Abstract
Background:
Behavioral and psychological symptoms of dementia (BPSD) are a primary manifestation of brain dysfunction in dementia and a great challenge in caregiving. While BPSD are historically associated with caregiver distress, it is unclear whether there is an identifiable point where BPSD number is associated with heightened caregiver distress. The purpose of this study was to determine if such a tipping point exists to assist clinicians in identifying caregiver compromise.
Methods:
Analyses were performed with three datasets totaling 569 community-dwelling persons with dementia and their caregivers. Each included identical demographic, BPSD, cognitive, and caregiver well-being measures. Linear regression was performed with 16 BPSD symptoms on caregiver well-being measures and predictive values determined with receiver operating characteristic (ROC) curves and pre-defined scores for clinically significant distress.
Results:
Of the 569 persons with dementia, 549 (96%) displayed at least one BPSD, mean of 5.7 (SD = 3.06) symptoms in the past month. After controlling for covariates, BPSD symptom number was significantly associated with caregiver depression and burden (p < 0.01 for both models). Findings indicate ≥ 4 BPSD has strong predictive values for depression (sensitivity 85%, specificity 44%, area under ROC curve 0.62, p < 0.01), and burden (sensitivity 84%, specificity 43%, area under ROC curve 0.67, p < 0.01).
Conclusions:
Caring for persons with four or more BPSD appears to reflect a tipping point for clinically meaningful distress. Findings have implications for clinicians working with persons with dementia and their caregivers and suggest need for continuous monitoring of BPSD and identification of at risk caregivers.
Keywords: Alzheimer’s disease, dementia, neuropsychiatric symptoms, behavioral and psychological symptoms of dementia (BPSD), carers
Background
Behavioral and psychological symptoms of dementia (BPSD) are a primary manifestation of brain dysfunction in Alzheimer’s disease (Lyketsos et al., 2011). Symptoms including delusions, hallucinations, agitation, aggression, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability, and aberrant motor behaviors, occur for all disease etiologies and across all stages of dementia (Mega et al., 1996). Symptoms are nearly universal, can cause considerable distress to caregivers (Ornstein and Gaugler, 2012), and have been cited as one of the most challenging aspects of caregiving (Gitlin et al., 2012).
Decreased cognition, coupled with BPSD may result in challenging situations for caregivers faced with their own lifestyle/role changes. There are many studies testing pharmacological (Van De Glind et al., 2013) and non-pharmacological (Livingston et al., 2014) management of BPSD, though less preliminary research has been reported regarding the quantifiable influence of BPSD on caregiver well-being. As BPSD is a clinical feature of dementia that will likely occur along the disease trajectory, it is important to understand the point at which the presence of behavioral symptoms becomes too challenging for caregivers to manage. This can inform clinical and research stakeholders to when intervention is necessary to help caregivers better manage BPSD and to assume a preventive stance.
There are 15.7 million informal caregivers of persons with dementia (Alzheimer’s Association, 2015) largely furloughing their lives to provide such support, with many facing a most difficult aspect of caregiving: BPSD. Caregiver depressive symptoms primarily involve mood disturbances resulting from caregiver demands. Alternatively, caregiver burden may be described as a culminating negative reaction from the provision of care and impact on the caregiver’s social, occupational, and personal roles (Sherwood et al., 2005) which generally makes it a broader construct. Depressive symptoms and caregiver burden have been described as interrelated and part of overall well-being, particularly for family caregivers of persons with dementia (Clyburn et al., 2000).
Research suggests that as many as 80% of caregivers of persons with Alzheimer’s disease experience high levels of stress (Schulz et al., 2002) and nearly 40% experience depressive episodes (Robinson et al., 2009). Care demands paired with caregiver resources (or lack thereof) can lead to psychological distress, increased medical susceptibility, and for stressed caregivers, a greater risk of mortality compared to age and gender-matched non-caregiving controls and non-stressed caregivers (Schulz and Beach, 1999). Many caregivers of persons with dementia experience financial challenges and social isolation leading to a decreased quality of life, increased risk of abuse, and an increased likelihood of institutionalization (Cooper et al., 2015).
