Central Message
DCD heart donation has the potential to significantly increase the pool of available donor allografts in the US, by as much as 30%, and will lead to a substantial decrease in waiting list mortality.
Heart transplantation remains the gold standard therapy for patients with advanced heart failure; however, the utilization of heart transplantation is constrained by the limited supply of donor organs. To further expand the pool of available organ donors, there has recently been renewed interest in the use of donation after circulatory death (DCD) donors for heart transplantation. Kidney, liver, pancreas, and lung transplants are currently being performed in the US with organs procured from these donors; however, there are no DCD heart transplants being performed. Programs in Europe and Australia, however, have adopted selective DCD donation for heart transplantation and report short-term survival comparable to those obtained with use of donation after brain death (DBD) donors [1–3]. The aim of this study was to estimate the potential maximal increase in the US heart transplant donor pool should selective DCD heart transplantation be adopted using a large national heart transplantation registry. This study was deemed exempt by Duke University’s Institutional Review Board.
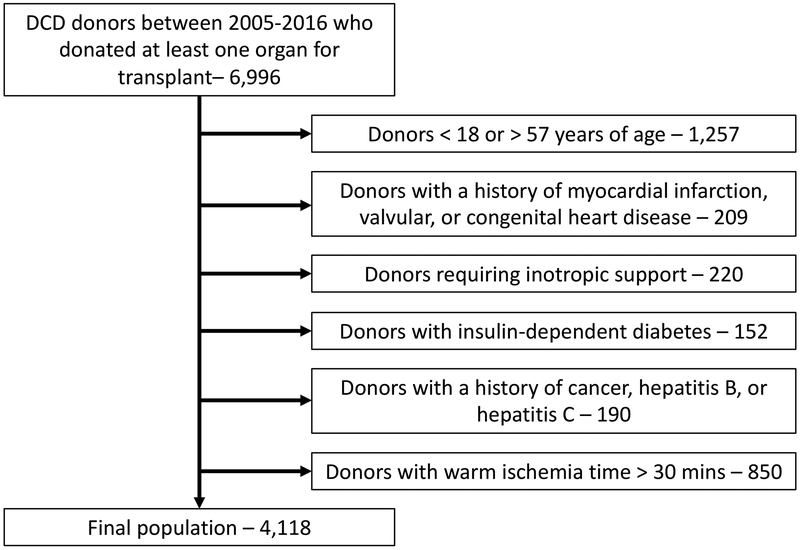
The 2005–2014 United Network for Organ Sharing (UNOS) deceased donor database was queried for all controlled (Maastricht III) DCD donors who donated at least one organ for transplant. The criteria for DCD heart transplant donor selection as published in 2017 by Messer and colleagues from Papworth Hospital in the United Kingdom were then applied to the pool of US DCD donors [1]. This included donor age 18–57 without a history of myocardial infarction, insulin dependent diabetes, hepatitis B and C, or valvular or congenital heart disease. While data regarding active malignancy, malignant melanoma, secondary intracerebral tumors, and intracerebral lymphoma were not specifically available in the UNOS registry, all donors with a history of cancer were excluded. Further, a 30-minute limit for warm ischemia time was applied, consistent with the window determined to be safest in porcine models of DCD heart transplantation [4].
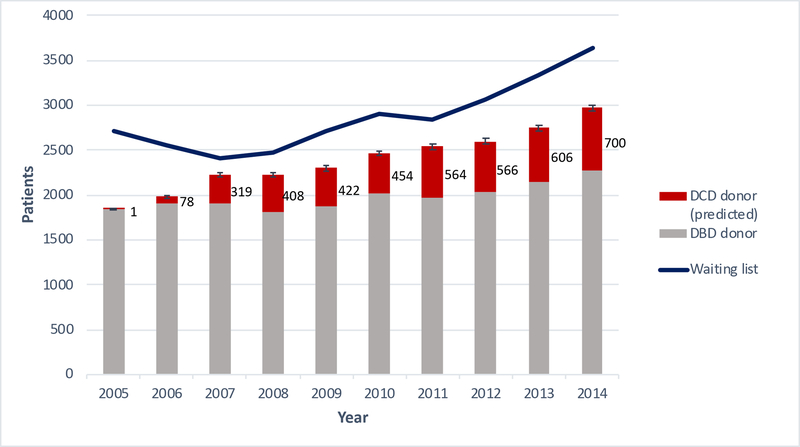
After application of the Messer criteria to the 2005–2014 pool of US DCD donors, a total of 4,118 potential DCD heart transplant donors were identified (Figure 1). Of these donors, 68.0% (n=2,800) were male and the median age was 40 (IQR 27–48). The cause of death was anoxia, cerebrovascular/stroke, and head trauma for 39.2%, 18.6%, and 37.3% of donors, respectively. 96.5% (n=3,972) donated at least one kidney for transplant, 40.4% (n=1,665) donated a liver for transplant, and 2.7% (n=112) donated at least one lung for transplant. The number of potential DCD heart transplant donors increased each year, with 700 donors identified in 2014, alone (Figure 2). This equates to an approximately 30% increase in adult heart transplants that could have been performed in 2014, which is comparable to the increase in the UK heart transplant donor pool as projected by Messer and colleagues in their 2015 study [5]. These data suggest that the DCD donor pool may be growing faster than the DBD pool. This is the first study, to our knowledge, that quantifies the potential expansion of the donor pool with use of DCD organs for heart transplantation in the US and demonstrates a significant potential decrease in waiting list mortality.
