Abstract
Enhancing skin allograft longevity lessens the need for new allografts before optimal intervention is available. Reduced activity of ADAMTS13 (an enzyme that cleaves the pro-thrombotic and proinflammatory von Willebrand factor) and presence of neutrophil extracellular traps (NETs) have been implicated in liver and lung allograft failures. The effect of ADAMTS13 treatment and the impact of NETs on skin allografts, however, remain unexplored. Here, we adopted a murine model of complete mismatch full thickness skin transplant by grafting dorsal skin from BALB/c mice to C57BL/6J background mice. Recombinant human ADAMTS13 (rhADAMTS13) treatment of graft recipients increased allograft survival. Western blot and immunofluorescence microscopy revealed the presence of NETs in allografts of vehicle, but surprisingly, not rhADAMTS13-treated mice, 3 days after surgery. Recapitulating the observations in mice, NETs were also observed in all the examined allografts from burn patients. Intriguingly, knocking out peptidylarginine deiminase 4 (PAD4, a key enzyme for NET formation) or DNase 1 treatment (which cleaves NETs) also prolonged allograft survival. In summary, rhADAMTS13 lessens inflammation in allografts by reducing NET burden, resulting in enhanced allograft survival. RhADAMTS13 and anti-NET treatments could be new therapeutic strategies to promote skin allograft longevity and, hence, the survival of patients with severe burns.
1. INTRODUCTION
Allogeneic skin transplant is a preferred first-line therapy for burn patients, in particular for those whose wounds do not have sufficient blood vessels to take autologous split-thickness skin grafts.1 Allografts not only serve as temporary dressings for burn wounds, they also prepare the wound bed for replacement with autografts later, such as by providing the wound bed with critical growth factors and cytokines for vascularization.2 In addition, unlike the civilian population who can be transported to a burn center within 24 hours after injury, combat-related burn wounds are not easily treated in a timely way. The average time for injured soldiers to receive optimal wound treatment is around 5 days.3,4 Immediate proper wound care, including skin allograft transplantation from deceased donors on location, is of paramount value before further medical or surgical intervention is available. Early excision of major burn wounds and temporary coverage with human cadaveric allografts have markedly improved survival of burn patients.5,6 Prolonging skin allograft survival or tolerance could reduce the need of re-allografting and hence the medical cost. It would also alleviate the challenge of skin donor shortage.
In addition to alloimmunity conferred by activated lymphocytes,7 microthrombosis may also be a key factor contributing to allograft rejection.8 ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) is a plasma metalloprotease that cleaves ultra-large von Willebrand factor (UL-VWF), which is highly proinflammatory and pro-thrombotic.9,10 Patients receiving liver transplants had significantly reduced plasma ADAMTS13 activity and elevated levels of UL-VWF multimers in the circulation.11 These changes were associated with post-transplant liver dysfunction, such as ischemia/reperfusion injury and acute rejection.11 Because organ deterioration was restricted to the transplanted liver, it could possibly be due to severe local microthrombosis. Indeed, VWF expression was elevated in grafted livers that were acutely rejected due to allogeneic immune response.12
VWF also promotes neutrophil recruitment13 and binds to neutrophil extracellular traps (NETs).14,15 VWF may therefore help NETs to anchor on the microthrombi in situ. Formation of NETs (NETosis) is mediated by peptidylarginine deiminase 4 (PAD4). By citrullinating arginine residues on histones, PAD4 massively decondenses the chromatin prior to NET release.16,17 NETs are lined with cytotoxic proteins that cause tissue damage, delay wound healing, and even aggravate sepsis.18–20 Intriguingly, NETs were present in the lungs of mice subjected to orthotopic lung transplant and were at higher levels in the bronchoalveolar lavage fluid of patients suffering severe primary graft dysfunction after lung transplant compared to patients free of the dysfunction.21 Disruption of NETs by DNase 1 rescued the function of the lung allografts in mice,21,22 indicating a pathogenic role of NETs in allograft failure.
Based on the above observations, we tested the hypothesis that limiting NET-mediated inflammation and microthrombosis could delay skin allograft rejection. We showed that NETs are present in the skin allografts of wild-type (WT) mouse recipients and burn patients, and that rhADAMTS13 treatment is an anti-NET strategy, which, similar to PAD4 deficiency (NETosis inhibition) or administration of DNase 1 (NET degradation), enhances skin allograft longevity.
