Abstract
Regeneration of articular cartilage is of great interest in cartilage tissue engineering since articular cartilage has a low regenerative capacity. Due to the difficulty in obtaining healthy cartilage for transplantation, there is a need to develop an alternative and effective regeneration therapy to treat degenerative or damaged joint diseases. Stem cells including various adult stem cells and pluripotent stem cells are now actively used in tissue engineering. Here, we provide an overview of the current status of cord blood cells and induced pluripotent stem cells derived from these cells in cartilage regeneration. The abilities of these cells to undergo chondrogenic differentiation are also described. Finally, the technical challenges of articular cartilage regeneration and future directions are discussed.
Keywords: cord blood, induced pluripotent stem cell, cartilage, chondrogenesis, tissue regeneration, differentiation
Introduction
Research on tissue regeneration using various types of stem cells is ongoing. The regenerative capacities of adult, tissue, and pluripotent stem cells are constantly being tested. Technologies are urgently needed to regenerate several tissues, including skeletal muscle, neuronal tissue, and cardiac tissue. Moreover, a regenerative technique is required to repair articular cartilage defects.
Cartilage has a poor regenerative capacity1,2. The dense extracellular matrix located between chondrocytes prevents movement of these cells and explains why cartilage is avascular, aneural, and alymphatic. Consequently, it is difficult for chondrocytes and nutrients to reach the defected cartilage. Progressive wear on articular cartilage causes a loss of cartilage tissue, which eventually leads to degenerative joint diseases such as osteoarthritis (OA)1. According to the Global Burden of Disease study, OA was the third most prevalent musculoskeletal disease in 2010 and affected the knee joints in 83% of cases3. The OA prevalence of the knee joints in South Korea is one of the highest in the Asia-Pacific region. In the United States (US), OA is reported to affect 33.6% (12.4 million) of adults aged 65 years or older. Considering the increases in population size and life expectancy, the worldwide occurrence of degenerative joint diseases is predicted to increase1. Therefore, a method to efficiently regenerate cartilage would greatly improve the treatment of these diseases.
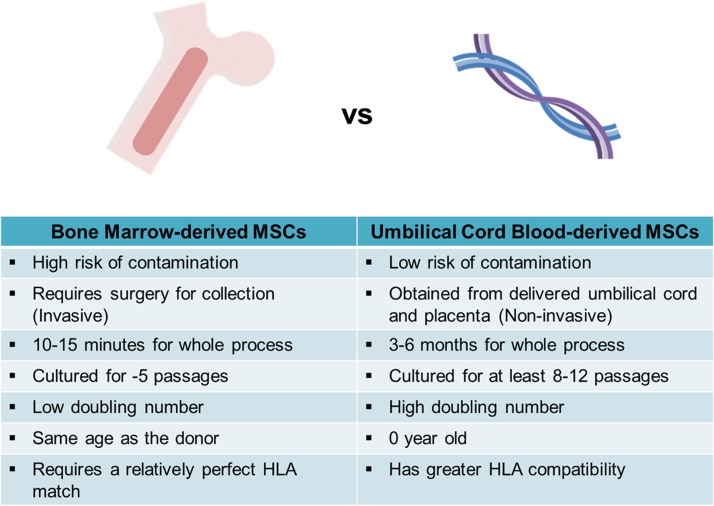
Attempts have been made to regenerate cartilage using cells from various sources. Adult stem cells, especially human mesenchymal stem cells (hMSCs), obtained from different regions (i.e. bone marrow, cord blood (CB), and adipose tissue) and articular chondrocytes (ACs) are widely used due to their innate regenerative capacities. However, the limited proliferation and differentiation potentials of these cells hamper treatment. To overcome this, researchers are attempting to regenerate cartilage using pluripotent stem cells. This review focuses on the abilities of human umbilical cord blood (hUCB) cells and human induced pluripotent stem cells (hiPSCs) derived from these cells to regenerate cartilage, as well as the advantages of these cells in this context (Fig. 1).
Fig 1.
Comparison of bone marrow-derived MSCs and cord blood-derived MSCs. The characteristics of each cell during harvest, culture are shown.
HLA: human leukocyte antigen; MSC: mesenchymal stem cell.
