Abstract
The rat partial optic nerve transection (PONT) model has been used for studying secondary degeneration of retinal ganglion cells (RGCs) in recent years. In this study, we carried out PONT of the temporal side of rat optic nerves, whereas PONT was carried out of the superior side in the previous publication. We found that this surgery is better and easier than the previous method and can produce a repeatable and reliable model. We detected significant changes in the polarization of microglia/macrophages and the level of autophagy in optic nerves after PONT. We also used this model to detect the effects of the polysaccharides extracted from Lycium barbarum (LBP) on the survival of RGCs and the changes in the polarization of microglia/macrophages and the level of autophagy after PONT. We find that LBP can delay secondary degeneration of RGCs after temporal injury of optic nerves, promote the M2 polarization of microglia/macrophages, and down-regulate the level of autophagy after PONT. In conclusion, we find that the polarization of microglia/macrophages and the autophagy level change after PONT; LBP treatment delays secondary degeneration of RGCs; and the polarization of microglia/macrophages and the level of autophagy are also altered after LBP treatment.
Keywords: Lycium barbarum, partial optic nerve transection, secondary degeneration, polarization of microglial/macrophages, autophagy
Introduction
Glaucoma and Partial Optic Nerve Transection Model
In terms of patient numbers, glaucoma ranks just below cataract as one of the leading causes of blindness in the world1. Glaucoma is also less readily treatable than cataract. Recent research has highlighted that glaucoma patient number in China will reach 6 million, accounting for 7% of the total glaucoma patients in the world, by 20201. One main pathological feature of glaucoma is the gradual degeneration of retinal ganglion cells (RGCs)2. Clinical studies have shown that even after reducing the intraocular pressure via surgery, the vision of some patients continues to deteriorate3. Therefore, secondary injury of RGCs is believed to exist in glaucoma4. The partial optic nerve transection (PONT) model is a new method for studying glaucoma and was established in the first decade of this century. Compared with the complete optic nerve transection model and optic nerve crush model, in both of which all the axons are damaged simultaneously, the merit of the PONT model is that instead of damaging all the axons inside the optic nerves, the transection damages only a portion of the axons. This model, therefore, can separate primary injury (the death of RGC bodies whose axons having been cut off) from secondary injury (the death of RGC bodies whose axons are intact). Many studies have used this model4–13. In these previous studies, PONT in rats was administrated from the superior side of optic nerves; however, in this study we cut the optic nerves from the temporal side and found that this operation was much easier to conduct than from the superior side due to the lack of obstruction from rectus superior and obliquus superior eye muscles (Fig. 1A).
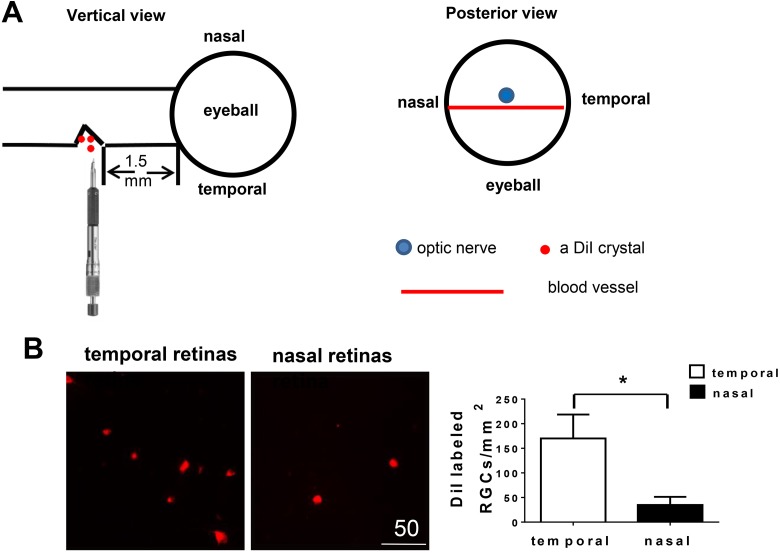
Fig. 1.
Partial optic nerve transection (PONT) model from the temporal side of optic nerves. (A) Schematic diagram showing the cut site of optic nerve and the structure (blood vessel) assisting the localization of the cut site during the surgery of PONT. After PONT, several crystals of DiI were immediately put into the cut site. (B) Retinal ganglion cells (RGCs) labeled with DiI in vivo. Photographs about 1.5 mm from the optic disc showed the different densities of DiI labeled RGCs in both the temporal and nasal retinas. More RGCs in the temporal retinas were labeled with DiI than in the nasal retinas 4 days after PONT (n = 6, Mean ± SEM, Student pair t-test, *p < 0.05).
Mechanisms of RGC Death in Glaucoma
RGC death mechanisms are very complicated in glaucoma, and include: activation of microglia/macrophages14–23, autophagy23–25, calcium regulation disorder26–28, apoptosis8,29,30, oxidative stress4,10,26, expression of pro-apoptosis proteins31–33, and neurotropic deprivation34. All of these mechanisms have been studied extensively in order to find ways to delay the death of RGCs and stop the progression of glaucoma. In this study, we focused on the changes of microglia/macrophages and autophagy after PONT.
Macrophages are distributed throughout the body and are called microglia in the central nervous system35,36. We used the term “microglia/macrophages” in this study rather than “microglia” or “macrophages” because we could not confirm whether activated cells originated from microglia only or also originated from macrophages in the blood, since the sheath of the optic nerves was broken and some blood cells might enter the optic nerve after PONT. Microglia/macrophages are active in glaucoma14. In recent years diverse terms have been used to describe microglia/macrophage activation and “polarization” means a status where a stimulus such as cytokines induces distinct patterns of gene and protein expression. Based on gene and protein expression and function, activated microglia/macrophages are divided into two extremes of status: M1 type that aggravates tissue damage, and M2 type that promotes tissue repair and cell proliferation37,38. M1-type cells express pro-inflammatory cytokines including inducible nitric oxide synthase (iNOS) to eliminate pathogenic microorganisms, to inhibit cell proliferation, and to induce tissue damage. On the other hand, M2-type microglia/macrophages express anti-inflammatory factors such as Arginase-1 to down-regulate inflammation, to promote angiogenesis, and to participate in tissue remodeling and repair37–40. Therefore, we used the expression levels of iNOS and Arginase-1 as indicators of the numbers of activated M1 and M2 types of microglia/macrophages in this study.
