-AuNP/UCNPs may be used as dual-modality imaging nanoprobes due to their combined plasmonic resonance of AuNPs and fluorescent properties of UCNPs. Moreover, the approach imparts polyvalent DNA on the superstructure surface, which can display specific molecular recognition ability and thus enable targeted bioimaging. Using this system, we constructed aptamer-functionalized satellite assemblies for targeted imaging of cancer cells. The cells treated with AS1411-functionalized superstructures (Apt-Au/UCNPs) showed both strong upconversion luminescence and light scattering signals, while the superstructures modified with control DNA (Rad-Au/UCNPs) showed much less signals in the cancer cells.
AuNPs possess many unique optical properties that make them well-suited not only for colorimetric biosensing described in Section 2.1, but also for bioimaging, including two-photon luminescence imaging, photothermal imaging, as well as photoacoustic imaging.3 A critical element in such applications is controlled synthesis of AuNPs with different shapes and surface properties, as the resulting morphologies dictate optical properties of AuNPs. To achieve the goal of controlling AuNP morphologies, molecular capping agents such as surfactants have been used to direct nanocrystal growth in a face selective fashion. Nevertheless, efficient conjugation chemistry methods are often required for further surface engineering of AuNPs for imaging application. Recently, we have explored using DNA molecules to modulate the morphologies and thus the optical properties of AuNPs (Figure 2D).33 The results showed that DNA can be used to tune morphology of AuNPs in a sequence-dependent manner. For example, synthesis of AuNPs in the presence of 30-mer oligo-A or -C resulted in formation of flower-shaped NPs (AuNFs), while the same synthesis in the presence of 30-mer oligo-T resulted in normal gold nanospheres. Furthermore, while the common way to conjugate DNA to AuNPs has been the use of thiolated DNA, such an attachment is known to be unstable in biofluids, since thiolated DNA may detach from the AuNPs in the presence of glutathione and other thio-containing molecules within hours or less. Therefore, such DNA-AuNPs are not suitable for long-term imaging and therapeutic applications in living cells or in vivo. Interestingly, even though we used unmodified DNA in the above DNA controlled synthesis of AuNPs with different morphologies, the DNA was found to be mostly retained on the AuNPs surface even after overnight incubation with mercaptothiol, probably because the DNA was integrated into the AuNPs during the synthesis. In addition, we found that there was sufficient DNA exposed on the DNA-AuNP surface that the DNA strands retained their biorecognition capability by complementary hybridization. Such high DNA stability and biorecognition ability make the DNA-conjugated AuNPs an excellent choice for bioimaging applications. We showed that DNA-AuNFs can be readily uptaken by cells for light scattering imaging and that DNA-AuNFs are much brighter than DNA-Au nanospheres on a per Au atom basis.33 Encouraged by the results that DNA of different sequences can control the morphologies of AuNPs, we have applied this DNA-mediated strategy to the controlled synthesis of various other metal NPs with different morphologies,34,35 including Au,36,37 Ag38 and Pd@Au39 core-shell NPs. The Kelley group reported the DNA-templated synthesis of QDs,40 which yields DNA-functionalized QDs for controlled assembly of QDs and targeted imaging of cancer cells.40,41 The exploration of this synthesis strategy provides not only deeper insight into the molecular basis of morphological control of NPs by capping ligands that bind different facets, but it also opens a new avenue for various biomedical applications.
2.2.2. Optogenetic control of FDNA engineered NPs for biosensing in living cells
Even though DNAzymes with high sensitivity and specificity for many metal ions have been selected from a large DNA library, the majority of them detect metal ions in environmental samples, such as water and soil, and applications of DNAzymes for imaging metal ions in living cells have been demonstrated only recently.42 We designed a DNAzyme-AuNP probe for imaging metal ions in living cells (Figure 3A) that consists of an AuNP core functionalized with a uranyl-specific 39E DNAzyme whose substrate strand is modified with a quencher at the 3’ end and a Cy3 fluorophore at the 5’ end. The Cy3 fluorescence is quenched by both the molecular quencher and AuNP. In the presence of uranyl, the nanoprobe was shown to cleave the fluorophore-labeled substrate and subsequently resulted in the release of the Cy3-contained shorter strand and an increase in fluorescence signals. Furthermore, the nanoprobes can readily enter HeLa cells and serve as a metal ion sensor in live cells.42 Based on the integration of FDNA with inorganic NPs, other groups also developed diverse nanoprobes for biosensing and imaging of specific targets in living cells.8,13 However, a potential downside of these nanoprobes is that the intracellular sensing cannot be spatiotemporally controlled. The FDNA can bind and react with targets encountered during the delivery process, which limits their applications in complex biological systems. To address this issue, we have developed a photochemical caging strategy to achieve light-activatable DNAzymes for metal ion sensing in living cells.43,44 The 2’-OH group of the RNA cleavage site was modified with a 2’-nitrobenzyl group, preventing it from being cleaved during the delivery process. Once the caged DNAzymes are uptaken into the cells, the DNAzyme activity can be restored upon irradiation with UV light.
Figure 3.
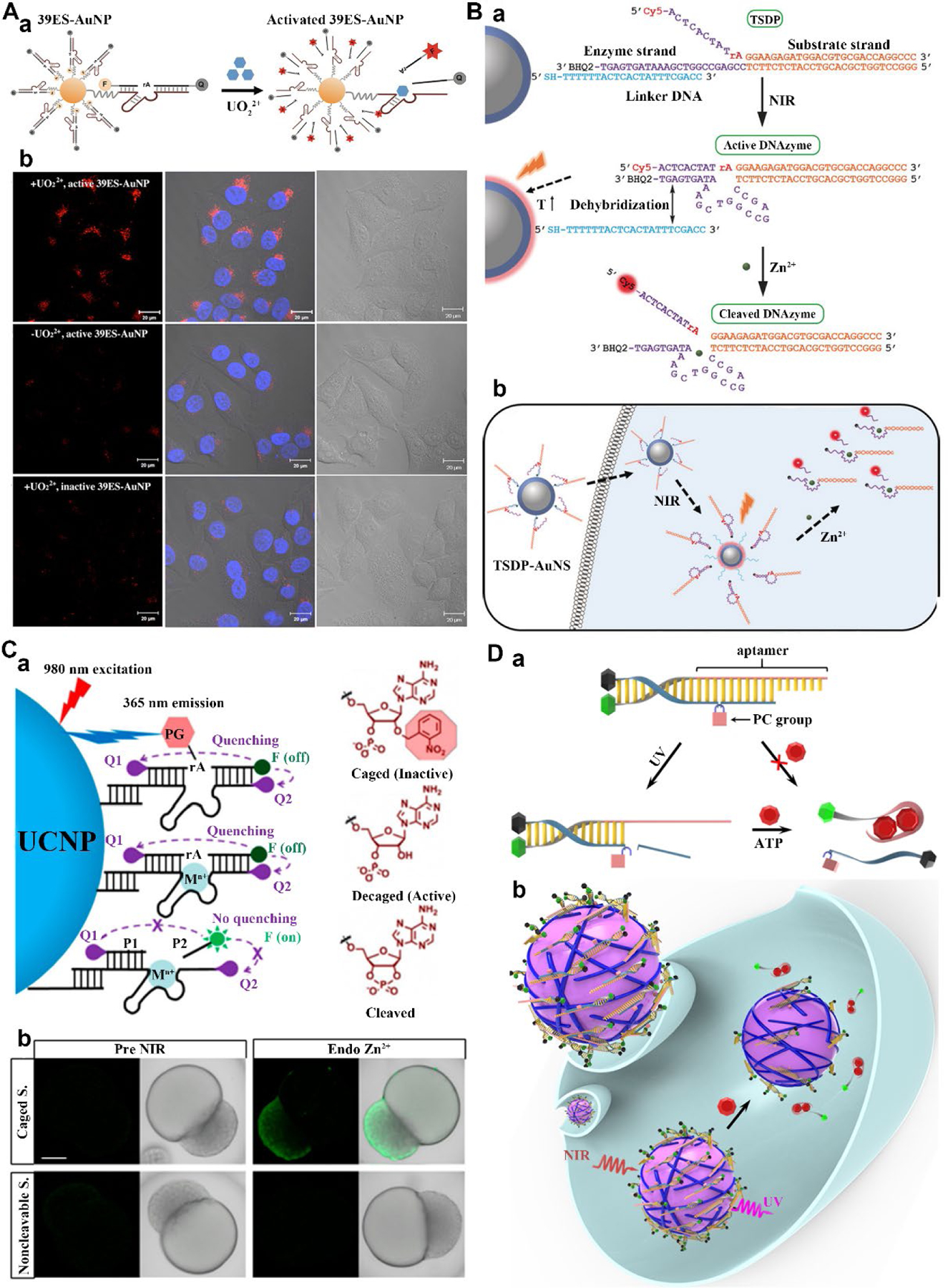
(A) (a) Design of the DNAzyme-AuNP probe for imaging of uranyl ions in living cells. (b) Confocal microscop images of HeLa cells with different treatment. Reproduced from refs 42. Copyright 2013 American Chemical Society. (B) (a) Design of NIR-activated TSDP-AuNS probe. (b) The intracellular Zn2+ imaging with TSDP-AuNS probe. Reproduced with permission from refs 45. Copyright 2017 Wiley. (C) Design of the NIR-controlled DNAzyme-UCNP probe for sensing of Zn2+. (b) Confocal microscopy images of NIR-activated Zn2+ imaging by the DNAzyme-UCNP probe. Reproduced from refs 46. Copyright 2018 American Chemical Society. (C) (a) Design of NIR-activated nanodevice for ATP sensing based on the integration of the UV light-activatable aptamer probe with UCNPs. (b) NIR-activated ATP imaging in live cells. Reproduced from refs 47. Copyright 2018 American Chemical Society.