Application of CB for Cartilage Regeneration
Umbilical CB Cells
hUCB is a common source of cells for transplantation and is readily available. hUCB-derived cells are routinely collected and banked worldwide4. CB has several advantages over other cell sources (Fig. 1). First, CB is easily collected and is reported to contain a higher percentage of stem cells than red bone marrow. Second, there is a lower risk of contamination during the collection of hUCB. CB consists of hematopoietic stem cells and mesenchymal stem cells (MSCs)5. CD34+ cells are usually transplanted because they have the highest proliferative and stemness potentials6. Third, the hUCB-derived cells have a low level of immunogenicity because of the decreased functionality of fetal lymphocytes, which is one of the major advantages of CB. These cells are reportedly less likely to cause graft-versus-host disease because they are collected from newborns, who are in a relatively immunodeficient state5. Due to these characteristics, hUCB-MSCs have been permissive to allogeneic transplantation and are now considered an alternative source to be examined in long-term clinical trials7,8. Fourth, the human leukocyte antigen (HLA) type is determined for all banked hUCB-derived cells, which facilitates the selection of cells of the appropriate HLA type.
Human UCB-derived MSCs (hUCB-MSCs) have several benefits compared with other tissue-derived hMSCs. Clones of hUCB-MSCs were cultured for at least 8–12 passages, while human bone marrow stem cells (hBMSCs) were only able to make it to five population doublings9. Both hUCB-MSCs or hBMSCs did not show any expression of hematopoietic markers such as CD14, CD34, CD45, and CD133. Expression of CD29, CD44, and CD73 was similar between the two cell types, however, the expression of CD105 was lower than that of hBMSCs. Both cell sources expressed HLA class I and were negative for HLA class II.
However, when compared with hBMSCs or human adipose-derived MSCs (hASCs), Kern and colleagues confirmed that it was more difficult to obtain hUCB-MSCs10. When seeded with an initial plating density of 1 × 106 cells per cm2, fibroblastoid cells derived from both hBMSCs and hASCs were able to induce a monolayer of outgrowth cells after only 4–5 days, while it took 2–4 weeks after plating in the case of hUCB-MSCs. The proliferation capacity was lowest in hBMSCs, which had the lowest doubling number through passages 4–6. The doubling rate of hASCs after passage 3 was followed, while hUCB-MSCs had the highest doubling numbers in all passages analyzed. MSCs are known to have a limited life span and to undergo cell replicative senescence that is characterized by loss of proliferation and original morphology. The lowest senescence ratio within early passages was that of hASCs, however, hUCB-MSCs were able to be cultured the longest, followed by hASCs and hBMSCs.
Immunomodulation and Anti-Inflammatory Effect of hUCB-MSCs
Several groups attempted to treat OA and rheumatoid arthritis with hUCB-MSCs, since MSCs are best known for differentiation into mesodermal lineages including cartilage and bone. MSCs, including hUCB-MSCs, are also reported to have immunomodulation properties. Studies were usually done with hBMSCs, however, relatively few studies were reported on the immunomodulation of hUCB-MSCs in arthritis11. Shawi and colleagues observed the effect of hUCB-MSCs on allogenic lymphocytes treated with phytohemagglutinin for mitogen-induced proliferation. To examine the effect of hUCB-MSCs on lymphocyte activity, Shawki and colleagues measured the protein levels of interferon (IFN)-γ, transforming growth factor (TGF)β1, and interleukin (IL)-10 in the culture media of lymphocytes, hUCB-MSCs, and hUCB-MSCs co-cultured with lymphocytes12. TGFβ1 and IL-10 increased in the cultured supernatant when lymphocytes were co-cultured with hUCB-MSCs, while the concentration of IFN-γ decreased significantly. This finding suggested that the immunomodulatory effect of hUCB-MSCs might be related to the regulation on IFN-γ expression.
