Abstract
Background
Cortical dopaminergic systems are critically involved in prefrontal cortex (PFC) functions, especially in working memory and neurodevelopmental disorders such as schizophrenia (SZ). Glycogen synthase kinase-3β (GSK-3β) is highly associated with cAMP-independent dopamine (DA) D2 receptor (D2R)-mediated signaling to affect DA-dependent behaviors. However, the mechanisms underlying the GSK-3β modulation of cognitive function via D2Rs remains unclear.
Methods
We explored how conditional cell-type specific ablation of GSK-3β in D2R+ neurons (D2R-GSK-3β−/−) in the brain affects synaptic function in the medial PFC. Both male and female (P60-P90) mice, including 140 D2R−, 24 D1R−, and 38 DISC1-mice were used.
Results
We found that NMDAR function was significantly increased in layer V pyramidal neurons in mPFC of D2R-GSK-3β−/− mice, along with increased DA modulation of NMDAR-mediated current. Consistently, NR2A and NR2B protein levels were elevated in mPFC of D2R-GSK-3β−/− mice. This change was accompanied by a significant increase in enrichment of activator histone mark H3K27ac at the promoters of both Grin2a and Grin2b genes. In addition, altered short- and long-term synaptic plasticity, along with an increased spine density in layer V pyramidal neurons were detected in D2R-GSK-3β−/− mice. Indeed, D2R-GSK-3β−/− mice also exhibited a resistance of working memory impairment induced by acute injection of NMDAR antagonist MK-801. Notably, either inhibiting GSK-3β or disrupting the D2R-DISC1 complex was able to reverse the mutant DISC1-induced decrease of NMDAR-mediated currents in the mPFC.
Conclusions
Our study demonstrates that GSK-3β modulates cognition via D2R-DISC1 interaction and epigenetic regulation of NMDAR expression and function.
Keywords: GSK-3β, prefrontal cortex, NMDA receptors, dopamine D2 receptors, histone modification, epigenetic, cognition
Introduction
The prefrontal cortex (PFC)-associated cognition is controlled by subpopulations of neurons that differentially express dopamine (DA) D1 receptors (D1Rs) and D2 receptors (D2Rs) with distinct projection targets (1–3). Dopaminergic control of PFC functions is also differentiated by the opposing D1R-mediated activation and D2R-mediated inhibition of the cAMP/PKA/DARPP-32 cascade (4, 5).
Recent studies have also identified glycogen synthase kinase 3β (GSK-3β) as a central signal integrator for DA-dependent responses, particularly D2R-mediated hyperdopaminergic behaviors (6–8). This cAMP-independent signaling pathway is critical for DA-targeting psychostimulants and antipsychotic drugs (6–9) and schizophrenia (SZ) pathophysiology (10, 11). However, a key impediment in understanding GSK-3β in DA-dependent responses is that almost nothing is known about the cell-type specific consequences of disrupted GSK-3β signaling in the brain. Recently, we found that deletion of GSK-3β in D2R+, but not D1R+, neurons regulates antipsychotic-sensitive behaviors, as well as working memory (12); however, the mechanism of these divergent responses is unclear.
DA plays an essential role in modulating glutamatergic function, especially NMDAR-mediated neurotransmission (13, 14) which is highly associated with synaptic plasticity relevant to SZ (15, 16). In particular, working memory and long-term recognition memory, both are impaired in SZ, depend on the activation of NMDARs (14). Importantly, we reported that activation of GSK-3β is required for hyperdopamine and D2R-induced inhibition of NMDAR-mediated transmission in the PFC (17). GSK-3β also plays critical roles in both NMDAR-dependent long-term potentiation (LTP) and depression (LTD). Specifically, activation of GSK-3β impairs LTP and promotes LTD (18, 19). This raises the possibility that deficiency of GSK-3β in D2R+ neurons may alter DA modulation of glutamatergic signaling, particularly NMDAR-mediated responses. We found that global deletion of GSK-3β in D2R+ neurons is accompanied by epigenetic-mediated enhancement of NMDAR subunit expression concomitant with increased NMDAR function, alterations in LTP and LTD, and increased spine density in the mPFC. The GSK-3β knockout effects in D2R+ neurons were recapitulated with drug treatment, viral knockdown, and in DISC1 mutant mice. Our findings reveal how D2R-GSK-3β signaling regulates cognitive function and provide a possible mechanism underlying NMDAR dysfunction in SZ pathophysiology.
Materials and Methods
Detailed methods and materials are described in the Supplemental Materials. Briefly, both male and female mice were used. For physiological recording and protein assay, see our previous study (17). Golgi-Cox impregnation was conducted with FD Rapid GolgiStain Kit (20). The set-shifting task was described in (21) and the dose of MK801 was referred from previous work (22).
Results
Genetic ablation of GSK-3β in D2R+ neurons increases NMDAR-, but not AMPAR-EPSCs
To examine how D2R-GSK-3β signaling affects excitatory synaptic transmission in the mPFC, we used transgenic mice in which GSK-3β is deleted in D2R+ neurons by crossing GSK-3βflox/flox mice with Drd2-Cre mice. This generated D2R-GSK-3β−/− mice and their GSK-3βflox/flox littermate controls (D2R-GSK-3β+/+). The expression patterns of Cre recombinase in D2Cre/+mice and the deletion of GSK-3β in D2R+ neurons were confirmed by immunostaining [refer (12) and Figure S1].
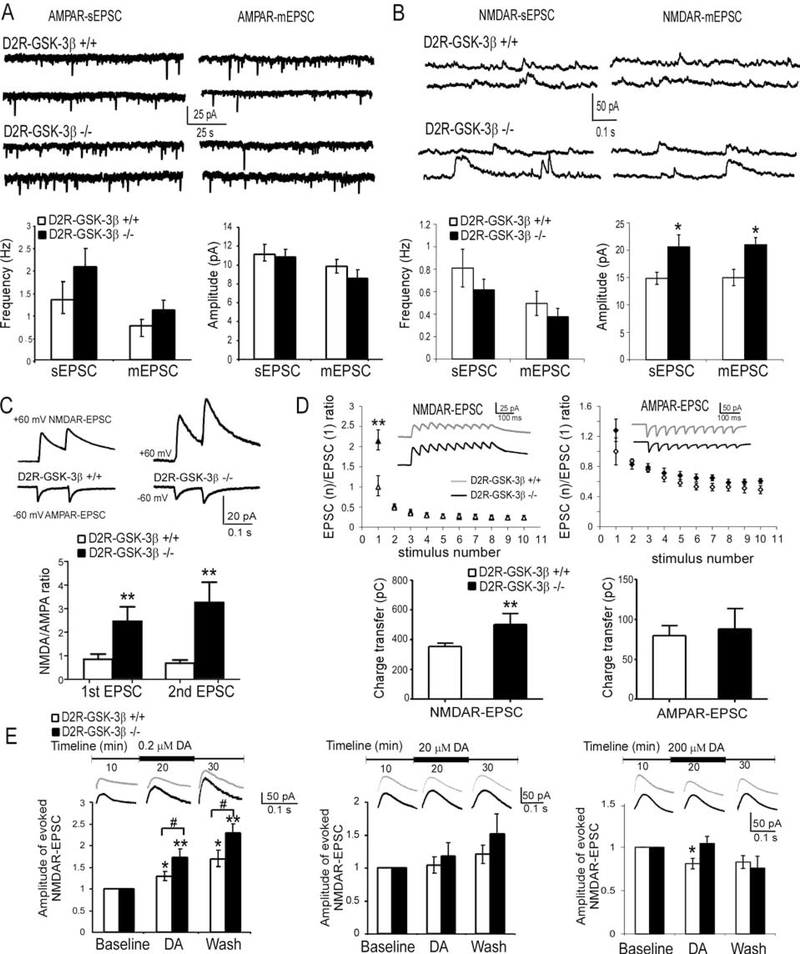
DA receptors express in all cortical layers (23, 24), particularly in layer V pyramidal neurons in the rodent PFC (25, 26). We thus focused on deep-layer pyramidal cells in the prelimbic region to study the effect of GSK-3β ablation on glutamatergic signaling. AMPAR- and NMDAR-sEPSCs and mEPSCs were recorded from P60–90 mice. Unexpectedly, neither AMPAR-sEPSCs nor mEPSCs was altered in D2R-GSK-3β−/− mice compared with control mice [(p > 0.05; Figure 1A, detailed statistics see Supplemental Results (SR)]. In contrast, the amplitudes, but not frequencies, of both NMDAR-sEPSCs and -mEPSCs, were increased in D2R-GSK-3β−/− mice (*p < 0.05; Figure 1B, SR). Consistently, the amplitudes of evoked NMDAR-EPSCs were significantly increased in D2R-GSK-3β−/− vs control mice (*p < 0.05; Figure S2, SR). In contrast, no alteration in NMDAR-sEPSCs and NMDAR-mEPSCs was detected in D1R-GSK-3β−/− mice (p > 0.05; Figure S3, SR), suggesting that deletion of GSK-3β specifically in D2R+ neurons affects NMDAR function in mPFC layer V pyramidal neurons.
