Abstract
Inverted colloidal crystal (ICC) hydrogel scaffolds represent unique opportunities in modeling lymphoid tissues and expanding hematopoietic-lymphoid cells. Fully interconnected spherical pore arrays direct the formation of stromal networks and facilitate interactions between stroma and hematopoietic-lymphoid cells. However, due to the intricate architecture of these materials, release of expanded cells is restricted and requires mechanical disruption or chemical dissolution of the hydrogel scaffold. One potent biomaterials strategy to release pore-entrapped hematopoietic-lymphoid cells without breaking the scaffolds apart is to transiently increase the dimensions of these materials using stimuli-responsive polymers. Having this mindset, we have developed thermoresponsive ICC scaffolds that undergo rapid (< 1 min) and substantial (> 300%) diameter change over a physiological temperature range (4 – 37 °C) by using poly(N-isopropylacrylamide) (PNIPAM) with nanogel crosslinkers. For a proof-of-concept study, we first replicated the stromal niche by creating osteospheroids, aggregates of osteoblasts and bone chips, and subsequently introduced Nalm-6 model hematopoietic-lymphoid cells. A 6-fold increase in cell count was harvested when ICC hydrogel scaffolds were expanded without termination of the established 3D stromal cell culture. We envision that thermoresponsive ICC hydrogel scaffolds will enable for scalable and sustainable ex vivo expansion of hematopoietic-lymphoid cells.
Thermoresponsive inverted colloidal crystal hydrogel scaffolds with rapid response rate and large volumetric change enable selective retrieval of expanded model hematopoietic-lymphoid cells from co-culture with osteospheroids via a temperature switch in a repeatable fashion.
Keywords: thermoresponsive, PNIPAM, hydrogel scaffold, inverted colloidal crystal, lymphoid tissue
Graphical Abstract

Introduction
Three-dimensional (3D) cell culture matrices, also known as scaffolds, have played a central role in creating functional tissue analogs outside of the body.[1] Each tissue consists of distinct populations of cells and extracellular matrix (ECM) proteins which are spatially organized in a highly specific manner.[2] Thus, both biomaterial selection and structural design of scaffolds need to be carefully considered in order to reproduce tissue-specific 3D architecture, biochemical complexity, and associated key cellular processes found in vivo. Inverted colloidal crystal (ICC) hydrogel scaffolds have been previously engineered to mimic lymphoid tissues including the bone marrow, thymus, and lymph nodes.[3–6] Recent studies have considered the liver as a lymphoid tissue as it supports prenatal hematopoiesis and postnatal extramedullary hematopoiesis under the bone marrow stress, in addition to harboring antigen presenting cells and coordinating immune responses.[7–9] ICC scaffolds have also demonstrated unique advantages to creating liver tissue in vitro.[10–14] A common anatomical feature across these lymphoid tissues is a stromal network embedded within a microporous 3D ECM meshwork. [15] This network directs intimate interactions between stromal cells and hematopoietic-lymphoid cells, a core cellular process that supports hematopoietic-lymphoid cell survival, expansion, maturation, and activation.[16–18] ICC hydrogel scaffolds consist of fully interconnected and tunable spherical pore arrays which lend two favorable characteristics in mimicking lymphoid tissues: (i) a large surface area to support stromal cell adhesion and subsequent stromal network formation, and (ii) due to the spherical geometry of pore cavities, hematopoietic-lymphoid cells entrapped within are directed to be within close proximity of nearby stromal cells, facilitating multicellular communication.
An important next step for lymphoid tissue engineering with ICC hydrogel scaffolds is the scale-up and manufacturing of clinical-grade hematopoietic-lymphoid cells. While ICC hydrogel scaffolds have improved the ability to expand hematopoietic-lymphoid cells, retrieval of these cells is difficult due to the intricate 3D microporous structure. Alternatively, hydrogel scaffolds can be mechanically dismantled or chemically dissociated to release pore-entrapped hematopoietic-lymphoid cells; however, this disruptive procedure has a high likelihood of damaging cells and requires another purification step to separate cells from biomaterial debris.[19, 20] Furthermore, the fabrication of ICC scaffolds and establishment of stromal networks requires substantial time and effort. Therefore, destruction of 3D lymphoid tissue mimics presents a critical barrier for scalable and sustainable expansion of hematopoietic-lymphoid cells.
One potential solution to release pore entrapped hematopoietic-lymphoid cells within ICC hydrogel scaffolds is to transiently increase the pore size by utilizing stimuli-responsive polymers - materials which dynamically respond to changes within the microenvironment - thereby enabling spatiotemporal control over previously static 3D tissue constructs. There has been substantial progress in creating thermoresponsive hydrogels using poly(N-isopropyl-acrylamide) (PNIPAM) which undergoes a reversible coil-to-globule conformational change at its lower critical solution temperature (LCST), resulting in a change of volume.[21–23] The goal of the present study was to fabricate thermoresponsive ICC hydrogel scaffolds that undergo rapid and substantial volumetric change over a physiological temperature range. The biological relevance of thermoresponsive ICC scaffolds was demonstrated in the context of the trabecular bone marrow through the creation of osteospheroids - aggregates composed of mouse osteoblasts and bovine trabecular bone chips - and their subsequent co-culture with Nalm-6 model hematopoietic-lymphoid cells.