Despite of its enhanced spatiotemporal control, this approach requires the use of high-energy UV light that has poor tissue penetration and can potentially cause phototoxicity to living cells. In addition, caged DNAzymes generally need cationic transfection reagents for cellular delivery. Such reagents cannot be applied for bioassays in vivo. To address this issue, we have developed a NIR photothermal activated nanoprobe for imaging of metal ions in living cells (Figure 3B).45 In this design, a three-stranded DNAzyme precursor (TSDP) was conjugated to gold nanoshells (AuNS) through thiol-gold chemistry. In the TSDP, the linker DNA strand (in blue) hybridizes with the left arm of the enzyme strand, inhibiting the hybridization of the enzyme strand with the left arm of the substrate strand and thus inactivating the DNAzyme. Upon NIR irradiation, AuNS can absorb NIR light and convert it into heat, leading to increased local temperature and hence a release of the enzyme strand from the linker strand that is conjugated to the AuNS. Once the dehybridization with the linker strand occurs, the enzyme strand can form an active DNAzyme upon the hybridization with the substrate strand, resulting in Zn2+-dependent cleavage and an increase in the fluorescence signal. Using this TSDP-AuNS probe, we demonstrated NIR-triggered Zn2+imaging in HeLa cells.
We use NIR-mediated photothermal effect to control DNAzymes activity because NIR light provides deeper tissue-penetration and lower photo-toxicity compared to UV light. However, photothermal effects can also damage cells. To overcome this limitation, we chose to use UCNPs, which can convert NIR irradiation into high-energy UV emissions, to develop a NIR-light-controlled DNAzyme nanoprobe for metal ion imaging in early embryos and larvae of zebrafish (Figure 3C).46 A photocaged Zn2+-specific DNAzymes was conjugated to UCNPs to yield the activatable nanoprobe. With 980 nm light irradiation, the UCNPs emits 365 nm emission locally, which then activate the photocaged Zn2+-specific DNAzymes for imaging. Upon injection into zebrafish, the nanoprobe can diffuse into any part of the zebrafish without Zn2+-induced cleavage of photocaged DNAzymes. Upon NIR irradiation, a significant fluorescence increase was observed inside zebrafish embryos compared with that without NIR irradiation. In contrast, fluorescence enhancement was not observed when using a noncleavable substrate for control experiments. Since Zn2+ is related to many diseases, such as prostate cancer, the method demonstrated in this work paves a new way for understanding the roles of this metal ions in many physiological processes.
Recently, we have also constructed a NIR-controlled DNA nanoprobe for detection of biomolecules in live cells based on the combination of UCNPs with aptamer (Figure 3D).47,48 The nanoprobe was constructed via assembly of a UV light-triggered aptamer probe on the surface of UCNP which acts as the NIR-to-UV transducer. We chose ATP as the analyte to demonstrate the feasibility of our design. The ATP aptamer strand was initially hybridized with a photocleavable (PC) bond-containing DNA to prevent its ATP binding activity. Upon UV light irradiation, the photolysis of PC bond will decrease the hybridization affinity of the complementary DNA to the aptamer strand, and thus the aptamer could switch its structure to bind ATP, leading to the dehybridization of the double stranded DNA and a fluorescent signal increase. Moreover, UCNPs can function as the transducer to realize NIR-controlled activation of a DNA probe. We showed that the nanodevice facilitates efficient cellular uptake of the aptamer probe, allowing NIR light-mediated temporal control over its ATP sensing activity in living cells. Ultimately, the platform will allow NIR-controlled imaging of various targets by integrating different aptamers.
2.3. FDNA engineered inorganic NPs and their applications in targeted drug delivery
Despite the severe side effects, chemotherapy still plays an important role for cancer therapy. Targeted drug delivery by multivalent nanomedicine allows the selective accumulation and uptake of therapeutics in the tumor and thus can mitigate undesired side effects of conventional chemotherapy. Recently, considerable attention has been paid to mesoporous silica nanoparticle (MSN) for drug delivery due to its good biocompatibility, and tunable nanopores for the controlled encapsulation and release of diverse imaging and therapeutic agents.49 By introducing the cancer-specific aptamer into mesoporous silica nanocarriers, we have developed a universal strategy to construct targeted drug delivery systems (Figure 4A).50 The AS1411 aptamer was modified onto the surface of MSN for targeting nucleolin-overexpressed breast cancer cells, while the mesoporous structure allowed high drug loading. The aptamer-mediated cellular targeting of the bioconjugates demonstrated more efficient cancer killing than the non-targeting NPs.
Figure 4.
