Abstract
Clonal hematopoiesis (CH) has emerged as an important factor linked with adverse health conditions in the elderly. CH is characterized by an overrepresentation of genetically distinct hematopoietic stem cell clones in the peripheral blood. Whereas the genetic mutations that underlie CH have been closely scrutinized, relatively little attention has been paid to the environmental factors that may influence the emergence of one dominant stem cell clone. Since there is huge individual variation in latency between acquisition of a genetic mutation and emergence of CH, environmental factors likely play a major role. Indeed, environmental stressors such as inflammation, chemotherapy, and metabolic syndromes are known to affect steady state hematopoiesis. To date, epidemiologic studies point towards smoking and prior chemotherapy exposure as likely contributors to some forms of CH, though the impact of other environmental factors are also being investigated. Mechanistic studies in murine models indicate that the role of different environmental factors in CH emergence may be highly specific to the mutation that marks each stem cell clone. For instance, recent studies have shown that clones with mutations in the PPM1D gene are more resistant to genotoxic stress induced by chemotherapy. These clones thus have a competitive advantage in the setting of chemotherapy, but not in other types of stress. Here we review currently available literature on the interplay between environmental and the genetic landscapes in CH and highlight critical areas for future study. Improved understanding of the effects of environmental stress on emergence of CH with mutation-specific clarity will guide future efforts to provide preventive medicine to individuals with CH.
Graphical Abstract
Introduction
Clonal hematopoiesis (CH) is a condition in which one or a few individual hematopoietic stem cells (HSCs) contribute disproportionately to peripheral blood production (Genovese et al., 2014; Jaiswal et al., 2014; Xie et al., 2014). Not only are individuals with CH at significantly greater risk of developing hematologic malignancies compared to their non-CH counterparts, but importantly they have greater all-cause mortality largely from heart disease and stroke (Jaiswal et al., 2014; Jaiswal et al., 2017). The prevalence of clonal hematopoiesis increases with age and is thought to occur at some level in virtually all people (Young et al., 2016), such that around 20% of individuals above the age of 70 have at least one HSC clone that is contributing to around 20% of their blood and that fraction increases steadily in each decade of life (Genovese et al., 2014; Jaiswal et al., 2014). Though it is believed that the vast majority of 50-year-olds harbor clones with mutations in the genes most commonly associated with CH, only a fraction of these individuals goes on to develop CH. Thus, while CH is clearly associated with mortality, the interaction between environmental and genetic factors that drive its emergence remains unclear (Bowman et al., 2018; Steensma, 2018).
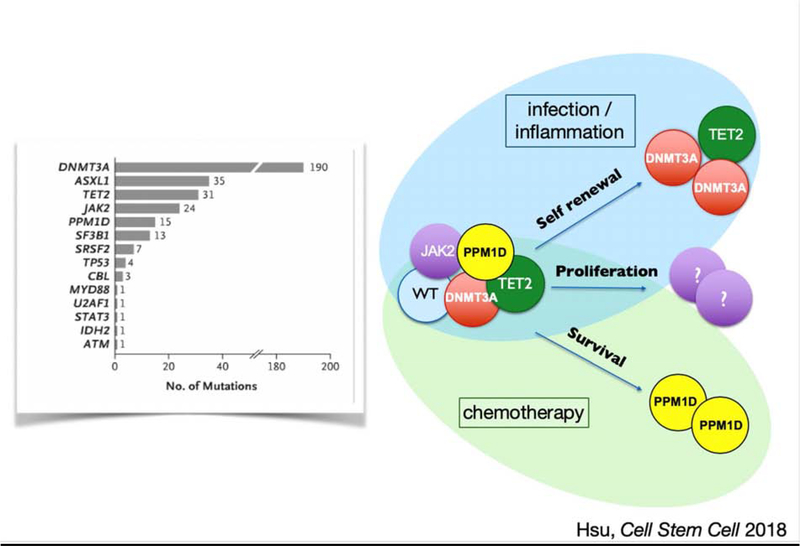
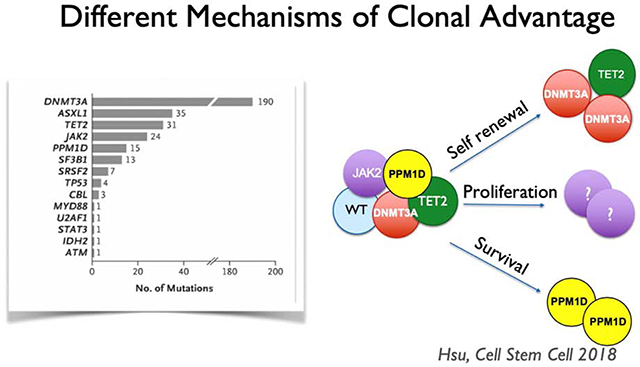
Heterozygous somatic mutations in about 20 genes are recurrently associated with CH, and some of these mutations are associated with cancer development (Steensma, 2018). Of the most frequent mutations, a few are known to confer a growth or self-renewal advantage to HSCs, enabling those mutant clones to attain numerical advantage (Figure 1). However, CH is unlikely to be driven by genetics alone (Loh et al., 2018). The pace at which CH emerges in individuals with known mutations may differ by decades, suggesting that environmental conditions are a critical driver of CH. Indeed, environmental conditions may provide the necessary backdrop for a survival advantage for certain mutant clones.
