Abstract
Organophosphate esters (OPEs) are a group of chemicals used as flame retardants and plasticizers that replaced polybrominated diphenyl ethers in consumer products such as furniture and electronics. To characterize exposure to OPEs during fetal development, we measured urinary OPE metabolite concentrations in women twice during pregnancy (16 and 26 weeks’ gestation) and at delivery (n=357). We also previously quantified house dust OPE parent compound concentrations at 20 weeks’ gestation (n=317). Diphenyl phosphate (DPHP) had the highest geometric mean urinary concentrations (1.5-2.3 μg/g creatinine), followed by bis(1,3-dichloro-2-propyl) phosphate (BDCIPP; 0.75-0.99 μg/g creatinine), and bis(2-chloroethyl) phosphate (BCEP; 0.72-0.97 μg/g creatinine), while dibutyl phosphate (DNBP) had the lowest concentrations (0.25-0.28 μg/g creatinine). Urinary OPE metabolites were moderately correlated with each other at 26 weeks (rs: 0.23-0.38, p<0.001) while the correlations at 16 weeks and delivery were slightly weaker. Intra-class correlations for urinary metabolites measured at three time points were poor (0.16-0.34), indicating high variability within individuals. Dust concentrations of OPE parent compounds were associated with BCEP, BDCIPP, and DPHP concentrations in urine at some but not all time points. In linear mixed models of urinary OPE metabolite concentrations, household size was inversely associated with BCEP concentrations, and being non-white was associated with lower BDCIPP and DPHP concentrations. Urine samples collected in the summer had the highest OPE metabolite concentrations. This study highlights the need to collect multiple urine samples during pregnancy to define exposure patterns and investigate potential periods of susceptibility.
Keywords: organophosphate esters, pregnancy, cohort study, urinary metabolites
1. Introduction
Polybrominated diphenyl ethers (PBDE) were one of the most common flame retardant chemicals added to consumer products such as furniture, electronics, and textiles to delay ignition times and slow burn rates. Starting in the mid-2000s, manufacturers began a voluntary phase-out of Penta-BDE and Octa-BDE formulations due to evidence of toxicity in experimental animals and emerging concerns of human toxicity (US Environmental Protection Agency, 2006). Since then, replacement chemicals, such as organophosphate esters (OPEs), have been added to consumer products (van der Veen and de Boer, 2012). OPEs are now routinely detected in indoor dust, migrating from the products on which they are applied and originating from some flooring types (Dodson et al., 2012; Wei et al., 2015). Accidental ingestion of dust is thought to be the primary exposure pathway for OPEs, followed by inhalation and dermal absorption (Kim et al., 2019; Schreder et al., 2016a). Dust concentrations of OPEs have previously been correlated to internal concentrations of urinary OPE metabolites (Carignan et al., 2013; Dodson et al., 2014), however, there is limited data on exposure patterns during pregnancy.
Pregnancy is a potential period of susceptibility to environmental toxicants for both women and their fetuses (Selevan et al., 2000). Although there is scarce human toxicity data for OPEs, experimental studies of these chemicals have garnered concern for potential endocrine disruption (Kojima et al., 2013), reproductive toxicity (Liu et al., 2013), and neurotoxicity (Dishaw et al., 2014). Tris (1,3,-dichloro-2-propyl) phosphate (TDCIPP) is one of the most commonly used OPEs and a suspected carcinogen (Gold et al., 1978; W. Li et al., 2019).
Additionally, we recently observed another OPE, triphenyl phosphate (TPHP), was detectable in 100% of the homes of pregnant women (Percy et al., 2020). In a small study from Massachusetts, higher concentrations of the metabolite of TPHP, diphenyl phosphate (DPHP), was detected in women than men, and it was associated with thyroid hormone disruption (Preston et al., 2017).
While other studies have assessed OPE exposure in pregnant women (Castorina et al., 2017; Hoffman et al., 2017; Romano et al., 2017), significant gaps in our knowledge remain about exposure routes and potential health impact. The relationship of dust OPE levels, and sociodemographic and housing factors with urinary OPE concentrations has not been well-characterized in pregnant women and most existing studies have focused on white women. Moreover, the within- and between-person variability of OPE metabolites is still unclear.
The goals of this study were to: 1) characterize pregnant women’s exposure to OPEs by measuring metabolites of these chemicals in urine samples during pregnancy and at delivery, 2) quantify the within- and between-person variation in urinary OPE metabolite concentrations, and 3) identify factors associated with urinary OPE metabolite concentrations, including levels of corresponding parent OPEs in house dust.
2. Materials and methods
2.1. Subjects
In Cincinnati, Ohio, between March 2003 and February 2006, 468 pregnant women were enrolled in a prospective pregnancy and birth cohort: the Health Outcomes and Measures of the Environment (HOME) Study. Detailed enrollment criteria are described elsewhere (Braun et al., 2017). We considered women eligible if they were 1) ≥18 years of age, 2) at 16 ±3 weeks’ gestation, and 3) living in a home built before 1978 (related to the original randomized trial to reduce residential lead and injury hazards) (Braun et al., 2018). We excluded women if they were: HIV positive; taking medication for seizures or thyroid disorders; not fluent in English; planning to move outside the Greater Cincinnati area; or having a diagnosis of bipolar disorder, schizophrenia, diabetes, or cancer that required radiation or chemotherapy. Our final sample included participants who provided at least one urine specimen during pregnancy that was analyzed for flame retardant metabolites (n=357). All women gave informed consent for themselves and their children, and the Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) approved the study protocol. The Centers for Disease Control and Prevention (CDC) deferred to the CCHMC IRB as the IRB of record.
2.2. Urinary OPE Metabolites
At approximately 16 and 26 weeks of gestation, and within 48 hours of delivery, women provided urine samples in polypropylene specimen cups. All samples were refrigerated for up to 24 hours before processing when they were frozen, stored, and shipped on dry ice to the CDC’s National Center for Environmental Health Laboratory where they were stored at −80°C until analysis. OPEs, like many other environmental chemicals, appear to be stable in urine when stored at subfreezing temperatures (Carignan et al., 2017; Gika et al., 2008; Laparre et al., 2017; Rotter et al., 2017). Therefore, OPE concentrations measured for this study are expected to accurately reflect the concentrations at the time the urine was collected.