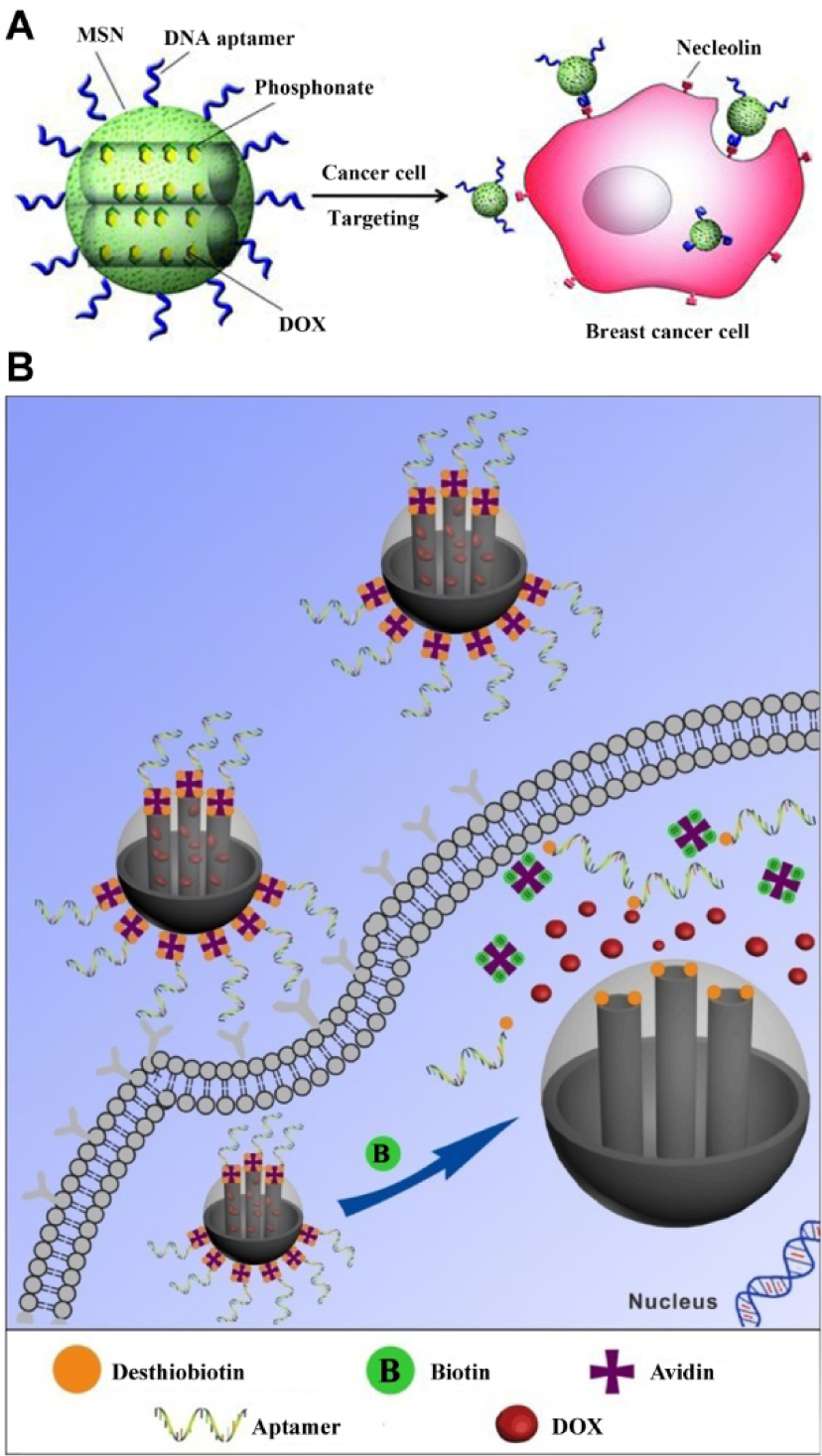
(A) Polyvalent aptamer functionalized MSNs for targeted drug delivery. Reproduced with permission from refs 50. Copyright 2012 Wiley. (B) Aptamer-MSN targeted delivery system with vitamin-responsive drug release. Reproduced with permission from refs 51. Copyright 2013 Royal Society of Chemistry.
In addition to targeting, responsive drug release is another key issue for improving drug delivery efficacy. The combination of active targeting with responsive drug release could maximize therapeutic efficiency and minimize side effects. Toward this goal, we have developed a new generation of MSN-based systems for simultaneous cancer-targeting and intracellular activatable drug release (Figure 4B).51 In this system, we loaded MSNs with an anticancer drug doxorubicin (DOX) and capped the pores of the silica with avidins through the strong desthiobiotin-avidin interaction. In addition, a DNA aptamer sgc8 that is specific to the cell membrane receptor protein tyrosine kinase 7 was conjugated to the surface of MSNs for active targeting. There is no drug release during the targeted delivery process since the pores of the MSNs remain capped. Upon entering the specific cells, the systems will be uncapped by intracellular vitamin H, which is present at higher levels in cancer cells than that in normal cells, accelerating drug release and killing the targeted cancer cells. The system could prevent deleterious off-target effects and demonstrates a significant enhancement in the anticancer activity over the drug alone.
3. FDNA ENGINEERED ORGANIC NPs AND THEIR APPLICATIONS IN BIOSENSING, IMAGING AND TARGETED DRUG DELIVERY
3.1. FDNA engineered organic NPs and their applications in biosensing
While FDNA-engineered inorganic NPs have shown enormous potentials as novel agents for applications in biosensing, bioimaging, and targeted drug delivery as described above, their long-term effects in the human body remain largely unknown. Until these health effects are clarified, using organic NPs can be a good alternative for nanomedicine applications. A primary example of organic NPs is the liposome, as many theranostic agents based on liposomes have already received regulatory approval.5 Nevertheless, synthesis methods and the targeting ability of liposomes need to be improved. Toward this goal, we have developed an enzyme-responsive liposome strategy to achieve sensitive and diverse functional DNA sensors (Figure 5).52 In this strategy, phospholipase A2 (PLA2), which specifically recognizes the sn-2 acyl bond of phospholipids and cleaves fatty acids, was selected as the trigger to induce liposomal rupture. A PLA2-responsive liposomal formulation was used as the reporter carrier. The conjugation of functional DNA and PLA2 establishes a direct relationship between target recognition and subsequent release of signal molecules from the broken liposomes. We demonstrated the effectiveness of this strategy by achieving multimodal detection of cocaine using fluorescence and MRI methods. Cocaine aptamer and PLA2 conjugates were both immobilized onto magnetic beads. The liposomes were loaded with uranin and gadopentetic acid as fluorescence and MRI signal molecules. The existence of cocaine triggers the release of the DNA-PLA2 conjugates, resulting in liposomal rupture and releasing of two signal reporters. As signal molecules are loaded in a high concentration, the induced release can be considered as signal amplification process, resulting in the dual-modal detection of cocaine in a dynamic range from 5 to 1000 μM. In addition to using the FDNA-liposome system for fluorescence and MRI detection, we have reported a sensing application for point-of-care (POC) diagnostics using a simple and widely available thermometers (Figure 5).53 In this case, a NIR dye, indocyanine green (ICG), was loaded into liposomes as signal reporter. Like the process described above, target binding leads to the release of ICG. Upon NIR-laser irradiation, the released ICG converts photon energy into heat, resulting in a temperature increase which can be detected by a thermometer. Using this strategy, a LOD of cocaine is estimated to be 3.8 μM, which is comparable to that of commercial cocaine test kits, such as AccuStik, Easy@Home, and Instant-View cocaine dipstick test (300 ng/L). However, the LOD is much higher than the LOD achieved by commercial ELISA kits (3.3 nM).54 This strategy has also been extended to detect UO22+ using 39E DNAzyme with a LOD of 25.7 nM, which is lower than that of US EPA regulated level (113 nM) for drinking water. In comparison to other FDNA-based methods, the sensitivity of our strategy is similar to those using other portable devices (e.g. personal glucose meters)14 or assembled hydrogel as the reporter carrier.65 Promising approaches to further improve the sensitivity and selectivity of this strategy include screening new FDNAs or using antibodies that possess stronger affinity to target molecules.
Figure 5.