Figure 1.
A variety of mutations are associated with clonal hematopoiesis. Clonal dominance may occur through several mechanisms, either via increased self-renewal, a proliferative advantage, or improved survival. DNMT3A and TET2 mutations are thought to offer a competitive advantage through increased self-renewal at the stem cell level. The mechanism through which JAK2 mutations confer and advantage are unknown but are speculatively indicated here as proliferation of the stem and progenitor cell population. PPM1D mutations likely confer an advantage through enhancing survival under adverse circumstances such as chemotherapy.
Age is the strongest epidemiologic predictor of clonal hematopoiesis, and increased inflammation is a common driver of many of the pathologies of older age, frequently termed “inflammaging” (Franceschi et al., 2007). Inflammatory stress may thus be a critical driver of CH. Indeed, a recent study showed that intestinal permeability and elevated inflammatory signaling are necessary for CH in a mouse model of somatic Tet2 mutation, demonstrating that interactions between genes and environment drive CH (Jaiswal et al., 2017; Meisel et al., 2018). On the other hand, not all environmental conditions affect genetically variant HSC clones the same way, and environmental drivers may be quite specific to certain CH clones. For example, PPM1D-mutant stem cells have been shown to have a clonal advantage only in the setting of chemotoxic stress (Figure 1) (Hsu et al., 2018). Thus, investigating potential environmental drivers of CH is likely to shed light on the role of certain driver mutations in HSC biology and provide insight into individual variation in CH emergence.
Aside from age, other environmental factors including former tobacco use or diseases that are linked to tobacco use such as lung cancer and chronic obstructive pulmonary disease are strongly associated with CH (Coombs et al., 2017), as is radiation exposure (Boucai et al., 2018). However, the full range of epidemiologic factors associated with CH is not known. The impact of these mutations on CH emergence likely depends on specific mutations and the mechanisms by which the two interact biologically.
In this article, we review our current understanding of the impact of environmental conditions, including inflammation, microbiome, and DNA damage due to various sources, on the emergence of CH. We propose that some types of clones will be particularly prone to expand under specific conditions and propose a framework for viewing the different types of drivers.
Inflammation as a driver of clonal hematopoiesis
The strong epidemiologic association of CH with age contributes to speculation that inflammation is a critical driver of this process. Inflammation increases with age, and is attributable to decayed regulatory mechanisms (Rea et al., 2018). For example, a recent study suggests that derepression of retrotransposable elements with age triggers interferon (IFN) responses that drive inflammation (De Cecco et al., 2019). These factors contribute to a wide variety of age-associated diseases including diabetes, Alzheimer’s, cardiovascular disease, and cancer.
In the blood system, genetic variants associated with pleiotropic peripheral blood counts are also associated with inflammatory and autoimmune conditions (Tajuddin et al., 2016). Inflammatory and autoimmune conditions are present in up to 25% of myelodysplastic syndrome (MDS) patients (Wolach and Stone, 2016), and a subset of MDS patients are highly sensitive to immunomodulatory medication (Glenthoj et al., 2016). Thus, inflammation is a well-known contributor to age-associated disease processes including in the hematopoietic system.
A significant and growing body of literature provides a conceptual understanding for how inflammation may contribute to clonal hematopoiesis. Infections are a common hematologic stress that generate inflammatory cytokines that impact bone marrow function and demand increased production of blood and immune cells. Numerous studies have demonstrated that infections promote HSC division and impair self-renewal. Increased stem cell division and hematopoietic progenitor prevalence have been recorded in the setting of a variety of infections including viruses such as lymphochoriomeningitis virus and cytomegalovirus, bacteria including mycobacteria, Ehrlichia (Baldridge et al., 2010; Smith et al., 2018), and parasites such as Plasmodium, which causes malaria (Vainieri et al., 2016). Infections may affect hematopoietic progenitors through pathogen-associated molecular patterns (PAMPs) such as LPS or TLR2 agonist (Herman et al., 2016; Takizawa et al., 2017) or cytokines induced during the infection, as previously reviewed (King and Goodell, 2011). Indeed, several studies have shown that cell division and differentiation programs in HSCs can be activated by inflammatory cytokines, including IFNα, IFNγ, IL1β, IL6, and TNFα (Essers et al., 2009; Pietras et al., 2016; Schürch et al., 2014; Yamashita and Passegue, 2019). Since infections naturally occur over the course of life, it is reasonable to view aging as a state of survival past an increasing number of infections.
The long-term consequences of infection and inflammatory signaling on HSCs can be severe. Indeed, excessive IFNγ has long been recognized to be an etiologic driver of acquired aplastic anemia (Nisticò and Young, 1994) whereas increased inflammation is also significantly associated with MDS (Barreyro et al., 2018). Where HSC activation and bone marrow dysfunction may at first appear contradictory, it is now recognized that stem cell divisions are often associated with a loss of self-renewal (Esplin et al., 2011). Indeed, an increase in the differentiation rate of HSCs leads to a loss of HSC reserves. Using a mouse model, we demonstrated that chronic infection depletes HSCs via excessive terminal differentiation (Matatall et al., 2016). Increased stress-induced apoptosis may also contribute to HSC loss (Pietras et al., 2014), whereas HSCs that survive long term inflammatory stress must do so by downregulating their responses to stress (Pietras et al., 2014). Whereas Rantes and CCL5 were previously reported to strongly influence HSC skewing with age (Ergen et al., 2012), the relative importance of various inflammatory cytokines in driving age-associated changes in hematopoiesis has yet to be fully defined (Kovtonyuk et al., 2016).
