Abstract
Frailty, a measure of physiologic reserve, is associated with poor outcomes and mortality among kidney transplant (KT) candidates and recipients. There are no national estimates of frailty in this population, which may help patient counseling and resource allocation at transplant centers. We studied 4,616 KT candidates and 1,763 recipients in our multi-center prospective cohort of frailty from 2008–2018 with Fried frailty measurements. Using SRTR data (KT candidates=560,143 and recipients=243,508), we projected the national prevalence of frailty (for KT candidates and recipients separately) using standardization through inverse probability weighting, accounting for candidate/recipient, donor, and transplant factors. In our multi-center cohort, 13.3% KT candidates were frail at evaluation; 8.2% of LDKT recipients and 17.8% of DDKT recipients were frail at transplantation. Projected nationally, our modeling strategy estimated 91,738 KT candidates or 16.4% (95%CI:14.4–18.4%) of all KT candidates during the study period were frail, and 34,822 KT recipients or 14.3% (95%CI:12.3–16.3%) of all KT recipients were frail (LDKT=8.2%; DDKT=17.8%). Given its estimated national prevalence, transplant programs should consider assessing frailty during KT evaluation to improve patient counseling and resource allocation along with identification of recipients at risk for poor outcomes.
INTRODUCTION
Frailty is characterized by decreased physiologic reserve and resistance when confronted with a stressor, such as transplantation. The Fried physical frailty phenotype, a common measurement of frailty, is comprised of unintentional weight loss, slowed walking speed, decreased grip strength, decreased physical activity, and exhaustion, and was initially identified in community-dwelling older adults (1). Based on previous cohort studies, frailty is prevalent in 12–20% of kidney transplant (KT) candidates and is associated with decreased listing for KT (2), waitlist mortality (2, 3), decreased transplantation rates after listing (2), and poor health-related quality of life (4). Furthermore, frailty in KT recipients is associated with poor outcomes following KT such as delirium (5), longer length of stay (6), early hospital readmission (7), immunosuppression intolerance (8), poor health-related quality of life (9), cognitive decline (10), and mortality (11). Yet these estimates are from multi-center cohort studies, and national prevalence estimates of frailty may vary from these studies due to differences in KT candidate and recipient populations across the United States. National frailty estimates may help centers with waitlist management, resource allocation, and planning at transplant centers, as well as with patient counseling regarding waiting time and outcomes prior to KT.
Frailty is not commonly assessed at the time of evaluation, transplant, or collected in national registries, despite its association with poor outcomes in KT candidates and recipients (12). In 2018 in the United States, 94,970 adults were listed for KT and 21,167 underwent KT (13), and likely a large percentage of those KT recipients were frail. Additionally, frailty is more common in older (age≥65) KT candidates (2, 3) and KT recipients (14), and the number of older adults undergoing KT is increasing over time, with more than 19% of KT recipients over the age of 65 (13, 15). Thus, national estimates of frailty across all states, donor service areas (DSAs), and transplant centers may help guide interventions to reduce or lessen the burden of frailty in the growing population of vulnerable KT candidates and recipients.
Understanding the prevalence of frailty among KT candidates and recipients can inform candidate expectations and waitlist management at centers across the United States. In this study, we estimated the prevalence of frailty in a prospective, longitudinal, multi-center cohort of KT candidates and recipients and developed a predictive statistical model using characteristics captured by the national transplant registry. Then, using a novel statistical approach, we estimated the national prevalence of frailty among transplant candidates and recipients in the United States over the two decades. Finally, we explored geographic difference in the prevalence of frailty across the United States.
METHODS
Prospective Cohort Data Source: KT Candidates
This study used data from a prospective, longitudinal multi-center cohort study at the Johns Hopkins Hospital (N=2,217), Baltimore, Maryland; the University of Michigan Hospital (N=97), Ann Arbor, Michigan; and the Methodist Specialty and Transplant Hospital (N=2,217), San Antonio, Texas, and has been described elsewhere (7, 16–18). Briefly, study participants were enrolled prior to KT and consented to medical record abstraction to allow for the identification of demographics and co-morbidities. KT candidates underwent a battery of exams to assess frailty (as described below) at KT evaluation in clinic. The clinical and research activities being reported are consistent with the Declaration of Helsinki and Declaration of Istanbul. The Institutional Review Boards of Johns Hopkins Hospital, the University of Michigan, and the Methodist Specialty and Transplant Hospital approved this study, and all participants provided written informed consent.
Prospective Cohort Data Source: KT Recipients
The data for KT recipients was collected from the same longitudinal, prospective cohort studies at Johns Hopkins Hospital (n=952), University of Michigan (n=82), and the Methodist Specialty and Transplant Hospital (n=729). At Johns Hopkins Hospital and the University of Michigan, study participants were enrolled at the time of KT and consented to medical record abstraction to allow for the identification of demographics and co-morbidities. KT recipients underwent a battery of exams to assess frailty (as described below) at admission for KT. At the Methodist Specialty and Transplant Hospital, participants were enrolled at KT evaluation and frailty was assessed at every visit prior to KT; the measure of frailty prior to KT was used to estimate the prevalence of frailty among KT recipients.