Results
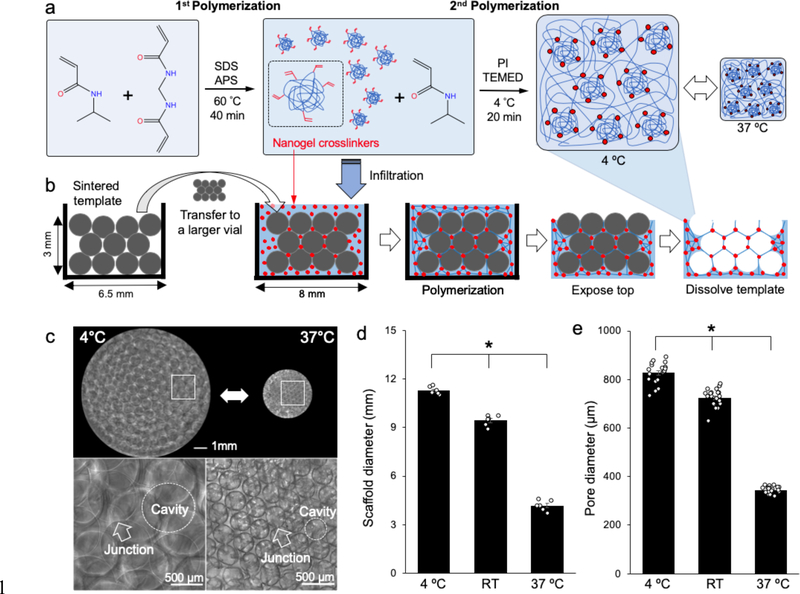
We fabricated thermoresponsive ICC hydrogel scaffolds by utilizing a previously reported PNIPAM hydrogel with nanogel crosslinkers that responds rapidly to temperature change and adapting it into colloidal crystal template-based hydrogel fabrication.[24] PNIPAM hydrogel scaffolds with nanogel crosslinkers were made by two-step polymerization: (i) synthesis of PNIPAM nanogels that retain unsaturated double bonds and (ii) polymerization of these nanogels which served as crosslinkers to create macroscale PNIPAM hydrogels (Figure 1a). A colloidal crystal template (6.5 mm inner diameter and 3 mm height) made with glass beads (550 ± 65 μm diameter) was placed in a larger shell vial (8 mm inner diameter) and then infiltrated with precursor solution containing nanogels for the second polymerization step. Subsequent hydrogel formation was conducted at 4 °C to maintain the expanded state of the nanogels during exothermic radical polymerization. After complete polymerization, the top face of the hydrogel in the colloidal crystal template was removed and the template beads were chemically dissolved using an acid solution. At the end of the process, a thin layer (< 1 mm) of hydrogel remained on the sides and bottom face, leaving behind a “pocket” hydrogel scaffold (Figure 1b). ICC PNIPAM scaffolds with nanogel crosslinkers (ICC PNIPAM-NG) exhibited substantial and reversible temperature-dependent volume change while maintaining ICC architecture, suggesting that hydrogel expansion and shrinkage occurs homogeneously in an isotropic manner (Figure 1c). Specifically, the overall diameters of ICC PNIPAM-NG at 4 °C, room temperature (RT), and 37 °C were 11.3 ± 0.1 mm, 9.4 ± 0.1 mm, and 4.2 ± 0.1 mm, respectively (Figure 1d). Similarly, the pore diameters of ICC PNIPAM-NG at 4 °C, RT, and 37 °C were 827 ± 9.7 μm, 704 ± 8.3 μm, and 343 ± 2.9 μm, respectively (Figure 1e). The diameter of the interconnecting junctions between the spherical pores were 181 ± 7.4 μm, 144 ± 5.7 μm, and 55.1 ± 3.1 μm at 4 °C, RT, and 37 °C, respectively. Overall, ICC PNIPAM-NG scaffolds undergo about 300% isotropic change in diameter between 4 °C and 37 °C.
Figure 1.
Fabrication of thermoresponsive ICC hydrogel scaffolds. a) Illustration of chemical structures during two-step polymerization to synthesize PNIPAM-NG. b) Schematic procedure of ICC PNIPAM-NG scaffold fabrication via application of PNIPAM-NG synthesis into colloidal crystal template-based ICC pocket scaffold fabrication. c) Optical microscopy images of thermoresponsive ICC PNIPAM-NG scaffolds (upper panels) and zoomed-in images of spherical pore arrays and interpore junctions (lower panels) at 4 °C and 37 °C. (d-e) Comparison of d) overall scaffold diameters (n = 6) and e) ICC pore diameters (n = 20) at 4 °C, room temperature (RT), and 37 °C, respectively. (*P < 0.05)
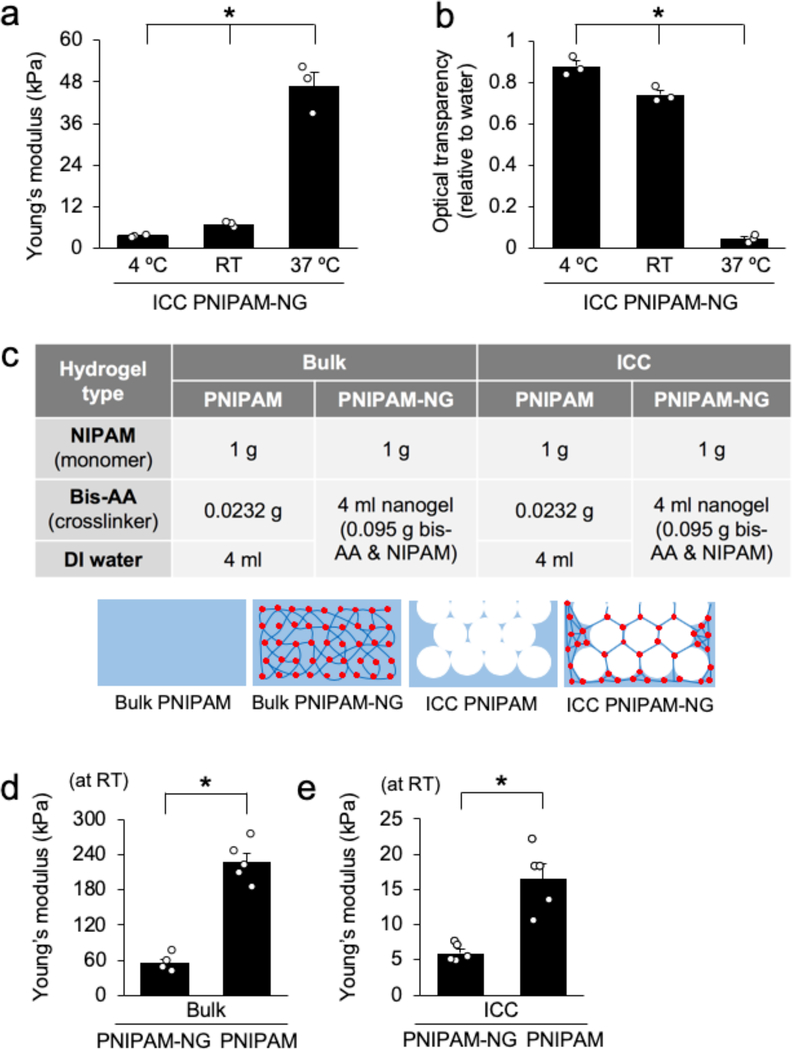
Temperature-dependent volumetric change of ICC PNIPAM-NG hydrogels are accompanied by changes in mechanical and optical properties. The compressive Young’s modulus of ICC PNIPAM-NG was 47 ± 4.1 kPa, 7.0 ± 0.53 kPa, and 3.4 ± 0.14 kPa at 37 °C, RT, and 4 °C, respectively (Figure 2a). Changes in hydrophobicity of PNIPAM directs optical transparency changes within the hydrogel. The relative optical transparency for ICC PNIPAM-NG based on water was 0.88 ± 0.028, 0.74 ± 0.021, and 0.04 ± 0.011 at 4 °C, RT, 37 °C, respectively (Figure 2b). Importantly, ICC PNIPAM-NG still exhibited sufficient mechanical integrity at 4 °C for experimental handling during 3D cell culture and optical transparency at RT for microscopic imaging.