A modification of a previously described technique was used to quantify four urinary OPE metabolites: bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), bis-2-chloroethyl phosphate (BCEP), DPHP, and di-n-butyl phosphate (DNBP). Briefly, after enzymatic hydrolysis of the metabolites’ conjugates in 200 μL of urine, the target metabolites underwent automated off-line solid-phase extraction, separation via reversed-phase high-performance liquid chromatography, and detection by isotope dilution-electrospray ionization tandem mass spectrometry (Jayatilaka et al., 2019, 2017). The limit of detection (LOD) was 0.10 μg/L for all metabolites, accuracy ranged from 98 to 108%, and intra- and inter-day imprecision was <10%. The CDC laboratory is certified by CLIA. For example, besides the study samples, each analytical run included calibration standards, two high- and two low-concentration quality control (QC) materials (prepared using pooled human urine), and blanks to ensure data accuracy and reliability. The duplicate QC concentrations were averaged and evaluated using standard statistical probability rules (Caudill et al., 2008). If the QC samples failed the statistical evaluation, all of the study samples in the run were re-extracted. We quantified creatinine using a modified Jaffe-kinetic method and adjusted for inter-individual differences in urine dilution by dividing each urinary metabolite concentration by urinary creatinine concentration.
2.3. Dust OPEs
To collect house dust samples, staff used a High Volume Surface Sampler during a home visit at approximately 20 weeks’ pregnancy. Samples were collected from a 1 m2 area of the main activity room floor. Staff also noted visible cleanliness of the room (appears clean, some evidence of housecleaning, or no evidence of housecleaning) and recorded flooring type (carpet: low, medium, or high pile; wood; tile; vinyl or linoleum) in the room.
Archived samples were shipped to the Virginia Institute of Marine Sciences, William & Mary for analysis of TPHP, TDCIPP, and tris(2-chloroethyl) phosphate (TCEP) concentrations. We sieved dust at 300 μm, and ~100 mg was subjected to accelerated solvent extraction. Extracts were purified using size exclusion and solid-phase chromatography. Purified extracts were analyzed by ultra high-performance liquid chromatography mass spectrometry to determine concentrations of individual analytes. The LOD for all analytes was 100 ng/g. Additional details about analytical and quality control methods for house dust samples are described elsewhere (La Guardia and Hale, 2015; Percy et al., 2020).
2.4. Predictors of Urinary OPE Concentrations
We obtained pregnant women’s weight and time of weight measurement data via chart review, which we used to estimate women’s weight at the time of dust sample collection. Staff collected sociodemographic information such as race, income, and education via standardized questionnaires and interviews administered during the 2nd or 3rd trimester.
2.5. Statistical analysis
For urinary metabolites, we replaced concentrations below the LOD (9.7%) with (Hornung and Reed, 1990). BCEP, BDCIPP, DNBP, and DPHP were measured <LOD in 135 (13.3%), 74 (7.3%), 266 (26.2%), and 15 (1.5%) samples, respectively. For house dust, we also replaced concentrations <LOD with (n=70 or 3.9% of all values). TCEP, TDCIPP, and TPHP were measured <LOD in 48 (15.1%), 8 (2.5%), and 3 (0.9%) samples, respectively. All urinary metabolite and dust analyte values were natural log transformed to reach approximate normal distribution.
For data analysis, we calculated univariate statistics of urinary OPE metabolite concentrations in pregnant women at three time points to characterize exposure patterns and variability. We also used multivariable linear regression models to identify factors associated with OPE metabolite concentrations, including the corresponding OPE parent compound in house dust samples.
Intraclass correlation (ICC) for urinary metabolite concentrations across the three time points were calculated using crude and creatinine-standardized values from linear mixed effect models. We applied ICC cut-offs described by Rosner to assess reproducibility: ≤0.4 is poor, 0.4-0.75 is fair to good, and ≥0.75 is excellent (Rosner, 2011). Additionally, we calculated pairwise Spearman correlation coefficients between urinary metabolites at each time point.
In addition to dust OPE concentrations, we calculated floor dust loading (ng/m2) by multiplying the concentration of each parent compound in the dust by the sieved dust (< 300 μm) weight and dividing by the sampling area. Further, we estimated daily personal exposure to parent compounds (ng OPE /kg body weight/day) for each participant using their weight and home sampling data assuming US Environmental Protection Agency estimates of adult average daily dust ingestion at 20 mg,(U S Environmental Protection Agency, 2017) assuming 100% bioavailability (Jones-Otazo et al., 2005). When we did not have a weight taken within one week of the dust sample collection, we created an estimate using the slope of weight gain during pregnancy, which is assumed to be linear after 12 weeks of gestation (National Research Council 2009, 2009).
To estimate the relation between dust OPEs parent compounds and corresponding urinary metabolites across multiple time points, we used linear mixed effect models with the main effect of dust OPEs and its interaction term with the time points of urinary metabolite measurement. For each urinary metabolite, we examined dust as a predictor three ways: 1) concentration in ng/g, 2) loading in ng/m2, and 3) estimated daily ingestion in ng/kg/day. We tested interaction terms for dust parent compound measurement and time point of urinary metabolite measurement at a significance level of p<0.2. Covariates were chosen a priori based on our previous analysis of dust flame retardant exposure: number of people living in the home, season of urine collection, race (white or non-white), year of delivery, education, smoking status, age, income, visible cleanliness (clean or not clean), and floor type (hard floor or carpet) (Percy et al., 2020).
We used R version 3.6.1 (R Core Team, Vienna, Austria) for all analyses, including packages EnvStats (Millard, 2013), lme4 (Bates et al., 2015), sjstats (Lukecke, 2019), and emmeans (Lenth, 2019).
3. Results and Discussion
3.1. Urinary OPE metabolite concentrations
Among HOME Study women, 357 provided at least one urine sample during pregnancy, and 86% provided all three samples. The cohort was 65.5% white, 97.8% non-Hispanic, 77% of women obtained education beyond high school, and they were an average of 29.7 years old at delivery (Supplemental Table 1). The participants eligible for this study were not significantly different from the complete cohort of 398 women (Braun et al., 2017).
