Abstract
Factors contributing to donor-specific HLA antibody (DSA) development after lung transplantation have not been systematically evaluated. We hypothesized that the isolation of Pseudomonas aeruginosa in respiratory specimens would increase the risk of DSA development. Our objective was to determine the risk of DSA development associated with the isolation of Pseudomonas aeruginosa after lung transplantation. We conducted a single-center retrospective cohort study of primary lung transplant recipients and examined risk factors for DSA development using Cox regression models. Of 460 recipients, 205 (45%) developed DSA; the majority developed Class II DSA (n = 175, 85%), and 145 of 205 (71%) developed DSA to HLA-DQ alleles. Univariate time-dependent analyses revealed that isolation of Pseudomonas from respiratory specimens, acute cellular rejection and lymphocytic bronchiolitis are associated with an increased risk of DSA development. In multivariable analyses, Pseudomonas isolation, acute cellular rejection, and lymphocytic bronchiolitis remained independent risk factors for DSA development. Additionally, there was a direct association between the number of positive Pseudomonas cultures and the risk of DSA development. Our findings suggest that proinflammatory events including acute cellular rejection, lymphocytic bronchiolitis, and Pseudomonas isolation after transplantation are associated with an increased risk of DSA development.
1. INTRODUCTION
Lung transplantation is the ultimate treatment for patients with advanced lung disease. However, long-term outcomes remain disappointing, and the median survival after transplantation is 6.5 years 1. Chronic lung allograft dysfunction (CLAD) is the leading cause of death beyond the first year after transplantation and is attributed to immune-mediated injury 1-4. The development of donor specific antibodies (DSA) to mismatched human leukocyte antigens (HLA) has been increasingly recognized as an independent risk factor for CLAD and death 5-11. Furthermore, multiple studies have reported a high incidence of DSA after lung transplantation 10,11. Indeed, in a prospective multicenter observational study, 36% of patients developed DSA within 4 months of transplantation 12. These data underscore the role of humoral immune responses in lung allograft rejection. Previous studies have identified risk factors for the development of DSA including pre-transplant allosensitization, re-transplantation, the lung allocation score (LAS) and primary graft dysfunction (PGD) 12-15. The associations of LAS and PGD with DSA suggest that pro-inflammatory events early after transplantation increase the risk of DSA development. However, these studies have not examined the role of infection as a risk factor for the development of DSA, yet infections are common complications after lung transplantation and have been linked to the development of CLAD 16-18. Emerging data support the paradigm that infections may augment or trigger alloimmune responses that promote rejection 19. Although various mechanisms have been proposed to explain this association, most of which involve analyzing T cell mediated immune responses, a role for humoral immune activation, especially DSA, has not been defined. Pseudomonas aeruginosa is known to damage the lung epithelium, and can facilitate proliferation of T and B cell subsets.20 Hence, we hypothesized that the isolation of Pseudomonas aeruginosa in respiratory specimens after lung transplantation increases the risk of DSA development independent of other risk factors including the underlying diagnosis. To that end, we sought to identify risk factors for the development of DSA. Some of the results from this manuscript have previously been reported in an abstract form 21.
2. MATERIALS AND METHODS
2.1. Study Design, Settings and Participants
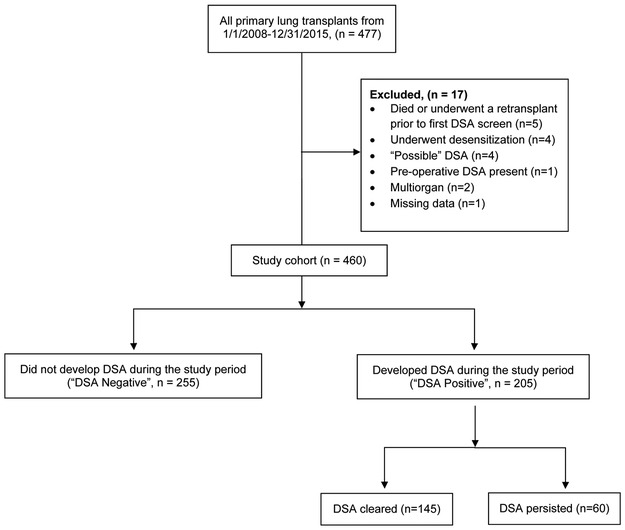
We performed a retrospective single-center cohort study and included all 477 primary lung transplant recipients at Barnes-Jewish Hospital between January 1, 2008 and December 31, 2015, with follow-up through December 31, 2018. We excluded multi-organ transplant recipients (n = 2), those who underwent desensitization before transplantation (n = 4), those who had pre-transplant DSA (n = 1), those who developed HLA antibodies but donor specificity could not be established (n = 4), and those who died or underwent a retransplant prior to first DSA screen (n = 5) (Figure 1). The Washington University School of Medicine Institutional Review Board for Human Studies approved the study protocol (ID # 201811073).
Figure 1: Flow diagram: study screening and eligibility.
Derivation of the study cohort. Flowchart of lung transplant recipients and application of eligibility criteria that resulted in the final study cohort stratified by whether they developed donor-specific antibodies (DSA), and whether they cleared it.
2.2. Clinical Management
As part of our routine clinical protocol, most patients were treated with anti-thymocyte globulin or basiliximab for induction immunosuppression. All recipients were treated with three-drug maintenance immunosuppression. In the immediate post-transplant period, all patients were treated with broad-spectrum antibacterial antibiotics that were tailored according to recipient and donor cultures for at least 14 days. DSA screens were performed at 10 days, 1, 2, 3, 6, and 12 months after transplantation and every 3 months thereafter. Additional DSA screens were performed during episodes of allograft dysfunction. Similarly, bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial lung biopsies was performed at 1, 2, 3, 6, and 12 months after transplantation and to evaluate episodes of allograft dysfunction. Bronchial washings and BAL fluid were sent for routine bacterial cultures and additional microbiological studies. All positive bacterial cultures were treated with appropriate oral or intravenous antibiotics for at least 14 days. Additional details regarding our program’s clinical management protocols are provided in the online data supplement.
2.3. Variables
HLA antibodies were detected using the LABScreen™ Single Antigen assay, and DSA was considered positive if the mean fluorescence intensity (MFI) was ≥ 2000. In cases where the donor was not high-resolution typed and DSA could not be definitively identified, the patients were excluded from the study (Figure 1). The time from transplantation to the first detection of DSA was defined as time to DSA positivity. DSA clearance was defined as all DSA tests being negative after an initial positive result, and the time between the first positive and the negative result after which all other results remained negative was defined as the time to DSA clearance. A positive bacterial culture from a bronchial wash or BAL was defined as bacterial isolation 22-26. We did not attempt to distinguish between infection and colonization. In other words, we considered Pseudomonas aeruginosa isolation if Pseudomonas aeruginosa was detected in the bronchial wash, BAL or sputum, and the first time it was detected post-transplantation was considered as the time to Pseudomonas isolation. However, based on the a priori defined objectives of our study, we did not distinguish whether bacterial isolation was on a surveillance bronchoscopy or during a hospital admission for deterioration of respiratory status or lung function. One of the reasons to do this was that we wanted to identify the risk of developing DSA in the event certain bacteria were isolated from the lungs; and our clinical interpretation of the risk would be unlikely to change in either setting. Rather, we focused on the cumulative number of isolates, and whether they were de novo or persistent (i.e., present prior to transplantation in the recipient). PGD and acute cellular rejection were defined based on the International Society of Heart and Lung Transplantation (ISHLT) criteria 27,28. CLAD was defined as a persistent decline in FEV1 ≤ 80% for at least 3 weeks without a specific cause 29. CLAD-free survival was defined as the earliest occurrence of either CLAD or death, whichever came first 2.