Figure 2.
Characterization of the mechanical and optical properties of PNIPAM hydrogels. a) Compressive Young’s modulus of ICC PNIPAM-NG at 4 °C, RT, and 37 °C (n = 3). b) Relative optical transparency of ICC PNIPAM-NG based on water (set as 1) at 4 °C, RT, and 37 °C (n = 3). c) Compositions of four different PNIPAM hydrogels with schematic illustrations. (d-e) Comparison of the characteristic Young’s modulus at RT d) between Bulk PNIPAM-NG and Bulk PNIPAM (n = 5), and e) between ICC PNIPAM-NG and ICC PNIPAM (n = 5). (*P < 0.05).
We next interrogated the effects of nanogel crosslinking and ICC geometry on the mechanical properties at RT by preparing four different hydrogel samples: (i) Bulk PNIPAM, (ii) Bulk PNIPAM-NG, (iii) ICC PNIPAM, and (iv) ICC PNIPAM-NG (Figure 2c). With respect to nanogel crosslinking, despite a 4-fold higher crosslinking density, (1.86 wt% for PNIPAM-NG and 0.46 wt% for PNIPAM) PNIPAM-NG exhibited a 4-fold lower Young’s modulus (E = 54.6 ± 6.4 kPa) compared to PNIPAM (E = 227 ± 15 kPa) (Figure 2d). As expected, ICC geometry with the introduction of macroscopic pores significantly decreased the Young’s modulus by 10-fold when compared to corresponding bulk hydrogels. Similarly, inclusion of nanogel crosslinkers in ICC scaffolds decreased the Young’s modulus by about 3fold (E = 5.98 ± 0.56 kPa) compared to ICC PNIPAM scaffolds without nanogel crosslinkers (E = 16.98 ± 2.0 kPa) (Figure 2e). These results demonstrate that nanogel crosslinking and ICC geometry independently decrease the mechanical properties of these hydrogels. When both properties are combined in ICC PNIPAM-NG, mechanical pliability is cooperatively increased as confirmed by the significantly lower Young’s modulus compared to the hydrogels having either one of these factors.
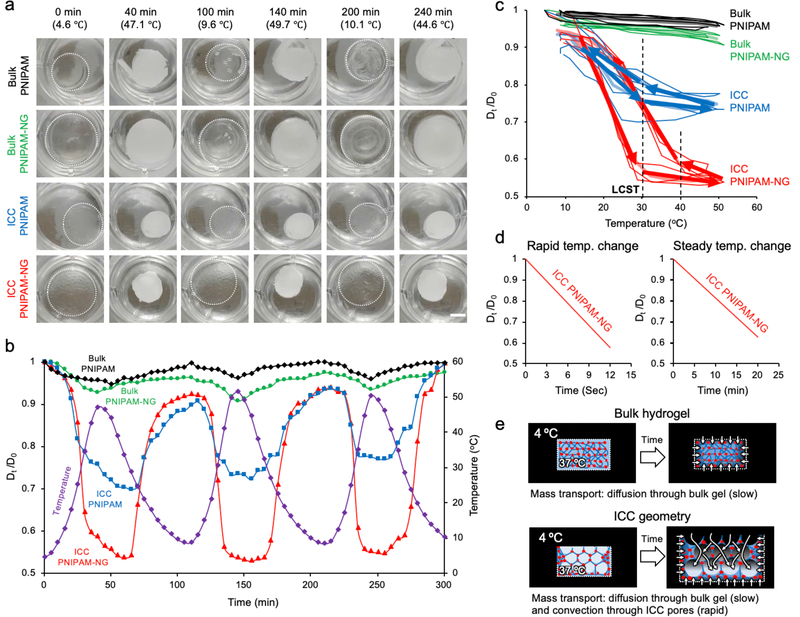
We next examined the impacts of nanogel crosslinking, ICC geometry, and the rate of temperature change on the kinetics and extent of volume change in PNIPAM hydrogels. To decouple the effect of temperature change on these variables, we set programmed temperature cycles between 4 °C and 50 °C at a rate of 1 °C per minute. Aforementioned PNIPAM hydrogels were placed in the same well-plate and their diameter changes monitored over 3 temperature cycles. Regardless of the presence of nanogels or ICC geometry, all PNIPAM hydrogels underwent sudden phase transition at LCST, 30 °C as evidenced by changes in optical transparency; however, their temperature-dependent volume change was significantly different from each other suggesting intrinsically different kinetics (Figure 3a, Supplementary Video 1). By plotting the size of the hydrogels at 5-minute intervals over 5 hours, we quantitatively visualized the dynamic size change of hydrogels as a ratio of the initial diameter at 4 °C (D0) to the diameter at a given temperature (Dt) with corresponding temperature cycles (Figure 3b). Bulk PNIPAM, Bulk PNIPAM-NG, ICC PNIPAM and ICC PNIPAM-NG exhibited about 4%, 8%, 25% and 48% changes in diameter, respectively. Nanogel crosslinking increases the diameter by 2-fold in bulk and ICC hydrogels whereas introduction of ICC geometry improves the diameter change in PNIPAM and PINPAM-NG up to 6-fold. In ICC PNIPAM-NG, these properties together showed a 12-fold diameter size change compared to Bulk PNIPAM hydrogels. These results quantitatively substantiate the contributions of nanogel crosslinking (2-fold) and ICC geometry (6-fold) to the response rate, and their additive effects when combined. ICC geometry introduces macroscale interconnected pores which accommodate convective mass transport of water molecules in addition to molecular diffusion. Furthermore, the empty macroscopic cavities presented by ICC geometry decreases the bulk mechanical properties while increasing mechanical pliability. These effects together direct more effective conformational change of the thermosensitive polymer chains and result in greater volume change than Bulk PNIPAM hydrogels with the same dimensions.
Figure 3.
