Abstract
Split liver transplantation (SLT) is one strategy for maximizing the number of deceased donor liver transplants. Recent reports suggest that utilization of SLT in the US remains low. We examined deceased donor offers that were ultimately split between 2010-2014. SLTs were categorized as “primary” and “secondary” transplants. We analyzed allocation patterns and used logistic regression to evaluate factors associated with secondary split discard. 418 livers were split: 54% from adult, 46% from pediatric donors. Of the 227 adult donor livers split, 61% met UNOS “optimal” split criteria. A total of 770 recipients (418 primary and 352 secondary) were transplanted, indicating 16% discard. 92% of the 418 primary recipients were children, and 47% were accepted on the first offer. 87% of the 352 secondary recipients were adults, and 7% were accepted on the first offer. Of the 352 pairs, 99% were transplanted in the same region, 36% at the same center. In logistic regression, shorter donor height was associated with secondary discard (OR 0.97 per cm, 95%CI 0.94-1.00, p=0.02. SLT volume by center was not predictive of secondary discard. Current policy proposals that incentivize SLT in the US could increase the number of transplants to children and adults.
1. Introduction:
Deceased donor split liver transplantation (SLT) was pioneered in the late 1980s when Pichlmayr et al reported the first case of utilizing one donor liver for transplantation of two recipients.(1) SLT offers pediatric patients and adults of short stature increased access to transplant by making size-appropriate livers available from a liver donor pool comprised of predominantly adult men.(2,3) Given that pediatric waitlist mortality has remained stubbornly high and a substantial number of ideal pediatric donor livers are diverted to adult candidates, there has been a greater urgency for policy solutions to address the unmet needs of pediatric and small adult transplant candidates.(4,5) However, despite improvements in surgical techniques and expertise, SLT remains underutilized with stagnant case volumes.(6,7) While the slow adoption of SLT has historically been attributed to higher rates of post-operative biliary and vascular complications leading to lower survival,(8) more recent studies have demonstrated similar rates of survival in recipients of SLT compared to whole liver transplantation (WLT).(6,7,9) These improvements in post-transplant mortality, with continued waitlist mortality amongst children and adults, suggest that expanded use of SLT could benefit both children and adults.(10–12)
As such, Perito et al recently reported that there were more potentially “split-able” livers than pediatric waitlist deaths.(3) Recognizing the potential value of SLT, policymakers and transplant centers have recently advocated for a “split liver variance” to incentivize SLT by allowing transplant centers to have preferential access to a “secondary split,” defined as the portion available after the primary segment is utilized for a recipient.(13) While there is ample opportunity for increased utilization of SLT, the current allocation patterns of split organs in the United States remain unclear, especially with regards to the fate of secondary splits, such as whether they remain within the same region or transplant center as primary splits. Characterization of secondary split allocation patterns may help inform policy revisions as the “split liver variance” is being implemented. In this study, we examine the complex SLT allocation patterns with attention to the secondary splits, or the liver segments that are returned to the donor pool after the initial segments are allocated.
2. Materials and Methods:
We examined all pediatric and adult United States deceased liver donor offers that were utilized for SLT from January 1, 2010 through December 31, 2014. Donors were followed until removal from the offer list or the end of the study period (December 31, 2014). We limited our analyses to this time period due to timely availability of data from the Potential Transplant Recipient (PTR) database due to its requiring additional programming. Data on recipients, deceased liver donors, and match run information were obtained from the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation (OPTN) Standard Transplant Analysis and Research (STAR) and PTR files made public as of March 31, 2016.
Deceased liver donor offers allocated for SLT were detected by the variables “split_liver” and “lityp.” Livers allocated as “partial” or “reduced,” as indicated by the “lityp” variable, were not included in our tabulation of split liver offers. An SLT was categorized as “primary” if it was the first segment offered and “secondary” if it was the second segment offered from one donor, based on the organ offer sequence numbers extracted from the PTR database. The Institutional Review Board at the University of California, San Francisco approved this study.
2.1. Deceased Donor Characteristics
Split liver donors were categorized into two cohorts: donors whose livers were split but ultimately transplanted into one recipient, “primary without secondary split,” and donors whose livers were split and transplanted into two recipients, “primary paired with secondary split.” Donors were characterized by the variables defined by the Donor Risk Index: age, sex, race/ethnicity, height, HCV antibody status, Public Health Service (PHS) increased risk donors, cause of death, donation after cardiac death, and donor location.(14)
We calculated the number of adult donors qualifying as “optimal” split candidates based on criteria utilized by UNOS since November 2007: 1. Less than 40 years old; 2. On zero or one vasopressor ; 3. Transaminases no greater than three times the upper limit of normal; 4. Body mass index (BMI) 28 or less.(15) We defined three different donor age categories (pediatric age < 12 years, pediatric age 12-17 years, and adult ≥ 18 years) following UNOS-designated allocation policies governing donors in these age groups. Characteristics of each donor liver were available only at transplant, therefore, we obtained these data by matching the donor identification number at offer with that at transplantation.