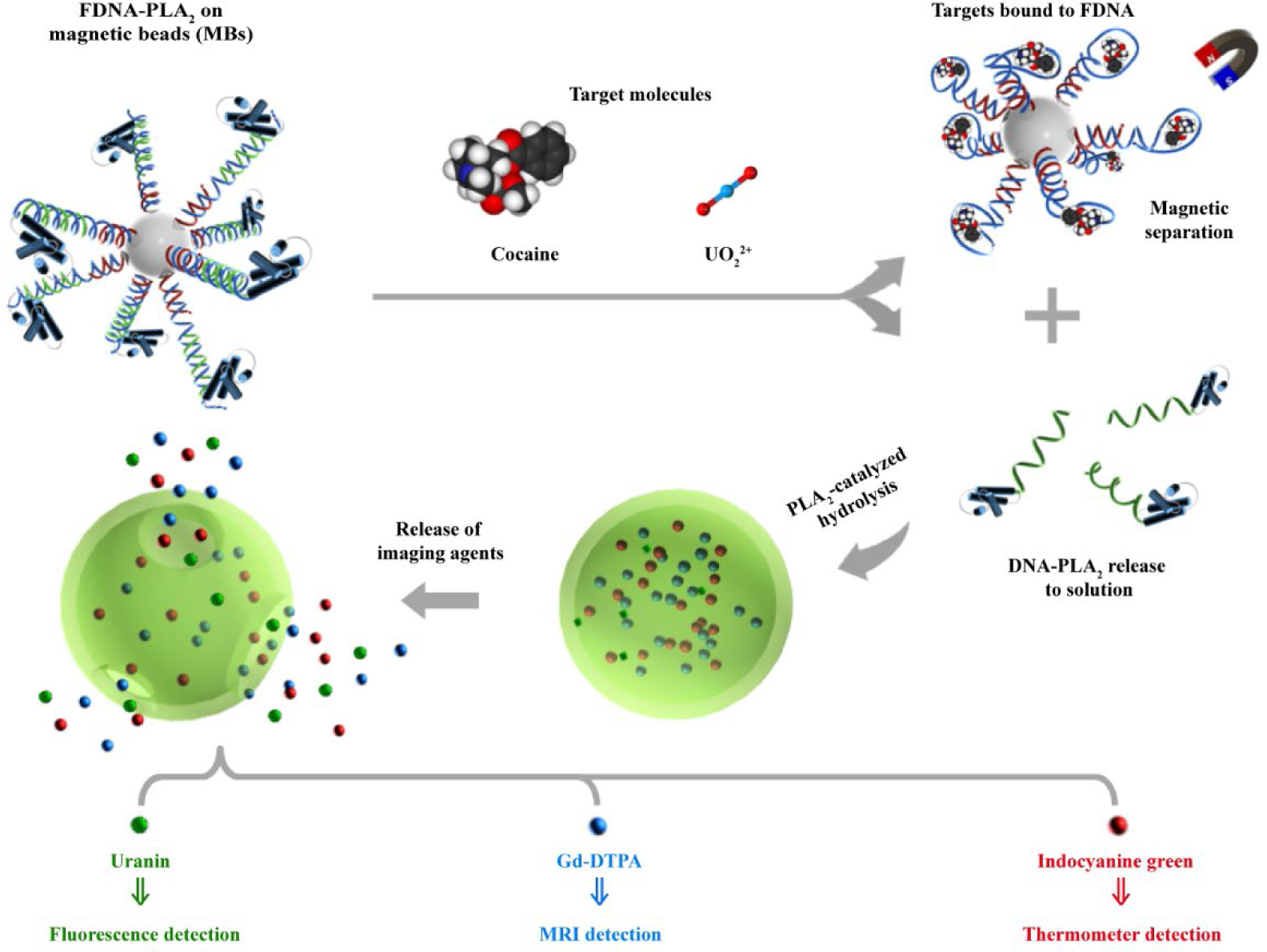
The working principle of the stimuli-responsive liposome strategy for multimodal detection of small molecular targets. Reproduced from refs 52 and 53. Copyright 2016 American Chemical Society. Copyright 2018 Royal Society of Chemistry.
3.2. FDNA engineered organic NPs and their applications in bioimaging
For FDNA-based nanomedicine applications involving interactions at the bio-nano interface, one key factor limiting the development process is the difficulty in precisely controlling the chemistry inside and on the surface of the carrier. In collaboration with the Prof. Steve C. Zimmerman group, we recently reported a bottom-up method to prepare polymeric organic NPs with diameters between ca. 5 and 50 nm using a sequential intramolecular ring-opening metathesis polymerization and ring-closing metathesis (ROMP-RCM) process.56 The choice of appropriated norbornene monomers and chain-transfer agents (CTAs) leads to NPs containing a broad range of functional groups with controlled valency. This strategy offers a particularly appealing NP structure from a single-chain block copolymer, allowing facile incorporation of different functional groups with controlled numbers, including macromolecules such as PEG and DNA. Using the single-chain polymer NP strategy, we have reported the preparation of NPs with a single covalently linked DNA strand (Figure 6A).57 The parent linear block copolymer consists of two blocks, i.e., a cross-linkable block forming the organic NP framework and an inert block as a spacer, which allows accessibility of the reactive azide end-group. DNA can be conjugated to the monovalent organic NP through strain-promoted azide-alkyne cycloaddition. The organic NP also serves as template for the formation of mono-DNA-functionalized AuNPs. The formed mono-DNA-AuNP preserves base-pair recognition properties, which can be used to quantitatively titrate complimentary DNAs and form plasmonic dimers. This strategy has allowed simultaneous control over several essential parameters of DNA-NP and could possibly be extended to other NPs as bioimaging agents or building blocks for nanoscale assembly.
Figure 6.
