Abstract
Prenatal arsenic exposure has been associated with reduced fetal growth and increased risk for preterm birth, but most studies have been conducted in highly exposed populations outside the U.S. or in non-Hispanic populations in the rural U.S. The objectives of the current study were to: 1) examine the impact of early pregnancy exposure to arsenic on birth weight and gestational age at birth in a predominately lower income Hispanic pregnancy cohort in urban Los Angeles and 2) compare multiple biomarkers of arsenic exposure (blood, urine, and hair) assessed in early pregnancy (mean ± SD gestational age at biospecimen collection: 14 ± 4 weeks). Total arsenic (blood, hair) was measured by ICP-MS and speciated arsenic (urine) was measured by HPLC coupled to ICP-MS. Associations between log2-transformed arsenic measures and birth outcomes were evaluated using multivariable linear regression. A doubling in hair arsenic was associated with a 72.2 g (95% CI: −144.3, −0.1, P=0.05) lower birth weight, after adjusting for potential confounders and gestational age at birth. A similar but non-significant trend was observed for blood arsenic, but not urine arsenic. The inverse association between hair arsenic and birth weight was more pronounced among infants whose mothers gained greater amounts of weight during pregnancy (Pinteraction=0.02). The association between urinary monomethyl arsenic and GA at birth differed by pre-pregnancy BMI (Pinteraction<0.01). This study provides evidence that even at relatively low levels of exposure, arsenic exposure (measured in hair samples collected in early pregnancy) may adversely affect fetal growth in this understudied population, particularly in combination with greater gestational weight gain. Future studies with larger sample sizes are needed to confirm these findings and to further investigate some of the inconsistencies observed for the different arsenic biomarkers evaluated.
Keywords: arsenic, birth outcomes, gestational weight gain, low-income, Hispanic, urban
1. Introduction:
Rates of low birth weight and preterm birth have been increasing in the U.S. (1). This may have profound impacts on public health, as both outcomes have been related to greater risks of morbidity and mortality across the life course (2–14). A diverse set of environmental toxicant exposures, including persistent organic pollutants, endocrine disruptors, and air pollution, have been associated with reduced fetal growth and gestational length (15–17). Growing evidence indicates that prenatal exposure to arsenic, a toxic metalloid that readily crosses the placenta (18), may also reduce fetal growth and increase risk for preterm birth (19–23).
Certain subpopulations may be more susceptible to the toxic effects of in utero arsenic exposure. Several studies have observed differential associations between arsenic exposure and birth outcomes depending on the sex of the newborn (19, 24–28), maternal pre-pregnancy BMI (24), and maternal smoking status during pregnancy (28). There is also evidence that inefficient arsenic metabolism increases risk for adverse birth outcomes (25, 29). Arsenic metabolism consists of two sequential methylation reactions: 1) the methylation of inorganic arsenic (iAs) to monomethyl arsenic (MMA), a toxic intermediate of arsenic metabolism, and 2) the methylation of MMA to dimethyl arsenic (DMA), which is rapidly eliminated into urine (30). The percentages of these three major metabolites in blood or urine (calculated as (iAs/(iAs+MMA+DMA))*100, (MMA/iAs+MMA+DMA)*100), and DMA/(iAs+MMA+DMA)*100) are thought to reflect arsenic methylation capacity and have been associated with numerous adverse health outcomes (31, 32).
The majority of previous studies examining prenatal arsenic exposure or arsenic metabolism in relation to birth outcomes have been conducted in highly exposed populations outside the U.S. or in predominately non-Hispanic populations in the rural U.S. (23, 33). Much less is known about these relationships in minority populations in the urban U.S. Additionally, few studies have compared multiple biomarkers of arsenic exposure, which represent different time windows of exposure and arsenic species. The objective of the current study was to examine the impact of prenatal arsenic exposure, measured in urine, blood, and hair, and specific urine arsenic metabolites, on birth weight and gestational age (GA) at birth in the Maternal and Developmental Risks from Environmental and Social Stressors (MADRES) pregnancy cohort (34), which represents a mostly lower income Hispanic population in urban Los Angeles.
2. Methods:
2.1. Study Participants
MADRES is an ongoing, prospective pregnancy cohort, which began in November 2015 and has been described previously (34). Briefly, participants are recruited from four prenatal care providers in Los Angeles, California, which include two community health clinics, one county hospital prenatal clinic, and one private obstetrics and gynecology practice. A small number of participants are also recruited through self-referral from community meetings and local advertisements. Most of the participating clinics serve predominately lower income Hispanic populations. Women are eligible to participate in MADRES if they are less than 30 weeks gestation at the time of recruitment, ≥18 years of age, and can speak either English or Spanish fluently. The current paper focused on participants who enrolled prior to 20 weeks gestation, given our interest in early pregnancy exposure to arsenic. Exclusion criteria for the study include: 1) HIV positive status; 2) having a physical, mental, or cognitive disability that would prevent participation in the study or the ability to provide informed consent, 3) current incarceration, and 4) multiple gestation. Informed consent was obtained from each participant at study entry, and the study was approved by the University of Southern California’s Institutional Review Board.
Laboratory analyses for hair arsenic assessment occurred in Fall 2018. At this time, a total of 366 MADRES participants had enrolled in the study prior to 20 weeks gestation, 233 (63.7%) of whom had reached their first trimester study visit window and provided a first trimester hair sample. Of these 233 first trimester hair samples, 167 were selected for hair arsenic testing, because these participants had not withdrawn from the study, had participated in at least one additional study visit, and had mostly complete covariate information. Laboratory analyses for blood arsenic assessment occurred in Summer 2018. At the time of sample selection, a total of 358 participants had enrolled in the MADRES study prior to 20 weeks gestation, 191 (53.4%) of whom had reached their first trimester study visit window and provided a blood sample. We prioritized 176 of these participants for blood arsenic assessment, because they had not withdrawn and also had a paired third trimester blood sample. Laboratory analyses for urine arsenic assessment were completed in July 2019. By that time, a total of 444 MADRES participants had enrolled in the study prior to 20 weeks gestation, 304 (68.5%) of whom had reached their first trimester study visit window and provided a urine sample. After excluding participants who had withdrawn prior to delivery, all remaining trimester 1 urine samples (N=296) were sent out for arsenic testing. Of the 167, 176, and 296 MADRES participants with hair, blood, and urine arsenic measurements, 116 (69.5%), 100 (56.8%), and 167 (56.4%) participants, respectively, had reached delivery and had high-quality birth outcomes data abstracted from electronic medical records (described in more detail below) and relevant covariate information at the time the statistical analyses were conducted (Fall 2019).
2.2. Biospecimen Collection
Maternal hair, blood, and urine samples were collected from participants between 6 and 24 weeks gestation (mean ± SD: 14 ± 4 weeks gestation). Hair samples (~50 strands) were collected at the base of the skull and stored in labeled paper envelopes at −80 °C. Up to 50 mL fasting blood were collected by a trained phlebotomist, using standard venipuncture protocols, in BD Vacutainer (blue top) EDTA collection tubes (Fisher Scientific) designed for trace element testing. Blood samples were transported on ice to the laboratory within one hour and stored at −80 °C. Spot urine samples were collected by participants in a 90 mL sterile specimen container and transported on ice to the laboratory within one hour. 1.5 mL aliquots were then stored at −80 °C in 2 mL sterile cryovials (VWR).