National Registry Data Source
This study also used data from the Scientific Registry of Transplant Recipients (SRTR) external release made available in December 2018. The SRTR data system includes data on all donors, waitlist candidates, and transplant recipients in the United States (US), submitted by members of the Organ Procurement and Transplantation Network (OPTN), and has been previously described (19). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. Using SRTR, we identified 560,143 adult (age≥18) candidates listed and 243,508 adult recipients who underwent KT between January 2000 and June 2018 to include the KT listing dates of candidates at the three prospective cohort centers.
Frailty
We studied the Fried physical frailty phenotype as defined (1) in older adults as well as in end-stage renal disease and KT populations (2, 4–8, 10, 11, 17, 20–26). The Fried physical frailty phenotype was based on 5 components: shrinking (self-report of unintentional weight loss of more than 10 pounds in the past year based on dry weight); weakness (grip-strength below an established cutoff based on gender and BMI); exhaustion (self-report); low activity (Kcals/week below an established cutoff); and slowed walking speed (walking time of 15 feet below an established cutoff by gender and height) (1). Each of the 5 components was scored as 0 or 1 representing the absence or presence of that component. The aggregate frailty score was calculated as the sum of the component scores (range 0–5); frail was defined as a score of ≥3, prefrail as a score of 2, and nonfrail as a score of <2. The physicians at Johns Hopkins Hospital and University of Michigan were not aware of the frailty assessment results at time of evaluation, but the physicians at Methodist Specialty and Transplant Hospital were aware of the frailty assessments at the time of evaluation.
Estimating National Prevalence
To estimate the national prevalence of frailty, we mapped data from our prospective cohort to the national transplant population using variables captured by both databases. We used standardization using inverse probability of selection weights (IPSW). Standardization is a common approach in public health that uses known characteristics about a target population to inform estimation in a sampled population or vice versa. Weighting approaches to standardization allow for additional covariates to be considered.
We used a two-stage approach to estimate the national prevalence of frailty using IPSW. This method seeks to provide unbiased estimates by adjusting for variables that may have affected the selection of our study population relative to the general transplant population. The first stage calculated restricted IPSW weights using baseline characteristics. For candidates, we adjusted for female sex, African-American race, Hispanic ethnicity, age at listing, time on dialysis, college education, body mass index (BMI), hypertension status, history of previous transplant, employment status, public insurance status, and PRA at listing. For recipients, we adjusted for female sex, African-American race, Hispanic ethnicity, age at transplant (splines with knots at 35 and 65), ≥2 years on dialysis, college education, BMI, hypertension status, history of previous transplant, employment status, public insurance status, PRA at transplant, and donor BMI. Potential variables were selected based on prior literature and parsimonious models were built to maximize the Akaike information criterion (AIC) and Bayesian information criterion (BIC). These weights were then restricted to the range 10% to 90% to avoid bias due to extreme weights (27) and converted to inverse probability of selection weights. The second stage used linear risk regression to examine the prevalence of frailty weighted by the IPSW.
Frailty prediction model
We constructed a prediction model for frailty using data from our prospective cohort. The model building approach maximized AUC and adjusted for recipient (age, sex, African-American race, Hispanic ethnicity, diabetes, hypertension, history of transplant, college education, PRA at transplant, preemptive transplant, years on dialysis, BMI, public insurance status, and employment status), donor (age, sex, African-American race, Hispanic ethnicity, kidney donor profile index [KDPI] for deceased donors, BMI, and eGFR), transplant (HLA mismatch, year of transplant, cold ischemia time), and outcome (all-cause graft loss) characteristics. Separate models were used for KT candidates and recipients; donor and outcome variables were only included in the KT recipient model.
Visualizing Prevalence Differences
Using the national prevalence estimates, we estimated the number of frail and prefrail candidates and transplant recipients across 58 DSAs and across 288 transplant centers. We estimated the ratio of the number of frail recipients divided by the number of frail candidates as a measure of access to transplantation for those that are frail (ratio >1 indicates greater access) for each DSA. We then compared the frail transplant recipient/candidate ratio in each DSA to the median frail transplant recipient/candidate ratio to demonstrate differences in access by geographic boundary. We presented a similar measure for candidates and recipients that are prefrail.
Statistical Analysis
For participants in the prospective cohort, differences in recipient, donor, and transplant characteristics by frailty status were assessed using the χ2 (categorical variables) and Mann-Whitney rank-sum (continuous variables) tests. We used a two-sided α of 0.05 to indicate a statistically significant difference. All analyses were performed using Stata 15/MP for Linux (College Station, Texas).
Sensitivity analyses
We built prediction models that were used to inform a multiple imputation approach. All models were run after multiple imputation for baseline characteristics. Conceptually, we used multiple imputation by chained equation (MICE) to predict the frailty status of individuals in the general transplant population by treating the frailty status of those not in our prospective cohort as missing-at-random. A prediction model for frailty was built in our prospective cohort to maximize area-under-the-curve (AUC). The variables from this prediction model were then used to generate 100 imputed datasets (after 10 run-in datasets) using MICE and Rubins’ rules for pooled estimation.
