Abstract
Introduction:
Type 1 hepatorenal syndrome (HRS) is a fatal complication of cirrhosis. Treatments trend towards HRS reversal, but few show clear mortality benefit. We sought to quantify the progress – or lack thereof – in improving outcomes of type 1 HRS over time.
Methods:
We performed a systematic review and meta-analysis for randomized controlled trials (RCTs) comparing type 1 HRS outcomes including a) overall survival (liver transplant-free survival if reported) and b) HRS reversal. Each study arm was analyzed separately to look at changes in outcomes over time. RCTs published comparing medical treatments for type 1 HRS were searched using several databases through July 31st, 2019.
Results:
Fourteen RCTs (28 arms) involving 778 participants enrolled between 2002 and 2018 were included. Twelve RCTs measured HRS reversal. In conjunction with albumin (or plasma expander), the most common medications used were terlipressin (13 arms), antibiotics (7), norepinephrine (6), dopamine (4) and midodrine/octreotide (3). Pooled survival rate was 34.6% (95% CI 26.4–43.8) and pooled HRS reversal rate was 42.8% (95% CI 34.2–51.9). Regression analyzing the incremental effect of the year the RCT was initiated showed that more recent studies were not associated with improved survival (OR 1.02, 95% CI 0.94–1.11, p=0.66) or HRS reversal rates (OR 1.01, 95% CI 0.93–1.09, p=0.41). There was no survival improvement when RCTs with endpoints assessed ≤ or > 1 month were analyzed separately with respective OR of 1.07 (95% CI 0.95–1.20, p=0.26) and 0.97 (95% CI 0.85–1.12, p=0.79).
Conclusion:
Outcomes have not improved for patients with type 1 HRS since 2002. There is a need to improve prevention and treatment of type 1 HRS.
Keywords: Renal Failure, Cirrhosis, Albumin, Vasopressors, Antibiotics, hepatorenal syndrome, liver cirrhosis, hypertension, portal, acute kidney injury
Introduction:
Hepatorenal syndrome (HRS) is the most fatal complication of cirrhosis with a median survival ranging between two weeks and two months (1, 2). First described in the 1800’s, it is a rapidly progressive, functional or hemodynamic renal failure without parenchymal injury on explant or autopsy (3, 4). The first formal definition and diagnostic criteria for HRS were proposed in 1978 (5) and subsequently refined in successive meetings of the International Ascites Club as a 50% (or ≥0.3 mg/dL) increase in serum creatinine after excluding other causes of renal injury in patients with cirrhosis (6, 7). Type 1 HRS occurs within a two week period with generally more marked renal impairment while Type 2 HRS occurs over a longer period of time (8). HRS physiology is complex but largely related to renal vasoconstriction which is needed to balance the impact of arterial vasodilation and its consequent cardiac under filling. When this balance is upset after, for example, a severe infection, renal perfusion is jeopardized (2). Medical therapies studied to combat this mechanism of renal injury uniformly include volume expansion (generally with intravenous albumin) and vasoactive medications including midodrine, octreotide, terlipressin, norepinephrine, and dopamine (9–22).
While several interventions appear to trend towards HRS reversal, few show clear survival benefit (10, 14). Compared to other therapies, prior meta-analyses have demonstrated no improvement or modest improvement in survival associated with terlipressin (23–25). Despite a modest number of trials focused on the treatment of type 1 HRS over the last decade, it is unclear if progress has been made. Temporal trends in HRS outcomes, or any complication of cirrhosis, may also represent improvement in supportive care rather than directed therapy. This was illustrated by the STOPAH trial in alcoholic hepatitis demonstrating higher rates of survival in the placebo arm compared to historical controls (26). To this end, we performed a systematic review and meta-analysis of randomized clinical trials in order to evaluate trends in outcomes of type 1 HRS.
Materials and Methods:
We reported this systematic review and meta-analysis according to the Preferred Reported Items for Systematic Review and Meta-Analyses Statement (PRISMA) (27).
Eligibility Criteria:
We included randomized controlled trials (RCTs) comparing the effect of any medical therapy: vasopressin, ornipressin, terlipressin, octreotide, norepinephrine, midodrine, dopamine, pentoxifylline, and albumin; on clinical outcomes of type 1 HRS. We examined each treatment arm separately. Outcomes of interest were overall survival, liver transplant (LT) free survival, and HRS reversal. We excluded studies that did not include at least one of these outcomes or analyzed type 1 and type 2 HRS together. We contacted authors to attempt to obtain the results for type 1 HRS separately from type 2 HRS for studies that grouped them together, in addition to requesting missing information for type 1 HRS studies. Only full text articles of RCTs published in English were included. Table 1 describes the population, intervention, comparison, and outcomes (PICO) criteria.
Table 1:
PICO Criteria
| Population | Adults (≥ 18 years) with Type 1 HRS |
| Intervention | Medical therapy evaluated in a RCT |
| Comparison | Medical therapy evaluated in a RCT |
| Outcome |
|
Search Strategy:
Published articles of RCTs in English language comparing treatment versus placebo or different treatments for type 1 HRS were searched via MEDLINE, EMBASE, SCOPUS, and Cochrane library from each database inception until July 31st, 2019. Search strings were created with the assistance of a trained librarian. MeSH terms were searched if available for corresponding terms. Citations were cross-checked through review of bibliographies of relevant published papers. Supplementary Table S1 shows the detailed search strategy.