2. MATERIAL AND METHODS
2.1. Animals
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Boston Children’s Hospital (Protocol number: 16–05-3199). Male mice aged 8–12 weeks, either bred in-house or purchased from the Jackson Laboratory, were used in the experiments. Mice were housed individually after skin transplant surgery to prevent them from disturbing each other’s grafts. All mice were fed a standard lab diet and maintained under standard laboratory conditions free of specific pathogens.
2.1.1. Skin transplant surgery
Complete mismatch skin allograft transplant model was adopted in the present study. Briefly, BALB/c WT mice were used as skin donors, and mice in C57BL/6J background were employed as graft recipients. Donor mice were euthanized by cervical dislocation. Their dorsal skin was rapidly shaved, sterilized alternatively with 70% ethanol wipes and betadine three times, and harvested. Connective tissue, fat tissue, and panniculus carnosus were removed from the donor’s skin, which was then cut into 1 cm x 1 cm grafts and kept in PBS on ice.23 After graft preparation, the dorsal skin of the recipient mice was shaved and sterilized. With proper anesthesia using isoflurane inhalation, a piece of full-thickness skin (1 cm x 1 cm) was excised from the back of the recipient mice, followed by suturing of the allograft to the wound. The mice were then bandaged for 7 days, upon which the sutures were removed. Acute graft rejection began with swelling and erythema, followed by graft desiccation, shrinkage, and scab formation. Complete rejection was defined as complete hardening of the graft with loss of hair.23
2.1.2. Administration of rhADAMTS13 and DNase 1
To examine whether rhADAMTS13 enhances skin allograft survival, BALB/c WT mice were used as skin donors and C57BL/6J WT as allograft recipients. RhADAMTS13 (Baxalta/Shire) was given at 3500 U/kg via retro-orbital intravenous injection immediately after grafting, and then once daily up to 7 days post transplantation (Figure 1A). Sterile saline was used as the vehicle control. The experiment was repeated using C57BL/6J Padi4−/− mice as allograft recipients.
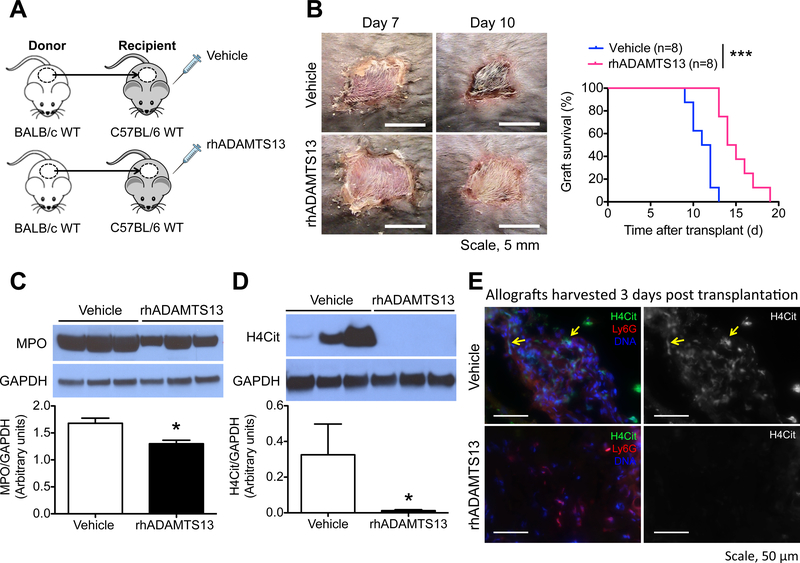
Figure 1.
Recombinant human ADAMTS13 (rhADAMTS13) enhances skin allograft survival in mice by reducing inflammation and NET burden in the graft. (A) RhADAMTS13 treatment of mice subjected to the complete mismatch skin transplant model, where BALB/c wild type (WT) served as skin donors and C57BL/6J WT as graft recipients. Mice were given rhADAMTS13 once daily for 7 days after skin transplant surgery. (B) Representative photographs of the allografts from vehicle- and rhADAMTS13-treated mice on day 7 and day 10 post transplantation and the allograft survival curves of these mice. n = 8 per group; ***P<0.001, Log-rank test. (C, D) Representative Western blots and summarized data of the levels of (C) myeloperoxidase (MPO) and (D) citrullinated histone H4 (H4Cit) in allografts harvested from vehicle- and rhADAMTS13-treated mice 3 days post transplantation. n = 6 per group; *P<0.05, Mann-Whitney test. (E) Immunofluorescence images showing sections of allografts from vehicle- and rhADAMTS13-treated mice 3 days after grafting. The yellow arrows depict the presence of NETs in the allograft of the vehicle-treated mice.