Figure 1.
Application of the 2017 Messer DCD heart transplant donor selection criteria to the US DCD donor pool.
Figure 2.
Predicted suitable DCD heart transplants (red) using 2017 Messer criteria and annual number of DBD heart transplants (gray). End-of-year waiting list volume (blue) provided by United Network for Organ Sharing (UNOS). Error bars indicate 95% confidence interval, estimated assuming binomial distribution and 65% DCD heart utilization rate from 2017 Messer study. DBD, donation after brain death; DCD, donation after circulatory death.
It should be noted, however, that a proportion of DCD hearts will be deemed unsuitable for transplant after a functional assessment is performed, which cannot be accounted for in this retrospective registry review. In their 2017 study, Messer and colleagues noted ~11% (2/18) of hearts instrumented using ex vivo perfusion were discarded for cardiac-specific reasons (rising lactate and left ventricular hypertrophy) [1]. Chew and colleagues reported discarding ~21% (7/33) of hearts (3 additional hearts were discarded, however, the investigators state that these organs would have been transplanted under their updated protocol) [3]. Therefore, assuming generalizability of these experiences, our projected DCD heart transplantation maximum is likely an overestimate by at least 10–20%.
Limitations of this study include the retrospective nature of the analysis using registry data and the resulting lack of availability of several variables of interest. This includes donor ejection fraction (EF), which is missing for DCD donors in the UNOS database since these donors have not been used for heart transplantation in the US previously and thus do not undergo a full cardiovascular evaluation. EF must be greater than 50% prior to withdrawal of life-sustaining measures according to European criteria, although greater than 89% of comparable DBD donors in the UNOS database met this requirement. In addition, donors requiring epinephrine, or dobutamine infusions as well as norepinephrine >0.3 μg/kg/min were excluded from the Messer study, however this degree of granularity pertaining to donor support was not available in the UNOS database. Overall inotropic support was available for analysis, however, it was only required for 5 donors in the identified cohort. Next, while we utilized the published criteria by Messer and colleagues indicating an acceptable donor age range of 18–57, the Messer study ultimately only included donors age 38 and below. Similarly, Chew and colleagues utilized a donor age cutoff of 40 [3]. Applying the Chew criteria (age less than 40 without a history of cardiac disease with a warm ischemia time less than 30 minutes) to the same UNOS dataset, we would have identified 2,860 potential DCD heart donors from 2006–2014, 493 of which in the year 2014, still representing a significant increase in the donor pool. Lastly, the purpose of this study was to estimate the potential maximal increase in the donor pool should all eligible DCD donors donate a heart for transplant. In reality, the introduction of DCD heart transplantation in the US would likely be adopted gradually and it would take several years to maximally utilize the DCD donor allograft pool.
In conclusion, the utilization of DCD heart donation has the potential to significantly increase the pool of available donor heart allografts in the United States, especially with the introduction of normothermic ex vivo perfusion techniques. Adoption of DCD heart transplantation in the US could result in a substantial decrease in waiting list mortality. Further research is needed to understand and optimize the use of DCD organs in heart transplantation in the US.
Funding:
Funding for this study was provided by NIH T-32 grant 5T32HL069749
Footnotes
Disclosures: The authors have nothing to disclose
References
- 1.Messer S, et al. , Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant, 2017. 36(12): p. 1311–1318. [DOI] [PubMed] [Google Scholar]
- 2.Dhital KK, et al. , Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet, 2015. 385(9987): p. 2585–91. [DOI] [PubMed] [Google Scholar]
- 3.Chew HC, et al. , Outcomes of Donation After Circulatory Death Heart Transplantation in Australia. J Am Coll Cardiol, 2019. 73(12): p. 1447–1459. [DOI] [PubMed] [Google Scholar]
- 4.White CW, et al. , Transplantation of Hearts Donated after Circulatory Death. Front Cardiovasc Med, 2018. 5: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messer S, et al. , The Potential of Transplanting Hearts From Donation After Circulatory Determined Death (DCD) Donors Within the United Kingdom. Journal of Heart and Lung Transplantation, 2015. 34(4): p. S275–S275. [Google Scholar]