Given that colonization with Pseudomonas aeruginosa (as well as other infections) and the occurrence of acute rejection and lymphocytic bronchiolitis could occur simultaneously with the development of donor-specific antibodies (DSA), we treated these risk factors as time-dependent covariates in a Cox proportional hazards model. Thus, our analyses accounted for the time of occurrence of the explanatory variables when determining the risk conferred by these variables on the development of DSA. In such analyses (say, for the development of DSA), the “time to outcome” was identified as occurring when the first DSA was detected, and the risk factor was treated in a time-dependent manner by selecting the first time after transplant that Pseudomonas was isolated in the bronchial wash, bronchoalveolar lavage or sputum. Therefore, in all cases in these analyses, the explanatory variables occurred before the outcome30,31.
2.4. Statistical Analysis
Differences in patient demographics were evaluated using the Pearson Chi-Square test when comparing categorical variables and the Mann-Whitney test for continuous variables. Results were reported as median (interquartile range) unless otherwise specified. Survival analysis included Kaplan-Meier and Cox proportional hazard models using events after transplantation as time-dependent covariates after conducting regression diagnostics. Specifically, acute rejection, lymphocytic bronchiolitis and bacterial isolation were treated as time-dependent covariates when evaluating risk factors for the development of DSA and DSA clearance. To minimize confounding, we defined the covariates for each of the three multivariable models a priori based on review of the literature12,13,32, and the focus of the study:
Model 1: Pseudomonas aeruginosa isolation post-transplantation as a time-dependent covariate, CPRA, PGD grade 3 at any point, LAS,
Model 2: Acute cellular rejection (Grade ≥ A2) as a time-dependent covariate, CPRA, PGD grade 3 at any point, LAS,
Model 3: Lymphocytic bronchiolitis (Grade ≥ B1R) as a time-dependent covariate, CPRA, PGD grade 3 at any point, LAS.
As a result, certain variables (for example, recipient age) were not included even if the p value was < 0.1 on univariate analysis 33. We included only one time-dependent variable in each multivariable model to avoid overinflation of risk; as a result, we present three multivariable models34. Given the interaction between PGD and cardiopulmonary bypass (CPB), the results were presented before and after adjusting for CPB. Because of the risk of DQ-DSA on adverse outcomes, we performed a subgroup analysis to identify risk factors for developing DQ-DSA 8,10,15,35. Similar multivariable models were evaluated as described above, for the development of DQ-DSA. An interval-censored survival analysis was also performed to handle the timing of screening when calculating the time of bacterial isolation and time of DSA-positivity. Statistical analysis was performed using SPSS 26.0 (SPSS Inc., Chicago, IL) and p values <0.05 were considered statistically significant.
We confirmed the proportional hazards assumption for all time-independent variables in the Cox proportional hazards models, using the log-negative-log method. We performed regression diagnostics for the multivariable models by identifying outliers using the DFBETA test, ran the time-dependent Cox proportional hazards models and plotted the residuals against the study ID (Supplementary Figure 1). Finally, we excluded nonlinearity for all variables in the Cox proportional hazards models, using partial residual plots.
3. RESULTS
3.1. Demographic characteristics of the cohort
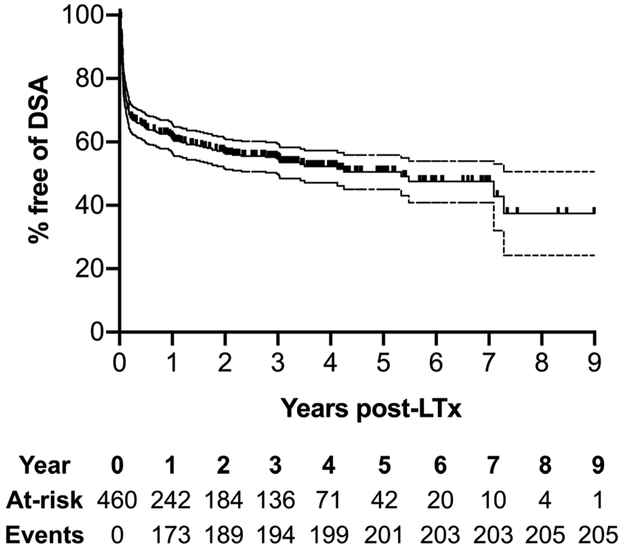
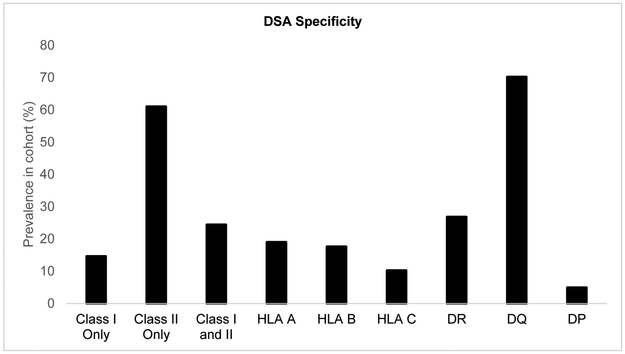
The 460 patients in our cohort were followed up for a mean of 4.82 ± 2.86 years (range: 0.03 – 10.99 years). We had 4,500 DSA results, with each patient having a mean of 9.74 ± 4.55 DSA tests. We had 3,110 culture results for bacterial isolates, with each patient having a mean of 6.73 ± 3.13 isolates. We had 2,549 transbronchial biopsy results, with each patient having a mean of 5.52 ± 2.34 biopsies. Among the 460 patients included in this cohort, 205 (45%) developed DSA during the study period (Figure 2). Those who developed DSA were more likely to be younger, have a higher CPRA prior to transplantation, and have required cardiopulmonary bypass during surgery (Table 1 and 2). Of note, there was no difference in the distribution of recipient gender or underlying diagnosis between the 2 groups. Additional baseline characteristics of the 2 groups are shown in Table 1. DSA-free survival was 4.26 years (Figure 2, 95% CI: 3.83 – 4.69 years). Among the 205 who developed DSA, 175 (85%) developed DSA to Class II HLA, and 145 (71%) developed DSA to HLA-DQ alleles (Figure 3). Pseudomonas aeruginosa was the most frequently isolated bacterial organism from bronchoscopy specimens after transplantation; among the 460 recipients, 147 (32%) had at least one positive culture for Pseudomonas aeruginosa. 87 of the 147 recipients (59%) who had Pseudomonas aeruginosa isolation after transplantation did not have Pseudomonas aeruginosa isolation before transplantation. While the DSA appeared before Pseudomonas isolation in 10.7% of our cohort, these cases were excluded from the time-dependent Cox proportional hazards analyses and did not contribute to the risk conferred by Pseudomonas isolation on DSA development30,31. Gram-negative bacilli other than Pseudomonas aeruginosa were isolated at least once in 182 (40%) recipients, and gram-positive cocci were isolated in 103 (22%) recipients. Candida and Aspergillus species were cultured more frequently; 358 (78%) recipients had at least one positive culture for Candida species, and 158 (34%) had at least one positive culture for Aspergillus species. Among the 147 who had at least one positive culture for Pseudomonas aeruginosa after transplantation, 70 (47%) had cystic fibrosis, 38 (26%) had interstitial lung disease, 23 (16%) had COPD, and 16 (11%) had other underlying diagnoses (Table S1). Patients with cystic fibrosis in whom Pseudomonas was isolated comprised of 15% (70/460) of the total cohort (Table S1). Among these 70 patients, 38 (54%) had DSA and 32 (46%) did not.