Comparative characterization of temperature-dependent volume changes in PNIPAM hydrogels. a) Snapshots of Bulk PNIPAM, Bulk PNIPAM-NG, ICC PNIPAM, and ICC PNIPAM-NG at discrete time points in pure water during 3 cycles of steady temperature change between 4 °C and 50 °C at 1 °C per min. b) Quantitative representation of temperature cycles (purple) and normalized deswelling ratios of Bulk PNIPAM (black), Bulk PNIPAM-NG (green), ICC PNIPAM (blue), and ICC PNIPAM-NG (red) with respect to time. (D0 = initial hydrogel diameter at 4 °C, Dt = hydrogel diameter at a given temperature). c) Characteristic hysteresis curves of Bulk PNIPAM, Bulk PNIPAM-NG, ICC PNIPAM, and ICC PNIPAM-NG as a function of temperature and normalized swelling ratio during 3 cycles of steady temperature change. Arrows identify two distinct kinetic regimes for the temperature-dependent normalized deswelling ratios. Dotted lines represent critical temperatures in which deswelling ratio changes significantly. d) Comparison of thermoresponsive kinetics of ICC PNIPAM-NG under sudden (left) and steady (right) temperature changes between 4 °C and 37 °C. e) Schematic explaining the differences in response rate between Bulk PNIPAM-NG and ICC PNIPAM-NG as a function of mass transport. Mass transport in ICC PNIPAM-NG is considerably faster than bulk PNIPAM-NG as ICC geometry facilitates convective transport whereas bulk hydrogels only support transport via diffusion.
To determine the thermoresponsive rate as a function of nanogel crosslinking and ICC geometry, we overlaid changes in the deswelling ratios during repeated temperature cycles with respect to temperature that revealed characteristic hysteresis loops in each PNIPAM hydrogel. Of note, not all hydrogels recovered their original size in the given heating and cooling rate (1 °C per min) (Figure 3c). Hysteresis loops appear linear in bulk hydrogels regardless of nanogel crosslinking whereas there are two distinct regimes with ICC geometry below and above the LCST. To quantitatively compare deswelling and reswelling kinetics, we introduced a characteristic slope index (CSI) defined as the ratio of the change in temperature over the change in diameter. When the temperature was below the LCST (4 – 30 °C), ICC PNIPAM-NG (CSI = 50) had 2-fold faster kinetics compared to ICC PNIPAM (CSI = 100), indicating that the impact of nanogel crosslinking was amplified by ICC geometry. However, when the temperature was above the LCST (30 – 50 °C), both ICC PNIPAM-NG and ICC PNIPAM exhibited about 10-fold slower but comparable kinetics (CSI = 550). Interestingly, while both ICC PNIPAM-NG and ICC PNIPAM displayed a shift in their CSI at the same temperature at 30 °C, when deswelling with increasing temperature, upon reswelling with decreasing temperature, ICC PNIPAM-NG transitioned from its slow, large CSI to its fast, small CSI at 40 °C whereas ICC PNIPAM underwent this transition at 30 °C, the same critical temperature for deswelling.
Last, we examined the impact of the rate of temperature change on the deswelling and reswelling kinetics of ICC PNIPAM-NG by comparing steady (1 °C per minute) and sudden temperature changes from 4 °C to 37 °C. Under steady temperature changes, the deswelling time took approximately 20 minutes to reach 60% size reduction (Dt/D0 = 0.6) whereas it took about 12 seconds under sudden temperature changes (Figure 3d, Supplementary Video 2). Collectively these results explain the mechanism behind rapid and substantial volume changes of ICC PNIPAM-NG. From the aspect of polymer chemistry, nanogel crosslinking amplifies the intrinsic temperature-dependent conformational change of PNIPAM chains while increasing mechanical flexibility that significantly improves the extent and kinetics of volumetric change. In terms of mass transport, influx and efflux of water molecules through the hydrogels occur via either diffusion or convection. Bulk hydrogels containing only sub-micron scale pores through the polymer meshwork support only diffusion. Introducing interconnected pores via ICC geometry accommodates convective mass transport in addition to diffusion through the polymer matrix, significantly enhancing mass transport and resultantly improves the response kinetics (Figure 3e). Our results suggest that structural design of hydrogels is of equivalent importance to optimizing polymer chemistries in order to maximize the dynamics and kinetics of ICC PNIPAM-NG in response to temperature stimuli.
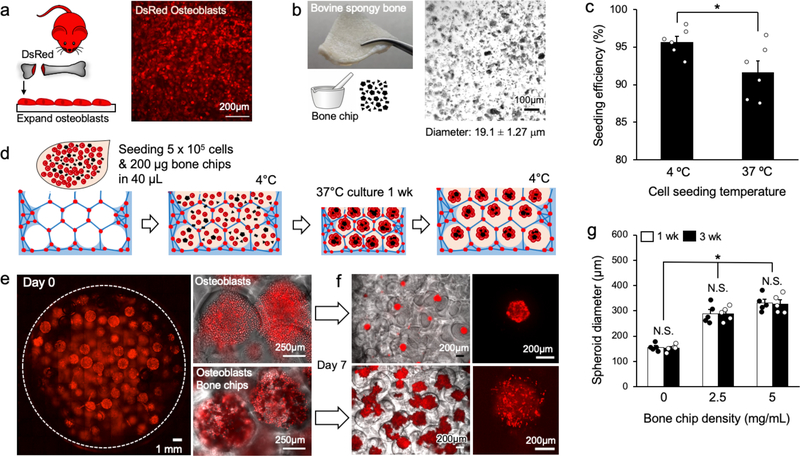
We next applied thermoresponsive ICC hydrogel scaffolds to simulate hematopoietic-lymphoid cell expansion in trabecular bone cavities. The large surface area in ICC scaffolds has previously been utilized to form a stromal network similar to those found in lymphoid organs.[4, 19] However, this feature cannot be exploited because the pores in thermoresponsive ICC scaffolds should remain stromal cell-free in order to undergo reversible expansion and contraction. Alternatively, we exploited another unique feature of ICC scaffold - spherical pores made with cell-repulsive hydrogel material directs the formation of 3D cell aggregates known as spheroids.[10, 25] Recent studies demonstrated that 3D aggregates of bone marrow stromal cells provide better support for hematopoietic stem cell culture expansion compared to stroma when cultured on 2D.[26, 27] Here, we leveraged these properties to create osteospheroids that consists of osteoblasts and bone chips in ICC PNIPAM-NG. Primary murine osteoblasts were retrieved from the femurs and tibias of DsRed reporter mice and expanded in tissue culture (Figure 4a). Decellularized bovine trabecular bone was used to make bone chips due to its ability to generate a large quantity of bone chips. Grounded trabecular bone chips had an average diameter of 19.1 ± 1.27 μm (Figure 4b). Next, DsRed osteoblasts (3 × 105 per scaffold) and bone chips were mixed and inoculated on top of fully expanded ICC PNIPAM-NG. Resultantly, we achieved a cell seeding efficiency of 95 ± 0.57% using scaffolds pre-equilibrated at 4 °C compared to a seeding efficiency of 91 ± 1.4% for scaffolds preequilibrated at 37 °C (Figure 4c). The reduced cell seeding efficiency at 37 °C was mainly due to the decreased size of the scaffolds when compared to the scaffolds at 4 °C. Pocket type ICC hydrogel scaffolds have been previously shown to exhibit more than 90% cell seeding efficiency, which is significantly higher than traditional open porous ICC scaffolds (Figure 4d).[28]
Figure 4.