Autophagy, a metabolic process of intracellular material degradation, is widely present in eukaryotic cells41. When subjected to external stimuli (starvation, cessation of growth factors, hypoxia, or accumulation of protein aggregates), cells respond immediately to remove damaged and malfunctioning proteins and organelles to maintain survival via autophagy42. LC3-II is the lapidated form of LC3 and its amount increases after autophagy. Therefore, the amount of LC3-II on a Western blot is commonly used as an indicator of autophagy level. Kim et al. reported elevated levels of LC3-II in the retina after optic nerve transection in rats24. After optic nerve crush, the number of LC3-positive vacuolar structures (autophagosomes) increased in RGCs25. The increase in the number of autophagosomes and in LC3-II expression was found in RGCs in rats with chronic ocular hypertension induced by scleral vein cauterization43. However, the activation of autophagy indicated by LC3-II level after PONT has not been studied before.
The Effects of Lycium barbarum on RGCs, Microglia/ Macrophages and Autophagy
Lycium barbarum is a Solanaceous defoliated shrub and widely distributed in northwestern China, which has arid and semi-arid regions. This plant is also distributed in southeastern Europe and Mediterranean areas. Lycium barbarum has a sweet small fruit, which is called a wolfberry or goji berry. The fruit has been used as a vital component in traditional Chinese medicine and as a food supplement for a long time, and is believed to have beneficial effects on the liver and eyes. The usage of Lycium barbarum in daily life has also been accepted outside China, including in North America, Europe, Oceania, and Southeast Asia44.
In order to test the Chinese traditional theory regarding wolfberry, we have tried to obtain experimental data in the laboratory using modern technology. The polysaccharides extracted from Lycium barbarum (LBP) are used in the present study. LBP are a water-soluble powder. Our studies have shown that LBP modulate retinal crystallin expression and activity of microglia in the chronic ocular hypertension model and in the PONT model to delay the degeneration of RGCs45–47. The data from our colleagues have shown that LBP modulate the autophagy level to exert protective effects both in a rat non-alcoholic fatty liver disease model48 and in an in vitro microglial (BV-2 cell line) culture model49. These results show that LBP modulate the function of microglia/macrophages and autophagy. However, whether LBP take part in the polarization of microglia/macrophages and their role on autophagy in glaucoma in vivo had not been studied. In this study, we will focus on these two aspects of LBP.
Materials and Methods
This study was approved by the Animal Protection and Use Committee of Jinan University in Guangzhou, China.
Animals and Procedures
Adult female Sprague Dawley (SD) rats (9–10 weeks of age, weighing 220–240 g, provided by Guangdong Provincial Medical Laboratory Animal Center, Guangdong, China) were used in the study. All animals were housed in a temperature-controlled room subjected to a 12-hour light/12-hour dark cycle and were supplied with food and water ad libitum. All experimental protocols were approved by the Animal Protection and Use Committee of Jinan University. All efforts were taken to minimize the number of animals used and their suffering.
For the surgery and sacrifice, all rats were anesthetized with 10% Chloral hydrate (0.38 ml/100 g for surgery and 0.5 ml/100 g for sacrifice).
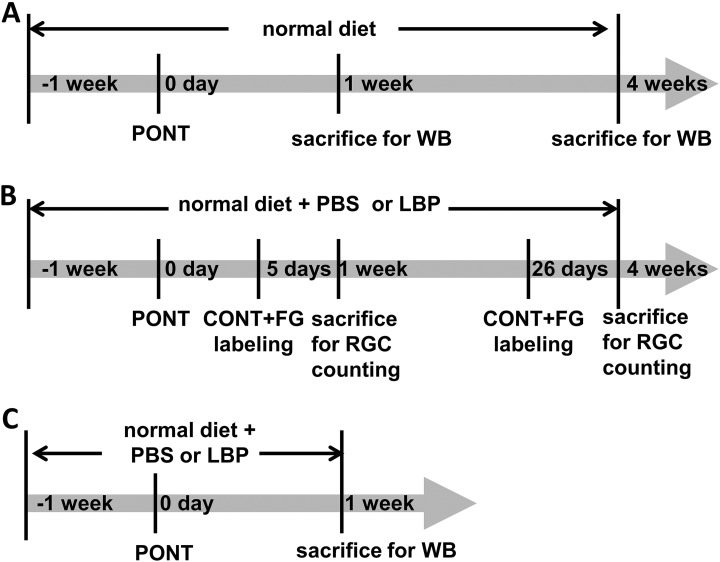
A total of 72 rats were used in our experiments: 6 rats were used for DiI labeling; 18 rats for detecting microglia/macrophage activation and autophagy alteration after PONT; and 48 for detecting the effects of LBP on RGC survival, microglia/macrophage activation and autophagy alteration after PONT. To further clarify the usage of animals, for detecting microglia/macrophage activation and autophagy alteration after PONT (total 18 rats), there were three groups: normal, 1-week PONT, and 4-week PONT groups (6 rats in each group; the time line is listed in Fig. 2A). For estimating the effects of Lyicum barbarum, a total of 48 rats was used (the time line is listed in Fig. 2B and 2C): 36 rats used for RGC counting in 4 groups: phosphate buffered saline (PBS)+1-week PONT, PBS+4-week PONT, LBP +1-week PONT, and LBP + 4-week PONT groups (nine rats in each group, Fig. 2B); 12 rats used for Western blot analysis (6 rats in PBS group and 6 rats in LBP group 1 week after PONT, Fig. 2C).
Fig. 2.
The time lines of different experiments. (A) Schematic diagrams showing the procedure for the estimation of RGC survival. Rats were fed with PBS or LBP 1 week before partial optic nerve transection (PONT) until sacrifice. On day 0, PONT was performed. Two days before sacrifice, rats received complete optic nerve transection (CONT) and a piece of gelatin soaked with Fluoro-gold (FG) was placed close to the optic nerve stump to label RGCs. Rats were sacrificed 1 week or 4 weeks after PONT. (B) Schematic diagrams showing the procedure for the estimation of microglia/macrophages activation and autophagy alteration after PONT with normal diet (without feeding PBS or LBP). Rats were sacrificed 1 week or 4 weeks after PONT. (C) Schematic diagrams showing the procedure for estimating the effects of LBP on microglia/macrophages activation and autophagy alteration after PONT. Rats were sacrificed 1 week after PONT.
