Abstract
Objectives:
Optimal prostate cancer (PCa) screening strategies will focus on men likely to have potentially lethal disease. Age-specific incidence rates (ASIRs), by modern clinical risk groups, could inform risk-stratification efforts for screening.
Methods:
We identified all men (n=20,356) diagnosed with PCa in Norway from 2014–2017, in this cross-sectional, population study. Age, Gleason score (primary+secondary), and clinical stage were extracted. Patients were assigned to clinical risk groups: low, favorable-intermediate, unfavorable-intermediate, high, regional, and metastatic. Chi-squared tests analyzed the independence of Gleason scores and modern PCa risk groups with age. ASIRs for each risk group were calculated as the product of Norwegian ASIRs for all PCa and the proportions observed for each risk category.
Results:
Older age was significantly associated with higher Gleason score and more advanced disease. The percentage of men with Gleason 8–10 disease among men aged 55–59, 65–69, 75–79, and 85–89 years was 16.5%, 23.4%, 37.2%, and 59.9%, respectively (p<0.001); the percentage of men in the same age groups with at least high-risk disease was 29.3%, 39.1%, 60.4%, and 90.6%, respectively (p<0.001). Maximum ASIRs (per 100,000 men) for low-risk, favorable-intermediate-risk, unfavorable-intermediate-risk, high-risk, regional, and metastatic disease were: 157.1 for ages 65–69, 183.8 for ages 65–69, 194.8 for ages 70–74, 408.3 for ages 75–79, 159.7 for ages 85+, and 314.0 for ages 85+, respectively. At age 75–79 years, the ASIR of high-risk disease was approximately 6 times greater than at 55–59 years.
Conclusions:
Risk of clinically-significant, localized PCa increases with age. Healthy older men may benefit from screening.
Keywords: prostate cancer, risk stratification, Gleason score, age-specific incidence, diagnosis, age
Precis
In a population-based study of 20,356 Norwegian men with prostate cancer, both the proportion and absolute incidence rate of advanced disease increase with age. At age 75–79, the absolute incidence of high-risk disease is 6 times greater than at 55–59, suggesting healthy older men may benefit from prostate cancer screening.
Introduction
Effective prostate cancer (PCa) screening seeks to identify clinically-significant and potentially-lethal cases requiring treatment, while avoiding overdiagnosis of more indolent, lower-risk cases eligible for active surveillance1,2. Disease aggressiveness is accounted for in clinical decision-making with widely-used risk stratification schemes3,4. Men diagnosed with PCa may be followed with active surveillance5 or treated aggressively with surgery, radiation therapy, androgen deprivation therapy, or a combination thereof3. Understanding the age-specific incidence of each modern clinical risk group could greatly inform effective screening strategies aimed to detect potentially lethal, localized PCa.
Prior studies have suggested associations between older age and higher Gleason score6,7, as well as between age and higher disease risk group8. Associations with age in PCa have important implications for pre-test probability of screening and, therefore, diagnostic accuracy for clinically significant PCa. Modern risk stratification includes an important distinction between unfavorable- and favorable-intermediate-risk cancer9. Unfavorable-intermediate-risk and high-risk disease have similar rates of developing distant metastases and of prostate-cancer-specific mortality10. This differentiation has been incorporated into clinical management3 and the American Joint Committee on Cancer 8th edition staging guidelines for PCa11. The difference between Gleason 4+3 and 3+4 is also included in these guidelines3,9–11. However, age associations have not been reported for these modern groupings. Additionally, age-specific incidence rates (ASIRs) for PCa have not been reported for clinical risk groups.
Here, we used data from the Cancer Registry of Norway to assess associations between age and modern PCa risk-stratification schemes used in clinical decision-making. We also estimated absolute ASIRs for each PCa risk category using population statistics from Norway. We hypothesized that older men are more likely to have higher grade and more advanced risk group, using contemporary definitions (including favorable- vs. unfavorable-intermediate-risk and distinguishing Gleason 3+4 vs. 4+3).
Methods
Patient Population
We identified all men with PCa diagnosed from 2014–2017 from the Cancer Registry of Norway, which has been reported to have >99% validity and completeness, overall, with regard to PCa reporting12,13. Information potentially available included: age at diagnosis, Gleason score (including primary and secondary Gleason score, allowing for a determination of Gleason grade group for separation of cases into favorable- and unfavorable-intermediate-risk14), and the clinical TNM stage. Patients were placed into 5-year age groups for analysis.