2.3. Arsenic Measurements
Hair arsenic concentrations were measured in the trace metal facility at the University of California, Santa Cruz. As described previously (35), cleaning and processing of hair samples was conducted in a HEPA filtered-air trace metal clean room, using acid-cleaned labware and ultrapure trace metal grade reagents. Two centimeters of hair were trimmed from the scalp end of each sample and cleaned of exogenous contamination, as described previously (36). Briefly, samples were placed in acid-cleaned 5 mL polypropylene syringe tubes and sonicated (20 min) in 0.5% Triton, rinsed five times with ultrapure Milli-Q water, sonicated (10 min) in 1 N trace metal grade nitric acid (Fisher Scientific, Santa Clara, CA, USA), rinsed with 1 N nitric acid, and rinsed five-times with Milli-Q water, and then dried at 65 °C for 48 h. Subsequently, samples were digested in 0.5 mL 15.7 N quartz-distilled nitric acid (Fisher Scientific, optima grade) at 80 °C for 6 h in a Class-100 HEPA filtered-air fume ho od, and then diluted with 5 mL Milli-Q water. For analyses, 0.25 mL of digestate was transferred to an acid-cleaned polyethylene microfuge tube, diluted with 0.25 mL Milli-Q water, and centrifuged at 13,000 × g for analysis. Rhodium and thallium were added to samples as internal standards, and samples were analyzed for total arsenic by magnetic sector inductively coupled plasma mass spectrometry (ICP-MS) (Thermo Element XR ICP-MS, Waltham, MA, USA). Methane was added to the argon (Ar) carrier gas to minimize ArCl formation. The analytical limits of detection (LOD) over four runs ranged from 0.001 to 0.008 ng/mL. For all hair samples, arsenic concentrations exceeded the LOD. The National Institute for Environmental Studies (Japan) Standard Reference Material (SRM) NIES 13 (human hair) was used to assess analytical accuracy. The mean SRM recovery of arsenic (%recovery ± %relative standard deviation) based on the non-certified reference value was 76% ± 2%.
Blood arsenic concentrations were measured in William Funk’s laboratory at Northwestern University. 50 μL of whole blood was added directly into 15 mL metal-free polypropylene centrifuge tubes containing 2.5 mL extraction solution (5% ultrapure acetic acid and 0.01% ultrapure Triton X-100 in deionized water) using acid-washed pipette tips. Blood extracts were centrifuged at 3,600 x g for 3 minutes and incubated at room temperature on a shaker table at 300 rpm for 90 minutes. Total arsenic was then quantified using a Thermo Scientific iCAP Q ICP-MS. Samples were measured across seven instrument runs. The LODs ranged from 0.0018 to 0.0071 μg/L. None of the blood arsenic concentrations were below the LOD.
Urine arsenic metabolites, including arsenite (AsIII), arsenate (AsV), MMA, DMA, and arsenobetaine (AsB) were measured by High Performance Liquid Chromatography (HPLC) coupled to ICP-MS at the University of Arizona Hazard Identification Core. Total urinary arsenic was calculated by summing inorganic (AsIII + AsV) arsenic metabolites, MMA, and DMA. AsB, a form of arsenic found in fish and seafood, was excluded from the total urinary arsenic variable, since it is thought to be non-toxic (37). Percent arsenic metabolites were calculated as follows: %inorganic arsenic (%iAs) = ((AsIII + AsV)/(AsIII + AsV + MMA + DMA) × 100), %MMA = ((MMA/)/(AsIII + AsV + MMA + DMA)) × 100), and %DMA = (DMA/(AsIII + AsV + MMA + DMA)) × 100. LODs across four analytical runs ranged from: 0.011 to 0.040 μg/L for AsIII, 0.020 to 0.143 μg/L for AsV, 0.020 to 0.086 μg/L for MMA, and 0.014 to 0.169 μg/L for DMA. Values below the detection limit were imputed as the dilution-corrected LOD divided by the square root of 2. The number (%) of samples below the LOD were 70 (41.9) for AsIII, 84 (50.3) for AsV, 38 (22.8) for MMA, 2 (1.2) for DMA, and 48 (28.7) for AsB. Urine specific gravity was measured by a refractometer (Itago), and urine arsenic concentrations were adjusted for specific gravity to account for urine dilution, using the following formula: Ac = A × [(SGmean−1)/(SG−1)], where Ac = the SG-adjusted arsenic or arsenic metabolite concentration, SGmean = the mean SG value for the study sample, and SG = the SG value of the participant (38).
2.4. Birth Outcomes
Birth weights were abstracted from electronic medical records. GA at birth was estimated for each participant based on ultrasound measures, which were abstracted from electronic medical records. If available, crown-rump length measures from an early ultrasound (<14 weeks gestation) were used to determine the GA at birth. However, if a first trimester ultrasound was not obtained, fetal measures from a second trimester ultrasound were used instead.
2.5. Covariate Information
Questionnaires were administered in either English or Spanish, depending on the participant’s preferred language, during the first study visit (first or second trimester). Maternal self-reported pre-pregnancy weight, race, ethnicity, birth country, ever smoking status, and education level (less than 12th grade, completed 12th grade, some college or technical school, completed 4 years of college, some graduate training after college) were determined. Maternal standing height was measured twice by stadiometer (Perspectives Enterprises Model PE-AIM-101). Maternal pre-pregnancy BMI was calculated using the self-reported weight and measured height values (kg/m2). Each participant’s age was determined using the date that she completed the questionnaire and her birth date. A combined variable indicating ethnicity by birth place was created based on the participant’s self-reported ethnicity (Hispanic vs. non-Hispanic) and birth country (U.S. versus other), which had 3 categories: non-Hispanic, Hispanic born in the U.S., and Hispanic born outside the U.S. Information on newborn sex and delivery type (normal spontaneous vaginal, planned cesarean section, unplanned/emergency cesarean section, vaginal birth after cesarean, vacuum assisted vaginal, and forceps assisted vaginal) were abstracted from electronic medical records; if this information was missing from the maternal medical records, it was filled in using reports from a questionnaire administered to the mothers 7–14 days after birth.
Information on maternal fish/seafood and rice consumption during the pregnancy was obtained by a questionnaire administered during the participant’s second visit (second trimester or early third trimester). Participants were asked if they had ever consumed any of the following types of fish or seafood during the pregnancy: fish sticks, fresh oily fish, other fresh fish, canned tuna, shellfish, or fried shellfish. A combined variable was then created with 2 categories: ever versus never consumed any type of fish or seafood during the pregnancy. Participants were also asked if they had ever consumed rice during the pregnancy. If a participant reported consuming rice, she was additionally asked how frequently she typically consumed rice (1–6 times per year, 7–11 times per year, 1 time per month, 2–3 times per month, 1 time per week, 3–4 times per week, 5–6 times per week, 1 time per day, or 2 or more times per day). These categories were then collapsed into a binary variable: frequent rice consumption (≥5–6 times per week) versus infrequent rice consumption (<5–6 times per week).
Total weight gain in pregnancy (in kg) was calculated by subtracting the participant’s self-reported pre-pregnancy weight from her last recorded weight taken during the pregnancy (if obtained within two weeks prior to delivery), which was abstracted from the medical records. If a participant was missing a self-reported pre-pregnancy weight, the first weight measurement obtained during pregnancy (at <10 weeks gestation) was used. Net weight gain in pregnancy (in kg) was also calculated by subtracting the infant’s birth weight from the total weight gained by the mother during her pregnancy. Since a subset of participants was missing information on gestational weight gain in pregnancy (N=11 for urinary arsenic subset, N=8 for blood arsenic subset, and N=9 for hair arsenic subset), we compared two different methods when utilizing this variable: 1) a complete case analysis and 2) multivariate imputation by chained equations to obtain gestational weight gain values for individuals missing this information, conducted using the MICE package in R (39), specifying 100 imputations and 100 iterations.
2.6. Statistical Analyses
The relationships between total blood arsenic, total hair arsenic, total urinary arsenic, and all urinary arsenic metabolites were examined using Pearson correlations. Associations between arsenic measures of interest (hair arsenic, blood arsenic, urinary arsenic, iAs, MMA, DMA, AsB, %iAs, %MMA, %DMA) and birth outcomes (birth weight, GA at birth) were examined using linear regression models. Arsenic measures were right-skewed and therefore log2-transformed to reduce the influence of extreme values and to improve linearity between the exposures and outcomes to meet linear regression model assumptions. All models were adjusted for hypothesized confounders, determined using directed acyclic graphs (40), and known predictors of the outcomes. These variables included recruitment site, maternal age, ethnicity by birth place, education (collapsed into two categories: completed high school or did not complete high school), pre-pregnancy BMI (continuous), parity (primiparous or multiparous), ever smoking status, delivery type (collapsed into three categories: normal spontaneous vaginal delivery, cesarean section, or other), newborn sex, and GA at birth (for birth weight models).