Treatment with LBP
LBP were extracted by Shanghai Institute of Material Medica as previously described47,50. Briefly, the aqueous extract of dried fruits of Lycium barbarum (Ningxia, China) was prepared sequentially by decoloration and delipidation in alcohol, and boiling in distilled water. The extract was then freeze-dried into powder for storage.
The LBP were stored in a dry-box and freshly dissolved in 0.01 mol/L phosphate-buffered saline (PBS; 0.01 M, pH 7.4) before use. The related studies had reported that 1 mg/kg for each rat had a neuroprotective effect for RGCs both in the chronic ocular hypertension model and the PONT model4,47. Therefore, we used the dosage of 1 mg/kg LBP in this study. The control group received PBS only. The treatment (LBP or PBS) began 1 week before surgery (PONT) until sacrifice at the scheduled time-points. The treatment was achieved by gavage feeding with needle once daily.
PONT Surgery
The PONT model used in previous studies (cut in the dorsal parts of the optic nerves)4,12,51 is different from the model used in this experiment (Fig. 1A). In this experiment, the right optic nerve was transected partially in the temporal part (1.5 mm from the optic disc). The surgery was undertaken using a diamond radial keratotomy knife (G-31480, Geuder AG, Hertzstrasse, Heidelberg, Germany) with the blade fixed to a length of 150 μm. The depth of the cut was determined by the protrusion of the blade beyond a surrounding precision-calibrated guard, allowing precise and reproducible injury. The reason for cutting the optic nerve from the temporal part is that the procedure is easy to carry out and avoids damaging surrounding structures. In addition, the position of the cut site can be localized more precisely because there is a blood vessel just below the optic nerve of SD rats across the posterior external surface of the entire eyeball (Fig. 1A).
Retrograde DiI Tracing in vivo After PONT
The method has been published previously4,15. Briefly, the optic nerve was partially cut from the temporal side and several crystals of DiI (Molecular Probes, Eugene, OR, USA) were placed precisely into the cut site to label the RGC bodies in the retinas whose axons were transected. Therefore, DiI labeled only the RGCs that would die from primary degeneration (Fig. 1A). The rats were sacrificed 4 days after DiI labeling. The retinas were processed as flat-mounts for RGC counting.
Retrograde Fluoro-Gold Tracing in vivo after PONT
For Fluoro-Gold (FG) labeling, 2 days before sacrifice, rats received a complete optic nerve transection (CONT) in the right optic nerves approximately 0.5 mm away from the optic disc without damaging blood vessels. A gelatin sponge soaked with 6% FG (Invitrogen, Carlsbad, CA, USA) was placed proximal to the transected optic nerve stump.
Quantification of RGCs
After sacrifice, retinas were collected and post-fixed in 4% paraformaldehyde (PFA) for 60 min. Then flat-mounted retinas were prepared for RGC counting. Retinas were divided into temporal and nasal halves and both halves were separated into three roughly equal sectors. Six to nine photographs (200 × 200 μm2) in each sector were captured along the median line, starting from the optic disc to the edge at 500 μm intervals under 400× magnification4. Surviving RGCs were counted separately in temporal and nasal retinas because the degeneration speeds were different in temporal and nasal retinas after PONT. The counting of both DiI and FG-labeled cells was conducted using a double-blind method by two persons, and the data were averaged (numbers per mm2, mean ± standard error of the mean (SEM)).
Western Blot Analysis
All the Western blot analysis in this study was done with optic nerve samples but not retinas. To detect the changes of microglia/macrophages after PONT, the levels of CD68, iNOS, and Arginase-1 were measured with western blotting analysis. The rats were sacrificed at scheduled time-points with 10% Chloral hydrate and optic nerves collected in cold PBS on ice. The optic nerves were then homogenized in RIPA lysis buffer supplemented with 1% protease inhibitor cocktail and 1% phosphatase inhibitor cocktail. The tissues were broken up with an ultra-sonication machine and placed in tubes in ice for 30 min. After centrifugation with a speed of 14,000 rpm for 10 min, the supernatants were collected and kept at –80°C. Before SDS-polyacrylamide gel electrophoresis, the concentration of protein in the supernatant was detected using Beyotime BCA protein assay kit. According to the property of different antibodies, different aliquots of proteins from each individual sample were used for Western blot analysis. After proteins were transferred onto polyvinylidene difluoride (PVDF) membranes, the membranes were blocked with 5% non-fat dry milk in Tris-buffered saline with 0.05% Tween 20 (TBST) for 2 h. Incubation with anti-actin (1:5000, 12620, rabbit monoclonal, Cell Signaling, Beverly, MA, USA), anti-CD68 (1:1000, MCA341GA, mouse monoclonal, AbD Serotec, Hercules, CA, USA), anti-iNOS (1:1000, ab15323, rabbit monoclonal, Abcam, Cambridge, UK), anti-Arginase-1 (1:1000, ab91279, rabbit monoclonal, Abcam) and anti-LC3 A/B (1:5000,12741, rabbit monoclonal, Cell Signaling) antibodies in TBST were performed overnight at 4°C. After washing with TBST 6 times for 10 min each time, membranes were incubated with Horseradish Peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) with the dilution of 1:5000 for CD68 and 1:8000 for other antibodies in TBST for 2 h at room temperature. The enhanced chemiluminescence (ECL) method was used for the detection of immunoreactive proteins. The images were acquired by Bio-Rad ChemiDoc Touch imaging system and densitometric analysis of the bands of proteins was achieved by Image J software. The densitometric values obtained from different target proteins were normalized with respect to beta-actin loading controls in the same blot to obtain the final ratios. All experiments for Western blot analysis were performed with 6 animals in each group and both optic nerves from each animal were collected as one individual sample.
Statistical Analysis
All comparisons were between two groups (student's t-test). All data were expressed as the mean ± SEM. The level of p=0.05 was considered to be statistically significant.
Results
DiI Labeled More RGC bodies in the Temporal Retinas than in the Nasal Retinas
DiI labeled the cell bodies of RGCs whose axons were transected after PONT and which would be expected to die from primary degeneration. The densities of DiI labeled RGCs were 170 ± 49 RGCs/mm2 and 35 ± 17 RGCs/mm2 in the temporal and nasal retinas, respectively. The difference was significant (Fig. 1B, p <0.05, Student’s pair t-test) and the ratio was about 4.9:1 between temporal and nasal retinas. These findings indicated that RGCs in both temporal and nasal retinas were vulnerable to primary degeneration after PONT; however, primary degeneration of RGCs mainly occurred in the temporal retinas.