Creation of osteospheroids in thermoresponsive ICC hydrogel scaffolds. a) Schematic of isolation and expansion of osteoblastic cells from a DsRed transgenic reporter mouse (left) and fluorescent microscopy image of DsRed osteoblasts on tissue culture plastic surface (right). b) Gross image of bovine trabecular bone (left) and optical microscopy image of bone chips after mechanical grinding (right). c) Comparison of osteoblast seeding efficiency in ICC PNIPAM-NG scaffolds between 4 °C and 37 °C. (n = 6). d) Schematic procedure of creating osteospheroids consisting of osteoblasts and bone chips in ICC PNIPAM-NG. (e-f) Confocal microscopy images e) after osteoblast seeding (left) and zoomed-in images of ICC pores with and without bone chips (left) and f) after 1 week of culture with and without bone chips (right). g) Quantitative comparison of osteospheroid diameters at 1 and 3 weeks of culture in ICC PNIPAM-NG as a function of increasing bone chip concentration (n = 5). (N.S.: not significant, *P < 0.05)
At RT, ICC PNIPAM-NG recovered sufficient optical transparency for microscopic imaging. Confocal imaging revealed homogeneous cell seeding throughout the ICC PNIPAM-NG scaffold (Figure 4e, Supplementary Video 3). Osteospheroid formation became evident with integration of osteoblasts and bone chips (Figure 4f). Quantitative imaging analysis after 1 week of culture revealed that osteospheroids without bone chips had an average diameter of 156 ± 6.57 μm whereas osteospheroids with bone chips exhibited increased diameter solely as a function of loaded bone chip amount. Osteospheroids with 2.5 mg/ml and 5 mg/ml of bone chips displayed diameters of 288 ± 16.8 μm and of 331 ± 14.4 μm, respectively. When osteospheroids became larger than the interconnecting junctions in the scaffold in an expanded state (diameter = 300 ± 14.4 μm), they became geometrically entrapped, preventing their release upon scaffold reswelling and deswelling via a ship-in-bottle effect. Culture of osteospheroids was continued for up to 3 weeks, with no significant size change between 1 and 3 weeks (Figure 4g), suggesting that osteospheroids stabilize within 1 week of culture in ICC PNIPAM-NG. Taken together, these data demonstrate the formation and culture of osteospheroids in ICC PNIPAM-NG of defined size in a simple, robust, and tunable manner that reproduces the anatomical, mechanical and stromal microenvironment of trabecular bone marrow.
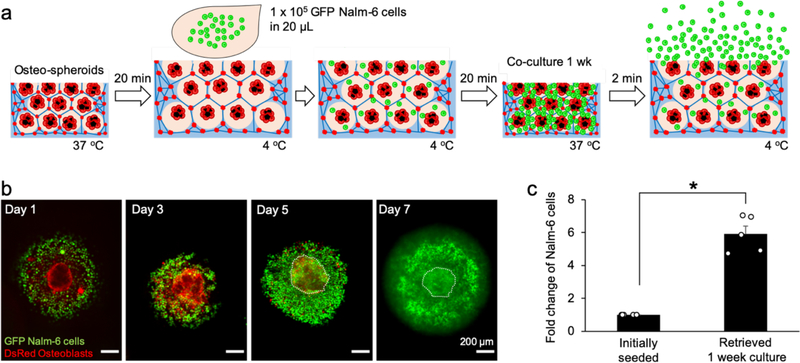
Finally, we applied thermoresponsive ICC scaffolds to expand and retrieve co-cultured Nalm-6 model hematopoietic-lymphoid cells. We introduced 1 × 105 Nalm-6 cells expressing green fluorescent protein (GFP) into ICC PNIPAM-NG embedded with osteospheroids created using a bone chip density of 5 mg/mL. Before cell seeding, the hydrogel scaffolds were stabilized at 4 °C to maximize cell seeding efficiency, and after cell seeding, the temperature was gradually returned to 37 °C to minimize spontaneous release of Nalm-6 cells during volumetric contraction (Figure 5a). Confocal images were taken every 2 days up to 1 week by detecting endogenous DsRed and GFP in osteoblasts and Nalm-6 cells, respectively. Reconstructed confocal images showed progressive expansion of Nalm-6 cells and subsequent densification in ICC pores (Figure 5b, Supplementary Video 4). At the end of the study, the temperature was reduced abruptly by adding ice-cold PBS to release pore-entrapped Nalm-6 cells while leaving behind stromal osteospheroids. The temperature was then suddenly increased by adding pre-warmed PBS to further release Nalm-6 cells during ICC pore contraction. This abrupt temperature change was repeated 3 cycles. Few osteospheroids smaller than the interconnecting junctions were released during this process. Quantification of released Nalm-6 cells confirmed about a 6-fold increase in cell number with 84 ± 6% viability over a 1-week period (Figure 5c). Our proof-of-concept study demonstrated that thermoresponsive ICC hydrogel scaffolds with osteospheroids recapitulate the anatomical and stromal microenvironment of trabecular bone marrow, supporting expansion and retrieval of hematopoietic-lymphoid cells without mechanical destruction via spatiotemporal control with temperature changes. The established 3D stromal culture is reusable for repeated cell expansion and retrieval, which is expected to advance ex vivo manufacturing hematopoietic-lymphoid cells.
Figure 5.