Study Selection:
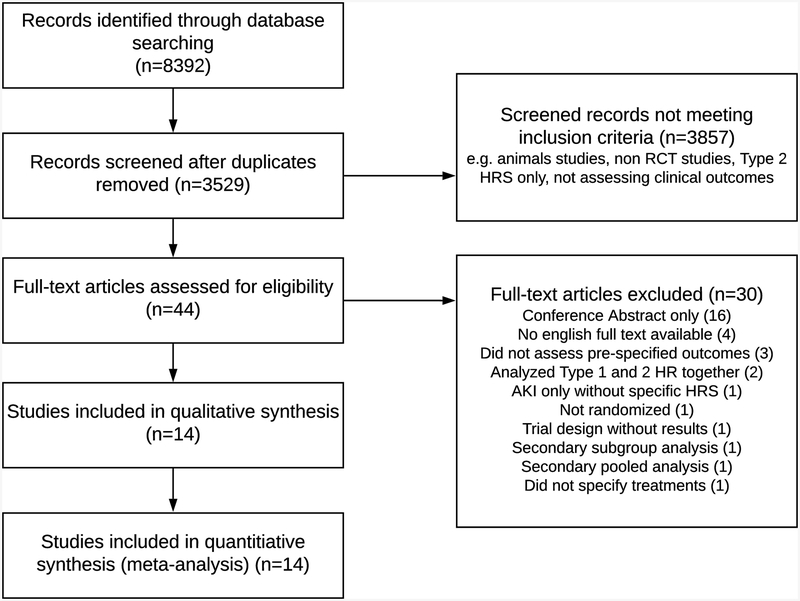
Using a reference management system (EndNote X7, Thomson Reuters), two reviewers (MJT, AT) independently screened the titles and abstracts for potential eligibility. Abstracts were retrieved and screened in duplicate with discrepancies resolved by a third party (EBT). Given the level of detail needed for this analysis, only full-text articles were included. A PRISMA (27) flowchart describing the exclusion criteria and the review process is provided in Figure 1.
Figure 1:
The Process of Study Selection.
Data Extraction:
We extracted the following variables from each study: study characteristics, including primary author and year study started (year of publication if not available), location, single vs. multicenter study; patient characteristics and number of patients, baseline differences, type 1 HRS definition; intervention characteristics including medication, dose, frequency, and concurrent albumin use; and outcome characteristics including HRS reversal, survival, and LT free survival.
Methodological Quality and Risk of Bias Assessment:
We used a Cochrane risk of bias tool to assess the methodological quality of RCTs. We used the following criteria to evaluate quality of evidence: risk of bias, inconsistency or heterogeneity, and publication bias.
Statistical Analysis:
Each study arm was analyzed separately to look at changes in HRS reversal and survival over time. We extracted outcome event rates or probability of the event and number of patients in each arm of the RCT. We then pooled the event rates using the random effects model. Temporal trends were analyzed using logistic meta-regression using the year the RCT was initiated (or published if this was not available) to determine the incremental effect start date had on outcomes. Odds ratios for survival or HRS reversal trends over time were for each incremental year since 2002 (the year the first RCT started). Pooled graphs were created from cumulative meta-analyses where each analysis is repeated with the addition of each weighted study arm. I2 was used to assess heterogeneity. Statistical analyses were conducted using Comprehensive Meta-Analysis software (version 3; Biostat, Inc, Englewood, NJ). The initial search yielded 8,392 citations, 3,529 citations after duplicates were removed. We eventually included 14 RCTs with a total of 28 medication treatment arms. All studies used versions of International Ascites Club definition of Type 1 HRS. Five used the 1996 version (28), eight used the 2007 version (29), and one used the 2015 version (30). Table 2 lists the components and characteristics of each RCT and the individual arms. All study arms included albumin (or a plasma expander). Studies used different protocols for albumin, ranging from 20 grams/day throughout the treatment period to 1 gram/kg on day one with reduced dosing afterwards. The weighted average albumin dose was 43 grams/day during treatment for the five studies (10 arms) that reported albumin dosing. The most common medications used as components in each of the arms were terlipressin (13 arms) norepinephrine (6 arms), dopamine (4 arms), and midodrine/octreotide (3 arms). Seven arms used antibiotics as adjunctive therapy in the absence of infection. Overall, 778 patients were included with a pooled mean Model for End Stage Liver Disease (MELD) score of 31. Patients were enrolled between 2002 and 2018.