Liu and colleagues first confirmed the therapeutic effect of hUCB-MSCs in rheumatoid arthritis13. Fibroblast-like synoviocytes and matrix metalloproteinases (MMPs) play an important role in inflammation and cartilage destruction. Liu confirmed proliferation of synoviocytes induced with tumor necrosis factor (TNF)α were inhibited by hUCB-MSCs in a cell-to-cell contact Transwell system in a dose-dependent manner. The production of MMP9 was significantly reduced by hUCB-MSCs in synoviocytes. When hUCB-MSCs were injected in collagen induced arthritis mice, the arthritic symptoms were reduced and the levels of TNFα and IL-6 were also downregulated. The migration of hUCB-MSCs in mice spleens shifted T helper 1 cells towards T helper 2 cells and T regulatory cells.
In 2012, Greish and colleagues confirmed the immunoregulatory effect of hUCB-MSCs in a rheumatoid arthritis rat model14. The overall arthritis signs were reduced 34 days after injection. Inflammatory cytokine serum levels of TNFα, IFN-γ, and IL-1 were decreased significantly in the groups injected with hUCB-MSCs. On the other hand, IL-10 levels were increased.
Chondrogenic Differentiation Ability of CB Cells
CB-derived cells can differentiate into a wide variety of cell types in vitro and have thus been proposed for therapeutic applications15–20. Several studies reported chondrogenic differentiation using hUCB-MSCs9,10,21–25 (Table 1). hUCB-MSCs are reported to have much higher chondrogenic potentials than other cell sources20. Early studies confirmed the chondrogenic differentiation ability of hUCB-MSCs by performing safranin O staining, which labels cartilage-specific proteoglycans. Lee and colleagues confirmed the mRNA expression of collagen type II and other extracellular matrix markers on day 14 of differentiation, whereas aggrecan (ACAN) was detected on day 2121. Wang and colleagues compared the chondrogenic differentiation ability of hUCB-MSCs with hBMSCs23. Increasing glycosaminoglycan (GAG) content in hUSC-MSCs was confirmed for over 6 weeks, while it was decreased in hBMSCs between week 3 to 6. Also, a hUSC-MSC-derived chondrogenic construct showed 1.7 and 2.1 times higher hydroxyproline per unit DNA compared with the hBMSC group.
Table 1.
Chondrogenic differentiation using hUCB-MSCs.
| No. | Author | Negative marker of hUCB-MSCs | Positive marker of hUCB-MSCs | Culture method | Growth factor | Reference |
|---|---|---|---|---|---|---|
| 1 | Bieback et al. | CD14, CD34, CD45, CD133, HLA class II | CD29, CD44, CD73, HLA class I | Pellet culture | TGFβ3 | 9 |
| 2 | Kern et al. | CD14, CD34, CD45, CD133, HLA Class II, CD144 | CD44, CD73, CD90, CD105, CD29, HLA class I, CD106 | Pellet culture | TGFβ3 | 10 |
| 3 | Lee et al. | CD3, CD7, CD19, CD31, CD33, CD34, CD45, CD62, CD90, CD117, CD133, CD135, HLA-DR | CD29, CD44, CD49, CD51, CD58, CD105, HLA-ABC, SH-2, SH-3, SH-4 | Pellet culture | TGFβ1 | 21 |
| 4 | Chang et al. | Spindle shape: CD26, CD31, CD34, CD45, HLA-DR Flatten shape: CD26, CD31, CD34, CD45, HLA-DR, CD90 |
Spindle shape: CD29, CD44, CD90, HLA-ABC, SH-2, SH-3, SH-4 Flatten shape: CD29, CD44, HLA-ABC, SH-2, SH-3, SH-4 |
Pellet culture | TGFβ1 | 22 |
| 5 | Wang et al. | - | - | Scaffolds | TGFβ1 | 23 |
HLA: human leukocyte antigen; TGF: transforming growth factor; hUCB: human umbilical cord blood; MSC: mesenchymal stem cell.
In 2013, Jeong and colleagues reported that hUCB-MSCs promoted the differentiation of chondroprogenitor cells via paracrine actions26. Interestingly, this effect was stronger when treatment was performed with synovial fluid from OA patients than when treatment was performed with synovial fluid obtained from fracture patients. A biotin label-based antibody array revealed that the level of thrombospondin-2 (TSP-2) was significantly increased in hUCB-MSCs treated with synovial fluid from OA patients, but not in those treated with synovial fluid from fracture patients. Treatment of mouse limb bud chondrogenitor cells with Thrombospondin-2 during chondrogenic differentiation via a micromass culture dose-dependently increased the levels of chondrogenic markers. Moreover, rat cartilage defects were not repaired following implantation of TSP-2-deleted hUCB-MSCs.
