Abstract
We first compared long-term clinical outcomes in treating critical limb ischemia (CLI) and foot ulcer in patients with diabetes between autologous bone marrow mesenchymal stem cell (BMMSC) and bone-marrow-derived mononuclear cell (BMMNC) transplants. Forty-one patients were enrolled and followed up for 3 years. They received an 18-day standard treatment before stem cell transplantation. Patients with bilateral CLI and foot ulcer were injected intramuscularly or basally with BMMSC, BMMNC, or normal saline (NS). Cox model analysis showed significant differences in the hazard ratio (HR) for amputation with treatment by BMMSC (HR 0.21 [95% CI (0.05, 0.95)], P = 0.043), infection of foot (HR 5.30 [95% CI (1.89, 14.92)], P = 0.002), and age ≥64 (HR 3.01 [95% CI (1.11, 8.15)], P = 0.030), but no significant differences by BMMNC at 9 months after transplantation. Regarding ulcer healing and recurrence rate, the BMMSC group demonstrated a significant difference from the NS group during the 3–6 months after transplantation or healing, but the BMMNC group did not. This trial suggests that, compared with BMMNC treatment, BMMSC treatment leads to a longer time of limb salvage and blood flow improvement, and, when compared with conventional therapy, it can promote limb blood flow and ulcerative healing, and reduce ulcer recurrence and amputation within 9 months.
Keywords: autologous transplantation, cellular therapy, clinical trial, critical limb, ischemia, diabetes
Introduction
Critical limb ischemia (CLI), which is at the end of the peripheral artery disease (PAD) spectrum, is developed with a four-times higher risk in patients with diabetes mellitus (DM) than in patients without DM1. Diabetic patients with CLI are more likely to suffer from foot pain, non-healing ulcer, or even amputation2,3. A population-based cohort study showed that once the patients have developed CLI, even with revascularization (peripheral angioplasty (PAT) or bypass graft (BPG)), 8.2–21.5% of patients still undergo major amputation within 6 years, and 14.6% of the patients needed secondary revascularization after a first surgery (4); 4.9% of these patients were not eligible for revascularization4. On the other hand, approximately 10–25% of patients with diabetes will develop foot ulcers in their lifetime, which is another independent risk factor for amputation in diabetic patients5.
In recent years, more and more investigators have conducted clinical and preclinical studies on treatment of CLI and foot ulcer using stem cells6,7. The most common stem cells used include bone marrow-derived mononuclear cells (BMMNCs), CD34+ bone marrow cells, and bone marrow-derived mesenchymal stem cells (BMMSCs). Initial pilot studies have suggested that most stem cell therapies can increase blood flow to the transplantation site in patients by promoting lower limb ischemic angiogenesis and neovascularization6. At the same time, stem cells are capable of targeting and bypassing normal pathways, and abnormal underlying healing mechanisms derange cell signaling in the wounds of patients with diabetes, thereby promoting healing7. However, although promising, most randomized controlled trials on the use of stem cells for the treatment of CLI have been small scale and have short follow-up times (average time of follow-up was 7.5 months)8; none of these trials had a follow-up > 12 months8. A few clinical studies have aimed to compare the efficacy and safety of different stem cells in the treatment of CLI and foot ulcer8.
In this study, we compared short-term clinical efficacy and long-term clinical outcomes of BMMSCs and BMMNCs in the treatment of CLI and foot ulcer patients with diabetes9.
Materials and Methods
Subjects, Study Design, and Procedures
This clinical study was approved by the Ethical Committee Board of Southwest Hospital affiliated to the Third Military Medical University, and complied with the recommendations of the Declaration of Helsinki. Written informed consent was obtained from all subjects. Patients with Type 2 DM enrolled in this single-center, double-blinded, randomized, placebo-controlled trial were admitted to Southwest Hospital (Chongqing, China) from October 2009 to January 2010, and participated in this trial on a voluntary basis. They had refused standard revascularization techniques for treating CLI because of fear of complications. Inclusion criteria were as follows: 1) patients aged 40–70 years; 2) patients with a diagnosis of Type 2 DM made according to WHO Type 2 Diabetes Diagnosis Standard (1999; https://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf); 3) patients with bilateral lower limb ischemia (ankle brachial index (ABI) = 0.30–0.60); and 4) patients with at least one foot ulcer. Exclusion criteria included dry gangrene above the ankle or moist gangrene, malignant tumors, as well as severe coronary, cerebral, and/or renal vascular diseases.