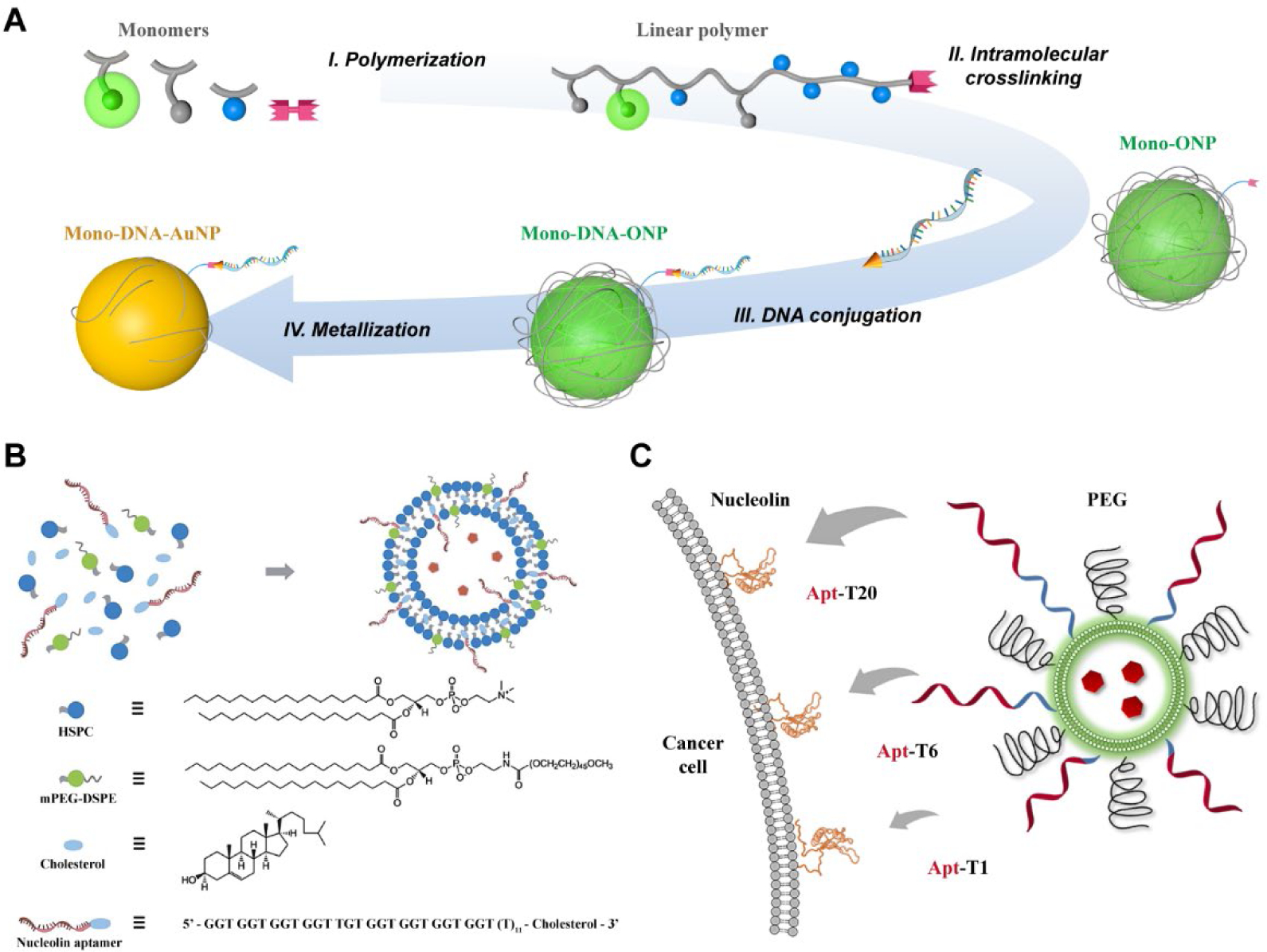
(A) The stepwise, bottom-up synthesis of monovalent DNA-NP conjugate using single-chain block copolymer as template. Reproduced from refs 57. Copyright 2017 American Chemical Society. (B) Synthesis of aptamer-conjugated liposome with encapsulated cargo. Reproduced with permission from refs 63. Copyright 2013 Royal Society of Chemistry. (C) Targeting ability of the aptamer-liposomes can be modified by using oligo-T sequences of different lengths as spacer. Reproduced with permission from refs 65. Copyright 2016 Wiley.
Taking advantage of the modularity and easy-synthesis of single-chain polymers, we have further synthesized a library of fluorescent organic NPs with different surface functional groups as bioimaging probes to study NP-cell interactions as a function of surface properties.58 When correlating the monomer lipophilicity with cell uptake ability, we have found that with an increase in the lipophilicity of repeating units, the internalization rate of the corresponding NP increased. A similar trend was observed in serum-containing biological environments, as organic NPs still show enhanced cellular internalization efficiency as lipophilicity is increased. In addition, the concept of surface post-functionalization of organic NPs was demonstrated using a NP with surface thiol groups, allowing the tuning of cell uptake rate upon maleimide conjugation. Moreover, the single-chain polymer NP strategy was also used to prepare organic NPs with coordinative copper-centers which can efficiently catalyzed intracellular click reactions to directly synthesize imaging probes inside living cells.59 The Cu-containing organic NPs were prepared by Cu2+-mediated intramolecular crosslinking of aspartate-bearing parent polymers. In vitro experiments showed that the Cu-NP entered cells efficiently and lit up cells by intracellularly synthesizing fluorescent probes using non-fluorescent precursors. An antimicrobial experiment was performed by coupling non-toxic azide and alkyne precursors to form a bisamidine antibiotic drug inside E. coli that killed the bacteria. The internal functionalization of organic NP allowed the intracellular synthesis of bioactive compounds through intermolecular coupling.
Our findings demonstrate the use of single-chain polymer NP strategy to achieve a more controlled and tunable degree of functionalization both inside and on the surface of NPs. Other methods have been reported to use molecularly pure core structures (e.g. Cg catalase, T8 polyoctahedral silsesquioxane, and buckminsterfullerene C60) to synthesize NPs with precise numbers of surface DNA strands for bioimaging and therapeutic applications.60,61 Compared to these methods, the single-chain polymer NP strategy is more likely to achieve larger scale synthesis and incorporation of multiple components with stoichiometry control, especially inside the structure. Taken together, our strategy has demonstrated the potential of using organic NPs to produce a promising DNA-NP platform for functional, tunable, and biocompatible imaging probes.
3.3. FDNA engineered organic NPs and their applications in targeted drug delivery
In addition to sensing and imaging applications, FDNA-liposome systems have also been developed for targeted drug delivery (Figure 6B).62,63 In our design, liposomal formulation using hydrogenated soy L-a-phosphatidylcholine (HSPC), cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-#-PEG2000 (mPEG2000-DSPE) was used as the delivery vehicle, mimicking the lipid composition of commercial PEGylated liposomal DOX, such as Doxil and Caelyx.64 An aptamer AS1411 was modified onto liposome surface as the target agent. The resulting AS1411-liposome was ca. 200 nm with cisplatin or DOX loaded as payloads. Cell studies showed that the AS1411-liposome enters NCL-positive MCF-7 cells much faster than NCL-negative LNCaP cells. The aptamer-modified liposome also showed higher cell uptake than a control DNA-modified particle, demonstrating the targeting capability. Animal studies showed that the AS1411-liposomal DOX showed an earlier tumor inhibition and overall enhanced efficacy.63 More importantly, the drug releasing process can be successfully controlled by introducing complementary strands of AS1411, serving as potential antidote for overdosing.62 To gain further mechanistic insights into how DNA behaves on the liposome surface, we investigated the interactions between PEG backfilling and AS1411 sequence (Figure 6C),65 and revealed that a spacer group of appropriate length is crucial for the recognition sequence to be fully exposed from neighboring PEGs to achieve cell-selective targeting.
4. CONCLUSIONS AND PERSPECTIVE
In this Account, we have discussed progress made toward engineering functional DNA-NPs for various biomedical applications. We summarized several strategies developed by our group for combining the biorecognition ability of FDNA with unique optical, magnetic, and electronic properties of inorganic and organic NPs for sensitive and rapid detection of analytes, bioimaging, and targeted drug delivery. We also described the development of novel nanoscale reporters and transducers, functional DNA design, and the controlled immobilization of DNA on very challenging NP surfaces, all of which are crucial to achieve the high performance demands of modern nanomedicine. Despite recent advances, there are still challenges that require further investigation and improvement:
First, with the rapid development of NPs with fascinating optical, magnetic, and electronic properties, a variety of FDNA-NPs have been designed and explored for POC diagnostics. However, a clear “bench to bedside” translation is still missing. Possible reasons could be that the well-developed commercial infrastructure of antibodies and their demonstrated effectiveness toward many clinically relevant targets has made it difficult for FDNA to penetrate into the POC market.9,11 One strategy to materialize the FDNA-based diagnostic revolution is the development of highly desirable homogeneous “bind and detect” assays where antibodies function poorly or cannot perform at all.9 Since it is difficult to generate antibodies selective for small molecular targets (such as metal ions), the clear advantages of FDNA-based sensors in terms of small molecule detection may bring FDNA closer to commercial product development. Some successes have been found in FDNA-based commercially available products for environmental monitoring of metal ions,66,67 which is encouraging for translating FDNA-based nanomedicine, at least in the area of diagnostics. In addition, FDNA-based sensors should be developed for more clinically relevant targets, such as vitamin D, which will certainly attract the attention of clinicians. Another strategy would be to combine FDNA-based sensors with existing, well-accepted devices. Recently, we combined an FDNA-based sensor with a routinely used Personal Glucose Monitor (PGM) device to detect various targets, which will certainly accelerate the speed of translation by taking advantage of many product development and scale-up manufacturing steps that the current POC devices have already established.14
Furthermore, most reported biosensors for molecular imaging in living cells possess little spatial and temporal control over their activity and thus may turn on their signals during the delivery process. To address this issue, light-activatable nanoprobes have been developed for sensing and imaging analytes in complex biological environments with enhanced spatial-temporal precision. In particular, NIR light-controlled bioimaging was realized though coupling functional DNA with photon upconversion technology. However, light-activatable sensing systems are still limited to organisms that light can easily penetrate deeply, such as zebrafish. Other strategies for spatiotemporal control need to be developed for imaging in animals and human beings.