Ability of CB Cells to Regenerate Cartilage
The non-invasive collection method and hypo-immunogenicity of hUCB-MSCs have encouraged research studies and preclinical trials aimed at determining whether these cells can treat various diseases, including stroke, Alzheimer’s disease, and myocardial infarction. Furthermore, much work has been expended on regenerating articular cartilage using hUCB-MSCs.
There is a representative large group in South Korea when it comes to clinical application of hUCB-MSCs. This group successfully took hUCB-MSCs to clinical trial and developed a therapeutic drug based on this cell famously known as ‘Cartistem’. Before applying the cells to clinical trials, the research group checked the regenerative ability of hUCB-MSCs in various animals including rat, rabbit, and minipig. In 2014, Chung and colleagues directly transplanted hUCB-MSCs with different types of hydrogels into a full-thickness articular cartilage defect created in the trochlear groove of the femur27. hUCB-MSCs were first treated in the cartilage defect with four different hydrogels (i.e. hyaluronic acid (HA), alginate, pluronic individually and also various mixtures with different ratios). With the use of HA, hUCB-MSCs were able to show their highest recovery rate in rat articular cartilage defect. Defects were generated in both knees, and the corresponding hydrogel alone was transplanted as a negative control. At 16 weeks after surgery, the gross appearance score was higher in knees transplanted with hUCB-MSCs than in knees transplanted with hydrogels alone. Cartilage regeneration was confirmed by Masson’s trichrome and safranin O staining. All knees transplanted with hUCB-MSCs showed better rescue of the defect. Collagen type II expression in the regenerated tissue was confirmed by immunostaining. Regenerated cartilage with a high collagen type II content was detected in more than half of knees transplanted with hUCB-MSCs but was not observed in knees transplanted with hydrogels alone. Notably, the results differed according to the type of hydrogel used. The group transplanted with 4% HA showed the highest levels of recovery and accumulation of collagen type II. Histological analysis of Sirius red staining using a polarized microscope showed that collagen organization in knees transplanted with hUCB-MSCs and 4% HA was similar to that in adjacent undamaged articular cartilage.
In 2016, hUCB-MSCs reconstituted in HA were applied to rabbit articular cartilage defect models28. Cells were treated with four different cell doses (i.e. 0.1, 0.5, 1.0, and 1.5 × 107 cells/ml) into the cartilage defect and the recovery was observed for 4, 8, and 16 weeks. The implanted hUSB-MSCs showed regenerative properties inside the defect generated in the articular cartilage. Interestingly, the authors observed that a high cell concentration was unfavorable in cartilage repair compared with a low cell concentration. Cell doses of 0.1, 0.5, and 1.0 × 107 cells/ml were more effective than higher doses (1.5 × 107 cells/ml), however, higher doses showed inferior cartilage repair capacity. The authors suggested two theories. First, the reduced viability of cells caused by excessively high concentrations and aggregation might reduce the efficiency. Second, loss or damage of cells by xenogenic immune rejection was thought as a result of a less effective cartilage repair.
The regenerative potential of hUCB-MSCs was also confirmed in larger animals by the same group29. Using six minipigs, implanted hUCB-MSCs with a HA hydrogel composite showed no abnormal findings 12 weeks after transplantation. A total of three different cell lines were compared in the cartilage defect and all three cell lines successfully induced regeneration. The mode of action is not yet certain, however, the study worked as a milestone on the way to human clinical trials and suggested that hUCB-MSCs have regenerative ability in full-thickness articular cartilage defects.