Figure 1:
Deletion of GSK-3β in D2R+ (D2R-GSK-3β−/−) neurons in the brain enhanced NMDAR function and DA sensitivity in layer V pyramidal neurons of mouse mPFC. A, sample traces of AMPAR-sEPSCs and AMPAR-mEPSCs recorded from layer V pyramidal neurons in the PFC (upper panel). D2R-GSK-3β−/− mice did not show significant changes in AMPAR-EPSCs compared with their littermate controls (n = 25, p > 0.05 for both; lower panel). B, sample traces of NMDAR-sEPSCs and NMDAR-mEPSCs recorded from layer V pyramidal neurons in the PFC (upper panel). Summary histograms in the lower panel exhibited significant increases in amplitude (n = 20, *p < 0.05 for both sEPSCs and mEPSCs) but not the frequency of either NMDAR-sEPSCs or NMDAR-mEPSCs (n = 20, p > 0.05) in D2R-GSK-3β−/− mice. C, sample traces of evoked AMPAR-EPSC (−60 mV) and NMDAR-EPSC (+60 mV) recorded from the same neuron in the mPFC, and the histogram showed that deletion of GSK-3β in D2R+ neurons significantly increases both 1st and 2nd NMDA/AMPA ratios (vs. D2R-GSK-3β+/+, n = 8, **p < 0.01 for both 1st and 2nd EPSC). D, upper: NMDAR-EPSC or AMPAR-EPSC trains in D2R-GSK-3β−/− mice and their littermate controls. The EPSC currents elicited in the train were normalized to the amplitude of the first EPSC and were plotted against the stimulus numbers. The amplitude of the 1st NMDAR-EPSC but not AMPAR-EPSC was dramatically increased in D2R-GSK-3β−/− mice compared with their littermate controls (n = 10 for each group, ** P < 0.01 for NMDAR-EPSC and p > 0.05 for AMPAR-EPSC), but paired-pulse ratios (PPR) of neither NMDAR-EPSCs nor AMPAR-EPSCs elicited by repetitive pulses were changed (i.e., EPSC2–10, p > 0.05 for all). Lower: the charger transfer of NMDAR-EPSCs but not AMPAR-EPSC were significantly increased in D2R-GSK-3β−/− mice (n = 10 for each group, **p < 0.01 for NMDAR and p > 0.05 for AMPAR). E, Top panel: samples traces of NMDAR-EPSCs. Lower panels: left: at low dose of 0.2 μM, the enhancing effects of DA on NMDAR-EPSCs were potentiated during 10 min DA application and washing period in D2R-GSK-3β−/− mice (n = 8, *p < 0.05 for both DA wash-in and wash-out in D2R-GSK-3β+/+ mice; n = 8, **p < 0.01 in D2R-GSK-3β−/− mice; # p < 0.05 for both DA wash-in and wash-out in D2R-GSK-3β+/+ mice vs. D2R-GSK-3β−/− mice). Middle: at a higher dose of 20 μM, DA didn’t show any significant effects on NMDAR-EPSCs in both D2R-GSK-3β+/+ mice and D2R-GSK-3β−/− mice (n = 8 for both groups, p > 0.05). Right: at a high dose of 200 μM, the suppressive effect of DA found in D2R-GSK-3β+/+ mice was blunted in D2R-GSK-3β−/− mice (n = 8, *p < 0.05 for DA application in D2R-GSK-3β+/+ mice; n = 8, p > 0.05 for DA application in D2R-GSK-3β−/− mice; p > 0.05 for both DA wash-in and wash-out in D2R-GSK-3β+/+ mice vs. D2R-GSK-3β−/− mice).
We further examined NMDA/AMPA ratio change by recording evoked AMPAR- and NMDAR-EPSCs at −60 mV and +60 mV, respectively, from mPFC layer V pyramidal neurons with a paired-pulse stimulation of layer II-III in the presence of GABAAR antagonist picrotoxin (50 μM). We found that D2R-GSK-3β−/− mice exhibited a larger NMDA/AMPA ratio vs. D2R-GSK-3β+/+ mice (** p < 0.01 for all, Figure 1C; SR), further confirming that deletion of GSK-3β in D2R+ neurons increases synaptic NMDAR function.
To determine how the increase in NMDAR-EPSCs in D2R-GSK-3β−/− mice affects short-term plasticity, we evoked either AMPAR- or NMDAR-EPSCs with a 10-pulse 20-Hz train and calculated the paired-pulse ratios (PPRs). The amplitude of both AMPAR- and NMDAR-EPSCs exhibited short-term depression in D2R-GSK-3β−/− and control mice. The amplitude of the 1st NMDAR-EPSC was significantly increased in D2R-GSK-3β−/− mice vs. D2R-GSK-3β+/+ mice (**p < 0.01; Figure 1D, SR). However, the PPRs were not altered (p > 0.05 for all; Figure 1D). Furthermore, neither amplitude of the 1st AMPAR-EPSC nor PPRs from the 2nd to 10th AMPAR-EPSCs showed a significant difference between D2R-GSK-3β−/− and controls (p >0.05 for all; Figure 1D), indicating no change in presynaptic release. The increase in NMDAR-EPSC amplitude was further evidenced by measuring the integrated areas of EPSCs as charge transfer. The NMDAR-, but not AMPAR-dependent, charge transfer was significantly enhanced in D2R-GSK-3β−/− mice (**p < 0.01 for NMDA and p > 0.05 for AMPA; Figure 1D, SR). These findings suggest that deletion of GSK-3β in D2R+ neurons increases NMDAR, but not AMPAR, function, which is likely due to postsynaptic mechanisms.
These findings in a global knockout of GSK-3β in D2R+ neurons were further confirmed by employing a selective deletion of GSK-3β in PFC neurons through bilaterally injecting a Cre-dependent AAV8-hSyn-Cre-mCherry or control AAV8-hSyn-GFP (27, 28) into the prelimbic region of GSK-3βflox/flox mice at P25 when brain is still developing. The GSK-3β protein levels were significantly reduced by ~40% in the mPFC with Cre-virus expressed in GSK-3βflox/flox mice for three weeks (Figure S4B)(29–31). We then recorded NMDAR-EPSCs from fluorescence-labeled layer V pyramidal neurons from both Cre- and control virus-injected mice at P60. The amplitude of NMDAR-mEPSCs was significantly increased (*p < 0.05; Figure S4A and C, SR) in mCherry-labeled neurons, but the amplitude of sEPSCs and frequency of both sEPSCs and mEPSCs were unchanged (p > 0.05 for all; Figure S4 A and C). This data suggest that local ablation of GSK-3β in the PFC similarly potentiates NMDAR function.
NMDAR-mediated EPSC sensitivity to DA is altered in D2R-GSK-3β−/− mice
We and others have shown that DA exerts a bidirectional effect on NMDAR-EPSCs in the mPFC. Typically, NMDARs are enhanced by low-dose DA via activation of D1Rs, but are depressed by high-dose DA via D2Rs (17, 32–34). Since deletion of GSK-3β in D2R+ neurons caused an increase of NMDAR-EPSC amplitude, we examined how DA regulation of NMDARs is altered by investigating the sensitivity of NMDAR-EPSCs to different concentrations of DA. Baseline evoked NMDAR-EPSCs were elicited by stimulating layer II/III. As shown in Figure 1E, NMDAR-EPSCs were significantly increased by low dose DA (0.2 μM; *p < 0.05) and were further increased 10 min after washout (**p < 0.01; SR), whereas a medium dose of DA (20 μM) induced a slight increase in EPSC amplitudes (p >0.05). A high dose of DA (200 μM) significantly decreased the amplitude of NMDAR-EPSCs (*p < 0.05). Surprisingly, the bidirectional effect of DA was significantly changed in D2R-GSK-3β−/− mice compared with their littermate controls; 0.2 μM DA induced a more robust enhancing effect than control animals (**p < 0.01 for both; #p < 0.05 for both), whereas the 200 μM DA-induced depressive effect completely disappeared in knockout animals and even showed a small, but not significant increase in NMDAR-EPSCs (p > 0.05). The effect of 20 μM DA on NMDAR-EPSCs was, however, unchanged in D2R-GSK-3β−/− mice (p > 0.05). These results indicate that blocking GSK-3β signaling selectively in D2R+ neurons increases NMDAR sensitivity to D1R-dependent responses while decreasing the sensitivity of NMDAR-mediated responses to D2R-dependent signaling, thus augmenting NMDAR-mediated synaptic transmission.