There is a notable lack of understanding to the existence of a clinically meaningful tipping point were presence of BPSD becomes too overwhelming for caregivers. The purpose of this study was to determine whether there was a discernable cut-off point as to the number of BPSD caregivers manage that leads to clinical consequences. The identification of a tipping point may lead to clinical guidance addressing when caregivers are at the highest risk for depressive symptoms and excessive burden, warranting intervention.
Methods
This study was approved by the University of Florida Institutional Review Board (IRB # 16U0053). The observational study employed a sample from three trials consisting of 569 caregivers and persons with dementia who had participated in completed clinical trials: Project ACT (n = 272) (Gitlin et al., 2010a), Project COPE (n = 237) (Gitlin et al., 2010b), and Project TAP (n = 60) (Gitlin et al., 2008). A cross-sectional design using baseline values (e.g. prior to random assignment and intervention) from the three trials was employed to examine the association between the risk factor (BPSD) and the outcomes of interest (caregiver depression and burden), while controlling for common covariates. Datasets from the studies were merged to create a single analytic sample to determine which risk factors independently or jointly were significantly predictive of caregiver well-being.
Measures
Detailed information about measures is found in Table 1. Information regarding caregiver well-being was collected using two psychometrically-sound measures (Gitlin et al., 2010b). These included the 10-Item Center for Epidemiologic Studies Depression Scale (CES-D) (Irwin et al., 1999) and the Zarit Short-Form Burden Scale (Bédard et al., 2001). Caregiver health was derived from four investigator-developed items. Caregiver’s reported: (1) their health at baseline, (2) their health compared to 3 years prior, (3) the impact of their health on participation in desired activities, and (4) their health compared to peers. The Agitated Behavior in Dementia Scale (ABID) (Logsdon et al., 1999) was used to collect care recipient BPSD. The ABID measures 16 BPSD including: (1) verbal aggression, (2) physical aggression, (3) self-harm, (4) screaming/crying out, (5) destroying property, (6) refusing help, (7) wandering, (8) arguing, (9) inappropriate sexual behavior, (10), inappropriate social behavior, (11), restlessness, (12) anxiety, (13) agitation, (14) waking up at night, (15) delusions, and (16) hallucinations. Available participant demographic/contextual variables included age, race/ethnicity, formal education, functional dependence, cognitive level, socioeconomic status, relationship type, hours spent in caregiving, gender, health, and behavioral medication use.
Table 1.
Assessment measures
| MEASURE | PURPOSE | RESPONSE FORMAT | CRONBACH’S a |
|---|---|---|---|
| The agitated behavior in dementia scale (Modified) (ABID). (Logsdon et al., 1999) | Measures presence, frequency of, and caregiver reaction from agitated symptoms in persons with dementia | For each behavior (0–16), caregivers note “yes or no;” counted as a score from 0–16 | 0.72 |
| Zarit Short-Form Burden Scale (Bedard et al., 2001) | Measures burden when caring for cognitively impaired adults with 12 items. | Scores range from 0–48, with scores over 17 indicating high levels of CG burden | 0.87 |
| The 10-item center for epidemiologic studies depression scale (CES-D). (Irwin et al., 1999) | Measures depressive symptomology in caregivers with 10 items. | Scores range from 0–30, where high scores imply greater depressive symptoms and scores of 10 or greater are predictive of significant depressive symptoms | 0.81 |
| The mini mental state exam (MMSE). (Folstein et al., 1975) | Measures cognitive function in persons with dementia and other disorders with five domains: orientation, registration, attention and calculation, recall, and language. | Scores range from 0–30, with scores less than 24 suggesting cognitive impairment (10–20 moderate; 0–10 severe) | Individual items not available in dataset |