Figure 2: Freedom from donor-specific antibodies (DSA) after lung transplantation (LTx).
Kaplan-Meier survival methods estimated the proportion of recipients in whom DSA had not occurred at follow-up after lung transplantation (LTx). The numbers below the x-axis time points represent those at risk. Cohorts consisted of primary LTx reported from January 1, 2008 and December 31, 2015, with follow-up through December 31, 2018. Analyses censored for end of study follow-up and loss to follow-up.
Table 1:
Demographics
| Variable | DSA negative (n=255) |
DSA positive (n=205) |
|---|---|---|
| Female recipient, n (%) | 104 (40.8) | 85 (41.5) |
| Recipient age at first transplant | 59 (16.8) | 55 (22.3) |
| Diagnosis, n (%) | ||
| Interstitial lung disease/pulmonary fibrosis | 115 (45.1) | 87 (42.4) |
| COPD | 65 (25.5) | 44 (21.5) |
| Cystic fibrosis (CF)/non-CF bronchiectasis | 40 (15.7) | 44 (21.5) |
| Pulmonary hypertension | 4 (1.6) | 3 (1.5) |
| Other | 31 (12.2) | 27 (13.2) |
| Lung allocation score at transplant | 40.5 (16) | 42.4 (24) |
| GERD | 113 (44.3) | 77 (37.6) |
| Pre-transplant CPRA | 10.5 (21.3) | 15.2 (24.7) |
| Total HLA mismatch number | 8 (2) | 8 (2) |
| Transplant type, n (%) | ||
| Single | 6 (2.4) | 7 (3.4) |
| Bilateral | 249 (97.6) | 198 (96.6) |
| Intraoperative CPB, yes n (%) | 113 (44.3) | 111 (54.1) |
| Ischemic time, min | 267.5 (97) | 277.5 (110) |
| CMV mismatch (yes), n (%) | 73 (28.9) | 59 (28.9) |
| Allosensitized, n (%) | 91 (35.7) | 72 (35.1) |
| Pre-operative ECMO, n (%) | 2 (0.8) | 2 (1.0) |
| Post-operative ECMO, n (%) | 9 (3.5) | 5 (2.4) |
| Induction immunosuppression | ||
| Basiliximab | 207 (81.2) | 150 (73.2) |
| ATGAM | 43 (16.9) | 44 (21.5) |
| Other | 3 (1.2) | 5 (2.4) |
| None | 1 (0.4) | 3 (1.5) |
| Unknown | 1 (0.4) | 3 (1.5) |
| PGD grade 3 at any time, n (%) | 64 (25.1) | 60 (29.3) |
Abbreviations: COPD: chronic obstructive pulmonary disease; CF: cystic fibrosis; CPRA: calculated panel reactive antibodies; HLA: human leukocyte antigen; CPB: cardiopulmonary bypass; CMV: cytomegalovirus; ECMO: extracorporeal membrane oxygenation; GERD: gastroesophageal reflux disease; ATGAM: antithymocyte globulin; PGD: primary graft dysfunction. Continuous variables are represented as median (interquartile range) except for cPRA, which is represented as mean (standard deviation) as the median was 0 in both groups.
Table 2:
Univariable Cox proportional hazards models of risk factors for posttransplant donor-specific HLA antibody (DSA) development.
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Recipient age | 0.99 | 0.98-1.00 | 0.01 |
| Diagnosis, CF | 1.03 | 0.95-1.12 | 0.43 |
| LAS | 1.00 | 1.00-1.01 | 0.61 |
| GERD | 0.83 | 0.63-1.11 | 0.83 |
| Pre-transplant CPRA | 1.01 | 1.00-1.01 | 0.02 |
| HLA mismatch number | 1.06 | 0.97-1.17 | 0.19 |
| Transplant type, bilateral | 0.81 | 0.38-1.72 | 0.58 |
| Intraoperative CPB | 1.46 | 1.11-1.92 | 0.008 |
| Induction, Basiliximab | 0.75 | 0.55-1.03 | 0.07 |
| PGD grade 3 at any time | 1.28 | 0.94-1.72 | 0.114 |
| Acute rejection Grade ≥ A2* | 1.97 | 1.39-2.78 | <0.001 |
| Lymphocytic bronchiolitis Grade ≥ B1R* | 1.69 | 1.18-2.41 | 0.004 |
| Cumulative A rejection score post-LTx | 1.09 | 1.03-1.15 | 0.001 |
| Cumulative B rejection score post-LTx | 1.15 | 1.06-1.25 | 0.001 |
| Gram-positive bacteria isolation post-LTx* | 0.99 | 0.66-1.48 | 0.96 |
| Gram-negative bacteria isolation post-LTx* | 1.23 | 0.90-1.68 | 0.20 |
| Pseudomonas isolation post-LTx* | 1.68 | 1.15-2.45 | 0.007 |
| Number of positive Pseudomonas cultures | 1.08 | 1.04-1.13 | <0.001 |
| CARV isolation post-LTx* | 1.05 | 0.65-1.69 | 0.86 |
| Pseudomonas isolation pre-LTx | 1.10 | 0.77-1.55 | 0.61 |
| Aspergillus isolation post-LTx* | 1.34 | 0.88-2.05 | 0.17 |
| CMV in blood post-LTx* | 0.88 | 0.63-1.22 | 0.45 |
| CMV in BAL post-LTx* | 0.88 | 0.60-1.28 | 0.49 |
Abbreviations: BAL: bronchoalveolar lavage; CARV: community-acquired respiratory viruses; CF: cystic fibrosis; CPRA: calculated panel reactive antibodies; CPB: cardiopulmonary bypass; CMV: cytomegalovirus; GERD: gastroesophageal reflux disease; LTx: lung transplant; PGD: primary graft dysfunction. p value is for Cox proportional hazards model with “time to DSA positivity” as time to event.
indicates variables which were evaluated using a time-dependent Cox proportional hazards model.
Figure 3: Characteristics of donor-specific antibodies (DSA).
This graph represents the specificity of donor-specific antibodies (DSA) present in those recipients transplanted between January 1, 2008 and December 31, 2015, with follow-up through December 31, 2018 (n=205), represented as a proportion (out of 100%). Each recipient may have more than one DSA and thus, may have contributed to more than one bar.