Given prior evidence of interactions between arsenic exposure and newborn sex (19, 24, 25, 27, 28), maternal pre-pregnancy BMI (24), and smoking status (28) in relation to birth outcomes, we examined potential interactions between each of these variables and the arsenic measures. We also explored potential interactions between arsenic measures and both total and net gestational weight gain. Since a previous study has observed that arsenic is associated with lower gestational weight gain and that arsenic-associated reductions in birth weight are partially mediated by this decrease in weight gain (41), we also investigated associations between arsenic exposure and maternal weight gain in pregnancy.
Sensitivity analyses were conducted for the main models to determine whether results were similar after: 1) excluding preterm pregnancies (GA at birth <37 weeks), 2) adjusting for frequency of rice consumption during the pregnancy, 3) adjusting for fish/seafood consumption during the pregnancy, 4) excluding participants who reported fish/seafood consumption during the pregnancy, and 5) additionally adjusting for urinary AsB. We also investigated the influence of AsB using residual regression, whereby each arsenic measure was regressed on urinary AsB. The residuals from these models were then extracted and evaluated as the exposures of interest in relation to each birth outcome.
3. Results:
3.1. Participant Characteristics
Characteristics of each subset of participants with hair, blood, or urinary arsenic measures are presented in Table 1. These characteristics were generally similar for each subset. Overall, participants were between 18 and 45 years of age. The median pre-pregnancy BMI was ~28 kg/m2 with a range of 18.6 to 45.5 kg/m2, and >60% of participants were overweight or obese. Approximately 80% or more of participants were Hispanic, and 45% or more were Hispanic and born outside the U.S. Median (range) arsenic concentrations were 0.010 (0.001, 0.060) μg/g in hair, 0.63 (0.23, 3.46) μg/L in blood, and 5.66 (1.96, 28.75) μg/L in urine (iAs+MMA+DMA). Median (range) urinary AsB concentrations were 0.50 (0.04, 478.82) μg/L.
Table 1.
Characteristics of Participants with Birth Outcomes Data and Urine, Blood, or Hair Arsenic Measures
| Hair Arsenic Subset (N=116) | Blood Arsenic Subset (N=100) | Urine Arsenic Subset (N=167) | |
|---|---|---|---|
| Characteristic | Median (Range) or N (%) | Median (Range) or N (%) | Median (Range) or N (%) |
| Birthweight, grams | 3310 (678, 4755) | 3315 (678, 4450) | 3288 (1650, 4755) |
| GA at Birth, weeks | 39.1 (27.1, 42.4) | 39.2 (27.1, 42.4) | 39.1 (31.9, 42.4) |
| Maternal Age, years | 29.4 (18.7, 45.5) | 29.6 (18.6, 41.0) | 29.0 (18.6, 45.5) |
| Maternal Pre-Pregnancy BMI, kg/m2 | 27.0 (17.6, 53.6) | 26.7 (17.6, 53.6) | 27.1 (17.6, 53.6) |
| Maternal Pre-Pregnancy BMI Category | |||
| Underweight or Normal Weight | 39 (33.6) | 38 (38.0) | 58 (34.7) |
| Overweight | 40 (34.5) | 34 (34.0) | 50 (29.9) |
| Obese | 37 (31.9) | 28 (28.0) | 59 (35.3) |
| Total Weight Gain in Pregnancya, kg | 11.3 (−4.6, 25.0) | 12.7 (−4.6, 24.5) | 11.3 (−5.0, 28.1) |
| Maternal Ethnicity by Birth Place | |||
| Non-Hispanic | 18 (15.5) | 21 (21.0) | 28 (16.8) |
| U.S. Born Hispanic | 44 (37.9) | 34 (34.0) | 63 (37.7) |
| Foreign Born Hispanic | 54 (46.6) | 45 (45.0) | 76 (45.5) |
| Maternal Parity | |||
| Primiparous | 44 (37.9) | 40 (40.0) | 60 (35.9) |
| Multiparous | 72 (62.1) | 60 (60.0) | 107 (64.1) |
| Maternal Education | |||
| Completed High School | 81 (69.8) | 72 (72.0) | 115 (68.9) |
| Did Not Complete High School | 35 (30.2) | 28 (28.0) | 52 (31.1) |
| Maternal Lifetime Smoking Status | |||
| Ever | 30 (25.9) | 27 (27.0) | 45 (26.9) |
| Never | 86 (74.1) | 73 (73.0) | 122 (73.1) |
| Delivery Type | |||
| Normal Spontaneous Vaginal | 69 (59.5) | 67 (67.0) | 105 (62.9) |
| Cesarean Section | 33 (28.4) | 25 (25.0) | 47 (28.1) |
| Otherb | 14 (12.1) | 8 (8.0) | 15 (9.0) |
| Fish/Seafood Consumption in Pregnancyc | |||
| Ever | 75 (67.0) | 65 (68.4) | 106 (67.5) |
| Never | 37 (33.0) | 30 (31.6) | 51 (32.5) |
| Rice Consumption in Pregnancyd | |||
| Ever | 112 (99.1) | 94 (97.9) | 156 (98.7) |
| Never | 1 (0.9) | 2 (2.1) | 2 (1.3) |
| Frequency of Rice Consumption in Pregnancyd | |||
| ≥5–6 Times Per Week | 11 (9.7) | 8 (8.3) | 15 (9.5) |
| <5–6 Times Per Week | 102 (90.3) | 88 (91.7) | 143 (90.5) |
| Hair Arsenic, μg/g | 0.010 (0.001, 0.060) | ||
| Blood Arsenic, μg/L | 0.63 (0.23, 3.46) | ||
| Urine Arsenic, μg/L | 5.66 (1.96, 28.75) | ||
| Urinary iAs, μg/L | 0.93 (0.17, 9.82) | ||
| Urinary MMA, μg/L | 0.44 (0.12, 4.96) | ||
| Urinary DMA, μg/L | 4.24 (0.82, 21.11) | ||
| Urinary AsB, μg/L | 0.50 (0.04, 478.82) | ||
| Urinary %iAs | 15.3 (2.6, 63.4) | ||
| Urinary %MMA | 8.0 (1.5, 26.5) | ||
| Urinary %DMA | 75.1 (22.5, 94.1) |
N=107 for subset with hair arsenic data, N=92 for subset with blood arsenic data, N=156 for subset with urinary arsenic data
Vaginal birth after cesarean section, vacuum assisted vaginal delivery, or forceps assisted vaginal delivery
N=112 for subset with hair arsenic data, N=95 for subset with blood arsenic data, N=157 for subset with urinary arsenic data
N=113 for subset with hair arsenic data, N=96 for subset with blood arsenic data, N=158 for subset with urinary arsenic data
3.2. Correlations between Blood, Hair, and Urine Arsenic Measures
Pearson correlations between arsenic measures are shown in Figure 1 for the 82 participants who had arsenic measures for all three sample types (hair, blood, and urine). Hair arsenic was weakly, but positively and significantly, correlated with all arsenic measures, except %iAs and %DMA. Total blood and urinary arsenic were weakly and significantly positively correlated. Blood arsenic was moderately and significantly positively correlated with urinary DMA. Total urinary arsenic was strongly and significantly positively correlated with urinary DMA, moderately and significantly positively correlated with urinary iAs and MMA, and weakly and significantly positively correlated with %MMA. Urinary AsB was positively and significantly correlated with all arsenic measures, except urinary MMA and the %arsenic metabolites.
Figure 1.