Both long-term synaptic plasticity and spine density are altered in D2R-GSK-3β−/− mice
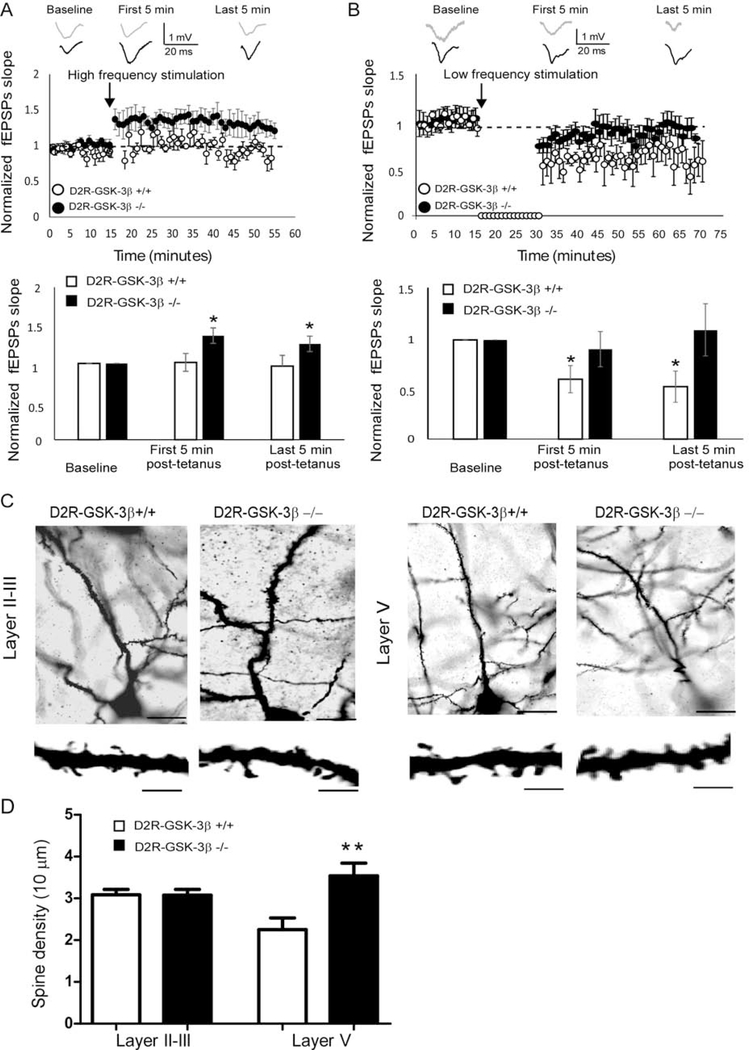
Synaptic plasticity contributes to learning and memory, whereas GSK-3β plays a critical role in the regulation of both NMDAR-dependent LTP and LTD (18, 19, 35). Since both NMDAR function and NMDA/AMPA ratio were enhanced in D2R-GSK-3β−/− mice, we hypothesized that LTP is enhanced and/or LTD is decreased in the mPFC by knockout of GSK-3β in D2R+ cells. We utilized a 100 Hz, 100-pulse tetanus stimulation paradigm that has been shown to elicit LTP, and compared the slope of field excitatory postsynaptic potentials (fEPSPs) recorded from D2R-GSK-3β−/− mice and their littermate controls. As we and others reported previously (36, 37), the fEPSP slope was unchanged and thus no LTP was evident in control mice when recorded in the presence of intact GABAergic neurotransmission (p > 0.05 for both first and last 5 min post-tetanus). However, this high-frequency stimulation paradigm significantly increased the slope of fEPSPs in D2R-GSK-3β−/− mice (*p < 0.05; Figure 2A; SR), demonstrating that LTP was able to be induced and maintained in the mPFC.
Figure 2:
Neuronal plasticity and dendritic spine were altered in D2R-GSK-3β−/− mice. A, LTP induction. Upper: representative fEPSP traces were recorded from mPFC layer V neurons of D2R-GSK-3β+/+ mice and D2R-GSK-3β−/− mice and graphical representation showing normalized fEPSP slope during baseline recording and following high-frequency (six trains of 100 pulse at 100 Hz) stimulation. Lower: summary histogram showed that the fEPSP slopes in both first 5 min and last 5 min after high-frequency stimulation were significantly increased in D2R-GSK-3β−/− mice, but not in D2R-GSK-3β+/+ mice, suggesting an increased LTP induction in D2R-GSK-3β−/− mice (n = 10, D2R-GSK-3β+/+: p > 0.05 for both the first and last 5 min post-tetanus; n = 10, D2R-GSK-3β−/−: * p < 0.05, both the first and last 5 min post-tetanus). B, LTD induction. Upper: representative fEPSP traces were recorded from mPFC layer V pyramidal neurons of D2R-GSK-3β+/+ mice and D2R-GSK-3β−/− mice and graphical representation showing normalized fEPSP slope during baseline recording and following low-frequency stimulation (900 pulses at 1Hz). Lower: summary histogram showed that the fEPSP slopes in both the first 5 min and last 5 min of low-frequency stimulation were decreased in wild-type D2R-GSK-3β+/+ mice, but the no LTD was induced in D2R-GSK-3β−/− mice, suggesting a terminated LTD in D2R-GSK-3β−/− mice (n = 8, D2R-GSK-3β+/+: *p < 0.05 for both the first and last 5 min post-tetanus; n = 8, D2R-GSK-3β−/−: p > 0.05, both the first and last 5 min post-tetanus). C, upper: Golgi–Cox-stained individual layers II-III and layer V pyramidal neurons in the mPFC from D2R-GSK-3β+/+ and D2R-GSK-3β−/− mice. High-magnification images of apical dendritic spines were shown in the lower panels. Scale bars = 50 μm for the upper panel and 10 μm for the lower panel. D, summary histogram showed that spine density in layer II-III and layer V pyramidal neurons of D2R-GSK-3β+/+ and D2R-GSK-3β−/− mice (n = 10 from 4 mice for each group, p > 0.05 for layer II-III and *p < 0.05 for layer V).
Next, we tested whether LTD is also affected by using a low-frequency stimulation. The fEPSP slope was significantly decreased in D2R-GSK-3β+/+, demonstrating the sensitivity of these cells to LTD induction (*p < 0.05 for all; Figure 2B). In contrast, LTD could not be induced in D2R-GSK-3β−/− mice (p > 0.05 for all; Figure 2B; SR). We thus wondered whether the increase in LTP and disruption in LTD is accompanied by a change in spine density, which may contribute to enhanced cognitive function in D2R-GSK-3β−/− mice. The spine density in the apical dendrites of layer V, but not layers II-III, pyramidal neurons in D2R-GSK-3β−/− mice were significantly increased (p > 0.05 for layer II-III; **p < 0.01 for layer V; Figure 2C and 2D; SR). These results suggest that GSK-3β in D2R+ neurons play an important role in neuronal plasticity in the mPFC.