2.2. Offeree and Recipient Characteristics
All recipients who received a split donor liver were defined as either “primary transplant,” indicating the primary split offered and transplanted, or “secondary transplant,” indicating the secondary split offered and transplanted. Primary transplant without a secondary split were deemed to have a secondary split discard – this was reconciled with the STAR Deceased Donor Database to determine disposition and rationale for discard, if given. Location of primary versus secondary transplants were calculated by matching UNOS region and transplant center codes extracted from transplant data.
Baseline demographic data of waitlist candidates who received offers of split livers, “offerees,” and recipients who were ultimately transplanted with split livers included age, sex, race/ethnicity, height, weight, and BMI. Clinical variables included offeree and recipient ABO blood type, etiology of liver disease, and if any exception points were ever granted. Initial allocation Model for End-Stage Liver Disease (MELD) or Pediatric End-Stage Liver Disease (PELD) score was considered for waitlist offerees while MELD or PELD at transplantation was considered for recipients. UNOS regions were categorized into “high” (regions 1, 5, 7, and 9), “medium” (regions 2, 4, and 6), and “low” (regions 3, 8, 10, and 11) regions based on median allocation MELD/PELD at transplantation. Race/ethnicity was classified into the following categories: Non-Hispanic White, Hispanic, Black, Asian, or Other/Multiracial. Etiologies of liver disease were categorized as: chronic liver diseases (including diseases such as primary biliary cirrhosis and primary sclerosing cholangitis), pediatric cholestatic diseases (including biliary atresia, hypoplasia, and familial cholestasis), malignancy/tumor, inborn errors of metabolism, acute hepatic necrosis, graft failure, and others.
2.3. Statistical Analyses:
Clinical characteristics and laboratory data for donors and recipients were summarized by medians and interquartile ranges (IQR) for continuous variables or numbers and percentages (%) for categorical variables. Comparisons among groups were performed using chi-square and Kruskal-Wallis tests. Primary and secondary allocation offer rates and acceptance rates were calculated through matching of deceased donor data with match-run data featured in the PTR database.
We used univariate and multivariate logistic regression models to assess for predictors of secondary split discard rates – i.e. primary splits without an associated secondary split allocated. Covariables evaluated in the logistic models included age, sex, race/ethnicity, height, HCV antibody status, PHS-increased risk donors, cause of death, donation after cardiac death, and donor location. Two-sided p-values <0.05 were considered statistically significant in all analyses. Analyses were performed using STATA statistical software, version 13.0 (StataCorp, College Station, TX, USA).
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and should in no way be seen as an official policy of or interpretation by the OPTN or the United States Government.
3. Results:
In the 5-year study period between January 1, 2010 and December 31, 2014, there were 8,931 deceased donors who met “optimal” criteria for splitting, but only 418 (4.7%) deceased donors whose livers were utilized for SLT. Of these 418 deceased donors: 54% were adults ≥ 18 years (n=227), 40% (167) were pediatric donors between 12-17 years (n=167), and 6% (24) were pediatric donors < 12 years. These 418 donors led to 770 recipient transplants, 418 primary transplants and 352 secondary paired transplants. Sixty-six (16%) of the primary transplants did not have a secondary paired allocation, implying discard. Of these 66 implied discards, 56 had disposition variables entered for the secondary split in the deceased donor database.
Of the 418 recipients of primary transplants, 90% (375) were pediatric recipients age < 12 years, 2% (8) were pediatric recipients between ages 12-17 years, and 8% (35) were adults ≥ 18 years. In contrast, of the 352 recipients of secondary transplants, 7% (26) were pediatric recipients age < 12 years, 6% (20) were pediatric recipients between ages 12-17 years, and 87% (306) were adults ≥ 18 years.