Expansion and retrieval of Nalm-6 model hematopoietic-lymphoid cells in ICC PNIPAM-NG scaffolds co-cultured with osteospheroids. a) Schematic procedure of experiment to seed, expand, and retrieve GFP Nalm-6 model hematopoietic-lymphoid cells in ICC PNIPAM-NG scaffolds. b) Confocal 3D microscope images of expanding GFP Nalm-6 cells in ICC pores with co-cultured osteospheroids and subsequent in situ densification during 1-week of culture. c) Fold increase of Nalm-6 cells retrieved after 1-week culture in ICC PNIPAM-NG hydrogel scaffolds by leveraging its thermoresponsive properties and rapid response rate. (*P < 0.05)
Discussion
Stimuli-responsive reversible hydrogels have emerged as powerful tools to actively exert spatiotemporal control over cell culture microenvironments, generating new opportunities and applications.[29, 30] For example, thermoresponsive PNIPAM hydrogels allow for detachment of epithelial layers without disrupting cellular junctions and deposited ECM proteins by switching the interactions cells experience at the cell-biomaterial interface as a function of temperature.[31, 32] Retrieved intact corneal epithelial cell sheets rapidly adhered to host tissue without the need for sutures and were able to be functionally integrated.[33] Zwitterionic hydrogels containing metalloproteinase-cleavable motifs have demonstrated improved ex vivo expansion of hematopoietic stem cells (HSCs) by reducing reactive oxygen species generation while simultaneously facilitating the retrieval of expanded cells by degradation of the crosslinked hydrogel matrix.[34] Inspired by these successes, we have created thermoresponsive ICC hydrogel scaffolds that undergo temperature-dependent rapid, substantial, and reversible volume change to facilitate the retrieval of expanded hematopoietic- lymphoid cells without mechanical destruction. The key success of our approach is the strategic integration of nanostructured PNIPAM hydrogel synthesis into colloidal crystal template-based ICC scaffold fabrication. Nanogel-based crosslinking and open porous ICC geometry substantially increased mechanical flexibility and response rate by permitting convective mass transport and diffusion together. This in turn greatly improved percolation of water molecules throughout the scaffold and accelerated the response rate. Our results demonstrate that optimizing 3D architecture and internal structural design of PNIPAM hydrogels provides an orthogonal opportunity to improve the kinetics and dynamics via highly interconnected macropores.
Establishment of osteospheroids using bone chips represents two distinct advantages in modeling the trabecular bone marrow niche in ICC PNIPAM-NG. First, bone chips provide relevant ECM complexity to reproduce trabecular bone nodules. Bone chips have been previously demonstrated to have high osteoinduction, osteoconduction, and are commercially implanted as grafts to provide a scaffold for bone growth and remodeling.[35] Second, inclusion of bone chips increases the size of osteospheroids in a simple and robust manner while reducing the burden of expanding large numbers of osteoblasts for 3D culture in order to reach a critical size necessary for their geometric entrapment in ICC spherical pore cavities. Geometric entrapment of osteospheroids within the spherical pore cavities allows for vigorous liquid handling to retrieve expanded hematopoietic-lymphoid cells while allowing for reusability of the established tissue microenvironment platform.
Bone marrow stromal cells sense and respond to their surrounding biophysiochemical milieu.[36, 37] Similarly, HSCs sense and adapt to their local mechanical microenvironments in the trabecular bone marrow which has been shown to be instrumental in determining early lineage commitment.[38, 39] Furthermore, it has been well documented that HSC activity is functionally linked to bone forming osteoblasts that reside mainly on unmineralized local bony surfaces known as the osteoid.[40] Expanding hematopoietic cells experience changes in their mechanical environment due to their proximity to the osteoid, which could be an important route to potentiate hematopoietic cellular processes including quiescence, proliferation, and differentiation.[41, 42] In this line, the similar characteristic Young’s modulus of ICC PNIPAM-NG at 37 °C (E = 47 kPa) to the osteoid (E = 34 kPa) is expected to have functional correlation to direct the fate of hematopoietic cells, which is an important topic for future studies.[43]
Our proof-of-concept study demonstrates the feasibility of expansion and retrieval of hematopoietic-lymphoid cells without termination of established trabecular bone marrow tissue models and resultant reusability for repeated expansion. This ability opens the door for advanced biomanufacturing of a wide array of hematopoietic-lymphoid cells. Ex vivo expansion of HSCs, T and B lymphocytes has long been pursued to broaden their therapeutic potential.[44–50] For example, HSC transplantation is the most advanced and successful stem cell therapy. However, it is limited due to insufficient number of donors and clinical-grade quantities available for therapeutic use. Thus, intense efforts have been dedicated to expand them ex vivo.[51, 52] Activation and expansion of T lymphocytes while preserving their intrinsic immune function would benefit cell-based therapies used in the clinic, such as chimeric antigen receptor T lymphocytes which have demonstrated efficacy to treat hematological cancers and immunological disorders.[53–56] Ex vivo manipulation and expansion of B-lymphocytes that synthesize immunoglobulins and antibodies is another important target to leverage biomaterial-directed clinical research.[57, 58] The recapitulation of 3D tissue architecture and tissue-specific stromal cells found in the niche are essential to capturing key cellular processes which support hematopoietic-lymphoid cells and are necessary to further the development of blood-based therapeutics.[59–61] The present study demonstrates the potential of biomaterial strategies in the engineering of scalable, well-defined 3D co-culture platforms to expand hematopoietic-lymphoid cells.
In summary, ICC PNIPAM-NG scaffolds with geometrically entrapped osteospheroids mimic several structural and mechanical aspects that permits recapitulation of bone marrow niche functions, providing an environment which enables the expansion and retrieval of model hematopoietic cells via a transient temperature switch. This platform allows for continuation of the established stromal culture for repeated multicellular co-culture and expansion. We envision that thermoresponsive ICC hydrogel scaffolds will advance biomimetic translational lymphoid tissue engineering by supporting efficient, scalable, and sustainable biomanufacturing of hematopoietic-lymphoid cells to realize their therapeutic potential and widespread accessibility to patients.
Supplementary Material
Supplementary Video 2. Rapid swelling and deswelling of ICC PNIPAM-NG under abrupt temperature changes between 37 °C and 4 °C.
Supplementary Video 1. Cyclic deswelling and swelling of PNIPM-NG, ICC PNIPAM, ICC PNIPAM-NG under steady-state temperature changes between 50 °C and 4 °C.
Supplementary Video 3. Confocal z-series of homogenously seeded osteoblasts in ICC PNIPAM-NG.
Supplementary Video 4. Confocal z-series of DsRed osteospheroids and expanding eGFP Nalm-6 cells in ICC PNIPAM-NG at Day 1, 3, 5 and 7.