Ablation of GSK-3β in D2R+ neurons increases NMDAR protein levels via enrichment of H3K27ac and HDAC2 at the proximal promoter region of NMDARs subunit genes
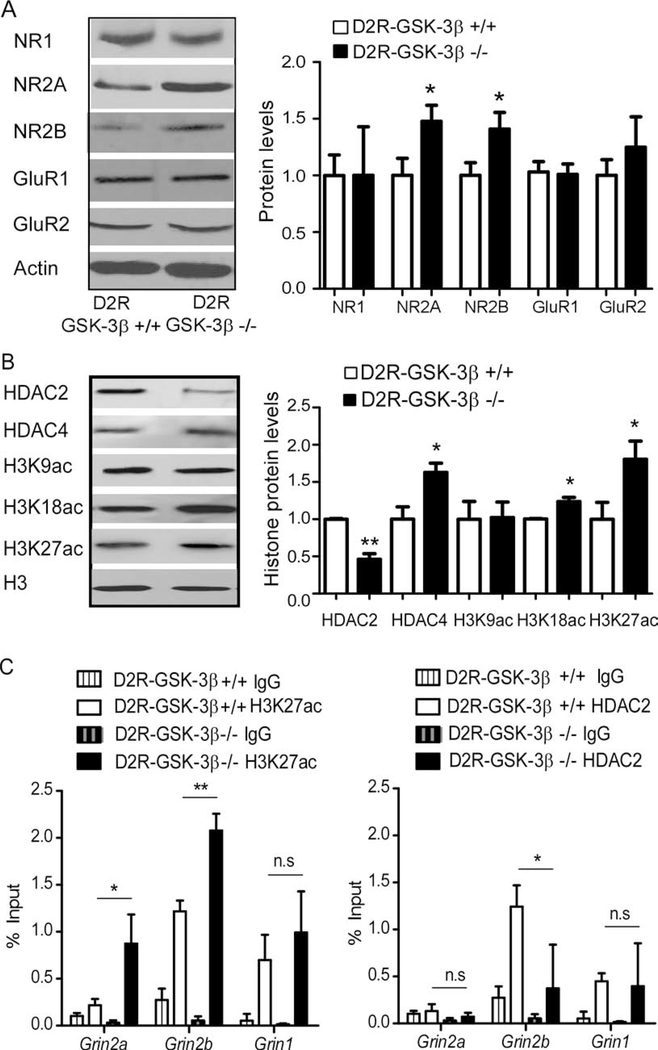
To determine whether functional changes in NMDAR neurotransmission are accompanied by alterations in receptor expression, we examined total protein levels of both NMDARs and AMPARs. Both NR2A and NR2B subunits (*p < 0.05), but not NR1 subunit (p > 0.05), were significantly increased in D2R-GSK-3β−/− mice (Figure 3A; SR). Interestingly, GluR1 and GluR2 subunits were also unaffected in D2R-GSK-3β−/− mice (p > 0.05 for both; Figure 3A). We and others have reported that GSK-3β is involved in AMPAR tracking in PFC cultured neurons (38, 39). However, we did not detect significant changes of either surface or intracellular GluR1 and GluR2 subunits analyzed with cross-linker BS3 in D2R-GSK-3β−/− mice, indicating that AMPAR subunit trafficking was not affected (p > 0.05 for all; Figure S5; SR). Consistently, bath application of selective NR2B antagonist Ro25–6981 (0.5 μM) decreased evoked NMDAR-EPSC amplitude by ~50% in D2R-GSK-3β−/− mice compared with a ~30% decrease in control mice, indicating more NR2B subunits in D2R-GSK-3β−/− mice. Additionally, the remaining NMDAR-EPSC amplitude after Ro 25–6981 treatment was significantly higher in D2R-GSK-3β−/− mice vs. control mice (*p < 0.05 for both; Figure S6; SR). These results suggest that NR2A and NR2B expression were specifically enhanced in D2R-GSK-3β−/− mice, consistent with the NMDAR functional change, and alterations detected in spine morphology.
Figure 3:
Histone modification is involved in the increase of NMDAR protein expression in mPFC of D2R-GSK3−/− mice. A, representative Western blots and summary histograms showed that total protein levels of NMDAR and AMPAR subunits from the mPFC of D2R-GSK-3β+/+ and D2R-GSK-3β−/− mice. Both NR2A and NR2B were significantly increased in D2R-GSK-3β−/− mice (n = 8 for each group, *p < 0.05 for both), but NR1, GluR1, and GluR2 were unchanged (n = 8 for each group, p > 0.05 for all). B, representative Western blots and summary histograms showed that total protein levels of HDAC2 & 4, as well as three acetylation sites of H3K from mPFC tissue of D2R-GSK-3β+/+ mice and D2R-GSK-3β−/− mice. HDAC2 was decreased while HDAC4 was increased in D2R-GSK-3β−/− mice (vs. D2R-GSK-3β−/− mice, n = 6 for each group, *p < 0.05 for both). Both H3K18ac and H3K27ac, but not H3K9ac, were increased in D2R-GSK-3β−/− mice (vs. D2R-GSK-3β−/− mice, n = 6 for each group, *p < 0.05). C, chromatin was pooled from the PFC of 2 animals from D2R-GSK-3β+/+ or D2R-GSK-3β−/− mice and immunoprecipitated using antibodies against H3K27ac (left) and HDAC2 (right). qPCR was performed using a primer set specific to the promoter region of Grin1, Grin2a, and Grin2b. The signal of the amplified DNA was normalized to input. H3K27ac enrichment at either Grin2a or Grin2b but not Grin1 were significantly enhanced in D2R-GSK-3β−/− (vs. D2R-GSK-3β+/+, n = 6 mice yielding 3 data points for each group, *p < 0.05, **p < 0.01). On the contrary, HDAC2 enrichment at Grin2b, but not Grin1 and Grin2a, was significantly reduced in D2R-GSK-3β−/− mice (vs. D2R-GSK-3β+/+, n = 6 mice yield 3 data points for each group, *p < 0.05).
GSK-3β is reported to be involved in gene expression via histone modifications. Specifically, GSK-3β promotes phosphorylation of several histone deacetylases (HDACs) to modulate their activity (40, 41) that is associated with cognitive processes (42, 43). We thus evaluated whether epigenetic mechanism is involved in up-regulating NMDAR subunit expression in D2R-GSK-3β−/− mice by examining the protein levels of HDAC2 and HDAC4 as both are closely related to synaptic plasticity (42, 44). We found that HDAC2 was decreased while HDAC4 was increased in D2R-GSK-3β−/− mice (*p < 0.05 for both; Figure 3B, SR). HDACs regulate histone acetylation by removing acetyl groups from histones, resulting in reduced permissivity of gene expression. Thus, we further examined levels of acetylation at specific sites on the histone H3 tail, which, when elevated at certain residues, can enhance gene expression. As shown in Figure 3B, D2R-GSK-3β−/− mice exhibited increased levels in H3K27ac and H3K18ac (*p < 0.05 for both), but not H3K9ac (p > 0.05), compared with control mice. This data indicate that the increase of NMDAR subunit expression in D2R-GSK-3β−/− mice may be attributed in part to site-specific enrichment of acetylation on the histone H3 tail.
To examine whether enhanced H3 acetylation and altered HDAC protein expression are specific to NMDAR subunit expression, we carried out ChIP-qPCR assays to measure the enrichment levels of H3K27ac and HDAC2 at the proximal promoter regions (PR1) of the Grin1 (NR1-encoding), Grin2a (NR2A-encoding) and Grin2b genes (NR2B-encoding). We found a significant increase in enrichment of H3K27ac at both the Grin2a and Grin2b, but not Grin1, PR1 sites in D2R-GSK-3β−/− mice mPFC compared with D2R-GSK-3β+/+ (*p < 0.05; **p < 0.01; Figure 3C left; SR). In addition, the PR1 site of Grin2b, but not Grin2a or Grin1, had a significant decrease in HDAC2 enrichment in D2R-GSK-3β−/− mice mPFC (*p < 0.05; Figure 3C right, SR). The reduced enrichment of HDAC2 protein is specifically associated with increased H3K27ac levels at the Grin2b PR1 site, but not at the Grin2a PR1 site. Together, these results suggest that increased enrichment of activation marker H3K27ac at both the Grin2a and Grin2b promoter regions may contribute to elevated NR2A and NR2B protein levels in D2R-GSK-3β−/− mice.