3.1. Baseline Characteristics of Donors
Baseline characteristics of SLT donors are presented in Table 1, categorized by whether donor livers were utilized for one recipient (“primary without secondary split”) or two recipients (“primary paired with secondary split).” Sixteen percent (66) of the 418 donor livers were split but only one portion of the liver was transplanted (for one recipient) – the remaining 84% (352) were transplanted into two recipients. The two groups of donors did not differ significantly by gender, race, donor ALT, inotrope use, positive HCV antibody, positive HBV core antibody, PHS high risk designation, donor ABO status, or mechanism of death. Of the 227 adult donors whose livers were split, only 61% (140) met criteria for “optimal” donor for splitting per UNOS guidelines defined above.(15)
Table 1 –
Baseline Characteristics of the 418 Liver Donors between 2010-2014
| Primary w/o Secondary (n = 66) | Primary Paired w/ Secondary (n = 352) | P-Value | |
|---|---|---|---|
| Pediatric Donors Age < 12 | 15 (23) | 9 (3) | <0.01 |
| Pediatric Donors Ages 12-17 | 13 (27) | 149 (42) | |
| Adult Donors Age >= 18 | 33 (50) | 194 (55) | |
| Adult Donors Qualifying Optimal Split | 16 (48) | 124 (64) | 0.083 |
| Age in Years (IQR) | 17.5 (12-23) | 19 (16-27) | <0.01 |
| Female | 28 (42) | 115 (33) | 0.13 |
| African-American Race (%) | 10 (15) | 59 (17) | 0.75 |
| Donor Height, cm (IQR) | 165.1 (152.4-175) | 172.7 (163.5-180) | <0.01 |
| Donor Weight, kg (IQR) | 61.5 (50.4-71.4) | 69.4 (59.9-79.4) | <0.01 |
| Donor BMI, kg/m2 (IQR) | 22.2 (19.6-25.7) | 23.4 (21.2-25.7) | 0.06 |
| Body Surface Area, m2 (IQR) | 1.68 (1.52-1.84) | 1.83 (1.67-1.96) | <0.01 |
| Donor ALT (IQR) | 32 (23-60) | 32 (21-56.5) | 0.45 |
| Donor on Inotropes (%) | 38 (58) | 190 (54) | 0.53 |
| Positive HCV Antibody (%) | 0 (0) | 1 (0.2) | 0.67 |
| Positive HBV Core Antibody (%) | 0 (0) | 2 (0.6) | 0.54 |
| CDC High Risk (%) | 7 (11) | 28 (8.0) | 0.48 |
| Donor ABO (%) | 0.29 | ||
| O | 45 (68) | 210 (60) | |
| A | 18 (27) | 99 (28) | |
| B | 3 (4.5) | 40 (11) | |
| AB | 0 (0) | 3 (8.5) | |
| Mechanism of Death (%) | 0.33 | ||
| Anoxia | 19 (29) | 67 (19) | |
| Cerebrovascular Accident | 8 (12) | 56 (16) | |
| Trauma | 32 (48) | 222 (63) | |
| Other | 1 (1.5) | 7 (2.0) | |
| Donation After Cardiac Death (%) | 0 (0) | 0 (0) | |
| Cold Ischemia Time, hrs (IQR) | 6 (5-8) | 7.4 (5.9-9.1) | <0.01 |
| Donor/Recipient Distance, per 100 miles ( IQR) | 0.62 (0.11-1.70) | 1.29 (0.22-2.85) | 0.04 |
Compared to donors whose livers were ultimately utilized for two recipients, donors only utilized for one recipient were more likely to be pediatric donors < 12 years (23% vs 3%, p<0.01) and less likely to be from a pediatric donor between ages 12-17 (27% vs 42%, p<0.01) or from an adult donor ≥ 18 years (50% vs 55%, p<0.01). Almost all indications of donor size (donor height, weight, and body surface area) were significantly smaller for donors utilized for only one recipient. Compared to donors whose livers were allocated to two recipients, those donors whose livers were transplanted for only one recipient had shorter cold ischemic times (6 vs 7 hours, p<0.01), and distances between donor and primary recipient (62 vs 129 miles, p=0.04).
3.2. Baseline Characteristics of Candidates who Received Split Offers and Recipients
The baseline characteristics of candidates who were offered split livers are shown in Table 2, categorized by whether candidates were offered the first (“primary split offerees”) or the second (“secondary split offerees”) split from a deceased donor liver. In total, 1,329 candidates received 1,483 primary split offers, and 7,518 candidates received 9,364 secondary split offers. Compared to secondary split offerees, primary split offerees were more likely to be pediatric patients < 12 years (44% vs 6%, p<0.01).
Table 2 –
Baseline Characteristics of Partial and Split Offerees 2010-2014
| Primary Split Offerees (n = 1329) | Secondary Split Offerees (n = 7518) | P-Value | |
|---|---|---|---|
| Total Partial/Split Offers Received | 1483 | 9364 | |
| Age at listing yr. (IQR) | 30 (1-55) | 55 (47-61) | <0.01 |
| Pediatric Patients < 12 years | 583 (44) | 462 (6.1) | <0.01 |
| Female | 573 (43) | 2732 (36) | <0.01 |
| Race/Ethnicity, no. (%) | 0.05 | ||
| White | 823 (62) | 4875 (65) | |
| Black | 133 (10) | 666 (8.9) | |
| Hispanic | 286 (22) | 1465 (19) | |
| Asian | 59 (4.4) | 402 (5.3) | |
| Other | 28 (2.1) | 108 (1.4) | |
| Height, cm (IQR) | 157.5 (81.1-172.7) | 170.2 (161.0-177.8) | <0.01 |
| Weight, kg (IQR) | 57.0 (11.3-83.0) | 80 (67.1-93.9) | <0.01 |
| BMI, kg/m2 (IQR) | 21.4 (16.8-28.0) | 27.4 (23.7-31.6) | <0.01 |
| Waitlist Candidate ABO, no. (%) | <0.01 | ||
| O | 895 (67) | 5005 (67) | |
| A | 273 (21) | 1937 (26) | |
| B | 119 (9.0) | 459 (6.1) | |
| AB | 42 (3.2) | 116 (1.5) | |
| Etiology of Liver Disease, no. (%) | <0.01 | ||
| Chronic Liver Diseases | 188 (14) | 2001 (27) | |
| Pediatric Cholestatic Diseases | 228 (17) | 203 (2.7) | |
| Malignancy/Tumor | 140 (11) | 1390 (18) | |
| Inborn Errors of Metabolism | 128 (9.6) | 164 (2.2) | |
| Acute Hepatic Necrosis | 73 (5.5) | 49 (0.7) | |
| Graft Failure | 19 (1.4) | 43 (0.6) | |
| Other | 553 (42) | 3668 (49) | |
| Any exception points, no. (%) | 437 (33) | 2470 (33) | <0.01 |
| Initial MELD | 13 (7-19) | 14 (10-18) | 0.14 |
| MELD Region | <0.01 | ||
| Low MELD | 702 (53) | 2069 (28) | |
| Medium MELD | 227 (17) | 1920 (26) | |
| High MELD | 400 (30) | 3528 (47) |
Table 3 displays the characteristics of split liver recipients based on whether they were transplanted with the first (“primary recipient”) or the second (“secondary recipient”) allocation. Primary recipients were overwhelmingly children < 12 years (90%), with a median age of only 1 year old. As such, they were significantly shorter (76cm), weighed less (57kg), and had a lower BMI (21 kg/m2). Of note, 64% of primary recipients received their transplants under regional allocation.