3.2. Risk factors for DSA development
Among the variables that we selected for inclusion into multivariable models a priori, Pseudomonas aeruginosa isolation (HR = 1.68; 95%CI: 1.15 – 2.45, p = 0.007), acute rejection grade ≥ A2 (HR = 1.97; 95%CI: 1.39 – 2.78, p = <0.001), and lymphocytic bronchiolitis grade ≥ B1R (HR = 1.69; 95%CI: 1.18 – 2.41, p = 0.004) were associated with a significantly increased risk of DSA development in univariate analyses (Table 2). Additionally, Pseudomonas aeruginosa isolation was associated with an increased risk of DSA development (HR 1.9; 95%CI: 1.13 – 3.19, p = 0.015) even after excluding cases (n=80) in whom Pseudomonas aeruginosa was isolated pretransplant. The cumulative acute rejection A score and the cumulative lymphocytic bronchiolitis B score after transplantation were also associated with an increased risk of DSA development (Table 2). PGD grade 3 at any time point within the first 72 hours was not a significant risk factor (HR = 1.28; 95%CI: 0.94 – 1.72, p = 0.114). In a subgroup analysis of recipients in whom transfusion data were available (n=168), the number of packed red blood cells received at the time of transplantation did not confer an increased risk of DSA development (HR = 1.01, 95%CI: 0.95 – 1.08, p = 0.720). However, there was no association between the LAS at the time of transplantation and DSA development (HR = 1.00; 95%CI: 0.99 – 1.01, p = 0.607). Post-hoc analysis of baseline characteristics that were different between those who developed DSA and those who did not revealed that the use of cardiopulmonary bypass was associated with an increased risk of DSA development (HR = 1.46; 95%CI: 1.11 – 1.92, p = 0.008), while recipient age was inversely associated with the risk of DSA development (HR = 0.99; 95%CI: 0.98 – 1.00, p = 0.010, Table 2). CMV viremia or CMV isolation in bronchoscopy specimens, the isolation of Pseudomonas aeruginosa in pre-transplant respiratory specimens, the isolation of gram-positive bacteria post-transplant, the isolation of Aspergillus post-transplant and the isolation of CARV post-transplant were not associated with an increased risk of DSA development (Table 2).
In multivariable models, each of the time-dependent covariates (1) Pseudomonas aeruginosa isolation (2) acute rejection grade ≥ A2, and (3) lymphocytic bronchiolitis grade ≥ B1R, was associated with a significantly increased risk of DSA development (Table 3 and S2). CPRA remained a significant and independent risk factor in the 3 models. PGD grade 3 at any time point within the first 72 hours was not a significant risk factor on multivariable models. This association also held true as a part of a sensitivity analysis when considering the excluded patients with “possible DSA” (Table S3) and on an interval-censored survival analysis (Table S4). The magnitude of the risk remained independently associated with DSA development when stratified on cardiopulmonary bypass (Table 3). When restricted to the development of DSA to HLA-DQ, the three time-dependent covariates (1) Pseudomonas aeruginosa isolation, (2) acute rejection grade ≥ A2, and (3) lymphocytic bronchiolitis grade ≥ B1R were also independently associated with an increased risk in both univariate and multivariable analyses (Table 4 and Table 5). In contrast, CPRA was not associated with an increased risk of DSA development to HLA-DQ (Table 4).
Table 3:
Multivariable Cox proportional hazards models of risk factors for posttransplant donor-specific HLA antibody (DSA) development
| Variable | Non-stratified | Stratified for CPB | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Pseudomonas isolation post-LTx | 1.75 | 1.18-2.60 | 0.005 | 1.76 | 1.19-2.61 | 0.005 |
| Pre-transplant CPRA | 1.01 | 1.00-1.01 | 0.009 | 1.008 | 1.00-1.01 | 0.008 |
| PGD grade 3 at any point | 1.25 | 0.91-1.71 | 0.176 | 1.173 | 0.85-1.62 | 0.331 |
| LAS | 1.00 | 1.00-1.01 | 0.397 | 1.00 | 0.99-1.01 | 0.879 |
| Variable | Non-stratified | Stratified for CPB | ||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Acute rejection Grade ≥ A2* | 2.06 | 1.43-2.97 | <0.001 | 2.01 | 1.4-2.9 | <0.001 |
| Pre-transplant CPRA | 1.01 | 1.00-1.01 | 0.017 | 1.01 | 1.00-1.01 | 0.015 |
| PGD grade 3 at any point | 1.27 | 0.92-1.75 | 0.141 | 1.2 | 0.87-1.65 | 0.276 |
| LAS | 1.00 | 1.00-1.01 | 0.430 | 1.00 | 0.99-1.01 | 0.927 |
| Variable | Non-stratified | Stratified for CPB | ||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Lymphocytic bronchiolitis Grade ≥ B1R* | 1.72 | 1.18-2.51 | 0.005 | 1.72 | 1.18-2.51 | 0.005 |
| Pre-transplant CPRA | 1.01 | 1.00-1.01 | 0.012 | 1.01 | 1.00-1.01 | 0.01 |
| PGD grade 3 at any point | 1.3 | 0.94-1.78 | 1.109 | 1.21 | 0.88-1.68 | 0.238 |
| LAS | 1.00 | 1.00-1.01 | 0.389 | 1.00 | 0.99-1.01 | 0.876 |
Abbreviations: CPRA: calculated panel reactive antibodies; CPB: cardiopulmonary bypass; HR: hazards ratio; CI: confidence interval; LAS: lung allocation score; LTx: lung transplant; PGD: primary graft dysfunction.
Table 4:
Univariable Cox proportional hazards models of risk factors for posttransplant donor-specific HLA antibody (DSA) development restricted to DQ-DSA
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Recipient age | 0.98 | 0.97-0.99 | 0.001 |
| Diagnosis, CF | 1.04 | 0.94-1.14 | 0.49 |
| LAS | 1.00 | 0.99-1.01 | 0.93 |
| Pre-transplant CPRA | 1.00 | 0.99-1.01 | 0.81 |
| Transplant type, bilateral | 0.64 | 0.28-1.45 | 0.28 |
| Intraoperative CPB | 1.32 | 0.95-1.83 | 0.1 |
| Induction, Basiliximab | 0.66 | 0.46-0.94 | 0.02 |
| PGD grade 3 at any time | 1.08 | 0.74-1.56 | 0.70 |
| Acute rejection Grade ≥ A2* | 2.05 | 1.38-3.05 | <0.001 |
| Lymphocytic bronchiolitis Grade ≥ B1R* | 2.07 | 1.39-3.08 | <0.001 |
| Cumulative A rejection score post-LTx | 1.11 | 1.04-1.18 | 0.001 |
| Cumulative B rejection score post-LTx | 1.17 | 1.07-1.29 | 0.001 |
| Gram-positive bacteria isolation post-LTx* | 0.99 | 0.62-1.58 | 0.95 |
| Gram-negative bacteria isolation post-LTx* | 1.41 | 0.98-2.02 | 0.06 |
| Pseudomonas isolation post-LTx* | 2.16 | 1.43-3.26 | <0.001 |
| Number of positive Pseudomonas cultures | 1.11 | 1.06-1.16 | <0.001 |
| Pseudomonas isolation pre-LTx | 1.17 | 0.78-1.77 | 0.44 |
| Aspergillus isolation post-LTx* | 1.38 | 0.84-2.26 | 0.20 |
| CMV in blood post-LTx* | 0.77 | 0.52-1.14 | 0.19 |
| CMV in BAL post-LTx* | 0.83 | 0.53-1.30 | 0.42 |
Abbreviations: BAL: bronchoalveolar lavage; CF: cystic fibrosis; CPRA: calculated panel reactive antibodies; CPB: cardiopulmonary bypass; CMV: cytomegalovirus; LTx: lung transplant; PGD: primary graft dysfunction. p value is for Cox proportional hazards model with “time to DSA positivity” as time to event.
indicates variables which were evaluated using a time-dependent Cox proportional hazards model.
Table 5:
Multivariable Cox proportional hazards models of risk factors for posttransplant donor-specific HLA antibody (DSA) development restricted to DQ-DSA.