Urinary OPE metabolite concentrations were log-normally distributed, and DPHP consistently had the highest concentration of all the analytes (Figure 1). DNBP had the lowest detection frequencies (59.9-83.0%), and DPHP had the highest (97.4-99.4%). Geometric mean (GM) concentration ranges at the three collection times were 0.72-0.97 μg/g creatinine (BCEP), 0.75-0.99 μg/g creatinine (BDCIPP), 0.25-0.28 μg/g creatinine (DNBP), and 1.50-2.32 μg/g creatinine (DPHP) (Figure 1, Supplemental Table 2). Results for BDCIPP and DNBP are similar to those reported for the 2013-2014 National Health and Nutrition Examination Survey (NHANES) for females and participants 20-59 years old; however, we found approximately two times higher concentrations of BCEP and DPHP in our subjects (Ospina et al., 2018). Our detection rates are higher than those reported in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort from California (Castorina et al., 2017), and similar to those reported in women residing in Rhode Island and North Carolina (Hoffman et al., 2014; Romano et al., 2017) (Supplemental Table 3).
Figure 1:
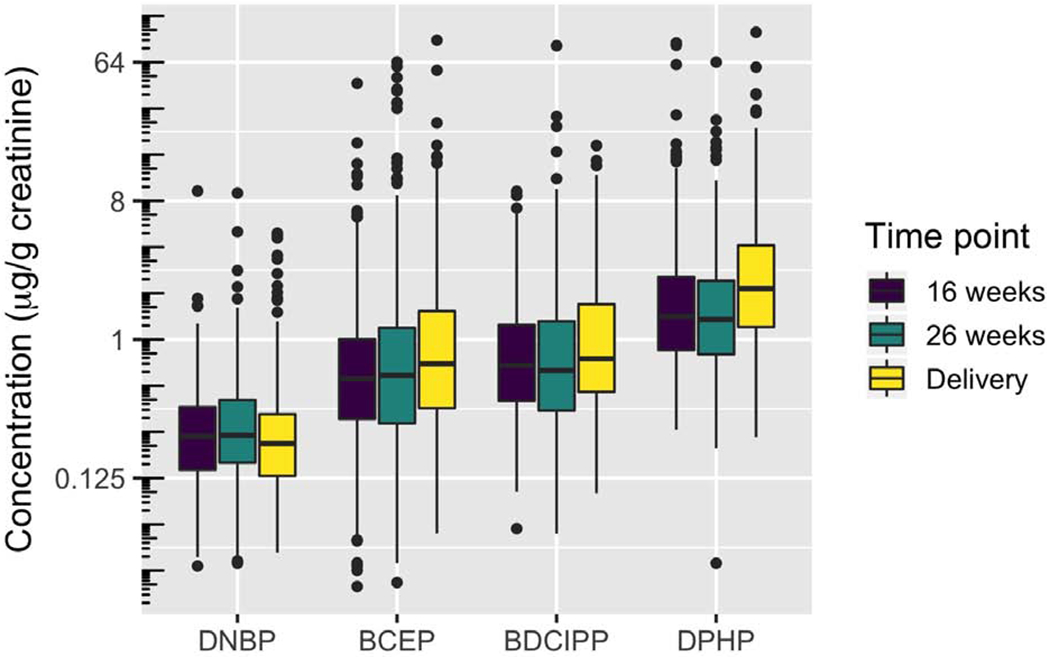
Creatinine-standardized distributions of OPFR urinary metabolites by time point, ordered by analyte median.
Compared to the California women, our subjects had 2.7-3.5 times higher urinary GM concentrations of BDCIPP and 1.6-2.5 times higher GM concentrations of DPHP (Castorina et al., 2017). The discrepancies may be due to differences in the sampling time frame—our recruitment took place about four years after the CHAMACOS Study. Housing and floor characteristics, cleaning frequencies, and dust exposure amount may also differ between the two studies. California also has had historically stricter flame retardancy standards than other parts of the United States, which could result in regional consumer products that had different OPE profiles than those in Ohio, and different potential exposures for the women in each study. Additionally, the differences could be due to genetic variations in OPE metabolism. The CHAMACOS cohort is predominantly comprised of Hispanic women, while the HOME Study consists of about 65% non-Hispanic white women, with most of the remainder identifying as non-Hispanic black. It is not currently known how much genetic variation contributes to OPE metabolism and urinary concentrations of metabolites. However, there is evidence that polymorphisms in human liver enzymes can change the metabolism of phthalates, another class of environmental toxicant (Ito et al., 2014).
3.2. OPE temporal variability
The ICCs for urinary metabolites of OPEs indicate low reproducibility in the latter two-thirds of pregnancy (Table 1). Creatinine-standardized ICCs were slightly lower than crude values for all metabolites, and they ranged from 0.16 (DPHP) to 0.34 (BCEP). Poor reproducibility indicates that a single measurement during pregnancy may not accurately estimate exposure to OPEs throughout pregnancy (Perrier et al., 2016).
Table 1:
Intra-class correlations (ICC) of OPE urinary metabolites. Measurements were taken at 16 weeks pregnancy, 26 weeks pregnancy, and delivery. Crude values represent non-creatinine standardized concentrations.
| Urinary metabolite | Crude ICC | Creatinine-standardized ICC |
|---|---|---|
| BCEP | 0.40 | 0.34 |
| BDCIPP | 0.38 | 0.36 |
| DNBP | 0.25 | 0.20 |
| DPHP | 0.24 | 0.16 |
Studies on the toxicokinetic properties of OPEs are limited, but they are consistent with the half-lives of these compounds being only about a few hours (van der Veen and de Boer, 2012). Rapid metabolism and excretion would mean that a single urinary measurement would capture only recent exposure, and poor reliability between measurements suggests episodic exposure. Additionally, pregnant women experience plasma volume expansion and altered renal function, including increased renal blood flow and glomerular filtration rate and differences in selective filtration and reabsorption of waste substances (Cheung and Lafayette, 2013). These changes in the way urine is produced throughout pregnancy and in comparison to the non-pregnant state could lead to increased variation in OPE metabolite concentrations. For example, Meeker et al. reported moderate to strong reliability between measurements of urinary OPE metabolites, but those subjects were adult men who were not experiencing significant physiological changes between sampling times (Meeker et al., 2013). Both Romano et al. (Rhode Island) and Hoffman et al. (North Carolina) studied pregnant women and found fair to good reproducibility for urinary OPE concentrations (Hoffman et al., 2014; Romano et al., 2017). These findings of better reproducibility could be due to more stable exposure sources in their populations, later years of sampling, or higher population homogeneity. Additionally, the sample sizes were comparatively small in both the Romano et al. and Hoffman et al. studies (n=59 and n=8, respectively), which limits the dependability of those calculations.