A variety of studies have demonstrated that individual subclasses of HSCs are differentially affected by inflammatory signaling. We showed that myeloid- versus lymphoid-biased HSCs respond differentially to IFNγ signaling (Matatall et al., 2014). In other studies, IFNα has been shown to preferentially stimulate a stem cell-like megakaryocyte progenitor (Haas et al., 2015), whereas histamine signaling affects a certain subclass of HSCs (Chen et al., 2017). Furthermore, a subclass of HSCs marked by CCR2 is activated to divide after the stress of myocardial infarction (Dutta et al., 2015). Collectively these studies indicate that not all HSCs are equally responsive to cytokine stress, leading to the concept that environmental conditions can provide a selection advantage to some subclasses over others. HSCs harboring CH-associated mutations may be considered as competing HSC subclasses, but there are already examples wherein a differential response to inflammation by these genetically variant HSCs leads to CH (Cai et al., 2018; Meisel et al., 2018).
Aside from age, epidemiologic factors associated with CH include smoking, smoking related-diseases, treatment of addiction, psychiatric disease, and chronic pulmonary disease, many or all of which are related to smoking (Zink et al., 2017). Smoking is strongly correlated with inflammation (Kianoush et al., 2017), but it remains to be determined whether smoking and inflammation contribute additively or synergistically to CH, or if they are one and the same.
Environmental stress and TET2 mutations in CH
Environmental impacts on Ten-eleven Translocation 2 (TET2)-mutant CH are particularly well studied. TET2 is an epigenetic modifier that is frequently mutated across myeloid malignancies. In CH, TET2 is the second most-frequently mutated gene, and growing evidence suggests that factors including secondary genetic alterations (Muto et al., 2013; Zhang et al., 2016), inflammation (Cai et al., 2018; Shen et al., 2018; Zhang et al., 2015) and changes in the microbiota (Meisel et al., 2018) may contribute to the clonal expansion and pre-leukemic condition of TET2-mutant hematopoietic stem and progenitor cells (HSPCs). Here, we will discuss the biological studies investigating environmental influences on HSPCs with TET2 loss-of-function (LOF) in CH.
The TET family of dioxygenases are able to successively oxidize 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) in the mammalian genome (He et al., 2011; Ito et al., 2011; Tahiliani et al., 2009). Among the three TET genes, somatic mutations of TET2 are the most frequently observed in individuals with CH (Busque et al., 2012; Jaiswal et al., 2014) and hematological malignancies (Couronne et al., 2012; Delhommeau et al., 2009; Langemeijer et al., 2009). Although TET2 mutations contribute to the selective advantage of HSPCs through increased self-renewal, not all the individuals with somatic TET2 mutations in HSPCs develop hematological neoplasms. In mouse models, Tet2 ablation leads to variable outcomes. Some studies documented only mild phenotypes with increased HSPC self-renewal and myeloid bias in Tet2 knockout mice (Ko et al., 2011; Moran-Crusio et al., 2011). However, several other groups reported that Tet2-deficient mice displayed hypermutagenicity and developed myeloid or lymphoid malignancies (Li et al., 2011; Pan et al., 2017; Quivoron et al., 2011).
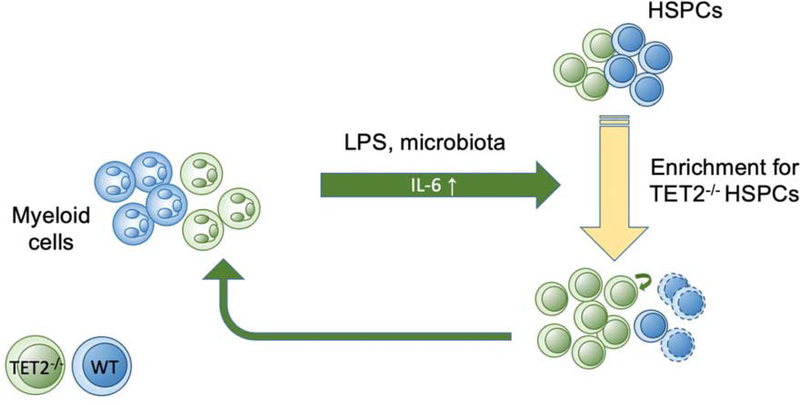
Multiple reports have shown that TET2-deficient HSPCs tend to expand relative to WT HSPCs in response to environmental stress, such as inflammation arising from stress stimulation or pathogenic organisms. In the bone marrow, Tet2-ablated HSPCs exhibited strong proliferation advantages and myeloid bias in response to lipopolysaccharide (LPS)-induced acute inflammation (Cai et al., 2018). Mechanistically, Tet2-deficient HSPCs tend to produce high levels of proinflammatory cytokines, such as interleukin-6 (IL-6), to maintain HSPC survival and suppress apoptosis through the upregulation of a novel anti-apoptotic long non-coding RNA, Morrbid during inflammation (Cai et al., 2018). In parallel, the progeny of Tet2-deficient HSPCs, particularly innate myeloid cells, also produce high levels of IL-6 during LPS challenge (Zhang et al., 2015). The upregulation of IL-6 in Tet2 deficient myeloid cells is due to the loss of transcriptional suppression at the Il6 promoter mediated by HDAC2. The up-regulation of IL-6 in Tet2-deficient innate myeloid cells might evoke a positive feedback to HSPCs during infection-induced inflammation and further promote the expansion of HSPCs to cause CH (Figure 2).