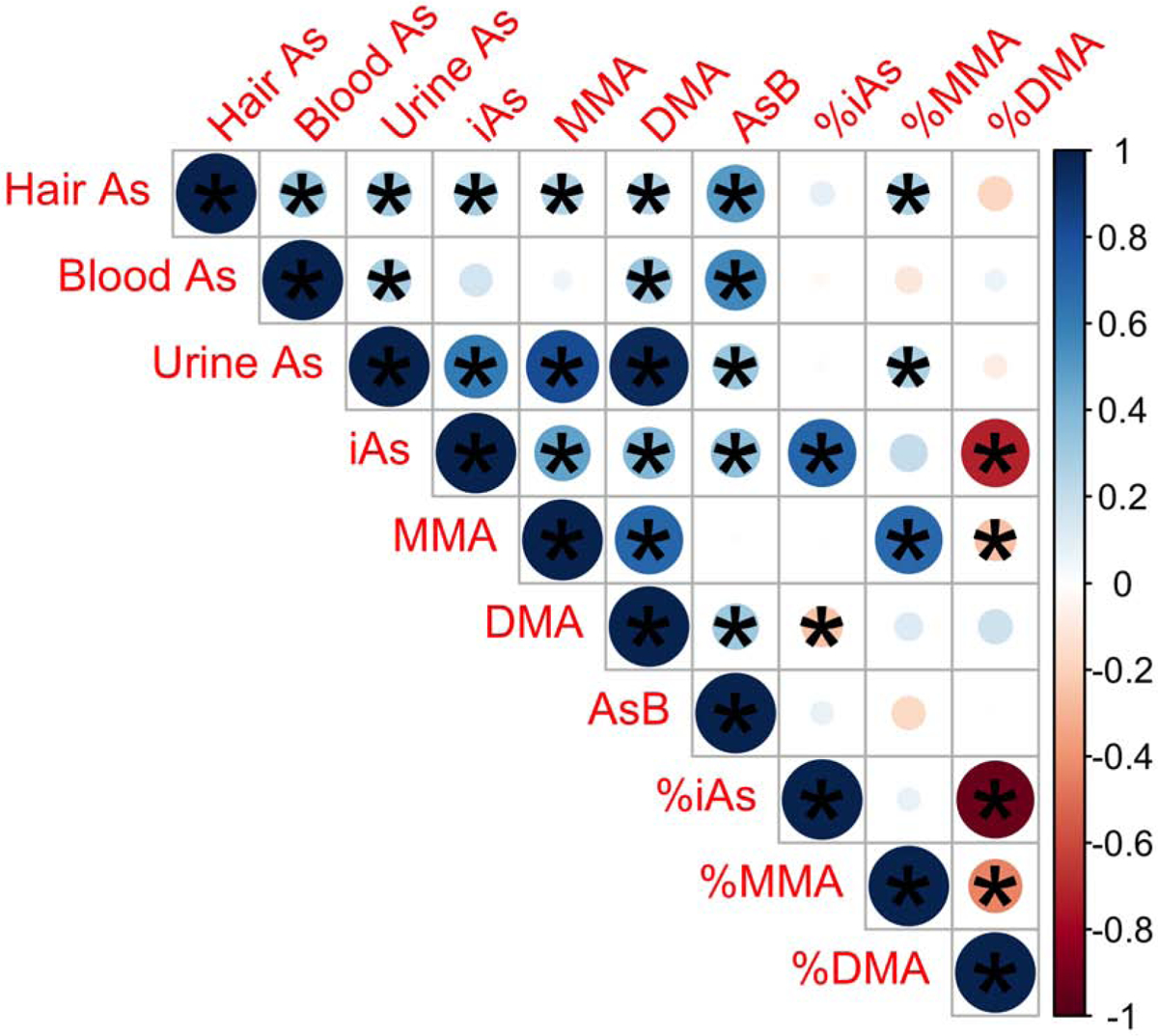
Pearson Correlations Between Arsenic Measures for Participants with Hair, Blood, and Urine Arsenic Measures (N=82). Positive correlations are indicated in blue shades. Negative correlations are indicated in red shades. Larger circles correspond to stronger correlations, as do darker shades, as indicated in the corresponding key. *P<0.05. Abbreviations used: As, arsenic; DMA, dimethyl arsenical species; iAs, inorganic arsenic arsenical species; MMA, monomethyl arsenical species
3.3. Associations between Arsenic Measures and Birth Outcomes
A doubling in maternal hair arsenic was associated with a 72.2 g (95% CI: −144.3, −0.1 g) lower birth weight (P=0.05) (Table 2). A similar but non-significant trend was observed for blood arsenic, but not for urinary arsenic or the urinary arsenic metabolites (Table 2). Participants with hair arsenic measures were generally representative of the larger MADRES cohort (Table S1), although there were slightly more overweight and Hispanic women and slightly fewer high-school educated women, fish/seafood consumers, and spontaneous vaginal deliveries. None of the arsenic measures were significantly associated with GA at birth in primary analyses (Table 2). Associations were similar after 1) excluding preterm births (GA at birth < 37 weeks) (Table S2), 2) additionally adjusting for fish/seafood consumption during the pregnancy (Table S3), and additionally adjusting for urinary AsB (Table S4). Results were also similar in the AsB residual regression sensitivity analyses (Table S5). However, the inverse association between hair As and birth weight was slightly stronger after additionally adjusting for the frequency of rice consumption during pregnancy (Table S6) and was much stronger after excluding participants who reported consuming fish/seafood during the pregnancy (Table S7). Additionally, urinary iAs was associated with a 0.6 (95% CI: −1.0, −0.1, P=0.03) week shorter gestational length after excluding participants who reported any fish/seafood consumption during the pregnancy (Table S7).
Table 2.
Difference in Birth Weight and GA at Birth for Doubling in Arsenic Exposurea
| Arsenic Measureb | Birth Weight | GA at Birth | ||
|---|---|---|---|---|
| Difference in Birth Weight, g (95% CI) | P | Difference in GA at Birth, wks (95% CI) | P | |
| Hair As (N=116) | −72.2 (−144.3, −0.1) | 0.05 | 0.1 (−0.2, 0.5) | 0.42 |
| Blood As (N=100) | −51.9 (−149.8, 46.0) | 0.30 | −0.1 (−0.6, 0.5) | 0.75 |
| Urinary As (N=167) | 18.4 (−72.3, 109.1) | 0.69 | −0.1 (−0.4, 0.3) | 0.76 |
| Urinary iAs (N=167) | 9.1 (−52.6, 70.7) | 0.77 | 0.0 (−0.3, 0.2) | 0.77 |
| Urinary MMA (N=167) | −11.8 (−82.0, 58.4) | 0.74 | 0.0 (−0.2, 0.3) | 0.76 |
| Urinary DMA (N=167) | 28.4 (−53.4, 110.1) | 0.49 | −0.1 (−0.4, 0.3) | 0.64 |
| Urinary AsB (N=167) | −14.4 (−39.5, 10.8) | 0.26 | 0.0 (−0.1, 0.1) | 0.60 |
| Urinary %iAs (N=167) | 0.7 (−69.9, 71.4) | 0.98 | 0.0 (−0.3, 0.3) | 0.93 |
| Urinary %MMA (N=167) | −37.8 (−127.9, 52.4) | 0.41 | 0.1 (−0.2, 0.5) | 0.48 |
| Urinary %DMA (N=167) | 109.0 (−124.0, 341.9) | 0.36 | −0.3 (−1.2, 0.7) | 0.59 |
Results are from linear regression models, which were adjusted for newborn sex, delivery type, maternal age, ethnicity by birth place, recruitment site, education, parity, pre-pregnancy BMI, ever smoking status, and GA at birth (for the birth weight analyses). Arsenic measures were log2-transformed, so results are expressed as the difference in the birth outcome for a doubling in the exposure.
Urine As excludes arsenobetaine. Urine As, iAs, MMA, DMA, and AsB concentrations were adjusted for specific gravity.