For DNase 1 treatment, which cleaves extracellular DNA including NETs, C57BL/6J WT were allograft recipients, and DNase 1 (dornase alfa, Genentech) was administered at 10 μg by retro-orbital intravenous injection 10 min before allograft transplant and 50 μg by intraperitoneal injection immediately after the surgery. DNase 1 was then given at 50 μg by intraperitoneal injection twice daily for 7 days post surgery (Figure 2F). Sterile saline was used as the vehicle control.
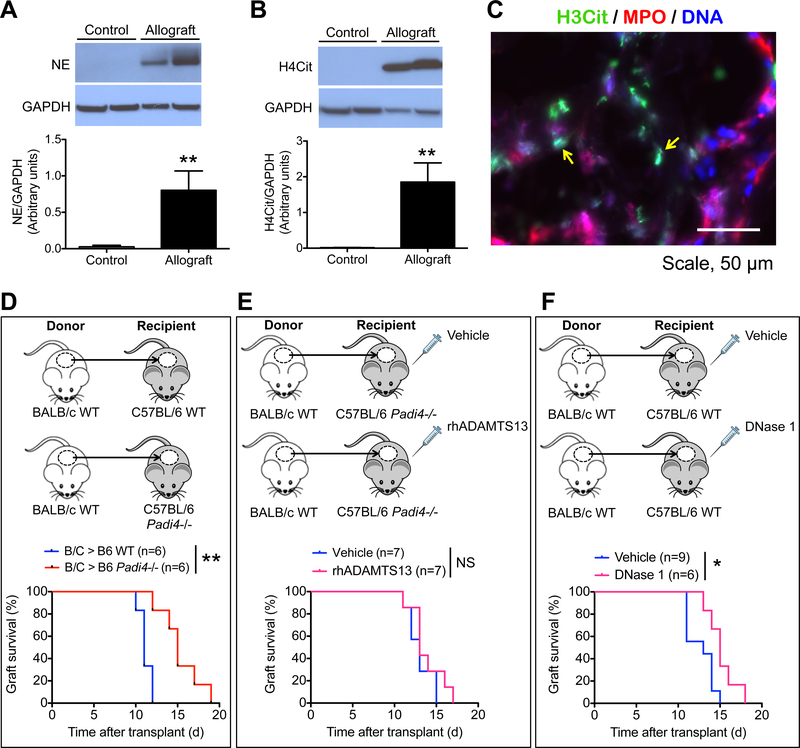
Figure 2.
NETs are present in the allografts of burn patients, and anti-NET strategies promote allograft survival in mice. (A, B) Representative Western blots and summarized data of the levels of (A) neutrophil elastase (NE) and (B) H4Cit in control skin (intact skin adjacent to burn wounds or skin collected for autografts) and allografts from burn patients. n = 4 for control, n = 6 for allograft, **P<0.01, Mann-Whitney test. (C) Representative immunofluorescence image showing the presence of NETs (yellow arrows) in the allograft of a burn patient. (D-F) Allograft survival curves of (D) WT versus Padi4−/− mouse recipients, (E) Padi4−/− allograft recipients treated with vehicle or rhADAMTS13, and (F) WT allograft recipients treated with vehicle or DNase 1. n = 6–9 per group as indicated on the graphs; *P<0.05, **P<0.01, NS, non-significant, Mann-Whitney test.
2.2. Human specimens
Control skin (i.e., intact skin adjacent to burn wounds or skin collected for autografts) and allografts were procured from burn patients in collaboration with Jeremy Goverman with Institutional Review Board approval from Massachusetts General Hospital (Protocol number: 2015P002056) and patient consent. The age range of the burn patients was 21–63 years old. The allografts were from multiple deceased donors. Skin and allograft samples were retrieved 7–15 days post burn injury. Samples included in the analysis were free from infection upon procurement. The tissues were snap frozen for Western blotting or embedded in Optimal Cutting Temperature (OCT) compound for immunofluorescence staining.