Additionally, we estimated the national prevalence of frailty among kidney transplant candidates, living donor kidney transplant (LDKT) recipients, and deceased donor kidney transplant (DDKT) recipients living outside the Stroke Belt using IPSW and MICE. We defined states in the Stroke Belt to include Alabama, Arkansas, Georgia, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee.
We also compared the distribution of impairment characterized by the Karnofsky Performance Score (KPS) and frailty across DSAs among KT candidates and KT recipients using tetrachoric correlation. KPS impairment was defined as at or below 70%.
RESULTS
Prospective Cohort Characteristics
Among 4,616 KT candidates in our prospective cohort, 612 (13.3%) were frail. Frail participants were more likely to be older (55.0 years vs. 52.0 years, p<0.001), African-American (41.2% vs. 25.8%, p<0.001), have diabetes (41.3% vs. 35.9%, p<0.01), and have a history of previous transplant (19.8% vs. 15.1%, p<0.01) and less likely to be Hispanic (22.4% vs. 37.5%, p<0.001) and employed at the time of listing (24.8% vs. 37.1%, p<0.001) compared to nonfrail participants (Table 1).
Table 1.
Characteristics of prospective cohort of kidney transplant candidates listed from 2000–2018 by frailty status (n=4,616).
| Not Frail | Pre-frail | Frail | p | |
|---|---|---|---|---|
| N (%) | 2862 (62.0%) | 1142 (24.7%) | 612 (13.3%) | |
| Recipient Characteristics | ||||
| Age at Listing* | 52.0 (42.0–60.0) | 54.0 (44.0–62.0) | 55.0 (45.0–63.0) | <0.001 |
| Female, % | 39.1 | 41.1 | 41.3 | 0.3 |
| African-American, % | 25.8 | 31.3 | 41.2 | <0.001 |
| Hispanic, % | 37.5 | 30.8 | 22.4 | <0.001 |
| BMI, kg/m2* | 29.1 (25.1–33.3) | 29.6 (25.3–33.5) | 29.5 (25.3–33.5) | 0.7 |
| Dialysis vintage, years* | 1.0 (0.4–2.4) | 1.0 (0.5–2.4) | 0.9 (0.4–2.2) | 0.2 |
| College educated, % | 55.8 | 52.9 | 53.8 | 0.4 |
| Employed, % | 37.1 | 30.5 | 24.8 | <0.001 |
| Public insurance, % | 52.0 | 58.2 | 56.2 | 0.05 |
| Diabetes, % | 35.9 | 38.4 | 41.3 | <0.01 |
| Hypertension, % | 23.6 | 23.4 | 24.7 | 0.6 |
| Previous transplant, % | 15.1 | 17.8 | 19.8 | <0.01 |
| PRA>80 at listing, % | 1.4 | 1.2 | 2.3 | 0.1 |
Median (interquartile range)
Among the 817 living donor kidney transplant (LDKT) recipients in our prospective cohort, 67 (8.2%) were frail. Frail participants were less likely to be Hispanic (17.9% vs. 35.3%, p<0.01) but were similar to nonfrail participants in other characteristics (Table 2).