Figure 2.
Positive feedback loop between TET2-deficient HSPCs and their innate myeloid cell progeny during the response to pathogen-associated molecular patterns. Inflammatory cytokines such as IL-6 are excessively produced by TET2-deficient myeloid cells, leading to further expansion of the HSPC compartment and perpetuation of the cycle.
Interestingly, increased IL-6 production is also observed in microbiota-dependent inflammation in a Tet2-deficient mouse model. Meisel et al showed increased intestinal permeability and spontaneous bacterial translocation (e.g., Lactobacillus) into the blood of Tet2-deficient mice, thereby resulting in an increase of plasma and spleen IL-6 levels (Meisel et al., 2018). These studies provide strong evidence to support a positive feedback loop between Tet2-deficient HSPCs and their progeny innate myeloid cells during the response to inflammation-induced cytokine production (Figure 2). Given that myeloid cells derived from Tet2-deficient HSPCs can exert non-cell-autonomous effects on HSPCs, it is interesting to speculate whether such cells could also promote expansion of HSPC clones carrying other mutations. In other words, would presence of a Tet2-deficient clone which produces Tet2-deficient macrophages accelerate the expansion of a Dnmt3a-mutant clone? Since people are likely to harbor a variety of genetically variant HSC clones, such cross-cutting effects may exist.
TET2-mutant HSPCs produce immune cells that contribute to abnormal adaptive and innate immune responses not only in the hematopoietic system, but also in the peripheral tissues. Indeed, it has been reported that Tet2-KO mice displayed worse tissue damage in both lung and gut after immune challenge (Zhang et al., 2015). Furthermore, previous reports point to a strong correlation between TET2 mutations and cardiovascular disease progression (Jaiswal et al., 2017). The causal relationship between Tet2 deletion in HSPCs and the increased risk of cardiovascular diseases has been further demonstrated in animal models (Fuster et al., 2017; Sano et al., 2018). Mechanistically, deletion of Tet2 in HSPCs leads to the upregulation of NLRP3 inflammasome and IL1▪ production in myeloid cells, thereby leading to increased plaque sizes and impaired cardiac repair. TET2 mutations are also detected in patients with COPD/asthma and neurodegenerative disorders (Buscarlet et al., 2017; Keogh et al., 2018), but the causal relationship between TET2 mutations and these diseases are yet to be defined.
Clonal hematopoiesis driven by chemotoxic exposures
While epigenetic regulators such as DNMT3A and TET2 sit at the top of the list for genes commonly mutated in clonal hematopoiesis, there are also a number of CH genes that are involved in DNA repair. The selective advantage their mutations impart is likely through entirely different mechanisms. Among this class of genes are PPM1D and TP53 (Coombs et al., 2017). PPM1D mutations are among the top ten CH mutations in individuals with an identified driver, representing on the order of 5% of CH cases. PPM1D mutations have recently been studied in some depth and likely represent a paradigm for this class of mutations.
PPM1D had not been described as a major participant in hematopoiesis previously so its frequent mutation in CH was of particular interest. PPM1D is a protein phosphatase that acts to down-modulate the DNA damage response by de-phosphorylating p53, ATM, CHEK1 and other damage response proteins(Le Guezennec and Bulavin, 2010). PPM1D mutations are typically clustered in the C-terminus of the protein and result in a truncated and highly stabilized protein. This stabilized protein results in an enhanced phosphatase activity that constitutively reduces the stress response. Normally, cells exposed to chemotoxic stress will pause to allow DNA repair to occur, with many cells undergoing apoptosis. However, cells with the PPM1D mutations are less sensitive to stress, and exhibit a lower rate of apoptosis(Hsu et al., 2018; Kahn et al., 2018). The net result of their stress resistance is that HSCs bearing a PPM1D mutation are more resistant to chemotherapeutic insults than WT cells. While chemotoxic treatment results in apoptosis in both WT and mutant cells, the rate of cell death in mutant cells is lower. At the end of each round of chemotherapy, a greater proportion of mutant cells have survived compared to WT cells. We found that this larger number of surviving cells, even if relatively small, was sufficient to give a competitive advantage to the mutant cells in the context of repeated rounds of chemotherapy.
In mice, when Ppm1d-mutant stem cells were transplanted in competition with WT cells, they were able to engraft and contribute to blood production with equivalent efficiency. However, when mice were exposed to DNA damaging agents, the mutant cells quickly out-competed their WT counterparts, and affect that was maintained for months after cessation of chemotherapy treatment. However, not all types of stress gave the PPM1D mutant cells a selective advantage; DNA damaging agents were the most powerful, while stress such as serial transplantation did not offer any advantage to the mutant cells. While PPM1D mutant cells may exhibit a slight proliferative advantage (Kahn et al., 2018), a moderate difference in apoptosis can explain most of the differential expansion of PPM1D mutant cells in the blood.