Risk Stratification
PCa risk stratification was based on modern definitions included in National Comprehensive Cancer Network (NCCN) guidelines3. Very-low-risk and low-risk cases were combined into low-risk PCa (Gleason score ≤6, clinical stage T1–T2, PSA<10 ng/mL), as they are managed similarly. Clinical stage T2 was not specified in the registry as T2a, T2b, or T2c, so T2b and T2c could not be used as intermediate-risk criteria. High-risk and very-high-risk cases were combined into high-risk PCa (clinical stage T3a or higher, or Gleason score ≥8, or PSA>20 ng/mL), as these cases are managed similarly. Regional and metastatic cases were those with clinical lymph node involvement or clinical/pathologic evidence of metastatic disease, respectively. Intermediate-risk cases were any that did not fit the above definitions. Favorable-intermediate-risk cases were those intermediate-risk cases with percentage of positive biopsy cores <50%, and only one of the following: (1) Gleason score 3+4 with PSA<10 ng/mL or (2) Gleason score 3+3 with PSA 10–20 ng/mL. Unfavorable-intermediate-risk cases were intermediate-risk cases with any of the following: (1) Gleason score 4+3, (2) Gleason score 3+4 with PSA 10–20 ng/mL, (3) Gleason score 3+3 with PSA 10–20 ng/mL with ≥50% positive biopsy cores, or (4) ≥50% positive biopsy cores.
Statistical Analyses
Chi-squared tests analyzed the independence of distributions between age and Gleason scores at diagnosis, with the separation of Gleason 7 disease into 3+4 and 4+3. Among those with low-grade (e.g., Gleason 6), we additionally assessed an association between age and higher clinical risk group due to other factors: PSA ≥10 ng/mL and/or high clinical stage (T3–T4 or nodal or distant metastasis). These latter cancers are often not eligible for active surveillance, despite the low Gleason score3.
The independence of distributions between age groups and modern PCa risk groups was also analyzed via Chi-squared tests; this included separation of the intermediate-risk group category into favorable and unfavorable disease. We analyzed the independence of distributions between age groups and having clinically significant PCa via Chi-squared tests. Clinically-significant disease (those generally less appropriate for active surveillance3) was defined two ways: first, all cases of at least intermediate-risk, and second, all cases of at least unfavorable-intermediate-risk.
The above Chi-squared tests were subsequently repeated, while limiting analyses to only cases diagnosed between ages 50–743,15. Significance was set at alpha=0.05 for two-tailed tests. We used the R environment for statistical computing for all analyses16.
PCa age-specific incidence, by risk group
We calculated PCa ASIRs, stratified by modern clinical risk groups, in the Norwegian population. ASIRs of any PCa in Norway 2015 were obtained from NORDCAN, a comprehensive cancer statistics database for the Nordic countries17,18. The overall Norway ASIR was multiplied by the proportion of cases meeting criteria for high-risk PCa to estimate the ASIR of high-risk PCa, specifically. This was repeated to obtain ASIRs for each risk group, which are reported per 100,000 males.
Prostate-cancer-specific mortality
The above analyses draw from a recent, narrow time window to give insights into a modern population; thus, long-term survival data are not yet available. Nevertheless, we calculated PCa-specific mortality (and overall mortality) for patients in each age group, as of January 1, 2019.
Results