Table 2.
Characteristics of prospective cohort of living donor kidney transplant recipients from 2008–2018 by frailty status (n=817).
| Not Frail | Prefrail | Frail | P value | |
|---|---|---|---|---|
| N (%) | 574 (70.3%) | 176 (21.5%) | 67 (8.2%) | |
| Recipient Characteristics | ||||
| Age, years* | 50.0 (38.0–60.0) | 51.0 (40.0–62.0) | 53.0 (41.0–60.0) | 0.4 |
| Female, % | 40.5 | 46.6 | 46.3 | 0.4 |
| African-American, % | 10.8 | 14.8 | 19.4 | 0.03 |
| Hispanic, % | 35.3 | 28.4 | 17.9 | <0.001 |
| BMI, kg/m2* | 28.0 (24.4–31.9) | 28.1 (24.3–31.7) | 27.5 (22.6–31.1) | 0.3 |
| Dialysis vintage, years* | 0.5 (0.0–1.9) | 0.7 (0.0–2.4) | 0.9 (0.0–3.1) | 0.1 |
| HCV, % | 2.5 | 1.1 | 0 | 0.2 |
| College educated, % | 67.9 | 65.7 | 70.8 | 0.6 |
| Employed, % | 45.8 | 46 | 47.0 | 0.9 |
| Public insurance, % | 42.0 | 40.9 | 41.8 | 1.0 |
| Diabetes, % | 27.6 | 28.4 | 31.3 | 0.5 |
| Hypertension, % | 16.0 | 13.6 | 11.9 | 0.4 |
| Previous transplant, % | 14.5 | 19.9 | 20.9 | 0.2 |
| PRA>80 at listing, % | 20.1 | 20 | 15.4 | 0.5 |
| Donor Characteristics | ||||
| Age, years* | 42.0 (33.0–52.0) | 44.5 (35.5–52.0) | 45.0 (35.0–54.0) | 0.1 |
| Female, % | 64.8 | 63.1 | 64.2 | 0.9 |
| African-American, % | 7.6 | 10.2 | 13.4 | 0.09 |
| Hispanic, % | 32.3 | 25.6 | 17.9 | 0.01 |
| BMI, kg/m2* | 27.3 (24.1–30.5) | 26.9 (23.9–29.7) | 26.9 (23.9–30.1) | 0.5 |
| eGFR* | 104.4 (87.7–118.3) | 103.0 (88.7–117.3) | 99.9 (88.0–112.3) | 0.3 |
| Biologically related, % | 39.9 | 44.3 | 32.8 | 0.3 |
| Transplant Characteristics | ||||
| ABO Incompatible, % | 5.5 | 6.8 | 6 | 0.9 |
| Zero HLA mismatch, % | 5.9 | 4.6 | 4.5 | 0.6 |
| Cold ischemia time, hours* | 1.3 (1.1–1.9) | 1.3 (1.0–2.0) | 1.6 (1.0–4.0) | 0.02 |
BMI= body mass index, HCV= hepatitis c virus, PRA= panel reactive antibody, GFR= glomerular filtration rate, HLA= human leukocyte antigen
Median (interquartile range)
Among the 946 deceased donor kidney transplant (DDKT) recipients in our prospective cohort, 168 (17.8%) were frail. Frail participants were more likely to be African-American (52.4% v. 39.2%, p<0.01), older (59 years vs. 54 years, p<0.001), have higher BMI (29.6 v. 28.1, p=0.03), and have HCV infection (17.4% vs. 7.4%, p<0.001) and were less likely to be Hispanic (4.8% vs. 26.3%, p<0.001) or have public insurance (59.5% vs. 72.0%, p<0.01) at the time of KT (Table 3) compared to nonfrail participants. Additionally, frail DDKT recipients were more likely to receive higher median KDPI donors than nonfrail DDKT recipients (52.8 vs. 42.7, p<0.001) and have a longer cold ischemia time (25.0 hours vs. 22.1 hours, p=0.04).