Table 3 –
Baseline Characteristics of Partial and Split Liver Recipients 2010-2014
| Primary Recipients (n = 418) | Secondary Recipients (n = 352) | P-Value | |
|---|---|---|---|
| Age at transplant, yr. (IQR) | 1 (0-5) | 56 (42-62) | <0.01 |
| Pediatric Patients < 12 years | 375 (90) | 26 (7.4) | <0.01 |
| Female | 203 (49) | 198 (56) | 0.04 |
| Race/Ethnicity, no. (%) | <0.01 | ||
| White | 213 (51) | 210 (60) | |
| Black | 64 (15) | 26 (7.4) | |
| Hispanic | 107 (26) | 72 (20) | |
| Asian | 26 (6.2) | 39 (11) | |
| Other | 8 (1.9) | 5 (1.4) | |
| Height, cm (IQR) | 76 (65-105) | 163 (157-173) | <0.01 |
| Weight, kg (IQR) | 10 (6.8-18) | 68 (56-81) | <0.01 |
| BMI, kg/m2 (IQR) | 17 (15-18) | 25 (22-29) | <0.01 |
| Waitlist Candidate ABO, no. (%) | |||
| O | 203 (49) | 195 (55) | |
| A | 125 (30) | 101 (29) | |
| B | 71 (17) | 46 (13) | |
| AB | 19 (4.5) | 10 (2.8) | |
| Etiology of Liver Disease, no. (%) | <0.01 | ||
| Chronic Liver Diseases | 36 (8.6) | 168 (4.7) | |
| Pediatric Cholestatic Diseases | 186 (44) | 25 (7.1) | |
| Malignancy/Tumor | 59 (14) | 121 (34) | |
| Inborn Errors of Metabolism | 57 (14) | 11 (3.1) | |
| Acute Hepatic Necrosis | 45 (11) | 13 (3.7) | |
| Graft Failure | 9 (2.1) | 1 (0.3) | |
| Other | 26 (6.2) | 13 (3.7) | |
| Any exception points, no. (%) | 215 (51) | 194 (55) | 0.22 |
| Allocation Match Category (%) | <0.01 | ||
| Local/State | 136 (33) | 243 (69) | |
| Regional | 268 (64) | 87 (25) | |
| National | 9 (2.2) | 5 (1.4) | |
| Status 1 | <0.01 | ||
| Non-Status 1 | 253 (61) | 329 (98) | |
| Status 1A | 62 (15) | 4 (1.2) | |
| Status 1B | 98 (24) | 3 (0.9) | |
| PELD/MELD at Transplantation, IQR | 30 (22-38) | 25 (22-31) | <0.01 |
| MELD Region | <0.01 | ||
| Low MELD | 132 (32) | 101 (29) | |
| Medium MELD | 80 (19) | 67 (19) | |
| High MELD | 206 (49) | 184 (52) |
In comparison, secondary recipients were overwhelmingly adults ≥ 18 years of age (87%) with a median age of 56 years. In addition, they were taller (163cm), weighed more (68kg), and had higher BMIs (25 kg/m2). Chronic liver diseases (47%) and malignancy/tumor (34%) were the leading indications for transplantation in secondary recipients. 69% of secondary recipients received their transplants under local allocation. 98% of secondary recipients were not Status 1.
3.3. Split Liver Allocation and Acceptance Patterns
Allocation patterns of split livers are presented in table 4, divided by primary split and secondary split transplants. Of the 418 primary transplants, 47% (198) were accepted on the first offer. Primary splits were accepted at a median of 3 offers. 66 (16%) primary transplants did not have a paired secondary split, implying discard of the remaining portion of the split donor organ. Of these 66 implied discards, 56 had disposition variables reconciled from the Deceased Donor Database: nine organs were not recovered, six were recovered not for transplantation, and 41 recovered for transplantation but not transplanted. Of the 352 secondary transplants, only 7% (24) were accepted on the first re-offer. Secondary split remained on the offer list for longer, going through a median of 12 offers prior to being accepted.