| The caregivers assessment of functional dependence and upset (CAFU). (Gitlin et al., 2005). | Measures physical dependence of persons with dementia and caregiver reaction. | Scores based on binary level of assistance for 15 activities of daily living. Scores range from 0–15 with higher scores indicating greater dependence | 0.85 |
| Caregiver health | Caregiver self-assessment of their health | 4-itmes with scores ranging from 3–13, with higher scores indicating better health | 0.74 |
| Person with dementia health | Measures caregiver assessment of person with dementia health | Single item with score ranging 1–5, with 1 being poor, 5 being excellent | Single item |
| Person with dementia pain | Measures caregiver assessment of the pain in person with dementia | Scores range from 4–20, with higher scores indicating greater impact on daily activity (due to pain) | 0.89 |
| Person with dementia behavioral medications | Measures number of behavioral medications | Range from 0–3 collected | Single item |
Data analysis
Demographic information was analyzed using descriptive statistics and frequencies for variables described above. Univariate linear regression was used to identify demographic and contextual variables that were least significant (highest p values) to exclude them from the analysis. Backwards selection criteria were used to identify covariates with an entry criteria of p = 0.20 to allow a parsimonious model to test our primary independent variable (IV) with. Historically signficant variables including cognition, health (person with dementia and caregiver), and behavioral medication number were included in all models regardless of their qualification in the backwards selection. Stratification (dummy) variables were included to differentiate possible effect location shifts among the three combined studies (ACT, COPE, and TAP). To determine if regression was appropriate, seven assumptions were considered: dependent variable (DV) measured a continuous level, IV measured at continuous or categorical levels, linear relationships between IV and DV, no significant outliers, independence of observations (Durbin–Watson statistic), homoscedasticity, and normal distribution of residuals. Upon meeting assumptions, separate linear regression analyses were performed with both DVs (caregiver well-being measures) along with parsimonious covariate models and BPSD.
Our primary aims were to examine the relationship of the number of BPSD and caregiver well-being, and to determine if a cut-off, or tipping point could be identified linking BPSD number with negative caregiver well-being. Testing for the primary covariate of interest, BPSD number, was performed as added-last tests of significance at the alpha = 0.05 level within post-selection multivariable models. We hypothesized that caregiver well-being would be negatively impacted by an increase in BPSD. However, we did not have a hypothesis as to the specific cut-off point predictive of a clinical level of depressive symptoms and perceived burden, or that point would vary by outcome. The analysis used the itemized number of BPSD, as reported in the ABID, and a cumulative representation for each caregiver measure of well-being. To determine a cut-off point, we transformed and dichotomized depressive symptoms (CES-D) based on previous report with scores ≥ 10 implying significant depression (Andresen et al., 1994) and burden (Zarit) scores were transformed and dichotomized for scores >17 implying significant burden (Bédard et al., 2001).
Results
Caregivers
The characteristics of caregivers of persons with dementia are found in Tables 2 and 3. Caregivers mean self-reported health was 8.5 (SD = 2.4), with a range of 3–13, suggesting good health and few recent health changes. Caregivers reported spending an average of 27.8 (SD = 26.1) hours per week caring for persons with dementia (range 0–126). Caregiver burden, as measured by the Zarit Short Form was reported at a mean of 21.5 (SD = 9.3), median 21.0, suggesting that the average caregiver was at a significant level of burden (criteria >17). Caregiver depression, as measured by the CES-D was at an average of 9.1 (SD = 5.7), median 9.0, with 252 (44%) of caregivers meeting the criteria for significant depression.
Table 2.