Table 3.
Characteristics of prospective cohort of deceased donor kidney transplant recipients from 2008–2018 by frailty status (n=946).
| Not Frail | Prefrail | Frail | P value | |
|---|---|---|---|---|
| N | 533 (56.3%) | 245 (25.9%) | 168 (17.8%) | |
| Recipient Characteristics | ||||
| Age, years* | 54.0 (42.0–62.0) | 58.0 (46.0–64.0) | 59.0 (50.0–66.5) | <0.001 |
| Female, % | 37.4 | 37.6 | 40.5 | 0.5 |
| African-American, % | 39.2 | 42.9 | 52.4 | <0.01 |
| Hispanic, % | 26.3 | 15.5 | 4.8 | <0.001 |
| BMI, kg/m2* | 28.1 (24.5–32.0) | 28.7 (25.1–32.4) | 29.6 (25.3–33.2) | 0.03 |
| Dialysis vintage, years* | 4.0 (1.8–6.6) | 3.0 (1.3–6.2) | 2.8 (0.9–4.9) | <0.001 |
| HCV, % | 7.9 | 11.5 | 17.4 | <0.001 |
| College educated, % | 54.8 | 55.9 | 55.2 | 0.9 |
| Employed, % | 32.2 | 34.2 | 25 | 0.07 |
| Public insurance, % | 72.0 | 65.3 | 59.5 | <0.01 |
| Diabetes, % | 25.4 | 26.1 | 24.4 | 0.8 |
| Hypertension, % | 34.2 | 32.7 | 37.5 | 0.4 |
| Previous transplant, % | 16.7 | 17.1 | 12.5 | 0.2 |
| PRA>80 at listing, % | 13.2 | 11.8 | 13.9 | 0.8 |
| Donor Characteristics | ||||
| Age, years* | 34.0 (23.0–47.0) | 35.0 (25.0–50.0) | 36.0 (26.0–49.0) | 0.10 |
| Female, % | 41.1 | 42.4 | 41.7 | 0.9 |
| African-American, % | 18.0 | 22 | 20.8 | 0.4 |
| Hispanic, % | 19.2 | 14.7 | 5.4 | <0.001 |
| BMI, kg/m2* | 26.6 (23.0–31.2) | 26.8 (23.3–32.3) | 25.7 (23.0–30.0) | 0.3 |
| KDPI* | 42.7 (23.7–63.5) | 46.1 (27.9–67.7) | 52.8 (36.8–71.1) | <0.001 |
| Transplant Characteristics | ||||
| Zero HLA mismatch, % | 5.1 | 4.1 | 5.4 | 0.9 |
| Cold ischemia time, hours* | 22.1 (15.3–29.1) | 24.0 (16.3–30.0) | 25.0 (15.8–32.0) | 0.04 |
BMI= body mass index, HCV= hepatitis c virus, PRA= panel reactive antibody, HLA= human leukocyte antigen
Median (interquartile range)
National Prevalence of Frailty
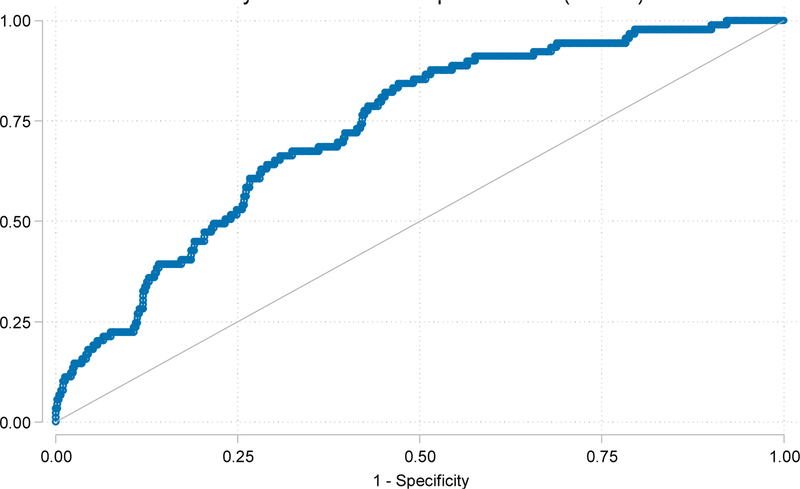
In our prospective cohort, the frailty prediction model had an AUC of 0.731 for KT recipients (Figure 1). 560,143 KT candidates were listed during our study period (01/2000–06/2018). We estimated that 16.4% (N=91,738, 95% CI: 14.4–18.4%) of transplant candidates were frail (Table 4). Additionally, there were a total of 243,508 transplant recipients during our study period, and we estimated that 14.3% (N=34,822, 95%CI: 12.3–16.3%) of transplant recipients were frail; 8.2% of LDKT recipients and 17.8% of DDKT recipients were frail.