3.3. Factors associated with urinary OPE metabolites
For all models, results without creatinine standardization were not different from creatinine-standardized results, therefore we only present the creatinine-standardized models. We chose to retain the interaction term between dust parent compound concentration and urine collection time point as p-values for the term were <0.2 and ICCs for the urine measurements were poor. Retaining the interaction term enabled us to separately estimate associations between a single dust measurement and urinary OPE concentrations at each time during pregnancy and at delivery.
Table 2 shows results for the models that include concentration of parent compound in dust, urine collection time points, their interactions, and select sociodemographic and housing characteristics. Each natural log-unit TCEP concentration increase in house dust was associated with a 15% higher concentration of urinary BCEP at 26 weeks (95% CI: 4-27%) and a 12% higher concentration at delivery (95% CI: 2-24%). Each natural log-unit increase in TDCIPP in house dust was associated with a 14% higher concentration of urinary BDCIPP at 26 weeks’ gestation (95% CI: 4-26%). Each natural log-unit increase in TPHP house dust concentrations was associated with 11% higher concentration of urinary DPHP at 16 weeks of gestation (95% CI: 1-22%). Recent work has shown that OPE parent compounds and their metabolites can co-exist in both house dust and food products (He et al., 2018; Tan et al., 2019), which may indicate that the source of urinary OPE metabolites in our participants were either environmental parent compounds or environmental metabolites. Additionally, the sporadic associations between house dust OPEs and urinary OPE metabolites may indicate that OPE exposure in this population is from multiple sources such as drinking water, indoor air, or food (Kim and Kannan, 2018; J. Li et al., 2019; Wong et al., 2018). Our results suggest that house dust is likely one source of exposure to TCEP, TDCIPP, and TPHP, although direct exposure to metabolites via house dust or other sources may explain why associations with urinary OPE metabolites were not stronger.
Table 2:
Adjusted associations between creatinine-standardized urinary OPE metabolite concentrations and personal characteristics, including parent compound dust concentration.
| Model Variables | BCEP |
BDCIPP |
DPHP |
|||
|---|---|---|---|---|---|---|
| CR | 95% CI | CR | 95% CI | CR | 95% CI | |
| Number of people residing in home | 0.89 | 0.81-0.98 | 0.94 | 0.87-1.03 | 0.99 | 0.92-1.07 |
| Natural log increase in dust concentration of parent compound | ||||||
| 16 week urine | 1.02 | 0.93-1.12 | 1.07 | 0.97-1.18 | 1.11 | 1.01-1.22 |
| 26 week urine | 1.15 | 1.04-1.27 | 1.14 | 1.04-1.26 | 1.03 | 0.93-1.14 |
| Delivery urine | 1.12 | 1.02-1.24 | 1.05 | 0.95-1.16 | 0.98 | 0.88-1.09 |
| Season of urine collection | ||||||
| Winter (Dec-Feb) | -- | -- | -- | -- | -- | -- |
| Spring (Mar-May) | 1.23 | 1.00-1.52 | 1.27 | 1.08-1.49 | 1.21 | 1.00-1.46 |
| Summer (Jun-Aug) | 2.39 | 1.94-2.96 | 2.59 | 2.20-3.06 | 1.45 | 1.20-1.76 |
| Fall (Sep-Nov) | 1.52 | 1.24-1.86 | 1.44 | 1.23-1.69 | 1.06 | 0.89-1.28 |
| Race | ||||||
| White | -- | -- | -- | -- | -- | -- |
| Non-white | 1.03 | 0.78-1.38 | 0.76 | 0.59-0.98 | 0.77 | 0.61-0.97 |
| Year | ||||||
| 2003 | -- | -- | -- | -- | -- | -- |
| 2004 | 1.45 | 0.94-2.24 | 0.88 | 0.60-1.29 | 0.91 | 0.65-1.28 |
| 2005 | 1.53 | 0.98-2.39 | 1.03 | 0.70-1.51 | 0.88 | 0.62-1.25 |
| 2006 | 1.51 | 0.92-2.46 | 1.16 | 0.75-1.78 | 0.92 | 0.62-1.35 |
| Education | ||||||
| High school or less | -- | -- | -- | -- | -- | -- |
| Some college | 1.06 | 0.75-1.49 | 0.93 | 0.69-1.26 | 1.00 | 0.75-1.34 |
| Bachelor’s degree | 1.13 | 0.77-1.66 | 0.97 | 0.70-1.35 | 1.05 | 0.77-1.44 |
| Graduate degree | 1.07 | 0.71-1.61 | 0.99 | 0.70-1.42 | 1.07 | 0.77-1.48 |
| Smoking status | ||||||
| Non-smoker | -- | -- | -- | -- | -- | -- |
| Smoker | 0.87 | 0.56-1.34 | 1.02 | 0.70-1.49 | 1.08 | 0.75-1.54 |
BCEP model includes TCEP, BDCIPP model includes TDCIPP, and DPHP model includes TPHP as dust concentration predictor. Models are also adjusted for age, income, cleanliness, and floor type. CR: concentration ratio by independent variables (i.e., exponentiated regression coefficient), CI: confidence interval
In all models, the season of urine collection was a strong predictor of OPE metabolite urinary concentrations. Compared to winter, urinary BCEP, BDCIPP, and DPHP concentrations were 2.39 times (95% CI: 1.94-2.96), 2.59 times (95% CI: 2.20-3.06), and 1.45 times (95% CI: 1.20-1.76) higher than in the summer, respectively. Hoffman et al. reported a similar pattern of results in North Carolina women: samples taken in summer months had a ~4-fold higher BDCIPP and a 1.6-fold higher DPHP compared to winter months. They speculated that increased outdoor temperatures caused the chemicals to reside in the air to a greater degree and thus be inhaled more readily than in the winter (Hoffman et al., 2017). Average summer temperatures in Chapel Hill, North Carolina from 2002-2005 were 5.2 °F higher than Cincinnati, Ohio from 2003-2005; however, the difference between summer and winter temperatures was greater in Cincinnati (Supplemental Table 4). Higher summer temperatures in North Carolina may explain the greater degree of seasonal variation of OPE urinary metabolites seen in that study. However, one would expect the larger difference in summer-to-winter temperatures that we observed in Cincinnati to also produce seasonal variation in urinary metabolites. The larger effect sizes reported from North Carolina could suggest that OPEs volatilize more at a higher temperature.