2.3. Immunofluorescence microscopy
Localization of citrullinated histone H3 or citrullinated histone H4 (H3Cit and H4Cit, NET biomarkers) and neutrophils in the allografts were examined by immunofluorescence microscopy. Human skin and allograft specimens, as well as allografts of vehicle- or rhADAMTS13-treated mice harvested on day 3, were cryosectioned into 10 μm sections. The sections were post-fixed in zinc fixative (100 mM Tris-HCl, 37 mM zinc chloride, 23 mM zinc acetate, 3.2 mM calcium acetate), permeabilized with 0.1% Triton-X and 0.1% sodium citrate for 10 min at 4°C, blocked with 3% BSA or 2.5% BSA/5% donkey serum in PBS, and incubated with combinations of primary antibodies against H3Cit (Abcam, ab5103, 1:1,000), H4Cit (Millipore, Cat. no. 07–596, 1:250), Ly6G (BD Pharmingen, Cat. no. 551459, 1:500) or myeloperoxidase (MPO, Abcam, Cat. no. ab25989, 1:1,000) at 4°C overnight. After washes, the sections were incubated with appropriate Alexa Fluor-conjugated secondary antibodies (Invitrogen, 1:1,500) for 2 h at room temperature. Hoechst 33342 (Invitrogen, Cat. no. H3570, 1:10,000) was used to counterstain for DNA. Images were acquired on an Axiovert 200M wide-field fluorescence microscope (Zeiss) coupled to an AxioCam MR3 monochromatic CCD camera (Zeiss) using a Zeiss Ph2 Neofluar 40x/0.75 objective lens with the Zeiss AxioVision software (version 4.6.3.0).
2.4. Western blot analysis
Levels of H4Cit, MPO, and neutrophil elastase of allografts were quantified by Western blotting. Samples were snap frozen upon retrieval and homogenized in RIPA buffer supplemented with protease inhibitor cocktails (Sigma) on ice. After centrifugation at 20,000 g for 20 min at 4°C, the protein content of the supernatant was determined by bicinchoninic acid protein assay, and an equal amount of protein per sample was resolved on gradient gels (Bolt 4–12% Bis-Tris Plus gels, Life Technologies) and electrobloted on PVDF membranes. After blocking, the membranes were incubated with primary antibodies against H4Cit (Millipore, Cat. no. 07–596, 1:500), MPO (DAKO, A0398, 1:500), or neutrophil elastase (Abcam, ab68672, 1:500) at 4°C overnight, and subsequently with goat anti-rabbit IgG (H+L)-HRP (BioRad, 1:10,000) for 2 h at room temperature. Blots were developed with enhanced chemiluminescence substrate (Thermo Scientific, Cat. no. 32106). Equal loading was confirmed by probing for GAPDH (Ambion, Cat. no. AM4300, 1:40,000). Blots were quantified using ImageJ software by dividing the intensity of protein-of-interest by the intensity of GAPDH.
2.5. Statistical analysis
Data are presented as mean ± S.E.M. At least two independent animal experiments were performed, and they were analyzed using Mann-Whitney test or Log-rank test (for graft survival). All analyses were performed using GraphPad Prism software (Version 5.0). Results were considered significant when P<0.05. Mice that disturbed or injured their grafts post surgery were sacrificed immediately and not included.
3. RESULTS
3.1. rhADAMTS13 treatment enhances allograft survival
To examine the effect of exogenous ADAMTS13 on skin allograft survival, C57BL/6J WT allograft recipients were treated daily with either rhADAMTS13 or vehicle for 7 days post surgery (Figure 1A). The grafts of vehicle- and rhADAMTS13-treated mice were similar in appearance and remained soft on day 7 upon suture removal. Strikingly after this period, the allografts of the vehicle-treated mice showed signs of rejection (erythema, graft desiccation, and shrinkage) much earlier than those of the rhADAMTS13-treated recipients (Figure 1B). Median allograft survivals of vehicle- and rhADAMTS13-treated mice were 11.5 and 14.5 days, respectively. RhADAMTS13 was, therefore, beneficial to allograft survival and significantly enhanced median survival of the allografts by 26%, compared to the vehicle-treated control.