Dyad demographics
| CAREGIVERS (N = 569) |
PERSONS WITH DEMENTIA (N = 569) | |
|---|---|---|
| Age | ||
| Mean (SD) | 64.35(12.5) | 81.4 (12.4) |
| Range | 25–100 | 53–102 |
| Gender, n (%) | ||
| Male | 94 (16.5) | 231 (40.6) |
| Female | 475 (83.5) | 338 (59.4) |
| Hispanic | 13 (2.3) | 11 (1.9) |
| Race, n (%) | ||
| White | 408 (71.7) | 409 (71.9) |
| Black | 148 (26.0) | 148 (26.0) |
| Native American/Alaskan Native | - | 2 (0.4) |
| Asian | 1 (0.2) | 1 (0.2) |
| Other | 12 (2.1) | 9 (1.6) |
| Marital Status, n (%) | ||
| Married | 390 (68.5) | 285 (50.1) |
| Widowed | 19 (3.3) | 228 (40.1) |
| Divorced | 76 (13.4) | 42 (7.4) |
| Never Married | 75 (13.2) | 13 (2.3) |
| Separated | 9 (1.6) | 1 (0.2) |
| Health Status | 8.5 (2.4) | 3.0(1.2) |
| Pain | 9.89(4.2) | |
| MMSE | 12.89(8.1) | |
| Function | 12.2 (2.9) | |
| Behavioral medication use | 0.40 (.638) | |
| CG Relationship to CR (%) | ||
| Spouse | 260 (45.7) | |
| Non-Spouse | 309 (54.3) | |
| Education (%) | ||
| Less than high school | 32 (5.6) | |
| High school graduate | 151 (26.5) | |
| Some college/associates | 172 (30.2) | |
| College | 126 (22.1) | |
| Post-graduate | 87 (15.3) | |
| Refused | 1 (0.1) | |
| Employment (%) | ||
| Yes | 206 (36.2) | |
| No | 363 (46.5) | |
| Employment Type (% of employed) | ||
| Full-time | 126 (61.2) | |
| Part-time | 76 (36.9) | |
| Unknown | 4 (0.02) | |
| Financial Difficulty (%) | ||
| Not at all | 196 (34.4) | |
| Not very | 133 (23.4) | |
| Somewhat | 198 (34.8) | |
| Very | 42 (7.4) | |
| Hours spent caring per week | 27.7 (26.1) | |
| Significant Depression | 252 (44.3) | |
| Significant Burden | 397 (69.9) | |
Note: Significant depression; ≥ 10-item Center for Epidemiologic Studies Depression Scale score 10; Significant burden Zarit Short-Form score >17.
Table 3.
BPSD counts
| (N = 569) | |
|---|---|
| BPSD reported (%) | |
| 0 | 20 (3.9) |
| 1 | 31 (5.4) |
| 2 | 41 (7.2) |
| 3 | 54 (9.5) |
| 4 | 63 (11.1) |
| 5 | 66 (11.6) |
| 6 | 76 (13.4) |
| 7 | 53 (9.3) |
| 8 | 47 (8.3) |
| 9 | 48 (8.4) |
| 10 | 38 (6.7) |
| 11 | 15 (2.6) |
| 12 | 9 (1.6) |
| 13 | 7 (1.2) |
| 14 | 0 (0.0) |
| 15 | 1 (0.2) |
| 16 | 0 (0.0) |
Note: Behavioral and Psychological Symptoms of Dementia (BPSD).
Persons with dementia
Characteristics of persons with dementia are located in Table 2. Of 16 BPSD, persons with dementia displayed an average of 5.7 (SD = 3.1) symptoms within the past month (Table 3). BPSD were normally distributed, with six BPSD being the mode (13.4% of sample) and an observed range of 0–15. The most commonly occurring BPSD were: arguing (67.3% of sample), anxiety (66.6%), restlessness (64.5%). verbal aggression (53.6%), refusal of care (53.1%), agitation (53.1%), and waking and getting up at night (51.1%) (Table 3).
BPSD and caregiver well-being
Table 4 shows the results of the linear regression with the depression variable (CES-D) alongside identified covariates and BPSD. The adjusted R2 value was 0.34. When controlling for the selected covariates, BPSD was significantly associated with the depressive outcome (p = 0.001). One-unit change in the number of BPSD predicted 0.24 points higher on the CES-D.
Table 4.
Regression analysis for BPSD and independent variables associated with depression (CES-D)
| VARIABLE | UNSTANDARDIZED B | SE B |
|---|---|---|
| Constant | 21.19 | 2.46 |
| MMSE score (cognition) | 0.03 | 0.03 |
| CG health | − 0.89 | 0.09 |
| CR health | 0.23 | 0.19 |
| CG sleep hours (x 3 days) | − 0.23 | 0.05 |
| CG education (<HS-post grad) | − 0.33 | 0.18 |
| Financial difficulty | 0.30 | 0.23 |
| CG age years | − 0.04 | 0.02 |
| CG married | − 0.83 | 0.51 |
| CR white | − 1.35 | 0.48 |
| CG male | − 1.53 | 0.59 |
| Daily hours spent in CG | 0.09 | 0.04 |
| CR pain | 0.08 | 0.05 |
| Study | 0.04 | 0.33 |
| Behavioral medications | 0.50 | 0.33 |
| BPSD number | 0.21 | 0.07 |
Note: R2 = 0.34; Behavioral and Psychological Symptoms of Dementia (BPSD).