Although nanomedicine has received major attention in the past decade, its clinical translation is still in the early stages, and its progress has been hindered by many hurdles.1 As targeted drug delivery research moves towards the clinical arena, very few actively targeted NP platforms have reached phase III trials, and no targeted nanomedicine has received clinical approval yet.64 While the aptamer-mediated targeted NPs have shown promise to improve the delivery efficacy and reduce the side effects from off-target accumulation of diagnostic agents or therapeutic drugs, current research on targeted drug delivery is far from clinical applications. Several key issues have delayed the clinical development of aptamer-based targeted drug delivery: lack of aptamers for clinically relevant targets with comparable affinity and selectivity to antibodies, lack of large-scale manufacturing infrastructure for these materials, biocompatibility, and safety. A potential available direction is to use cancer-targeted aptamers to improve the pharmacokinetic profiles and targeting specificities of FDA-approved nanocarriers (e.g., liposomes), which provides a highly promising route to lower development costs and facilitate new development cycles. Other ways to improve targeted liposomal therapy may include the use of external stimuli such as ultrasound or the photothermal effect to control drug release;68 or checkpoint inhibitors (such as CTLA-4 or PD-L1 inhibitors) as accompanying immunotherapy could improve the efficacy of liposomal treatment.69 In addition, toxicity assessment of FDNA-NPs deserves more attention for their potential clinical translation. By overcoming these challenges, FDNA-NPs will play a key role in advancing both fundamental science and clinical applications of nanomedicine.
ACKNOWLEDGMENT
We thank Dr. Evan N. Mirts for proof-reading, the NSFC (Nos. 21822401 and 21771044) and U.S. National Institutes of Health (Grants GM124316 and MH110975) for financial support.
Biographies
Lele Li is a professor in National Center for Nanoscience and Technology (NCNST). He received his Ph.D. degree in Chemistry from Peking University in 2010 under Prof. Chun-Hua Yan. He then worked as a postdoctoral research fellow with Prof. Yi Lu (at UIUC), Prof. Daniel S. Kohane (at Harvard Medical School), and Prof. Robert Langer (at MIT) before joining the NCNST in 2016. His current research interests focus on the development of nanobiotechnologies for analysis and imaging in vitro and in vivo with high precision both temporally and spatially.
Hang Xing is a professor in the Institute of Chemical Biology and Nanomedicine (ICBN) in Hunan University. He received his B.S. and M.S. degree from Nanjing University with Prof. Junfeng Bai, and Ph.D. degree from UIUC in 2014 with Prof. Yi Lu. He then moved to Northwestern University working with Prof. Chad A. Mirkin as a postdoctoral research fellow before joining the ICBN in 2017. His research interests focus on developing bio-inspired nanomaterials to study quorum-sensing effect and cell social interactions.
Jingjing Zhang received his PhD degree in chemistry under the supervision of Prof. Jun-Jie Zhu from the School of Chemistry and Chemical Engineering at Nanjing University in 2010. Currently, he works as a postdoctoral research associate in the group of Prof. Yi Lu at UIUC since March 2012. His research interests focus on interfacing carbon and gold nanomaterials with biological systems, and developing novel tools for imaging, sensing and diagnostics.
Yi Lu is Jay and Ann Schenck Professor in the Departments of Chemistry, Biochemistry, Material Science and Engineering, and Bioengineering. He received his B.S. degree from Peking University in 1986 and his Ph.D. degree from UCLA in 1992 under Dr. Joan S. Valentine. After 2 years of postdoctoral research in Dr. Harry B. Gray’s group at Caltech, Dr. Lu started his own independent career at UIUC in 1994. He is also a member of the Center for Biophysics and Quantitative Biology, Beckman Institute for Advanced Science and Technology and Carl R. Woese Institute for Genomic Biology. His group interests are in bioinorganic chemistry, biomaterial chemistry, and bioanalytical chemistry.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Chen G; Roy I; Yang C; Prasad PN Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev 2016, 116, 2826–2885. [DOI] [PubMed] [Google Scholar]
- (2).Jain RK; Stylianopoulos T Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol 2010, 7, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dreaden EC; Alkilany AM; Huang X; Murphy CJ; El-Sayed MA The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev 2012, 41, 2740–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Dong H; Du S-R; Zheng X-Y; Lyu G-M; Sun L-D; Li L-D; Zhang P-Z; Zhang C; Yan C-H Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy. Chem. Rev 2015, 115, 10725–10815. [DOI] [PubMed] [Google Scholar]
- (5).Pillai G Nanomedicines for Cancer Therapy: An Update of FDA Approved and Those under Various Stages of Development. SOJPharm. Pharm. Sci 2014, 1, 1–13. [Google Scholar]
- (6).Xing H; Hwang K; Lu Y Recent Developments of Liposomes as Nanocarriers for Theranostic Applications. Theranostics 2016, 6, 1336–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Liu J; Cao Z; Lu Y Functional Nucleic Acid Sensors. Chem. Rev 2009, 109, 1948–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Tan W; Donovan MJ; Jiang J Aptamers from Cell-Based Selection for Bioanalytical Applications. Chem. Rev 2013, 113, 2842–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Dhiman A; Kalra P; Bansal V; Bruno JG; Sharma TK Aptamer-Based Point-of-Care Diagnostic Platforms. Sens. ActuatorsB: Chem 2017, 246, 535–553. [Google Scholar]
- (10).Liu M; Zhang W, Chang D; Zhang Q; Brennan JD; Li Y Integrating Graphene Oxide, Functional DNA and Nucleic-Acid-Manipulating Strategies for Amplified Biosensing. Trends Anal. Chem 2016, 74, 120–129. [Google Scholar]
- (11).Baird GS Where Are All the Aptamers? Am. J. Clin. Pathol 2010, 134, 529–531. [DOI] [PubMed] [Google Scholar]