3.2. NET burden is reduced in allografts of rhADAMTS13-treated mice
Neutrophils and NETs have been shown in allografts of other solid-organ transplants, such as lungs,21 as well as in skin wounds.20 Therefore, we examined whether NETs are generated in skin allografts. PAD4 citrullinates histones to decondense chromatin before NETosis.16,17 Thus, citrullinated histones H4 or H3 (H4Cit and H3Cit) have been widely adopted as biomarkers for NETosis.20,24–26 Western blot analysis showed that neutrophils (using MPO as the marker) and NETs (indicated by H4Cit) were present in the allografts of vehicle-treated mice 3 days post transplantation (Figure 1C,D). RhADAMTS13 reduced neutrophil infiltration into the allografts by 23% (Figure 1C); however, remarkably, NETs were undetectable in these allografts of the rhADAMTS13-treated mice (Figure 1D). Immunofluorescence microscopy revealed H4Cit+ extracellular DNA in areas with neutrophil accumulation (Ly6G+ areas, membrane marker of neutrophils) (Figure 1E) throughout the allograft. In contrast, there were fewer neutrophils and an absence of H4Cit+ cells in the allografts of rhADAMTS13-treated mice (Figure 1E), substantiating the findings of Western blotting (Figure 1C,D). Thus, rhADAMTS13 decreases inflammation/pro-thrombotic potential in the allografts by reducing neutrophil content and NET burden.
3.3. NETs are present in human skin allografts
To evaluate whether the mouse findings are relevant to humans, we examined NET presence in human skin allografts. Western blot of surgically removed specimens showed that neutrophils (indicated by neutrophil elastase) and NETs (indicated by H4Cit) were non-detectable in the control skin, while there were high levels of both in the allografts of the burn patients (Figure 2A, B). Immunofluorescence staining showed that NETs (H3Cit+ and MPO+ extracellular DNA) were dispersed across the allograft tissue (Figure 2C), corroborating the observations of the mouse study.
3.4. PAD4 deficiency and NET cleavage prolong allograft longevity
Since rhADAMTS13 enhanced allograft longevity and reduced allograft NET burden, we asked next whether inhibition of NET production offers benefits to the graft. The dorsal skin of BALB/c mice was transplanted to C57BL/6J WT and C57BL/6J Padi4−/− mice, with the latter being deficient in NETosis. Complete graft rejection occurred significantly later in Padi4−/− recipients. The median allograft survivals of WT and Padi4−/− recipients were 11 and 15 days, respectively (Figure 2D). The 36% increase in median survival indicates that absence of PAD4 is highly beneficial to allograft longevity. Interestingly, when rhADAMTS13 was administered to the Padi4−/− allograft recipients, there was no additional impact on allograft survival compared to vehicle-treated Padi4−/− (median survival of vehicle and rhADAMTS13-treated Padi4−/− were both 13 days in this experiment) (Figure 2E), indicating that the rhADAMTS13 effect is mediated through a reduction of NET burden or, possibly, reduction in PAD4 activation.
Since DNase 1 cleaves the DNA backbone of NETs, we tested whether 7-day DNase 1 treatment can also increase allograft longevity (Figure 2F). The median allograft survival of vehicle- and DNase 1-treated mice were 13 and 15 days, respectively. Compared to complete NET inhibition, DNase 1 treatment only offered a 15% enhancement in median survival of the allografts.
4. DISCUSSION
Plasma enzymes that cleave UL-VWF (ADAMTS13) and NETs (DNase 1) have been used clinically,27,28 but their therapeutic potential, as well as the role of UL-VWF and NETs, has not been explored in allogeneic skin transplant. The current study combined preclinical animal studies for drug testing and burn patient specimen analysis to evaluate whether NETs can serve as a biomarker that indicates early skin allograft rejection. We found that administration of exogenous rhADAMTS13 or DNase 1 enhanced skin allograft longevity in mice; and, importantly, inhibition of NET formation by knocking out PAD4 in the allograft recipient mice also markedly delayed allograft rejection. Thus, the present findings suggest that NETosis inhibition, as well as NET degradation after they are formed, are both beneficial in enhancing allograft survival. NET production in skin allografts was not confined to the mouse models, as we also observed a profound increase in the levels of neutrophils and NET biomarker (H3Cit and H4Cit) in the allografts of burn patients. In WT allograft mouse recipients, NETs were detectable 3 days post allograft transplant, where no signs of rejection can be observed macroscopically. In mice treated with rhADAMTS13, NETs were rare, and grafts had a longer half-life. Thus, early emergence of NETs in the skin allograft may be an indicator of later graft rejection.
