Abstract
Angiogenesis-mediated neovascularization correlates with recovery after intracerebral implantation of neural stem cells (NSCs) in stroke. To elucidate NSCs’ mechanism of action, it is essential to understand how these interact with the brain’s vasculature after implantation. Using an all-human endothelial cell (EC, D3 cell line) and NSC (STROC05 and CTXOE03) co-culture model, fluorescently activated cell sorting (FACS) was used to isolate each cell type for a comparison of gene expression between monocultures of undifferentiated proliferating and differentiated non-proliferating cells. Gene expression for angiogenic factors (vascular endothelial growth factor, platelet derived growth factor, angiopoietin), as well as cell survival (brain derived neurotrophic factor, fibroblast growth factor) and migration (stromal cell-derived factor-1a) were measured and contrasted with the corresponding receptors on each cell type. The cellular source of extracellular matrix defining the basement membrane (vitronectin, fibronectin, laminin, collagen I and IV) and neuropil (hyaluronic acid, aggrecan, neurocan, thrombospondin, nidogen and brain associated link protein-1) was evaluated for NSCs and ECs. Co-culturing dramatically changed the expression profiles of each cell type in comparison to undifferentiated, but also differentiated cells. These results indicate that monocultures provide a poor model to investigate the cellular signaling involved in a tissue repair response. Co-cultures of NSCs and ECs forming vasculature-like structures (VLS) provide a more complex model to investigate NSC-induced neovascularization. These in vitro studies are essential to tease out individual cell signaling in NSCs and ECs to develop a mechanistic understanding of the efficacy of NSCs as a therapeutic for stroke.
Keywords: Neural Stem Cell, Endothelial Cell, Neurovascular Unit, Extracellular Matrix, Basement Membrane, Angiogenesis
Introduction
The neurovascular environment of the brain is severely affected after a stroke. Implantation of neural stem cells (NSCs) into the damaged brain tissue improves behavioral impairments [1, 2], but it remains unclear how NSCs exert these beneficial effects. A correlation between behavioral recovery, astrocytes differentiation and vasculature has been reported after NSCs implantation [1]. NSCs are known to exert angiogenic effects thought to contribute to some of the therapeutic effects observed after NSC implantation [3]. Blocking of angiogenesis after NSC implantation has further been show to prevent behavioral recovery [4] indicating its importance in achieving a therapeutic response. However, angiogenesis also affects neurogenesis, emphasizing the interdependence of these compartments and their constituent cells [5, 6]. Understanding the signaling interaction between endothelial cells (ECs) and NSCs is therefore crucial to develop a mechanistic understanding of the therapeutic effects NSCs exert in stroke.
To examine the interaction between ECs and NSCs, a stringently controlled in vitro environment is favourable to dissect molecular changes in each cell type [7, 8]. Co-culturing of ECs and NSCs produced a neurovascular environment [9] that allowed us to determine that both juxtacrine and autocrine/paracrine factors are required to induce endothelial morphogenesis [10]. However, establishment of vasculature-like structures (VLS) was highly dependent on NSC “dosing”. Striatal NSCs (STROC05) were more potent inducers of stable VLS compared to cortical NSCs (CTXOE03) [10]. Nevertheless, cortical NSCs increased VEGF-A release 4x higher in the presence of ECs than striatal NSCs, highlighting the complex interplay between NSCs and ECs, as well as the multi-faceted nature of their signalling interactions. It further indicates that not all NSC lines will exert the same effects and that these signalling molecules are dramatically altered in the presence of other cells, such as ECs. By identifying key signals and how these change in response to other cells relevant to therapeutic efficacy (i.e. ECs), it is possible to develop a potency assay to screen cell lines for markers of putative efficacy.
Extensive mechanistic studies are difficult to perform in vivo due to the brain’s complexity. In vivo it is difficult to eliminate the contribution of individual cell types, but it is also challenging to administer factors that cross the blood-brain barrier to enhance, inhibit or block specific signalling pathways in the neuropil. In vitro studies hence provide an alternative approach, where cellular composition can be controlled and a relative high throughput of different factors and doses can be evaluated. Using this approach, we established that VEGF-R2 (VEGF-A receptor), PDGF-Rβ (PDGF-B receptor), αvβ3 (vitronectin receptor), and α6 (laminin receptor) signalling are necessary to form NSC-induced VLS [10], suggesting that these factors are crucial for NSCs to induce angiogenesis in vivo. This is consistent with NSCs inducing angiogenesis after implantation in stroke-damaged tissue [3] and Avastin, which blocks human VEGF-A, eliminating this pro-angiogenic effect [4].
Using immunocytochemistry, we further established that the presence of ECs shifted NSCs’ differentiation into neurons and astrocytes, rather than oligodendrocytes [10]. However, other cellular and molecular changes were not readily attributable to either ECs or NSCs using immunocytochemistry. We therefore here investigated molecular changes by means of gene expression in either cell type to reveal how ECs and NSCs influence each other. A separation of both cell types was achieved using fluorescently activated cell sorting (FACS) followed by analysis of gene expression changes using quantitative reverse transcription polymerase chain reaction (qRT-PCR). Specifically, we investigated whether ECs increase the production of angiogenic proteins, such as vascular endothelial growth factors (VEGF-A, VEGF-C, VEGF-D), platelet-derived growth factors (PDGF-A, PDGF-B), angiopoietins (ANGPT1, ANGPT2), and transforming growth factor (TGFb1) in NSCs. Conversely, we hypothesized that ECs may upregulate the expression of pro-survival factors (BDNF), as well as substrates required for NSC migration (SDF-1a). As NSCs and ECs form VLS, they also produce extracellular matrix (ECM) proteins that define both compartments. We therefore further investigated which cell type produced ECM molecules forming the basement membrane (vitronectin, fibronectin, laminin, collagen I and IV), as well as ECM proteins predominantly found in the neuropil (hyaluronic acid, aggrecan, nidogen, brain associated link protein-1, neurocan, thrombospondin). These gene expression profiles provide a more detailed understanding of how NSCs and ECs regulate each other to promote a neurovascular environment in vivo.