D2R-GSK-3β−/− mice show resistance to MK801-induced cognitive deficits
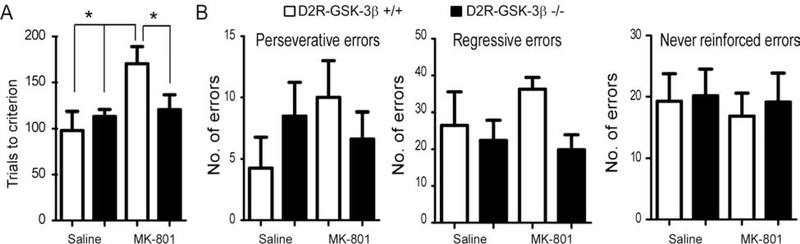
We further evaluated whether the increase in NMDAR function and expression induced by deletion of GSK-3β in D2R+ neurons contributes to an improvement in cognitive function. MK-801 as an NMDAR antagonist has been widely used to model the cellular and behavioral effects of NMDAR hypofunction reported in SZ (45, 46). MK-801 impaired learning and memory in different tasks (47–50). Importantly, DA and GSK-3β are both involved in regulating the NMDAR antagonist-induced impairment in learning and memory (13, 51). Therefore, we used MK-801 to induce cognitive dysfunction as measured by an attentional set-shifting task (21) and then examined whether deletion of GSK-3β in D2R+ neurons decreases cognitive deficits known to be induced by MK-801. D2R-GSK-3β−/− mice and their littermate controls were treated with MK-801 (D2R-GSK-3β−/− MK-801 and D2R-GSK-3β+/+ MK-801) or saline (D2R-GSK-3β−/− saline and D2R-GSK-3β+/+ saline). The number of trials to reach criterion for D2R-GSK-3β+/+ mice showed a significant group-treatment interaction effect (two-way ANOVA, F = 6.28, *p < 0.05; Figure 4A). Further main effect analysis also showed significantly increased trial number in D2R-GSK-3β+/+ MK-801 mice vs. saline-treated D2R-GSK-3β+/+ and D2R-GSK-3β−/− mice (*p < 0.05 for both; Figure 4A; SR), demonstrating an expected MK-801-induced cognitive deficit in wild-type animals. As we hypothesized, MK-801-treated D2R-GSK-3β−/− mice required fewer trials to reach criterion compared to MK-801-treated D2R-GSK-3β+/+ mice (*p < 0.05; Figure 4A). We did not detect significant differences in perseverative, regressive and never reinforced errors between D2R-GSK-3β−/− mice and their littermate controls (p > 0.05 for all; Figure. 4B; SR). In contrast, there was no difference in trial number between D1R-GSK-3β+/+ and D1R-GSK-3β−/− mice, neither treated with MK-801 nor saline (p > 0.05 for both; Figure S7; SR). These results demonstrate that deletion of GSK-3β in D2R+, but not D1R+, neurons ameliorated MK801-induced cognitive impairment.
Figure 4:
D2R-GSK-3β−/− mice resisted MK-801-induced working memory deficits. A, two-way ANOVA analysis of the number of trials for D2R-GSK-3β+/+ mice and D2R-GSK-3β−/− mice to reach criteria revealed a significant difference in interaction effect (n = 8 for each group, F = 6.28, *p < 0.05). The further simple effect test analysis showed that the number of trials significantly increased after injection of MK-801 in D2R-GSK-3β+/+ mice compared with either injection of saline in D2R-GSK-3β+/+ mice or D2R-GSK-3β−/− mice (*p < 0.05 for both). However, injection of MK-801 in D2R-GSK-3β−/− mice exhibited less number of trials compared with that in D2R-GSK-3β+/+ mice (n = 8 for each group, *p < 0.05). B, there were no significant differences in three types of error analyzed among D2R-GSK-3β+/+ saline, D2R-GSK-3β+/+ MK-801, D2R-GSK-3β−/− saline and D2R-GSK-3β−/− MK-801 (Perseverative errors: interaction F = 1.78, p > 0.05; treatment F = 0.46 p > 0.05; genotypes F = 0.02, p > 0.05. Regressive errors: interaction F = 2.66, p > 0.05; treatment F = 0.12, p > 0.05; genotypes F = 2.89, p >0.05. Never reinforced errors: interaction F = 0.02, p > 0.05; treatment F = 0.14, p > 0.05; genotypes F = 0.12, p > 0.05).
Disrupting D2R-DISC1 complex-mediated GSK-3β signaling underlies up-regulating NMDAR in D2R-GSK-3β−/− mice
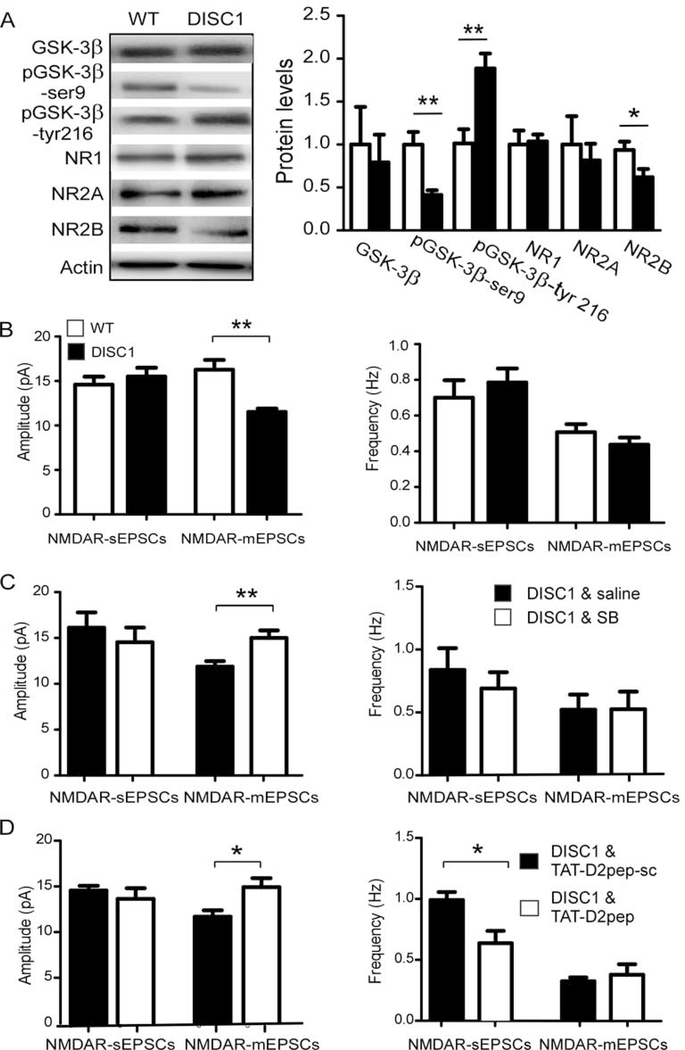
The selective alteration of NMDARs in D2R-, but not D1R-, GSK-3β−/− mice is of particular interest. It has reported that DISC1 forms a complex with D2Rs, but not D1Rs, to modulate GSK-3β signaling (52). Further, DISC1 knockdown significantly increased NMDAR-EPSCs and protein expression (53), suggesting a negative regulation of NMDARs by DISC1. Therefore, we proposed that genetic ablation of GSK-3β in D2R+ neurons will disrupt the D2R-DISC1 complex, which in turn results in upregulation of NMDAR expression and function. We utilized an inducible Myc-fused mutant human DISC1 mouse (hDISC1) (54) to test the hypothesis. Mutant hDISC1 protein was detected by anti-Myc antibody in mutant mice but not in wild-type mice (Figure S8). We then examined both GSK-3β activity and NMDAR expression in these animals. Interestingly, we found a decreased pGSK-3β-Ser9, increased pGSK-3β-Tyr216 (**p < 0.01 for both), and no change in total GSK-3β level (p > 0.05; Figure 5A; SR) in mutant hDISC1 mice. Since GSK-3β activity is down-regulated by pGSK-3β-Ser9 and up-regulated by pGSK-3β-Tyr216, these results indicate an enhanced GSK-3β activity in mutant hDISC1 mice (55). Consistently, there was a significantly decreased NR2B protein (*p < 0.05), but no change in NR2A and NR1 levels (p > 0.05 both; Figure 5A; SR) in mutant hDISC1 mice.
Figure 5:
Inhibiting GSK-3β or interrupting the interaction of D2R and DISC1 restored NMDAR function in mutant hDISC1 mice. A, representative Western blots and summary histograms showed that total protein levels of GSK-3β, pGSK-3β, and NMDAR subunits from mPFC tissue of mutant hDISC1 mice and their littermate controls. pGSK-3β at serine 9 (pGSK-3β-Ser9) was decreased while pGSK3β tyrosine 216 (pGSK-3β-Tyr216) was increased in mutant hDISC1 overexpressing mice compared with control mice (n = 6 for each group, **p < 0.01 for both). NR2B was reduced in mutant hDISC1 mice compared with control mice (n = 6 for each group, *p < 0.05). There were no significant differences in GSK-3β, NR1, and NR2A detected between mutant hDISC1 mice and control mice (n = 6 for each group, p > 0.05 for all). B, NMDAR-sEPSCs, and NMDAR-mEPSCs were recorded from layer V pyramidal neurons in the mPFC of both mutant hDISC1 mice and their littermate controls. The amplitude but not the frequency of NMDAR-mEPSCs was significantly decreased in mutant hDISC1 mice compared with control mice (n = 6 for each group, *p < 0.05). C, treatment with selective GSK-3β inhibitor SB216763 (2 mg/kg/day, i.p., once a day for 5 days) during juvenile period prevented a decrease in NMDAR-mEPSC amplitude compared with saline injection in mutant hDISC1 mice (n = 12 for each group, *p < 0.05). D, similarly, treatment with TAT-D2pep (10 μM, i.p., once a day for 5 days) during the juvenile period also prevents a decrease in NMDAR-mEPSC amplitude (but not frequency) compared with scrambled control peptide (TAT-D2pep-sc) treated mutant hDISC1 mice (n = 10 for each group, *p < 0.05). However, treatment with TAT-D2pep also caused a significant decrease in the frequency of NMDAR-sEPSC compared with scrambled control peptide (TAT-D2pep-sc) treated mutant hDISC1 mice (n = 10 for each group, *p < 0.05).