Table 2:
Study Characteristics
| Author and Publication Date | Study Timeline | Treatment Arms | MELD | No. of patients | Follow up period | Responders analyzed separately for survival? | Outcomes measured? | |
|---|---|---|---|---|---|---|---|---|
| Survival | HRS Reversal | |||||||
| Solanki et al 2003(18) | 2002 | Terl + Alb + FFP + DA | NR | 12 | 15 days | No | Yes | No |
| Plc +Alb + FFP + DA | NR | 12 | ||||||
| Neri et al 2008(13) | 2002–2005 | Terl + Alb | CTP 12 | 26 | 180 days | Yes | Yes | Yes |
| Alb | CTP 11 | 26 | ||||||
| Sanyal et al 2008(14) | 2004–2006 | Terl + Alb | 33 | 56 | 180 days | Yes | Yes | Yes |
| Plc + Alb | 33 | 56 | ||||||
| Sharma et al 2008(15) | 2005–2006 | NE + Alb + Abx | 32 | 20 | 15 days | Yes | Yes | Yes |
| Terl + Alb + Abx | 30 | 20 | ||||||
| Srivastava et al 2015(19) | 2005–2010 | Terl + Alb + Abx | NR | 20 | 1 month | No | Yes | No |
| DA + Furos + Alb + Abx | NR | 20 | ||||||
| Alessandria et al 2007(9) | 2006 | NE + Alb | 26 | 4 | 6 months | Yes | Yes | Yes |
| Terl + Alb | 26 | 5 | ||||||
| Cavallin et al 2016(11) | 2007–2014 | Terl inf + Alb | 29 | 34 | 6 months | Yes | Yes | Yes |
| Terl + Alb | 30 | 37 | ||||||
| Silawat et al 2011(16) | 2009–2010 | Terl+ Alb | NR | 30 | 7 days | No | Yes | Yes |
| “Symptomatic Treatment”** | NR | 30 | ||||||
| Singh et al 2012(17) | 2009–2011 | Terl + Alb | 26 | 23 | 15 days | Yes | Yes | Yes |
| NE + Alb | 25 | 23 | ||||||
| Boyer et al 2016(10) | 2010–2013 | Terl + Alb | 34 | 97 | 90 days | Yes | Yes | Yes |
| Plc + Alb | 33 | 99 | ||||||
| Tavakkoli et at 2012(20) | 2011–2012 | NE + Alb | 33 | 6 | 1 month | No | Yes | Yes |
| M/O + Alb | 35 | 9 | ||||||
| Stine et al 2018 (22) | 2014–2016 | PTX + M/O + Alb | 26 | 6 | 180 days | No | Yes | Yes |
| M/O + Alb | 27 | 6 | ||||||
| Goyal et al 2016 (12) | 2016 | NE + Alb +/− Furos + Abx | 29 | 21 | 2 weeks | No | Yes | Yes |
| Terl + Alb + Abx | 30 | 20 | ||||||
| Saif et al 2018 (21) | 2018 | NE + Alb | 30 | 30 | 90 days | No | Yes | Yes |
| Terl + Alb | 29 | 30 | ||||||
Treatment Arm Key: Abx: Antibiotics, Alb: Albumin, DA: Dopamine, FFP: Fresh Frozen Plasma, Furos: Furosemide, M/O: Midodrine/Octreotide, NE: Norepinpehrine, Plc: Placebo, PTX: Pentoxifylline, Terl: Terlipressin
NR: Not reported CTP: Child Turcotte Pugh
Symptomatic treatment consisted of dopamine, furosemide, antibiotics, “plasma expanders”
Methodological Quality and Publication Bias:
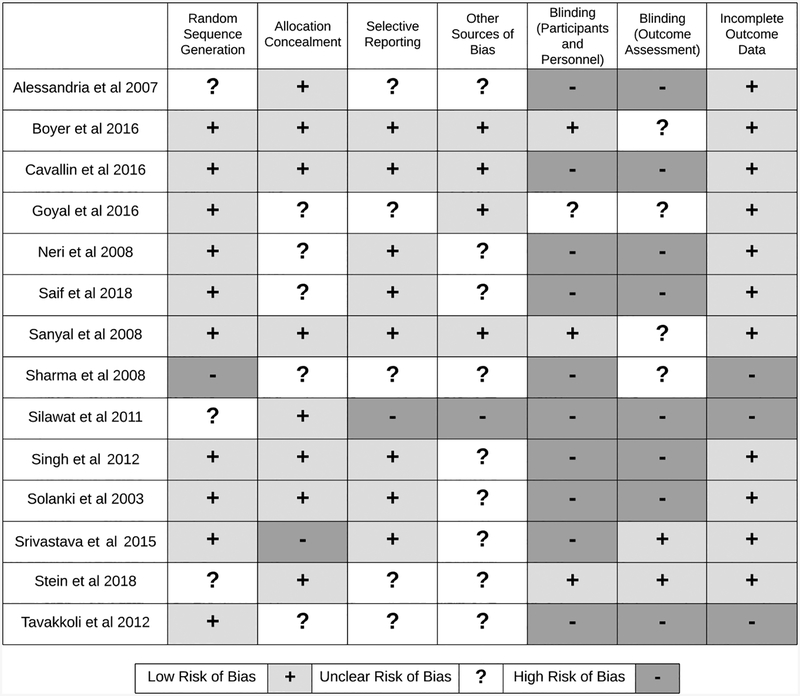
Three of the 14 RCTs had incomplete outcome data. Six RCTs had high risk or unclear selective reporting biases. Only three studies reported blinding participants and study personnel. Only two studies reported blinding the outcome assessors from knowledge of which intervention a patient received. Assessment of the methodological quality for studies included is shown in Figure 2 (31). All of the studies had one or more Cochrane bias domains with unclear or high risk of bias, indicating a high risk of bias for each study. We evaluated publication bias using funnel plot (Supplementary Fig S2). While there was substantial heterogeneity of effects, there was no significant asymmetry to suggest publication bias.