- (12).Zhou J; Rossi J Aptamers as Targeted Therapeutics: Current Potential and Challenges. Nat. Rev. Drug Discov 2017, 16, 181–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).McGhee CE; Loh KY; Lu Y DNAzyme Sensors for Detection of Metal Ions in the Environment and Imaging Them in Living Cells. Curr. Opin. Biotechnol 2017, 45, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Xiang Y; Lu Y Using Personal Glucose Meters and Functional DNA Sensors to Quantify a Variety of Analytical Targets. Nat. Chem 2011, 3, 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Rosi NL; Mirkin CA Nanostructures in Biodiagnostics. Chem. Rev 2005, 105, 1547–1562. [DOI] [PubMed] [Google Scholar]
- (16).Liu J; Lu Y A Colorimetric Lead Biosensor Using DNAzyme-Directed Assembly of Gold Nanoparticles. J. Am. Chem. Soc 2003, 125, 6642–6643. [DOI] [PubMed] [Google Scholar]
- (17).Lee JH; Wang Z; Liu J; Lu Y Highly Sensitive and Selective Colorimetric Sensors for Uranyl (UO22+): Development and Comparison of Labeled and Label-Free DNAzyme-Gold Nanoparticle Systems. J. Am. Chem. Soc 2008, 130, 14217–14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Liu J; Lu Y Non-Base Pairing DNA Provides a New Dimension for Controlling Aptamer-Linked Nanoparticles and Sensors. J. Am. Chem. Soc 2007, 129, 8634–8643. [DOI] [PubMed] [Google Scholar]
- (19).Liu J; Lu Y Fast Colorimetric Sensing of Adenosine and Cocaine Based on a General Sensor Design Involving Aptamers and Nanoparticles. Angew. Chem., Int. Ed 2006, 45, 90- [DOI] [PubMed] [Google Scholar]
- (20).Chen B; Wang Z; Hu D; Ma Q; Huang L; Xv C; Guo Z; Jiang X Scanometric Nanomolar Lead (II) Detection Using DNA-Functionalized Gold Nanoparticles and Silver Stain Enhancement. Sens. Actuators B: Chem 2014, 200, 310–316. [Google Scholar]
- (21).Mazumdar D; Liu J; Lu G; Zhou J; Lu Y Easy-To-Use Dipstick Tests for Detection of Lead in Paints Using Non-Cross-Linked Gold Nanoparticle-DNAzyme Conjugates. Chem. Comm 2010, 46, 1416–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Liu J; Mazumdar D; Lu Y A Simple and Sensitive “Dipstick” Test in Serum Based on Lateral Flow Separation of Aptamer-Linked Nanostructures. Angew. Chem., Int. Ed 2006, 45, 7955–7959. [DOI] [PubMed] [Google Scholar]
- (23).Liu J; Lee JH; Lu Y Quantum Dot Encoding of Aptamer-Linked Nanostructures for One-Pot Simultaneous Detection of Multiple Analytes. Anal. Chem 2007, 79, 4120–4125. [DOI] [PubMed] [Google Scholar]
- (24).Yigit MV; Mazumdar D; Lu Y: MRI Detection of Thrombin with Aptamer Functionalized Superparamagnetic Iron Oxide Nanoparticles. Bioconjugate Chem. 2008, 19, 412–417. [DOI] [PubMed] [Google Scholar]
- (25).Yigit MV; Mazumdar D; Kim H-K; Lee JH; Odintsov B; Lu Y Smart “Turn-On” Magnetic Resonance Contrast Agents Based on Aptamer-Functionalized Superparamagnetic Iron Oxide Nanoparticles. ChemBioChem 2007, 8, 1675–1678. [DOI] [PubMed] [Google Scholar]
- (26).Bamrungsap S; Chen T; Shukoor MI; Chen Z; Sefah K; Chen Y; Tan W Pattern Recognition of Cancer Cells Using Aptamer-Conjugated Magnetic Nanoparticles. ACS Nano 2012, 6, 3974–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wang F; Han Y; Lim CS; Lu YH; Wang J; Xu J; Chen HY; Zhang C; Hong M; Liu X Simultaneous Phase and Size Control of Upconversion Nanocrystals through Lanthanide Doping. Nature 2010, 463, 1061–1065. [DOI] [PubMed] [Google Scholar]
- (28).Zhou J; Liu Q; Feng W; Sun Y; Li F Upconversion Luminescent Materials: Advances and Applications. Chem. Rev 2015, 115, 395–465. [DOI] [PubMed] [Google Scholar]
- (29).Li Y; Di Z; Gao J; Cheng P; Di C; Zhang G; Liu B; Shi X; Sun L-D; Li L; Yan C-H Heterodimers Made of Upconversion Nanoparticles and Metal-Organic Frameworks. J. Am. Chem. Soc 2017, 139, 13804–13810. [DOI] [PubMed] [Google Scholar]
- (30).Li L; Zhang R; Yin L; Zheng K; Qin W; Selvin PR; Lu Y Biomimetic Surface Engineering of Lanthanide-Doped Upconversion Nanoparticles as Versatile Bioprobes. Angew. Chem., Int. Ed 2012, 51, 6121–6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Li L; Wu P; Hwang K; Lu Y An Exceptionally Simple Strategy for DNA-Functionalized Up-Conversion Nanoparticles as Biocompatible Agents for Nanoassembly, DNA Delivery, and Imaging. J. Am. Chem. Soc 2013, 135, 2411–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Li L; Lu Y Regiospecific Hetero-Assembly of DNA-Functionalized Plasmonic Upconversion Superstructures. J. Am. Chem. Soc 2015, 137, 5272–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Wang Z; Zhang J; Ekman JM; Kenis PJA; Lu Y DNA-Mediated Control of Metal Nanoparticle Shape: One-Pot Synthesis and Cellular Uptake of Highly Stable and Functional Gold Nanoflowers. Nano Lett. 2010, 10, 1886–1891. [DOI] [PubMed] [Google Scholar]
- (34).Wang Y; Satyavolu NSR; Lu Y Sequence-Specific Control of Inorganic Nanomaterials Morphologies by Biomolecules. Curr. Opin. Colloid. Interface 2018, 38, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Tan LH; Xing H; Lu Y DNA as a Powerful Tool for Morphology Control, Spatial Positioning, and Dynamic Assembly of Nanoparticles. Acc. Chem. Res 2014, 47, 1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Wang Z; Tang L; Tan LH; Li J; Lu Y Discovery of the DNA “Genetic Code” for Abiological Gold Nanoparticle Morphologies. Angew. Chem., Int. Ed 2012, 51, 9078–9082. [DOI] [PubMed] [Google Scholar]
- (37).Song T; Tang L; Tan LH; Wang X; Satyavolu NSR; Xing H; Wang Z; Li J; Liang H; Lu Y DNA-Encoded Tuning of Geometric and Plasmonic Properties of Nanoparticles Growing from Gold Nanorod Seeds. Angew. Chem., Int. Ed 2015, 54, 8114–8118. [DOI] [PubMed] [Google Scholar]
- (38).Wu J; Tan LH; Hwang K; Xing H; Wu P; Li W; Lu Y DNA Sequence-Dependent Morphological Evolution of Silver Nanoparticles and Their Optical and Hybridization Properties. J. Am. Chem. Soc 2014, 136, 15195–15202. [DOI] [PubMed] [Google Scholar]
- (39).Satyavolu NSR; Tan LH; Lu Y DNA-Mediated Morphological Control of Pd-Au Bimetallic Nanoparticles. J. Am. Chem. Soc 2016, 138, 16542–16548. [DOI] [PubMed] [Google Scholar]
- (40).Ma N; Sargent EH; Kelley SO One-Step DNA-Programmed Growth of Luminescent and Biofunctionalized Nanocrystals. Nat. Nanotechnol 2009, 4, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Wei W; He X; Ma N DNA-Templated Assembly of a Heterobivalent Quantum Dot Nanoprobe for Extra- and Intracellular Dual-Targeting and Imaging of Live Cancer Cells. Angew. Chem. Int. Ed 2014, 53, 5573–5577. [DOI] [PubMed] [Google Scholar]