Figure 1.
Prediction model performance (AUC = 0.731) for frailty among kidney transplant recipients.
Table 4.
National estimates of frailty among kidney transplant candidates, living donor kidney transplant (LDKT) recipients, and deceased donor kidney transplant (DDKT) recipients. Predicted national prevalence was estimated using inverse-probability of selection weights (IPSW).
| N | IPSW (95% Confidence Interval)* |
|
|---|---|---|
| KT candidates | 560,143 | 16.4% (14.4%–18.4%) |
| LDKT | 81,322 | 8.2% (6.3%–10.1%) |
| DDKT | 162,186 | 17.8% (15.3%–20.2%) |
| All KT recipients | 243,508 | 14.3% (12.3%–16.3%) |
Geographic Distribution
Among KT candidates, the median (IQR) prevalence of frailty across 58 DSAs was 13.9% (12.6%−14.6%) and across 288 transplant centers was 13.4% (11.6%−14.7%). Among KT recipients, the median (IQR) prevalence of frailty across 58 DSAs was 18.8% (18.0%−20.5%) and across 288 transplant centers was 18.2% (16.4%−20.5%).
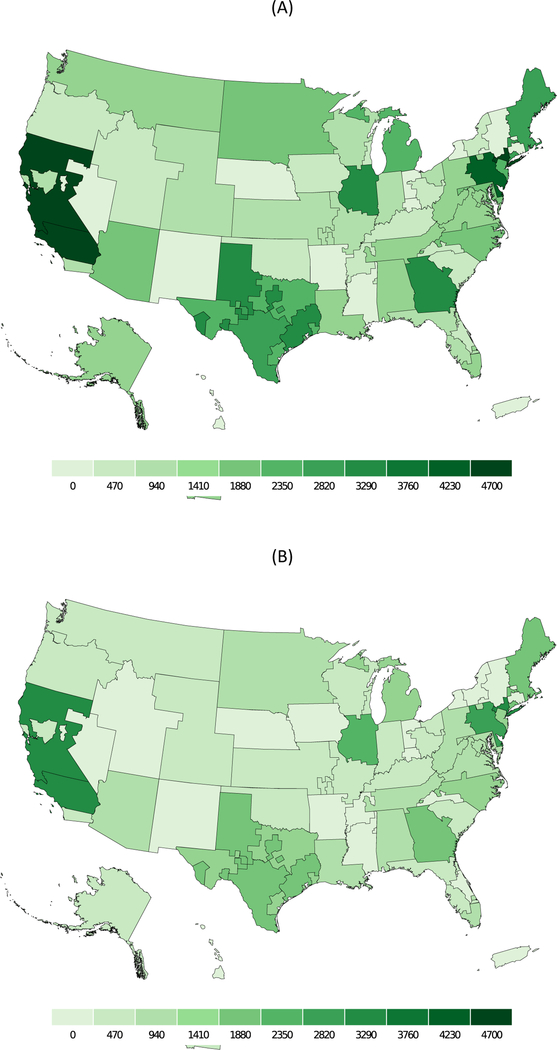
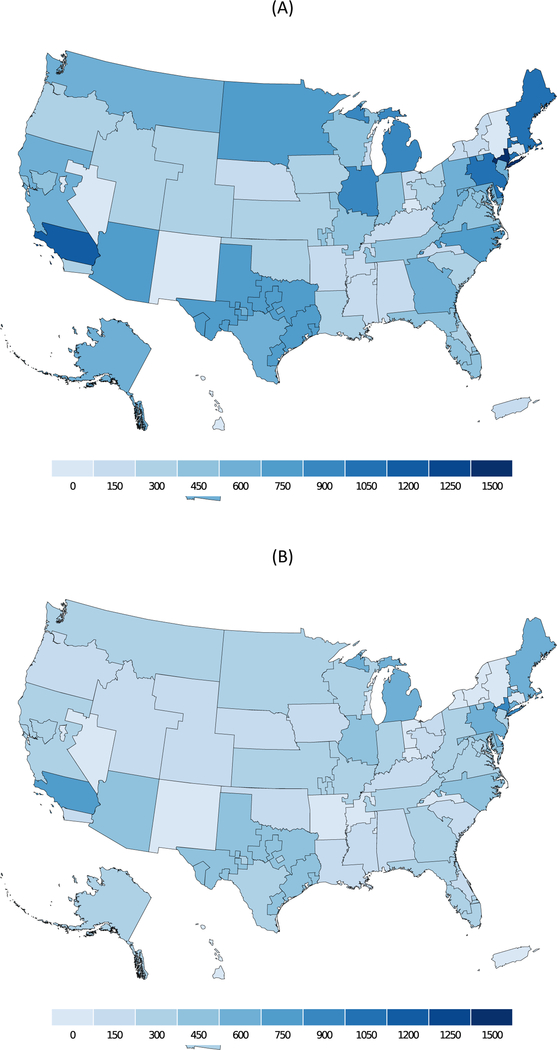
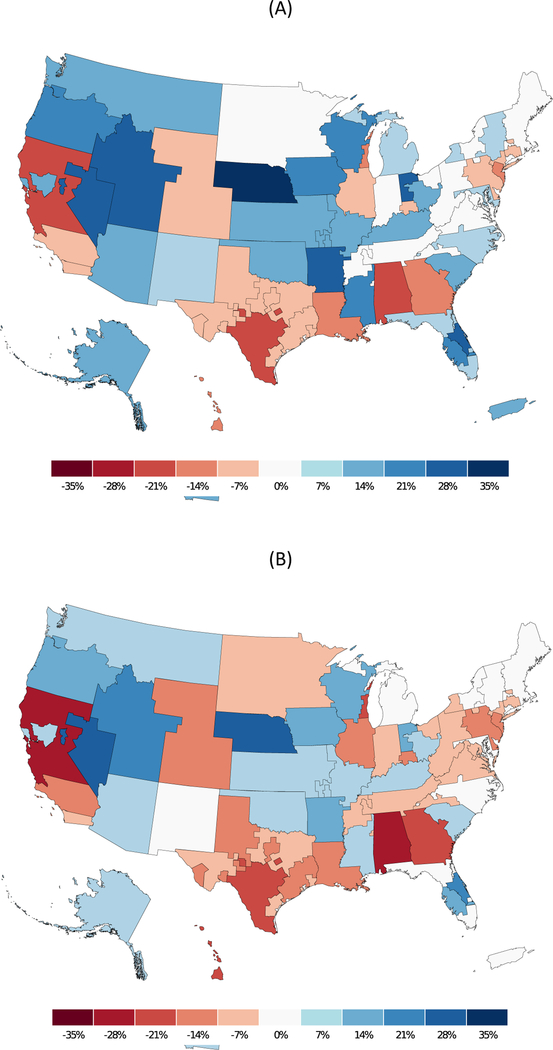
Based on our prediction model for frailty, we estimated the number of frail and prefrail candidates (Figure 2) and recipients (Figure 3) by DSA. The highest concentration of prefrail and frail candidates were in California and New York. Our estimates also suggest variation in the transplant/candidate ratio for frail and prefrail individuals by DSA (Figure 4). The lowest access to transplant in frail candidates were in California, Texas, Alabama, and Georgia.
Figure 2.
Number of (A) prefrail and (B) frail KT candidates by donor service area from 2000–2018.
Figure 3.
Number of (A) prefrail and (B) frail kidney transplant recipients by donor service area from 2000–2018.
Figure 4.
Number of (A) prefrail and (B) frail percentage difference from median transplant/candidate ratio to assess access to transplantation for among frail and nonfrail candidates from 2000–2018. The lowest access to transplant in frail candidates were in California, Texas, Alabama, and Georgia.
Sensitivity analyses
Using MICE, we estimated that 20.5% (N=49,904, 95%CI: 14.5–26.5%) of transplant recipients were frail, and we estimated that 16.4% (N=92,003, 95% CI: 15.4–17.5%) of transplant candidates were frail.