3.4. Interactions between Arsenic Measures and Maternal Smoking, Pre-Pregnancy BMI, Gestational Weight Gain, and Newborn Sex
Statistically significant interactions were observed between hair arsenic and both total (Pinteraction=0.02) and net (Pinteraction=0.02) gestational weight gain in relation to birth weight, such that the inverse association between hair arsenic and birth weight was stronger among women who gained more weight during their pregnancies (Figure 2). Results were similar when gestational weight gain values were imputed for nine participants who were missing this information (Pinteraction=0.04 for both total and net gestational weight gain). Cross-product terms for gestational weight gain and other arsenic measures were not statistically significant (p-values > 0.05). However, significant interactions were observed between pre-pregnancy BMI and both total urinary arsenic (Pinteraction=0.05) and urinary MMA (Pinteraction<0.01) in relation to GA at birth; an inverse association was observed between MMA and GA at birth among infants whose mothers had low pre-pregnancy BMIs (less than ~25 kg/m2), while a positive association was observed among infants whose mothers had high pre-pregnancy BMIs (greater than ~30 kg/m2) (Figure S1). A similar trend was seen for total urinary arsenic (Figure S1). No significant interactions were observed between any of the arsenic measures and either infant sex or maternal smoking status (p-values > 0.05).
Figure 2.
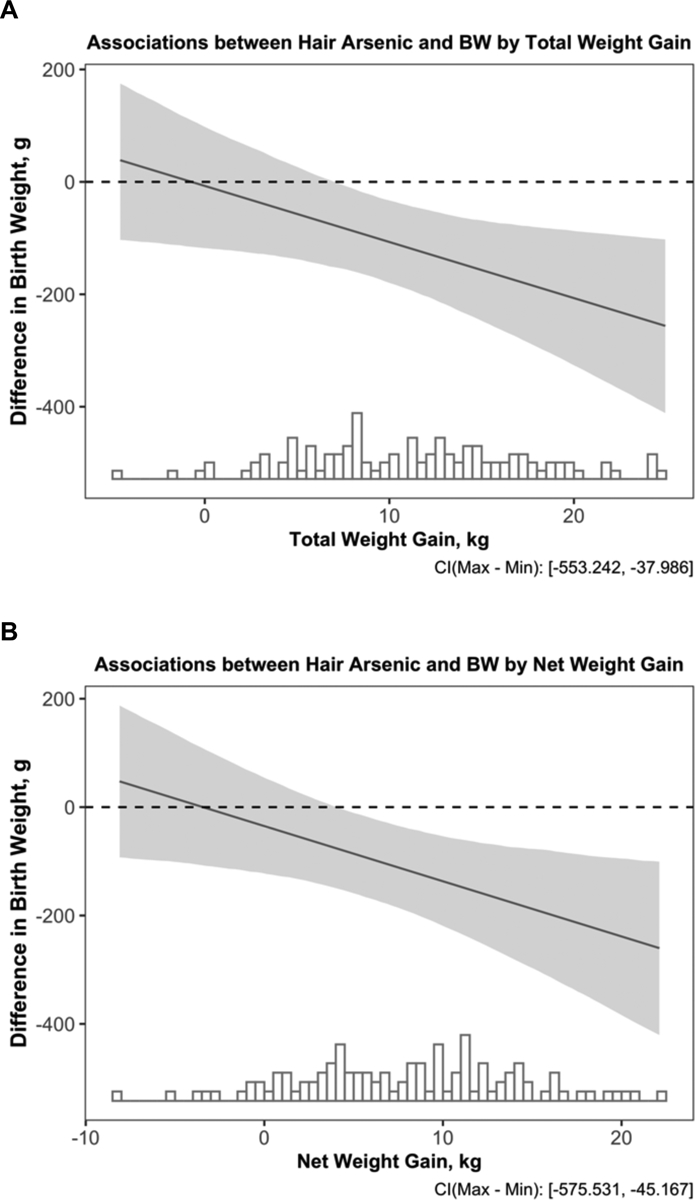
Associations between Hair Arsenic and Birth Weight by Total (A) and Net (B) Gestational Weight Gain (N=107). The x-axis indicates the total or net gestational weight gain (total weight gain-birth weight) in kg with an overlaying histogram. The y-axis shows the difference in birth weight in g and the corresponding 95% confidence interval for a doubling in hair arsenic. Results are from linear regression models, which were adjusted for gestational age at birth, newborn sex, delivery type, maternal age, ethnicity by birth place, recruitment site, education, parity, pre-pregnancy BMI, and ever smoking status.
3.5. Associations between Hair Arsenic and Gestational Weight Gain
Given previous evidence that gestational weight gain may mediate arsenic’s impact on birth weight (41), we additionally evaluated associations between arsenic exposure and both total and net gestational weight gain. Since the inverse association between hair arsenic and birth weight was robust, we focused on hair arsenic for these analyses. Results from a complete case analysis were compared with results from an analysis that imputed gestational weight gain for the nine participants who were missing this information. In the complete case analysis (N=107), a doubling in hair arsenic was associated with a 1.3 kg (95% CI: 0.2, 2.4 kg) higher total weight gain in pregnancy (P=0.02) and a 1.4 kg (95% CI: 0.3, 2.4 kg) higher net weight gain in pregnancy (P=0.01), after adjusting for the length of the pregnancy, newborn sex, ethnicity by birth place, recruitment site, age, education, parity, pre-pregnancy BMI, and ever smoking status (Table 3). Results from models using imputed gestational weight gain values (N=116) were very similar (Table 3).
Table 3.
Difference in Gestational Weight Gain for Doubling in Hair Arsenic Concentrationa
| Analysis Type | Total GWG | Net GWG | ||
|---|---|---|---|---|
| Estimated Difference in kg (95% CI) | P | Estimated Difference in kg (95% CI) | P | |
| Complete Case Analyses (N=107) | 1.3 (0.2, 2.4) | 0.02 | 1.4 (0.3, 2.4) | 0.01 |
| Multiple Imputation for Missing GWG Values (N=116) | 1.2 (0.2, 2.3) | 0.02 | 1.3 (0.3, 2.3) | 0.01 |
Abbreviations Used: GWG, gestational weight gain; kg, kilograms
Results are from linear regression models, which were adjusted for the length of the pregnancy, newborn sex, ethnicity by birth place, recruitment site, age, education, parity, pre-pregnancy BMI, and ever smoking status. A log2-transformation was applied to hair arsenic. Results are therefore expressed as the difference in GWG (in kg) for a doubling in hair arsenic concentration.
4. Discussion
Previous studies have observed that arsenic exposure adversely affects fetal growth and increases risk for preterm birth (23, 33). Although the majority of studies have focused on populations outside the U.S., such as in Bangladesh, India, Taiwan, and Chile, where arsenic concentrations in drinking water can reach extremely high levels (20, 23, 42), several studies in the U.S. have found that even at relatively low levels of exposure, arsenic may adversely affect birth outcomes (24, 43, 44). For example, an inverse association between urinary arsenic and birth weight was observed among girls born to overweight/obese women in the New Hampshire Birth Cohort study (24). Additionally, blood arsenic was inversely associated with birth weight in a study of pregnant women living near the Tar Creek Superfund Site in Oklahoma (43). To our knowledge, this study is among the first in the U.S. to examine associations between arsenic and birth outcomes in a predominately Hispanic population, which experiences higher rates of preterm birth and low birth weight (1), and in urban Los Angeles, where the levels and sources of exposure may differ from those of rural populations. The median urinary arsenic concentration in MADRES was slightly higher (5.7 μg/L) than in the New Hampshire Birth Cohort study (3.4 μg/L), but the median blood arsenic concentration was lower (0.6 μg/L) than in the Tar Creek study (1.4 μg/L). Despite generally low levels of exposure, we observed that a doubling in hair arsenic was associated with a 72.2 g lower birth weight, which is similar in magnitude to the associations observed in these two previous U.S. studies. In the New Hampshire Birth Cohort Study, a doubling in urinary arsenic was associated with a 62.9 g lower birth weight among infants of overweight/obese mothers (24), and in the Tar Creek Study, an interquartile range increase in blood arsenic was associated with a 77.5 g lower birth weight (43). Although the previous U.S. studies did not evaluate hair arsenic, an inverse association between hair arsenic and birth weight has also been observed in a much more highly exposed population in Bangladesh (45).