Year of sample collection, education, smoking status, age, income, cleanliness, and floor type were not statistically associated with creatinine-standardized BCEP, BDCIPP, or DPHP concentrations. Each additional person living in the home was associated with a 12% decrease in urinary BCEP concentrations, which may be due to sharing of dust OPE burden by additional members in the family, lowering individual exposures to parent compounds. BDCIPP and DPHP concentrations were ~30% lower among non-white women, which we speculate reflects the composition of consumer products present in non-white women’s homes during the study period. In 2004, the technical products Penta- and Octa-PBDEs were being phased out of consumer products, and OPEs were being added at increasing rates so products purchased during or after this time would have been more likely to contain OPEs than older products. We observed a trend for increasing concentrations of urinary BCEP by year of sample collection from 2003-2006, despite its non-significance in models.
Results from models using parent compound dust loading were similar to the dust concentration models (Supplemental Table 5). We created a third set of models in which the estimated daily intake of parent compounds via dust was a predictor (Supplemental Table 6). Estimated daily ingestion values considered participant body weight, and these calculations were used for risk estimation associated with the exposure. However, we found that they were just as predictive of urinary OPE metabolite concentrations as parent compound dust concentrations.
Because dust concentration, dust loading, and estimated daily dust ingestion are measured on different scales and are not directly comparable, we calculated the expected ratio change in urine metabolite concentration per interquartile range (IQR) increase in dust parent compound concentration, loading, and ingestion (Table 3). We observed more significant associations between dust concentration and estimated daily ingestion with urinary OPE metabolites compared to dust loading as a predictor.
Table 3:
Ratio difference in urinary OPE metabolite concentration per interquartile range (IQR) increase in dust OPE parent compound concentration and loading
| Compounds | Time point | Dust concentration (95% CI) | Dust loading (95% CI) | Estimated Ingestion (95% CI) |
|---|---|---|---|---|
| TCEP ➔ BCEP | (Dust IQR) | 8.2-fold increase (237-1950 ng/g) | 10.6-fold increase (53-562 ng/m2) | 9.6-fold increase (0.06-0.57 ng/kg/day) |
| 16 week metabolite | 1.04 (0.84-1.28) | 1.03 (0.85-1.23) | 1.01 (0.81-1.26) | |
| 26 week metabolite | 1.39 (1.12-1.72) | 1.10 (0.92-1.33) | 1.32 (1.08-1.70) | |
| Delivery metabolite | 1.30 (1.04-1.63) | 1.21 (0.99-1.48) | 1.27 (1.01-1.60) | |
| TDCIPP ➔ BDCIPP | (Dust IQR) | 4.4-fold increase (880-3890 ng/g) | 5.2-fold increase (193-1011 ng/m2) | 4.6-fold increase (0.23-1.04 ng/kg/day) |
| 16 week metabolite | 1.10 (0.96-1.26) | 0.99 (0.86-1.13) | 1.09 (0.95-1.26) | |
| 26 week metabolite | 1.21 (1.05-1.39) | 0.99 (0.86-1.15) | 1.18 (1.03-1.37) | |
| Delivery metabolite | 1.07 (0.92-1.23) | 1.07 (0.87-1.17) | 1.03 (0.90-1.20) | |
| TPHP ➔ DPHP | (Dust IQR) | 4.0-fold increase (532-2120 ng/g) | 6.8-fold increase (92-632 ng/m2) | 6.2-fold increase (0.14-0.57 ng/kg/day) |
| 16 week metabolite | 1.16 (1.01-1.32) | 1.26 (1.06-1.49) | 1.17 (1.02-1.34) | |
| 26 week metabolite | 1.04 (0.91-1.20) | 1.08 (0.91-1.29) | 1.04 (0.90-1.19) | |
| Delivery metabolite | 0.97 (0.84-1.13) | 0.94 (0.78-1.13) | 0.96 (0.83-1.11) | |
Fold increases represent differences from the 25th to 75th percentile of dust concentrations.
Ratio changes are from fully-adjusted regression models presented in Table 2.
CI: confidence interval
As shown in Table 4, we observed statistically significant correlations (p<0.001) between all urinary metabolites at the 26 week time point (rs = 0.23-0.38); at birth, only DNBP and BDCIPP are uncorrelated. Consistent correlations among OPE metabolites may indicate that they are used together in consumer products and thus shed into the environment in a similar fashion. Various OPEs are often combined for use in products (Abbasi et al., 2016), although tributyl phosphate—the parent compound of DNBP—is typically used for different types of applications than the halogenated OPEs. While the halogenated OPEs are used in polyurethane foams and textiles, the non-halogenated OPEs are more commonly used in lacquers, antifoam agents, and as plasticizers (Van den Eede et al., 2011). This difference in parent compound usage patterns may explain why DNBP is less correlated with the other OPEs in urine specimens.
Table 4:
Spearman correlation coefficients between maternal urinary OPE metabolite concentrations assessed at 16 weeks’ gestation, 26 weeks’ gestation, and delivery (creatinine-standardized).
| 16 weeks | ||||
|---|---|---|---|---|
| BCEP | BDCPP | DNBP | DPHP | |
| BCEP | 1 | 0.20* | 0.07 | 0.10 |
| BDCPP | 1 | −0.03 | 0.16* | |
| DNBP | 1 | 0.20* | ||
| DPHP | 1 | |||
| 26 weeks | ||||
| BCEP | BDCPP | DNBP | DPHP | |
| BCEP | 1 | 0.38* | 0.23* | 0.24* |
| BDCPP | 1 | 0.31* | 0.33* | |
| DNBP | 1 | 0.24* | ||
| DPHP | 1 | |||
| Birth | ||||
| BCEP | BDCPP | DNBP | DPHP | |
| BCEP | 1 | 0.31* | 0.22* | 0.22* |
| BDCPP | 1 | 0.06 | 0.25* | |
| DNBP | 1 | 0.20* | ||
| DPHP | 1 | |||
p<0.05
3.4. Strengths and Limitations
The main strength of this study is the ability to assess urinary OPE metabolites at three time points during pregnancy. Pregnancy is a sensitive period for both maternal and fetal health (Alonso-Magdalena et al., 2010; Cohn et al., 2012; Ettinger et al., 2009; Lambert et al., 2017), and thorough characterization of environmental exposures experienced during this time is critical to policymaking and public health messaging. However, the house dust samples in this study were not collected concurrently with any of the three urine samples or repeatedly during pregnancy; this makes it challenging to infer the variability of dust concentrations over that period and thus the degree of correlation between residential dust OPE levels and internal urinary OPE metabolite biomarkers. Although sampling for this study took place from 2003-2006 and may not capture current exposure levels, this time period represents the PBDE phase-out and is a good reference point for when OPEs began to be incorporated in consumer products in larger quantities.