Neutrophils are the main leukocytes that are rapidly recruited to wounds upon injury, and they have been implicated in delayed wound healing (Wong et al., 2015).20 The current study showed, for the first time, that NETs are generated in skin allografts in both mice and humans and contribute to allograft rejection. This corroborates the findings on orthotopic lung transplant, where NETs are present in the graft, and disruption of NETs improves primary graft function.21,22 We also showed that rhADAMTS13, which effectively dampens inflammatory responses post skin allograft transplant, profoundly enhances allograft longevity, as well. The exact mechanism through which rhADAMTS13 has this effect is not known, but it likely implicates the disruption of NET retention. Alternatively, NET formation could be reduced in the absence of neutrophil adhesion to UL-VWF.
Considerable research effort has been devoted to increasing skin allograft survival.29,30 Combined treatment of IL-2 and rapamycin was shown to maintain skin allograft survival for at least 60 days, however, the success is restricted to mouse strains with genetic backgrounds that differ only in minor histocompatibility antigens.31 This treatment has no beneficial effect when the donor and recipient are completely mismatched, such as C57BL/6 and BALB/c in our current setting.31 Our study suggests that anti-UL-VWF and anti-NET treatments are more effective in enhancing skin allograft survival and open new avenues in the search for strategies to delay allograft rejection. How exactly NETosis modulates alloimmune responses warrants further investigation. Proliferation and proinflammatory response of CD8+ T cells mediate skin allograft rejection after burn injuries in mice.32 Since NETting neutrophils can prime T cells for proliferation and cytokine production,33 it is possible that NETs contribute to the development of alloimmune responses via T cell activation.
In contrast to our findings that disruption of NETs increases skin allograft survival, Scozzi et al. reported that the release of NET fragments by DNase 1 stimulates innate immune responses that prevent tolerance of the lung transplant.22 Such a discrepancy could be due to the difference in the type of organ that is transplanted. While alloimmune response to the skin allograft is attributed to the infiltration of T cells preactivated in the draining lymph node,7 T cell priming occurs in the allograft tissue of the lung transplant34 where NET fragments prolong the interaction between antigen-presenting cells and T cells.22 To the general field of organ transplant, inhibiting NET generation, rather than cleaving NETs, may represent an unequivocal beneficial approach to enhance allograft survival and tolerance. Indeed, apart from chromatin decondensation for NETosis, PAD4 also augments the expression of proinflammatory cytokines, IL-1β and TNF-α, by citrullinating NF-κB p65 subunit, thereby promoting its nuclear translocation in neutrophils.35 In macrophages, PAD2 and PAD4 are required for the assembly of NLRP3 inflammasome and IL-1β release.36 Interestingly, recent results indicate that NLRP3 inflammasome in neutrophils is important in NET formation, as well.37 Thus, specific PAD4 inhibitors and, perhaps, NLRP3 inhibitors would be highly valuable for this new line of research, with the potential for clinical application.
PAD4 inhibition may also provide benefits beyond the salvage of allografts. Burn patients have increased NETs in the circulation after burn injuries,38 and it has been reported that thrombotic thrombocytopenic purpura and hemolytic uremic syndrome can develop after a burn injury.39,40 Both of these conditions could be attributed to an impairment of UL-VWF cleavage by ADAMTS13.41,42 Recently, it was reported that endogenous ADAMTS13 activity is reduced when the enzyme is citrullinated by PAD4, which remains active after its release from neutrophils in processes such as NETosis.43 Therefore, preventing NETosis or inhibiting PAD4 may also protect burn patients from developing burn-induced thrombotic microangiopathies.
To conclude, the present study demonstrates that anti-UL-VWF and anti-NET strategies increase the viability of allogeneic skin grafts in mice. Potentially, these treatments could expand the treatment window for optimal intervention of burn injuries in patients. Recently, NETs have been shown to serve as an important link between innate and adaptive immunity, and their impact in transplantation medicine is emerging. Investigations on other solid-organ transplant may further unveil the clinical significance of targeting NETs and UL-VWF in enhancing allograft tolerance and patient survival.