The patients were assigned randomly to group A and group B; one of the lower limbs in each patient in group A or B was selected randomly for BMMSC or BMMNC transplantation (BMMSC or BMMNC group), and the other lower limb of the same patients was selected for placebo (normal saline, NS) injection (NS group). All patients received the same standard treatment in the duration of this trial (from participation to the end of follow up), including control of blood glucose, blood pressure and blood lipid, debridement to remove extensive callus and necrotic tissues, pressure-relief after wound dressing, and the application of antibiotics.
For preparation of BMMSCs, the detailed process was reported previously9. Briefly, after 30 ml bone marrow was extracted under sterile conditions, mononuclear cells were obtained by density gradient centrifugation, and then cultured in flasks containing alpha-modified minimum essential medium (α-MEM; Invitrogen-Life Technologies Corp., Carlsbad, CA, USA) supplemented with 10% autologous serum. The cells were passaged once every 4–5 days. When the targeted number of expanded BMMSCs was attained, the cells were thoroughly washed and resuspended in NS, and identified by flow cytometry9. For preparation of BMMNCs, the following procedure was carried out: after 18 days of ordinary treatment, bone marrow (∼300 mL) from patients in group B was aspirated from the ileum under epidural anesthesia, and then processed by density gradient centrifugation. The resulting fraction of BMMNCs was suspended in NS.
After 18 days of standard treatment (the run-in period), only patients still in line with the inclusion criteria and without any exclusion criteria were allowed to enter this clinical trial and be treated with cell therapy9.
Under strict aseptic conditions, 100 mg tramadol hydro-chloride was injected intramuscularly. After 20 min, the cells (BMMSCs 9.3 ± 1.1 × 108 or BMMNCs 9.6 ± 1.1 × 108) suspended in 20 mL NS were injected intramuscularly into the lower limb (20 sites, 3 cm × 3 cm in interval, 1–1.5 cm in depth, and 0.5–1 mL BMMSCs or BMMNCs per site); 2 mL cells were injected into the basilar part of each foot ulcer and the surrounding subcutaneous tissues9. In a similar manner, the control limbs in NS group were injected with an equal volume of NS.
The rationale, randomization, and study procedures of this trial were reported previously9. The trial was registered with a number of NCT00955669 at www.ClinicalTrials.gov.
Follow-Up and Endpoints
The primary endpoint was amputation (including minor and major amputation). The secondary endpoints included ulcer healing, ulcer recurrence, rest pain, pain-free walking time, ABI, transcutaneous oxygen pressure (TcPO2) and angiogenesis score as judged by magnetic resonance angiography (MRA). Most clinical and laboratory data were collected prospectively, and follow-up visits were performed at 1 day before and 2, 6, 9, 12, 24, and 36 months after transplantation. Ulcer healing after transplantation and ulcer recurrence after ulcer healing were recorded throughout. Amputation and censored endpoints (including death, loss to follow-up, and revascularization) were recorded at any time after transplantation. To better understand the dynamics of amputation, we analyzed amputation and its risks by the Kaplan-Meier method and Cox model every month during the transition period (from 7 to 12 months after transplantation). The follow up was extended to 3 years (maximum) for this additional analysis, and performed using patient medical records or through contacting patients by phone.