A clinical trial study was conducted in South Korea to confirm the safety and efficacy of articular cartilage regeneration using allogeneic hUCB-MSCs in OA patients30. This study was the first-in-human clinical trial of Cartistem (Medipost, Seongnam-si, Gyeonggi-do, South Korea), which is composed of culture-expanded allogeneic hUCB-MSCs and a HA hydrogel. The study followed up patients for 7 years. Overall, seven participants were divided into two groups based on the defect size and received a transplantation of hUCB-MSCs and HA hydrogel in 2005. Group A, in which the defect size was 4.9 cm2, received 1.15–1.25 × 107 hUCB-MSCs, and group B, in which the defect size was 7.3 cm2, received 1.65–2.00 × 107 hUCB-MSCs. Different numbers of cells were transplanted to determine the maximum tolerated dose based on dose-limiting toxicity. No severe events occurred over 7 years; however, mild-to-moderate adverse events (i.e. arthralgia, back pain, bladder distension, and elevated antithyroglobulin antibody levels) were reported in five participants. In addition, no participant showed dose-limiting toxicity; therefore, the authors concluded that the highest dose delivered was the maximum tolerated dose. Recovered lesions in two participants were further examined by biopsy at 1 year after transplantation. The regenerated tissue was thick, glossy, white hyaline-like cartilage with a smooth surface and was strongly stained with Masson’s trichrome stain, safranin O, and an anti-type II collagen antibody. Upon observation of the lesions of five participants by magnetic resonance imaging, the mean relative ΔR1 index was 1.44, which indicated the regenerated cartilage had high glycosaminoglycan content.
Application of Induced Pluripotent Stem Cells for Cartilage Regeneration
Application of Induced Pluripotent Stem Cells for Tissue Regeneration
Genetic reprogramming restores the differentiation potential of adult somatic cells and leads to the generation of hiPSCs. Thus, it is possible to convert fully differentiated somatic cells into multipotent stem cells. There are several advantages and disadvantages associated with the future clinical use of hiPSCs.
Reprogramming of CB Cells
Cells from various sources have been reprogrammed31–45. Fibroblasts are representative cells used for reprogramming. However, it is important to generate hiPSCs from a more affordable and easily obtained cell source if they are to be clinically used in regenerative medicine. One such suggested cell source is hUCB. The collection of CB cells does not require any surgical procedures, and these cells can be obtained from any private or public cell bank.
Wang and colleagues successfully reprogrammed hUCB-MSCs using a dox-inducible lentiviral system46. Although reprogramming using lentiviral systems is more efficient than that of using episomal vectors, it is unsuitable for clinical use. Therefore, the authors promised to focus on increasing the efficiency of hUCB-MSC reprogramming using episomal vectors or mRNAs without oncogenes in the future.
Reprogramming using lentiviral and retroviral systems has the highest transduction efficacy. Consequently, this approach was widely used during the early years of hiPSC studies to confirm the reprogramming ability of various types of somatic cells47–57 and was also employed to generate patient-specific hiPSCs, which is usually difficult58,59. However, this method carries the risk of incorporation of the viral vector into the hiPSC genome and is thus unsuitable for clinical applications60. Consequently, reprogramming using non-integrating viral or non-viral vectors is being studied.
In 2009, Giorgetti and colleagues attempted to reprogram hUCB cells by transducing a pCEP4 EBNA1/OriP-based episomal vector containing various combinations of OCT4, SOX2, KLF4, and/or c-Myc61,62. After 12–15 days, colonies containing cells with a similar morphology as human embryonic stem cells (hESCs) started to appear. An average of five colonies formed using 8 × 104 CD133+ hUCB cells. Reprogramming was achieved using only two factors: OCT4 and SOX2.
Sendai virus, an RNA virus, has several advantages for reprogramming. First, it does not enter the nucleus and thus does not integrate into the host genome. Second, transduced Sendai virus is reported to be eliminated from cells after ∼10 passages60. Third, although the transduction efficacy of Sendai virus may be lower than that of lentiviruses, it can produce a large amount of the protein of interest and is commercially available. Blood cells are more difficult to reprogram than fibroblasts; however, hiPSCs have been generated from neonatal and adult fibroblasts and blood cells via infection of Sendai virus expressing Yamanaka factors37,63,64.