Figure 2:
Methodological Quality of Studies using the Cochrane Risk of Bias Tool.
Results:
Overall and LT Free survival
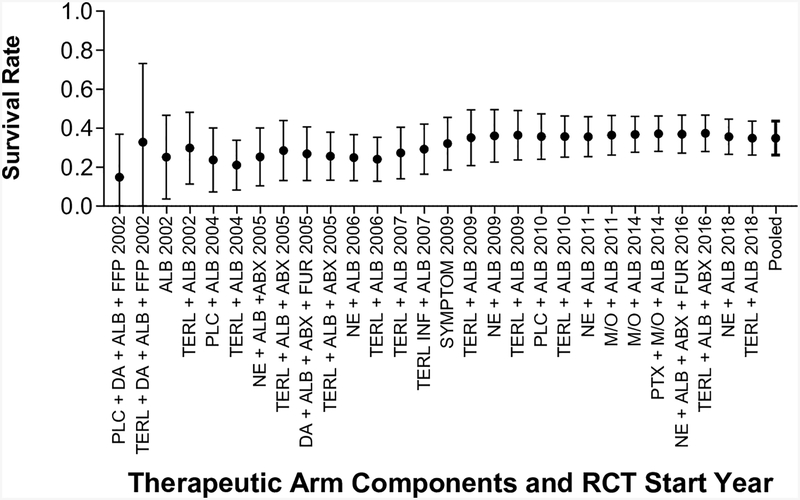
The duration of follow up in the included studies ranged between 7 and 180 days. All 14 RCTs (28 treatment arms) measured either overall survival or LT-free survival. If both were reported, LT-free survival was used for analysis. If survival was measured at multiple time points, the longest follow-up period was used. Between study variance for survival effect size, as measured by I2, was 78.29%. Overall survival was analyzed for nine studies and LT-free survival was analyzed for five studies. The pooled survival rate was 34.6% (95% CI 26.4–43.8%) for all arms of the RCTs. Survival assessed at ≤ 1 month (7 studies) was 45.5% (95% CI 34.2–57.4) was lower than survival assessed at > 1 month (7 studies) 23.6% (95% CT 12.7–40.0), but these two groups overlapped.
Determinants of Survival
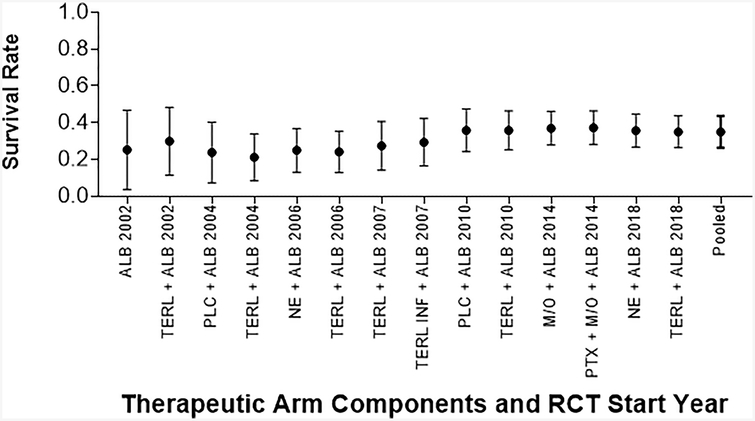
As the cohorts included had uniformly advanced cirrhosis, MELD was not significantly associated with survival (OR 0.93, 95% CI 0.81–1.07, p=0.32). Analysis of the impact of the year the RCT was initiated showed that more recent studies trended towards improved survival but the difference was not statistically significant (OR 1.02, 95% CI 0.94–1.11, p=0.66, Figure 3). Given the wide range in follow up for survival endpoints, we separately compared studies with outcomes assessed at ≤ 1 month (7 studies) versus three or six months (7 studies). No improvement was observed in more recent studies evaluating ≤ 1 month survival (OR 1.07, 95% CI 0.95–1.20, p=0.26) and studies evaluating > 1 month survival (OR 0.97, 95% CI 0.85–1.12, p=0.70).
Figure 3: Pooled Survival of Patients with Type 1 HRS Over Time.
Survival as reported in published papers, assessed at varying time points. Pooled survival calculated with the addition of each arm.
(A): Survival Measured at Any Time Point from Diagnosis
(B): Survival Measured ≤ 1 Month from Diagnosis
(C): Survival Measured > 1 Month from Diagnosis
Key: Abx: Antibiotics, Alb: Albumin, DA: Dopamine, FFP: Fresh Frozen Plasma, Furos: Furosemide, Inf: Infusion, M/O: Midodrine/Octreotide, NE: Norepinephrine, Plc: Placebo, Symptom: DA + Furos + Abx + “Plasma Expander”, Terl: Terlipressin.