20,356 men were diagnosed with PCa in Norway from 2014–2017 (eTable 1). Gleason score was available for 18,665 cases (91.7%). 17,920 patients (88.0%) could be placed into the broad risk group categories of low-risk vs. intermediate-risk and higher; 14,303 (70.3%) had sufficient available clinical data for complete PCa risk stratification (including favorable/unfavorable-intermediate-risk).
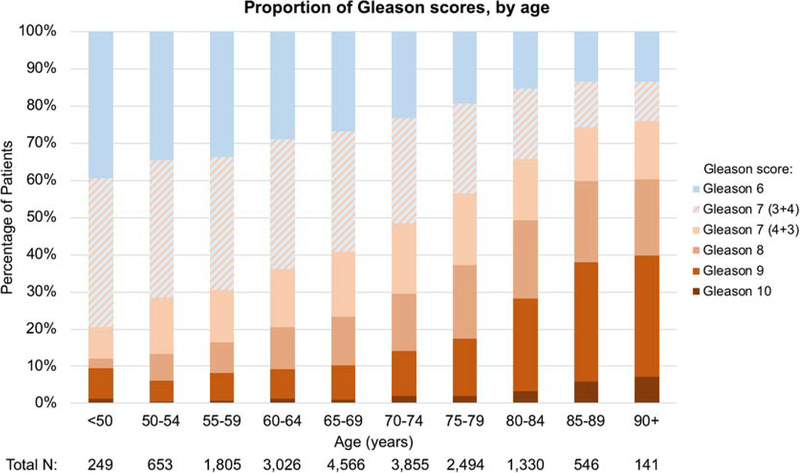
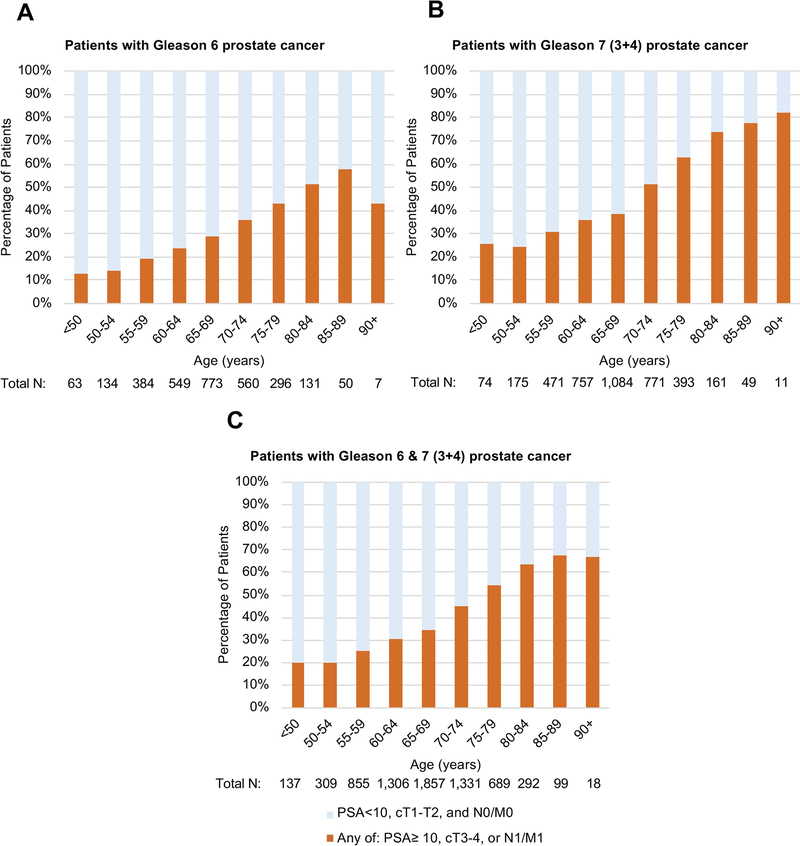
Older age at diagnosis was associated with a higher Gleason score at diagnosis. Figure 1 shows age-stratified proportions of cases by Gleason score (proportions are listed in eTable 2). The percentage of men with Gleason 8–10 disease among men aged 55–59, 65–69, 75–79, and 85–89 years was 16.5%, 23.4%, 37.2%, and 59.9%, respectively (p<0.001). Of those with Gleason 7 disease, older patients were more likely to be diagnosed with grade group 3 (Gleason 4+3) disease compared to grade group 2 (Gleason 3+4) disease. The percentage of Gleason 3+4 disease among men aged 55–59, 65–69, 75–79, and 85–89 years was 35.7%, 32.5%, 24.0%, and 12.4%, respectively, while the percentage of Gleason 4+3 disease across the same age groups was 14.0%, 17.3%, 19.3%, and 14.3%, respectively (p<0.001). When evaluating only those with Gleason 6, older men were still more likely to be diagnosed with disease ineligible for active surveillance (Figure 2). The percentage of men aged 55–59, 65–69, 75–79, and 85–89 years with Gleason 6 disease and one or more of these risk factors (PSA ≥10 ng/mL, clinical stage ≥T3a, or nodal/metastatic disease) was: 19.5%, 28.7%, 42.9%, and 58.0%, respectively (p<0.001). Similar trends were seen for those men with Gleason 3+4 disease, and when men with Gleason 6 and 3+4 disease were combined (Figure 2).
Figure 1.
Stratification of prostate cancer patients in Norway by Gleason scores and age, 2014–2017. Patients with Gleason 7 disease were divided into Gleason 3+4 (grade group 2) and Gleason 4+3 (grade group 3). Total number of patients with Gleason score data available: n=18,665 (91.7% of all prostate cancer cases in Norway).
Figure 2.
Proportion of men with Gleason 6 (Panel A, n=2,947), Gleason 7 (3+4; Panel B, n=3,946), and Gleason 6 or 7 (3+4) prostate cancer (panel C, n=6,893) who meet one or more of the following criteria: PSA ≥10 ng/mL, clinical T3–4 stage, or N1/M1 disease, by age group.
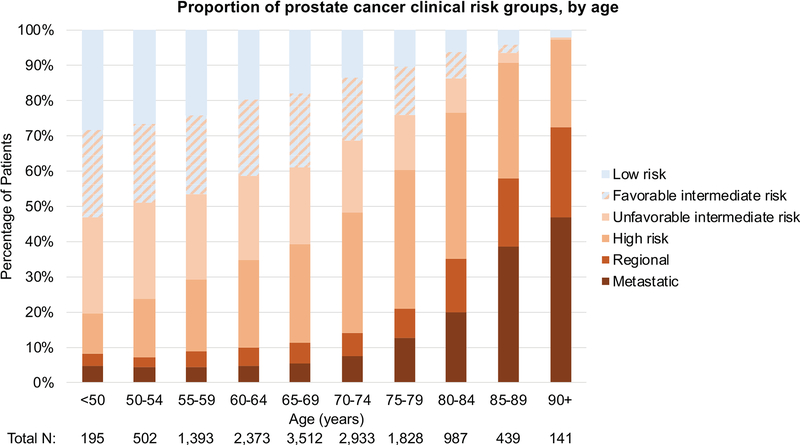
There was also an association of PCa risk groups with age. Older patients with PCa were more likely to have more advanced disease. The percentage of men aged 55–59, 65–69, 75–79, and 85–89 years with at least high-risk PCa was 29.3%, 39.1%, 60.4%, and 90.6%, respectively. The percentage of men with low-risk PCa across the same age groups was 24.0%, 17.9%, 10.2%, and 4.1%, respectively (p<0.001). Figure 3 shows age-stratified proportions of cases by PCa risk group. Older men were more likely to be diagnosed with clinically-significant disease: p<0.001 when counting at least intermediate-risk as clinically-significant (the percentage of men aged 55–59, 65–69, 75–79, and 85–89 years with at least favorable-intermediate-risk disease was 76.0%, 82.1%, 89.8%, and 95.9%, respectively) and p<0.001 when counting at least unfavorable-intermediate-risk PCa as clinically-significant (the percentage of men aged 55–59, 65–69, 75–79, and 85–89 years with at least unfavorable-intermediate-risk disease was 53.3%, 61.2%, 75.8%, and 93.6%, respectively). When the analyses were restricted to men aged 50–74, all of the above associations remained significant (p<0.001). Numerical proportions are listed in eTable 3.