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Pseudomonas isolation post-LTx | 2.26 | 1.46-3.50 | <0.001 |
| Pre-transplant CPRA | 1.00 | 1.00-1.01 | 0.55 |
| PGD grade 3 at any point | 1.06 | 0.71-1.58 | 0.77 |
| LAS | 1.00 | 0.99-1.01 | 0.82 |
| Variable | HR | 95% CI | p |
| Acute rejection Grade ≥ A2* | 2.21 | 1.45-3.37 | <0.001 |
| Pre-transplant CPRA | 1.00 | 0.99-1.01 | 0.78 |
| PGD grade 3 at any point | 1.09 | 0.73-1.62 | 0.67 |
| LAS | 1.00 | 0.99-1.01 | 0.82 |
| Variable | HR | 95% CI | p |
| Lymphocytic bronchiolitis Grade ≥ B1R* | 2.06 | 1.35-3.15 | 0.001 |
| Pre-transplant CPRA | 1.00 | 0.99-1.01 | 0.68 |
| PGD grade 3 at any point | 1.13 | 0.76-1.69 | 0.54 |
| LAS | 1.00 | 0.99-1.01 | 0.79 |
Abbreviations: CPRA: calculated panel reactive antibodies; CPB: cardiopulmonary bypass; LAS: lung allocation score; LTx: lung transplant; PGD: primary graft dysfunction.
In addition, there was a direct relationship between the number of positive cultures for Pseudomonas aeruginosa and the risk of DSA development in both univariate (HR = 1.08 for every +”n” positive culture, 95%CI: 1.04 – 1.13, <p=0.001, Table 2) and multivariable analyses when adjusted for CPRA, LAS and PGD (HR = 1.08 for every +”n” positive culture, 95%CI: 1.04 – 1.13, p=<0.001). The isolation of other Gram-negative bacteria, or other organisms (i.e., Gram-positive bacteria, Aspergillus, CMV, CARV) was not associated with an increased risk of DSA development (Table 2 and 4). After accounting for CPRA, LAS and PGD in the multivariable analysis, the magnitude of risk for developing DSA to HLA-DQ remained high in recipients who had Pseudomonas aeruginosa isolation after transplantation (Table 5).
3.3. Pseudomonas aeruginosa isolation and long-term outcomes
Among the 460 recipients, 306 (67%) developed CLAD or died during the study period. The development of DSA was associated with worse CLAD-free survival (HR = 1.80, 95%CI: 1.43 – 2.25, p<0.001, Table 6). Among those who developed DSA (n=205), clearance of DSA was associated with better CLAD-free survival (HR = 0.39, 95%CI: 0.28 – 0.55, p < 0.001, Table 6). In other words, there was a 71% reduction in the risk of CLAD or allograft failure in those who cleared DSA compared to those who did not clear DSA. Given that isolation of Pseudomonas aeruginosa has been associated with worse long-term outcomes after transplantation, we examined its association with CLAD-free survival. Isolation of Gram-positive and Gram-negative bacteria, including Pseudomonas, was associated with worse CLAD-free survival on univariate analysis (Table 6). The cumulative number of positive cultures for Pseudomonas aeruginosa after transplantation was also associated with worse CLAD-free survival (HR = 1.06 for every +”n” positive culture, 95%CI: 1.03 – 1.10, p=<0.001), but the isolation of Pseudomonas aeruginosa before transplantation was not. We also found that lymphocytic bronchiolitis was associated with worse CLAD-free survival on univariate and multivariable analysis (HR = 1.29, 95%CI 1.02 – 1.62, p = 0.035, Table 6 and 7). On multivariable analysis of a priori defined variables, Pseudomonas aeruginosa isolation after transplantation remained a risk factor for CLAD-free survival after adjusting for PGD Grade 3 at any time point and LAS (HR = 1.42, 95%CI: 1.11 – 1.81, p=0.005, Table 7) as well as after adding the cumulative acute rejection A score post-transplantation (Table S5); and in a separate model after adding the cumulative lymphocytic bronchiolitis B score post-transplantation (Table S5).
Table 6:
Univariable Cox proportional hazards models of risk factors for chronic lung allograft dysfunction-free survival (CLAD-free survival).
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Recipient age | 0.99 | 0.98-1.00 | 0.004 |
| Diagnosis, CF | 1.00 | 0.94-1.07 | 0.92 |
| LAS | 1.00 | 1.00-1.01 | 0.18 |
| cPRA | 1.00 | 0.99-1.00 | 0.27 |
| Transplant type, bilateral | 1.08 | 0.56-2.10 | 0.82 |
| Intraoperative CPB | 1.16 | 0.92-1.45 | 0.21 |
| Induction, Basiliximab | 1.10 | 0.84-1.43 | 0.50 |
| PGD grade 3 at any time | 1.24 | 0.97-1.59 | 0.09 |
| A2 rejection or greater* | 1.15 | 0.91-1.45 | 0.23 |
| Lymphocytic bronchiolitis,>=B1R* | 1.29 | 1.02-1.63 | 0.03 |
| Cumulative A rejection score post-LTx | 1.04 | 0.99-1.09 | 0.15 |
| Cumulative B rejection score post-LTx | 1.09 | 1.00-1.18 | 0.05 |
| Gram-positive bacteria isolation post-LTx* | 1.46 | 1.14-1.87 | 0.003 |
| Gram-negative bacteria isolation post-LTx* | 1.48 | 1.18-1.85 | 0.001 |
| Pseudomonas isolation post-LTx* | 1.42 | 1.11-1.81 | 0.005 |
| Number of positive Pseudomonas cultures | 1.06 | 1.03-1.10 | <0.001 |
| Pseudomonas isolation pre-LTx | 1.07 | 0.80-1.43 | 0.64 |
| Aspergillus isolation post-LTx* | 1.50 | 1.19-1.90 | 0.001 |
| CARV isolation post-LTx* | 0.98 | 0.76-1.27 | 0.90 |
| CMV in blood post-LTx* | 1.27 | 1.00-1.61 | 0.05 |
| CMV in BAL post-LTx* | 1.07 | 0.85-1.35 | 0.55 |
| Development of DSA* | 1.80 | 1.43-2.25 | <0.001 |
| DSA Class | 0.28 | ||
| DSA Class II only | 1.56 | 0.90-2.72 | 0.17 |
| DSA Class II DR | 1.36 | 0.98-1.89 | 0.07 |
| DSA Class II DQ | 1.65 | 1.31-2.07 | <0.001 |
| DSA Class II DP | 1.04 | 0.49-2.21 | 0.91 |
| DSA developing < 30 days post-LTx | 0.69 | 0.50-0.95 | 0.02 |
| DSA developing < 90 days post-LTx | 0.90 | 0.64-1.27 | 0.54 |
| Clearance of DSA* | 0.39 | 0.28-0.55 | <0.001 |
Abbreviations: BAL: bronchoalveolar lavage; CARV: community-acquired respiratory viruses; CF: cystic fibrosis; CPRA: calculated panel reactive antibodies; CPB: cardiopulmonary bypass; CMV: cytomegalovirus; DSA: donor-specific antibodies; LTx: lung transplant; PGD: primary graft dysfunction. p value is for Cox proportional hazards model with “time to CLAD-free survival” as time to event.
indicates variables which were evaluated using a time-dependent Cox proportional hazards model. Note that those variables in italics were applicable only to those patients who were DSA positive (n=205).