Changes of Microglia/Macrophages and Autophagy after PONT
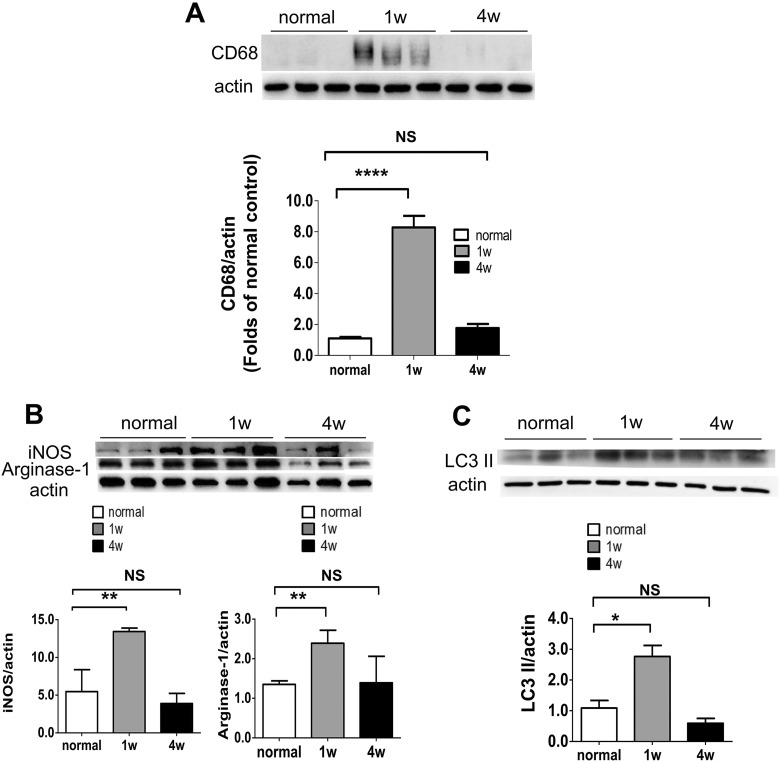
Cluster of differentiation 68 (CD68) is a protein highly expressed by microglia/macrophages after activation52,53. Therefore, the anti-CD68 antibody was used to detect the change of microglia/macrophages in this study. Using western blotting analysis, the results showed that the expression of CD68 increased significantly 1 week after PONT, but not 4 weeks after PONT compared with the normal group (Fig. 3A). The effect of PONT on M1 and M2 polarization of microglia/macrophages was evaluated by the expression levels of iNOS and Arginase-1, respectively. More microglia/macrophages were polarized to both M1 and M2 directions 1 week after PONT, but went back to normal 4 weeks after PONT (Fig. 3B). In this study, the effect of PONT on autophagy was evaluated based on changes in LC3 II expression. The expression level of LC3 II was higher in rats 1 week after PONT and went back to normal 4 weeks after PONT (Fig. 3C).
Fig. 3.
Examination of the changes in microglia/macrophages and autophagy in optic nerves after PONT. (A) Effects of PONT on the activation of microglia/macrophages. Western blot analysis shows that the expression level of CD68 increases significantly 1 week after PONT in the injured optic nerves (student t-test, ***p < 0.001), but there was no significant difference between optic nerves from rats 4 weeks after PONT and that of normal retinas (student t-test, p > 0.05). (B) Effects of PONT on the polarization of microglia/macrophages. The expression levels of iNOS and Arginase-1 increase significantly 1 week after PONT (Student t-test, **p < 0.01); but not 4 weeks after PONT (Student t-test, p > 0.05). (C) Activation of autophagy after PONT. The LC3 II expression increased significantly 1 week after PONT (Student t-test, *p < 0.05); but not 4 weeks after PONT (Student t-test, p > 0.05). (mean ± SEM; NS: not significant; n = 6 in each group for Western blot analysis, two optic nerves in one sample.).
Effects of LBP on Survival of RGCs after PONT
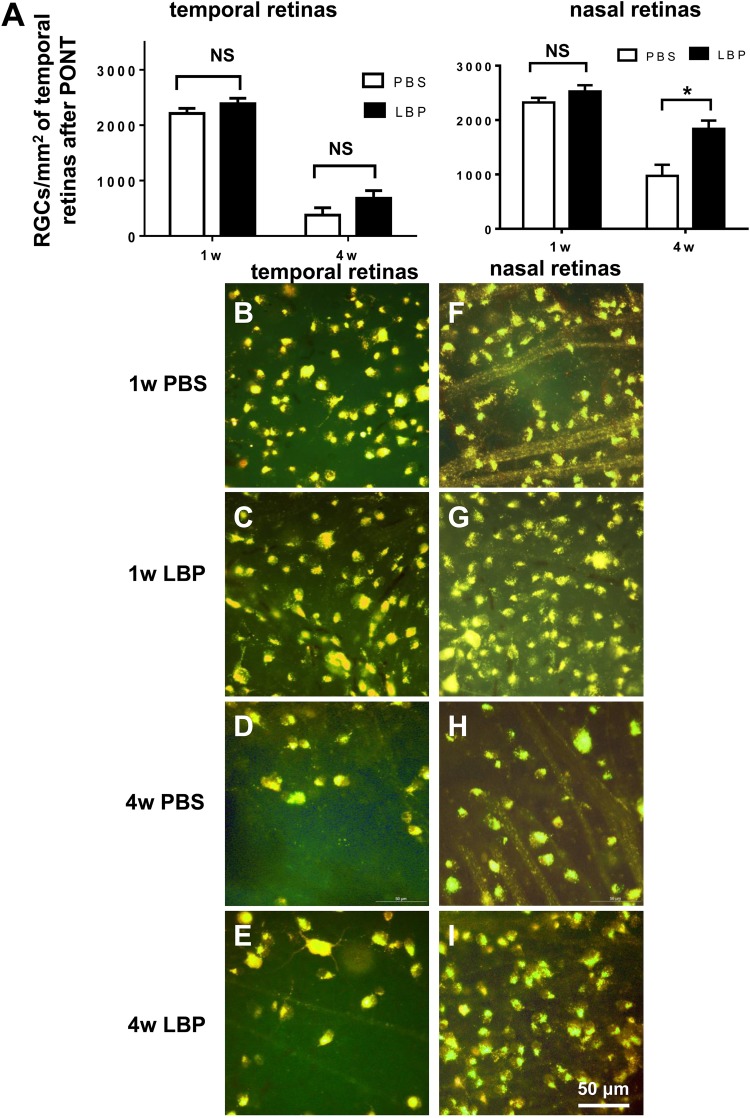
LBP had no effects on the survival of RGCs in the temporal retinas either 1 week or 4 weeks after PONT (Fig. 4A–E) (1-week PBS: 2212 ± 92 RGCs/mm2; 1-week LBP: 2386 ± 98 RGCs/mm2; 4-week PBS: 378 ± 133 RGCs/mm2; 4-week LBP: 680 ± 139 RGCs/mm2). In the nasal retinas, LBP delayed the degeneration of RGCs 4 weeks after the PONT but not 1 week after PONT (Fig. 4A, 4F–I) (1-week PBS: 2323 ± 85 RGCs/mm2; 1-week LBP: 2326 ± 116 RGCs/mm2; 4-week PBS: 975 ± 203 RGCs/mm2; 4-week LBP: 1839 ± 152 RGCs/mm2).