Table 4 –
Primary and Secondary Offer Data for Split Livers
| Primary Transplants | Secondary Transplants | |
|---|---|---|
| Splits Transplanted | 418 | 352 |
| Accepted on First Offer (Re-Offer for Secondary Transplants) | 198 (47) | 24 (6.8) |
| Median Offer Numbers for Splits not Accepted on First Offer | 3 (2-4) | 12 (6-29) |
| Primary Transplants without Paired Secondary Allocation | 66 (16) |
Analyses of regional distribution of the 352 secondary transplants showed that 99% (350) of the split organs were transplanted in the same region as the primary transplant. Only 36% (127) of the 352 secondary transplants remained in the same transplant center as the primary allocation (Figure 1). 76 transplant centers conducted at least one SLT during the 5-year study period. Of these 76 centers, 40 conducted ≥ 5 SLTs and 10 conducted ≥ 20 SLTs. As such, SLTs (both primary and secondary transplants) were heavily concentrated in the 10 transplant centers that conducted ≥ 20 SLTs. They were responsible for 49% (378) of the 770 total SLTs. In the 431 pediatric recipients < 18 years of primary and secondary splits, these 10 centers were responsible for 55% of the total pediatric SLT volume. Secondary split discard rates in these 10 centers ranged from 0 to 19%, with a median of 10%.
Figure 1 –
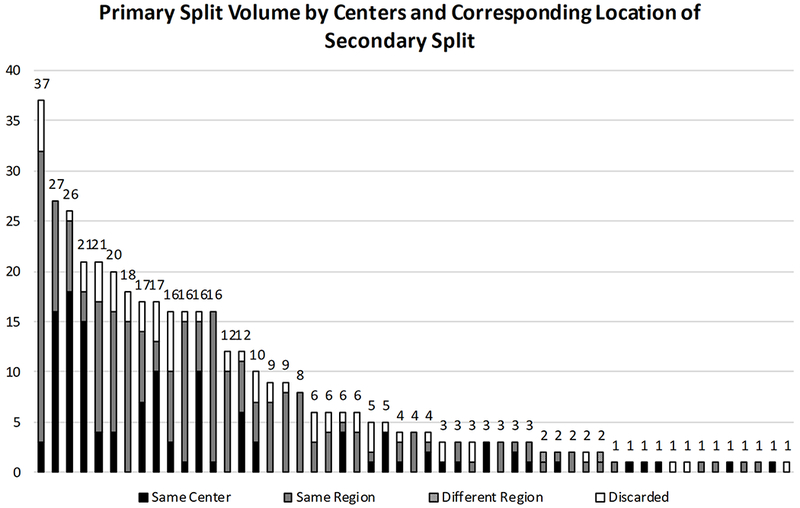
Distribution of Primary Split Liver Volume and Corresponding Secondary Split Locations
3.4. Factors Associated with “Secondary” Discard
Finally, we analyzed donor factors associated with secondary split discard, as 16% (66) of primary transplants did not have a paired secondary transplant (Table 5). In univariable logistic regression, variables associated with donor size were primarily associated with secondary split discard: donor age < 12 years (OR 9.80, 95%CI 3.92-24.3, p-value <0.01), age in years (OR 0.95, 95%CI 0.92-0.99, p-value <0.01), donor height (OR 0.95, 95%CI 0.93-0.97, p-value <0.01), donor weight (OR 0.97, 95%CI 0.95-0.98, p-value <0.01). In addition, each 1 hour decrease in cold ischemic time (OR 0.84, 95%CI 0.75-0.94, p-value <0.01) and every 100 mile increase in distance between donor and recipient (1.14, 95%CI 1.01-1.29, p-value = 0.04) were also associated with secondary split discard.