- (42).Wu P; Hwang K; Lan T; Lu Y A DNAzyme-Gold Nanoparticle Probe for Uranyl Ion in Living Cells. J. Am. Chem. Soc 2013, 135, 5254–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Hwang K; Wu P; Kim T; Lei L; Tian S; Wang Y; Lu Y Photocaged DNAzymes as a General Method for Sensing Metal Ions in Living Cells. Angew. Chem., Int. Ed 2014, 53, 13798–13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Torabi S-F; Wu P; McGhee CE; Chen L; Hwang K; Zheng N Cheng J; Lu Y In Vitro Selection of a Sodium-Specific DNAzyme and Its Application in Intracellular Sensing. Proc. Natl. Acad. Sci. U. S. A 2015, 112, 5903–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Wang W; Satyavolu NSR; Wu Z; Zhang J-R; Zhu J-J; Lu Y Near-Infrared Photothermally Activated DNAzyme-Gold Nanoshells for Imaging Metal Ions in Living Cells. Angew. Chem., Int. Ed 2017, 56, 6798–6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Yang Z; Loh KY; Chu Y-T; Feng R; Satyavolu NSR; Xiong M; Huynh SMNH; Hwang K; Li L; Xing H; Zhang X; Chemla YR; Gruebele M; Lu Y; Optical Control of Metal Ion Probes in Cells and Zebrafish Using Highly Selective DNAzymes Conjugated to Upconversion Nanoparticles. J. Am. Chem. Soc 2018, 140, 17656–17665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Zhao J; Gao J; Xue W; Di Z; Xing H; Lu Y; Li L Upconversion Luminescence-Activated DNA Nanodevice for ATP Sensing in Living Cells. J. Am. Chem. Soc 2018, 140, 578–581. [DOI] [PubMed] [Google Scholar]
- (48).Li M; Zhao J; Chu H; Mi Y; Zhou Z; Di Z; Zhao M; Li L Light-Activated Nanoprobes for Biosensing and Imaging. Adv. Mater 2018, 1804745. [DOI] [PubMed] [Google Scholar]
- (49).Slowing II; Vivero-Escoto JL; Wu CW; Lin VSY Mesoporous Silica Nanoparticles as Controlled Release Drug Delivery and Gene Transfection Carriers. Adv. Drug Delivery Rev 2008, 60, 1278–1288. [DOI] [PubMed] [Google Scholar]
- (50).Li L; Yin Q; Cheng J; Lu Y Polyvalent Mesoporous Silica Nanoparticle-Aptamer Bioconjugates Target Breast Cancer Cells. Adv. Healthcare Mater 2012, 1, 567–572. [DOI] [PubMed] [Google Scholar]
- (51).Li L; Xie MY; Wang J; Li XY; Wang C; Yuan Q; Pang DW; Lu Y; Tan WH A Vitamin-Responsive Mesoporous Nanocarrier with DNA Aptamer-Mediated Cell Targeting. Chem. Commun 2013, 49, 5823–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Xing H; Zhang CL; Ruan G; Zhang J; Hwang K; Lu Y; Multimodal Detection of a Small Molecule Target Using Stimuli-Responsive Liposome Triggered by Aptamer-Enzyme Conjugate. Anal. Chem 2016, 88, 1506–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Zhang J; Xing H; Lu Y Translating Molecular Detections into a Simple Temperature Test Using a Target-Responsive Smart Thermometer. Chem. Sci 2018, 9, 3906–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Garcia Y; Smolinska-Kempisty K; Pereira E; Piletska E; Piletsky S Development of Competitive ‘Pseudo’-ELISA Assay for Measurement of Cocaine and Its Metabolites Using Molecularly Imprinted Polymer Nanoparticles. Anal. Methods 2017, 9, 4592–4598. [Google Scholar]
- (55).Zhu Z; Wu C; Liu H; Zou Y; Zhang X; Kang H; Yang CJ; Tan W An Aptamer Cross-Linked Hydrogel as a Colorimetric Platform for Visual Detection. Angew. Chem. Int. Ed 2010, 49, 1052–1056. [DOI] [PubMed] [Google Scholar]
- (56).Bai Y; Xing H; Vincil GA; Lee J; Henderson EJ; Lu Y; Lemcoff NG; Zimmerman SC Practical Synthesis of Water-Soluble Organic Nanoparticles with a Single Reactive Group and a Functional Carrier Scaffold. Chem. Sci 2014, 5, 2862–2868. [Google Scholar]
- (57).Xing H; Bai Y; Bai Y; Tan LH; Tao J; Pedretti B; Vincil GA; Lu Y; Zimmerman SC Bottom-Up Strategy to Prepare Nanoparticles with a Single DNA Strand. J. Am. Chem. Soc 2017, 139, 3623–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Bai Y; Xing H; Wu P; Feng X; Hwang K; Lee JM; Phang XY; Lu Y; Zimmerman SC Chemical Control over Cellular Uptake of Organic Nanoparticles by Fine Tuning Surface Functional Groups. ACS Nano 2015, 9, 10227–10236. [DOI] [PubMed] [Google Scholar]
- (59).Bai Y; Feng X; Xing H; Xu Y; Kim BK; Baig N; Zhou T; Gewirth AA; Lu Y; Oldfield E; Zimmerman SC A Highly Efficient Single-Chain Metal-Organic Nanoparticle Catalyst for Alkyne-Azide “Click” Reactions in Water and in Cells. J. Am. Chem. Soc 2016, 138, 11077–11080. [DOI] [PubMed] [Google Scholar]
- (60).Brodin JD; Auyeung E; Mirkin CA DNA-Mediated Engineering of Multicomponent Enzyme Crystals. Proc. Natl. Acad. Sci. U. S. A 2015, 112, 4564–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Li H; Zhang B; Lu X; Tan X; Jia F; Xiao Y; Cheng Z; Li Y; Silva DO; Schrekker HS; Zhang K; Mirkin CA Molecular Spherical Nucleic Acids. Proc. Natl. Acad. Sci. U. S. A 2018, 115, 4340–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Cao ZH; Tong R; Mishra A; Xu WC; Wong GCL; Cheng JJ; Lu Y Reversible Cell-Specific Drug Delivery with Aptamer-Functionalized Liposomes. Angew. Chem., Int. Ed 2009, 48, 6494–6498. [DOI] [PubMed] [Google Scholar]
- (63).Xing H; Tang L; Yang XJ; Hwang K; Wang WD; Yin Q; Wong NY; Dobrucki LW; Yasui N; Katzenellenbogen JA; Helferich WG; Cheng J; Lu Y Selective Delivery of an Anticancer Drug with Aptamer-Functionalized Liposomes to Breast Cancer Cells in Vitro and in Vivo. J. Mater. Chem. B 2013, 1, 5288–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Barenholz YC Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [DOI] [PubMed] [Google Scholar]
- (65).Xing H; Li J; Xu W; Hwang K; Wu P; Yin Q; Li Z; Cheng J; Lu Y The Effects of Spacer Length and Composition on Aptamer-Mediated Cell-Specific Targeting with Nanoscale PEGylated Liposomal Doxorubicin. ChemBioChem 2016, 17, 1111–1117. [DOI] [PubMed] [Google Scholar]
- (66).Liu J; Brown AK; Meng X; Cropek DM; Istok JD; Watson DB; Lu Y A Catalytic Beacon Sensor for Uranium with Parts-per-Trillion Sensitivity and Millionfold Selectivity. Proc. Natl. Acad. Sci. USA 2007, 104, 2056–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).ANDalyze Home Page. http://andalyze.com/ (accessed July 14, 2019)
- (68).Rwei AY; Paris JL; Wang B; Wang W; Axon CD; Vallet-Regi M; Langer R; Kohane DS Ultrasound-Triggered Local Anaesthesia. Nat. Biomed. Eng 2017, 1, 644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Rios-Doria J; Durham N; Wetzel L; Rothstein R; Chesebrough J; Holoweckyj N Zhao W; Leow CC; Hollingsworth R Doxil Synergizes with Cancer Immunotherapies to Enhance Antitumor Responses in Syngeneic Mouse Models. Neoplasia 2015, 17, 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]


