Abstract
Laryngopharyngeal reflux (LPR) induces a differential damage effect on several anatomic sites within the larynx and hypopharynx; therefore, an in vitro model is needed for each anatomic site. This study aimed to establish a primary culture method for human laryngeal and hypopharyngeal epithelial cells derived from multiple anatomic sites. Surgical mucosa specimens were treated with a two-step enzymatic strategy to establish a primary culture. Of the 46 samples, primary cultivation was achieved successfully with 36 samples, and the positive ratio was 78.3%. In addition, flow cytometry revealed that these primary cells were epithelial cells with a purity of 94.9%. The proliferative ability was confirmed by positive staining for Ki-67. Laryngeal and hypopharyngeal epithelial cells from multiple sites exhibited similar epithelial morphology and positive cytokeratin expression. These cells can be cultured to passage 4. In summary, we successfully established the in vitro epithelial model of larynx and hypopharynx subsites, which may potentially be used as a platform for reflux research, especially for site-specific damage effect.
Keywords: epithelial cell, larynx, hypopharynx, laryngopharyngeal reflux, site-specific heterogeneity
Introduction
Laryngopharyngeal reflux (LPR) refers to exposure of gastric refluxate in extra-oesophageal regions, extending from the larynx, hypopharynx to middle ear1. In Otolaryngology clinics, approximately 10% of patients have LPR-related disorder2,3. Clinical manifestations are caused by injuries from gastric refluxate, including acid4, pepsin5, bile acids6, and pancreatic proteases3.
It has been shown that different anatomic regions have specific mucosal resistance to LPR. Posterior commissure (PC) is the most resistant site in LPR, while the subglottic site is the most vulnerable area7. In 2018, Wood et al. reported a site-specific LPR genotype, with the majority present in the medial arytenoid region8. The vulnerability to damage and transforming potential is unique to each anatomic region. Hence, a more comprehensive and sophisticated exploitation of LPR-associated pathogenesis requires an in vitro model for each anatomic region.
Currently, no epithelial cell line of larynx or hypopharynx is commercially available, so primary culture offers an ideal option, especially for a site-specific model. Primary cells have been cultured successfully from supraglottis9, postcricoid10, PC11, vocal cord12, and hypopharynx13. The purpose of this study was to describe a versatile approach for expansion and characterization of human epithelial cells from multiple sites within the laryngopharynx.
Materials and Methods
Isolation and Expansion
After sample collection, mucosa specimens were kept on ice until further processed. Tissue was minced finely and treated with 10mg/ml Dispase II (Sigma-Aldrich, St. Louis, MO, USA) for 48 h at 4°. The cell pellet was then dissociated enzymatically in 0.05% Trypsin/EDTA (Life Technologies, Carlsbad, CA, USA) at 37°C for 2–3 mins. Tissue was isolated mechanically by passing through a strainer. The final pellet was resuspended in serum-free keratinocyte media KGMTM-2 (LONZA, Basel, Switzerland) and seeded at 37°C, 5% CO2. Cultures were left for 48 h for cell adherence, and the medium was replaced every 2 days. After 10–14 days, cells reached 90% confluence and could be expanded using Accutase (Life Technologies, Carlsbad, CA, USA).
Immunofluorescence Staining and Confocal Microscopy
Immunofluorescence staining was performed as described previously14. Briefly, epithelial cells were fixed in 4% paraformaldehyde (PFA, Sigma-Aldrich, St. Louis, MO, USA) at room temperature overnight and permeabilized with 0.4% Triton X-100 (Sigma-Aldrich) in PBS for 20 min. Cells were blocked for 1 h with normal goat serum (Beyotime Biotechnology, Haimen, China), and incubated with primary antibodies: mouse anti-pan cytokeratin (ab7753, 1:125dilution, Abcam, Cambridge, MA, USA) and rabbit anti-Ki-67(ab92742, 1:250dilution, Abcam) overnight at 4°C. Secondary antibodies Alexa-488 (#4408, 1:500dilution, Life Technologies, Carlsbad, CA, USA) and Alexa-555 (#4413, 1:500dilution, Life Technologies) were applied for 1 h at room temperature. Fluorescent images were captured using an Olympus microscope (IX71; Olympus, Tokyo, Japan) and an Olympus confocal microscope (FV3000) and processed using Image software (DP controller).