Materials and methods
Neural stem cells (NSCs)
Human NSC lines STROC05 and CTXOE03 (ECACC accession numbers 04110301 and 04091601, ReNeuron), respectively isolated from the whole ganglionic eminence and the cortex of a 12-week gestation human fetal brain, were cultured on plates coated with laminin (Sigma-Aldrich) in serum free medium (Supplementary Table S1) [2, 11]. Both cell lines were produced by transduction with the retroviral vector pLNCX-2 (Clontech) encoding the c-mycERTAM gene. Differentiation was induced by withdrawal of basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), and 4-hydroxytamoxifen (4-OHT) [12]. For the NSCs only condition, cell lines were seeded at 20,000 cells/cm2 in a T75 flask and differentiated in co-culture media, consisting of a 50:50 mix of NSC and EC media (Supplementary Table S1), for 7 days prior to being detached with Accutase. Cells were re-suspended for fluorescently activated cell sorting (FACS).
Endothelial cells (ECs)
The human cerebral microvascular endothelial cell (EC) line D3 (kindly provided by Dr. Pierre-Olivier Couraud, Institut Cochin) was isolated from microvessel fragments of an adult temporal lobe. The D3 EC line was derived from adult primary cells through co-expression of human telomerase reverse transcriptase (hTERT) and the SV40 large T antigen via a lentiviral vector transduction system [13]. D3 cells were cultured on glass coverslips coated with rat tail collagen type 1 (BD Biosciences) at a concentration of 150 μg/mL at 37°C in 5% CO2, using EBM-2 basal medium supplemented with 5% fetal bovine serum and additional components (Supplementary Table S1) [13]. For the ECs only condition, the D3 cell line was seeded at 20,000 cells/cm2 in a T75 flask and differentiated in co-culture media, consisting of a 50:50 mix of NSC and EC media (Supplementary Table S1), for 7 days prior to being detached with Accutase. Cells were re-suspended for FACS.
Co-culturing NSCs and ECs
To investigate the interaction between NSCs and differentiated ECs (Figure 1A), NSCs were added to a confluent layer of differentiated ECs to produce vascular-like structure (VLS, Figure 1B), as previously described in detail [9]. To determine an appropriate ratio of NSCs and ECs, D3 ECs were first seeded at 20,000 cells/cm2 on collagen I in a plate and maintained for 7 days in co-culture medium, consisting of a 50:50 mix of NSC and EC media (Supplementary Table S1). This produces approximately 200,000 ECs/cm2. STROC05 (12,500 cells/cm2) and CTXOE03 (50,000 cells/cm2) NSCs, were added to the EC layer and differentiated for 7 days. We have previously established that this approach induces endothelial morphogenesis that compartmentalizes a vascular and neural compartment, modeling the neurovascular environment in vitro using human cells [9]. Both juxtacrine and paracrine/autocrine signaling are required to establish these neurovascular structures [10]. After co-culturing for 7 days, cells were detached with Accutase and re-suspended for FACS.
Figure 1. Experimental Design.
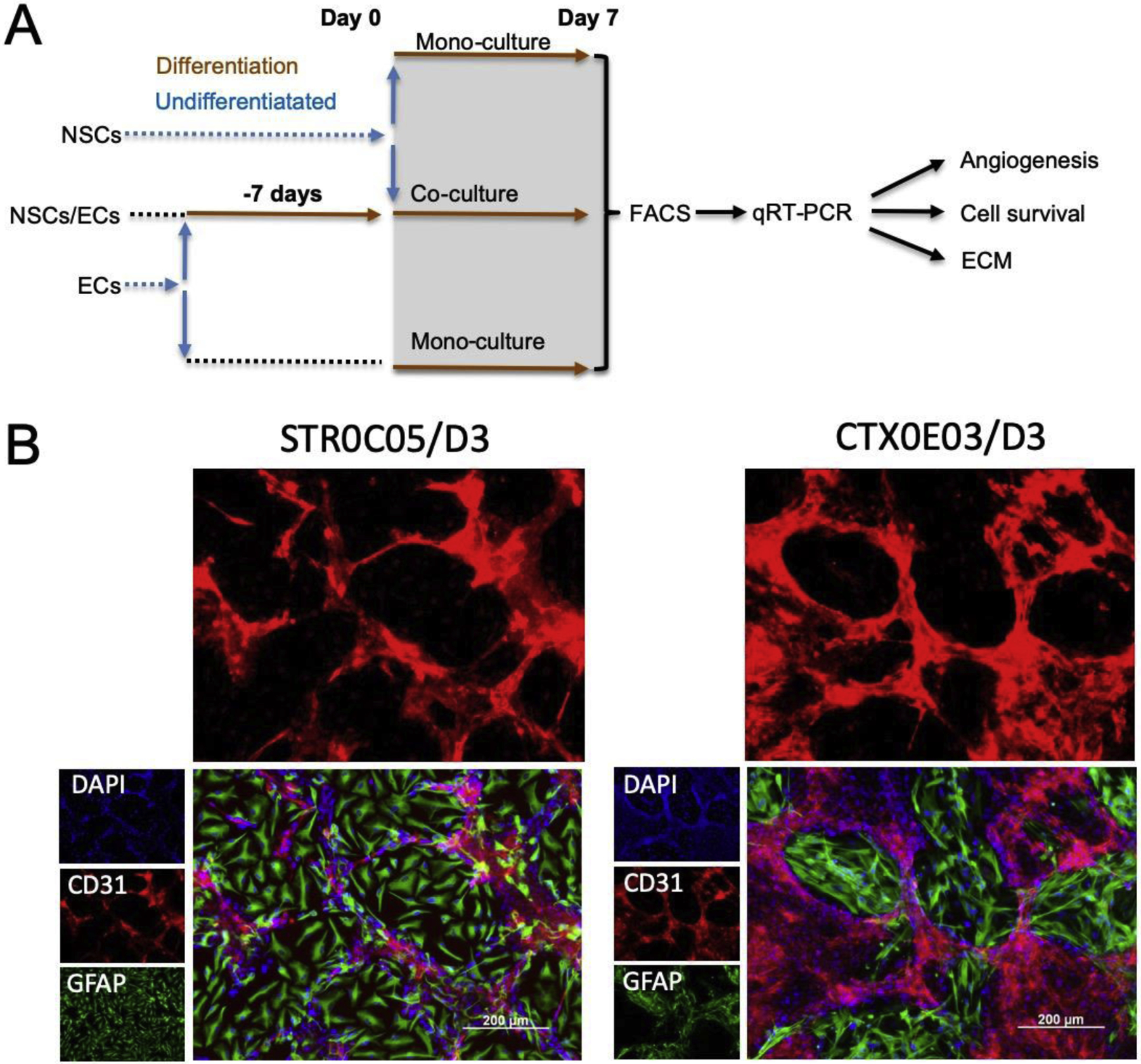
A. Neural stem cells (NSCs) and endothelial cells (ECs) were expanded in monoculture in their undifferentiated state. For differentiation, both cell types were grown for 7 days. For co-culture, ECs were plated first and allowed to differentiate for 7 days before undifferentiated NSCs were added for co-culture under differentiation conditions. After 7 days of co-culture, cells underwent fluorescently activated cell sorting (FACS) to separate NSCs and ECs for individual cell analysis using quantitative reverse transcription polymerase chain reaction (qRT-PCR). B. Vasculature-like structures (VLS) are formed through endothelial morphogenesis in which ECs (CD31+) organize in a plexus with NSCs (GFAP+) forming a neuropil-like patch.