Figure 3.
Clinical risk group stratification of prostate cancer patients in Norway, 2014–2017. Total number of patients with risk stratification data available: n=14,303 (70.3% of all prostate cancer cases in Norway).
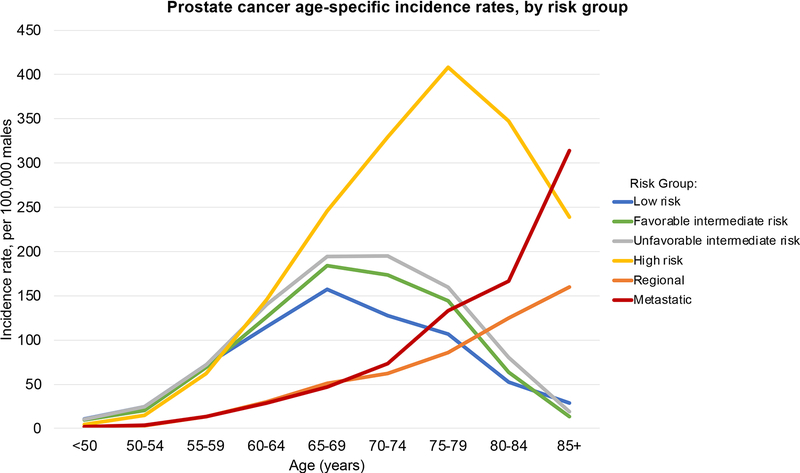
ASIRs by risk groups are shown in Table 1 and Figure 4. As men aged across the Norwegian population, they were increasingly likely to have more advanced PCa. Qualitatively, the PCa ASIRs by risk groups demonstrate an increase in incidence rates across all risk groups until ages 65–69. At ages 65–69, the rates of low and intermediate-risk cases (including both favorable and unfavorable) begin to level off or decrease. The maximum ASIR for low-risk, favorable-intermediate-risk, and unfavorable-intermediate-risk PCa are: 157.1 for ages 65–69, 183.8 for ages 65–69, and 194.8 for ages 70–74, respectively. Meanwhile, incidence rates of high-risk disease in men over 65–69 continue to increase sharply; the ASIRs of high-risk disease surpasses those of the low and intermediate-risk categories until ages 75–79—when the ASIR is 408.3—at which point they begin to decrease. The incidence of regional and metastatic disease always increases with age.
Table 1.
Prostate cancer age-specific incidence rates (ASIRs) in Norway per 100,000 men by clinical risk group stratification. Intermediate-risk prostate cancer is subdivided into favorable and unfavorable risk.
| ASIRs (per 100,000 men) by prostate cancer risk group | ||||||
|---|---|---|---|---|---|---|
| Age (years) | Low-risk | Favorable intermediate-risk | Unfavorable Intermediate-risk | High-risk | Regional | Metastatic |
| <50 | 10.4 | 9.3 | 10.0 | 4.2 | 1.3 | 1.7 |
| 50–54 | 24.1 | 20.5 | 24.8 | 15.0 | 2.7 | 3.8 |
| 55–59 | 72.2 | 68.3 | 72.2 | 61.6 | 13.2 | 13.6 |
| 60–64 | 115.1 | 126.2 | 139.5 | 145.2 | 29.5 | 28.3 |
| 65–69 | 157.1 | 183.8 | 194.6 | 245.6 | 50.2 | 47.0 |
| 70–74 | 127.0 | 173 | 194.8 | 328.7 | 61.4 | 72.5 |
| 75–79 | 106.0 | 144.6 | 159.9 | 408.3 | 85.1 | 132.7 |
| 80–84 | 51.5 | 63.3 | 80.2 | 346.9 | 124.9 | 166.3 |
| 85+ | 27.9 | 13.3 | 18.6 | 238.2 | 159.7 | 314.0 |
Figure 4.