Flow Cytometry Analysis
For intracellular protein labeling, cells were fixed and permeabilized using the Fix and Perm Cell Kit according to manufacturer’s protocol (MultiSciences Biotech Co, Shanghai, China). The cells were then incubated with mouse anti-pan cytokeratin (ab7753, 0.5μg/ml, Abcam, Cambridge, MA, USA), followed by the Alexa-488 anti-mouse conjugate (#4408, 1:500dilution, Life Technologies, Carlsbad, CA, USA). The cells were analyzed using a BD flow cytometer (Becton Dickinson, San Jose, CA, USA). Cells stained with secondary antibody alone were used as a negative control.
Western Blot
Cells derived from multiple anatomic sites (epiglottis, ventricular fold, vocal fold, subglottis, postcricoid, and pyriform sinus) were ex-vivo expanded, and protein concentrations were measured using a BCA protein assay kit (Life Technologies, Carlsbad, CA, USA). Protein samples were separated by SDS-PAGE (Bio-Rad Laboratories, Hercules, CA, USA), followed by transferred to PVDF membranes (Millipore, Billerica, MA, USA) and blocked with 5% non-fat milk. The membranes were incubated with primary antibody anti-pan cytokeratin (ab7753, 1:1000 dilution, Abcam, Cambridge, MA, USA) overnight at 4°C. The secondary antibodies were incubated at 1:10,000 dilutions for 1 h at room temperature and detected by chemiluminescence (Millipore).
Senescence-Associated β-Galactosidase Staining
Senescence detection was performed using a Senescence-associated β-galactosidase (SA-β-gal) staining kit (Beyotime Biotechnology, Haimen, China) according to the manufacturer’s protocol. Cells were fixed with 4% PFA for 15 min and stained with the mixture according to the protocol overnight at 37°C. After washing with PBS, cells were analyzed by an Olympus microscope (IX71).
Statistical Analysis
All statistical analyses were performed using SPSS statistical software 17.0. Data were presented as mean ± standard deviation and analyzed by Chi-Square analysis of variance. A p < 0.05 was considered statistically significant.
Results
This study was approved by Southern Medical University Nanfang Hospital Institutional Review Board (NO. NFEC-201607-K2-01). Biopsy specimens were obtained from 46 patients (39 males, 7 females) who underwent laryngeal surgery between February 2017 and January 2018. These patients had a mean age of 53 years, and all signed informed consent.
Patients were divided into three groups based on the type of surgery. Group 1 comprised 19 patients with malignancy receiving a total or partial laryngectomy. Group 2 consisted of 10 patients with malignant tumor receiving a transoral laser cordectomy or partial laryngectomy. Group 3 included 17 patients requiring direct laryngoscopy for treatment of the nonmalignant lesion. After surgery, a macroscopically normal specimen was taken from the larynx (epiglottis, ventricular fold, vocal cord, and subglottis) and hypopharynx (postcricoid and pyriform sinus).
Of the 46 cases, cellular growth occurred in samples obtained from 36 patients, and positive ratio reached up to 78.3%. Data, including age, gender, specimen location and surgical procedure, are summarized in Table 1. The relationship between surgical procedure, pathology, age, gender, and culture results are analyzed in Table 2. Although positive ratios were 91.7% for younger patients (<45 years), 77.8% for middle-aged donors (45–59 years) and 68.8% for old-aged donors (>59 years), no significant differences were found in culture results between subgroups stratified by age, as well as other variables (surgical procedure, pathology and gender).
Table 1.