Table 5 shows the results of the linear regression with the burden variable (Zarit) alongside selected covariates and BPSD. The adjusted R2 value was 0.27. BPSD was significantly associated with burden (p < 0.001), indicating a one-unit change in the number of BPSD predicted 0.7 points higher on the burden score.
Table 5.
Regression analysis BPSD and independent variables associated with Burden (Zarit)
| VARIABLE | UNSTANDARDIZED B | SE B |
|---|---|---|
| Constant | 8.12 | 9.07 |
| MMSE score (cognition) | 0.14 | 0.05 |
| Function score | 0.16 | 0.14 |
| CG health | − 0.68 | 0.15 |
| CR health | − 0.09 | 0.32 |
| CG sleep hours (× 3 days) | − 0.19 | 0.08 |
| CG employed | 1.03 | 0.82 |
| CG age years | − 0.11 | 0.04 |
| CR married | − 1.28 | 1.01 |
| CR white | − 5.75 | 0.81 |
| Related to CR | 22.16 | 8.06 |
| CG married | − 0.26 | 0.95 |
| CG male | − 2.61 | 0.99 |
| CR pain total | 0.10 | 0.09 |
| Study | 0.50 | 0.54 |
| Behavioral medications | 0.92 | 0.57 |
| BPSD number | 0.69 | 0.13 |
Note: R2 = 0.27; Behavioral and Psychological Symptoms of Dementia (BPSD); Zarit Short-Form (Zarit).
Identifying a tipping point
We found that the presence of ≥ 4 BPSD had strong predictive value for clinical depression with sensitivity 85%, and specificity 44% (Figure 1). This cut point, indicated by the red circles in Figure 1, had the highest Youden Index (a measure of overall diagnostic value calculated as Sensitivity+Specificity-1), or 0.51. The area under ROC curve for this model was 0.62 (p = 0.01 vs. a null of 0.5), showing significant predictive ability. Of importance is that the presence of ≥ 4 BPSD also had strong (and best) predictive values for burden, with sensitivity 84% and specificity of 43%. The Youden index was 0.41. The area under the ROC curve for this model was 0.67 (p = 0.01).
Figure 1.
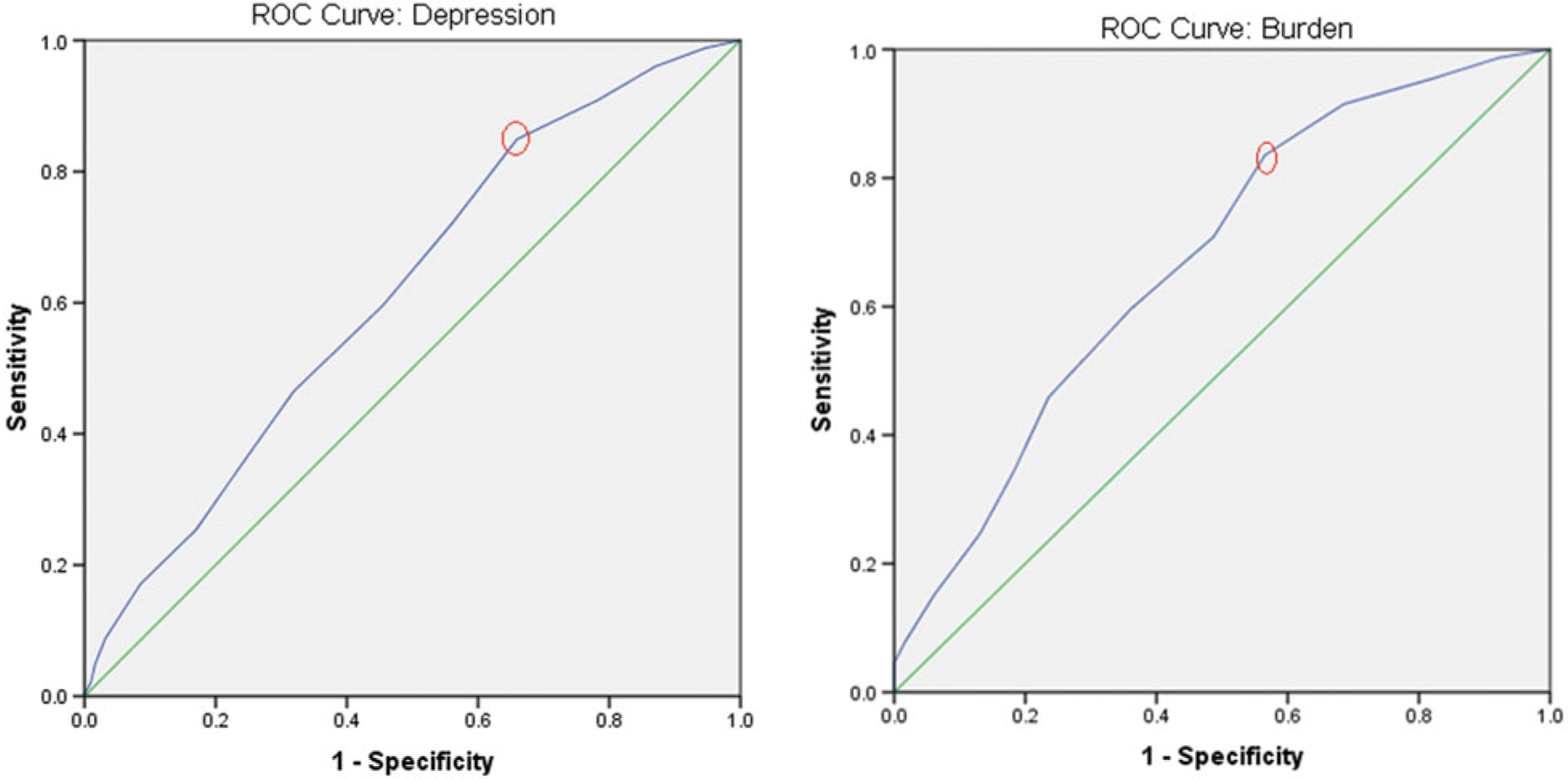
(Colour online) ROC curves.
Discussion
We report findings on the relationship between BPSD and two dimensions of caregiver well-being: depression and burden. Results indicate that BPSD are significantly associated with caregiver well-being as measured by depressive symptoms and burden, when controlling for covariates, a finding supported by a number of previous studies (Dukle et al., 2014; Fauth and Gibbons, 2014). Of importance, is that we show that a unit change in BPSD predicted a gradual linear decrease in the caregiver well-being outcomes, suggesting identification of a tipping point may be feasible.