Norway prostate cancer age-specific incidence rates (per 100,000 males) by clinical risk group stratification. Intermediate-risk prostate cancer is subdivided into favorable and unfavorable risk.
Median follow-up for the analyzed cohort was 1.9 years (range 0–4.0 years). PCa-specific (and overall) mortality increased with age (eTable 4). Most of the 931 deaths from PCa occurred in men initially diagnosed with advanced disease: 364 in those with metastatic (39.1%), 75 in those with regional (8.0%), and 48 in those with high-risk (5.2%) disease.
Discussion
This is the first study to report PCa ASIRs by modern clinical risk groups. We additionally demonstrate the age dependence of PCa Gleason scores, when including the clinically relevant separation of grade groups 2 (Gleason 3+4) and 3 (Gleason 4+3). Older men are more likely to be diagnosed with higher-grade disease. We further found an age dependence of clinical risk stratification of PCa at diagnosis, using modern distinctions between favorable- and unfavorable-intermediate-risk disease11. Not only does the proportion of metastatic PCa increase with age (which could be simply due to less screening), but so does a man’s absolute risk of potentially lethal, localized PCa. At age 75–79 years, the absolute incidence of high-risk PCa is roughly 6 times greater than at age 55–59 years.
While detailed data on screening patterns by age were unavailable, it is likely that screening rates decreased after age 70, which could explain the decrease in incidence of low-risk disease. However, it is important to note that the absolute incidence rates of high-risk, localized disease substantially increased with age. This effect cannot be attributed to decreased screening in older men.
The overall pattern of greater proportions of regional and metastatic PCa in elderly is an expected finding, at least in part: older men are less likely to be screened for PCa compared to their younger counterparts4 and are more likely to receive PSA testing because of urinary symptoms. However, older men may also be more likely to present with metastatic disease because they were already at increased risk of having an undetected potentially-lethal cancer, making them more prone to metastases10. Detecting these cancers at an earlier stage may have permitted curative therapy and avoidance of metastatic spread.
These PCa ASIRs for modern risk groups have implications for screening and public health. Overall, older men were increasingly likely to present with a clinically-significant PCa in the Norwegian population, highlighting the relevance of age-specific screening decisions for individual men1. Rates of high-risk, localized disease increased dramatically from ages 55 to 74 (while rates for low and intermediate-risk disease decreased), leading to a maximum ASIR over twice as high as that of favorable intermediate-risk disease. Some guidelines discourage any PCa screening above age 7019, while others recommend consideration in healthy older men3,20,21. The ASIRs shown here indicate that the absolute incidence of potentially lethal disease actually increases in men older than 70. The 10-year metastasis rates for men with unfavorable-intermediate-risk and high-risk disease are quite high10. Men aged 70–75 typically have estimated life expectancies over 10 years22–24, and healthy men up to age 80 can have life expectancies exceeding 10 years24. PCa screening trials may have underestimated the potential mortality benefit of screening by including many men under 60, who have long life expectancies but relatively low incidence of unfavorable-intermediate-risk or high-risk disease15,25. Screening mostly younger men (barring other risk factors) will yield higher relative rates of false positives and of low-risk cancer overdiagnosis19,26,27. Meanwhile, early treatment of intermediate-risk or high-risk disease reduces PCa morbidity and mortality5,28–30.
Age could be combined with other risk factors to further optimize screening strategies1,31–34. Though follow up time in this study is short, as expected, advanced disease at diagnosis was associated with increased likelihood of PCa death. While our data suggest that screening older men may identify more clinically significant PCa, it is acknowledged that our data does not prove that screening could influence important clinical outcomes like survival or quality of life. Additionally, the risks of low-risk disease overdiagnosis must be considered. Nonetheless, the absolute incidence of high-risk disease is greatest in this older age group; there are some healthy men in this age group who could plausibly benefit from screening, given their high risk of aggressive disease and otherwise good life expectancy. The selection of patients for PCa screening remains an individualized one, and average life expectancy must be weighed against each individual’s other comorbidities. Ultimately, screening might be reasonable in a patient with a high risk of aggressive disease and a life expectancy long enough to benefit from treatment of localized PCa—the NCCN suggests 10 years for this life expectancy cutoff3,24.
Prior reports, using older Gleason and clinical risk categories, have also found associations between age and more advanced PCa6–8,35. Additionally, previous work has reported population PCa stage-specific incidence rates and trends over eras in time36, mainly by dividing cases into localized, regional, or distant disease37,38. The introduction of opportunistic PSA screening in Norway led to increased incidence rates of localized and regional PCa in younger men over time37, but no further subdivision was previously made for the localized disease cases that were potentially-lethal versus eligible for active surveillance. Because prior studies did not calculate absolute ASIRs, it was difficult to tell whether increased proportions of advanced PCa in older men were solely due to relatively fewer screen-detected diagnoses.