Fluorescence-activated cell sorting (FACS)
To establish gene expression changes in NSCs and ECs due to co-culture, both cell types were separated using FACS. For this, cells were pelleted (1,500 rpm at 10 cm, i.e. 252 g, for 5 min) and approximately 1.0 × 106 cells were re-suspended in 2 ml of PBS (500 cells/μL). ECs were tagged for FACS using an allophycocyanin-conjugated mouse monoclonal anti-human CD31/PECAM-1 antibody (FAB3567A; 1/200 R&D Systems). Propidium iodide (P4170; 1/500 Sigma-Aldrich) was used to determine cell viability. Cells were incubated for 30 min at 22°C prior to being washed with ice-cold PBS (3x) to remove unbound labels. Cells were analyzed and sorted using a Becton Dickinson FACS Aria II flow cytometer (BD Biosciences). All analyses consisted of 3 technical replicates (i.e. 3 readings from each preparation) for 3 biological replicates (i.e. 3 separate culture preparations).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
After FACS, total RNA was extracted from CD31+ and CD31- cells collected using RNase Inhibitor (Qiagen) and the RNeasy Mini Kit (Qiagen). The concentration of RNA was determined using a Qubit 2.0 fluorometer (Invitrogen). Samples were reversely transcribed in a GS4 thermal cycler (G-storm) using the high capacity cDNA reverse transcription kit with RNase inhibitor (Invitrogen). With a real-time PCR system (StepOne™, Applied Biosystems), forward and reverse primers (Supplementary Table 2) were applied with power SYBR® green PCR master mix (Invitrogen) to produce a quantitative read-out of the threshold cycle (CT), reflecting the level of gene expression. Expression of the target genes was normalized using the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as reference. A log 2 transformation was undertaking to equally scale gene expression increases and decreases. Changes in gene expression were compared to undifferentiated cells to determine the impact of differentiation and co-culture. Each biological sample (n=3) was tested in triplicates.
Statistical analysis
Graphs were drawn in Prism 5 (GraphPad) with data points representing the mean ± standard error of mean. Biological variability of log2 transformed gene expression data was normally distributed and therefore parametric analyses, notably analysis of variance (ANOVA), comparing multiple groups were computed. Tukey’s post-hoc testing determined statistical significance between experimental conditions. Statistical significance was set at p<0.01, with a magnitude of change >2-fold compared to baseline.
Results
FACS robustly separates NSC and EC after co-culture
To dissect gene expression of individual cell types in EC/NSC cocultures, FACS was performed after cells were detached from the cell culture plate. Total cell counts and viability measures were performed for each cell type in their undifferentiated and differentiated state to ensure a high sample quality (Figure 2A). Approximately 5.6 × 106 cells were collected from D3/STROC05 coculture, and 4.3 × 106 CD31- cells from D3/CTXOE03 coculture. Using an antibody against CD31/PECAM-1, an endothelial marker, ECs were separated from NSCs to produce samples with each cell type (Figure 2B). CD31 was highly specific to isolate ECs >97% of D3 cells and less than 0.3% of NSCs being positive (Figure 2C). Among the live cells collected from D3/STROC05 coculture, 42.9% were positive for CD31 (i.e. D3 cells), and 49.2% were negative (i.e. NSCs). Among the live cells collected from D3/CTXOE03 coculture, 78.6% were CD31+ D3 cells and 10.2% were CD31- NSCs.
Figure 2. Fluorescently activated cell sorting (FACS) of co-cultures.

A. Scattergrams of total cell counts, as well as live/dead cells for all 3 cell lines. B. The same analysis was performed for co-cultured cells after cell sorting using CD31 as a marker to separate ECs (CD31+ cells) from NSCs (CD31- cells). C. The percentage of CD31+ cells in D3 monoculture was 97.1%. Only 0.3% of NSCs expressed CD31 making this a highly selective cell separation marker. D. Based on FACS, gene expression in ECs and NSCs for endothelial and neural markers were measured and normalized using GAPDH. A log2 transformation of data was computed to afford an equal scaling between increases and decreases in gene expression. E. To contextualize gene expression changes due to differentiation and co-culture, gene expression in undifferentiated cells was considered as baseline. Co-culture dramatically increased the expression of endothelial and neural markers in ECs and NSCs.
Separation of individual cell types afforded the isolation of RNA from both cell type to investigate how co-culture affected gene expression using the comparative CT method for qRT-PCR. To account for different numbers of cells in each sample, gene expression was normalized to GAPDH expression and further log2 transformed to equally space increased and decreased expression profiles (Figure 2D). Expression of CD31 in D3 cells was 512 times higher than in STROC05 cells (p<0.001) and 1,176 times higher than in CTXOE03 cells (p<0.001), but there was no significant difference in undifferentiated and differentiated ECs. In contrast, gene expression of GFAP was 24,833 times higher in STROC05 (p<0.001) and 244,589 times higher in CTXOE03 monoculture (p<0.001) than D3 ECs. Microtubule associated protein 2 (MAP2) gene expression was upregulated in differentiating STROC05 and CTX0E03 cells, but not ECs. Co-culturing only upregulated MAP2 in STROC05 cells, but not CTX0E03.