In contrast with the findings for hair arsenic, blood and urine arsenic measures were not significantly associated with birth weight in MADRES. One possible explanation may be that hair arsenic represents previous exposure, whereas urine and blood arsenic primarily reflect recent exposure over the past several days (46). While rates of hair growth differ between individuals, the average rate is estimated to be one centimeter per month (47). Given the methods used to collect hair samples in MADRES (i.e., 2 cm of hair cut close to the scalp), the hair arsenic values in the current study represent exposure over the past ~2 months. Hair arsenic may therefore be a better biomarker of integrated exposure in this urban population, which is likely exposed to arsenic from dietary and/or industrial sources that may vary temporally. In addition to representing different windows of exposure, each biospecimen reflects different arsenic species. For example, hair is thought to primarily accumulate iAs, whereas blood and urine represent all of the major arsenic species, with DMA being the predominant species in urine (46). Blood and urine arsenic biomarkers may also reflect arsenic from fish/seafood. For example, total blood arsenic can include AsB, a metabolite derived from fish/seafood that is rapidly eliminated from the body and considered non-toxic (46). While we excluded AsB from our total urinary arsenic measure, arsenosugars and arsenolipids are also derived from fish/seafood and can be metabolized to DMA (48). The blood and urine arsenic measures in our study may therefore reflect arsenosugars and arsenolipids in addition to iAs exposure. However, the toxicity of these complex organic arsenicals is currently unclear (48). Nevertheless, the null associations between the blood and urine arsenic measures and birth weight were robust in a series of sensitivity analyses, which included additionally adjusting for fish/seafood consumption; additionally adjusting for AsB, a biomarker of fish/seafood consumption (46); using residual regression models to remove variance from urinary AsB; and restricting to participants who reported that they did not consume fish/seafood. In contrast, the inverse association between hair arsenic and birth weight increased in magnitude after restricting to non-fish consumers. While this was somewhat unexpected, since hair is thought to primarily accumulate iAs (46), other arsenic metabolites including DMA have been identified in human hair (49). Consistent with this, we observed significant, albeit weak, positive correlations between hair arsenic and all of the urinary arsenic metabolites.
Interestingly, we observed a significant interaction between hair arsenic and gestational weight gain, whereby the magnitude of the inverse arsenic-birth weight association increased as weight gain increased. While this finding may seem counterintuitive, as birth weight tends to increase as maternal weight gain increases (50), one possible hypothesis may be that arsenic diverts nutrients from the fetus to the mother, leading to a higher maternal weight gain, but restrictions in fetal growth. While this hypothesized mechanism has been largely untested and would need to be investigated further, we did observe a positive association between hair arsenic and gestational weight gain, which is consistent with this hypothesis and also prior evidence that arsenic may be an obesogen (51). Although a previous study of CD-1 mice did not observe arsenic-associated alterations in gestational weight gain (52), exposure was restricted to the second half of gestation and the dams were lean. It is therefore unclear how results would have compared if early pregnancy exposures had been evaluated or if the experimental conditions more closely represented our study population. Our results are also inconsistent with previous studies in Bangladesh, which observed inverse associations between arsenic exposure (measured in drinking water and maternal toenails) and gestational weight gain (20, 41). However, these discrepancies may be explained by population differences, such as the high arsenic exposures, low prevalence of overweight/obesity, and high prevalence of malnutrition observed in Bangladesh (20, 41). Evidence from Bangladesh also indicates that lower maternal weight gain may partially mediate arsenic-associated reductions in birth weight (41), but this was not supported in our study, since arsenic was associated with increased gestational weight gain.
Although numerous studies, many conducted in Bangladesh, have observed that arsenic is associated with a shorter gestational period (20, 27, 29, 41, 44, 53, 54), this was not observed in our primary analysis. The association between prenatal arsenic exposure and GA at birth was also null in the New Hampshire Birth Cohort Study (24). Potential explanations for the inconsistent findings observed between the current study and studies conducted in other countries may therefore be differences in exposure levels or population differences in the rates and causes of preterm birth; Bangladesh, for example, experiences one of the highest incidences of preterm birth in the world (55). It is also possible that our null results were driven by negative confounding by fish/seafood consumption. Although associations between arsenic measures and GA at birth remained null after additionally adjusting for AsB and also when using residual-regression to remove the influence of AsB, we did observe a significant inverse association between urinary iAs and GA at birth when we excluded participants who reported consuming fish/seafood during the pregnancy. However, the latter result needs to be considered with some caution, given the small number of participants (N=51) in the restricted analysis. Prior studies in Bangladesh have also observed that arsenic-associated reductions in birth weight are almost entirely mediated by reductions in GA at birth (41, 53). However, in MADRES the inverse association between hair arsenic and birth weight was independent of GA at birth, consistent with the New Hampshire Birth Cohort study (24). The mechanism by which arsenic impacts birth size may therefore depend on population-specific characteristics, such as exposure levels and the prevalence of overweight/obesity. Consistent with this, we observed a complex relationship between urinary MMA and GA at birth, such that an inverse association was observed when maternal pre-pregnancy BMI was low or normal (<25 kg/m2) while in contrast a positive association was observed when maternal pre-pregnancy BMI was high (>30 kg/m2).
Our study had several limitations. Most notably, speciated arsenic measures were not available for hair, so we could not investigate the arsenic metabolites responsible for the inverse association observed between hair arsenic and birth weight. While hair is thought to primarily accumulate iAs (46), other arsenic metabolites have also been detected in human hair samples (49). Furthermore, the stronger inverse association observed for hair arsenic and birth weight among non-fish consumers, and the positive correlations between hair arsenic and all urinary arsenic metabolites, suggest that arsenic species other than iAs may have been present. An additional limitation of our study was the potential for confounding by fish/seafood consumption, as a large portion of MADRES participants (67%) reported consuming some type of fish/seafood during the pregnancy. While we attempted to account for this in a series of sensitivity analyses, using information on both fish/seafood intake and urinary AsB, which is an objective biomarker of fish/seafood consumption, we cannot rule out residual confounding. Another important consideration for this study is that hair samples are susceptible to exogenous contamination (35). However, we used a rigorous cleaning method that successfully removes exogenous metal contamination (36) and observed positive and significant correlations between hair arsenic and both blood and urine arsenic measures, which are not susceptible to external contamination. This is consistent with prior studies that have also demonstrated that hair arsenic can reflect the internal body burden of arsenic (56). We also collected information on permanent hair treatments and hair dye use for a subset of participants (N=30), given evidence that these behaviors may impact certain metal concentrations in hair (57). While only two participants reported permanent hair treatments within the past six months, nearly 40% reported dyeing or highlighting their hair. However, hair arsenic concentrations were not higher among women reporting hair dye use. In fact, inverse associations were observed between hair dye use and all three of the As biomarkers evaluated (hair, blood, and urine).
Importantly, information on arsenic exposure from drinking water was not available for MADRES participants. While this population resides in urban Los Angeles and is therefore likely drinking from bottled or municipal water sources, rather than from private wells, participants may still be exposed to some arsenic through their drinking water. An additional consideration is that we may have been underpowered to identify possible differences by infant sex. Given limited statistical power, we were also unable to examine potential three-way interactions, such as between arsenic, pre-pregnancy BMI, and fetal sex, which has been seen previously (24). Furthermore, while we observed a positive association between hair arsenic and gestational weight gain, indicating that weight gain is likely not a mediator of the arsenic-birth weight association in our study, we were underpowered to formally evaluate this. We therefore cannot rule out the possibility that maternal weight gain may be an intermediate in this pathway. The interaction that we observed between hair arsenic and gestational weight gain therefore needs to be considered with caution, as conditioning on an intermediate can introduce collider bias (58). To address this, we attempted a sensitivity analysis proposed by VanderWeele et al. to examine the possibility of collider bias, using the predicted probability of gestational weight gain based on baseline covariates, rather than gestational weight gain itself (58). However, we were unable to specify a model that was sufficiently predictive of gestational weight gain to apply this method. Future studies are therefore needed to more formally investigate how maternal weight gain may contribute to arsenic-associated reductions in birth weight and how this role may vary in different populations.