We note that the diagnosis of localized PCa is typically made by biopsy, and the several decisions, by both clinician and patient, that might lead to a biopsy procedure also afford the potential for bias in who is diagnosed39. Additionally, some Norwegian men diagnosed with aggressive disease at an older age might have been diagnosed with less aggressive disease at a younger age, had they undergone frequent screening. It is unclear how much this hypothetical situation might have affected the actual results found here, but it would be consistent with the overall pattern demonstrated here of increased risk of more advanced disease with age.
As confirmed in the present study, age is a clear risk factor for development of PCa (and its more aggressive variants), but physicians are generally discouraged by guidelines19 from screening men older than 7021. Knowledge of PCa risk group age-specific incidence patterns may help better inform the quality metric decisions for PSA screening by highlighting the effect of age on risk of potentially lethal PCa. Ultimately, the decision of whether to check PSA must hinge on a net benefit, which increases when the probability of detecting a potentially lethal cancer increases, provided the patient is not expected to die from another cause before PCa progression leads to morbidity and mortality. Our data suggest that a man of average health in the 70–75 years age range has a high risk of potentially lethal PCa. Decision-making guidelines generally emphasize shared decision-making for men aged 50–69 and do not encourage screening of men aged 70–75, but perhaps the latter should be reconsidered for otherwise healthy men40.
Norway opted not to participate in the major European PCa screening trial15, citing concerns for the ethical implications of screening otherwise asymptomatic men and the trial’s overall clinical significance41. This decision was widely debated42 and screening remains controversial in Norway. Norway’s national healthcare system does not recommend population-based PCa screening in asymptomatic men without a family history of PCa. Rather, shared decision-making is encouraged between physicians and patients43, leading to approximately 45% of Norwegian men over 40 years receiving PSA testing (likely for screening and/or diagnostic reasons) in 201144. A survey of Norwegian primary care physicians showed that a minority include PSA testing in their routine lab work for men, but that 70% would order PSA testing if the patient requested, and 72% would find it “difficult” not to refer to a urologist if a patient’s PSA is elevated44.
Our work has some limitations. Very-low or very-high-risk PCa cases were not distinguished, as Gleason score for each biopsy core and PSA density were not available. These cases tend to be clinically managed similarly to low and high-risk PCa, respectively, and thus were combined with those categories3. Approximately 30% of the cohort could not be assigned a precise risk group due to incomplete PSA or clinical T stage information, but most of these had partial data and were available for analyses of Gleason score and clinically-significant PCa. Moreover, the rate of missing details for these clinical risk groups was approximately the same (~25%) for each age category from 50–74 years; after age 75, missing details were more common (eTable 1). Screening history, reason for diagnostic testing, and rationale for prostate biopsy were unavailable in the registry for most patients, leading to potential sampling bias. These nuanced decisions may have been influenced by an individual’s symptoms (or lack thereof), life expectancy, comorbidities, or age, and are not well characterized in registry data. In a screened population, we expect to find higher rates of lower-risk PCa cases that may be managed with active surveillance5. Conversely, in an unscreened population, we expect some of the missed lower-risk cases to progress to advanced disease. The Norwegian population is comprised of a mixture of unscreened individuals and those screened with varying degree of frequency. This is a confounder, but one likely present in most developed countries, even where screening is encouraged. In the older age ranges, biopsy may have been limited to older men who are very healthy or symptomatic—concordant with the principle that one would only screen healthy men who have reasonable life expectancy. Differential decisions by physicians to screen, and then to biopsy, could affect the overall prevalence of PCa: if physicians are limiting biopsies among older men (such as holding them to a higher threshold for biopsy), we are potentially missing more low-risk disease, particularly in the older age groups. The benefits and costs of detecting more lower-risk disease and delaying treatment in favor of active surveillance until time of disease progression needs further evaluation by policy makers who direct screening programs. Finally, we were able to obtain plausible estimates of age-specific incidence and proportion for an entire population by use of high-quality registry data, but the direct findings are limited to Norway. As the population of Norway remains relatively homogenous, the patterns described here may not be applicable to men from other countries or ancestries. Nevertheless, it is reasonable to posit that the patterns observed here could be similar in other western countries, a suggestion partially supported by prior work in the USA6,8.
Conclusions
Both the proportion and absolute incidence rates of clinically significant PCa increase with age. Notably, the absolute incidence of high-risk disease at ages 75–79 is over six times higher than that at ages 55–59. Older men also are more likely to be diagnosed with higher Gleason score. Efforts to optimize PCa screening for efficient detection of localized, potentially lethal disease should account for this strong age dependence.
Supplementary Material
Funding Sources:
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number K08EB026503. This study was funded in part by a grant from the United States Department of Defense (#W81XWH-13-1-0391), the Research Council of Norway (#223273), KG Jebsen Stiftelsen, and South East Norway Health Authority. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: TMS reports grant funding from Varian Medical Systems, unrelated to the present study, and honoraria from WebMD, Inc. and HealthLytix, Inc., unrelated to this work. OAA reports speakers’ honorarium from Lundbeck and is consultant for HealthLytix, Inc.