Acknowledgements
This work was supported by the National Cancer Institute (R00 CA163671 & R01 CA237171) and the Institute for Applied Life Sciences. We thank Dr. Shelly Peyton for access to her confocal microscope.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
References
- 1.Lee J; Cuddihy MJ; Kotov NA, Tissue Eng Pt B-Rev 2008, 14 (1), 61–86. [DOI] [PubMed] [Google Scholar]
- 2.Hussey GS; Dziki JL; Badylak SF, Nat Rev Mater 2018, 3 (7), 159–173. [Google Scholar]
- 3.Nichols JE; Cortiella JQ; Lee J; Niles JA; Cuddihy M; Wang SP; Bielitzki J; Cantu A; Mlcak R; Valdivia E; Yancy R; McClure ML; Kotov NA, Biomaterials 2009, 30 (6), 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J; Kotov NA, Small 2009, 5 (9), 1008–1013. [DOI] [PubMed] [Google Scholar]
- 5.Stachowiak AN; Irvine DJ, Journal of Biomedical Materials Research Part A 2008, 85a (3), 815–828. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YS; Zhu CL; Xia YN, Advanced Materials 2017, 29 (33). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim CH, J Blood Med 2010, 1, 13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crispe IN, Annual Review of Immunology 2009, 27, 147–163. [DOI] [PubMed] [Google Scholar]
- 9.Jenne CN; Kubes P, Nat Immunol 2013, 14 (10), 996–1006. [DOI] [PubMed] [Google Scholar]
- 10.Lee J; Cuddihy MJ; Cater GM; Kotov NA, Biomaterials 2009, 30 (27), 4687–4694. [DOI] [PubMed] [Google Scholar]
- 11.Shirahama H; Kumar SK; Jeon WY; Kim MH; Lee JH; Ng SS; Tabaei SR; Cho NJ, Jove-J Vis Exp 2016, (114). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y; Kim MH; Shirahama H; Lee JH; Ng SS; Glenn JS; Cho NJ, Sci Rep-Uk 2016, 6, 37427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng SS; Saeb-Parsy K; Blackford SJI; Segal JM; Serra MP; Horcas-Lopez M; No DY; Mastoridis S; Jassem W; Frank CW; Cho NJ; Nakauchi H; Glenn JS; Rashid ST, Biomaterials 2018, 182, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu LY; Ferracci G; Wang Y; Fan TF; Cho NJ; Chow PKH, Rsc Adv 2019, 9 (31), 17995–18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvi LM; Adams GB; Weibrecht KW; Weber JM; Olson DP; Knight MC; Martin RP; Schipani E; Divieti P; Bringhurst FR; Milner LA; Kronenberg HM; Scadden DT, Nature 2003, 425 (6960), 841–846. [DOI] [PubMed] [Google Scholar]
- 16.Morrison SJ; Scadden DT Nature 2014, (7483), 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagasawa T, Nature Reviews Immunology 2006, 6 (2), 107–116. [DOI] [PubMed] [Google Scholar]
- 18.Morrison SJ; Spradling AC, Cell 2008, 132 (4), 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols JE; Cordella J; Lee J; Niles JA; Cuddihy M; Wang S; Bielitzki J; Cantu A; Mlcak R; Valdivia E; Yancy R; McClure ML; Kotov NA, Biomaterials 2009, 30 (6), 1071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y; Xia YN, Advanced Functional Materials 2012, 22 (1), 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galperin A; Long TJ; Ratner BD, Biomacromolecules 2010, 11 (10), 2583–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y; Jiang H; Ye SH; Yoshizumi T; Wagner WR, Biomaterials 2015, 53, 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellati A; Kiamahalleh MV; Madani SH; Dai S; Bi JX; Jin B; Zhang H, Journal of Biomedical Materials Research Part A 2016, 104 (11), 2764–2774. [DOI] [PubMed] [Google Scholar]
- 24.Xia LW; Xie R; Ju XJ; Wang W; Chen QM; Chu LY, Nature Communications 2013, 4, 2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YC; Bahng JH; Kotov NA, J Mater Res 2019, 34 (8), 1371–1380. [Google Scholar]
- 26.Isern J; Martin-Antonio B; Ghazanfari R; Martin AM; Lopez JA; del Toro R; Sanchez-Aguilera A; Arranz L; Martin-Perez D; Suarez-Lledo M; Marin P; Van Pel M; Fibbe WE; Vazquez J; Scheding S; Urbano-Ispizua A; Mendez-Ferrer S, Cell Reports 2013, 3 (5), 1714–1724. [DOI] [PubMed] [Google Scholar]
- 27.Sart S; Tsai AC; Li Y; Ma T, Tissue Eng Pt B-Rev 2014, 20 (5), 365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J; Lilly GD; Doty RC; Podsiadlo P; Kotov NA, Small 2009, 5 (10), 1213–1221. [DOI] [PubMed] [Google Scholar]
- 29.Seliktar D, Science 2012, 336 (6085), 1124–1128. [DOI] [PubMed] [Google Scholar]
- 30.Stuart MAC; Huck WTS; Genzer J; Muller M; Ober C; Stamm M; Sukhorukov GB; Szleifer I; Tsukruk VV; Urban M; Winnik F; Zauscher S; Luzinov I; Minko S, Nature Materials 2010, 9 (2), 101–113. [DOI] [PubMed] [Google Scholar]
- 31.Da Silva RMP; Mano JF; Reis RL, Trends Biotechnol 2007, 25 (12), 577–583. [DOI] [PubMed] [Google Scholar]
- 32.Nash ME; Healy D; Carroll WM; Elvira C; Rochev YA, Journal of Materials Chemistry 2012, 22 (37), 19376–19389. [Google Scholar]
- 33.Yamato M; Okano T, Mater Today 2004, 7 (5), 42–47. [Google Scholar]
- 34.Bai T; Li J; Sinclair A; Imren S; Merriam F; Sun F; O’Kelly MB; Nourigat C; Jain P; Delrow JJ; Basom RS; Hung HC; Zhang P; Li B; Heimfeld S; Jiang S; Delaney C, Nat Med 2019, 25 (10), 1566–1575. [DOI] [PubMed] [Google Scholar]