By combining the data from three separate trials, we could examine these relationships in a relatively large community-dwelling dementia caregiver sample. The demographic profile of this combined sample was similar to those reported in other dementia caregiving studies (Steinberg et al., 2008), suggesting our results may be generalizable. Like other dementia caregiver studies, close to half of the sample (44%) were at risk for clinical depression, as reported on the CES-D, and approximately 70% were at risk for burden per their scores on the Burden Inventory using Bédard et al.’s (2001) conservative estimates. Despite caregivers reporting good physical health, it was evident that caregiving was a stressful emotional experience with negative psychological consequences.
The importance of this study lies in its identification of a tipping point by which BPSD overwhelm the caregiver, resulting in negative psychological consequences. While there is a linear relationship between number of BPSD and psychological well-being, this study is the first, to our knowledge, to show that four or more behavioral symptoms of any type are related to clinically meaningful depressive symptoms and burden. The possibility of predicting caregiver’s susceptibility to depression and burden by identifying BPSD number has great clinical utility. Identifying the threshold of BPSD that is most suggestive of caregiver distress may best allow for more resourceful and targeted interventions. Also, when confronted with time constraints, clinicians may not have the time or knowledge to identify caregivers at risk. Thus, our finding suggests that caregivers reporting four or more behavioral symptoms should be targeted for intervention or, at the minimum, referral to resources (support groups, respite care, clinical trials) to help manage such symptoms.
The design of this study was a secondary data analysis, naturally limiting our control of the collection and measurement variables. This limited our ability to examine all variables of interest, particularly as it pertained to BPSD, and understanding the context in which behaviors occur. This also limited our ability to identify exhaustive medical comorbidities that may influence caregiver well-being as well as robust pharmacologic histories. As the ABID does not measure severity, it may also be that just one BPSD, if severe enough, could influence caregiver well-being, though this risk should be minimal secondary to the large sample size.