Table 5 –
Association of Donor Characteristics with Secondary Split Discard
| Univariable Logistic Regression | Multivariable Logistic Regression | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P-Value | Odds Ratio | 95% CI | P-Value | |
| Donor Age | ||||||
| Adult Donors Age >= 18 | Ref | Ref | ||||
| Pediatric Donors Ages 12-17 | 0.71 | 0.38-1.31 | 0.27 | 0.63 | 0.33-1.17 | 0.14 |
| Pediatric Donors Age < 12 | 9.80 | 3.96-24.2 | <0.01 | 3.33 | 0.96-11.5 | 0.06 |
| Age in Years | 0.95 | 0.92-0.99 | <0.01 | |||
| Female | 1.52 | 0.89-2.60 | 0.13 | |||
| African-American Race (%) | 0.89 | 0.43-1.84 | 0.75 | |||
| Donor Height (cm) | 0.95 | 0.93-0.97 | <0.01 | 0.97 | 0.94-1.00 | 0.02 |
| Donor Weight (kg) | 0.97 | 0.95-0.98 | <0.01 | |||
| Donor BMI (kg/m2) | 0.94 | 0.87-1.01 | 0.09 | |||
| Body Surface Area (m2) | 0.09 | 0.03-0.26 | <0.01 | |||
| Donor ALT | 1.00 | 1.00-1.00 | 0.83 | |||
| Donor on Inotropes (%) | 1.19 | 0.69-2.03 | 0.54 | |||
| CDC High Risk (%) | 1.37 | 0.57-3.29 | 0.48 | |||
| Donor ABO (%) | ||||||
| O | Ref | |||||
| A | 0.85 | 0.47-1.54 | 0.59 | |||
| B | 0.35 | 0.10-1.18 | 0.09 | |||
| AB | 1 | |||||
| Mechanism of Death (%) | ||||||
| Trauma | Ref | |||||
| Anoxia | 1.66 | 0.90-3.06 | 0.11 | |||
| Cerebrovascular Accident | 0.83 | 0.37-1.89 | 0.66 | |||
| Other | 0.83 | 0.10-6.98 | 0.87 | |||
| Donation After Cardiac Death (%) | 1 | |||||
| Cold Ischemia Time (hours) | 0.84 | 0.75-0.94 | <0.01 | |||
| Donor/Recipient Distance (per 100 miles) | 1.14 | 1.01-1.29 | 0.04 | |||
After adjustment, the multivariable logistic regression showed shorter donor height (OR 0.97 per cm, 95%CI 0.94-1.00, p=0.02) was significantly associated with secondary discard while donor age <12y (OR 3.30, 95%CI 0.96-11.5, p=0.06) did not reach statistical significance. Donor weight and donor BMI were strongly associated with donor height; therefore these variables were dropped in the multivariable logistic regression. Of note, SLT volume by center was not predictive of secondary split discard in either univariable or multivariable logistic regression models.
4. Discussion:
In the United States, SLT is almost always initiated by allocation to a pediatric recipient (92%), with an adult recipient usually receiving the secondary transplant (87%). Primary split offers were readily accepted by their offerees – with 47% being accepted on the first offer to a candidate, while secondary split offers were turned down more frequently on the waitlist, with a median of 12 offers prior to acceptance. The profile of a secondary split recipient, however, skewed to adult waitlist candidates who were female (56%) and shorter than average (median height of 163cm). In contrast, the demographic profile of the entire adult waitlist pool was 36% female with a median height of 173cm.
While SLT were performed at least once in 76 of the 143 United States liver transplant centers during this 5-year study period, nearly half of SLTs were concentrated in 10 transplant centers. Not coincidentally, these were also the 10 transplant centers that performed ≥ 20 SLTs during the study period. While secondary split discard is not very common (16%) – our analyses suggested that donor size (as implied by height and age) rather than center volume (a surrogate for expertise) was the primary driver for this observed pattern. In small donors, the celiac artery is typically procured with the left segment leaving the right lobe with a small artery. The risk of hepatic artery thrombosis increases due to the vulnerability of the remaining blood supply to the right lobe. Vascular variations, therefore, appears to play a significant role in the decision to discard a secondary split. Secondary split transplants, for the most part, remained in the same region as their primary counterparts – however, only 36% remained at the same transplant center as the primary split. Of note, of the 227 adult donor livers that were ultimately split, only 61% (140) met the optimal donor criteria set by UNOS for splitting.
Our analyses revealed several notable facts about the current state of SLT allocation and have implications on current policy proposals to increase SLT. First, SLT almost always allowed for transplant of one child and one adult. Splits were rarely shared between two children or between two adults. Any expansion of SLT should be done to primarily promote the welfare of smallest pediatric candidates – who are at higher risk for waitlist mortality among children and have decreased access to liver transplantation.(16) A recent simulation conducted by Valentino et al noted that children who ultimately were transplanted with a whole liver transplant waited for an additional four months on the waitlist had they received a SLT.(17) The action of splitting in this circumstance does not “remove” a liver for the adult waitlist pool – unless the secondary segment is discarded. As donor height and age appear to be associated with secondary discard, this phenomenon could be avoided with increasing “optimal” donors selected for SLT.
Second, secondary splits largely benefit smaller adults – secondary recipients were majority women (56%) with a median height of 163cm (5 foot 4 inches). Women and men with short stature are two more populations currently disadvantaged and underserved by the allocation system due to appropriate size matching.(18) Secondary split livers could be prioritized for these vulnerable populations as they often languished on the waitlist waiting for a size-appropriate graft to become available.
Third, SLT expertise is concentrated in approximately 10 transplant centers. Most (64%) secondary splits are “exported” out of the transplant center that conducted the primary allocation. This is with the caveat that many adult transplant programs are affiliated with nearby pediatric transplant programs, but the affiliated programs are categorized as two separate transplant centers. A recent analysis conducted by Sasaki et al suggested that receiving SLT at a high-volume center and being a female adult recipient were protective against post-transplant graft loss. Any expansion of SLT by incentivizing the secondary split preferentially remaining in the same center will likely immediately benefit these “high-volume” centers. The introduction of split variance, however, may also induce “low-volume” centers to gain experience in SLT techniques and achieve outcomes currently seen by “high-volume” centers.