Fig. 4.
Effects of Lycium barbarum polysaccharides (LBP) on RGC survival 1 week and 4 weeks after PONT. (A) Both in temporal and nasal retinal halves, LBP treatment did not delay the degeneration of RGCs 1 week after PONT (Student's t-test; p > 0.05). However, LBP reduced the degeneration of RGCs in the nasal retinas 4 weeks after PONT (Student's t-test, *p < 0.05,); but not in the temporal retinas (Student's t-test; p > 0.05). (B–I) The photographs of RGCs labeled with Fluoro-Gold (FG) in both the temporal and nasal retinas are about 1.5 mm away from the optic disc: (B–E) In the temporal retinas, the densities of RGCs were similar between the PBS and LBP groups both 1 week and 4 weeks after PONT (Student's t-test, p > 0.05). (F–I) In the nasal retinas, the density of RGCs in the LBP group was higher than that in the PBS group 4 weeks after PONT (Student's t-test, *p < 0.05); but not 1 week after PONT (Student's t-test; p > 0.05). (Mean ± SEM; NS: not significant; n = 9 in both PBS and LBP groups both 1 week and 4 weeks after PONT).
Effects of LBP on Changes of Microglia/Macrophages and Autophagy after PONT
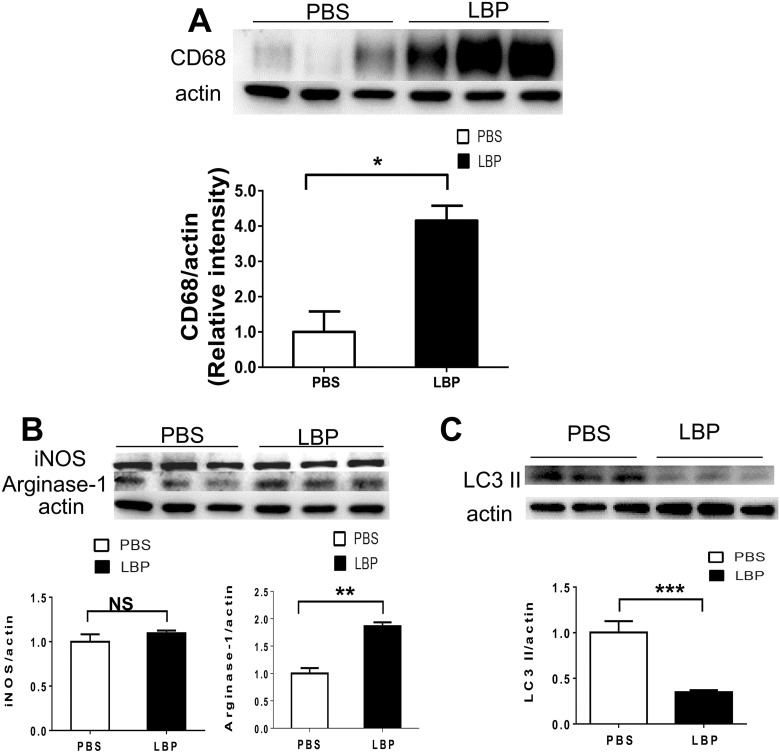
Using Western blot analysis to detect the expression levels of CD68, the results showed that the expression level of CD68 in the 1-week LBP group was higher than in the 1-week PBS group (Fig. 5A). For M1/M2 polarization examination, LBP could increase the Arginase-1 expression rather than the expression of iNOS (Fig. 5B). In addition, LBP could reduce the expression of LC3 II 1 week after PONT (Fig. 5C).
Fig. 5.
The effects of LBP on activation of microglia/macrophages, polarization of microglia/macrophages and activation of autophagy in optic nerves. (A) The Western blot analysis showed that LBP treatment significantly increased the expression of CD68 (Student's t-test, *p < 0.05). (B) Effects of LBP on polarization of microglia/macrophages. The expression level of Arginase-1 increased significantly 1 week after PONT in rats fed with LBP (Student's t-test, **p < 0.01); but the expression level of iNOS did not change (Student's t-test, p > 0.05). (C) Effects of LBP on autophagy. LBP led to decreased LC3 II expression (Student's t-test, ***p < 0.001) in the LBP group compared with the PBS group (Student's t-test, *p < 0.05). (Mean ± SEM; NS: not significant; n = 6 in both PBS and LBP groups for Western blot analysis, two optic nerves in one sample).
Discussion
The PONT model was established using monkeys in 2001 by Levkovitch-Verbin et al.6,54. It was a good model for separating primary degeneration from secondary degeneration of RGCs. In this study, we did partial injury from the temporal side of optic nerves, rather than from the superior parts as in previous studies. PONT was more accurate and convenient when performed from the temporal side because exposure of the optic nerves was much easier due to the lack of obstruction of the rectus superior and obliquus superior eye muscles. DiI labeling showed the densities of RGCs were 170 ± 49 RGCs/mm2 and 35 ± 17 RGCs/mm2 in the temporal and nasal retinas, respectively; the ratio was about 4.9:1. In a previous study optic nerves were cut from the superior side and DiI labeled 461±53 RGCs/mm2 in the superior retinas and 191±49 RGCs/mm2 in the inferior retinas, respectively; the ratio was about 2.4:14. This meant that cutting from the temporal side of optic nerves led to less primary degeneration in the nasal retinas. Therefore, the method used in this study was more suitable for detecting secondary degeneration of RGCs. Our results also showed that LBP could delay the death of RGCs on the nasal retinas where most secondary injury occurred 4 weeks after PONT; this result was consistent with the finding of the previous study that LBP mainly delayed secondary degeneration4,7.