These data can be extrapolated to explain at least some of the presence of PPM1D mutations in individuals with CH. In patients who have previously undergone chemotherapy for solid tumors, CH with PPM1D mutations is much more prevalent than without such exposures (Coombs et al.). Similarly, in therapy-related AML patients who have been exposed to DNA damaging agents, PPM1D mutations are particularly common (Hsu et al., 2018). Notably, PPM1D mutations do show up in the general population with CH (Genovese et al., 2014). It is possible that these individuals represent those in the general population who have been exposed to chemotherapy or other stresses, or that PPM1D mutations offer advantages in some additional contexts that are yet to be identified.
Diet, metabolism, and clonal hematopoiesis
While the effects of diet or metabolic milieu on CH remain to be studied, obesity and metabolic syndromes are known to influence the fate of HSPCs. Specifically, obesity and metabolic syndromes enhance myelopoiesis. Both hyperglycemia in type 1 diabetes models and obesity caused by high-fat diet increase myeloid progenitors and myelopoiesis (Lee et al., 2018; Luo et al., 2015; Nagareddy et al., 2013; Singer et al., 2014). These metabolic disorders affect myelopoiesis largely through cell extrinsic mechanisms involving the HSC niche or by causing an inflammatory state.
HSCs reside in the bone marrow niche consisting of several cell types such as endothelial cells and bone marrow mesenchymal stromal cells (BMSCs), which have the capacity to differentiate into adipocytes, osteoblast, and chondrocytes (Morrison and Scadden, 2014). Obesity not only expands subcutaneous and visceral fat mass, it also promotes differentiation of BMSCs into adipocytes (Ambrosi et al., 2017). Increased marrow adipocytes, in turn, reduce the number of HSCs (Ambrosi et al., 2017; Hu et al., 2019; Naveiras et al., 2009). In contrast, a recent study suggests that exercise reduces leptin, a hormone that governs energy expenditure, and reduction of leptin instructs BMSCs to express HSC niche factors to promote HSC quiescence (Frodermann et al., 2019). These studies illuminate the links between metabolic and physical conditions to the bone marrow microenvironment to support HSCs, with obesity and exercise negatively and positively affecting HSCs, respectively.
Obesity-induced changes in the microbiome have also been shown to alter the HSC niche and promote myelopoiesis (Luo et al., 2015; Yan et al., 2018). Whether these changes in the microbiome are responsible for age-related changes in HSCs and their niche, leading to the emergence of CH, remains to be tested. Thus, whether by regulation of adipocytes or through microbiome-related changes, the net effect of obesity is to reduce the HSC population. It is therefore tempting to speculate that some CH mutations confer mutant HSCs with resistance against the negative pressure imposed by fatty marrow in obese or aged populations.
Additionally, metabolic syndromes may promote CH indirectly by causing chronic inflammation. Obesity is a state of chronic inflammation characterized by expansion of proinflammatory immune cells in adipose tissues (Reilly and Saltiel, 2017). Adipocytes themselves also regulate inflammation by secreting a proinflammatory cytokine leptin and an anti-inflammatory cytokine adiponectin, the expression of which are increased and decreased in adipocytes of obese subjects, respectively. Obesity has been shown to increase intestinal permeability, causing a systemic endotoxemia state (Cani et al., 2008). Additionally, hyperglycemia in a mouse model of type 1 diabetes caused neutrophils to produce a sterile inflammatory signal S100A8/S100A9, which then instructed myeloid progenitor cells to secrete myelopoietic cytokines such as M-CSF (Nagareddy et al., 2013).The resulting pro-inflammatory state was characterized by increased secretion of cytokines such as TGFβ, IL-1, and IFNS, all of which act on HSPCs as discussed above.
The effects of metabolic syndromes on promoting myelopoiesis parallel the observation that HSCs and myeloid progenitors with CH mutations exhibit preferential expansion compared to lymphocytes (Arends et al., 2018). Intriguingly, some individuals with CH show preferential expansion of myeloid progenitors over the more immature HSCs, suggesting that some mutations, environmental selective pressure, or the combination of both may encourage clonal expansion of committed progenitors. It should be noted that neither diet nor metabolic syndromes have been demonstrated to be epidemiological factors associated with CH, and the links remains speculative. Nevertheless, identification of the mutations that allow CH clones to expand in the proinflammatory conditions associated with metabolic syndromes may lead to strategies to assess the risk of developing CH or to prevent the expansion of such clones in people with metabolic syndromes.
Unanswered questions and future directions
CH represents a pre-malignant status that provides an excellent opportunity to monitor patients for the early stages of malignant development. Current studies are focused on the genetic defects in CH. However, accumulating evidence suggests a strong functional interplay between genetic defects and environmental factors to promote the clonal expansion of HSPCs. Here we discussed several examples of how environmental cues, such as inflammatory stress, chemotherapy, or diet and metabolites, influence the clonal expansion of subsets of HSPCs bearing specific genetic defects. Based on these studies, we hope that environmental factors will be taken into consideration in addition to genetic defects as critical contributors to the pathogenesis of CH. From a clinical perspective, the lifestyle of the individual is likely to impact risk assessment during CH management. For the population with a high risk to develop CH, intervening in environment cues might provide an opportunity to reduce or prevent CH progression. In addition, many other environmental influences, such as anti-cancer radiation treatment, psychosocial stress, toxin exposure, and cardiac myocardial infarction, are yet to be fully explored with respect to their impact on CH. Further systematic studies are needed to elucidate the underlying mechanisms.