Characteristcs of Patients in the Series.
| Sample Location |
No. of patients | Gender male/ female |
Age, years | Surgical procedure | Successful culture | Positive ratio,% | ||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | ||||||
| Epiglottis | 12 | 12/0 | 51.67±12.37 | 10 | 2 | 0 | 12 | 100% |
| Ventricular fold | 15 | 14/0 | 53.33±12.36 | 8 | 5 | 2 | 15 | 100% |
| Vocal cord | 4 | 4/0 | 47.75±11.27 | 4 | 0 | 0 | 4 | 100% |
| Subglottis | 4 | 4/0 | 47.75±9.39 | 4 | 0 | 0 | 4 | 100% |
| Postcricoid | 41 | 34/7 | 53.68±12.75 | 15 | 10 | 16 | 31 | 76.8% |
| Pyriform sinus | 2 | 2/0 | 47.00±10.95 | 2 | 0 | 0 | 2 | 100% |
| Total | 46 | 39/7 | 53.04±12.75 | 19 | 10 | 17 | 36 | 78.3% |
Group 1 – total or partial laryngectomy. Group 2 – transoral endoscopic laryngectomy for laryngeal malignancy. Group 3 – direct laryngoscopy for treatment of benign lesion in laryngopharynx.
Table 2.
Correlation Between Clinical Variables and Culture Results.
| Variable | Total | Successful culture | Positive ratio,% |
P value | |
|---|---|---|---|---|---|
| Surgical procedure | Group 1 | 19 | 16 | 84.2% | 0.606 |
| Group 2 | 10 | 8 | 80.0% | ||
| Group 3 | 17 | 12 | 70.6% | ||
| Pathology | Malignant | 29 | 24 | 82.8% | 0.334 |
| Nonmalignant | 17 | 12 | 70.6% | ||
| Age, years | <45 | 12 | 11 | 91.7% | 0.346 |
| 45–59 | 18 | 14 | 77.8% | ||
| >59 | 16 | 11 | 68.8% | ||
| Gender | Male | 39 | 30 | 76.9% | 0.604 |
| Female | 7 | 6 | 85.7% | ||
Group 1 – total or partial laryngectomy. Group 2 – transoral endoscopic laryngectomy for laryngeal malignancy. Group 3 – direct laryngoscopy for treatment of benign lesion in laryngopharynx.
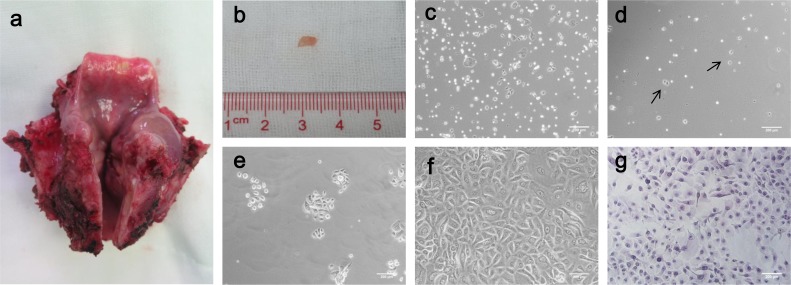
The freshly isolated cells were seeded on Petri dishes, and exhibited single cells and sphere-like clumps on Day 1 (Fig 1c). After 48 h, non-adherent cells remained suspended and were removed during the first change of medium. Only 10–20% of cells adhered and started to spread into a monolayer (Fig 1d), and most of them formed tiny clones after 7 days (Fig 1e). The cells reached confluence at 10–14 days after seeding, as illustrated in Fig 1f. The detailed process of in vitro expansion is described Fig 1. These cells can be cultured to passage 4.
Fig 1.
Detailed process of epithelial cell expansion. (a) Sample taken from a laryngectomy after the posterior side was opened. (b) Removal of macroscopically normal epithelial tissue for primary culture. (c–f) Phase contrast microscopy showed the process of expansion on Day 1(c), Day 2(d), Day 7(e) and Day 14(f) after seeding. Arrow indicates the adherent cells after first irrigation (Bar 200 μm). (g) Representative HE staining of epithelial cells (Bar 200 μm).