OAA has a patent application (US 20150356243) pending; AMD also applied for this patent application and assigned it to UC San Diego. AMD has stock options in Human Longevity, Inc, and is a founder and equity holder in CorTechs Labs, Inc., and HealthLytix, Inc. AMD reports research funding from General Electric Healthcare, unrelated to the present study. The other authors have no disclosures to report.
A preliminary version of this work was presented in poster form at the 61th Annual Meeting of the American Society for Radiation Oncology; September 15–18, 2019; Chicago, IL.
References
- 1.Seibert TM, Fan CC, Wang Y, et al. Polygenic hazard score to guide screening for aggressive prostate cancer: Development and validation in large scale cohorts. BMJ. 2018;360:1–7. doi: 10.1136/bmj.j5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pashayan N, Duffy SW, Chowdhury S, et al. Polygenic susceptibility to prostate and breast cancer: Implications for personalised screening. Br J Cancer. 2011;104(10):1656–1663. doi: 10.1038/bjc.2011.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer version 22018.
- 4.Parker C, Gillessen S, Heidenreich A, Horwich A, ESMO Guidelines Committee. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v69–v77. doi: 10.1093/annonc/mdv295 [DOI] [PubMed] [Google Scholar]
- 5.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
- 6.Muralidhar V, Ziehr DR, Mahal BA, et al. Association between older age and increasing gleason score. Clin Genitourin Cancer. 2015;13(6):525–530e3. doi: 10.1016/j.clgc.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 7.Draisma G, Postma R, Schröder FH, Van Der Kwast TH, De Koning HJ. Gleason score, age and screening: Modeling dedifferentiation in prostate cancer. Int J Cancer. 2006;119(10):2366–2371. doi: 10.1002/ijc.22158 [DOI] [PubMed] [Google Scholar]
- 8.Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101(18):1280–1283. doi: 10.1093/jnci/djp262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zumsteg ZS, Spratt DE, Pei I, et al. A New Risk Classification System for Therapeutic Decision Making with Intermediate-risk Prostate Cancer Patients Undergoing Dose-escalated External-beam Radiation Therapy. Eur Urol. 2013;64(6):895–902. doi: 10.1016/J.EURURO.2013.03.033 [DOI] [PubMed] [Google Scholar]
- 10.Spratt DE, Zhang J, Santiago-Jiḿenez M, et al. Development and validation of a novel integrated clinical-genomic risk group classification for localized prostate cancer. J Clin Oncol. 2018;36(6):581–590. doi: 10.1200/JCO.2017.74.2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual, Eighth Edition.; 2017. https://www.springer.com/us/book/9783319406176. Accessed August 7, 2018.
- 12.Harvei S, Tretli S, Langmark F. Quality of Prostate Cancer Data in the Cancer Registry of Norway. Eur J Cancer. 1996;32(1):104–110. doi:10.1016/0959–8049(95)00501–3 [DOI] [PubMed] [Google Scholar]
- 13.Larsen IK, Småstuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: An overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–1231. doi: 10.1016/j.ejca.2008.10.037 [DOI] [PubMed] [Google Scholar]
- 14.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2015;40(2):1. doi: 10.1097/PAS.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 15.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and Prostate-Cancer Mortality in a Randomized European Study. N Engl J Med. 2009;360(13):1320–1328. doi: 10.1016/j.eeh.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 16.R Core Team. R: A language and environment for statistical computing . In: Vienna, Austria: R Foundation for Statistical Computing. ; 2015. [Google Scholar]
- 17.Engholm G, Ferlay J, Christensen N, et al. NORDCAN - A Nordic tool for cancer information, planning, quality control and research. Acta Oncol (Madr). 2010;49(5):725–736. doi: 10.3109/02841861003782017 [DOI] [PubMed] [Google Scholar]
- 18.Engholm G, Ferlay J, Christensen N, et al. Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 8.1 (28.06.2018). Association of the Nordic Cancer Registries. Danish Cancer Society. http://www.ancr.nu. Accessed September 18, 2018. [Google Scholar]
- 19.Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(18):1901–1913. doi: 10.1001/jama.2018.3710 [DOI] [PubMed] [Google Scholar]
- 20.Vickers AJ, Eastham JA, Scardino PT, Lilja H. The Memorial Sloan Kettering Cancer Center Recommendations for Prostate Cancer Screening. Urology. 2016;91:12–18. doi: 10.1016/j.urology.2015.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Urological Association. Early Detection of Prostate Cancer (2018). https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Published 2018. Accessed October 13, 2019.
- 22.Social Security Administration. Actuarial Life Table 2014. https://www.ssa.gov/OACT/STATS/table4c6.html. Accessed March 27, 2019.