Statistical Methods
The continuous variables were presented as mean ± standard deviation (SD). We explored the roles of continuous variables across the groups with Student’s t test and ANOVA. The chi-square test was used to compare the rates and the likelihood ratio for the differences among these groups. The amputation rate was estimated with the Kaplan-Meier method, and differences in survival among the groups were analyzed with the log-rank test. Cox’s proportional hazards regression model was used to select the significant prognostic factors. The proportionality among the survival rates and attributable factors in the Cox model was assessed by plotting the log(–log [survival function])–time curve in each subgroup. The significant role of covariates was measured with the likelihood ratio test, and the role of each covariate entering the model was assessed by Wald statistic. Statistical significance was assumed at a value of p < 0.05. All statistical analyses were performed using SPSS (Version 19.0 for Windows, SPSS, Chicago, IL, USA).
Results
Subjects
Forty-one patients with Type 2 DM were enrolled (20 in group A and 21 in group B), with 20 limbs in the BMMSC group, 21 limbs in the BMMNC group and 41 limbs in the NS group. Each patient presented at least one diabetic foot ulcer (DFU), and six patients had two DFUs, with one located in each limb. All patients were accorded Fontaine grade IV, and were followed up for a mean time of 3.23 ± 0.31 years. The baseline demographics showed an equal distribution of selected variables in the two groups, including sex, smoking status, diabetic retinopathy, diabetic nephropathy, hypertension, hyperlipidaemia, coronary artery disease, stroke, medication, mean age, body mass index (BMI), waist/hip ratio, duration of diabetes, glycosylated hemoglobin, ulcer size, ulcer duration, rest pain, pain-free walking time, ABI, TcO2, angiographic score of MRA, and number of implanted cells. The detailed data of each patient’s clinical characteristics were reported previously9. During follow-up, 12/41 (29.3%) subjects died, including 6 (30%) in group A (n = 20) and 6 (28.76%) in group B (n = 21) (P = 0.92). Nine patients died of acute myocardial infarction, two of massive cerebral infarction, and one of lung cancer. None of the patients were lost to follow up or underwent revascularization surgery (including PAT and BPG). There was no malignant tumor related to the stem cell transplantation in these patients. Edema in three patients was observed after stem cell transplantation (two in the BMMNC group and one in the BMMSC group), but did not cause discomfort and disappeared spontaneously after 12 h.
Amputation
Out of 82 limbs, 47 (57.3%) underwent amputation, including 30 major and 17 minor amputation. Of 30 limbs undergoing major amputation, 7 (23.3%) were from the BMMSC group, 8 (26.7%) from the BMMNC group and 15 (50.0%) from the NS group. At 6 months, compared with the NS group, there was a significant reduction in the amputation rate in both the BMMSC group (P = 0.024) and the BMMNC group (P = 0.021)9. As analyzed using the Kaplan-Meier method, there was a significant difference in the amputation rate between BMMSC group and NS group only at 7 months after transplantation (Table 1). After follow-up for 3 years, the amputation-free survival curve was not significantly different between various groups (BMMSC vs. NS, P = 0.205; BMMNC vs. NS, P = 0.435; Fig. 1A). However, after treatment groups and potentially significant prognostic factors (including age, infection of foot, and smoking (obtained by univariate analysis)) were included into the Cox’s proportional hazards regression model, there were statistically significant differences in HR for amputation with treatment by BMMSCs (HR 0.21 [95% CI (0.05, 0.95)], P = 0.043), infection of foot (HR 5.30 [95% CI (1.89, 14.92)], P = 0.002) and age ≥64 years (HR 3.01 [95% CI (1.11, 8.15], P = 0.030) at 9 months after transplantation (Fig. 1B). At the same time, there was no statistically significant difference in HR for amputation with treatment by BMMNCs, as shown by Cox model (HR 0.41 [95% CI (0.13, 1.28], P = 0.124; Fig. 1B).
Table 1.