In 2016, Kim and colleagues reported a modified protocol to reprogram blood cells, including human peripheral blood mononuclear cells (hPBMCs) and human CB mononuclear cells (hCBMCs), using the Sendai virus37. Application of centrifugal force increased the efficacy of blood cell reprogramming and increased the number of hiPSC colonies obtained from 3 × 105 hPBMCs or hCBMCs. Overall, three hPBMC-derived and three hCBMC-derived hiPSC lines were reported to be pluripotent and to have a normal karyotype.
Attempts are continually being made to develop non-viral reprogramming methods with a higher efficacy. In 2013, Yamanaka’s group reported a reprogramming protocol using blood cells and episomal vectors65. They confirmed that a combination of reprogramming factors, including OCT4, SOX2, KLF4, L-MYC, LIN28, and TP53-targeting shRNA, had the highest efficacy, and applied this protocol to hCBMCs. Vectors containing the reprogramming factors were delivered into CD34+ hCBMCs via electroporation. The reprogramming efficiency of this protocol was around 0.06%, which is higher than that reported using episomal vectors and suspension blood cells.
Abilities of CB Cell-Derived hiPSCs to Undergo Chondrogenic Differentiation and Regenerate Cartilage
The generation of hiPSCs has impacted various research fields. These cells can be used to screen drugs, model diseases, and develop regenerative medicines in industrial and clinical studies (Fig. 2). Patient-matched and homozygous HLA-matched personalized hiPSCs are attractive for the development of regenerative therapies in various clinical fields. In vitro chondrogenesis of hMSCs, human adipose stem cells, human embryonic stem cells, and cells from other sources has been induced using various two-dimensional and three-dimensional methods (i.e. monolayer, micromass, and pellet culture)66–68. Chondrogenesis of hiPSCs derived from cells obtained from various sources has also been reported2,69–75. Several studies have reported chondrogenesis of hiPSCs generated from hCBMCs2,72.
Fig. 2.
Cartilage treatment using hUCB-derived cells. HLA-typed hUCB cells are stored in a private or public cord blood bank. MSCs isolated from hUCBs are commonly used for cartilage treatment. As a new cell source for cartilage regeneration, hiPSCs generated from hUCBs are a potentially new alternative to hUCB-derived MSC cells. Chondrogenic cells or tissues derived from hUCB-hiPSCs hold great potential for chondrogenesis and cartilage regeneration. The schema provides an overview of recent work on chondrogenesis and cartilage regeneration using hUCBs and hUCB-hiPSCs.
hiPSC: human induced pluripotent stem cell; HLA: human leukocyte antigen; hUCB: human umbilical cord blood; MSC: mesenchymal stem cell.
In 2014, Guzzo and colleagues induced chondrogenesis of hiPSCs derived from ACs and CB cells72. The efficiency of chondrogenesis was compared between hiPSCs generated from these cells and those generated from a well-established dermal fibroblast (DF) cell line. Chondrogenesis was induced via micromass culture and human recombinant bone morphogenetic protein 2 treatment. Cultures were maintained for 21 days prior to characterization. hiPSCs tend to differentiate more readily into their cell type of origin; therefore, it was hypothesized that hiPSCs generated from ACs would be more prone to undergo chondrogenesis. As predicted, the percentage of cells stained with Alcian blue was higher among AC-derived hiPSCs than among DF and CB cell-derived hiPSCs. However, it was also higher among CB cell-derived hiPSCs than DF-derived hiPSCs. Expression of cartilage-specific markers, such as SOX9, COL2A1, COL2B, and ACAN, was highest in micromass cultures of AC-derived hiPSCs and lowest in those of CB cell-derived hiPSCs. By contrast, expression of hypertrophic markers was highest in micromass cultures of DF-derived hiPSCs.