After exclusion of states in the Stroke Belt, the national prevalence of frailty estimates among KT candidates, LDKT recipients, and DDKT recipients were similar to our primary results using IPSW and MICE (Supplemental Table 1A, 1B).
Among our KT candidate cohort, 30 of 612 frail candidates had KPS impairment, and among our KT recipient cohort, 7 of 235 frail recipients had KPS impairment. Among national KT candidates, 24.3% had KPS impairment. KPS impairment poorly correlated with frailty measurements for KT candidates in our cohort (rho 0.07). Furthermore, among national KT recipients, 26.8% had KPS impairment. KPS impairment poorly correlated with frailty measurements for KT recipients in our cohort (rho 0.13).
DISCUSSION
Nationally, we estimated that 16.4% of KT candidates were frail, and 14.3% of KT recipients were frail from 2000–2018; 8.2% of LDKT recipients and 17.8% of DDKT recipients were frail. Furthermore, the prevalence of frailty among KT recipients and candidates varies across the United States. In our three-center prospective cohort study of frailty in more than 6,000 participants, we found that 8.2% of LDKT recipients, and 17.8% of DDKT recipients were frail. Frail KT candidates were more likely to be older (p<0.001) and African-American (p<0.001) and less likely to be Hispanic (p<0.001). Similarly, frail DDKT recipients were more likely to be older (p<0.001) and African-American (p<0.01) and less likely to be Hispanic (p<0.001).
Our finding that 16.4% of KT candidates were frail from national estimates were lower than those seen in other studies of hemodialysis patients using the Fried physical frailty phenotype with modification for weight loss (30–60% frail) (28, 29). However, this finding is not surprising considering that frail participants have nearly a 2-fold decreased chance of being listed for KT at evaluation compared to nonfrail participants. Additionally, our findings that frail participants were more likely to be older and have diabetes were similar to findings in the aforementioned study of hemodialysis patients (28). Identification of frail candidates at evaluation can help with patient counseling with regard to poor waitlist outcomes such as increased waitlist mortality and lower rate of KT (2), and also identify those who may benefit from closer follow-up and interventions.
Additionally, using national registry data, we estimated that 8.2% of LDKT recipients and 17.8% of DDKT recipients in the US were frail at the time of transplant. Our results highlight the importance of identification of recipients at the time of transplant, given that one in five KT recipients will be at an increased odds of delirium (5) and longer length of stay (6), increased risk of delayed graft function (21), early hospital readmission (7), immunosuppression intolerance (8), cognitive decline (10), and mortality (11). Identification of these vulnerable patients can potentially help clinicians target those patients to mitigate poor outcomes after KT (2), and quantifying the national prevalence of frailty in kidney transplant candidates and recipients is important for resource allocation planning.
Strengths of this study include a large sample of KT candidates and recipients from three transplant centers and prospective cohort study of frailty. Additionally, the use of the novel measurement of frailty and national projections to estimate national prevalence are not currently possible through use of registry data; frailty is not captured in national registries. There are several limitations to this study. One limitation is the selection bias of participants who were referred from the community to the three transplant centers; however, we have no way to measure frailty in participants who were not referred for evaluation. Furthermore, there is a selection bias of who is referred and listed for transplantation, but the goal of our study was to inform decision-making at two distinct times for two distinct populations: at KT evaluation/listing for candidates and at admission for KT for recipients. Another notable limitation is that our national estimates may not be accurate given the prospective study population characteristics, and these should be noted as estimates. However, demographics between the three transplant centers are quite different (age, race, time on dialysis, living donor) which is a strength of this study (2).
In conclusion, we estimated that 16.4% of KT candidates and 14.3% of KT recipients in the United States were frail from 2000–2018, and that the prevalence of frailty in KT candidates and recipients varied by geographic location. Given the high prevalence of frailty, transplant programs should consider assessing frailty during KT evaluation to improve informed consent and identify candidates for pre-KT interventions. Our findings can encourage centers to include frailty as part of their evaluation and help identify a vulnerable population of patients that may benefit from potential interventions like prehabilitation (30).
Supplementary Material
ACKNOWLEDGMENTS
Funding for this study was provided in part by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) and the National Institute on Aging (NIA) grant numbers F32AG053025 (PI: Christine Haugen), F30DK116658 (PI: Ashton Shaffer), K24DK101828 (PI: Dorry Segev), and R01AG055781 (PI: Mara McAdams-DeMarco). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
The data reported here have been supplied by the Hennnepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR, OPTN/UNOS, or the US Government.