Amputation rates between BMMSC and NS groups and between BMMNC and NS groups from 7 months to 12 months after transplantation analyzed by Kaplan-Meier method.
| 7 months | 8 months | 9 months | 10 months | 11 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | P | χ2 | P | χ2 | P | χ2 | P | χ2 | P | χ2 | P | |
| BMMSC | 4.289 | 0.038 | 3.473 | 0.062 | 3.057 | 0.080 | 3.057 | 0.080 | 2.322 | 0.128 | 2.792 | 0.095 |
| BMMNC | 2.503 | 0.114 | 2.124 | 0.145 | 1.051 | 0.305 | 0.463 | 0.496 | 0.700 | 0.403 | 0.458 | 0.499 |
Fig. 1.
(A) Kaplan-Meier curves representing the estimated cumulative incidence rates of amputation-free survival in BMMSC, BMMNC, and NS groups over 36 months of follow up, with censored data for patients who died. (B) After age, smoking, and infection were taken into account in a Cox proportional hazard regression model, the curves represent the estimated cumulative rates of amputation-free survival in BMMSC, BMMNC, and NS groups at 9 months after transplantation.
As analyzed using the Kaplan-Meier method and the log-rank test from 7 months to 12 months after transplantation, there was a significant difference in the amputation rate between the BMMSC and NS groups only at 7 months (P = 0.038).
Ulcer Healing and Recurrence
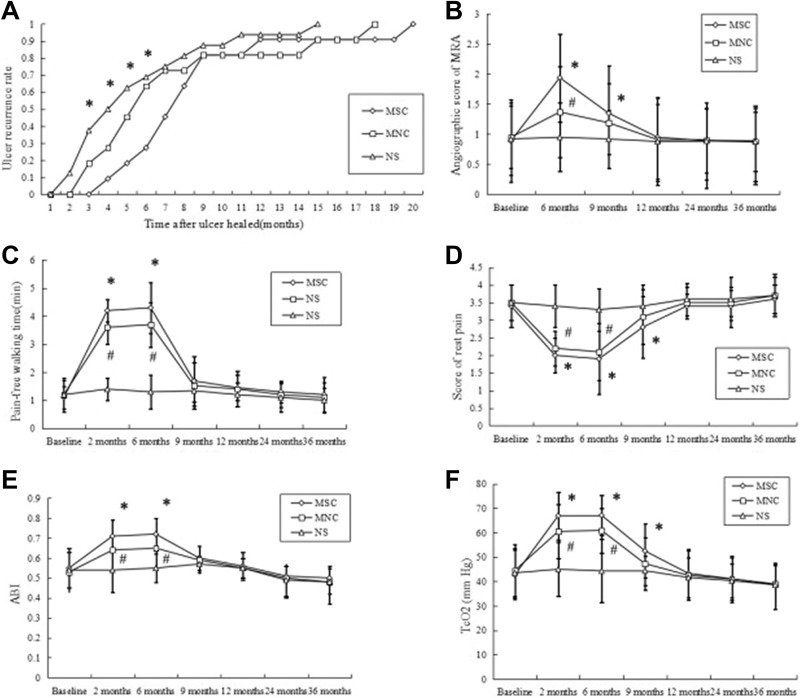
We previously reported that the healing rate of ulcers in the BMMSC group was significantly higher than that in the NS group at 1 month after transplantation, whereas this difference was observed between the NS group and the BMMNC group at 3 months; the healing rate of ulcers reached 100% in the BMMSC group at 1 month, i.e., earlier than the BMMNC group9. All of the ulcer recurrence happened within 20 months after treatment (Fig. 2A). The ulcer recurrence rate in the BMMSC group was significantly lower than that in the NS group during 3–6 months after ulcer healing (3 months: 0/11 in BMMSC group vs. 6/16 in NS group, P = 0.027; 6 months: 3/11 in the BMMSC group vs. 11/16 in NS group, P = 0.041; Fig. 2A). The ulcer recurrence rate in the BMMNC group was low, but there was no statistically significant difference between the BMMNC group and the NS group (3 months: 2/11 in the BMMNC group vs. 6/16 in the NS group, P = 0.261; 6 months: 7/11 in the BMMNC group vs. 11/16 in NS group, P = 0.551; Fig. 2A).
Fig. 2.