Another study limitation is that we did not have data on the number or type of furniture and electronics in the homes of participants or on handwashing behavior. This data would likely have been informative in our regression modeling, as others have reported that decorating and cleaning patterns as well as handwashing are associated with OPE levels in dust and urine (Phillips et al., 2018; Sugeng et al., 2018). Additionally, the dust OPE concentrations in the main activity room are not expected to be representative of all rooms of the house. We suggest that future studies collect more information about participant behaviors and home characteristics as well as obtaining bio-specimens during multiple points in pregnancy and environmental samples that correspond to at least one bio-specimen collection time point. Additionally, we were unable to assess inhalation as a potential route of exposure as we do not have data on air concentrations of OPEs, although inhalation is now considered another major source of exposure (Schreder et al., 2016b).
Despite lacking some information on handwashing and home furnishings, we were able to include a variety of potential predictors in regression models, including SES characteristics, housing factors, and house dust OPE data. These results further our understanding of how individual exposure to OPEs varies, and the data will help inform future studies of OPEs and health outcomes.
4. Conclusions
We found DPHP to have the highest urinary concentration of all four OPEs, followed by BDCIPP, BCEP, and DNBP in descending order. Reproducibility between repeated urinary OPE metabolite concentrations in latter two-thirds of pregnancy were low, suggesting that a single measurement may not accurately represent the entire pregnancy period. Additionally, house dust levels of parent OPEs concentrations were associated with maternal concentrations of urinary OPE metabolites at some but not all time points.
Supplementary Material
House dust is considered a major source of organophosphate ester (OPE) exposure.
We measured urinary OPE metabolites in pregnant women at three timepoints.
Intraclass correlations between measurement timepoints were poor.
Non-white race was associated with lower OPE metabolite concentrations.
House dust OPEs and household size were associated with urinary OPE metabolites.
Acknowledgements:
This work was supported by grants from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (NIEHS P01 ES11261, R01 ES014575, R01 ES020349, R01 ES027224, R01 ES028277, P30 ES006096; EPA P01 R829389), and the University of Cincinnati Medical Scientist Training Program Grant 2T32GM063483-1. This paper is contribution number 3877 from the Virginia Institute of Marine Science, William & Mary.
Abbreviations:
- PBDE
polybrominated diphenyl ether
- OPE
organophosphate ester
- TDCIPP
tris(1,2,-dichloro-2-propyl) phosphate
- TPHP
triphenyl phosphate
- DPHP
diphenyl phosphate
- BDCIPP
bis(1,3-dichloro-2-propyl) phosphate
- TCEP
tris(2-chloroethyl) phosphate
- BCEP
bis-2-chloroethyl phosphate
- DNBP
di-n-butyl phosphate
- LOD
limit of detection
- ICC
intraclass correlation
- GM
geometric mean
- CI
confidence interval
- IQR
interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. The authors declare no competing financial interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abbasi G, Saini A, Goosey E, Diamond ML, 2016. Product screening for sources of halogenated flame retardants in Canadian house and office dust. Sci. Total Environ 545-546, 299–307. 10.1016/j.scitotenv.2015.12.028 [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A, 2010. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ. Health Perspect 118, 1243–50. 10.1289/ehp.1001993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Machler M, Bolker B, Walker S, 2015. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Software2 67, 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Braun JM, Hornung R, Chen A, Dietrich KN, Jacobs DE, Jones R, Khoury JC, Liddy-Hicks S, Morgan S, Vanderbeek SB, Xu Y, Yolton K, Lanphear BP, 2018. Effect of Residential Lead-Hazard Interventions on Childhood Blood Lead Concentrations and Neurobehavioral Outcomes: A Randomized Clinical Trial. JAMA Pediatr. 172, 934–942. 10.1001/jamapediatrics.2018.2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K, Lanphear BP, 2017. Cohort profile: The Health Outcomes and Measures of the Environment (HOME) study. Int. J. Epidemiol 46 10.1093/ije/dyw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, McCleana MD, Cooper EM, Watkins DJ, Frasera AJ, Heiger-Bernaysa W, Stapleton HM, Webstera TF, 2013. Predictors of Tris(1,3-dichloro-2-propyl) phosphate Metabolite in the Urine of Office Workers. Environ. Int 5, 56–61. 10.1111/j.1743-6109.2008.01122.x.Endothelial [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, Minguez-Alarcon L, Hauser R, Butt CM, Stapleton HM, Meeker JD, Williams PL, Hauser R, Hauser R, 2017. Influence of storage vial material on measurement of organophosphate flame retardant metabolites in urine. Chemosphere 181, 440–446. 10.1016/j.chemosphere.2017.04.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorina R, Butt C, Stapleton HM, Avery D, Harley KG, Holland N, Eskenazi B, Bradman A, 2017. Flame retardants and their metabolites in the homes and urine of pregnant women residing in California (the CHAMACOS cohort). Chemosphere 179, 159–166. 10.1016/j.chemosphere.2017.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudill S, Schleicher R, Pirkle J, 2008. Multi-rule quality control for the age-related eye disease study. Stat Med 27, 4094–106. 10.1002/sim.3222 [DOI] [PubMed] [Google Scholar]