Table 7:
Multivariable Cox proportional hazards models of risk factors for chronic lung allograft dysfunction-free survival (CLAD-free survival).
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Pseudomonas isolation post-LTx | 1.42 | 1.11-1.81 | 0.005 |
| PGD grade 3 at any point | 1.22 | 0.94-1.57 | 0.14 |
| LAS | 1.00 | 1.00-1.01 | 0.41 |
| Variable | HR | 95% CI | p |
| Lymphocytic bronchiolitis,>=B1R* | 1.29 | 1.02-1.62 | 0.035 |
| PGD grade 3 at any point | 1.20 | 0.93-1.55 | 0.171 |
| LAS | 1.00 | 1.00-1.01 | 0.34 |
Abbreviations: DSA: donor-specific antibodies; LAS: lung allocation score; LTx: lung transplant; PGD: primary graft dysfunction.
4. DISCUSSION
The development of DSA portends poor outcomes after lung transplantation 5-10. In this study, we show that there are distinct risk factors associated with the development of DSA. Specifically, we confirm that acute rejection is associated with an increased risk of DSA, as previously reported, and identify a similar risk attributable to lymphocytic bronchiolitis 12. Moreover, we now identify Pseudomonas aeruginosa isolation from the respiratory tract as an independent risk factor for the development of DSA, which has not been previously reported. Furthermore, we identify a significant association between Pseudomonas aeruginosa isolation and the development of DSA to mismatched DQ alleles, which is independently associated with an increased risk of CLAD 10,35. These findings suggest that innate immune responses to Pseudomonas aeruginosa can activate humoral alloimmune responses after lung transplantation. The association of Pseudomonas aeruginosa isolation and DSA was independent of the underlying diagnosis. In addition, our findings corroborate previous studies by demonstrating that the development of DSA is a strong risk factor for CLAD and death 7-10, whereas DSA clearance mitigates this risk. Additionally, we report that Pseudomonas aeruginosa isolation is associated with worse CLAD-free survival, as is the cumulative number of positive cultures for Pseudomonas aeruginosa. Our multivariable analysis also identified Aspergillus isolation, and the development of DSA as risk factors for worse CLAD-free survival on multivariable analysis, which is consistent with reports of them being risk factors for deleterious long-term outcomes after lung transplantation 8,22,23. Our findings are consistent with those of the UCLA group who had previously reported that the likelihood of transition from transplant to chronic rejection and death is increased by the interactions between Pseudomonas aeruginosa, Aspergillus and ELR+ CXC chemokines derived from lung epithelial cells that can act via its receptor CXCR2 (IL-8 receptor β).22 Interestingly, CARV did not appear to be a risk factor for the development of DSA in our analyses. Our findings are consistent with the findings of the CTOTC-03 study, which although done in pediatric lung transplant recipients, also did not find a relationship between CARV infection and the development of alloimmune or autoimmune humoral or cellular responses36.
Previous studies have identified risk factors for the development of DSA, but these have not examined the role of microbial isolation from the respiratory tract although infection is a common pro-inflammatory complication after lung transplantation. Indeed, the focus of the study identifying PGD as a risk factor for DSA was to evaluate the role of pro-inflammatory cytokines, with the underlying hypothesis that PGD-induced inflammation augments alloimmunity, thereby predisposing to CLAD 13. Similarly, the HALT (HLA Antibodies after Lung Transplantation) study identified acute rejection and the lung allocation score as independent risk factors for DSA development; however, the primary focus of the study was to define the incidence of DSA and their characteristics early after transplantation in a prospective multi-center fashion 12. Our results complement these studies in identifying PGD and acute rejection as risk factors for DSA and expand the identified pro-inflammatory complications associated with DSA to Pseudomonas aeruginosa isolation. Taken together, these findings support the paradigm that pro-inflammatory events after transplantation increase the risk of DSA development, which has been proposed in both pre-clinical and clinical studies 18,22,37,38.
Over half of those who had Pseudomonas aeruginosa isolation after transplantation were culture-negative for Pseudomonas aeruginosa before transplantation. Moreover, the association between post-transplant Pseudomonas aeruginosa and DSA persisted even after excluding those who were culture-positive for Pseudomonas aeruginosa before transplantation. This suggests that the acquisition of Pseudomonas aeruginosa after transplantation promotes the development of donor-specific antibodies. Our data indicate that this relationship is unique to Pseudomonas aeruginosa, as we did not detect an association between other bacteria, fungi or viruses and DSA in our cohort. Several reports have demonstrated that Pseudomonas aeruginosa stimulates potent innate immune responses, including airway neutrophilia 39-41. Our group has also shown that Pseudomonas aeruginosa infection in a mouse model of lung transplantation promotes neutrophilia through a G-CSF-dependent mechanism that prevents established tolerance39. Notably, we also observed that Pseudomonas aeruginosa infection induced allograft-infiltrating neutrophils to upregulate the B7 molecules CD80 and CD86, which in turn could drive alloantigen-specific T cell responses through providing B7:CD28 co-stimulation in trans.42 Although in that study we did not measure DSA, it is interesting to speculate that Pseudomonas aeruginosa-mediated neutrophil B7 upregulation could bolster CD28-mediated CD4+ T cell help to B cells to promote DSA generation in a similar manner. Ultimately, the inflammatory environment induced by Pseudomonas aeruginosa may promote the clonal expansion of alloreactive T-cells and the polyclonal activation of B-cells 13,43,44, and upregulation of HLA molecules on airway epithelial cells 45,46 to amplify the humoral alloimmune response. Pseudomonas aeruginosa is also capable of facilitating affinity maturation and promoting isotype switching and somatic mutations in alloreactive B-cells, especially if these antigens are already exposed on the allograft 47,48. Moreover, Pseudomonas aeruginosa isolation from the respiratory tract or the resultant clinical treatment with antibiotics may modulate the gut microbiome and result in both protective and deleterious humoral responses 49,50. Thus, altering the microbiome in itself may modulate the severity of allograft injury 51,52. Together these results establish a precedent to investigate mechanistically how Pseudomonas aeruginosa may promotes alloimmune responses, and how these responses can be mitigated to improve long-term outcomes after lung transplantation.
There is a possibility that the abovementioned crosstalk between the innate and adaptive responses contribute to the association between Pseudomonas and CLAD, specifically through DSA driving antibody-mediated rejection (AMR). To that effect, we have previously reported in an abstract that Pseudomonas isolation may be a trigger for AMR.53 Since DSA is an integral component of the clinical definition of AMR and is thought to be the cause of AMR, one would expect a clear association between Pseudomonas, DSA, AMR and CLAD. However, most patients who have DSA do not develop AMR, although they are at a higher risk for CLAD.54 A mechanistic link to explain how Pseudomonas may trigger antibody-mediated rejection through DSA is currently being actively investigated by our research group.