To contextualize gene expression changes for each cell line, gene expression profiles were further compared to undifferentiated cells to highlight how differentiation and co-culture affected individual cell lines (Figure 2E). Interestingly CD31 was only upregulated in STROC05 and CTXOE03 cells after co-culture (p<0.001). GFAP was also dramatically upregulated >3000x in D3 cells compared to differentiated D3 in monoculture (p<0.001). GFAP was also increased in differentiating CTX0E03 (p<0.001), but less so in STROC05 (p<0.001). MAP2 expression in ECs was within baseline expression (i.e. −1 to 1-fold change), but differentiating STROC05 and CTX0E03 has higher expressions of MAP2 (p<0.001). However, co-culturing did not further increase MAP2 expression in NSCs, with CTXOE03 even exhibiting a significant decrease in expression (p<0.001). The gene expression profiles highlight major cellular changes that occur during differentiation and co-culture that also affect cell signaling.
Co-culturing reciprocally upregulates angiogenic signaling
Analysis of angiogenic factors in supernatant prevent the identification of the cellular source. However, FACS of cells after co-culture and analysis of gene expression provides novel insight to which cells upregulate the production of angiogenic factors, as well as their respective receptors. The VEGF gene family is a major secreted factor in the angiogenic cascade (Figure 3A). Co-culture here upregulated VEGF-A in ECs by more than 200x (p<0.001), but also increased VEGF-A gene expression in STROC05 by >1000 fold (p<0.001) and CTXOE03 by >100 fold (p<0.001). VEGF-C was also upregulated in STROC05 after differentiation and co-culture (p<0.001), whereas VEGF-D was only upregulated in co-cultured ECs (p<0.01). VEGFR2 was only upregulated (p<0.001) in CTX0E03 after co-culture (Figure 3B), whereas VEGFR1 was upregulated in differentiated (p<0.01) and co-cultured STROC05 (p<0.001) and co-cultured CTXOE03 (p<0.001). No marked VEGF receptor changes were evident for ECs (<1-fold change). Absolute gene expression changes are presented in Supplementary Figure S1.
Figure 3. Angiogenic factors and receptors.

A. The expression of VEGFA and VEGFC was dramatically altered in co-culture. Less marked changes in VEGFD were evident in ECs (CD31+) in coculture with NSCs. B. VEGF receptor expression was less altered by co-culture, although considerable changes in VEGFR1 were observed. C. Changes in PDGFA and PDGFB were markedly less than those observed for VEGFA. D. In contrast, PDGFRa receptor changes were considerable for ECs in co-culture. E. ANGPT1 was upregulated in all conditions, apart of ECs in co-culture with CTXOE03. ECs in co-culture with CTXOE03 instead upregulated ANGPT2. F. Only minor changes in the angiopoietin Tie2 receptor were evident due to differentiation or co-culture. G. No marked changes in gene expression in TGFb1 were evident. H. However, TGFbR1 was slightly upregulated in ECs in co-culture, as well as in differentiating STROC05 cells.
In contrast to VEGF-A, other angiogenic factors underwent far less dramatic changes. PDGFA was only slightly increased in ECs compared to undifferentiated cells (Figure 3C), with only differentiating CTXOE03 exhibiting an almost 4-fold increase in expression (p<0.001). PDGFB only revealed an increase in EC, especially when co-cultured with STROC05 (p<0.001). The PDGF receptors were only upregulated in ECs (Figure 3D). PDGFRa in ECs was highly upregulated (>40-fold increase) when co-cultured with NSCs, whereas PDGFRb was less affected by co-culture with STROC05 inducing the greatest increase (18-fold, p<0.001). ANGPT1 was significantly upregulated by at least a factor of 8 in all conditions (p<0.001), apart of the co-culture with CTXOE03 (Figure 3E). In contrast, this one condition was significantly upregulated for ANGPT2 (p<0.01). The Tie2 angiopoietin receptor, however, was only upregulated in ECs when co-cultured with STROC05 NSCs (p<0.001, Figure 3F). There was no differential gene expression of TGFb1 compared to baseline undifferentiated cells (Figure 3G). Gene expression for the TGFbR1 was upregulated in ECs and differentiated STROC05 (p<0.01), but there was no change in the TGFbR2 expression (Figure 3H). These results indicate that NSCs dramatically increase their pro-angiogenic function in the presence of ECs, especially VEGFA and VEGFR1. In ECs, gene expression for VEGFA was also very markedly increased, but this was accompanied by an increase in PDGFRa and not an increase in VEGF receptors. These differences between ECs and NSCs potentially indicate a differential regulation of the angiogenic cascade by both cell types.
Cell survival and migration
The cell survival factor BDNF was not increased with differentiation of ECs or NSCs, but was upregulated by >4 fold (p<0.001) in both cell types when cocultured with CTXOE03 NSCs (Figure 4A, see Supplementary Figure S2 for absolute gene expression). Coculture with STROC05 only upregulated BDNF in NSCs. The associated TrkB receptor was also upregulated in both ECs and STROC05 cells when co-cultured (p<0.001). Upon co-culture with CTXOE03 TrkB was only upregulated in ECs (Figure 4B). This condition saw a minor upregulation of p75 instead, whereas no other condition saw an expression change beyond baseline. An upregulation of bFGF (p<0.001) was only evident in ECs when co-cultured with STROC05 (Figure 4C), but the corresponding receptor was only upregulated (p<0.001) when NSCs were differentiated without ECs (Figure 4D). The migratory factor, SDF-1a, was upregulated (p<0.01) in co-cultured NSCs, as well as differentiated STROC05 (Figure 4E), but there was no change in the corresponding receptor CXCR4 expression levels beyond baseline (Figure 4F). These results indicate that ECs are beneficial to NSCs to produce pro-survival factors, such as BDNF and upregulate its receptor TrkB. An upregulation of bFGF in co-cultured ECs and STROC05 reflects an ongoing pro-angiogenic response, when ECs need to proliferate to produce more cells required for tubulogenesis. SDF-1a was mainly in co-cultured NSCs, potentially indicating that NSCs induce endothelial morphogenesis by secretion of this pro-migration factor.
Figure 4. Cell survival and migration factors and receptors.

A. BDNF was upregulated in NSCs in co-culture, as well as ECs when cocultured with CTXOE03. B. TrkB receptors were upregulated in ECs in coculture and in STROC05 under differentiation and coculture conditions. C. bFGF exhibited a more limited upregulation in ECs and STROC05 when co-cultured. D. The FGFR2 receptor in contrast was only upregulated in differentiating NSCs, not in coculture. E. Differentiation and co-culture of STROC05 upregulated SDF-1a, as well as in co-cultured CTXOE03. However, no change in ECs was evident. F. The receptor CXCR4 was downregulated in differentiating and cocultured STROC05, but upregulated in CTXOE03 cells in the same conditions.