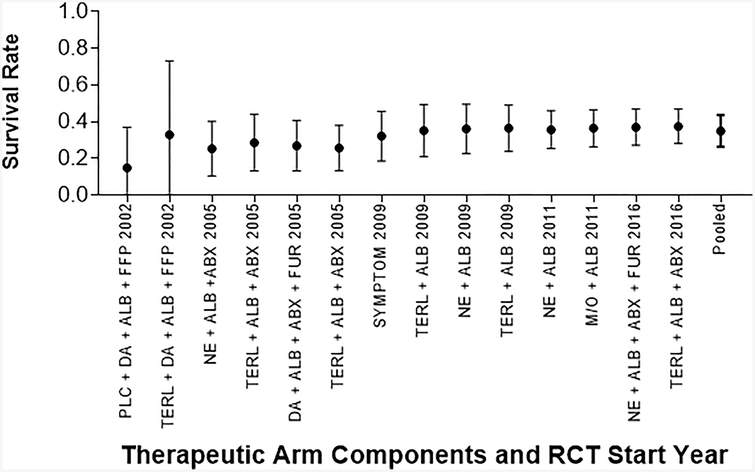
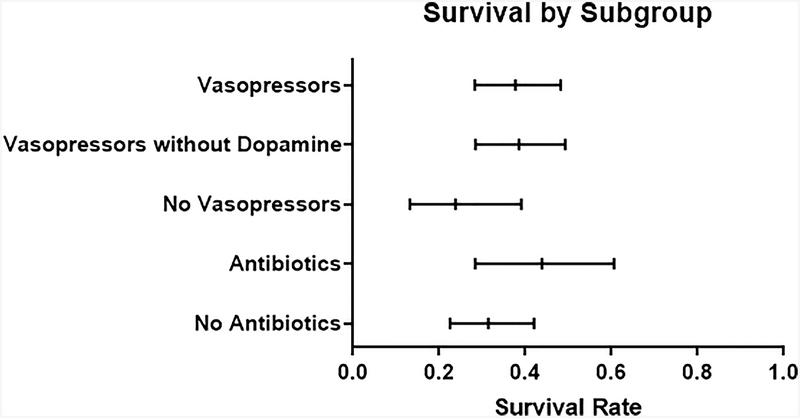
The pooled survival rate for the 22 treatment arms that contained intravenous vasopressors was 37.7% (95% CI 28.3–48.2). This group included terlipressin, norepinephrine, and dopamine. This was higher than, but overlapped with the pooled survival rate for the remaining six arms that contained midodrine/octreotide and/or albumin (survival rate 23.8%, 95% CI 13.2–39.1, Figure 4). Evaluating the arms that used intravenous vasopressors (terlipressin, norepinephrine, or dopamine) alone, there was no increase in survival over time (OR 0.99, 95% CI 0.90–1.09, p=0.85). The pooled survival for the 18 treatment arms that contained norepinephrine or terlipressin was 38.3% (95% CI 28.4–49.3%), and this cohort also did not have improved survival over time (OR 0.96, 95% CI 0.88–1.06, p=0.47). Seven arms included antibiotics as adjunctive medical treatment for HRS in the absence of bacterial infection. Survival rate for these seven arms was 43.8% (95% CI 28.3–60.6), which overlapped with survival rate of 31.4% (95% CI 22.5–42.0), in the 21 arms that did not use antibiotics (Figure 4). There was no increase in survival over time for the seven arms that used antibiotics (OR 1.05, 95% CI 0.90–1.22, p=0.53).
Figure 4: Survival by Subgroup.
Mean survival with 95% Confidence Intervals.
HRS Reversal
Twelve RCTs (24 treatment arms) measured HRS reversal. HRS reversal was most commonly reported as return of serum creatinine to less than 1.5 mg/dL. Between study variance for HRS reversal effect size, as measured by I2, was 76.40%. All arms administered albumin (or a plasma expander). For the five studies that reported albumin dose, the weighted albumin daily dose was 43 grams/day. The most common medications were terlipressin (11 arms), norepinephrine (6 arms), dopamine (4 arms), and midodrine/octreotide (3 arms). Five arms used adjunctive antibiotics. A total of 714 patients were included with a pooled mean MELD score of 31. MELD was not significantly associated with HRS reversal (OR 0.91, 95% CI 0.81–1.03, p=0.14).