While sensitivity for predictive values was high (85%, and 84%, respectively), specificity was quite low (44% and 43%) suggesting a heightened risk for false positives. While true, for clinical screening purposes, high sensitivity, and negative predictive value are more important than high specificity and positive predictive value (Strik et al., 2001). Despite a notable sample size, generalizability of the study may be questioned as study participation was voluntary and our sample was generally white, educated, and financially stable. Additionally, participants in this study may have higher incidences of BPSD and caregiver distress as the interventions tested addressed these manifestations. Future research is warranted in understanding the relationship of BPSD to caregiver well-being and the context in which behaviors occur and for diverse samples to confirm these findings.
Conclusion
By examining the interaction between the number of BPSD and caregiver well-being, this study adds incrementally to the understanding of the impact of this clinical feature. This study provides additional evidence to suggest that the well-being of caregivers of persons with dementia is compromised, particularly as the number of behavioral symptoms increases. The management of four or more behaviors appears to be a clinically meaningful tipping point, negatively effecting psychological health.
Clinicians should help caregivers prevent, manage and reduce BPSD regardless of the number occurring. However, those at most risk appear if four or more behaviors are occurring. There are effective intervention approaches to help caregivers better cope and manage these symptoms yet few families have access to them (Gitlin et al., 2010a; Gitlin, 2012). Our study suggests the importance of implementing these programs, particularly for those at most risk.
Acknowledgments
The research question for this paper was developed from the primary author’s doctoral dissertation. While some dissertation committee members are co-authors, we would like to acknowledge additional committee members including Dr. Mary Ellen Young, Dr. Orit Shechtman, and Dr. Jamie Pomeranz, all distinguished faculty members in the Department of Occupational Therapy at the University of Florida. Additionally, much of this work was completed while the first author was a faculty member at the University of Florida. Analyzed studies were funded by the National Institute on Aging and the National Institute on Nursing Research grant R01AG22254, the Pennsylvania Department of Health, Tobacco Funds grant 2401000272, and the National Institute of Mental Health grant R21MH069425 in which Dr. Gitlin was the principal investigator. Dr. Gitlin was further supported in part in the conduct of this study from the National Institute on Aging grants # R01AG041781; R01AG049692; P30AG048773.
The funders of analyzed projects had no role in the design, methods, data organization, analysis, or preparation of this paper.
Footnotes
Ethics approval and consent to participate
This study was approved by the University of Florida Institutional Review Board (IRB # 16U0053).
Conflict of interest
None.
References
- Alzheimer’s A (2015). 2015 Alzheimer’s disease facts and figures: Alzheimer’s & dementia. The Journal of the Alzheimer’s Association, 11, 332. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB and Patrick DL (1994). Screening for depression in well older adults: evaluation of a short form of the CES-D. American Journal of Preventative Medicine, 10, 77–84. doi: 10.1016/0091-7435(81)90008-6. [DOI] [PubMed] [Google Scholar]
- Bédard M, Molloy DW, Squire L, Dubois S, Lever JA and O’Donnell M (2001). The Zarit Burden interview a new short version and screening version. The Gerontologist, 41, 652–657. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- Clyburn LD, Stones MJ, Hadjistavropoulos T and Tuokko H (2000). Predicting caregiver burden and depression in Alzheimer’s disease. Journals of Gerontology Series B, 55, S2–S13. doi: 10.1093/geronb/55.1.S2. [DOI] [PubMed] [Google Scholar]
- Cooper C, Sommerlad A, Lyketsos CG and Livingston G (2015). Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. American Journal of Psychiatry, 172, 323–334. doi: 10.1176/appi.ajp.2014.14070878. [DOI] [PubMed] [Google Scholar]
- Dunkle RE, Feld S, Lehning AJ, Kim H, Shen HW and Kim MH (2014). Does becoming an ADL spousal caregiver increase the caregiver’s depressive symptoms? Research on Aging, 36, 655–682. doi: 10.1177/0164027513516152. [DOI] [PubMed] [Google Scholar]