Our study also had several notable strengths. We focused on a population that has a higher risk of preterm birth and low birth weight (1) that has been understudied in the context of arsenic toxicity. Although arsenic exposures were in the low range for most MADRES participants, we observed an inverse association between hair arsenic and birth weight that was similar in effect size to studies conducted in non-Hispanic populations in the rural U.S., including a study of private well users in New Hampshire (24) and a study of more highly exposed women in Oklahoma (43). We were also able to compare multiple biomarkers of arsenic exposure, which represent different exposure timeframes and arsenic species. Furthermore, we measured arsenic in biospecimens that were collected in the first or second trimester (representing pre-pregnancy through mid-pregnancy exposures), which is important given evidence that fetal growth trajectories may be largely determined by conditions in early pregnancy, or even as early as the periconceptional period (reviewed in (59)).
The findings from this study have important public health implications. The inverse association between hair arsenic and birth weight was comparable in magnitude to effect estimates observed for secondhand tobacco smoke exposure, which have ranged from a difference in birth weight of −6 g to −120 g, depending on the exposure metric evaluated (reviewed in (60)). This is concerning, since lower birth weight has been associated with increased risk for numerous adverse health outcomes later in life (2–14), including obesity and cardiometabolic disease, which are more prevalent in lower income and Hispanic populations (61–63). It will therefore be important to examine the subsequent health consequences of these arsenic-associated reductions in birth weight. Since the MADRES pregnancy cohort was designed to follow children through the first 5 years of life, impacts on early life growth and adiposity can be directly examined in future studies.
5. Conclusions
In a population of mostly lower income Hispanic women living in urban Los Angeles, hair arsenic (representing pre-pregnancy and early pregnancy exposure) was inversely associated with birth weight, independent of GA at birth. This association was more pronounced among women who gained greater amounts of weight during the pregnancy, which requires further investigation. Since reduced birth weight has been associated with adverse health outcomes later in life, identifying and reducing the major sources of arsenic exposure in this understudied population is essential.
Supplementary Material
Highlights:
Hair arsenic was inversely and significantly associated with birth weight
The hair arsenic-birth weight association differed by gestational weight gain
Hair arsenic was associated with increased gestational weight gain
Blood and urine arsenic were not significantly associated with birth weight
Acknowledgements:
We would like to thank the MADRES participants, the study staff, and our community clinic partners for their many contributions to this work. We would also like to thank the Arizona Laboratory for Emerging Contaminants (ALEC) at the University of Arizona, Tucson, AZ, which performed the urinary metals analyses.
Funding Support: Funding for this study was provided by NIH grants P50 ES026086 and 4UH3OD023287-03 and an EPA grant 83615801-0. Dr. Howe is supported by a NIEHS Pathway to Independence Award (K99 ES030400). Dr. Farzan is supported by a NIEHS Pathway to Independence Award (R00 ES024144) and a USC Provost’s Fellowship. Dr. Garcia is supported by a Research Supplement to Promote Diversity in Health-Related Research Award (P50 ES026086-04S1). The funding agencies that supported this work had no role in the design; collection, analysis, or interpretation of data; the writing of this report; or the decision to submit the article for publication.
Abbreviations Used:
- Ar
argon
- AsIII
arsenite
- AsV
arsenate
- AsB
arsenobetaine
- iAs
inorganic arsenic
- DMA
dimethyl arsenical species
- GA
gestational age
- HPLC
high performance liquid chromatography
- ICP-MS
inductively coupled plasma mass spectrometry
- LOD
limit of detection
- MADRES
Maternal and Developmental Risks from Environmental and Social Stressors Study
- MMA
monomethyl arsenical species
- SRM
standard reference material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest:
CGH, SFF, EG, TJ, RI, KB, TAC, TLH, BHG, DRS, TMB, and CVB declare no conflicts of interest. WEF is a founding partner in EnMed MicroAnalytics, a company that provides heavy metal screening for newborns and children.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final Data for 2017. Natl Vital Stat Rep. 2018;67(8):1–50. [PubMed] [Google Scholar]
- 2.Woythaler M, McCormick MC, Mao W-Y, Smith VC. Late preterm infants and neurodevelopmental outcomes at kindergarten. Pediatrics. 2015;136(3):424–31. [DOI] [PubMed] [Google Scholar]
- 3.Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. Bmj. 2012;344:e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr. 2017;106(9):1409–37. [DOI] [PubMed] [Google Scholar]
- 5.Kugelman A, Colin AA. Late preterm infants: near term but still in a critical developmental time period. Pediatrics. 2013;132(4):741–51. [DOI] [PubMed] [Google Scholar]
- 6.Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clinical obstetrics and gynecology. 2006;49(2):257–69. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ, Hales CN, Fall C, Osmond C, Phipps K, Clark P. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36(1):62–7. [DOI] [PubMed] [Google Scholar]
- 8.Barker DJ, Osmond C, Winter P, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. The Lancet. 1989;334(8663):577–80. [DOI] [PubMed] [Google Scholar]
- 9.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, et al. Fetal and infant growth and impaired glucose tolerance at age 64. Bmj. 1991;303(6809):1019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdez R, Athens M, Thompson G, Bradshaw B, Stern M. Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia. 1994;37(6):624–31. [DOI] [PubMed] [Google Scholar]
- 11.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. Journal of hypertension. 2000;18(7):815–31. [DOI] [PubMed] [Google Scholar]
- 12.Ramadhani MK, Grobbee DE, Bots ML, Cabezas MC, Vos LE, Oren A, et al. Lower birth weight predicts metabolic syndrome in young adults: the Atherosclerosis Risk in Young Adults (ARYA)-study. Atherosclerosis. 2006;184(1):21–7. [DOI] [PubMed] [Google Scholar]
- 13.Oken E, Gillman MW. Fetal origins of obesity. Obesity research. 2003;11(4):496–506. [DOI] [PubMed] [Google Scholar]
- 14.Shenkin SD, Starr JM, Deary IJ. Birth weight and cognitive ability in childhood: a systematic review. Psychological bulletin. 2004;130(6):989. [DOI] [PubMed] [Google Scholar]
- 15.Kamai EM, McElrath TF, Ferguson KK. Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environmental Health. 2019;18(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng T, Zhang J, Sommer K, Bassig BA, Zhang X, Braun J, et al. Effects of Environmental Exposures on Fetal and Childhood Growth Trajectories. Ann Glob Health. 2016;82(1):41–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson KK, O’Neill MS, Meeker JD. Environmental contaminant exposures and preterm birth: a comprehensive review. J Toxicol Environ Health B Crit Rev. 2013;16(2):69–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicol Sci. 1998;44(2):185–90. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Lu S, Zhang B, Xia W, Liu W, Peng Y, et al. Maternal arsenic exposure and birth outcomes: A birth cohort study in Wuhan, China. Environ Pollut. 2018;236:817–23. [DOI] [PubMed] [Google Scholar]
- 20.Rahman ML, Kile ML, Rodrigues EG, Valeri L, Raj A, Mazumdar M, et al. Prenatal arsenic exposure, child marriage, and pregnancy weight gain: Associations with preterm birth in Bangladesh. Environ Int. 2018;112:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullin AM, Amarasiriwardena C, Cantoral-Preciado A, Henn BC, Leon Hsu HH, Sanders AP, et al. Maternal blood arsenic levels and associations with birth weight-for-gestational age. Environ Res. 2019;177:108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao KW, Chang CH, Tsai MS, Chien LC, Chung MY, Mao IF, et al. Associations between urinary total arsenic levels, fetal development, and neonatal birth outcomes: A cohort study in Taiwan. Sci Total Environ. 2018;612:1373–9. [DOI] [PubMed] [Google Scholar]