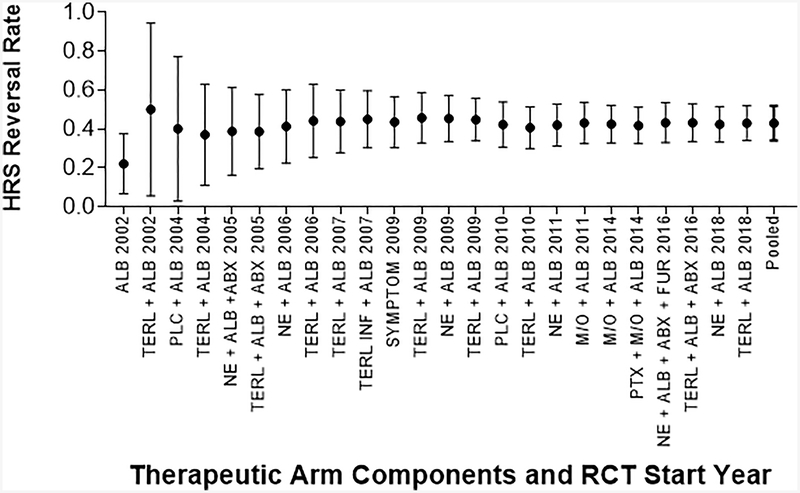
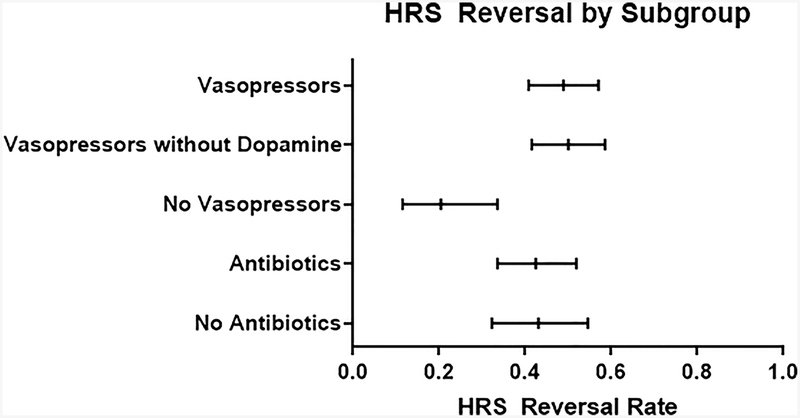
The pooled HRS reversal rate was 42.8% (95% CI 34.2–51.9%). More recent studies were not associated with higher rates of HRS reversal (OR 1.03, 95% CI 0.96–1.12, p=0.41, Figure 5). The pooled HRS reversal rate for the 18 treatment arms that contained intravenous vasopressors (48.9%, 95% CI 40.8–57.0), was higher than the remaining four arms without intravenous vasopressors (20.4%, 95% CI 11.5–33.6, Figure 6). Examining only studies that included intravenous vasopressors, HRS reversal did not increase over time (OR 0.99, 95% CI 0.92–1.06, p=0.78). The pooled HRS reversal for the 17 treatment arms that contained norepinephrine or terlipressin was 50% (95% CI 41.5–58.6%), and this cohort also did not have improve survival over time (OR 0.99, 95% CI 0.92–1.06). The pooled HRS reversal rate for the five arms that included adjunctive antibiotics in the absence of bacterial infection was 42.5% (95% CI 33.6–51.9), similar to 43.1% (95% CI 32.3–54.6) in 19 arms that did not include use of antibiotics (Figure 6). Evaluating only arms that included antibiotics did not show improved rates of HRS reversal over time (OR 1.01, 95% CI 0.93–1.09, p=0.77).
Figure 5: Pooled HRS Reversal with Type 1 HRS Over Time.
HRS Reversal as reported in published papers, assessed at varying time points. Pooled survival calculated with the addition of each arm.
Key: Abx: Antibiotics, Alb: Albumin, DA: Dopamine, FFP: Fresh Frozen Plasma, Furos: Furosemide, Inf: Infusion, M/O: Midodrine/Octreotide, NE: Norepinephrine, Plc: Placebo, Symptom: DA + Furos + Abx + “Plasma Expander”, Terl: Terlipressin.
Figure 6: HRS Reversal by Subgroup.
Mean survival with 95% Confidence Intervals.
Survival in patients who had reversal of HRS
Only seven studies examined survival in the subset of patients who had reversal of HRS. Given the small size, only descriptive analysis was done for this subset. In addition, some of the results were incomplete. Five studies found that response to any treatment was associated with an improved survival with four studies showing a statistically significant difference. Cavallin et al.’s study found a statistically higher probability of 90 day survival (70% vs. 41%, p = 0.004) in patients with a complete response than those who did not (11). Alessandria et al. found that patients who responded to either norepinephrine or terlipressin had a statistically significant improvement in survival compared to nonresponders (9). Singh et al. found that complete responders to terlipressin (77.8% vs. 14.3%) or norepinephrine (80% vs. 23.1%) had significantly higher rates of survival than nonresponders (17). Sharma et al. found that complete responders had significantly higher survival rates (100% for norepinephrine and antibiotics and 80% for terlipressin and antibiotics) compared to the entire cohort (55%) (15). By contrast, Sanyal et al. found a non-significantly improved 180 day survival for patients who responded to either terlipressin or placebo with albumin compared to nonresponders (50% vs. 38%, p = 0.07) (14).
The remaining two studies found improved survival among responders was restricted only to patients who received terlipressin. Boyer et al. found survival was improved in terlipressin patients who had confirmed HRS reversal and Neri et al. found an improved probability of survival in those who had improved renal function on terlipressin therapy (10, 13). Both studies compared terlipressin to albumin with or without placebo.
Discussion:
In this systematic review with meta-analysis, we confirm that patients with type 1 HRS are at high risk of mortality with a pooled survival rate of 34.6% across all studies. Consistent with the existing literature, we found that intravenous vasopressors were more effective in reversing HRS, but did not find improved HRS reversal over time for vasopressors. We extend the literature by demonstrating no significant improvement in HRS reversal or HRS-related mortality since 2002, irrespective of the therapy used. Therapy for type 1 HRS is therefore a persistent unmet need in the clinical care of patients with cirrhosis.