ACKNOWLEDGMENTS
We thank Ann Elizabeth Whalen (Massachusetts General Hospital) for collection and initial processing of human specimens, and Tiffany Frary (Boston Children’s Hospital) for the assistance in manuscript preparation. This work was funded by Baxalta US Inc., a member of the Takeda group of companies, Investigator Initiated Research Grant H15-30683 (to DDW), NIH R35 HL135765 (to DDW) and Pilot Research Grant from Plastic Surgeon Foundation (to JG).
ABBREVIATIONS
- ADAMTS13
a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13
- H3Cit
citrullinated histone H3
- H4Cit
citrullinated histone H4
- MPO
myeloperoxidase
- NETs
neutrophil extracellular traps
- PAD4
peptidylarginine deiminase 4
- Padi4−/−
mice deficient in peptidylarginine deiminase 4
- rhADAMTS13
recombinant human ADAMTS13
- UL-VWF
ultra-large von Willebrand factor
- VWF
von Willebrand factor
- WT
wild-type
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Kitala D, Kawecki M, Klama-Baryla A, et al. Allogeneic vs. Autologous Skin Grafts in the Therapy of Patients with Burn Injuries: A Restrospective, Open-label Clinical Study with Pair Matching. Adv Clin Exp Med. 2016;25(5):923–929. [DOI] [PubMed] [Google Scholar]
- 2.Landsman A, Rosines E, Houck A, et al. Characterization of a Cryopreserved Split-Thickness Human Skin Allograft-TheraSkin. Adv Skin Wound Care. 2016;29(9):399–406. [DOI] [PubMed] [Google Scholar]
- 3.Johnson BW, Madson AQ, Bong-Thakur S, et al. Combat-related facial burns: analysis of strategic pitfalls. J Oral Maxillofac Surg. 2015;73(1):106–111. [DOI] [PubMed] [Google Scholar]
- 4.Wolf SE, Kauvar DS, Wade CE, et al. Comparison between civilian burns and combat burns from Operation Iraqi Freedom and Operation Enduring Freedom. Ann Surg. 2006;243(6):786–792; discussion 792–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong YS, Samuel M, Song C. Meta-analysis of early excision of burns. Burns. 2006;32(2):145–150. [DOI] [PubMed] [Google Scholar]
- 6.Orgill DP. Excision and skin grafting of thermal burns. N Engl J Med. 2009;360(9):893–901. [DOI] [PubMed] [Google Scholar]
- 7.Benichou G, Yamada Y, Yun SH, et al. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy. 2011;3(6):757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanitakis J, Petruzzo P, Gazarian A, et al. Capillary Thrombosis in the Skin: A Pathologic Hallmark of Severe/Chronic Rejection of Human Vascularized Composite Tissue Allografts? Transplantation. 2016;100(4):954–957. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Chung DW. Inflammation, von Willebrand factor, and ADAMTS13. Blood. 2018;132(2):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawecki C, Lenting PJ, Denis CV. von Willebrand factor and inflammation. J Thromb Haemost. 2017;15(7):1285–1294. [DOI] [PubMed] [Google Scholar]
- 11.Ko S, Okano E, Kanehiro H, et al. Plasma ADAMTS13 activity may predict early adverse events in living donor liver transplantation: observations in 3 cases. Liver Transpl. 2006;12(5):859–869. [DOI] [PubMed] [Google Scholar]
- 12.Kiuchi T, Oldhafer KJ, Schlitt HJ, et al. Background and prognostic implications of perireperfusion tissue injuries in human liver transplants: a panel histochemical study. Transplantation. 1998;66(6):737–747. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan AK, Kisucka J, Brill A, et al. ADAMTS13: a new link between thrombosis and inflammation. J Exp Med. 2008;205(9):2065–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107(36):15880–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grassle S, Huck V, Pappelbaum KI, et al. von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arterioscler Thromb Vasc Biol. 2014;34(7):1382–1389. [DOI] [PubMed] [Google Scholar]