(A) Ulcer recurrence rate and blood flow in limbs injected with BMMSCs, BMMNCs, or NS. Ulcer recurrence rate (i.e., the number of limbs with ulcers recurrence/the total number of limbs with ulcers healed in a group) (*P < 0.05, BMMSC group vs. NS group). (B–F) The results of rest pain, pain-free walking time, ABI, TcO2, and angiography are presented as mean ±SD (*P < 0.05, BMMSC group vs. NS group; #P < 0.05, BMMNC group vs. NS group).
Clinical Parameters of Lower Limb Blood Flow
At 6 months after transplantation, the pain score, pain-free walking time, ABI, TcPO2, and angiogenesis score of MRA in the BMMSC or BMMNC groups were significantly different from those in the NS group (pain-free walking time: P < 0.001 [BMMSC and BMMNC]; rest pain: P < 0.001 [BMMSC and BMMNC]; TcO2: P < 0.001[BMMSC and BMMNC]; ABI: P < 0.001 [BMMSC and BMMNC]; angiogenesis score: P < 0.001 [BMMSC], P = 0.027 [BMMNC]; Fig. 2 BCDEF). At 9 months after transplantation, all of the above clinical parameters in the BMMNC group were decreased or increased to levels at which no significant differences were observed as compared with the NS group. Otherwise, there were statistically significant differences between the BMMSC group and the NS group (rest pain: P = 0.015; TcO2: P = 0.004; angiogenesis score: P = 0.032). There were no statistically significant differences in the clinical parameters of lower limb blood flow among the three groups at 9 months of follow-up (Fig. 2BCDEF). Representative results of angiography are shown in Fig. 3.
Fig. 3.

MRA analysis of collateral vessel formation in limbs injected with BMMSCs, BMMNCs, and NS. (A) Abundant increased collateral circulation in a limb of the BMMSC group from +0 (A1) to +3 (A2) appeared 9 months after implantation. (B) A moderate increase in collateral circulation in a limb of BMMNC group from +0 (B1) to +2 (B2) appeared 9 months after implantation. (c) In contrast, there was no collateral circulation (+0) in a limb from the NS group before (C1) and 9 months after (C2) implantation.
Discussion
CLI is the most serious clinical manifestation of PAD, and is more likely to occur in patients with diabetes10. Stem cell therapy for those patients, in particular those who are unsuitable for revascularization or for whom the procedure is unsuccessful for CLI, brings a new treatment option6.
We previously reported that, within 6 months, autologous transplantation of BMMSCs may be as safe as BMMNC therapy and with similar limb salvage rate, but that it is more effective in increasing lower limb perfusion and promoting foot ulcer healing in diabetic CLI9. However, how long can these effects last? The answer to this question may determine the timing of subsequent stem cell therapy. Another interesting question is whether stem cell therapy can also prevent ulcer recurrence.
In this study, the improvements in the clinical parameters of lower limb blood flow (including rest pain, pain-free walking time, ABI, TcO2 and angiogenesis score) suggested that BMMSCs might be better than BMMNCs in promoting lower limb blood flow and relieving the pain of the patients with diabetes and CLI. These improvements reached a peak at 2–6 months after transplantation, then decreased gradually. The decline rate in the BMMSC group was slower than that in the BMMNC group. The increased ABI of the BMMSC group at 6 months after transplantation in this trial is similar to the report of Gupta et al.11, but inconsistent with the report of Lee et al12. The rest pain score is broadly consistent with the results of both these clinical studies11,12, and the angiogenesis score is similar to that found by Lee et al.12. The different results in these trials may be related to the small sample size and the large individual variation. At 9 months follow up in this trial, unlike other parameters of blood flow, the ABI level in the BMMSC group was not significantly higher than that in the NS group, perhaps associated with the increased overall level of ABI in the NS group, which resulted from the amputation of extremely low ABI limbs in the NS group. The pain-free walking time might be similar to ABI. On the other hand, with BMMNC transplantation, the improvement of blood flow parameters in the BMMNC group at 6 months after transplantation in this trial is similar to most reports in recent years6,8. Also, there are only a few reports about the blood flow of lower limbs at 6 months after BMMNC transplantation. Possible mechanisms to explain the different effects of BMMSC and BMMNC treatments in this trial, may be suggested from preclinical studies, which suggested that, through secreting more angiogenic factors (vascular endothelial growth factor, fibroblast growth factor 2, and angiopoietin-1) and differentiating into angioblasts (endothelial cells and vascular smooth muscle cells), BMMSCs can better promote angiogenesis than BMMNCs, and increase more blood flow to the ischemic lower limbs13. Meanwhile, a recent study completed by Gremmels et al. indicated that the capability of BMMSCs from patients with diabetes and CIL to secret angiogenic factors and promote neovascularization was not significantly influenced14. In addition, BMMSCs might provide an additional benefit when used in an allogeneic approach; for example, off-the-shelf availability, and avoiding potentially damaging BM aspiration procedures in frail patients with CLI15.