Clinical and Research Implications
Our study found that comparisons between studies and across time are limited by significant heterogeneity in when and for whom the study endpoints were measured. First, survival endpoints were assessed at varying times between 7 days to 6 months. The clinical difference between surviving one week, which would generally fall within the same hospitalization HRS was diagnosed, versus six months cannot be ignored. While our findings showed a trend towards lower long term (> 1 month) survival, the confidence intervals between short term (≤ 1 month) and long term survival overlapped. No trend was seen in survival over time when examining all survival endpoints, as well as when short and long term survival were analyzed separately. Second, not all trials performed subgroup analysis for patients who responded to treatment or for those who underwent liver transplant. Both of these are competing risks for survival and should be adjusted for. Future studies would benefit from measuring endpoints at consistent short and longer term intervals (we propose one month and six months), performing additional separate analysis on responders, as well as including transplant free survival in the endpoints.
These data also highlight a role for alternative study approaches. First, given that patients who respond to therapy for HRS are often more likely to survive, further work should identify predictors of response. Second, although transjugular intrahepatic portosystemic shunt placement has been suggested as treatment in uncontrolled studies (32), randomized trials of this intervention in type 1 HRS are needed before expanded use, though feasibility of such trials will be challenging. Third, adjunct antibiotic use as treatment for HRS may improve outcomes. The lack of statistical significance is likely due to the small sample size with only seven arms using antibiotics. Randomized trials of adjunctive antibiotic therapy for the treatment of HRS are warranted because antibiotics may influence gut flora and inflammation and subsequent peripheral arterial vasodilation associated with bacterial translocation (33, 34). Indeed, a retrospective study demonstrated manipulating the microbiome through antibiotics may have a role in preventing HRS (35).
Contextual Factors
Our data must be interpreted in the context of the study design. First, while other systematic reviews on HRS outcomes have focused on one particular medication, terlipressin (23), or comparing individual medical therapies against each other (24, 25), our analysis is the first to compare outcomes over time. Second, in contrast to other studies which combined types 1 and 2 HRS (36, 37), we focused exclusively on type 1 HRS to provide specific assessment of type 1 HRS which is more urgent and associated with higher short-term mortality. Third, published trials were of heterogeneous design, potentially biasing the trends. However, we performed several sensitivity analyses of the data to disentangle the effect of variable endpoint ascertainment and did not find any differences in the overall trends of HRS outcomes. Finally, we limited our search to published, full text articles given the need for detailed results which are not reported in abstracts.
Conclusion:
Outcomes after type 1 HRS are poor. Despite advances in management of other complications of cirrhosis, medical therapy for type 1 HRS has not improved survival or HRS reversal rates since 2002. These findings are humbling for providers. In order to address this unmet need, trials of novel therapies and prevention efforts are needed. To allow for accurate interpretation of results, such studies should perform subgroup analysis of responders and adopt standard definitions of endpoints.
Supplementary Material
Supplementary Figure S1: Publication Bias Funnel Plot.
Acknowledgements:
We would like to acknowledge Carol Shannon, who assisted with creating the search strings for this review.
Financial Support: This study was funded in part by an NIH Training Grant in Epidemiology and Health Services (T32 DK062708 - MJT) and an NIH Grant from the Michigan Institute for Clinical and Health Research (KL2 TR002241 - EBT).
Abbreviations:
- CTP
Child-Turcotte-Pugh
- HRS
Hepatorenal Syndrome
- LT
Liver transplant
- Model for End Stage Liver Disease
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized Controlled Trial
Footnotes
Conflict of Interest: The authors have no conflict of interests that pertain to this work.