Basement membrane and neuropil associated ECM proteins
Deposition of a basement membrane is a crucial step in dividing the neurovascular environment into a vascular and neuropil compartment. RGD ECM molecules support NSCs migration, but also define the basement membrane of the vasculature. No major changes in vitronectin gene expression were evident (Figure 5A, see Supplementary Figure S3 for absolute gene expression). Fibronectin in contrast was markedly increased (18 fold) in co-cultured CTXOE03 (p<0.001), but not in ECs. Less marked increases (>3 fold) were also significant (p<0.01) for co-cultured STROC05 and differentiated NSCs. The most significant (p<0.001) upregulation of the ITGaV receptor was in co-cultured STROC05, but all co-cultured cells upregulated its gene expression (>3-fold, Figure 5B). The ITGb3 receptor was only significantly (p<0.001) upregulated 4.5-fold in co-cultured STROC05. The expression of laminin was only increased in co-cultured EC (p<0.001), with STROC05 exerting a 5x greater effect than CTXOE03 (Figure 5C). There was however only a minor upregulation (2–3 fold) of the laminin receptors in STROC05 co-cultured cells (p<0.01, Figure 5D). The deposition of collagen I was only associated with significant (p<0.001) gene expression changes in ECs, with STROC05 co-cultured ECs revealing a 35-fold increase (Figure 5E). Collagen IV production was mostly unchanged, with only ECs co-cultured with STROC05 exhibiting a 5-fold increased (p<0.001) gene expression compared to undifferentiated cells (Figure 5F).
Figure 5. Basement membrane associated ECM proteins.

A. Only differentiating CTXOE03 cells revealed a minor upregulation of vitronectin, whereas co-cultured CTXOE03 markedly upregulated fibronectin. B. In contrast, cocultured STROC05 upregulated the corresponding receptors for ITGaV and ITGb3. C. Laminin was only upregulated in ECs after co-culture. D. The laminin receptors ITGa6 and ITGb1 were mainly upregulated in EC and STROC05 co-cultures. E. Collagen I was most strongly upregulated in ECs when co-cultured with STROC05, but 7x less so when co-cultured with CTXOE03. F. Collagen IV was also mainly upregulated in ECs when co-cultured with STROC05.
The production of HA was upregulated in differentiating NSCs (p<0.001), as well as co-cultured CTXOE03 (p<0.001), but not STROC05 (Figure 6A, see Supplementary Figure S4 for absolute gene expression). Co-cultured EC also upregulated HA production (p<0.01), but to a lesser degree. Changes in aggrecan gene expression did not reach statistical significance (Figure 6B). Another neuropil-associated ECM protein, neurocan was most strongly upregulated in STROC05 co-cultured ECs (>230-fold, p<0.001), but less so (>10-fold) in differentiating and co-cultured CTXOE03 (Figure 6C). Thrombospondin was only upregulated (p<0.001) in differentiating STROC05 (Figure 6D), whereas nidogen1 was expressed at baseline for all conditions (Figure 6E). Bral1 was upregulate most (>7-fold) in co-cultured ECs (p<0.001), but was non-significantly different in other conditions (Figure 6F). These results indicate that NSCs are the main source of fibronectin, whereas laminin, collagen I and IV, neurocan and Bral1 are mainly produced by ECs. It is important to note that overall STROC05 co-culture exerted a greater effect on ECM production than CTXOE03.
Figure 6. Neuropil associated ECM proteins.

A. Hyaluronic acid (HA) was mainly upregulated in NSCs, but also co-cultured ECs upregulated HA. B. Aggrecan was upregulated in co-cultured ECs and CTXOE03, but not in STROC05. C. Neurocan was dramatically upregulated in STROC05 co-cultured ECs and CTXOE03 that were differentiated or co-cultured. D. Thrombospondin was only upregulated in differentiated STROC05 cells. E. Nidogen 1 was not upregulated in any condition. F. Co-culture upregulated brain associated link protein-1 (Bral1) in ECs, but not in NSCs.
Discussion
The interaction between NSCs and ECs in the brain remains poorly understood. Nevertheless, the implantation of NSCs is known to induce an angiogenic response that affects the brain’s neurovascular microenvironment and is thought to contribute to behavioral recovery [1, 3]. We established an in vitro co-culture model of NSCs and EC that produces a neurovascular environment [9, 10] and previously characterized the role of autocrine/paracrine and juxtacrine signaling in this system [10]. Using FACS, isolation of NSCs and ECs here afforded a characterization of gene expression changes in each cell type that underlie the angiogenic response, as well as the deposition of ECM proteins that define both the vascular and neural compartments of brain tissue.
NSC-induced angiogenesis
During brain development, there is substantial cross-talk between the vasculature and NSCs that drives proliferation, as well as differentiation of both cell types [6, 14, 15]. VEGF secreted from ECs, as well as NSCs, exerts diverse roles in the developing brain [16], but also plays a key role in angiogenesis after stroke [17]. NSCs induce angiogenesis when implanted in the peri-infarct stroke cavity [1, 3] through a VEGFA mediated process [4], which is a major signal involved in peri-infarct angiogenesis [18]. Co-culture of NSCs and ECs is known to upregulate the release of VEGF-A and thought to model the in vivo interaction between these two cell types [10]. However, it remained unclear if ECs, NSCs or both upregulated VEGFA. Our results here indicate that both NSCs and ECs jointly upregulate VEGFA. Interestingly, STROC05, which more efficiently produces endothelial morphogenesis [9], saw a 4-fold higher upregulation of VEGFA compared to CTXOE03 and ECs. Astrocyte-derived VEGFA has been shown to promote the survival of ECs and to stabilize tubular structures [19], potentially highlighting a key role for astrocytes tightly bound to VLS. STROC05 NSCs also upregulated VEGFC, which was not upregulated in CTXOE03 or ECs. VEGFC and VEGFR3 have been associated with the activation of NSCs to induce self-renewal [20]. This is also consistent here with the increase in bFGF in the STROC05 cell line, which produces a proliferative effect in both NSCs and ECs. In contrast to NSCs, ECs upregulated VEGFD, but only when co-cultured with NSCs. VEGFD is upregulated after brain injury [21] and a known EC mitogen involved in angiogenesis and lymphangiogenesis [22]. The EC-specific upregulation of VEGFD here indicates that NSCs can induce the release of secreted factors in other cell types that can produce additional therapeutic effects independent of the implanted cells.