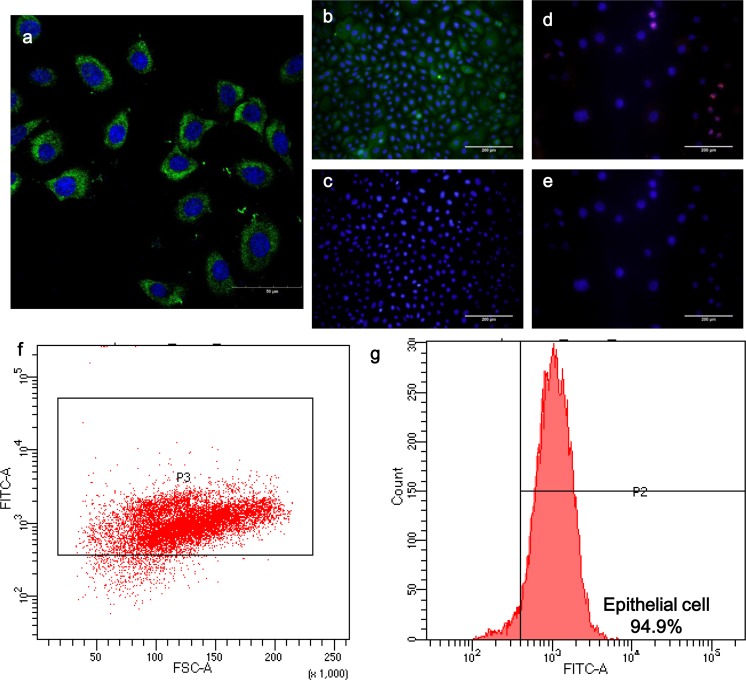
The cells were positively for pan-cytokeratin immunostainning and anti-pan cytokeratin can recognize a series of cytokeratin epitopes, thus the epithelial phenotype was identified (Fig 2a–c). Flow cytometry revealed that these primary cells were epithelial cells with a purity of 94.9% (Fig 2f–g). In addition, the proliferative ability was confirmed by positive staining for Ki-67 (Fig 2d–e).
Fig 2.
Cell phenotype, proliferative ability, and purity. (a) Representative confocal image of immunofluorescence staining of pan-cytokeratin (Bar 50 μm). (b–c) Immunofluorescence staining of pan-cytokeratin indicated its epithelial phenotype (Bar 200 μm). (d–e) Immunofluorescence staining of Ki-67 demonstrated its proliferative ability (Bar 200 μm). (f–g) Representation of flow cytometry of cultured epithelial cells from laryngopharynx staining with pan-cytokeratin. Nearly 95% of cells were epithelial cells.
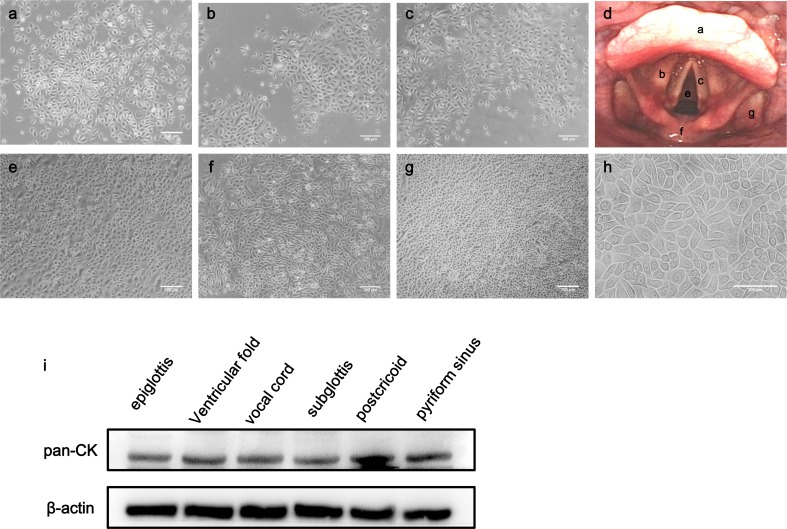
Tissues were derived from multiple sites of larynx and hypopharynx. Laryngeal biopsies consisted of supraglottis (laryngeal surface of the epiglottis and ventricular fold), glottis (vocal cord), and subglottis, while hypopharyngeal samples contained postcricoid and pyriform sinus (Fig 3a–c, e–g). All samples eventually yielded a morphologically similar population. These cells were cobblestone-shaped and arranged in a pavement sheet-like manner, which represented characteristic of epithelial cell morphology (Fig 3 h). Furthermore, pan-cytokeratin was expressed in the epithelial cells from all anatomic sites, and was relatively higher in postcricoid than in other anatomic sites (Fig 3i).