- 16.Lewis HD, Liddle J, Coote JE, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11(3):189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li P, Li M, Lindberg MR, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207(9):1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7(2):e32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong SL, Demers M, Martinod K, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21(7):815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayah DM, Mallavia B, Liu F, et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2015;191(4):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scozzi D, Wang X, Liao F, et al. Neutrophil extracellular trap fragments stimulate innate immune responses that prevent lung transplant tolerance. Am J Transplant. 2019;19(4):1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng CH, Lee CF, Fryer M, et al. Murine Full-thickness Skin Transplantation. J Vis Exp. 2017(119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borissoff JI, Joosen IA, Versteylen MO, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33(8):2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110(21):8674–8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savchenko AS, Martinod K, Seidman MA, et al. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J Thromb Haemost. 2014;12(6):860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scully M, Knobl P, Kentouche K, et al. Recombinant ADAMTS-13: first-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood. 2017;130(19):2055–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suri R The use of human deoxyribonuclease (rhDNase) in the management of cystic fibrosis. BioDrugs. 2005;19(3):135–144. [DOI] [PubMed] [Google Scholar]
- 29.Larocca RA, Moraes-Vieira PM, Bassi EJ, et al. Adipose tissue-derived mesenchymal stem cells increase skin allograft survival and inhibit Th-17 immune response. PLoS One. 2013;8(10):e76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mastroianni M, Ng ZY, Goyal R, et al. Topical Delivery of Immunosuppression to Prolong Xenogeneic and Allogeneic Split-Thickness Skin Graft Survival. J Burn Care Res. 2018;39(3):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilon CB, Petillon S, Naserian S, et al. Administration of low doses of IL-2 combined to rapamycin promotes allogeneic skin graft survival in mice. Am J Transplant. 2014;14(12):2874–2882. [DOI] [PubMed] [Google Scholar]
- 32.Maile R, Barnes CM, Nielsen AI, et al. Lymphopenia-induced homeostatic proliferation of CD8+ T cells is a mechanism for effective allogeneic skin graft rejection following burn injury. J Immunol. 2006;176(11):6717–6726. [DOI] [PubMed] [Google Scholar]
- 33.Tillack K, Breiden P, Martin R, et al. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. 2012;188(7):3150–3159. [DOI] [PubMed] [Google Scholar]
- 34.Gelman AE, Li W, Richardson SB, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182(7):3969–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun B, Dwivedi N, Bechtel TJ, et al. Citrullination of NF-kappaB p65 promotes its nuclear localization and TLR-induced expression of IL-1beta and TNFalpha. Sci Immunol. 2017;2(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra N, Schwerdtner L, Sams K, et al. Cutting Edge: Protein Arginine Deiminase 2 and 4 Regulate NLRP3 Inflammasome-Dependent IL-1beta Maturation and ASC Speck Formation in Macrophages. J Immunol. 2019;203(4):795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.nzer P, Negro R, Magupalli V, et al. Abstract 118: Assembly of the Nlrp3 inflammasome regulates NET formation and is promoted by the vimentin intermediate filament cytoskeletal system. Arterioscler Thromb Vasc Biol. 2019;39:A118. [Google Scholar]
- 38.Otawara M, Roushan M, Wang X, et al. Microfluidic Assay Measures Increased Neutrophil Extracellular Traps Circulating in Blood after Burn Injuries. Sci Rep. 2018;8(1):16983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Öztürk MA, Babacanlar N, Akkoz C, et al. Can burn injury cause thrombotic thrombocytopenic purpura? South Clin Ist Euras. 2019;30(2):175–177. [Google Scholar]
- 40.Emil S, Rockstad R, Vannix D. Hemolytic uremic syndrome in a child with burn injuries. J Burn Care Rehabil. 1998;19(2):135–137. [DOI] [PubMed] [Google Scholar]
- 41.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347(8):589–600. [DOI] [PubMed] [Google Scholar]
- 42.Nolasco LH, Turner NA, Bernardo A, et al. Hemolytic uremic syndrome-associated Shiga toxins promote endothelial-cell secretion and impair ADAMTS13 cleavage of unusually large von Willebrand factor multimers. Blood. 2005;106(13):4199–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorvillo N, Mizurini DM, Coxon C, et al. Plasma Peptidylarginine Deiminase IV Promotes VWF-Platelet String Formation and Accelerates Thrombosis After Vessel Injury. Circ Res. 2019;125(5):507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]