Changes in VEGF expression were the most dramatic changes occurring in co-culture, but the PDGFRa receptor also underwent a marked upregulating in ECs. Expression of this receptor in ECs has been associated with remodeling of tissues, especially neovascularization [23], which occurs after NSCs implantation in the peri-infarct stroke area [1]. However, changes in the angiogenic cascade also affect neurogenesis. For instance, the upregulation of angiopoietin-1, due to the presence of ECs here, can shift NSC differentiation from oligodendrocytes to neurons and astrocytes [10], as ANGPT1 has been shown to be pro-neurogenic [24]. ECs also exert an increased release of BDNF from NSCs that underlies an improved survival and neuronal differentiation [25–27]. NSCs are also the main source of SDF-1a, which promotes their migratory behavior [28, 29], even though migration of neuroblast is known to use the basement membrane of the vasculature for adhesion [30].
ECM proteins defining the neural and vascular compartments
In the neurovascular microenvironment, the basement membrane serves several functions, notably providing a substrate for NSCs to migrate to sites of injuries, but it also defines the junction between the vasculature and the neuropil [31]. We here demonstrated that fibronectin is mostly upregulated in NSCs, especially CTXOE03. Fibronectin is known to improve NSC survival and migration in vivo [32, 33] and is commonly used as a substrate for NSCs to attach to culture flasks, as is laminin [34]. However, laminin in co-culture was selectively upregulated in ECs, as was collagen I and collagen IV. These results suggest that different molecules forming the basement membrane might have distinct cellular origins. However, in the absence of typical mural cells, such as pericytes and vascular smooth muscle cells, cellular function in vitro might not fully reflect the cellular signaling interactions found in vivo in the neurovascular unit [35]. Nevertheless, our results here provide a greater understanding of the interactions between NSCs and ECs, which is required to further elucidate the roles of additional signaling partners, such as pericytes [36]. The temporal dynamics of ECM deposition forming the basement membrane might also affect which type of cell contributes to the basement membrane during vessel formation [10, 37].
The basement membrane separates the vascular environment from the neuropil. It can therefore be expected that ECM in the neuropil is mostly produced by neural cells derived from NSCs [38]. Although NSCs prefer vasculature-associated cell adhesion molecules, such as fibronectin and laminin, during proliferation and migration, a different set of molecules reflective of ECM molecules in the neuropil is required as these cells mature and position themselves within tissue [12]. We here demonstrated that, for instance, hyaluronic acid (HA), is upregulated in NSCs and to a lower degree also in co-cultured ECs. HA plays a pivotal role in the NSC niche, as well as during the cells differentiation [39]. HA is very abundant in brain tissue and provides it with its elasticity properties [40]. Thrombospondin, an ECM molecule associated with peri-neuronal nets, was singularly upregulated in differentiated STROC05. Thrombospondin is widely distributed in the striatum and cortex. Although commonly thought to be produced by astrocytes, it is mostly found around neurons in the brain [41], reflecting the key difference between cells producing an ECM molecule versus its structural and juxtacrine role in other cells. Interestingly, many neuropil-associated proteins, such as aggrecan, neurocan, and Bral1 were more upregulated in co-cultured ECs. Although neurocan was deemed to be dispensable during brain development [42], the roles of these molecules in development versus maintaining a mature tissue remain poorly understood. It is noteworthy that the ECM is not a static element of tissue [43], but contributes to cell differentiation through juxtacrine signaling in both development [44] and after brain injury [45]. The expression of ECM molecules here might hence not necessarily reflect their role in mature brain tissue, but could be specific to the individual cells exert when NSCs encounter a differentiated vasculature and induce a neovascularization response. The differentiation of NSCs into different lineages, as well as formation of synaptic connections, based on the microenvironmental context might further alter their gene expression profile and will need consideration in future studies [46, 47].
Conclusions
The CTXOE03 line has been shown to promote a cell dose-dependent recovery after stroke [48]. Recovery is associated with angiogenesis [3] and correlated with neovascularization [1]. A key challenge for in vivo and in vitro co-culture studies is to understand how individual cells signal to other cells to produce a recovery response. We here demonstrated that FACS of co-cultured cells can provide a deeper insight into gene expression related to angiogenesis and the neurovascular environment formed by ECs and NSCs. However, it is important to note that in vivo microglia [49, 50] and pericytes [51, 52] also contribute to the signaling within the 3 dimensional neurovascular niche [53]. The development of more complex models representing brain tissue are therefore desirable [54, 55]. It is expected that these studies will identifiy key signaling nodes, such as GSK3β [56], that regulate NSCs interaction with ECs to provide the basis for a more complete mechanistic understanding of how NSCs exert therapeutic effects directly and indirectly [57, 58]. We further predict that these assays paired with whole transcriptome analysis [59] will eventually also provide tools for high throughput in vitro screening of individual cell lines to predict in vivo efficaciousness and accelerate the development and translation of cell therapy products [60, 61].
Supplementary Material
Highlights.