Fig 3.
Comparative study of epithelial cells from multiple sites. (a–c, e–g) Phase contrast microscopy of cultured epithelial cells from different anatomic sites (a. laryngeal surface of the epiglottis; b. ventricular fold; c. vocal cord; e. subglottis; f. postcricoid; g. pyriform sinus) (Bar 200 μm). (d) Flexible laryngoscopy exhibited the mentioned anatomic sites that were marked with corresponding letters (a–c, e–g). (h) Phase contrast microscopy revealed a characteristic epithelial morphology (Bar 200 μm). (i) Epithelial cells from multiple sites exhibited positive pan-cytokeratin expression.
Consistent with previous reports, our primary cultured cells were also limited by senescence. At early passage, epithelial cells showed homogenous polygonal morphology with a prominent nucleus, assuming a cobblestone-like configuration (Fig 4a). Cytoplasmic vacuoles with increased cell size were observed later, which was an indicator of senescence (Fig 4b). Subsequently, a large population of cells exhibited enlarged and rounded phenotypes in addition to cytoplasmic vacuoles (Fig 4c). SA-β-gal staining was detected specifically in these cells (Fig 4d).
Fig 4.
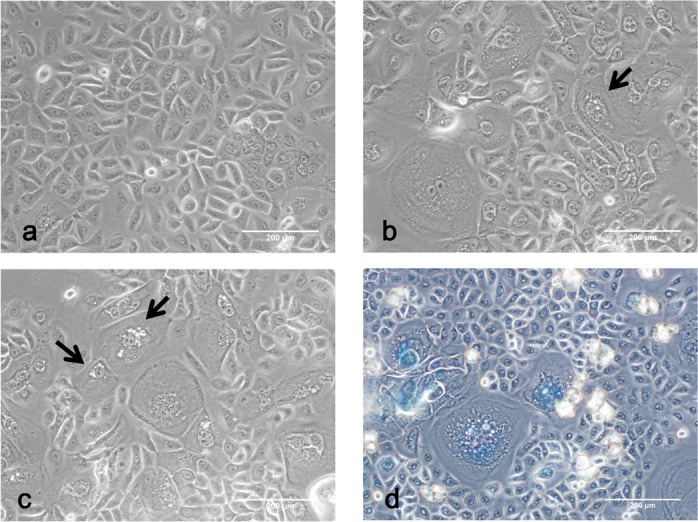
Phase contrast microscopy exhibited cellular senescence. (a) Cells of early passage were small and polygonal morphology (Bar 200 μm). (b) The onset of senescence included morphology change and vacuoles accumulation. Arrows indicate vacuoles in the cells (Bar 200 μm). (c) Late passage allowed increasing numbers of senescent cells. Arrows indicated increasing vacuoles (Bar 200 μm). (d) Senescent cells were positive for SA-β-gal staining (Bar 200 μm).
Discussion
The lining epithelium of the laryngopharynx has been reported to be histologically variable, with patches of squamous mucosa intermixed with ciliated epithelium15–18. The laryngeal surface of epiglottis, ventricular fold, and subglottis undergo a transition from ciliate to squamous epithelium19,20, while the vocal cord and hypopharyngeal epithelium are mainly squamous21,22. In addition to the histology derivation, these anatomic regions originate from different arches embryologically23. Due to mucosal heterogeneity among the subsites, differential impact secondary to reflux is evidenced and this has been unanimously found in all reflux-associated studies with respect to site-specific differences in LPR.
Site-specific heterogeneity of barrier function and gene profile within laryngopharynx has been recognized recently. The enhanced level of carbonic anhydrase III in PC, one of the self-defense mechanisms in laryngeal epithelium24,25, predisposes this specific location to be significantly resistant to reflux-associated damage. This is consistent with David M. Bulmer’s findings in the porcine model. Additively, LPR-associated gene profile exhibited significant differences in mucosal defense and inflammation, involving CRNN, CD1d, TGFβ-1, MUC2, and CDH1, whereas the most evident changes occurred in medial arytenoids8. Our results also revealed differential expression of cytokeratin among multiple subsites. These findings suggest the need for a site-specific vitro model for studying the pathogenic mechanism of LPR.