- Cheung KL, Lafayette RA, 2013. Renal Physiology of Pregnancy. Adv. Chronic Kidney Dis. 20, 209–214. 10.1053/j.ackd.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn BA, Terry MB, Plumb M, Cirillo PM, 2012. Exposure to polychlorinated biphenyl (PCB) congeners measured shortly after giving birth and subsequent risk of maternal breast cancer before age 50. Breast Cancer Res. Treat 136, 267–275. 10.1007/s10549-012-2257-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishaw LV, Hunter DL, Padnos B, Padilla S, Stapleton HM, 2014. Developmental exposure to organophosphate flame retardants elicits overt toxicity and alters behavior in early life stage zebrafish (danio rerio). Toxicol. Sci 142, 445–454. 10.1093/toxsci/kfu194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Brody JG, Rudel RA, Van Den Eede N, Covaci A, 2014. Urinary biomonitoring of phosphate flame retardants: Levels in california adults and recommendations for future studies. Environ. Sci. Technol 48, 13625–13633. 10.1021/es503445c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van Den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA, 2012. After the PBDE phase-out: A broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol 46, 13056–13066. 10.1021/es303879n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger AS, Zota AR, Amarasiriwardene CJ, Hopkins MR, Schwartz J, Hu H, Wright RO, 2009. Maternal arsenic exposure and impaired glucose tolerance during pregnancy. Environ. Health Perspect 117, 1059–1064. 10.1289/ehp.0800533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gika HG, Theodoridis GA, Wilson ID, 2008. Liquid chromatography and ultra-performance liquid chromatography-mass spectrometry fingerprinting of human urine. Sample stability under different handling and storage conditions for metabonomics studies. J. Chromatogr. A 1189, 314–322. 10.1016/j.chroma.2007.10.066 [DOI] [PubMed] [Google Scholar]
- Gold MD, Blum A, Ames BN, 1978. Another flame retardant, tris(1,3-dichloro-2-propyl) phosphate, and its expected metabolites are mutagenic. Science (80-. ). 200, 785–787. [DOI] [PubMed] [Google Scholar]
- He C, Wang X, Tang S, Thai P, Li Z, Baduel C, Mueller JF, 2018. Concentrations of Organophosphate Esters and Their Specific Metabolites in Food in Southeast Queensland, Australia: Is Dietary Exposure an Important Pathway of Organophosphate Esters and Their Metabolites? Environ. Sci. Technol 52, 12765–12773. 10.1021/acs.est.8b03043 [DOI] [PubMed] [Google Scholar]
- Hoffman K, Daniels JL, Stapleton HM, 2014. Urinary Metabolites of Organophosphate Flame Retardants and Their Variability in Pregnant Women. Environ. Int 2, 169–172. 10.1111/j.1743-6109.2008.01122.x.Endothelial [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Lorenzo A, Butt C, Adair L, Herring AH, Heather M, Daniels J, 2017. Predictors of urinary flame retardant concentration among pregnant women. Environ. Int 98, 96–101. 10.1016/j.envint.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung R, Reed L, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg 5, 46–51. [Google Scholar]
- Ito Y, Kamijima M, Hasegawa C, Tagawa M, Kawai T, Miyake M, Hayashi Y, Naito H, Nakajima T, 2014. Species and inter-individual differences in metabolic capacity of di(2-ethylhexyl)phthalate (DEHP) between human and mouse livers. Environ. Health Prev. Med 19, 117–125. 10.1007/s12199-013-0362-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatilaka NK, Restrepo P, Davis Z, Vidal M, Calafat AM, Ospina M, 2019. Quantification of 16 urinary biomarkers of exposure to flame retardants, plasticizers, and organophosphate insecticides for biomonitoring studies. Chemosphere 235, 481–491. 10.1016/j.chemosphere.2019.06.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatilaka NK, Restrepo P, Williams L, Ospina M, Valentin-Blasini L, Calafat AM, 2017. Quantification of three chlorinated dialkyl phosphates, diphenyl phosphate, 2,3,4,5-tetrabromobenzoic acid, and four other organophosphates in human urine by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem 409, 1323–1332. 10.1007/s00216-016-0061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Otazo HA, Clarke JP, Diamond ML, Archbold JA, Ferguson G, Harner T, Richardson GM, Ryan JJ, Wilford B, 2005. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ. Sci. Technol 39, 5121–5130. 10.1021/es048267b [DOI] [PubMed] [Google Scholar]
- Kim U-J, Wang Y, Li W, Kannan K, 2019. Occurrence of and human exposure to organophosphate flame retardants/plasticizers in indoor air and dust from various microenvironments in the United States. Environ. Int 125, 342–349. 10.1016/j.envint.2019.01.065 [DOI] [PubMed] [Google Scholar]
- Kim UJ, Kannan K, 2018. Occurrence and Distribution of Organophosphate Flame Retardants/Plasticizers in Surface Waters, Tap Water, and Rainwater: Implications for Human Exposure. Environ. Sci. Technol 52, 5625–5633. 10.1021/acs.est.8b00727 [DOI] [PubMed] [Google Scholar]
- Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, Yoshida T, 2013. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 314, 76–83. 10.1016/j.tox.2013.09.004 [DOI] [PubMed] [Google Scholar]
- La Guardia MJ, Hale RC, 2015. Halogenated flame-retardant concentrations in settled dust, respirable and inhalable particulates and polyurethane foam at gymnastic training facilities and residences. Environ. Int 79, 106–114. 10.1016/j.envint.2015.02.014 [DOI] [PubMed] [Google Scholar]
- Lambert JA, Carlisle MA, Lam A, Aggarwal S, Doran S, Ren C, Bradley WE, Dell’Italia L, Ambalavanan N, Ford DA, Patel RP, Jilling T, Matalon S, 2017. Mechanisms and Treatment of Halogen Inhalation-Induced Pulmonary and Systemic Injuries in Pregnant Mice. Hypertension 70, 390–400. 10.1161/HYPERTENSIONAHA.117.09466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laparre J, Kaabia Z, Mooney M, Buckley T, Sherry M, Le Bizec B, Dervilly-Pinel G, 2017. Impact of storage conditions on the urinary metabolomics fingerprint. Anal. Chim. Acta 951, 99–107. 10.1016/j.aca.2016.11.055 [DOI] [PubMed] [Google Scholar]
- Lenth R, 2019. emmeans:Estimated Marginal Means, aka Least-Squares Means.