Guzzo and colleagues demonstrated that micromass culture can induce chondrogenesis of CB cell-derived hiPSCs72. In 2017, Nam and colleagues generated chondrogenic pellets using hCBMC-derived hiPSCs2,76. In that study, expression of cartilage-specific genes increased as differentiation proceeded from day 10 to day 30. The extracellular matrix was detected by several staining methods. Levels of staining in chondrogenic pellets generated from hCBMC-derived hiPSCs were similar to those in chondrogenic pellets generated from hMSCs, which were used as a positive control. In addition, cartilage-specific markers, such as SOX9, ACAN, and COL2A1, were detected. Expression of COL2A1 and SOX9 was significantly increased in chondrogenic pellets generated from hCBMC-derived hiPSCs. Most importantly, expression of the hypertrophic cartilage marker COL10A1 was much lower in chondrogenic pellets generated from hCBMC-derived hiPSCs than in those generated from hMSCs. In a subsequent study by the same group, Rim and colleagues compared the differentiation efficiencies of hiPSCs generated from human dermal fibroblasts (hDFs), hPBMCs, hCBMCs, and human fibroblast-like synoviocytes (hFLS). While the morphological characteristics of chondrogenic pellets generated from hDF, hPBMC, and hCBMC-derived hiPSCs did not significantly differ, chondrogenic pellets generated from OA FLS-derived hiPSCs were smaller. Expression of the cartilage-specific markers ACAN and COL2A1 was significantly higher in chondrogenic pellets generated from hiPSCs derived from hCBMCs than in those generated from hiPSCs derived from other cell types. In contrast with the results of Guzzo and colleagues, chondrogenic pellets generated from CBMC-derived hiPSCs were of a better quality than those generated from DF-derived hiPSCs. In another study, chondrocyte-like cells were isolated from the generated chondrogenic pellets generated with hCBMC-hiPSCs77. The aim of the study was to generate an injectable cell source for treatment. Isolated chondrocyte-like cells showed similar levels of ACAN and COL2A1 expression, however, the levels of COL1A1 was significantly increased compared with the cells maintained inside the chondrogenic pellet. The expression of COL10A1 was yet lower than that of chondrogenic pellets. When delivered through intra-articular injection, single chondrocyte-like cells showed recovery through safranin O staining compared with the defected control. Based on various characterizations, however, the study concluded a smaller unit of a three-dimensional chondrogenic pellet that can pass through the needle might be more promising than the approach using isolated single cells to induce cartilage recovery.
Concluding Remarks
Regeneration of cartilage, which has poor regenerative ability, has been studied for several decades. Within this context, hUCB-derived hiPSCs hold great potential for the future treatment of degenerative joint diseases and joint damage. This review provides an overview of hUCB-derived cells and hiPSCs generated from these cells, with a focus on cartilage regeneration.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the Korea Healthcare Technology R&D project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (HI16C2177 and H18C1178).
References
- 1. Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng. 2009;37(1–2):1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nam Y, Rim YA, Jung SM, Ju JH. Cord blood cell-derived ipscs as a new candidate for chondrogenic differentiation and cartilage regeneration. Stem Cell Res Ther. 2017;8(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee S, Kim SJ. Prevalence of knee osteoarthritis, risk factors, and quality of life: the fifth korean national health and nutrition examination survey. Int J Rheum Dis. 2017;20(7):809–817. [DOI] [PubMed] [Google Scholar]
- 4. Roura S, Pujal JM, Galvez-Monton C, Bayes-Genis A. The role and potential of umbilical cord blood in an era of new therapies: a review. Stem Cell Res Ther. 2015;6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medhekar SK, Shende VS, Chincholkar AB. Recent stem cell advances: cord blood and induced pluripotent stem cell for cardiac regeneration- a review. Int J Stem Cells. 2016;9(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Theunissen K, Verfaillie CM. A multifactorial analysis of umbilical cord blood, adult bone marrow and mobilized peripheral blood progenitors using the improved ml-ic assay. Exp Hematol. 2005;33(2):165–172. [DOI] [PubMed] [Google Scholar]
- 7. Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384–1392. [DOI] [PubMed] [Google Scholar]