Fourth, Hsu et al and Perito et al previously found that while many adult deceased donor livers met “optimal” split criteria, few were actually split.(3,4) In addition, recent analyses showed that deceased donor organs suitable to be split most often went as whole transplants to adults with a median MELD score of 16.(17) International experiences have shown that countries that mandated splitting nearly eliminated pediatric liver waitlist deaths.(10) In our analyses, however, we found that nearly 2 out of 5 adult split liver donors did not meet UNOS criteria for “optimal” donors. This suggests that experienced centers and surgical teams may have a broader definition of which donor organs are acceptable for SLT than prescribed by the UNOS “optimal” criteria. This indicates that UNOS could revisit the “optimal” criteria and encourage further research into donor livers eligible for splitting.
Finally, previous research and our analyses suggest that SLT represents the greatest potential for creating value in liver transplantation. As Porter and Lee noted, “value is defined as the health outcomes achieved that matter to patients relative to the cost of achieving those outcomes.”(19) Value-based healthcare is the practice of medicine that incorporates evidenced-based data, patient-perceived value, and minimal resource expenditure in the intervention.(20) By expanding one scarce resource into two through splitting, and by providing early access to life saving interventions to vulnerable populations, such as children and adult women – SLT has the potential to create tremendous value for our patients.
This analysis has several limitations due to issues inherent in the UNOS registry STAR and PTR databases. As in previous analyses, we found that split liver segments reported in the registry were likely incorrectly categorized – e.g. right tri-segments were recorded to be the majority of allocation to children < 12 years and left lateral segments were recorded to be the majority of allocation to adults ≥ 18 years.(7) This is not known to reflect actual clinical practice in which children are most likely to receive left lateral segments. The possibly incorrect categorization of split segments meant that we could not analyze segment allocation patterns. This also limited our ability to discern left and right liver splits that may have been allocated to two adults. As these segment variables were also coded by in situ versus ex vivo splits, we also could not analyze the impact of in situ versus ex vivo splitting on allocation and discard patterns.
Our finding of 66 secondary splits discarded raises the concern that these donor livers were not split but were actually “paired-down” or reduced. We believed that this possibility was less likely as the “lityp” variable featured separate categories for “partial” or “reduced” livers that were different from splits. Due to limitations of match data available in the PTR database, we restricted our analyses to the five-year time period between January 1, 2010 through December 31, 2014 to define the precise order in which split liver segments were offered. While advancements in techniques and/or practice variations may have increased SLT in the time period since then, we found that 8,955 deceased donors met the “optimal” split criteria but only 384 donor livers were split resulting in 708 candidates transplanted with split livers in the time period from January 1, 2015 through December 31, 2018 from data available in the STAR database. This represented 4.3% of “optimal” deceased donor livers utilized for splits between 2015-2018, which is comparable to the 4.7% we found between 2010-2014.
We restricted our analyses to only donor livers that were ultimately successfully offered, allocated, and transplanted as these were the data available from the PTR and STAR databases. These analyses do not allow for any conclusions regarding “unsuccessful” donors whose organs could have been potentially split but were not offered to and/or were not transplanted into candidates.
In conclusion, these analyses suggest that expansion of SLT will benefit vulnerable populations, particularly children, adult women, and men with short stature. Our analyses, in combination with other recent analyses concerning SLT, indicate that “high-volume” centers will most likely immediately benefit from split variances that incentivize preferential allocation of the secondary split within the same transplant center. These incentives may, in turn, induce an increase in the number of “optimal” donors selected for SLT. Any expansion of SLT, however, must be balanced against the potential for unintended consequences. An increase in splitting might lead to an increase in discards of secondary split or additional sequestering of secondary splits within the same center as opposed to allocating to more medically urgent patients in nearby programs. The offering of secondary splits to high priority adults (Status 1 or MELD > 32) within the same acuity circle prior to being preferentially allocated in the same transplant center would mitigate this. However, a negative impact upon national waiting list mortality is unlikely as liver transplant numbers would be increased, those with an unmet need would be more often transplanted and all recipients of these offers would have transplant benefit as a function of existing allocation policies. Ultimately, policies to incentivize SLT in the United States could increase the number of pediatric and adult candidates transplanted and decrease deaths on the waitlist.
Acknowledgements:
Financial Support
This study was funded by R01AG059183/K23AG048337 (National Institute on Aging, Lai) and by 5T32DK060414-17 (National Institute of Diabetes and Digestive and Kidney Diseases, Ge). The funding agencies played no role in the analysis of the data or the preparation of this manuscript.