Coculture of human brain endothelial and neural stem cells
Fluorescent activated cell sorting (FACS) to isolate endothelial and neural stem cells after co-culture
Differential gene expression after monoculture and coculture for factors involved in angiogenesis, cell survival and migration
Identification of cellular source of extracellular matrix molecules in the basement membrane and neuropil
Acknowledgments
This work was supported by grants from the Biotechnology and Biological Sciences Research Council (BB/D014808/1) and the National Institute of Neurological Disorders and Stroke (R01 NS08226). CHC was supported by the Taiwanese Government Ministry of Science and Technology (MOST 104-2314-B-016-017-MY3; MOST 107-2314-B-016-017) and thanks the Tri-Service General Hospital (TSGH-C107-007-007-S05) for their generous support. NSC lines were kindly provided by Dr John Sinden (ReNeuron Ltd), whereas the D3 EC line was provided by Dr Pierre-Olivier Couraud (Institute Cochin).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Smith EJ, Stroemer RP, Gorenkova N, Nakajima M, Crum WR, Tang E, et al. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells. 2012;30(4):785–96. doi: 10.1002/stem.1024. [DOI] [PubMed] [Google Scholar]
- 2.Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, et al. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199(1):143–55. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Hicks C, Stevanato L, Stroemer RP, Tang E, Richardson S, Sinden JD. In Vivo and In Vitro Characterization of the Angiogenic Effect of CTX0E03 Human Neural Stem Cells. Cell Transplant. 2013;22(9):1541–52. doi: 10.3727/096368912X657936. [DOI] [PubMed] [Google Scholar]
- 4.Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29(2):274–85. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28(4):764–71. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madri JA. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60 Suppl 4:95–104. [PubMed] [Google Scholar]
- 7.Palmiotti CA, Prasad S, Naik P, Abul KM, Sajja RK, Achyuta AH, et al. In vitro cerebrovascular modeling in the 21st century: current and prospective technologies. Pharm Res. 2014;31(12):3229–50. doi: 10.1007/s11095-014-1464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ment LR, Stewart WB, Scaramuzzino D, Madri JA. An in vitro three-dimensional coculture model of cerebral microvascular angiogenesis and differentiation. In Vitro Cell Dev Biol Anim. 1997;33(9):684–91. doi: 10.1007/s11626-997-0126-y. [DOI] [PubMed] [Google Scholar]
- 9.Chou CH, Sinden JD, Couraud PO, Modo M. In vitro modeling of the neurovascular environment by coculturing adult human brain endothelial cells with human neural stem cells. PloS one. 2014;9(9):e106346. doi: 10.1371/journal.pone.0106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou CH, Modo M. Human neural stem cell-induced endothelial morphogenesis requires autocrine/paracrine and juxtacrine signaling. Scientific reports. 2016;6:29029. doi: 10.1038/srep29029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson S, Price J, Modo M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cells. 2008;26(9):2444–54. doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]
- 12.El-Akabawy G, Medina LM, Jeffries A, Price J, Modo M. Purmorphamine increases DARPP-32 differentiation in human striatal neural stem cells through the Hedgehog pathway. Stem Cells Dev. 2011;20(11):1873–87. doi: 10.1089/scd.2010.0282. [DOI] [PubMed] [Google Scholar]
- 13.Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. Faseb J. 2005;19(13):1872–4. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 14.Tata M, Ruhrberg C. Cross-talk between blood vessels and neural progenitors in the developing brain. Neuronal Signaling. 2018;2(1):NS20170139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J, Sha B, Zhou W, Yang Y. VEGF-mediated angiogenesis stimulates neural stem cell proliferation and differentiation in the premature brain. Biochem Biophys Res Commun. 2010;394(1):146–52. doi: 10.1016/j.bbrc.2010.02.132. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie F, Ruhrberg C. Diverse roles for VEGF-A in the nervous system. Development. 2012;139(8):1371–80. doi: 10.1242/dev.072348. [DOI] [PubMed] [Google Scholar]
- 17.Nag S, Eskandarian MR, Davis J, Eubanks JH. Differential expression of vascular endothelial growth factor-A (VEGF-A) and VEGF-B after brain injury. J Neuropathol Exp Neurol. 2002;61(9):778–88. doi: 10.1093/jnen/61.9.778. [DOI] [PubMed] [Google Scholar]
- 18.Geiseler SJ, Morland C. The Janus Face of VEGF in Stroke. Int J Mol Sci. 2018;19(5). doi: 10.3390/ijms19051362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chow J, Ogunshola O, Fan SY, Li Y, Ment LR, Madri JA. Astrocyte-derived VEGF mediates survival and tube stabilization of hypoxic brain microvascular endothelial cells in vitro. Brain Res Dev Brain Res. 2001;130(1):123–32. doi: 10.1016/s0165-3806(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 20.Han J, Calvo CF, Kang TH, Baker KL, Park JH, Parras C, et al. Vascular endothelial growth factor receptor 3 controls neural stem cell activation in mice and humans. Cell Rep. 2015;10(7):1158–72. doi: 10.1016/j.celrep.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nag S, Manias J, Eubanks JH, Stewart DJ. Increased Expression of Vascular Endothelial Growth Factor-D Following Brain Injury. Int J Mol Sci. 2019;20(7). doi: 10.3390/ijms20071594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A. 1998;95(2):548–53. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horikawa S, Ishii Y, Hamashima T, Yamamoto S, Mori H, Fujimori T, et al. PDGFRalpha plays a crucial role in connective tissue remodeling. Scientific reports. 2015;5:17948. doi: 10.1038/srep17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosa AI, Goncalves J, Cortes L, Bernardino L, Malva JO, Agasse F. The angiogenic factor angiopoietin-1 is a proneurogenic peptide on subventricular zone stem/progenitor cells. J Neurosci. 2010;30(13):4573–84. doi: 10.1523/JNEUROSCI.5597-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen SQ, Cai Q, Shen YY, Cai XY, Lei HY. Combined use of NGF/BDNF/bFGF promotes proliferation and differentiation of neural stem cells in vitro. Int J Dev Neurosci. 2014;38:74–8. doi: 10.1016/j.ijdevneu.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Pelegri NG, Gorrie CA, Santos J. Rat Hippocampal Neural Stem Cell Modulation Using PDGF, VEGF, PDGF/VEGF, and BDNF. Stem Cells Int. 2019;2019:4978917. doi: 10.1155/2019/4978917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84(8):1656–68. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 28.Cheng X, Wang H, Zhang X, Zhao S, Zhou Z, Mu X, et al. The Role of SDF-1/CXCR4/CXCR7 in Neuronal Regeneration after Cerebral Ischemia. Front Neurosci. 2017;11:590. doi: 10.3389/fnins.2017.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101(52):18117–22. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujioka T, Kaneko N, Sawamoto K. Blood vessels as a scaffold for neuronal migration. Neurochem Int. 2019;126:69–73. doi: 10.1016/j.neuint.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Thomsen MS, Routhe LJ, Moos T. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab. 2017;37(10):3300–17. doi: 10.1177/0271678X17722436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tate MC, Shear DA, Hoffman SW, Stein DG, Archer DR, LaPlaca MC. Fibronectin promotes survival and migration of primary neural stem cells transplanted into the traumatically injured mouse brain. Cell Transplant. 2002;11(3):283–95. [PubMed] [Google Scholar]