There are limitations that are inherent to this study’s design that should be considered when interpreting our data. First, this was a single-center retrospective study in patients transplanted between 2008–2015. Although the majority of patients were treated with a similar clinical protocol after transplantation, there may be patient-specific deviations that might impact alloimmune responses that have not yet been identified as modulating DSA development. Second, we did not differentiate colonization from infection when considering Pseudomonas aeruginosa isolation. We believe that this distinction would be fraught with complications in a retrospective analysis, particularly because it is often difficult to make this distinction prospectively in clinical practice. For this reason, we elected to use the term “isolation” to avoid implying that an invasive infection was present. Nevertheless, this does not detract from our findings that isolating Pseudomonas aeruginosa in respiratory specimens after transplantation is associated with the development of DSA. Third, we cannot exclude the possibility that some patients had DSA below the threshold that our laboratory has chosen (MFI ≤ 2000), which cross that threshold after the isolation of Pseudomonas aeruginosa. However, the prevalence of DSA in our cohort was consistent with or even higher than other centers that have used a similar or lower cutoff.8,10,35 Despite this, the interpretation that Pseudomonas aeruginosa increases the risk of DSA development needs to be tested in in vivo model systems with a lower threshold for reporting DSA. Fourth, as we used a Cox proportional hazards with time-dependent covariates, we did not consider waxing and waning status when determining how Pseudomonas (or acute rejection) affect the risk of developing DSA, as the “time to risk factor” and/or “time to event” was the first reported time to detection of the risk factor or event. As a result, we did not report on how the clearance of Pseudomonas alters the risk for DSA development or CLAD-free survival. Rather, we reported the number of Pseudomonas isolates from the same individual, and found this to be a significant risk factor for both DSA and CLAD-free survival. One of the reasons for approaching the analysis in this manner is to avoid introducing bias into the analysis. For example, our surveillance bronchoscopies are only done at 1, 2, 3, 6 and 12 months post-transplant (thus, within the first year) and only clinically indicated thereafter. Hence, there are more data points in the first 6 months, compared to thereafter. As a result, although the patient may clear Pseudomonas in month 13, that may not be documented. Nevertheless, we aimed to handle this issue by reporting number of positive Pseudomonas cultures and the risk that it confers. Similarly, when evaluating CLAD-free survival, we considered “time to DSA clearance” as time-dependent variable. This would allow us to account for when the DSA was first identified as being cleared; however, we did not include it in the analysis if it recurred after initial clearance. Fifth, our data suggest a strong association between Pseudomonas isolation and the development of DSA, but this does not imply causality. It creates a precedence for subsequent mechanistic studies to determine how Pseudomonas contributes to DSA development.
Through this manuscript, we propose that pro-inflammatory events after lung transplantation, such as acute rejection and Pseudomonas aeruginosa isolation are associated with an increased risk of DSA development, independent of the underlying diagnosis. These findings suggest that the isolation of Pseudomonas aeruginosa should be followed by close monitoring for the development of DSA. Additionally, our findings would need to be validated in an independent cohort and warrant in vivo verification. Results from such logical extension of our findings may then form the basis for clinical investigations focused on ameliorating the risk of alloimmunity associated with Pseudomonas aeruginosa isolation. Additionally, because DSA development is a critical component of antibody-mediated rejection (AMR), our work lays the foundation for studies evaluating whether Pseudomonas aeruginosa isolation is associated with AMR.
Supplementary Material
ACKNOWLEDGEMENTS/FUNDING
We thank Neil Anderson, MD and the Barnes Jewish Clinical Microbiology Laboratory for their assistance, members of our multidisciplinary lung transplant team for their feedback, and our patients for their participation.
R.H. and A.E.G. were funded by the Cystic Fibrosis Foundation. H.S.K. was supported by the American Lung Association and the National Heart, Lung, and Blood Institute under Award Number K08HL148510. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2 TR002346 (to H.S.K. and L.T., PI: Victoria J. Fraser, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- AMR
antibody-mediated rejection
- BAL
bronchoalveolar lavage
- CARV
community-acquired respiratory viruses
- CI
confidence interva
- CLAD
chronic lung allograft dysfunction
- CMV
cytomegalovirus
- CPB
cardiopulmonary bypass
- CPRA
calculated panel reactive antibody
- DSA
donor specific antibodies
- G-CSF
granulocyte-colony stimulating factor
- HALT
HLA Antibodies after Lung Transplantation (study)
- HLA
human leukocyte antigen
- HR
hazard ratio
- ISHLT
International Society of Heart and Lung Transplantation
- IQR
interquartile range
- LAS
Lung Allocation Score
- MFI
mean fluorescent intensity
- PGD
primary graft dysfunction
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DESCRIPTION OF SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
DATA SHARING POLICY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018;37(10):1169–1183. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni HS, Cherikh WS, Chambers DC, et al. Bronchiolitis obliterans syndrome-free survival after lung transplantation: An International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. J Heart Lung Transplant. 2019;38(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Royer P-J, Olivera-Botello G, Koutsokera A, et al. Chronic Lung Allograft Dysfunction: A Systematic Review of Mechanisms. Transplantation. 2016;100(9):1803–1814. [DOI] [PubMed] [Google Scholar]
- 4.Hachem RR. Humoral responses after lung transplantation. Curr Opin Organ Transplant. 2016;21(3):267–271. [DOI] [PubMed] [Google Scholar]
- 5.Morrell MR, Pilewski JM, Gries CJ, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. 2014;33(12):1288–1294. [DOI] [PubMed] [Google Scholar]
- 6.Safavi S, Robinson DR, Soresi S, Carby M, Smith JD. De novo donor HLA-specific antibodies predict development of bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2014;33(12):1273–1281. [DOI] [PubMed] [Google Scholar]
- 7.Kauke T, Kneidinger N, Martin B, et al. Bronchiolitis obliterans syndrome due to donor-specific HLA-antibodies. Tissue Antigens. 2015;86(3):178–185. [DOI] [PubMed] [Google Scholar]
- 8.Verleden SE, Vanaudenaerde BM, Emonds M-P, et al. Donor-specific and -nonspecific HLA antibodies and outcome post lung transplantation. Eur Respir J. 2017;50(5). [DOI] [PubMed] [Google Scholar]
- 9.Courtwright A, Diamond JM, Wood I, et al. Detection and clinical impact of human leukocyte antigen antibodies in lung transplantation: A systematic review and meta-analysis. HLA. 2018;91(2):102–111. [DOI] [PubMed] [Google Scholar]
- 10.Tikkanen JM, Singer LG, Kim SJ, et al. De Novo DQ Donor-Specific Antibodies Are Associated with Chronic Lung Allograft Dysfunction after Lung Transplantation. Am J Respir Crit Care Med. 2016;194(5):596–606. [DOI] [PubMed] [Google Scholar]
- 11.Le Pavec J, Suberbielle C, Lamrani L, et al. De-novo donor-specific anti-HLA antibodies 30 days after lung transplantation are associated with a worse outcome. J Heart Lung Transplant. 2016;35(9):1067–1077. [DOI] [PubMed] [Google Scholar]
- 12.Hachem RR, Kamoun M, Budev MM, et al. Human leukocyte antigens antibodies after lung transplantation: Primary results of the HALT study. Am J Transplant. 2018;18(9):2285–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharat A, Kuo E, Steward N, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86(1):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ius F, Sommer W, Tudorache I, et al. Early donor-specific antibodies in lung transplantation: risk factors and impact on survival. J Heart Lung Transplant. 2014;33(12):1255–1263. [DOI] [PubMed] [Google Scholar]