LBP may regulate the function of microglia/macrophages both in a chronic ocular hypertension model and a PONT model45,5. It is known that activated microglia/macrophages can be divided into pro-inflammatory M1 and pro-tissue-repair M2 types. In this study, we used Western blot analysis to detect changes in the expression levels of CD68, which indicates the activation of microglia/macrophages. We also detected changes of iNOS, which is expressed by M1-type microglia/macrophages; and Arginase-1, which is expressed by M2-type microglia/macrophages. Our results show that the expression levels of CD68, iNOS, and Arginase-1 increased 1 week after PONT. The increase of M1-type microglia/macrophages might contribute to the death of RGCs; on the other hand, the increase of M2-type microglia/macrophages might be neuroprotective for RGCs. We found that LBP increased the number of total activated microglia/macrophages and M2-type microglia/macrophages 1 week after PONT; this result indicates that LBP might modulate the activity of microglia/macrophages and promote the M2 polarization to delay the degeneration of RGCs. There are conflicting opinions about the influence of activation of microglia/macrophages in the nervous system. Some believe that this influence could contribute to cell death22 and others think it could be neuroprotective16,37,38,55. In this study we find more M2-type microglia/macrophages are present in the optic nerve together with less RGC death. In one previous study using the PONT model, LBP has been shown to decrease the activation of Iba-1-positive microglia/macrophages 4 weeks after PONT5. Why did LBP exert different effects in the same model? Firstly, the time-points detected are different: it was 4 weeks after PONT in the previous study and 1 week in this study. In the previous study the activated extent of microglia/macrophages was much lower 4 weeks after PONT than 1 week after PONT; therefore, the status of cell activation should be different and LBP had various effects on the cells. Secondly, CD68 was used in this study and Iba-1 was used in the previous study. These two different antibodies may not label exactly the same number or category of microglia/macrophages because not all CD68 positive cells are positive for Iba-1, a point that has been shown in different studies11.
In addition, we found that LC3 II expression was significantly elevated 1 week after PONT. This result is consistent with other studies using the optic nerve compression model14, NMDA-induced neurotoxic retinal injury model18–20, and a chronic ocular hypertension model in which the level of autophagy increased after injury43. Inhibition of autophagy with 3-methyladenine (3-MA) reduced the death of RGCs in a rat model of chronic elevated intraocular pressure43. Therefore, it is believed that activation of autophagy can lead to the death of RGCs43. However, there is also a lot of evidence showing that autophagy is neuroprotective for RGCs24,25,43. In this study, we have shown that the level of autophagy decreased 1 week after PONT after LBP treatment. However, we cannot conclude that LBP decreases autophagy as we cannot exclude the possibility that the decrease is a result of less RGC death after LBP treatment. We will inhibit or increase autophagy levels to see their effect on RGC survival after PONT in future experiments.
We have shown that LBP may decrease secondary RGC death 4 weeks after PONT but not 1 week after PONT. Why, then, does it take 4 weeks rather than 1 week for LBP to exert the neuroprotective effect for RGCs? The possible explanation is as follows: firstly, the extent of secondary cell loss of RGCs is not big enough to exhibit a significant difference between the PBS group and LBP group; alternatively, the effect of Chinese herbal medicine is mild and needs a longer time period, more than 1 week, for LBP to exert its neuroprotective effect for RGCs. Because LBP is a compound and contains various monomers, in future experiments we will detect which kind of monomer contributes to the neuroprotective effect of LBP. Following this, we may start to cooperate with pharmaceutical manufacturers to produce eye drops and an ointment which can be administrated to humans in order to preserve RGCs.
Based on these results, we conclude that cutting from the temporal side of optic nerves to conduct the PONT model is more suitable for the study of secondary degeneration of RGCs, and LBP can delay secondary degeneration of RGCs 4 weeks after PONT. In addition, increased polarization of microglia/macrophages to both M1 and M2 directions and increased autophagy were observed after PONT. Lastly, more M2-type polarization and a lower autophagy level were observed after LBP treatment, although their direct relationship with RGC survival will require further investigation.
Acknowledgements
We thank Dr. Greg Campbell for his constructive comments on the manuscript.
Author Contributions: Conceived and designed the experiments: H.Y.L., K.F.S.; Performed the experiments: H.Y.L., M.H., Q.Y.L., X.H.; Analyzed the data: H.Y.L., M.H., S.R. K.F.S.; Contributed reagents/materials/analysis tools: H.Y.L., S.R., K.F.S.; Writing and editing: M.H., H.Y.L., K.F.S.; Funding acquisition: H.Y.L., S.R., K.F.S.; All authors reviewed the manuscript.
Hong-Ying Li, Mi Huang: These authors contribute equally to this study.
Ethical Approval: This study was approved by the Animal Protection and Use Committee of Jinan University in Guangzhou, China.