Highlights.
Environmental factors influence development of clonal hematopoiesis.
Inflammation exerts a strong influence on hematopoietic stem cell biology and may serve as a unifying driver of CH.
Obesity, hyperglycemia, and the microbiome affect hematopoietic stem cell responses and may contribute to CH.
Acknowledgements
This work was supported by grants from National Institute of Health grants (R01HL136333 and R01HL134880 to KYK, R01HL134780 and R01HL146852 to YH, R01CA193235 and R01DK107413 to DN) the American Cancer Society (RSG-18-043-01-LIB to YH). This work was also supported by 1CA237291, CA183252, DK092883, AG036695, and the Edward P. Evans Foundation (MAG). DN is a scholar of the Leukemia and Lymphoma Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schurmann A, et al. (2017). Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 20, 771–784 e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends CM, Galan-Sousa J, Hoyer K, Chan W, Jager M, Yoshida K, Seemann R, Noerenberg D, Waldhueter N, Fleischer-Notter H, et al. (2018). Hematopoietic lineage distribution and evolutionary dynamics of clonal hematopoiesis. Leukemia 32, 1908–1919. [DOI] [PubMed] [Google Scholar]
- Baldridge MT, King KY, Boles NC, Weksberg DC, and Goodell MA (2010). Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature 465, 793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreyro L, Chlon TM, and Starczynowski DT (2018). Chronic immune response dysregulation in MDS pathogenesis. Blood 132, 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucai L, Falcone J, Ukena J, Coombs CC, Zehir A, Ptashkin R, Berger MF, Levine RL, and Fagin JA (2018). Radioactive Iodine-Related Clonal Hematopoiesis in Thyroid Cancer Is Common and Associated With Decreased Survival. J Clin Endocrinol Metab 103, 4216–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RL, Busque L, and Levine RL (2018). Clonal Hematopoiesis and Evolution to Hematopoietic Malignancies. Cell Stem Cell 22, 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscarlet M, Provost S, Zada YF, Barhdadi A, Bourgoin V, Lepine G, Mollica L, Szuber N, Dube M-P, and Busque L (2017). DNMT3Aand TET2dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood 130, 753–762. [DOI] [PubMed] [Google Scholar]
- Busque L, Patel JP, Figueroa ME, Vasanthakumar A, Provost S, Hamilou Z, Mollica L, Li J, Viale A, Heguy A, et al. (2012). Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nature Publishing Group 44, 1179–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Kotzin JJ, Ramdas B, Chen S, Nelanuthala S, Palam LR, Pandey R, Mali RS, Liu Y, Kelley MR, et al. (2018). Inhibition of Inflammatory Signaling in Tet2 Mutant Preleukemic Cells Mitigates Stress-Induced Abnormalities and Clonal Hematopoiesis. Cell Stem Cell 23, 833–849 e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, and Burcelin R (2008). Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481. [DOI] [PubMed] [Google Scholar]
- Chen X, Deng H, Churchill MJ, Luchsinger LL, Du X, Chu TH, Friedman RA, Middelhoff M, Ding H, Tailor YH, et al. (2017). Bone Marrow Myeloid Cells Regulate Myeloid-Biased Hematopoietic Stem Cells via a Histamine-Dependent Feedback Loop. Cell Stem Cell 21, 747–760.e747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, Hyman DM, Solit DB, Robson ME, Baselga J, et al. (2017). Therapy-Related Clonal Hematopoiesis in Patients with Non-hematologic Cancers Is Common and Associated with Adverse Clinical Outcomes. Cell Stem Cell 21, 374–382 e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couronne L, Bastard C, and Bernard OA (2012). TET2 and DNMT3A mutations in human T-cell lymphoma. N Engl J Med 366, 95–96. [DOI] [PubMed] [Google Scholar]
- De Cecco M, Ito T, Petrashen AP, Elias AE, Skvir NJ, Criscione SW, Caligiana A, Brocculi G, Adney EM, Boeke JD, et al. (2019). L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, Kosmider O, Le Couedic JP, Robert F, Alberdi A, et al. (2009). Mutation in TET2 in myeloid cancers. N Engl J Med 360, 22892301. [DOI] [PubMed] [Google Scholar]
- Dutta P, Sager HB, Stengel KR, Naxerova K, Courties G, Saez B, Silberstein L, Heidt T, Sebas M, Sun Y, et al. (2015). Myocardial Infarction Activates CCR2(+) Hematopoietic Stem and Progenitor Cells. Cell Stem Cell 16, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergen AV, Boles NC, and Goodell MA (2012). Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood 119, 2500–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, Humphrey MB, Yang Q, Borghesi LA, and Kincade PW (2011). Chronic Exposure to a TLR Ligand Injures Hematopoietic Stem Cells. The Journal of Immunology 186, 5367–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers MAG, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, and Trumpp A (2009). IFNα activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, et al. (2007). Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128, 92–105. [DOI] [PubMed] [Google Scholar]
- Frodermann V, Rohde D, Courties G, Severe N, Schloss MJ, Amatullah H, McAlpine CS, Cremer S, Hoyer FF, Ji F, et al. (2019). Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, et al. (2017). Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, et al. (2014). Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. New England Journal of Medicine 371, 2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenthoj A, Orskov AD, Hansen JW, Hadrup SR, O’Connell C, and Gronbaek K (2016). Immune Mechanisms in Myelodysplastic Syndrome. Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas S, Hansson J, Klimmeck D, Loeffler D, Velten L, Uckelmann H, Wurzer S, Prendergast ÁM, Schnell A, Hexel K, et al. (2015). Inflammation-Induced Emergency Megakaryopoiesis Driven by Hematopoietic Stem Cell-like Megakaryocyte Progenitors. Stem Cell 17, 422–434. [DOI] [PubMed] [Google Scholar]