- Fauth EB and Gibbons A (2014). Which behavioral and psychological symptoms of dementia are the most problematic? Variability by prevalence, intensity, distress ratings, and associations with caregiver depressive symptoms. International Journal of Geriatric Psychiatry, 29, 263–271. doi: 10.1002/gps.4002. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE and McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Gitlin LN et al. (2005). Caregiver appraisals of functional dependence in individuals with dementia and associated caregiver upset: psychometric properties of a new scale and response patterns by caregiver and care recipient characteristics. Journal of Aging and Health, 17, 148–171. doi: 10.1177/0898264304274184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Kales HC and Lyketsos CG (2012). Nonpharmacologic management of behavioral symptoms in dementia. JAMA, 308, 2020–2029. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN (2012). Good news for dementia care: caregiver interventions reduce behavioral symptoms in people with dementia and family distress. The American Journal of Psychiatry, 169, 894–897. doi: 10.1176/appi.ajp.2012.12060774. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP and Hauck WW (2008). Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. The American Journal of Geriatric Psychiatry, 16, 229–239. doi: 10.1097/01.JGP.0000300629.35408.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Dennis MP Hodgson N and Hauck WW (2010a). Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention. Journal of the American Geriatrics Society, 58, 1465–1474. doi: 10.1111/j.1532-5415.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Dennis MP, Hodgson N and Hauck WW (2010b). A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA, 304, 983–991. doi: 10.1001/jama.2010.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, Artin KH and Oxman MN (1999). Screening for depression in the older adult: criterion validity of the 10-item center for epidemiological studies depression scale (CES-D). Archives of Internal Medicine, 159, 1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- Livingston G et al. (2014). Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. The British Journal of Psychiatry, 205, 436–442. doi: 10.1192/bjp.bp.113.141119. [DOI] [PubMed] [Google Scholar]
- Logsdon RG et al. (1999). Assessment of agitation in Alzheimer’s disease: the agitated behavior in dementia scale. Journal of the American Geriatrics Society, 47, 1354–1358. doi: 10.1111/j.1532-5415.1999.tb07439.x. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG et al. (2011). Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers & Dementia, 7, 532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Fiorello T and Gornbein J (1996). The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 46, 130–135. doi: 10.1212/WNL.46.1.130. [DOI] [PubMed] [Google Scholar]
- Ornstein K and Gaugler JE (2012). The problem with “problem behaviors”: a systematic review of the association between individual patient behavioral and psychological symptoms and caregiver depression and burden within the dementia patient–caregiver dyad. International Psychogeriatrics, 24, 1536–1552. doi: 10.1017/S1041610212000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison J, Fortinsky R, Kleppinger A, Shugrue N and Porter M (2009). A broader view of family caregiving: effects of caregiving and caregiver conditions on depressive symptoms, health, work, and social isolation. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 64, 788–798. doi: [DOI] [PubMed] [Google Scholar]
- Schulz R and Beach SR (1999). Caregiving as a risk factor for mortality: the caregiver health effects study. JAMA, 282, 2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Schulz R et al. (2002). Dementia caregiver intervention research in search of clinical significance. The Gerontologist, 42, 589–602. doi: 10.1093/geront/42.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood PR, Given CW, Given BA and Von Eye A (2005). Caregiver burden and depressive symptoms: analysis of common outcomes in caregivers of elderly patients. Journal of Aging and Health, 2, 125–147. doi: 10.1177/0898264304274179. [DOI] [PubMed] [Google Scholar]
- Steinberg M et al. (2008). Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the cache county study. International Journal of Geriatric Psychiatry, 170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strik JJ, Honig A, Lousberg R and Denollet J (2001). Sensitivity and specificity of observer and self-report questionnaires in major and minor depression following myocardial infarction. Psychosomatics, 42, 423–428. doi: 10.1176/appi.psy.42.5.423. [DOI] [PubMed] [Google Scholar]
- Van De Glind EM et al. (2013). Pharmacological treatment of dementia: a scoping review of systematic reviews. Dementia and Geriatric Cognitive Disorders, 36, 211–228. doi: 10.1159/000353892. [DOI] [PubMed] [Google Scholar]