In recent years, preclinical studies have suggested that stem cells are promising in the treatment of DFUs, in which BMMSCs may achieve therapeutic effects through differentiation and angiogenesis7,16–18. A study by Falanga et al. demonstrated that, after topical application of bone-marrow-derived stem cells in chronic wounds (ulcer duration > 1 year), all treated wounds began to close within 2–4 weeks19. Meanwhile, two clinical studies with a small sample size also showed that BMMSC treatment for lower limb ischemia could promote ulcer healing12,20. The present trial indicates that both BMMSC and BMMNC therapies can promote the healing of DFU, and that transplantation of BMMSCs can delay ulcer recurrence, with the most significant effects observed within 6 months after ulcer healing.
In this trial, the 6-month amputation rate in the control (NS) group was 29.3% (in accordance with the assumption of the hypothetical patients to calculate, 41 limbs = 20.5 patients, 6/20.5 = 0.293), which is consistent with literature reports about the amputation rate of no-option CLI patients, in which reports, the 6-month major amputation rate ranged from 10% to 40%6. At 6 months after transplantation, BMMNCs did not significantly reduce the amputation rate, which is consistent with many reports8,21. For BMMSC transplantation, two recent clinical studies with a small sample size (n ≤ 20) suggested that, at 6 months after treatment, there was no significant difference between the BMMSC and control groups11,12, which is inconsistent with the results of this trial; in this trial, at 9 months after BMMSC transplantation, when age, smoking, and infection were taken into Cox’ model, the multivariate analysis showed that BMMSC treatment could significantly reduce the risk of amputation in diabetic patients with CLI and DFU. Reasons for the inconsistent results may include: firstly, we transplanted the stem cells not only into the lower limb muscles to improve circulation, but also into the basilar part of each foot ulcer and the surrounding subcutaneous tissues to promote ulcer healing and delay ulcer recurrence (most ulcer recurrence in the BMMSC group happened after 6 months of ulcer healing, i.e., 9 months after transplantation). As we know, ulcer is an important independent risk of amputation in patients with diabetes and CLI5. Secondly, we took the dominant prognostic factors (smoking, age, and infection) into the multivariate analysis model, and analyzed other factors comprehensively. In other two BMMSC trials, the sample size was too small to analyze other prognostic factors11,12. In addition, based on multivariate analysis, this trial also implies that infection and age ≥ 64 years are two important independent risk factors of amputation in patients with diabetes and CIL, and can increase the possibility of amputation. This means that, when studying the problem of amputation in patients with diabetes, these two important prognostic factors (infection and age) must be taken into account.
There were no serious adverse events (including malignant tumors) related to BMMSC or BMMNC injection during the 3-year follow-up period. Edema was observed in only three patients after stem cell transplantation, and disappeared spontaneously after 12 h. Conforming to our study, many trials have shown that BMMSC and BMMNC transplantations are safe in long-term follow-up22–24. Unfortunately, we did not observe hemangioma by color Doppler ultrasonography or other means, which needs to be included in future research.