- He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, et al. (2011). Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 333, 1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AC, Monlish DA, Romine MP, Bhatt ST, Zippel S, and Schuettpelz LG (2016). Systemic TLR2 agonist exposure regulates hematopoietic stem cells via cell-autonomous and cell-nonautonomous mechanisms. Blood cancer journal 6, e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JI, Dayaram T, Tovy A, De Braekeleer E, Jeong M, Wang F, Zhang J, Heffernan TP, Gera S, Kovacs JJ, et al. (2018). PPM1D Mutations Drive Clonal Hematopoiesis in Response to Cytotoxic Chemotherapy. Cell Stem Cell 23, 700–713.e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Kitano A, Luu V, Dawson B, Hoegenauer KA, Lee BH, and Nakada D (2019). Bmi1 Suppresses Adipogenesis in the Hematopoietic Stem Cell Niche. Stem Cell Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, and Zhang Y (2011). Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 333, 1300–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. (2014). Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371, 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. (2017). Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med 377, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JD, Miller PG, Silver AJ, Sellar RS, Bhatt S, Gibson C, McConkey M, Adams D, Mar B, Mertins P, et al. (2018). PPM1D-truncating mutations confer resistance to chemotherapy and sensitivity to PPM1D inhibition in hematopoietic cells. Blood 132, 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh MJ, Wei W, Aryaman J, Walker L, van den Ameele J, Coxhead J, Wilson I, Bashton M, Beck J, West J, et al. (2018). High prevalence of focal and multi-focal somatic genetic variants in the human brain. Nat Commun 9, 4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianoush S, Yakoob MY, Al-Rifai M, DeFilippis AP, Bittencourt MS, Duncan BB, Bensenor IM, Bhatnagar A, Lotufo PA, and Blaha MJ (2017). Associations of Cigarette Smoking With Subclinical Inflammation and Atherosclerosis: ELSA-Brasil (The Brazilian Longitudinal Study of Adult Health). Journal of the American Heart Association 6, 30483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KY, and Goodell MA (2011). Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nature Publishing Group, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M, Bandukwala HS, An J, Lamperti ED, Thompson EC, Hastie R, Tsangaratou A, Rajewsky K, Koralov SB, and Rao A (2011). Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc Natl Acad Sci U S A 108, 14566–14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtonyuk LV, Fritsch K, Feng X, Manz MG, and Takizawa H (2016). Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment. Frontiers in Immunology 7, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langemeijer SM, Kuiper RP, Berends M, Knops R, Aslanyan MG, Massop M, Stevens-Linders E, van Hoogen P, van Kessel AG, Raymakers RA, et al. (2009). Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet 41, 838–842. [DOI] [PubMed] [Google Scholar]
- Le Guezennec X, and Bulavin DV (2010). WIP1 phosphatase at the crossroads of cancer and aging. Trends in biochemical sciences 35, 109–114. [DOI] [PubMed] [Google Scholar]
- Lee J-M, Govindarajah V, Goddard B, Hinge A, Muench DE, Filippi M-D, Aronow B, Cancelas JA, Salomonis N, Grimes HL, et al. (2018). Obesity alters the long-term fitness of the hematopoietic stem cell compartment through modulation of Gfi1 expression. The Journal of Experimental Medicine 215, 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Cai X, Cai C, Wang J, Zhang W, Petersen BE, Yang FC, and Xu M (2011). Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh P-R, Genovese G, Handsaker RE, Finucane HK, Reshef YA, Palamara PF, Birmann BM, Talkowski ME, Bakhoum SF, McCarroll SA, et al. (2018). Insights into clonal haematopoiesis from 8,342 mosaic chromosomal alterations. Nature 559, 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Chen GL, Hannemann N, Ipseiz N, Kronke G, Bauerle T, Munos L, Wirtz S, Schett G, and Bozec A (2015). Microbiota from Obese Mice Regulate Hematopoietic Stem Cell Differentiation by Altering the Bone Niche. Cell Metab 22, 886–894. [DOI] [PubMed] [Google Scholar]
- Matatall KA, Jeong M, Chen S, Sun D, Chen F, Mo Q, Kimmel M, and King KY (2016). Chronic Infection Depletes Hematopoietic Stem Cells through Stress-Induced Terminal Differentiation. CellReports 17, 2584–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matatall KA, Shen C-C, Challen GA, and King KY (2014). Type II Interferon Promotes Differentiation of Myeloid-Biased Hematopoietic Stem Cells. Stem Cells 32, 3023–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel M, Hinterleitner R, Pacis A, Chen L, Earley ZM, Mayassi T, Pierre JF, Ernest JD, Galipeau HJ, Thuille N, et al. (2018). Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature 557, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, et al. (2011). Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer cell 20, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, and Scadden DT (2014). The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto T, Sashida G, Oshima M, Wendt GR, Mochizuki-Kashio M, Nagata Y, Sanada M, Miyagi S, Saraya A, Kamio A, et al. (2013). Concurrent loss of Ezh2 and Tet2 cooperates in the pathogenesis of myelodysplastic disorders. J Exp Med 210, 2627–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, et al. (2013). Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 17, 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, and Daley GQ (2009). Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisticò A, and Young NS (1994). gamma-Interferon gene expression in the bone marrow of patients with aplastic anemia. Annals of internal medicine 120, 463–469. [DOI] [PubMed] [Google Scholar]