The impact of LPR in laryngopharyngeal carcinogenesis may well be elucidated by its site-specific effect11. Unlike other locations, carcinoma in PC is correlated with exposure to gastric refluxate26, which is more aggressive in biological behavior27. The variation of LPR impact may explain the pathophysiological discrepancy of tumors between PC and other sites, which underscores the need for an in vitro model reflecting the genetic characteristic of different anatomic regions.
In vitro cell lines originating from larynx and hypopharynx are not yet widely available, and a site-specific model has been rarely reported in human study. David M. Bulmer et al. firstly applied a porcine larynx model for mirroring site-specific mucosal susceptibility after reflux7. Currently, an immortalized PC cell line has been established via transfection with lentivirus-encoding human papillomavirus E6/E7 protein11, while a subglottic epithelial cell line has just been reported in 201919. However, low successful establishment of stable normal cell lines is attributed to the tedious process of immortalization and minority of donor tissue, thereby reducing the utility of this methodology for wide application and a comparative study between subsites.
Our study described a versatile approach for generating primary laryngopharyngeal epithelial cells. Prior studies concerning primary cultivation were inevitably constrained to specific anatomic location9–11,13. We employed a two-step enzymatic strategy and demonstrated that this was applicable for multiple sites, thus potentially applying a robust model for performing site-specific research and comparative study in LPR-associated disorders. Additionally, our methodology is efficient, with a positive culture rate up to 78.3% and still offers us a high purity of epithelial cells, which has never been reported before.
In order to explore the potential factors affecting primary cell growth, we compared the culture results among a series of clinical variables. Although the positive ratios of successful culture were not significantly different among patients stratified by surgical procedure, pathology, age, and gender, we observed that the positive ratio was relatively higher in patients younger than 44 years and lower in those older than 60 years, indicating that age may be a critical factor affecting primary cultivation28. We proposed that this might be due to the presence of more cell vitality in mucosa samples from younger donors, but this should be validated by a study with a larger sample size.
Our current study focuses mainly on monolayer cultures, showing that the monolayer culture of epithelial cells from laryngopharynx allows for a similar cell population, both morphologically and immunohistochemically. Yet whether the cells we obtained possess their site-specific phenotype remains unknown, and an air-liquid interface (ALI) culture is capable of simulating typical epithelium structure of airway29, as well as barrier function and gene pattern21,30. Our group is in the process of transforming ALI cultures from different sites of larynx and pharynx.
Another limitation of our study is early senescence and limited expansion, which is in line with previous studies21. These appear to be a common problem in epithelial primary culture, whereas conditional reprogramming(CR) technology has unveiled its exciting potential in rapid propagation and long-term passage of normal epithelial tissue and epithelial tumor31,32, thus offering us a promising future in circumventing the hurdle during primary culture.
In conclusion, we describe a versatile approach for expansion and characterization of epithelial cells from multiple laryngopharyngeal sites, which can possibly reflect the genetic and cellular heterogeneity of mucosa from specific sites. This model renders it possible to explore site-specific differential damaging impact and mucosal transforming potential in the pathologic process of LPR.
Supplemental Material
Supplement_material for Optimized Generation of Primary Human Epithelial Cells from Larynx and Hypopharynx: A Site-Specific Epithelial Model for Reflux Research by Ting-Ting Mo, Jia-Jie Tan, Mei-Gui Wang, Yuan-Feng Dai, Xiong Liu and Xiang-Ping Li in Cell Transplantation
Footnotes
Ethical Approval: Approval was obtained from Southern Medical University Nanfang Hospital Institutional Review Board(NO. NFEC-201607-K2-01).
Statement of Human and Animal Rights: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement of Informed Consent: Informed consent was obtained from all individual participants included in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by National Natural Science Foundation of China (grant number 81400452).