Statement of Human and Animal Rights: This article contains animal studies approved by the Animal Protection and Use Committee of Jinan University in Guangzhou, China.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work described in this paper was supported by grant from the National Natural Science Foundation of China (81501091), the Natural Science Foundation of Guangdong Province of China (2015A030310201), the fund of Leading Talents of Guangdong Province (87014002), the fund of Ningxia Key Research and Development Program Grant (Yinchuan, Ningxia, China), Programme of Introducing Talents of Discipline to Universities (B14036), National Basic Research program (2015CB351800), the Fundamental Research Funds for the Central Universities Grant (21609101). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iD: Hong-Ying Li  https://orcid.org/0000-0002-1109-0799
https://orcid.org/0000-0002-1109-0799
References
- 1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta N, Kikkawa DO, Levi L, Weinreb RN. Severe vision loss and neovascular glaucoma complicating superior ophthalmic vein approach to carotid-cavernous sinus fistula. Am J Ophthalmol. 1997;124(6):853–855. [DOI] [PubMed] [Google Scholar]
- 3. McKinnon SJ, Goldberg LD, Peeples P, Walt JG, Bramley TJ. Current management of glaucoma and the need for complete therapy. Am J Manag Care. 2008;14(suppl 1):S20–S27. [PubMed] [Google Scholar]
- 4. Li H, Liang Y, Chiu K, Yuan Q, Lin B, Chang RC, So KF. Lycium barbarum (wolfberry) reduces secondary degeneration and oxidative stress, and inhibits JNK pathway in retina after partial optic nerve transection. Plos One. 2013;8(7):e68881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li HY, Ruan YW, Kau PW, Chiu K, Chang RC, Chan HH, So KF. Effect of Lycium barbarum (Wolfberry) on alleviating axonal degeneration after partial optic nerve transection. Cell Transplant. 2015;24(3):403–417. [DOI] [PubMed] [Google Scholar]
- 6. Li HY, Ruan YW, Ren CR, Cui Q, So KF. Mechanisms of secondary degeneration after partial optic nerve transection. Neural Regen Res. 2014;9(6):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan F, Guo S, Chai Y, Zhang L, Liu K, Lu Q, Wang N, Li S. Partial optic nerve transection in rats: a model established with a new operative approach to assess secondary degeneration of retinal ganglion cells. J Vis Exp. 2017;(128):56272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levkovitch-Verbin H, Dardik R, Vander S, Melamed S. Mechanism of retinal ganglion cells death in secondary degeneration of the optic nerve. Exp Eye Res. 2010;91(2):127–134. [DOI] [PubMed] [Google Scholar]
- 9. Levkovitch-Verbin H, Spierer O, Vander S, Dardik R. Similarities and differences between primary and secondary degeneration of the optic nerve and the effect of minocycline. Graefes Arch Clin Exp Ophthalmol. 2011;249(6):849–857. [DOI] [PubMed] [Google Scholar]
- 10. Fitzgerald M, Bartlett CA, Harvey AR, Dunlop SA. Early events of secondary degeneration after partial optic nerve transection: an immunohistochemical study. J Neurotrauma. 2010;27(2):439–452. [DOI] [PubMed] [Google Scholar]
- 11. Smith NM, Giacci MK, Gough A, Bailey C, McGonigle T, Black AMB, Clarke TO, Bartlett CA, Swaminathan Iyer K, Dunlop SA, Fitzgerald M. Inflammation and blood-brain barrier breach remote from the primary injury following neurotrauma. J Neuroinflammation. 2018;15(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giacci MK, Bartlett CA, Smith NM, Iyer KS, Toomey LM, Jiang H, Guagliardo P, Kilburn MR, Fitzgerald M. Oligodendroglia are particularly vulnerable to oxidative damage after neurotrauma in vivo. J Neurosci. 2018;38(29):6491–6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giacci MK, Bartlett CA, Huynh M, Kilburn MR, Dunlop SA, Fitzgerald M. Three dimensional electron microscopy reveals changing axonal and myelin morphology along normal and partially injured optic nerves. Sci Rep. 2018;8(1):3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mac Nair CE, Schlamp CL, Montgomery AD, Shestopalov VI, Nickells RW. Retinal glial responses to optic nerve crush are attenuated in Bax-deficient mice and modulated by purinergic signaling pathways. J Neuroinflammation. 2016;13(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fitzgerald M, Bartlett CA, Evill L, Rodger J, Harvey AR, Dunlop SA. Secondary degeneration of the optic nerve following partial transection: the benefits of lomerizine. Exp Neurol. 2009;216(1):219–230. [DOI] [PubMed] [Google Scholar]
- 16. Levkovitch-Verbin H, Quigley HA, Martin KR, Zack DJ, Pease ME, Valenta DF. A model to study differences between primary and secondary degeneration of retinal ganglion cells in rats by partial optic nerve transection. Invest Ophthalmol Vis Sci. 2003;44(8):3388–3393. [DOI] [PubMed] [Google Scholar]
- 17. Davies MH, Eubanks JP, Powers MR. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol Vis. 2006;12:467–477. [PubMed] [Google Scholar]
- 18. Wada Y, Nakamachi T, Endo K, Seki T, Ohtaki H, Tsuchikawa D, Hori M, Tsuchida M, Yoshikawa A, Matkovits A, Kagami N. et al. PACAP attenuates NMDA-induced retinal damage in association with modulation of the microglia/macrophage status into an acquired deactivation subtype. J Mol Neurosci. 2013;51(2):493–502. [DOI] [PubMed] [Google Scholar]
- 19. Singhal S, Lawrence JM, Salt TE, Khaw PT, Limb GA. Triamcinolone attenuates macrophage/microglia accumulation associated with NMDA-induced RGC death and facilitates survival of Muller stem cell grafts. Exp Eye Res. 2010;90(2):308–315. [DOI] [PubMed] [Google Scholar]
- 20. Ebneter A, Casson RJ, Wood JP, Chidlow G. Microglial activation in the visual pathway in experimental glaucoma: spatiotemporal characterization and correlation with axonal injury. Invest Ophthalmol Vis Sci. 2010;51(12):6448–6460. [DOI] [PubMed] [Google Scholar]
- 21. Quigley HA. Experimental glaucoma damage mechanism. Arch Ophthalmol. 1983;101(8):1301–1302. [DOI] [PubMed] [Google Scholar]
- 22. Yuan L, Neufeld AH. Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res. 2001;64(5):523–532. [DOI] [PubMed] [Google Scholar]
- 23. Bosco A, Steele MR, Vetter ML. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol. 2011;519(4):599–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SH, Munemasa Y, Kwong JM, Ahn JH, Mareninov S, Gordon LK, Caprioli J, Piri N. Activation of autophagy in retinal ganglion cells. J Neurosci Res. 2008;86(13):2943–2951. [DOI] [PubMed] [Google Scholar]