- Li J, Zhao L, Letcher RJ, Zhang Y, Jian K, Zhang J, Su G, 2019. A review on organophosphate Ester (OPE) flame retardants and plasticizers in foodstuffs: Levels, distribution, human dietary exposure, and future directions. Environ. Int 127, 35–51. 10.1016/j.envint.2019.03.009 [DOI] [PubMed] [Google Scholar]
- Li W, Wang Y, Asimakopoulos AG, Covaci A, Gevao B, Johnson-Restrepo B, Kumosani TA, Malarvannan G, Moon HB, Nakata H, Sinha RK, Tran TM, Kannan K, 2019. Organophosphate esters in indoor dust from 12 countries: Concentrations, composition profiles, and human exposure. Environ. Int 133, 105178 10.1016/j.envint.2019.105178 [DOI] [PubMed] [Google Scholar]
- Liu X, Ji K, Jo A, Moon HB, Choi K, 2013. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat. Toxicol 134-135, 104–111. 10.1016/j.aquatox.2013.03.013 [DOI] [PubMed] [Google Scholar]
- Lukecke D, 2019. sjstats:Statistical Functions for Regression Models (Version 0.17.4). 10.5281/zenodo.1284472 [DOI]
- Meeker JD, Cooper EM, Stapleton HM, Hauser R, 2013. Urinary metabolites of organophosphate flame retardants: Temporal variability and correlations with house dust concentrations. Environ. Health Perspect 121, 580–585. 10.1289/ehp.1205907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard S, 2013. EnvStats: An R package for environmental statistics. Springer, New York. [Google Scholar]
- National Research Council 2009, 2009. Weight gain during pregnancy: reexamining the guidelines (2009). The National Academies Press, Washington, DC: 10.17226/12584 [DOI] [PubMed] [Google Scholar]
- Ospina M, Jayatilaka NK, Wong LY, Restrepo P, Calafat AM, 2018. Exposure to organophosphate flame retardant chemicals in the U.S. general population: Data from the 2013-2014 National Health and Nutrition Examination Survey. Environ. Int 110, 32–41. 10.1016/j.envint.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy Z, La MJ, Xu Y, Hale RC, Dietrich KN, Lanphear BP, Yolton K, Vuong AM, Cecil KM, Braun JM, Xie C, Chen A, 2020. Concentrations and loadings of organophosphate and replacement brominated flame retardants in house dust from the HOME study during the PBDE phase-out. Chemosphere 239. 10.1016/j.chemosphere.2019.124701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C, 2016. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 27, 378–388. 10.1097/eDe.0000000000000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Hammel SC, Hoffman K, Lorenzo AM, Chen A, Webster TF, Stapleton HM, 2018. Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ. Int 116, 176–185. 10.1016/j.envint.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston EV, McClean MD, Claus Henn B, Stapleton HM, Braverman LE, Pearce EN, Makey CM, Webster TF, 2017. Associations between urinary diphenyl phosphate and thyroid function. Environ. Int 101, 158–164. 10.1016/j.envint.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Hawley NL, Eliot M, Calafat AM, Jayatilaka NK, Kelsey K, McGarvey S, Phipps MG, Savitz DA, Werner EF, Braun JM, 2017. Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environ. Heal. A Glob. Access Sci. Source 16, 1–11. 10.1186/s12940-017-0247-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B, 2011. Fundamentals of Biostatistics. Cengage Learning, Boston, MA. [Google Scholar]
- Rotter M, Brandmaier S, Prehn C, Adam J, Rabstein S, Gawrych K, Bruning T, Illig T, Lickert H, Adamski J, Wang-Sattler R, 2017. Stability of targeted metabolite profiles of urine samples under different storage conditions. Metabolomics 13. 10.1007/s11306-016-1137-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreder ED, Uding N, La Guardia MJ, 2016a. Inhalation a significant exposure route for chlorinated organophosphate flame retardants. Chemosphere 150, 499–504. 10.1016/j.chemosphere.2015.11.084 [DOI] [PubMed] [Google Scholar]
- Schreder ED, Uding N, La Guardia MJ, 2016b. Inhalation a significant exposure route for chlorinated organophosphate flame retardants. Chemosphere 150, 499–504. 10.1016/j.chemosphere.2015.11.084 [DOI] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P, 2000. Identifying critical windows of exposure for children’s health. Environ. Health Perspect 108, 451–455. 10.1289/ehp.00108s3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugeng EJ, de Cock M, Leonards PEG, van de Bor M, 2018. Electronics, interior decoration and cleaning patterns affect flame retardant levels in the dust from Dutch residences. Sci. Total Environ 645, 1144–1152. 10.1016/j.scitotenv.2018.07.127 [DOI] [PubMed] [Google Scholar]
- Tan H, Yang L, Yu Y, Guan Q, Liu X, Li L, Chen D, 2019. Co-Existence of Organophosphate Di- and Tri-Esters in House Dust from South China and Midwestern United States: Implications for Human Exposure. Environ. Sci. Technol 53, 4784–4793. 10.1021/acs.est.9b00229 [DOI] [PubMed] [Google Scholar]
- U S Environmental Protection Agency, 2017. Exposure Factors Handbook: Chapter 5 (Soil and Dust Ingestion) 2017 update. Washington, DC. [Google Scholar]
- US Environmental Protection Agency, 2006. Polybrominated Diphenyl Ethers (PBDEs) Project Plan. [Google Scholar]
- Van den Eede N, Dirtu AC, Neels H, Covaci A, 2011. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ. Int 37, 454–461. 10.1016/j.envint.2010.11.010 [DOI] [PubMed] [Google Scholar]
- van der Veen I, de Boer J, 2012. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153. 10.1016/j.chemosphere.2012.03.067 [DOI] [PubMed] [Google Scholar]
- Wei GL, Li DQ, Zhuo MN, Liao YS, Xie ZY, Guo TL, Li JJ, Zhang SY, Liang ZQ, 2015. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut 196, 29–46. 10.1016/j.envpol.2014.09.012 [DOI] [PubMed] [Google Scholar]
- Wong F, de Wit CA, Newton SR, 2018. Concentrations and variability of organophosphate esters, halogenated flame retardants, and polybrominated diphenyl ethers in indoor and outdoor air in Stockholm, Sweden. Environ. Pollut 240, 514–522. 10.1016/j.envpol.2018.04.086 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