ORCID iD: Xiang-Ping Li  https://orcid.org/0000-0002-0238-3557
https://orcid.org/0000-0002-0238-3557
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Patel DA, Harb AH, Vaezi MF. Oropharyngeal reflux monitoring and atypical gastroesophageal reflux disease. Curr Gastroenterol Rep. 2016;18(3):12. [DOI] [PubMed] [Google Scholar]
- 2. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101(4 Pt 2 suppl 53):1–78. [DOI] [PubMed] [Google Scholar]
- 3. Johnston N, Ondrey F, Rosen R, Hurley BP, Gould J, Allen J, DelGaudio J, Altman KW. Airway reflux. Ann N Y Acad Sci. 2016;1381(1):5–13. [DOI] [PubMed] [Google Scholar]
- 4. Pearson JP, Parikh S, Orlando RC, Johnston N, Allen J, Tinling SP, Johnston N, Belafsky P, Arevalo LF, Sharma N, Castell DO. et al. Review article: reflux and its consequences--the laryngeal, pulmonary and oesophageal manifestations. Conference held in conjunction with the 9th International Symposium on Human Pepsin (ISHP) Kingston-upon-Hull, UK, 21-23 April 2010. Aliment Pharmacol Ther. 2011;33(suppl 1):1–71. [DOI] [PubMed] [Google Scholar]
- 5. Formanek M, Jancatova D, Kominek P, Tomanová R, Zeleník K. Comparison of impedance and pepsin detection in the laryngeal mucosa to determine impedance values that indicate pathological laryngopharyngeal reflux. Clin Transl Gastroenterol. 2017;8(10):e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aldhahrani A, Powell J, Ladak S, Ali M, Ali S, Verdon B, Pearson J, Ward C. The potential role of bile acids in acquired laryngotracheal stenosis. Laryngoscope. 2018;128(9):2029–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bulmer DM, Ali MS, Brownlee IA, Dettmar PW, Pearson JP. Laryngeal mucosa: its susceptibility to damage by acid and pepsin. Laryngoscope. 2010;120(4):777–782. [DOI] [PubMed] [Google Scholar]
- 8. Wood JM, Hussey DJ, Woods CM, Astill D, I, Watson D, Lee B, Carney AS. Does gene expression in laryngeal subsites differ between patients with laryngopharyngeal reflux and controls? Clin Otolaryngol. 2018;43(1):158–163. [DOI] [PubMed] [Google Scholar]
- 9. Rees LE, Gunasekaran S, Sipaul F, Birchall MA, Bailey M. The isolation and characterisation of primary human laryngeal epithelial cells. Mol Immunol. 2006;43(6):725–730. [DOI] [PubMed] [Google Scholar]
- 10. Johnston N, Yan JC, Hoekzema CR, Samuels TL, Stoner GD, Blumin JH, Bock JM. Pepsin promotes proliferation of laryngeal and pharyngeal epithelial cells. Laryngoscope. 2012;122(6):1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee SH, Samuels T, Bock JM, Blumin JH, Johnston N. Establishment of an immortalized laryngeal posterior commissure cell line as a tool for reflux research. Laryngoscope. 2015;125(2):E73–E77. [DOI] [PubMed] [Google Scholar]
- 12. Bonet M, Basterra J, Perez A, Zapater E. A novel method for culturing human glottic cells. Laryngoscope. 2013;123(12):E104–E108. [DOI] [PubMed] [Google Scholar]
- 13. Sasaki CT, Issaeva N, Vageli DP. In vitro model for gastroduodenal reflux-induced nuclear factor-kappaB activation and its role in hypopharyngeal carcinogenesis. Head Neck. 2016;38(suppl 1):E1381–E1391. [DOI] [PubMed] [Google Scholar]
- 14. Lim JJ, Seol DW, Choi KH, Shin DH, Kim HJ, Song SH, Lee DR. Spermatogonial stem cell enrichment using simple grafting of testis and in vitro cultivation. Sci Rep. 2014;4:5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tucker J, Vidic B, Tucker GJ, Stead J. Survey of the development of laryngeal epithelium. Ann Otol Rhinol Laryngol. 1976;85(5 suppl 30 PT 2):1–16. [PubMed] [Google Scholar]