References:
- 1.Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jimenez W, Arroyo V, Rodes J, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology 2005;41:1282–1289. [DOI] [PubMed] [Google Scholar]
- 2.Cardenas A Hepatorenal syndrome: a dreaded complication of end-stage liver disease. Am J Gastroenterol 2005;100:460–467. [DOI] [PubMed] [Google Scholar]
- 3.A F. Clinical Report on hydro-pneumoperitoneum based on an analysis of forty-six cases. Am J Med Sci;43:306–339. [Google Scholar]
- 4.Epstein M, Berk DP, Hollenberg NK, Adams DF, Chalmers TC, Abrams HL, Merrill JP. Renal failure in the patient with cirrhosis: the role of active vasoconstriction. The American journal of medicine 1970;49:175–185. [DOI] [PubMed] [Google Scholar]
- 5.Wong Patrick Y. M GC, Spielberg Alan, Milora Robert V., Balint John A.. The Hepatorenal Syndrome. Gastroenterology 1979;77:1326–1334. [PubMed] [Google Scholar]
- 6.Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut 2015:gutjnl-2014–308874. [DOI] [PubMed] [Google Scholar]
- 7.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of the hepatorenal syndrome in cirrhosis. A consensus workshop of the international ascites club. Gut 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tapper EB, Bonder A, Cardenas A. Preventing and Treating Acute Kidney Injury Among Hospitalized Patients with Cirrhosis and Ascites: A Narrative Review. Am J Med 2016;129:461–467. [DOI] [PubMed] [Google Scholar]
- 9.Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, Balzola F, et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. Journal of hepatology 2007;47:499–505. [DOI] [PubMed] [Google Scholar]
- 10.Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O’Leary JG, Ganger D, et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology 2016;150:1579–1589. e1572. [DOI] [PubMed] [Google Scholar]
- 11.Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, Gola E, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology 2016;63:983–992. [DOI] [PubMed] [Google Scholar]
- 12.Goyal O, Sidhu SS, Sehgal N, Puri S. Noradrenaline is as effective as terlipressin in hepatorenal syndrome type 1: a prospective, randomized trial. J Assoc Physicians India 2016;64:30–35. [PubMed] [Google Scholar]
- 13.Neri S, Pulvirenti D, Malaguarnera M, Cosimo BM, Bertino G, Ignaccolo L, Siringo S, et al. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Digestive diseases and sciences 2008;53:830–835. [DOI] [PubMed] [Google Scholar]
- 14.Sanyal AJ, Boyer T, Garcia–Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 2008;134:1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Kumar A, Shrama BC, Sarin SK. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. The American journal of gastroenterology 2008;103:1689. [DOI] [PubMed] [Google Scholar]
- 16.Silawat FN, Shaikh MK, Lohana RK, Devrajani B, Shah S, Ansari A. Efficacy of terlipressin and albumin in the treatment of hepatorenal syndrome. World Appl Sci J 2011;12:1946–1950. [Google Scholar]
- 17.Singh V, Ghosh S, Singh B, Kumar P, Sharma N, Bhalla A, Sharma A, et al. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. Journal of hepatology 2012;56:1293–1298. [DOI] [PubMed] [Google Scholar]
- 18.SOLANKI P, CHAWLA A, GARG R, GUPTA R, JAIN M, SARIN SK. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo‐controlled clinical trial. Journal of gastroenterology and hepatology 2003;18:152–156. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava S, Vishnubhatla S, Prakash S, Sharma H, Thakur B, Acharya SK. Randomized controlled trial comparing the efficacy of terlipressin and albumin with a combination of concurrent dopamine, furosemide, and albumin in hepatorenal syndrome. Journal of clinical and experimental hepatology 2015;5:276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavakkoli H, Yazdanpanah K, Mansourian M. Noradrenalin versus the combination of midodrine and octreotide in patients with hepatorenal syndrome: randomized clinical trial. International journal of preventive medicine 2012;3:764. [PMC free article] [PubMed] [Google Scholar]
- 21.Saif RU, Dar HA, Sofi SM, Andrabi MS, Javid G, Zargar SA. Noradrenaline versus terlipressin in the management of type 1 hepatorenal syndrome: A randomized controlled study. Indian J Gastroenterol 2018;37:424–429. [DOI] [PubMed] [Google Scholar]
- 22.Stine JG, Wang J, Cornella SL, Behm BW, Henry Z, Shah NL, Caldwell SH, et al. Treatment of Type-1 Hepatorenal Syndrome with Pentoxifylline: A Randomized Placebo Controlled Clinical Trial. Ann Hepatol 2018;17:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Israelsen M, Krag A, Allegretti AS, Jovani M, Goldin AH, Winter RW, Gluud LL. Terlipressin versus other vasoactive drugs for hepatorenal syndrome. The Cochrane Library 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Facciorusso A, Chandar AK, Murad MH, Prokop LJ, Muscatiello N, Kamath PS, Singh S. Comparative efficacy of pharmacological strategies for management of type 1 hepatorenal syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2017;2:94–102. [DOI] [PubMed] [Google Scholar]
- 25.Nanda A, Reddy R, Safraz H, Salameh H, Singal AK. Pharmacological Therapies for Hepatorenal Syndrome: A Systematic Review and Meta-Analysis. J Clin Gastroenterol 2018;52:360–367. [DOI] [PubMed] [Google Scholar]
- 26.Thursz MR, Forrest EH, Ryder S. Prednisolone or Pentoxifylline for Alcoholic Hepatitis. N Engl J Med 2015;373:282–283. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arroyo V, Gines P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 1996;23:164–176. [DOI] [PubMed] [Google Scholar]
- 29.Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007;56:1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut 2015;64:531–537. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song T, Rossle M, He F, Liu F, Guo X, Qi X. Transjugular intrahepatic portosystemic shunt for hepatorenal syndrome: A systematic review and meta-analysis. Dig Liver Dis 2018;50:323–330. [DOI] [PubMed] [Google Scholar]
- 33.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 2015;63:1272–1284. [DOI] [PubMed] [Google Scholar]
- 34.Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, Tsianos EV. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol 2012;10:815–818. [DOI] [PubMed] [Google Scholar]
- 35.Dong T, Aronsohn A, Gautham Reddy K, Te HS. Rifaximin Decreases the Incidence and Severity of Acute Kidney Injury and Hepatorenal Syndrome in Cirrhosis. Dig Dis Sci 2016;61:3621–3626. [DOI] [PubMed] [Google Scholar]
- 36.Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, Bernardi M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology 2015;62:567–574. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Llahi M, Pepin MN, Guevara M, Diaz F, Torre A, Monescillo A, Soriano G, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology 2008;134:1352–1359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Publication Bias Funnel Plot.