- 23.Statistisk sentralbyrå (Statistics Norway). Life Expectancy - Remaining Years for Males and Females at Selected Ages. Statistisk sentralbyrå; https://www.ssb.no/en/befolkning/statistikker/dode/aar. Accessed March 27, 2019. [Google Scholar]
- 24.NCCN. Older Adult Oncology v2.2018. NCCN. 2018. [Google Scholar]
- 25.Andriole GL, Crawford ED, Grubb RL, et al. Mortality Results from a Randomized Prostate-Cancer Screening Trial. N Engl J Med. 2009;360(13):1310–1319. doi: 10.1056/NEJMoa0810696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf AMD, Wender RC, Etzioni RB, et al. American Cancer Society Guideline for the Early Detection of Prostate Cancer: Update 2010. CA Cancer J Clin. 2010;60(2):70–98. doi: 10.3322/caac.20066 [DOI] [PubMed] [Google Scholar]
- 27.Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Database Syst Rev. 2013;(1):CD004720. doi: 10.1002/14651858.CD004720.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical Prostatectomy or Watchful Waiting in Prostate Cancer — 29-Year Follow-up. N Engl J Med. 2018;379(24):2319–2329. doi: 10.1056/NEJMoa1807801 [DOI] [PubMed] [Google Scholar]
- 29.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of Androgen Suppression in the Treatment of Prostate Cancer. N Engl J Med. 2009;360(24):2516–2527. doi: 10.1056/NEJMoa0810095 [DOI] [PubMed] [Google Scholar]
- 30.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and Short-Term Androgen Deprivation for Localized Prostate Cancer. N Engl J Med. 2011;365(2):107–118. doi: 10.1056/NEJMoa1012348 [DOI] [PubMed] [Google Scholar]
- 31.Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: case-control study. BMJ. 2013;346:f2023. doi: 10.1136/bmj.f2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ankerst DP, Straubinger J, Selig K, et al. A Contemporary Prostate Biopsy Risk Calculator Based on Multiple Heterogeneous Cohorts. Eur Urol. 2018;74(2):197–203. doi: 10.1016/j.eururo.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ankerst DP, Hoefler J, Bock S, et al. Prostate cancer prevention trial risk calculator 2.0 for the prediction of low- vs high-grade prostate cancer. Urology. 2014;83(6):1362–1367. doi: 10.1016/j.urology.2014.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huynh-Le M-P, Fan CC, Parsons JK, et al. A genetic risk score to personalize prostate cancer screening, applied to population data. J Clin Oncol. 2019;37(7_suppl):181–181. doi: 10.1200/jco.2019.37.7_suppl.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alibhai SMH, Krahn MD, Fleshner NE, Cohen MM, Tomlinson GA, Naglie G. The association between patient age and prostate cancer stage and grade at diagnosis. BJU Int. 2004;94(3):303–306. doi: 10.1111/j.1464-410X.2004.04883.x [DOI] [PubMed] [Google Scholar]
- 36.Cremers RGHM Karim-Kos HE, Houterman S, et al. Prostate cancer: Trends in incidence, survival and mortality in the Netherlands, 1989–2006. Eur J Cancer. 2010;46(11):2077–2087. doi: 10.1016/j.ejca.2010.03.040 [DOI] [PubMed] [Google Scholar]
- 37.Møller MH, Kristiansen IS, Beisland C, Rørvik J, Støvring H. Trends in stage-specific incidence of prostate cancer in Norway, 1980–2010: a population-based study. BJU Int. 2016;118(4):547–555. doi: 10.1111/bju.13364 [DOI] [PubMed] [Google Scholar]
- 38.Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004–2014. Ann Epidemiol. 2018;28(5):328–330. doi: 10.1016/j.annepidem.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tangen CM, Goodman PJ, Till C, Schenk JM, Lucia MS, Thompson IM. Biases in recommendations for and acceptance of prostate biopsy significantly affect assessment of prostate cancer risk factors: Results from two large randomized clinical trials. J Clin Oncol. 2016;34(36):4338–4344. doi: 10.1200/JCO.2016.68.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Medicare and Medicaid Services. AUA Measure Development: PSA Screening Shared Decision Making Measures. Measure Development Education & Outreach for Specialty Societies & Patient Advocacy Groups. [Google Scholar]
- 41.Fosså SD, Eri LM, Skovlund E, Tveter K, Vatten L, Norwegian Urological Cancer Group. No randomised trial of prostate cancer screening in Norway. Lancet Oncol. 2001;2(12):741–745. doi: 10.1016/S1470-2045(01)00588-5 [DOI] [PubMed] [Google Scholar]
- 42.Schröder FH, De Koning HJ. The Norwegian decision on screening for prostate cancer: A response. Lancet Oncol. 2001;2(12):746–749. doi: 10.1016/S1470-2045(01)00589-7 [DOI] [Google Scholar]
- 43.Norwegian Directorate of Health. Screening for prostatakreft i den generelle befolkningen - Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av prostatakreft. https://www.helsebiblioteket.no/retningslinjer/prostatakreft/3-screening-og-tidlig-påvisning/3.1-screening-for-prostatakreft. Accessed February 2, 2019.
- 44.Breidablik HJ, Meland E, Aakre KM, Førde OH. PSA measurement and prostate cancer – overdiagnosis and overtreatment ? Tidsskr Nor Legeforen. 2013;16(133):1711–1716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.