In this study, we reported and compared the long-term efficacy and safety of BMMSC and BMMNC treatments for patient with diabetes, CLI, and ulcer. Our study results suggest that both cell therapies are safe, and imply that, during the early period after transplantation when treating patients with diabetes, CLI, and foot ulcers (within 9 months), BMMSC treatment can significantly reduce the amputation risk. Moreover, the clinically significant effects (e.g., increasing lower limb blood flow, promoting ulcer healing, and reducing ulcer recurrence) of BMMSC treatment are maintained longer than those of BMMNC treatment. After 6 months of BMMNC transplantation or 9 months of BMMSC transplantation, stem cell treatment cannot significantly reduce the rate of amputation. In order to obtain a more accurate HR and confidence interval in the general population (patients with diabetes, CLI, and ulcer) and a more precise time range of significant curative effect after stem cell treatment, future study should focus on increasing numbers of samples and sample size, and introducing all kinds of possible risk factors, such as age, course of disease, smoking, cardiovascular and cerebrovascular events, blood lipid, blood pressure, infection, neuropathy, etc., into multivariate analysis. In further study, we will investigate the following considerations: within the time range of the significant curative effect, ensuring the quality of stem cells, increasing the injection frequency or combining with revascularization to enhance the curative effects and reduce the risk of amputation25,26. Since this study shows that the effects of BMMSCs and BMMNCs are transient, the forthcoming study also needs to address whether repeated administration (perhaps every 6 or 9 months) could greatly improve outcomes for patients.
Conclusions
This trial suggests that, compared with BMMNCs, BMMSC treatment has a longer time of limb salvage and blood flow improvement in patients with diabetes, CLI, and foot ulcer. Compared with conventional therapy, it can promote limb blood flow and ulcerative healing, and reduce ulcer recurrence and amputation within 9 months.
Footnotes
Author Contributions: DL and YJ analyzed the data, contributed to the discussion, and wrote the manuscript. ZL, YZ, WD and QW analyzed the data. ZL, FG and YC wrote the manuscript. DL, YX and BC analyzed the data, contributed to the discussion, reviewed and edited the manuscript. All authors read and approved the final manuscript. YX and BC take full responsibility for this study.
Ethical Approval: The ethical approval to report this work was obtained from “the Ethical Committee Board of Southwest Hospital affiliated to the Third Military Medical University”.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the protocol approved by “the Ethical Committee Board of Southwest Hospital affiliated to the Third Military Medical University” and complied with the recommendations of the Declaration of Helsinki.
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This study was supported by the National Natural Science Foundation of China (grant nos. 81200614 and 81400845). D.L. is the recipient of the grant.
ORCID iD: Yaoming Xue  https://orcid.org/0000-0003-1356-4780
https://orcid.org/0000-0003-1356-4780
References
- 1. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5–S67. [DOI] [PubMed] [Google Scholar]
- 2. Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361(9368):1545–1551. [DOI] [PubMed] [Google Scholar]
- 3. Johannesson A, Larsson GU, Ramstrand N, Turkiewicz A, Wirehn AB, Atroshi I. Incidence of lower-limb amputation in the diabetic and nondiabetic general population a 10-year population-based cohort study of initial unilateral and contralateral amputations and reamputations. Diabetes Care. 2009;32(2):275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faglia E, Clerici G, Clerissi J, Gabrielli L, Losa S, Mantero M, Caminiti M, Curci V, Quarantiello A, Lupattelli T, Morabito A. Long-term prognosis of diabetic patients with critical limb ischemia. Diabetes Care. 2009;32(5):822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, Ribeiro M, Barata P, Lima J, Soares R. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complicat. 2014;28(5):632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teraa M, Conte MS, Moll FL, Verhaar MC. Critical limb ischemia: current trends and future directions. J Am Heart Assoc. 2016;5(2):e002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blumberg SN, Berger A, Hwang L, Pastar I, Warren SM, Chen W. The role of stem cells in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract. 2012;96(1):1–9. [DOI] [PubMed] [Google Scholar]