Further, the decrease in NR2B expression was accompanied by an attenuation of NMDAR function. Specifically, the amplitude (**p < 0.01), but not frequency (p > 0.05) of NMDAR-mEPSCs was significantly decreased (Figure 5B; SR) in mutant hDISC1mice. However, there was no significant change in NMDAR-sEPSCs (p > 0.05 for both amplitude and frequency; Figure 5B and S9), nor in AMPAR-sEPSCs and AMPAR-mEPSCs between mutant hDISC1 mice and controls (data not shown, p > 0.05 for all; SR). Together, these results suggest that mutant hDISC1 specifically and negatively affects NMDAR neurotransmission due to an increase in GSK3β activity.
As we (20) and others (56) recently reported, GSK-3β activity exhibits a critical developmental window during the juvenile period and inhibition of GSK-3β effectively prevented both physiological changes and behavioral phenotypes in animal models for SZ. To further confirm that the mutant hDISC1-induced increase of GSK-3β activity also contributes to the down-regulation of NMDAR function during cortical development, we treated mutant hDISC1 mice with a selective GSK-3β inhibitor during juvenile period, as previously reported (20, 56). We then examined NMDAR-EPSCs in adult mice. Specifically, mutant hDISC1 mice received once-daily I.P. injections of SB216763 or saline beginning at P25 for 5 consecutive days (20). As shown in Figure 5C and S9, in vivo SB216763 injections rescued the reduced NMDAR-mEPSC amplitude in mutant hDISC1 mice (p > 0.05 for both sEPSCs frequency and amplitude; p > 0.05 for mEPSCs frequency; **p < 0.01 for mEPSCs amplitude; SR). This result suggests that reversing GSK-3β activity at early developmental period is key to normalize NMDA function.
D2R can interact with DISC1 through its third intracellular loop, and an increase of D2R-DISC1 complexes are detected in both postmortem brain tissues of SZ and Disc1-L100P mutant mice (52). Since D2R levels are increased in mPFC of mutant hDISC1 mice (57), we hypothesized that mutant hDISC1-induced decrease in NMDAR function was attributable to the increased D2R-DISC1 interaction, which resulted in enhanced GSK-3β signaling. We tested this possibility by disrupting D2R-DISC1 complex with TAT-D2pep [K211-T225], a peptide that effectively disrupted D2R interaction with DISC1 (9, 52). Mutant hDISC1 mice received once-daily I.P. injections of TAT-D2pep [K211-T225; 10 μM) or scrambled control peptide (D2pep-sc; 10 μM) beginning at P25 for 5 consecutive days. Similar to treatment with GSK-3β inhibitor described above, TAT-D2pep [K211-T225] reversed the decreased amplitude of NMDAR-mEPSCs in mutant hDISC1 mice compared with the scrambled control peptide (D2pep-sc) (**p < 0.01 for mEPSCs amplitude; Figure 5D and S6). In addition, TAT-D2pep [K211-T225] caused a significant decrease of NMDAR-sEPSC frequency (*p < 0.05) (p > 0.05; Figure 5D and S9; SR). This finding suggests that TAT-D2pep [K211-T225] disrupts the D2R-DISC1 complex at a critical developmental period, affecting glutamate release. Altogether, these results indicate that enhanced D2R-DISC1 complex formation regulates GSK-3β signaling and is specifically associated with down-regulation of NMDAR expression and function.
Discussion
We report several interesting and novel findings here (see Supplement for detail discussion). First, DA receptors, especially D2Rs-mediated modulation in NMDARs via GSK-3β signaling in the PFC, exert powerful effects on cognition by modulating synaptic transmission (8, 17). Interestingly, we found that global conditional deletion of GSK-3β significantly increased NMDAR-EPSCs in the mPFC of D2R-, but not D1R-, GSK-3β−/− mice. In addition, global ablation of GSK-3β in D2R+ neurons disrupts the balance of D1R- vs D2R-dependent modulation of NMDAR-EPSCs in the mPFC. Specifically, we revealed an enhanced sensitivity of NMDAR-mediated function and a shifted response to DA, which is caused by the loss of GSK-3β-D2R signaling-mediated regulation of NMDAR expression as we previously reported (17). Despite the prominent alterations in NMDAR-EPSCs, surprisingly, we didn’t detect any significant change of AMPAR-EPSCs in the mPFC of D2R-GSK-3β−/− mice. In contrary, previous studies reported that CRISPR/Cas9 or Cre-mediated knockout, or inhibition, of GSK3β in adult cortical or hippocampal region decreases AMPA-sEPSC (9, 38, 58, 59). The reason for this discrepancy is likely that we knocked out GSK3β during neurodevelopment in D2R-Cre mice, while other studies reporting AMPA-mediated effects by knockout GSK-3β in adult mice (9, 58), suggesting a possible system-wide neurodevelopmental effect of GSK-3β in the current study.
Second, consistent with the long-term plasticity changes detected in D2R-GSK-3β−/− mice, we found that spine number in apical dendrites of PFC layer V, but not layer II/III, pyramidal neurons, is significantly increased in D2R-GSK-3β−/− mice. However, it has been reported that deletion of GSK-3β in adult mPFC did not affect spine density (9), while knockout of GSK-3β in adult hippocampus decreased the spine density (58). These inconsistent effects on the spine quantity induced by deletion of GSK-3β at different ages or brain regions also suggest a potential developmental and cell-type specific effect of GSK-3β reported in our study. An increase in spine density in mPFC layer V pyramidal neurons as we report here may contribute to the improvement of cognitive function in D2R-GSK-3β−/− mice.
Third, we found increased both NR2A and NR2B, but not NR1 NMDAR subunits in the mPFC of D2R-GSK-3β−/− mice. What is the mechanism underlying the increase in NR2 expression? Epigenetic processes are increasingly implicated in developmental changes of NMDARs (60, 61), SZ pathophysiology (62, 63), neurodevelopment and memory formation (64–66). Thus, we asked whether deletion of GSK-3β leads to a decrease in HDAC2 activity and increased histone acetylation. We found a significant decrease in HDAC2 and a marked increase in HDAC4 protein levels in the mPFC of D2R-GSK-3β−/− mice. It has been reported that GSK-3β can trigger HDAC4 degradation (40). Consequently, knockout of GSK-3β can induce an increase of HDAC4 expression by decreasing its degradation. Notably, HDAC4 is a positive regulator of synaptic plasticity (44) while HDAC2 negatively regulates dendritic spine density (42). Thus, the increase in HDAC4 levels and decrease in HDAC2 levels in D2R-GSK-3β−/− mouse mPFC support the enhanced synaptic plasticity and increased spine density. Consistent with a decrease of HDAC2 levels, we also found a significant increase of overall histone acetylation at H3K27 and H3K18 residues of the histone H3 tail. ChIP assays further confirmed that activation mark H3K27ac was highly enriched at the promoter of both Grin2a and Grin2b. The pattern of HDAC2 enrichment in either control mice or mutant mice across promoters is Grin2b > Grin1 > Grin2a, suggesting that Grin2b expression is more tightly regulated than other NMDAR subunit proteins by HDAC2.
We and other’s studies indicated the important role of cortical GSK-3β in maintaining cognitive function (20, 67, 68). Our study shows that D2R-, but not D1R-, GSK-3β−/− mice, are resistant to MK-801-induced impairment in set-shifting, suggesting that NMDAR-dependent cognitive flexibility was not affected by MK-801 after deletion of GSK-3β in D2R+ neurons. Consistently, global inhibition of GSK-3β by GSK-3β inhibitor during development can rescue the spatial working memory deficit (20, 56).