Abbreviations:
- SLT
split liver transplantation
- OR
odds ratio
- CI
confidence interval
- WLT
whole liver transplantation
- UNOS
United Network for Organ Sharing
- OPTN
Organ Procurement and Transplantation Network
- STAR
Standard Transplant Analysis and Research
- PTR
Potential Transplant Recipient
- PHS
Public Health Service
- BMI
body mass index
- MELD
Model for End-Stage Liver Disease
- PELD
Pediatric End-Stage Liver Disease
- IQR
interquartile range
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and should in no way be seen as an official policy of or interpretation by the OPTN or the United States Government.
References
- (1).Pichlmayr R, Ringe B, Gubernatis G, Hauss J, Bunzendahl H. [Transplantation of a donor liver to 2 recipients (splitting transplantation)--a new method in the further development of segmental liver transplantation]. Langenbecks Arch Chir 1988;373(2):127–130. [PubMed] [Google Scholar]
- (2).Lai JC, Feng S, Roberts JP, Terrault NA. Gender differences in liver donor quality are predictive of graft loss. Am. J. Transplant 2011;11(2):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Perito ER, Roll G, Dodge JL, Rhee S, Roberts JP. Split Liver Transplantation and Pediatric Waitlist Mortality in the United States: Potential for Improvement. Transplantation 2019;103(3):552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hsu EK, Shaffer ML, Gao L, Sonnenday C, Volk ML, Bucuvalas J, et al. Analysis of Liver Offers to Pediatric Candidates on the Transplant Wait List. Gastroenterology 2017;153(4):988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ge J, Hsu EK, Bucuvalas J, Lai JC. Deceased Pediatric Donor Livers: How Current Policy Drives Allocation and Transplantation. Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Cauley RP, Vakili K, Fullington N, Potanos K, Graham DA, Finkelstein JA, et al. Deceased-Donor Split-Liver Transplantation in Adult Recipients: Is the Learning Curve Over? Journal of the American College of Surgeons 2013;217(4):67–684.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sasaki K, Firl DJ, McVey JC, Schold JD, Iuppa G, Diago Uso T, et al. Elevated Risk of Split-Liver grafts in adult liver Transplantation: Statistical Artifact or Nature of the Beast? Liver Transpl. 2019;25(5):741–751. [DOI] [PubMed] [Google Scholar]
- (8).Cescon M, Spada M, Colledan M, Torre G, Andorno E, Valente U, et al. Feasibility and limits of split liver transplantation from pediatric donors: an italian multicenter experience. Ann. Surg 2006;244(5):805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Cauley RP, Vakili K, Potanos K, Fullington N, Graham DA, Finkelstein JA, et al. Deceased donor liver transplantation in infants and small children: are partial grafts riskier than whole organs? Liver Transpl. 2013;19(7):721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hsu EK, Mazariegos GV. Global lessons in graft type and pediatric liver allocation: A path toward improving outcomes and eliminating wait-list mortality. Liver Transpl. 2017;23(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Battula NR, Platto M, Anbarasan R, Perera M Thamara PR, Ong E, Roll GR, et al. Intention to Split Policy: A Successful Strategy in a Combined Pediatric and Adult Liver Transplant Center. Ann. Surg 2017;265(5):1009–1015. [DOI] [PubMed] [Google Scholar]
- (12).Mogul DB, Luo X, Garonzik-Wang J, Bowring MG, Massie AB, Schwarz KB, et al. Expansion of the Liver Donor Supply Through Greater Use of Split-Liver Transplantation: Identifying Optimal Recipients. Liver Transpl. 2019;25(1):119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).OPTN/UNOS Liver, Intestinal Transplantation Committee. Split Liver Variance. [Google Scholar]
- (14).Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am. J. Transplant 2006;6(4):783–790. [DOI] [PubMed] [Google Scholar]
- (15).Organ Procurement and Transplantation Network. Split Versus Whole Liver Transplantation. 2016; Available at: https://optn.transplant.hrsa.gov/resources/ethics/split-versus-whole-liver-transplantation/ Accessed 12/15/, 2018.
- (16).Hsu EK, Horslen SP, Reyes JD. Pediatric End-stage Liver Disease Scores as a Method of Assessing Mortality Risk or Prioritization to Transplantability: Let Us Save the Children. JAMA Pediatr 2018;172(11):1015–1017. [DOI] [PubMed] [Google Scholar]
- (17).Valentino PL, Emre S, Geliang G, Li L, Deng Y, Mulligan D, et al. Frequency of whole-organ in lieu of split-liver transplantation over the last decade: Children experienced increased wait time and death. Am. J. Transplant 2019. [DOI] [PubMed] [Google Scholar]
- (18).Lai JC, Terrault NA, Vittinghoff E, Biggins SW. Height contributes to the gender difference in wait-list mortality under the MELD-based liver allocation system. Am. J. Transplant 2010;10(12):2658–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Porter ME, Thomas H. Lee MD The Strategy That Will Fix Health Care. Harvard Business Review 2013 -10-01T04:00:00Z(October 2013). [Google Scholar]
- (20).What Is Value in Health Care? | NEJM. Available at: https://www.nejm.org/doi/10.1056/NEJMp1011024 Accessed May 19, 2019.