- 33.Flanagan LA, Rebaza LM, Derzic S, Schwartz PH, Monuki ES. Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res. 2006;83(5):845–56. doi: 10.1002/jnr.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi Y, Furue MK. Biological Effects of Culture Substrates on Human Pluripotent Stem Cells. Stem Cells Int. 2016;2016:5380560. doi: 10.1155/2016/5380560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Gaviro MV, Lovell-Badge R, Fernandez-Aviles F, Lara-Pezzi E. The vascular stem cell niche. J Cardiovasc Transl Res. 2012;5(5):618–30. doi: 10.1007/s12265-012-9371-x. [DOI] [PubMed] [Google Scholar]
- 36.Lauschke K, Frederiksen L, Hall VJ. Paving the Way Toward Complex Blood-Brain Barrier Models Using Pluripotent Stem Cells. Stem Cells Dev. 2017;26(12):857–74. doi: 10.1089/scd.2017.0003. [DOI] [PubMed] [Google Scholar]
- 37.Nicosia RF, Madri JA. The microvascular extracellular matrix. Developmental changes during angiogenesis in the aortic ring-plasma clot model. Am J Pathol. 1987;128(1):78–90. [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnaswamy VR, Benbenishty A, Blinder P, Sagi I. Demystifying the extracellular matrix and its proteolytic remodeling in the brain: structural and functional insights. Cell Mol Life Sci. 2019;76(16):3229–48. doi: 10.1007/s00018-019-03182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Preston M, Sherman LS. Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front Biosci (Schol Ed). 2011;3:1165–79. doi: 10.2741/218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bignami A, Hosley M, Dahl D. Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat Embryol (Berl). 1993;188(5):419–33. doi: 10.1007/bf00190136. [DOI] [PubMed] [Google Scholar]
- 41.Liu JR, Modo M. Quantification of the Extracellular Matrix Molecule Thrombospondin 1 and Its Pericellular Association in the Brain Using a Semiautomated Computerized Approach. J Histochem Cytochem. 2018;66(9):643–62. doi: 10.1369/0022155418771677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou XH, Brakebusch C, Matthies H, Oohashi T, Hirsch E, Moser M, et al. Neurocan is dispensable for brain development. Mol Cell Biol. 2001;21(17):5970–8. doi: 10.1128/mcb.21.17.5970-5978.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840(8):2506–19. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long KR, Huttner WB. How the extracellular matrix shapes neural development. Open Biol. 2019;9(1):180216. doi: 10.1098/rsob.180216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonneh-Barkay D, Wiley CA. Brain extracellular matrix in neurodegeneration. Brain Pathol. 2009;19(4):573–85. doi: 10.1111/j.1750-3639.2008.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liauw J, Hoang S, Choi M, Eroglu C, Choi M, Sun GH, et al. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab. 2008;28(10):1722–32. doi: 10.1038/jcbfm.2008.65. [DOI] [PubMed] [Google Scholar]
- 47.Simao D, Silva MM, Terrasso AP, Arez F, Sousa MFQ, Mehrjardi NZ, et al. Recapitulation of Human Neural Microenvironment Signatures in iPSC-Derived NPC 3D Differentiation. Stem Cell Reports. 2018;11(2):552–64. doi: 10.1016/j.stemcr.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stroemer P, Patel S, Hope A, Oliveira C, Pollock K, Sinden J. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil Neural Repair. 2009;23(9):895–909. doi: 10.1177/1545968309335978. [DOI] [PubMed] [Google Scholar]
- 49.Diaz-Aparicio I, Paris I, Sierra-Torre V, Plaza-Zabala A, Rodriguez-Iglesias N, Marquez-Ropero M, et al. Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. J Neurosci. 2020. doi: 10.1523/JNEUROSCI.0993-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matarredona ER, Talaveron R, Pastor AM. Interactions Between Neural Progenitor Cells and Microglia in the Subventricular Zone: Physiological Implications in the Neurogenic Niche and After Implantation in the Injured Brain. Front Cell Neurosci. 2018;12:268. doi: 10.3389/fncel.2018.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ElAli A, Theriault P, Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci. 2014;15(4):6453–74. doi: 10.3390/ijms15046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geranmayeh MH, Rahbarghazi R, Farhoudi M. Targeting pericytes for neurovascular regeneration. Cell Commun Signal. 2019;17(1):26. doi: 10.1186/s12964-019-0340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uwamori H, Higuchi T, Arai K, Sudo R. Integration of neurogenesis and angiogenesis models for constructing a neurovascular tissue. Scientific reports. 2017;7(1):17349. doi: 10.1038/s41598-017-17411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sivandzade F, Cucullo L. In-vitro blood-brain barrier modeling: A review of modern and fast-advancing technologies. J Cereb Blood Flow Metab. 2018;38(10):1667–81. doi: 10.1177/0271678X18788769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sood D, Cairns DM, Dabbi JM, Ramakrishnan C, Deisseroth K, Black LD 3rd, et al. Functional maturation of human neural stem cells in a 3D bioengineered brain model enriched with fetal brain-derived matrix. Scientific reports. 2019;9(1):17874. doi: 10.1038/s41598-019-54248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Q, Michaud M, Canosa S, Kuo A, Madri JA. GSK-3beta: a signaling pathway node modulating neural stem cell and endothelial cell interactions. Angiogenesis. 2011;14(2):173–85. doi: 10.1007/s10456-011-9201-9. [DOI] [PubMed] [Google Scholar]
- 57.Baker EW, Kinder HA, West FD. Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain Behav. 2019;9(3):e01214. doi: 10.1002/brb3.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sinden JD, Hicks C, Stroemer P, Vishnubhatla I, Corteling R. Human Neural Stem Cell Therapy for Chronic Ischemic Stroke: Charting Progress from Laboratory to Patients. Stem Cells Dev. 2017;26(13):933–47. doi: 10.1089/scd.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Q, Canosa S, Flynn K, Michaud M, Krauthammer M, Madri JA. Modeling the neurovascular niche: unbiased transcriptome analysis of the murine subventricular zone in response to hypoxic insult. PloS one. 2013;8(10):e76265. doi: 10.1371/journal.pone.0076265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boltze J, Modo MM, Mays RW, Taguchi A, Jolkkonen J, Savitz SI. Stem Cells as an Emerging Paradigm in Stroke 4: Advancing and Accelerating Preclinical Research. Stroke. 2019;50(11):3299–306. doi: 10.1161/STROKEAHA.119.025436. [DOI] [PubMed] [Google Scholar]
- 61.Modo MM, Jolkkonen J, Zille M, Boltze J. Future of Animal Modeling for Poststroke Tissue Repair. Stroke. 2018;49(5):1099–106. doi: 10.1161/STROKEAHA.117.018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