- 35.Amini AR; Laurencin CT; Nukavarapu SP, Crit Rev BiomedEng 2012, 40 (5), 363408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swift J; Ivanovska IL; Buxboim A; Harada T; Dingal PCDP; Pinter J; Pajerowski JD; Spinler KR; Shin JW; Tewari M; Rehfeldt F; Speicher DW; Discher DE, Science 2013, 341 (6149), 1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brusatin G; Panciera T; Gandin A; Citron A; Piccolo S, Nat Mater 2018, 17 (12), 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holst J; Watson S; Lord MS; Eamegdool SS; Bax DV; Nivison-Smith LB; Kondyurin A; Ma LA; Oberhauser AF; Weiss AS; Rasko JEJ, Nature Biotechnology 2010, 28 (10), 1123–U168. [DOI] [PubMed] [Google Scholar]
- 39.Lee-Thedieck C; Spatz JP, Biomater Sci-Uk 2014, 2 (12), 1796–1796. [DOI] [PubMed] [Google Scholar]
- 40.Sacchetti B; Funari A; Michienzi S; Di Cesare S; Piersanti S; Saggio I; Tagliafico E; Ferrari S; Robey PG; Riminucci M; Bianco P, Cell 2007, 131 (2), 324–336. [DOI] [PubMed] [Google Scholar]
- 41.Engler AJ; Sen S; Sweeney HL; Discher DE, Cell 2006, 126 (4), 677–689. [DOI] [PubMed] [Google Scholar]
- 42.Shin JW; Buxboim A; Spinler KR; Swift J; Christian DA; Hunter CA; Leon C; Gachet C; Dingal PCDP; Ivanovska IL; Rehfeldt F; Chasis JA; Discher DE, Cell Stem Cell 2014, 14 (1), 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivanovska IL; Shin JW; Swift J; Discher DE, Trends Cell Biol 2015, 25 (9), 523532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee-Thedieck C; Spatz JP, Macromol Rapid Comm 2012, 33 (17), 1432–1438. [DOI] [PubMed] [Google Scholar]
- 45.Bourgine PE; Klein T; Paczulla AM; Shimizu T; Kunz L; Kokkaliaris KD; Coutu DL; Lengerke C; Skoda R; Schroeder T; Martin I, P Natl Acad Sci USA 2018, 115 (25), E5688–E5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortera-Blanco T; Mantalaris A; Bismarck A; Aqel N; Panoskaltsis N, Biomaterials 2011, 32 (35), 9263–9270. [DOI] [PubMed] [Google Scholar]
- 47.Leisten I; Kramann R; Ferreiraa MSV; Bovi M; Neuss S; Ziegler P; Wagner W; Knuchel R; Schneider RK, Biomaterials 2012, 33 (6), 1736–1747. [DOI] [PubMed] [Google Scholar]
- 48.Di Maggio N; Piccinini E; Jaworski M; Trumpp A; Wendt DJ; Martin I, Biomaterials 2011, 32 (2), 321–329. [DOI] [PubMed] [Google Scholar]
- 49.Ferreira MSV; Jahnen-Dechent W; Labude N; Bovi M; Hieronymus T; Zenke M; Schneider RK; Neuss S, Biomaterials 2012, 33 (35), 9165–9165. [DOI] [PubMed] [Google Scholar]
- 50.Gilchrist AE; Lee S; Hu YH; Harley BAC, Adv Healthc Mater 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Lima M; McNiece I; Robinson SN; Munsell M; Eapen M; Horowitz M; Alousi A; Saliba R; McMannis JD; Kaur I; Kebriaei P; Parmar S; Popat U; Hosing C; Champlin R; Bollard C; Molldrem JJ; Jones RB; Nieto Y; Andersson BS; Shah N; Oran B; Cooper LJN; Worth L; Qazilbash MH; Korbling M; Rondon G; Ciurea S; Bosque D; Maewal I; Simmons PJ; Shpall EJ, New England Journal of Medicine 2012, 367 (24), 2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boitano AE; Wang JA; Romeo R; Bouchez LC; Parker AE; Sutton SE; Walker JR; Flaveny CA; Perdew GH; Denison MS; Schultz PG; Cooke MP, Science 2010, 329 (5997), 1345–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poznansky MC; Evans RH; Foxall RB; Olszak IT; Piascik AH; Hartman KE; Brander C; Meyer TH; Pykett MJ; Chabner KT; Kalams SA; Rosenzweig M; Scadden DT, Nature Biotechnology 2000, 18 (7), 729–734. [DOI] [PubMed] [Google Scholar]
- 54.Cheung AS; Zhang DKY; Koshy ST; Mooney DJ, 2018, 36 (2), 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah NJ; Mao AS; Shih TY; Kerr MD; Sharda A; Raimondo TM; Weaver JC; Vrbanac VD; Deruaz M; Tager AM; Mooney DJ; Scadden DT, Nat Biotechnol 2019, 57 (3), 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.June CH; O’Connor RS; Kawalekar OU; Ghassemi S; Milone MC, Science 2018, 559 (6382), 1361–1365. [DOI] [PubMed] [Google Scholar]
- 57.Purwada A; Jaiswal MK; Ahn H; Nojima T; Kitamura D; Gaharwar AK; Cerchietti L; Singh A, Biomaterials 2015, 65, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Purwada A; Singh A, Nat Protoc 2017, 12 (1), 168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S; Shah SB; Graney PL; Singh A, Nat Rev Mater 2019, 4 (6), 355–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baryawno N; Przybylski D; Kowalczyk MS; Kfoury Y; Severe N; Gustafsson K; Kokkaliaris KD; Mercier F; Tabaka M; Hofree M; Dionne D; Papazian A; Lee D; Ashenberg O; Subramanian A; Vaishnav ED; Rozenblatt-Rosen O; Regev A; Scadden DT, Cell 2019, 177 (7), 1915–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aijaz A; Li M; Smith D; Khong D; LeBlon C; Fenton OS; Olabisi RM; Libutti S; Tischfield J; Maus MV; Deans R; Barcia RN; Anderson DG; Ritz J; Preti R; Parekkadan B, Nature Biomedical Engineering 2018, 2 (6), 362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carpenter RA; Kwak JG; Peyton SR; Lee J, Nature Biomedical Engineering 2018, 2 (12), 915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carpenter R; Oh HJ; Ham IH; Kim D; Hur H; Lee J, Acs Biomater Sci Eng 2019, 5 (12), 6667–6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 2. Rapid swelling and deswelling of ICC PNIPAM-NG under abrupt temperature changes between 37 °C and 4 °C.
Supplementary Video 1. Cyclic deswelling and swelling of PNIPM-NG, ICC PNIPAM, ICC PNIPAM-NG under steady-state temperature changes between 50 °C and 4 °C.
Supplementary Video 3. Confocal z-series of homogenously seeded osteoblasts in ICC PNIPAM-NG.
Supplementary Video 4. Confocal z-series of DsRed osteospheroids and expanding eGFP Nalm-6 cells in ICC PNIPAM-NG at Day 1, 3, 5 and 7.