Abbreviations:
- AUC
area under the curve
- DDKT
deceased donor kidney transplantation
- DSA
donor service area
- ESRD
end-stage renal disease
- HLA
human leukocyte antigen
- IPSW
inverse probability of selection weights
- KDPI
kidney donor profile index
- KT
kidney transplantation
- LDKT
living donor kidney transplantation
- MICE
multiple imputation by chain equation
- OPTN
Organ Procurement and Transplantation Network
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 2.Haugen CE, Chu NM, Ying H, Warsame F, Holscher CM, Desai NM et al. Frailty and Access to Kidney Transplantation. Clin J Am Soc Nephrol 2019;14(4):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAdams-DeMarco MA, Ying H, Thomas AG, Warsame F, Shaffer AA, Haugen CE et al. Frailty, Inflammatory Markers, and Waitlist Mortality Among Patients With End-stage Renal Disease in a Prospective Cohort Study. Transplantation 2018;102(10):1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAdams-DeMarco MA, Ying H, Olorundare I, King EA, Desai N, Dagher N et al. Frailty and Health-Related Quality of Life in End Stage Renal Disease Patients of All Ages. J Frailty Aging 2016;5(3):174–179. [PMC free article] [PubMed] [Google Scholar]
- 5.Haugen CE, Mountford A, Warsame F, Berkowitz R, Bae S, A GT et al. Incidence, Risk Factors, and Sequelae of Post-kidney Transplant Delirium. J Am Soc Nephrol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAdams-DeMarco MA, King EA, Luo X, Haugen C, DiBrito S, Shaffer A et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Annals of surgery 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAdams-DeMarco MA, Law A, Salter ML, Chow E, Grams M, Walston J et al. Frailty and early hospital readmission after kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2013;13(8):2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAdams-DeMarco MA, Law A, Tan J, Delp C, King EA, Orandi B et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation 2015;99(4):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAdams-DeMarco MA, Olorundare IO, Ying H, Warsame F, Haugen CE, Hall R et al. Frailty and Postkidney Transplant Health-Related Quality of Life. Transplantation 2018;102(2):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu NM, Gross AL, Shaffer AA, Haugen CE, Norman SP, Xue QL et al. Frailty and Changes in Cognitive Function after Kidney Transplantation. J Am Soc Nephrol 2019;30(2):336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N et al. Frailty and mortality in kidney transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2015;15(1):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobashigawa J, Dadhania D, Bhorade S, Adey D, Berger J, Bhat G et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant 2019;19(4):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.OPTN Kidney report. 2017. 5/9/2018]; Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#
- 14.McAdams-DeMarco MA, Ying H, Olorundare I, King EA, Haugen C, Buta B et al. Individual Frailty Components and Mortality in Kidney Transplant Recipients. Transplantation 2017;101(9):2126–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAdams-DeMarco MA, James N, Salter ML, Walston J, Segev DL. Trends in kidney transplant outcomes in older adults. J Am Geriatr Soc 2014;62(12):2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAdams-DeMarco MA, Law A, Tan J, Delp C, King EA, Orandi B et al. Frailty, Mycophenolate Reduction, and Graft Loss in Kidney Transplant Recipients. Transplantation 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Ying H, Olorundare I, King EA, Haugen C, Buta B et al. Individual Frailty Components and Mortality In Kidney Transplant Recipients. Transplantation 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nastasi A, McAdams-DeMarco M, Schrack J, Ying H, Haugen C, Fernandez MG et al. Pre-Kidney Transplant Lower Extremity Impairment and Post-Transplant Mortality. American Journal of Transplantation 2017;17:811–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massie AB, Kuricka LM, Segev DL. Big Data in Organ Transplantation: Registries and Administrative Claims. American Journal of Transplantation 2014;14(8):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. Journal of the American Geriatrics Society 2013;61(6):896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg 2012;147(2):190–193. [DOI] [PubMed] [Google Scholar]
- 22.McAdams-Demarco MA, Suresh S, Law A, Salter ML, Gimenez LF, Jaar BG et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC nephrology 2013;14(1):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAdams-DeMarco MA, Tan J, Salter ML, Gross A, Meoni LA, Jaar BG et al. Frailty and Cognitive Function in Incident Hemodialysis Patients. Clin J Am Soc Nephrol 2015;10(12):2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAdams-DeMarco MA, Isaacs K, Darko L, Salter ML, Gupta N, King EA et al. Changes in Frailty After Kidney Transplantation. Journal of the American Geriatrics Society 2015;63(10):2152–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAdams-DeMarco MA, Ying H, Thomas AG, Warsame F, Shaffer AA, Haugen CE et al. Frailty, Inflammatory Markers, and Waitlist Mortality Among Patients with End-Stage Renal Disease in a Prospective Cohort Study. Transplantation 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu NM, Deng A, Ying H, Haugen CE, Garonzik Wang JM, Segev DL et al. Dynamic Frailty Before Kidney Transplantation-Time of Measurement Matters. Transplantation 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Statistical methods in medical research 2017;26(4):1654–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansen KL, Dalrymple LS, Delgado C, Kaysen GA, Kornak J, Grimes B et al. Association between body composition and frailty among prevalent hemodialysis patients: a US Renal Data System special study. J Am Soc Nephrol 2014;25(2):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol 2007;18(11):2960–2967. [DOI] [PubMed] [Google Scholar]
- 30.McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S, Schrack J, Haugen CE, Chu NM et al. Prehabilitation prior to kidney transplantation: Results from a pilot study. Clin Transplant 2019;33(1):e13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.