Finally, DISC1 protein can form a complex with D2Rs to modulate GSK-3β signaling (52). Specifically, GSK-3β activity is enhanced by an increased interaction between D2Rs and DISC1 in Disc1-L100P mutant mice and SZ patients (52). Indeed, we found an enhanced GSK-3β activity that is accompanied by a decreased NMDAR function in mutant human DISC1 mice. Importantly, the reduced NMDAR-EPSCs were effectively reversed, not only by inhibiting GSK-3β activity with selective inhibitor but also by interrupting D2R-DISC1 interaction with TAT-D2 peptide during the adolescent period. These data provide strong evidence that GSK-3β modulation of NMDAR function is closely associated with D2R-DISC1 interaction.
In summary, GSK-3β regulates NMDAR function and expression via D2R-DISC1 interactions and epigenetic-mediated increases in acetylation, which in turn leads to spared NMDAR-dependent cognitive dysfunction (Figure S10). Our study thus reveals a novel mechanism at the molecular level within a genetically defined D2R+ neuronal subpopulation that underlies the unique role of GSK-3β in cognition.
Supplementary Material
| Primers for genotyping: | |
| Specific D2Cre primers: | |
| D2Cre-genotype-Forward: | 5’- GGG AAT TCT CAG CTC TGC TAG C -3’ |
| Cre-R improved (JT): | 5’- CAG CAT TGC TGT CAC TTG GTC G -3’ |
| Specific D1Cre primers: | |
| D1Cre-genotyp2-Forward: | 5’- GCA CTG AAC CCA GAA GAC AGG TGG -3’ |
| Cre- R improved (JT): | 5’- CAG CAT TGC TGT CAC TTG GTC G -3’ |
| Control primers: | |
| IL2-Forward: | 5’- CTA GGC CAC AGA ATT GAA AGA TCT -3’ |
| IL2-Reverse: | 5’- GTA GGT GGA AAT TCT AGC ATC ATC C- 3’ |
| GSK3bflox primers | |
| Forward: | 5’- GCC ATC AAG AAA GTT CTA CAG GA- 3’ |
| Reverse: | 5’- GCT GAA GTC CAG AGC AAG TCT- 3’ |
| Td tomato primers: | |
| Dtomato wild type Forward: | 5’-A A G GGA GCT GCA GT G GA G T A -3’ |
| Dtomato wild type Reverse: | 5’-CCG A A A A T C T GT GGG A A G T C -3’ |
| Dtomato mutant type Forward: | 5’- GGC A T T A A A GCA GCG T A T CC-3’ |
| Dtomato mutant type Reverse: | 5’-CT G T T C CT G T A C GGC A T G G-3’ |
| Primers to ChIP-qPCR: | |
| Grin2b primer | |
| Forward: | GGTCAAGCTGCCTCTCCAT |
| Reverse: | GCAGAGCAGAAGGAAATGTATTCG |
| Grin2a primer | |
| Forward: | TCCGGAGTGGAACAGAAAGC |
| Reverse: | CTCATCCAGCCCCATGCT |
| Grin1 primer | |
| Forward: | TCCCTGCTTCCTCTCTTGGA |
| Reverse: | AATGACTGCTGGGAGCAAGAC |
Significance statement.
Glycogen synthase kinase 3β (GSK-3β) is a central signal integrator for DA-dependent responses, particularly D2R-mediated hyperdopaminergic behaviors. However, the mechanism behind GSK-3β signaling in D2R+ cells in the regulation of cognitive function is still unclear. Here, we report that conditional ablation of GSK-3β in D2R+ neurons (D2R-GSK-3β−/−) affects NMDAR-mediated synaptic transmission and plasticity, which is accompanied by epigenetic modification of NMDAR expression, in the medial prefrontal cortex (mPFC). Further, these molecular and physiological changes result in sparing of cognitive dysfunction in an attentional set-shifting task. Finally, D2R-DISC1 interaction mediates GSK-3β-dependent regulation of NMDARs and cognitive function. Our study reveals a novel mechanism at the molecular level within a genetically defined D2R+ neuronal subpopulation that underlies the unique role of GSK-3β in cognition.
Acknowledgment
This study was supported by NIH R01MH085666 and R21MH110678 to W.J. Gao, Commonwealth Universal Research Enhancement (CURE) Program to Y.C. Li, and in part by NIH 5R37MH073853 to MGC.
Abbreviations
- DA
Dopamine
- D1Rs
Dopamine D1 receptors
- D2Rs
Dopamine D2 receptors
- GSK3-β
Glycogen synthase kinase-3β
- hDISC1
Human DISC1 mouse
- LTD
Long-term depression
- LTP
Long-term potentiation
- mEPSCs
Miniature excitatory postsynaptic currents
- mPFC
Medial prefrontal cortex
- PFC
Prefrontal cortex
- sEPSC
Spontaneous excitatory postsynaptic currents
- SZ
Schizophrenia
Footnotes
Conflict of Interests
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seong HJ, Carter AG (2012): D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 32:10516–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gee S, Ellwood I, Patel T, Luongo F, Deisseroth K, Sohal VS (2012): Synaptic activity unmasks dopamine D2 receptor modulation of a specific class of layer V pyramidal neurons in prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 32:4959–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seamans JK, Yang CR (2004): The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 74:1–58. [DOI] [PubMed] [Google Scholar]
- 4.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998): Dopamine receptors: from structure to function. Physiological reviews. 78:189–225. [DOI] [PubMed] [Google Scholar]
- 5.Greengard P (2001): The neurobiology of slow synaptic transmission. Science. 294:1024–1030. [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu JM, Gainetdinov RR, Caron MG (2007): The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 28:166–172. [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG (2005): An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 122:261–273. [DOI] [PubMed] [Google Scholar]
- 8.Li Y-C, Gao W-J (2011): GSK-3[beta] activity and hyperdopamine-dependent behaviors. Neuroscience & Biobehavioral Reviews. 35:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khlghatyan J, Evstratova A, Chamberland S, Marakhovskaia A, Bahremand A, Toth K, et al. (2018): Mental Illnesses-Associated Fxr1 and Its Negative Regulator Gsk3beta Are Modulators of Anxiety and Glutamatergic Neurotransmission. Front Mol Neurosci. 11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaulieu JM, Del’guidice T, Sotnikova TD, Lemasson M, Gainetdinov RR (2012): Beyond cAMP: The regulation of Akt and GSK3 by dopamine receptors. Front Mol Neurosci. 4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emamian ES (2012): AKT/GSK3 signaling pathway and schizophrenia. Front Mol Neurosci. 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urs NM, Snyder JC, Jacobsen JPR, Peterson SM, Caron MG (2012): Deletion of GSK3beta in D2R-expressing neurons reveals distinct roles for Î2-arrestin signaling in antipsychotic and lithium action. Proceedings of the National Academy of Sciences. 109:20732–20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma A, Moghaddam B (1996): NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 16:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang CR, Chen L (2005): Targeting prefrontal cortical dopamine d1 and N-methyl-d-aspartate receptor interactions in schizophrenia treatment. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 11:452–470. [DOI] [PubMed] [Google Scholar]
- 15.Lau CG, Zukin RS (2007): NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nature reviews Neuroscience. 8:413–426. [DOI] [PubMed] [Google Scholar]
- 16.Laruelle M, Kegeles LS, Abi-Dargham A (2003): Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 1003:138–158. [DOI] [PubMed] [Google Scholar]
- 17.Li YC, Xi D, Roman J, Huang YQ, Gao WJ (2009): Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 29:15551–15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, et al. (2008): The role of GSK-3 in synaptic plasticity. Br J Pharmacol. 153 Suppl 1:S428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, et al. (2007): Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. The Journal of neuroscience : the official journal of the Society for Neuroscience. 27:12211–12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing B, Li YC, Gao WJ (2016): GSK3beta hyperactivity during an early critical period impairs prefrontal synaptic plasticity and induces lasting deficits in spine morphology and working memory. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 41:3003–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li ML, Yang SS, Xing B, Ferguson BR, Gulchina Y, Li YC, et al. (2015): LY395756, an mGluR2 agonist and mGluR3 antagonist, enhances NMDA receptor expression and function in the normal adult rat prefrontal cortex, but fails to improve working memory and reverse MK801-induced working memory impairment. Exp Neurol. 273:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaCrosse AL, Burrows BT, Angulo RM, Conrad PR, Himes SM, Mathews N, et al. (2015): mGluR5 positive allosteric modulation and its effects on MK-801 induced set-shifting impairments in a rat operant delayed matching/non-matching-to-sample task. Psychopharmacology. 232:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Descarries L, Lemay B, Doucet G, Berger B (1987): Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience. 21:807–824. [DOI] [PubMed] [Google Scholar]
- 24.Khlghatyan J, Quintana C, Parent M, Beaulieu JM (2018): High Sensitivity Mapping of Cortical Dopamine D2 Receptor Expressing Neurons. Cereb Cortex.October 6. doi: 10.1093/cercor/bhy1261. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santana N, Mengod G, Artigas F (2009): Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cerebral cortex (New York, NY : 1991). 19:849–860. [DOI] [PubMed] [Google Scholar]