- 23.Milton AH, Hussain S, Akter S, Rahman M, Mouly TA, Mitchell K. A Review of the Effects of Chronic Arsenic Exposure on Adverse Pregnancy Outcomes. Int J Environ Res Public Health. 2017;14(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert-Diamond D, Emond JA, Baker ER, Korrick SA, Karagas MR. Relation between in Utero Arsenic Exposure and Birth Outcomes in a Cohort of Mothers and Their Newborns from New Hampshire. Environ Health Perspect. 2016;124(8):1299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drobna Z, Martin E, Kim KS, Smeester L, Bommarito P, Rubio-Andrade M, et al. Analysis of maternal polymorphisms in arsenic (+3 oxidation state)-methyltransferase AS3MT and fetal sex in relation to arsenic metabolism and infant birth outcomes: Implications for risk analysis. Reprod Toxicol. 2016;61:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Yokoyama K, Tian Y, Piao FY, Kitamura F, Kida H, et al. Decrease in birth weight and gestational age by arsenic among the newborn in Shanghai, China. Nihon Koshu Eisei Zasshi. 2011;58(2):89–95. [PubMed] [Google Scholar]
- 27.Wang H, Li J, Zhang X, Zhu P, Hao JH, Tao FB, et al. Maternal serum arsenic level during pregnancy is positively associated with adverse pregnant outcomes in a Chinese population. Toxicol Appl Pharmacol. 2018;356:114–9. [DOI] [PubMed] [Google Scholar]
- 28.Bloom MS, Neamtiu IA, Surdu S, Pop C, Anastasiu D, Appleton AA, et al. Low level arsenic contaminated water consumption and birth outcomes in Romania-An exploratory study. Reprod Toxicol. 2016;59:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laine JE, Bailey KA, Rubio-Andrade M, Olshan AF, Smeester L, Drobna Z, et al. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ Health Perspect. 2015;123(2):186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vahter M Mechanisms of arsenic biotransformation. Toxicology. 2002;181–182:211–7. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh RL, Huang YL, Shiue HS, Huang SR, Lin MI, Mu SC, et al. Arsenic methylation capacity and developmental delay in preschool children in Taiwan. Int J Hyg Environ Health. 2014;217(6):678–86. [DOI] [PubMed] [Google Scholar]
- 32.Kuo CC, Moon KA, Wang SL, Silbergeld E, Navas-Acien A. The Association of Arsenic Metabolism with Cancer, Cardiovascular Disease, and Diabetes: A Systematic Review of the Epidemiological Evidence. Environ Health Perspect. 2017;125(8):087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong Q, Cui Y, Wu H, Niu Q, Lu X, Wang L, et al. Association of maternal arsenic exposure with birth size: A systematic review and meta-analysis. Environ Toxicol Pharmacol. 2019;69:129–36. [DOI] [PubMed] [Google Scholar]
- 34.Bastain TM, Chavez T, Habre R, Girguis MS, Grubbs B, Toledo-Corral C, et al. Study Design, Protocol and Profile of the Maternal And Developmental Risks from Environmental and Social Stressors (MADRES) Pregnancy Cohort: a Prospective Cohort Study in Predominantly Low-Income Hispanic Women in Urban Los Angeles. BMC Pregnancy Childbirth. 2019;19(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jursa T, Stein C, Smith D. Determinants of hair manganese, lead, cadmium and arsenic levels in environmentally exposed children. Toxics. 2018;6(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eastman RR, Jursa TP, Benedetti C, Lucchini RG, Smith DR. Hair as a biomarker of environmental manganese exposure. Environmental science & technology. 2013;47(3):1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tseng CH. A review on environmental factors regulating arsenic methylation in humans. Toxicol Appl Pharmacol. 2009;235(3):338–50. [DOI] [PubMed] [Google Scholar]
- 38.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54(10):615–27. [DOI] [PubMed] [Google Scholar]
- 39.Buuren Sv, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. Journal of statistical software. 2010:1–68. [Google Scholar]
- 40.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC medical research methodology. 2008;8(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kile ML, Cardenas A, Rodrigues E, Mazumdar M, Dobson C, Golam M, et al. Estimating Effects of Arsenic Exposure During Pregnancy on Perinatal Outcomes in a Bangladeshi Cohort. Epidemiology. 2016;27(2):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin PD, Bromage S, Mostofa MG, Rahman M, Allen J, Oken E, et al. Mediating role of arsenic in the relationship between diet and pregnancy outcomes: prospective birth cohort in Bangladesh. Environ Health. 2019;18(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Claus Henn B, Ettinger AS, Hopkins MR, Jim R, Amarasiriwardena C, Christiani DC, et al. Prenatal Arsenic Exposure and Birth Outcomes among a Population Residing near a Mining-Related Superfund Site. Environ Health Perspect. 2016;124(8):1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almberg KS, Turyk ME, Jones RM, Rankin K, Freels S, Graber JM, et al. Arsenic in drinking water and adverse birth outcomes in Ohio. Environ Res. 2017;157:52–9. [DOI] [PubMed] [Google Scholar]
- 45.Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, et al. Maternal arsenic exposure associated with low birth weight in Bangladesh. J Occup Environ Med. 2007;49(10):1097–104. [DOI] [PubMed] [Google Scholar]
- 46.Council NR. Critical Aspects of EPA’s IRIS Assessment of Inorganic Arsenic. 2013.
- 47.LeBeau MA, Montgomery MA, Brewer JD. The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Sci Int. 2011;210(1–3):110–6. [DOI] [PubMed] [Google Scholar]
- 48.Molin M, Ulven SM, Meltzer HM, Alexander J. Arsenic in the human food chain, biotransformation and toxicology--Review focusing on seafood arsenic. J Trace Elem Med Biol. 2015;31:249–59. [DOI] [PubMed] [Google Scholar]
- 49.Katz SA. On the use of hair analysis for assessing arsenic intoxication. International journal of environmental research and public health. 2019;16(6):977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frederick IO, Williams MA, Sales AE, Martin DP, Killien M. Pre-pregnancy body mass index, gestational weight gain, and other maternal characteristics in relation to infant birth weight. Matern Child Health J. 2008;12(5):557–67. [DOI] [PubMed] [Google Scholar]
- 51.Ceja-Galicia ZA, Daniel A, Salazar AM, Panico P, Ostrosky-Wegman P, Diaz-Villasenor A. Effects of arsenic on adipocyte metabolism: Is arsenic an obesogen? Mol Cell Endocrinol. 2017;452:25–32. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez KF, Ungewitter EK, Crespo-Mejias Y, Liu C, Nicol B, Kissling GE, et al. Effects of in Utero Exposure to Arsenic during the Second Half of Gestation on Reproductive End Points and Metabolic Parameters in Female CD-1 Mice. Environ Health Perspect. 2016;124(3):336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahman ML, Valeri L, Kile ML, Mazumdar M, Mostofa G, Qamruzzaman Q, et al. Investigating causal relation between prenatal arsenic exposure and birthweight: Are smaller infants more susceptible? Environ Int. 2017;108:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, et al. Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect. 2001;109(6):629–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skröder H, Kippler M, Nermell B, Tofail F, Levi M, Rahman SM, et al. Major limitations in using element concentrations in hair as biomarkers of exposure to toxic and essential trace elements in children. Environmental health perspectives. 2017;125(6):067021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Registry AfTSaD. Hair Analysis Panel Discussion: Exploring the State of the Science. 2001.
- 58.VanderWeele TJ, Mumford SL, Schisterman EF. Conditioning on intermediates in perinatal epidemiology. Epidemiology (Cambridge, Mass). 2012;23(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bloomfield FH, Oliver MH, Harding JE. The late effects of fetal growth patterns. Arch Dis Child Fetal Neonatal Ed. 2006;91(4):F299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Misra DP, Nguyen R. Environmental tobacco smoke and low birth weight: a hazard in the workplace? Environmental Health Perspectives. 1999;107(suppl 6):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Q, Wang Y. Socioeconomic inequality of obesity in the United States: do gender, age, and ethnicity matter? Soc Sci Med. 2004;58(6):1171–80. [DOI] [PubMed] [Google Scholar]
- 62.Agbim U, Carr RM, Pickett-Blakely O, Dagogo-Jack S. Ethnic Disparities in Adiposity: Focus on Non-alcoholic Fatty Liver Disease, Visceral, and Generalized Obesity. Curr Obes Rep. 2019;8(3):243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daviglus ML, Pirzada A, Van Horn L. Ethnic disparities in cardiovascular risk factors in children and adolescents. Current Cardiovascular Risk Reports. 2014;8(3):376. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