- 16. Ali M, Bulmer DM, Dettmar PW, Pearson JP. Mucin gene expression in reflux laryngeal mucosa: histological and in situ hybridization observations. Int J Otolaryngol. 2014;2014:264075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dowdall JR, Sadow PM, Hartnick C, Vinarsky V, Mou H, Zhao R, Song PC, Franco RA, Rajagopal J. Identification of distinct layers within the stratified squamous epithelium of the adult human true vocal fold. Laryngoscope. 2015;125(9):E313–E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thibeault SL, Rees L, Pazmany L, Birchall MA. At the crossroads: mucosal immunology of the larynx. Mucosal Immunol. 2009;2(2):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Powell J, Verdon B, Wilson JA, Simpson AJ, Pearson J, Ward C. Establishment of an immortalized human subglottic epithelial cell line. Laryngoscope. 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juric-Lekic G, Radujkovic V, Kancijan V, Bulić-Jakus F, Lokosek V, Katusić A, Vlahović M, Serman L. Differentiation of epiglottal epithelia during prenatal and postnatal human development. Coll Antropol. 2008;32(4):1115–1120. [PubMed] [Google Scholar]
- 21. Ling C, Li Q, Brown ME, Kishimoto Y, Toya Y, Devine EE, Choi KO, Nishimoto K, Norman IG, Tsegyal T, Jiang JJ. et al. Bioengineered vocal fold mucosa for voice restoration. Sci Transl Med. 2015;7(314):314ra187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sasaki CT, Toman J, Vageli D. The in vitro effect of acidic-pepsin on nuclear factor kappab activation and its related oncogenic effect on normal human hypopharyngeal cells. Plos One. 2016;11(12):e168269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamamoto M, Honkura Y, Rodriguez-Vazquez JF, Murakami G, Katori Y, Cho BH, Abe S. Switching of the laryngeal cavity from the respiratory diverticulum to the vestibular recess: a study using serial sagittal sections of human embryos and fetuses. J Voice. 2016;30(3):263–271. [DOI] [PubMed] [Google Scholar]
- 24. Johnston N, Bulmer D, Gill GA, Panetti M, Ross PE, Pearson JP, Pignatelli M, Axford SE, Dettmar PW, Koufman JA. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112(6):481–491. [DOI] [PubMed] [Google Scholar]
- 25. Min HJ, Hong SC, Yang HS, Mun SK, Lee SY. Expression of CAIII and Hsp70 Is increased the mucous membrane of the posterior commissure in laryngopharyngeal reflux disease. Yonsei Med J. 2016;57(2):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rucci L, Bocciolini C, Franchi A, Ferlito A, Casucci A. Epidermal growth factor receptor and p53 expression in T1-T2 glottic cancer involving the anterior or posterior commissure. Acta Otolaryngol. 2004;124(1):102–106. [DOI] [PubMed] [Google Scholar]
- 27. Shvero J, Shvili I, Mizrachi A, Shpitzer T, Nageris B, Koren R, Hadar T. T1 glottic carcinoma involving the posterior commissure. Laryngoscope. 2009;119(6):1116–1119. [DOI] [PubMed] [Google Scholar]
- 28. Shi L, Lei ZJ, Zhao CY, Lv XX, Jiang L, Li J, Li XY. A modified culture strategy of human keratinocytes to shorten the primary culture time. Cell Biol Int. 2015;39(9):1073–1079. [DOI] [PubMed] [Google Scholar]
- 29. Fukahori M, Chitose S, Sato K, Sueyoshi S, Kurita T, Umeno H, Monden Y, Yamakawa R. Regeneration of vocal fold mucosa using tissue-engineered structures with oral mucosal cells. Plos One. 2016;11(1):e146151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walimbe T, Panitch A, Sivasankar MP. An in vitro scaffold-free epithelial-fibroblast coculture model for the larynx. Laryngoscope. 2017;127(6):E185–E192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR. et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180(2):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X, Krawczyk E, Suprynowicz FA, Palechor-Ceron N, Yuan H, Dakic A, Simic V, Zheng YL, Sripadhan P, Chen C, Lu J. et al. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat Protoc. 2017;12(2):439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement_material for Optimized Generation of Primary Human Epithelial Cells from Larynx and Hypopharynx: A Site-Specific Epithelial Model for Reflux Research by Ting-Ting Mo, Jia-Jie Tan, Mei-Gui Wang, Yuan-Feng Dai, Xiong Liu and Xiang-Ping Li in Cell Transplantation