- 25. Knoferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P, Tonges L, Stadelmann C, Bruck W, Bahr M, Lingor P. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A. 2010;107(13):6064–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wells J, Kilburn MR, Shaw JA, Bartlett CA, Harvey AR, Dunlop SA, Fitzgerald M. Early in vivo changes in calcium ions, oxidative stress markers, and ion channel immunoreactivity following partial injury to the optic nerve. J Neurosci Res. 2012;90(3):606–618. [DOI] [PubMed] [Google Scholar]
- 27. Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17(10):871–890. [DOI] [PubMed] [Google Scholar]
- 28. Begemann M, Grube S, Papiol S, Malzahn D, Krampe H, Ribbe K, Friedrichs H, Radyushkin KA, El-Kordi A, Benseler F, Hannke K. et al. Modification of cognitive performance in schizophrenia by complexin 2 gene polymorphisms. Arch Gen Psychiatry. 2010;67(9):879–888. [DOI] [PubMed] [Google Scholar]
- 29. Levkovitch-Verbin H, Dardik R, Vander S, Nisgav Y, Kalev-Landoy M, Melamed S. Experimental glaucoma and optic nerve transection induce simultaneous upregulation of proapoptotic and prosurvival genes. Invest Ophthalmol Vis Sci. 2006;47(6):2491–2497. [DOI] [PubMed] [Google Scholar]
- 30. Levkovitch-Verbin H, Harizman N, Dardik R, Nisgav Y, Vander S, Melamed S. Regulation of cell death and survival pathways in experimental glaucoma. Exp Eye Res. 2007;85(2):250–258. [DOI] [PubMed] [Google Scholar]
- 31. Hashimoto K, Parker A, Malone P, Gabelt BT, Rasmussen C, Kaufman PS, Hernandez MR. Long-term activation of c-Fos and c-Jun in optic nerve head astrocytes in experimental ocular hypertension in monkeys and after exposure to elevated pressure in vitro. Brain Res. 2005;1054(2):103–115. [DOI] [PubMed] [Google Scholar]
- 32. Lingor P, Koeberle P, Kugler S, Bahr M. Down-regulation of apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death in vivo. Brain. 2005;128(Pt 3):550–558. [DOI] [PubMed] [Google Scholar]
- 33. Sun H, Wang Y, Pang IH, Shen J, Tang X, Li Y, Liu C, Li B. Protective effect of a JNK inhibitor against retinal ganglion cell loss induced by acute moderate ocular hypertension. Mol Vis. 2011;17:864–875. [PMC free article] [PubMed] [Google Scholar]
- 34. Murphy JA, Clarke DB. Target-derived neurotrophins may influence the survival of adult retinal ganglion cells when local neurotrophic support is disrupted: implications for glaucoma. Med Hypotheses. 2006;67(5):1208–1212. [DOI] [PubMed] [Google Scholar]
- 35. Lannes N, Eppler E, Etemad S, Yotovski P, Filgueira L. Microglia at center stage: a comprehensive review about the versatile and unique residential macrophages of the central nervous system. Oncotarget. 2017;8(69):114393–114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheon SY, Kim EJ, Kim JM, Kam EH, Ko BW, Koo BN. Regulation of microglia and macrophage polarization via apoptosis signal-regulating kinase 1 silencing after ischemic/hypoxic injury. Front Mol Neurosci. 2017;10:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–6173. [DOI] [PubMed] [Google Scholar]
- 39. Parisi C, Napoli G, Pelegrin P, Volonte C. M1 and M2 functional imprinting of primary microglia: role of P2X7 activation and miR-125b. Mediators Inflamm. 2016;2016:2989548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17(4):654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kourtis N, Tavernarakis N. Autophagy and cell death in model organisms. Cell Death Differ. 2009;16(1):21–30. [DOI] [PubMed] [Google Scholar]
- 43. Park HY, Kim JH, Park CK. Activation of autophagy induces retinal ganglion cell death in a chronic hypertensive glaucoma model. Cell Death Dis. 2012;3:e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xing X, Liu F, Xiao J, So KF. Neuro-protective mechanisms of Lycium barbarum. Neuromolecular Med. 2016;18(3):253–263. [DOI] [PubMed] [Google Scholar]
- 45. Chiu K, Chan HC, Yeung SC, Yuen WH, Zee SY, Chang RC, So KF. Modulation of microglia by Wolfberry on the survival of retinal ganglion cells in a rat ocular hypertension model. J Ocul Biol Dis Infor. 2009;2(2):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chiu K, Zhou Y, Yeung SC, Lok CK, Chan OO, Chang RC, So KF, Chiu JF. Up-regulation of crystallins is involved in the neuroprotective effect of wolfberry on survival of retinal ganglion cells in rat ocular hypertension model. J Cell Biochem. 2010;110(2):311–320. [DOI] [PubMed] [Google Scholar]
- 47. Chan HC, Chang RC, Koon-Ching Ip A, Chiu K, Yuen WH, Zee SY, So KF. Neuroprotective effects of Lycium barbarum lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp Neurol. 2007;203(1):269–273. [DOI] [PubMed] [Google Scholar]
- 48. Xiao J, Xing F, Huo J, Fung ML, Liong EC, Ching YP, Xu A, Chang RC, So KF, Tipoe GL. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model. Sci Rep. 2014;4:5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bie M, Lv Y, Ren C, Xing F, Cui Q, Xiao J, So KF. Lycium barbarum polysaccharide improves bipolar pulse current-induced microglia cell injury through modulating autophagy. Cell Transplant. 2015;24(3):419–428. [DOI] [PubMed] [Google Scholar]
- 50. Liu F, Zhang J, Xiang Z, Xu D, So KF, Vardi N, Xu Y. Lycium barbarum polysaccharides protect retina in rd1 mice during photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2018;59(1):597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cummins N, Bartlett CA, Archer M, Bartlett E, Hemmi JM, Harvey AR, Dunlop SA, Fitzgerald M. Changes to mitochondrial ultrastructure in optic nerve vulnerable to secondary degeneration in vivo are limited by irradiation at 670 nm. BMC Neurosci. 2013;14(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Walker DG, Lue LF. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther. 2015;7(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Turtzo LC, Lescher J, Janes L, Dean DD, Budde MD, Frank JA. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J Neuroinflammation. 2014;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Levkovitch-Verbin H, Quigley HA, Kerrigan-Baumrind LA, D’Anna SA, Kerrigan D, Pease ME. Optic nerve transection in monkeys may result in secondary degeneration of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2001;42(5):975–982. [PubMed] [Google Scholar]
- 55. Brune B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, von Knethen A, Weigert A. Redox control of inflammation in macrophages. Antioxid Redox Signal. 2013;19(6):595–637. [DOI] [PMC free article] [PubMed] [Google Scholar]