- 15.Islam AK, Sinha N, DeVos JM, et al. Early clearance vs persistence of de novo donor-specific antibodies following lung transplantation. Clin Transplant. 2017;31(8). [DOI] [PubMed] [Google Scholar]
- 16.Burguete SR, Maselli DJ, Fernandez JF, Levine SM. Lung transplant infection. Respirology. 2013;18(1):22–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregson AL. Infectious Triggers of Chronic Lung Allograft Dysfunction. Curr Infect Dis Rep. 2016;18(7):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belperio J, Palmer SM, Weigt SS. Host-Pathogen Interactions and Chronic Lung Allograft Dysfunction. Ann Am Thorac Soc. 2017;14(Supplement_3):S242–S246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Orsogna L, van den Heuvel H, van Kooten C, Heidt S, Claas FHJ. Infectious pathogens may trigger specific allo-HLA reactivity via multiple mechanisms. Immunogenetics. 2017;69(8–9):631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curran CS, Bolig T, Torabi-Parizi P. Mechanisms and Targeted Therapies for Pseudomonas aeruginosa Lung Infection. Am J Respir Crit Care Med. 2018;197(6):708–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsui K, Aguilar P, Byers D, et al. Risk Factors for the Development of Donor-specific Antibodies and Their Impact on Outcomes After Lung Transplantation. J Heart Lung Transplant. 2018;37(4):S16. [Google Scholar]
- 22.Gregson AL, Wang X, Weigt SS, et al. Interaction between Pseudomonas and CXC chemokines increases risk of bronchiolitis obliterans syndrome and death in lung transplantation. Am J Respir Crit Care Med. 2013;187(5):518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregson AL, Wang X, Injean P, et al. Staphylococcus via an interaction with the ELR+ CXC chemokine ENA-78 is associated with BOS. Am J Transplant. 2015;15(3):792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botha P, Archer L, Anderson RL, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008;85(5):771–774. [DOI] [PubMed] [Google Scholar]
- 25.Gottlieb J, Mattner F, Weissbrodt H, et al. Impact of graft colonization with gram-negative bacteria after lung transplantation on the development of bronchiolitis obliterans syndrome in recipients with cystic fibrosis. Respir Med. 2009;103(5):743–749. [DOI] [PubMed] [Google Scholar]
- 26.Gandotra S, Ravichandran B, Lockman DK, et al. Impact of De-escalation of Antibiotic Surgical Prophylaxis in Lung Transplant Recipients. Open Forum Infect Dis. 2018;5(suppl_1):S629–S630. [Google Scholar]
- 27.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1097–1103. [DOI] [PubMed] [Google Scholar]
- 28.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229–1242. [DOI] [PubMed] [Google Scholar]
- 29.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33(2):127–133. [DOI] [PubMed] [Google Scholar]
- 30.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133(6):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas L, Reyes EM. Tutorial: Survival Estimation for Cox Regression Models with Time-Varying Coefficients Using SAS and R. Journal of Statistical Software. 2014;61(1):1–23. [Google Scholar]
- 32.Saini D, Weber J, Ramachandran S, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30(6):624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lederer DJ, Bell SC, Branson RD, et al. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Ann Am Thorac Soc. 2019;16(1):22–28. [DOI] [PubMed] [Google Scholar]
- 34.Poguntke I, Schumacher M, Beyersmann J, Wolkewitz M. Simulation shows undesirable results for competing risks analysis with time-dependent covariates for clinical outcomes. BMC Med Res Methodol. 2018;18(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roux A, Bendib Le Lan I, Holifanjaniaina S, et al. Characteristics of Donor-Specific Antibodies Associated With Antibody-Mediated Rejection in Lung Transplantation. Front Med. 2017;4:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweet SC, Chin H, Conrad C, et al. Absence of evidence that respiratory viral infections influence pediatric lung transplantation outcomes: Results of the CTOTC-03 study. Am J Transplant. 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobo LJ, Aris RM, Schmitz J, Neuringer IP. Donor-specific antibodies are associated with antibody-mediated rejection, acute cellular rejection, bronchiolitis obliterans syndrome, and cystic fibrosis after lung transplantation. J Heart Lung Transplant. 2013;32(1):70–77. [DOI] [PubMed] [Google Scholar]
- 38.Nayak DK, Zhou F, Xu M, et al. Zbtb7a induction in alveolar macrophages is implicated in anti-HLA-mediated lung allograft rejection. Sci Transl Med. 2017;9(398). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto S, Nava RG, Zhu J, et al. Cutting edge: Pseudomonas aeruginosa abolishes established lung transplant tolerance by stimulating B7 expression on neutrophils. J Immunol. 2012;189(9):4221–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borthwick LA, Suwara MI, Carnell SC, et al. Pseudomonas aeruginosa Induced Airway Epithelial Injury Drives Fibroblast Activation: A Mechanism in Chronic Lung Allograft Dysfunction. Am J Transplant. 2016;16(6):1751–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vos R, Vanaudenaerde BM, Geudens N, Dupont LJ, Van Raemdonck DE, Verleden GM. Pseudomonal airway colonisation: risk factor for bronchiolitis obliterans syndrome after lung transplantation? Eur Resp J. 2008;31(5):1037–1045. [DOI] [PubMed] [Google Scholar]
- 42.Mandelbrot DA, Kishimoto K, Auchincloss H, Sharpe AH, Sayegh MH. Rejection of mouse cardiac allografts by costimulation in trans. J Immunol. 2001;167(3):1174–1178. [DOI] [PubMed] [Google Scholar]
- 43.Bharat A, Chiu S, Zheng Z, et al. Lung-Restricted Antibodies Mediate Primary Graft Dysfunction and Prevent Allotolerance after Murine Lung Transplantation. Am J Respir Cell Mol Biol. 2016;55(4):532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilhelm I, Levit-Zerdoun E, Jakob J, et al. Carbohydrate-dependent B cell activation by fucose-binding bacterial lectins. Sci Signal. 2019;12(571). [DOI] [PubMed] [Google Scholar]
- 45.Cunningham AC, Zhang JG, Moy JV, Ali S, Kirby JA. A comparison of the antigen-presenting capabilities of class II MHC-expressing human lung epithelial and endothelial cells. Immunology. 1997;91(3):458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milne DS, Gascoigne AD, Wilkes J, et al. MHC class II and ICAM-1 expression and lymphocyte subsets in transbronchial biopsies from lung transplant recipients. Transplantation. 1994;57(12):1762–1766. [PubMed] [Google Scholar]
- 47.Jung S, Schickel J-N, Kern A, et al. Chronic bacterial infection activates autoreactive B cells and induces isotype switching and autoantigen-driven mutations. Eur J Immunol. 2016;46(1):131–146. [DOI] [PubMed] [Google Scholar]
- 48.Vu Van D, Beier KC, Pietzke L-J, et al. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat Commun. 2016;7:10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robak OH, Heimesaat MM, Kruglov AA, et al. Antibiotic treatment-induced secondary IgA deficiency enhances susceptibility to Pseudomonas aeruginosa pneumonia. J Clin Invest. 2018;128(8):3535–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuijt TJ, Lankelma JM, Scicluna BP, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alegre M-L, Bartman C, Chong AS. Microbes and allogeneic transplantation. Transplantation. 2014;97(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cribbs SK, Beck JM. Microbiome in the pathogenesis of cystic fibrosis and lung transplant-related disease. Transl Res. 2017;179:84–96. [DOI] [PubMed] [Google Scholar]
- 53.Sunder S, Witt C, Byers D, Hachem R. Risk Factors for the Development of Antibody-mediated Rejection (AMR) after Lung Transplantation. J Heart Lung Transplant. 2019;38(4):S408. [Google Scholar]
- 54.Kulkarni HS, Bemiss BC, Hachem RR. Antibody-mediated Rejection in Lung Transplantation. Curr Transplant Rep. 2015;2(4):316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.