- Pan F, Wingo TS, Zhao Z, Gao R, Makishima H, Qu G, Lin L, Yu M, Ortega JR, Wang J, et al. (2017). Tet2 loss leads to hypermutagenicity in haematopoietic stem/progenitor cells. Nat Commun 8, 15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras EM, Lakshminarasimhan R, Techner J-M, Fong S, Flach J, Binnewies M, and Passegué E (2014). Re-entry into quiescence protects hematopoietic stem cells from the killing effect of chronic exposure to type I interferons. The Journal of Experimental Medicine 211, 245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, Lakshminarasimhan R, Chin CP, Techner J-M, Will B, et al. (2016). Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nature Cell Biology 18, 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivoron C, Couronne L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, Do Cruzeiro M, Delhommeau F, Arnulf B, Stern MH, et al. (2011). TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer cell 20, 25–38. [DOI] [PubMed] [Google Scholar]
- Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, and Ross OA (2018). Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front Immunol 9, 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly SM, and Saltiel AR (2017). Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 13, 633–643. [DOI] [PubMed] [Google Scholar]
- Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, et al. (2018). Tet2-Mediated Clonal Hematopoiesis Accelerates Heart Failure Through a Mechanism Involving the IL-1beta/NLRP3 Inflammasome. J Am Coll Cardiol 71, 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürch CM, Riether C, and Ochsenbein AF (2014). Cytotoxic CD8+ T Cells Stimulate Hematopoietic Progenitors by Promoting Cytokine Release from Bone Marrow Mesenchymal Stromal Cells. Stem Cell, 1–13. [DOI] [PubMed] [Google Scholar]
- Shen Q, Zhang Q, Shi Y, Shi Q, Jiang Y, Gu Y, Li Z, Li X, Zhao K, Wang C, et al. (2018). Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature 554, 123–127. [DOI] [PubMed] [Google Scholar]
- Singer K, DelProposto J, Morris DL, Zamarron B, Mergian T, Maley N, Cho KW, Geletka L, Subbaiah P, Muir L, et al. (2014). Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Molecular metabolism 3, 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JNP, Zhang Y, Li JJ, McCabe A, Jo HJ, Maloney J, and MacNamara KC (2018). Type I IFNs drive hematopoietic stem and progenitor cell collapse via impaired proliferation and increased RIPK1-dependent cell death during shock-like ehrlichial infection. PLoS Pathogens 14, e1007234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensma DP (2018). Clinical Implications of Clonal Hematopoiesis. Mayo Clin Proc 93, 1122–1130. [DOI] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, et al. (2009). Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajuddin SM, Schick UM, Eicher JD, Chami N, Giri A, Brody JA, Hill WD, Kacprowski T, Li J, Lyytikainen LP, et al. (2016). Large-Scale Exome-wide Association Analysis Identifies Loci for White Blood Cell Traits and Pleiotropy with Immune-Mediated Diseases. Am J Hum Genet 99, 22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa H, Fritsch K, Kovtonyuk LV, Saito Y, Yakkala C, Jacobs K, Ahuja AK, Lopes M, Hausmann A, Hardt W-D, et al. (2017). Pathogen-Induced TLR4-TRIF Innate Immune Signaling in Hematopoietic Stem Cells Promotes Proliferation but Reduces Competitive Fitness. Cell Stem Cell 21, 225–240.e225. [DOI] [PubMed] [Google Scholar]
- Vainieri ML, Blagborough AM, MacLean AL, Haltalli ML, Ruivo N, Fletcher HA, Stumpf MP, Sinden RE, and Celso CL (2016). Systematic tracking of altered haematopoiesis during sporozoite-mediated malaria development reveals multiple response points. Open Biol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolach O, and Stone R (2016). Autoimmunity and Inflammation in Myelodysplastic Syndromes. Acta haematologica 136, 108–117. [DOI] [PubMed] [Google Scholar]
- Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, Schmidt HK, Yellapantula V, Miller CA, et al. (2014). Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nature Medicine 20, 1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, and Passegue E (2019). TNF-alpha Coordinates Hematopoietic Stem Cell Survival and Myeloid Regeneration. Cell Stem Cell 25, 357–372 e357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Baldridge MT, and King KY (2018). Hematopoiesis and the bacterial microbiome. Blood 132, 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AL, Challen GA, Birmann BM, and Druley TE (2016). Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun 7, 12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, Zhao D, Liu Y, Wang C, Zhang X, et al. (2015). Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Su J, Jeong M, Ko M, Huang Y, Park HJ, Guzman A, Lei Y, Huang YH, Rao A, et al. (2016). DNMT3A and TET2 compete and cooperate to repress lineage-specific transcription factors in hematopoietic stem cells. Nat Genet 48, 1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, Thorgeirsson TE, Sigurdsson A, Gudjonsson SA, Gudmundsson J, et al. (2017). Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 130, 742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]