- 26.Gaspar P, Bloch B, Le Moine C (1995): D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. The European journal of neuroscience. 7:1050–1063. [DOI] [PubMed] [Google Scholar]
- 27.Arango-Lievano M, Schwarz JT, Vernov M, Wilkinson MB, Bradbury K, Feliz A, et al. (2014): Cell-type specific expression of p11 controls cocaine reward. Biological psychiatry. 76:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R, et al. (2002): Adeno-associated virus effectively mediates conditional gene modification in the brain. Proceedings of the National Academy of Sciences of the United States of America. 99:2320–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, et al. (2017): Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. 20:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vajda F, Jordi N, Dalkara D, Joly S, Christ F, Tews B, et al. (2015): Cell type-specific Nogo-A gene ablation promotes axonal regeneration in the injured adult optic nerve. Cell death and differentiation. 22:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller OH, Bruns A, Ben Ammar I, Mueggler T, Hall BJ (2017): Synaptic regulation of a thalamocortical circuit controls depression-related behavior. Cell reports. 20:1867–1880. [DOI] [PubMed] [Google Scholar]
- 32.Zheng P, Zhang XX, Bunney BS, Shi WX (1999): Opposite modulation of cortical N-methyl-D-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience. 91:527–535. [DOI] [PubMed] [Google Scholar]
- 33.Li YC, Liu G, Hu JL, Gao WJ, Huang YQ (2010): Dopamine D(1) receptor-mediated enhancement of NMDA receptor trafficking requires rapid PKC-dependent synaptic insertion in the prefrontal neurons. Journal of neurochemistry. 114:62–73. [DOI] [PubMed] [Google Scholar]
- 34.Alaghband Y, Marshall JF (2013): Common influences of non-competitive NMDA receptor antagonists on the consolidation and reconsolidation of cocaine-cue memory. Psychopharmacology. 226:707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, et al. (2007): LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 53:703–717. [DOI] [PubMed] [Google Scholar]
- 36.Urban KR, Waterhouse BD, Gao WJ (2012): Distinct age-dependent effects of methylphenidate on developing and adult prefrontal neurons. Biological psychiatry. 72:880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu TX, Yao WD (2010): D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 107:16366–16371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei J, Liu W, Yan Z (2010): Regulation of AMPA receptor trafficking and function by glycogen synthase kinase 3. Journal of Biological Chemistry. 285:26369–26376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang MJ, Li YC, Snyder MA, Wang H, Li F, Gao WJ (2013): Group II metabotropic glutamate receptor agonist LY379268 regulates AMPA receptor trafficking in prefrontal cortical neurons. PloS one. 8:e61787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cernotta N, Clocchiatti A, Florean C, Brancolini C (2011): Ubiquitin-dependent degradation of HDAC4, a new regulator of random cell motility. Molecular biology of the cell. 22:278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen S, Owens GC, Makarenkova H, Edelman DB (2010): HDAC6 regulates mitochondrial transport in hippocampal neurons. PloS one. 5:e10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. (2009): HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 459:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris MJ, Mahgoub M, Na ES, Pranav H, Monteggia LM (2013): Loss of histone deacetylase 2 improves working memory and accelerates extinction learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 33:6401–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim MS, Akhtar MW, Adachi M, Mahgoub M, Bassel-Duby R, Kavalali ET, et al. (2012): An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 32:10879–10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moghaddam B, Jackson ME (2003): Glutamatergic animal models of schizophrenia. Ann N Y Acad Sci. 1003:131–137. [DOI] [PubMed] [Google Scholar]
- 46.Olney JW, Newcomer JW, Farber NB (1999): NMDA receptor hypofunction model of schizophrenia. Journal of psychiatric research. 33:523–533. [DOI] [PubMed] [Google Scholar]
- 47.Murray TK, Ridley RM (1997): The effect of dizocilpine (MK-801) on conditional discrimination learning in the rat. Behav Pharmacol. 8:383–388. [PubMed] [Google Scholar]
- 48.Wozniak D, Olney J, Kettinger III L, Price M, Miller J (1990): Behavioral effects of MK-801 in the rat. Psychopharmacology. 101:47–56. [DOI] [PubMed] [Google Scholar]
- 49.Pitkanen M, Sirvio J, MacDonald E, Niemi S, Ekonsalo T, Riekkinen P Sr. (1995): The effects of D-cycloserine and MK-801 on the performance of rats in two spatial learning and memory tasks. Eur Neuropsychopharmacol. 5:457–463. [PubMed] [Google Scholar]
- 50.van der Meulen JA, Bilbija L, Joosten RN, de Bruin JP, Feenstra MG (2003): The NMDA-receptor antagonist MK-801 selectively disrupts reversal learning in rats. Neuroreport. 14:2225–2228. [DOI] [PubMed] [Google Scholar]
- 51.Chan MH, Chiu PH, Lin CY, Chen HH (2012): Inhibition of glycogen synthase kinase-3 attenuates psychotomimetic effects of ketamine. Schizophrenia research. 136:96–103. [DOI] [PubMed] [Google Scholar]
- 52.Su P, Li S, Chen S, Lipina TV, Wang M, Lai TK, et al. (2014): A Dopamine D2 Receptor-DISC1 Protein Complex may Contribute to Antipsychotic-Like Effects. Neuron. 84:1302–1316. [DOI] [PubMed] [Google Scholar]
- 53.Wei J, Graziane NM, Wang H, Zhong P, Wang Q, Liu W, et al. (2014): Regulation of N-Methyl-D-Aspartate receptors by Disrupted-in-Schizophrenia-1. Biological psychiatry. 75:414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, et al. (2008): Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 13:173–186, 115. [DOI] [PubMed] [Google Scholar]
- 55.Jope RS, Johnson GV (2004): The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 29:95–102. [DOI] [PubMed] [Google Scholar]
- 56.Tamura M, Mukai J, Gordon JA, Gogos JA (2016): Developmental Inhibition of Gsk3 Rescues Behavioral and Neurophysiological Deficits in a Mouse Model of Schizophrenia Predisposition. Neuron. 89:1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, et al. (2013): Adolescent stress–induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 339:335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ochs SM, Dorostkar MM (2015): Loss of neuronal GSK3beta reduces dendritic spine stability and attenuates excitatory synaptic transmission via beta-catenin. 20:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen P, Gu Z, Liu W, Yan Z (2007): Glycogen synthase kinase 3 regulates N-methyl-D-aspartate receptor channel trafficking and function in cortical neurons. Molecular pharmacology. 72:40–51. [DOI] [PubMed] [Google Scholar]
- 60.Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, et al. (1996): Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. The Journal of neuroscience : the official journal of the Society for Neuroscience. 16:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang Y, Jakovcevski M, Bharadwaj R, Connor C, Schroeder FA, Lin CL, et al. (2010): Setdb1 histone methyltransferase regulates mood-related behaviors and expression of the NMDA receptor subunit NR2B. J Neurosci. 30:7152–7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grayson DR, Guidotti A (2013): The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 38:138–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grayson DR, Kundakovic M, Sharma RP (2010): Is there a future for histone deacetylase inhibitors in the pharmacotherapy of psychiatric disorders? Mol Pharmacol. 77:126–135. [DOI] [PubMed] [Google Scholar]
- 64.Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P (2008): Decoding the epigenetic language of neuronal plasticity. Neuron. 60:961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Day JJ, Sweatt JD (2011): Epigenetic modifications in neurons are essential for formation and storage of behavioral memory. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 36:357–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodenas-Ruano A, Chavez AE, Cossio MJ, Castillo PE, Zukin RS (2012): REST-dependent epigenetic remodeling promotes the developmental switch in synaptic NMDA receptors. Nature neuroscience. 15:1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen T, Fan T, George SR, Perreault ML (2017): Disparate Effects of Lithium and a GSK-3 Inhibitor on Neuronal Oscillatory Activity in Prefrontal Cortex and Hippocampus. Frontiers in aging neuroscience. 9:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Vaart A, Meng X, Bowers MS, Batman AM, Aliev F, Farris SP, et al. (2018): Glycogen synthase kinase 3 beta regulates ethanol consumption and is a risk factor for alcohol dependence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 43:2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.