- 8. Peeters Weem SM, Teraa M, de Borst GJ, Verhaar MC, Moll FL. Bone marrow derived cell therapy in critical limb ischemia: a meta-analysis of randomized placebo controlled trials. Eur J Vasc Endovasc Surg. 2015;50(6):772–783. [DOI] [PubMed] [Google Scholar]
- 9. Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92(1):26–36. [DOI] [PubMed] [Google Scholar]
- 10. Spreen MI, Gremmels H, Teraa M, Sprengers RW, Verhaar MC, Statius van Eps RG, de Vries JP, Mali WP, van Overhagen H; PADI and JUVENTAS Study Groups. Diabetes mellitus is associated with decreased limb survival in patients with critical limb ischemia: pooled data from two randomized controlled trials. Diabetes Care. 2016;39(11):2058–2064. [DOI] [PubMed] [Google Scholar]
- 11. Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, Krishnamurthy S, Anthony N, Pherwani A, Majumdar AS. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, Park JH, Lee SY, Kim SP, Kim YD, Chung SW. et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J Offic J Japan Circ Soc. 2012;76(7):1750. [DOI] [PubMed] [Google Scholar]
- 13. Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, Kangawa K, Kitamura S. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66(3):543–551. [DOI] [PubMed] [Google Scholar]
- 14. Gremmels H, Teraa M, Quax PH, den Ouden K, Fledderus JO, Verhaar MC. Neovascularization capacity of mesenchymal stromal cells from critical limb ischemia patients is equivalent to healthy controls. Mol Ther. 2014;22:1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gremmels H, Fledderus JO, Teraa M, Verhaar MC. Mesenchymal stromal cells for the treatment of critical limb ischemia: context and perspective. Stem Cell Res Ther. 2013;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–2659. [DOI] [PubMed] [Google Scholar]
- 17. Fu X, Fang L, Li X, Cheng B, Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen, 2006;14(3):325–335. [DOI] [PubMed] [Google Scholar]
- 18. Lara L, Ocean S, Afsha A, Shirley L, Haidi H, Toshihiko I, Haiyang L, Tun W, Shun O, Xiangjiang G, Bogdan Y. et al. Stem cell therapy for diabetic foot ulcers: a review of preclinical and clinical research. Stem Cell Res Ther. 2018;9(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13(6):1299–1312. [DOI] [PubMed] [Google Scholar]
- 20. Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA, Fleury S, Gadelorge M, Taurand M. et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16(2):245–257. [DOI] [PubMed] [Google Scholar]
- 21. Matoba S, Tatsumi T, Matsubara H, Murohara T, Kondo T, Imaizumi T, Katsuda Y, Toyama Y, Saito S, Komai H, Ito M. et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156(5):1010–1018. [DOI] [PubMed] [Google Scholar]
- 22. Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. [DOI] [PubMed] [Google Scholar]
- 23. Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, Monroy R, Kurtzberg J. Efficacy and safety of Ex Vivo cultured adult human mesenchymal stem cells (Prochymal™) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17(4):534–541. [DOI] [PubMed] [Google Scholar]
- 24. Duong Van Huyen JP, Smadja DM, Bruneval P, Gaussem P, Dal-Cortivo L, Julia P, Fiessinger JN, Cavazzana-Calvo M, Aiach M, Emmerich J. Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod Pathol. 2008;21:837–846. [DOI] [PubMed] [Google Scholar]
- 25. Reddy AM, Kwak BK, Shim HJ, Jang EC, Park AJ, Park E, Ahn C. A long-term outcome of therapeutic angiogenesis by transplantation of peripheral blood stem cells in critical limb ischemia after interventional revascularization. Diagn Interv Radiol. 2013;19(1):76–80. [DOI] [PubMed] [Google Scholar]
- 26. Kang WC, Oh PC, Lee K, Ahn T, Byun K. Increasing injection frequency enhances the survival of injected bone marrow derived mesenchymal stem cells in a critical limb ischemia animal model. Korean J Physiol Pharmacol. 2016;20(6):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]