- 8. Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord wharton’s jelly-derived cells. Stem Cells. 2008;26(11):2865–2874. [DOI] [PubMed] [Google Scholar]
- 9. Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22(4):625–634. [DOI] [PubMed] [Google Scholar]
- 10. Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. [DOI] [PubMed] [Google Scholar]
- 11. Kim JY, Jeon HB, Yang YS, Oh W, Chang JW. Application of human umbilical cord blood-derived mesenchymal stem cells in disease models. World J Stem Cells. 2010;2(2):34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shawki S, Gaafar T, Erfan H, El Khateeb E, El Sheikhah A, El Hawary R. Immunomodulatory effects of umbilical cord-derived mesenchymal stem cells. Microbiol Immunol. 2015;59(6):348–356. [DOI] [PubMed] [Google Scholar]
- 13. Liu Y, Mu R, Wang S, Long L, Liu X, Li R, Sun J, Guo J, Zhang X, Guo J, Yu P. et al. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther. 2010;12(6):R210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greish S, Abogresha N, Abdel-Hady Z, Zakaria E, Ghaly M, Hefny M. Human umbilical cord mesenchymal stem cells as treatment of adjuvant rheumatoid arthritis in a rat model. World J Stem Cells. 2012;4(10):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang XQ, Zang WJ, Bao LJ, Li DL, Song TS, Xu XL, Yu XJ. Fibroblast growth factor-4 and hepatocyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytes. World J Gastroenterol. 2005;11(47):7461–7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ali H, Bayatti N, Lindsay S, Dashti AA, Al-Mulla F. Directed differentiation of umbilical cord blood stem cells into cortical gabaergic neurons. Acta Neurobiol Exp (Wars). 2013;73(2):250–259. [DOI] [PubMed] [Google Scholar]
- 17. Tang XP, Zhang M, Yang X, Chen LM, Zeng Y. Differentiation of human umbilical cord blood stem cells into hepatocytes in vivo and in vitro. World J Gastroenterol. 2006;12(25):4014–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hutson EL, Boyer S, Genever PG. Rapid isolation, expansion, and differentiation of osteoprogenitors from full-term umbilical cord blood. Tissue Eng. 2005;11(9–10):1407–1420. [DOI] [PubMed] [Google Scholar]
- 19. Gomez-Leduc T, Hervieu M, Legendre F, Bouyoucef M, Gruchy N, Poulain L, de Vienne C, Herlicoviez M, Demoor M, Galera P. Chondrogenic commitment of human umbilical cord blood-derived mesenchymal stem cells in collagen matrices for cartilage engineering. Sci Rep. 2016;6:32786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marmotti A, Mattia S, Castoldi F, Barbero A, Mangiavini L, Bonasia DE, Bruzzone M, Dettoni F, Scurati R, Peretti GM. Allogeneic umbilical cord-derived mesenchymal stem cells as a potential source for cartilage and bone regeneration: an in vitro study. Stem Cells Int. 2017;2017:1732094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–1675. [DOI] [PubMed] [Google Scholar]
- 22. Chang YJ, Tseng CP, Hsu LF, Hsieh TB, Hwang SM. Characterization of two populations of mesenchymal progenitor cells in umbilical cord blood. Cell Biol Int. 2006;30(6):495–499. [DOI] [PubMed] [Google Scholar]
- 23. Wang L, Tran I, Seshareddy K, Weiss ML, Detamore MS. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15(8):2259–2266. [DOI] [PubMed] [Google Scholar]
- 24. Arufe MC, De la Fuente A, Fuentes I, Toro FJ, Blanco FJ. Umbilical cord as a mesenchymal stem cell source for treating joint pathologies. World J Orthop. 2011;2(6):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park YB, Song M, Lee CH, Kim JA, Ha CW. Cartilage repair by human umbilical cord blood-derived mesenchymal stem cells with different hydrogels in a rat model. J Orthop Res. 2015;33(11):1580–1586. [DOI] [PubMed] [Google Scholar]
- 26. Jeong SY, Kim DH, Ha J, Jin HJ, Kwon SJ, Chang JW, Choi SJ, Oh W, Yang YS, Kim G, Kim JS. et al. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31(10):2136–2148. [DOI] [PubMed] [Google Scholar]
- 27. Chung JY, Song M, Ha CW, Kim JA, Lee CH, Park YB. Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model. Stem Cell Res Ther. 2014;5(2):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park YB, Ha CW, Kim JA, Rhim JH, Park YG, Chung JY, Lee HJ. Effect of transplanting various concentrations of a composite of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel on articular cartilage repair in a rabbit model. PLoS One. 2016;11(11):e0165446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ha CW, Park YB, Chung JY, Park YG. Cartilage repair using composites of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel in a minipig model. Stem Cells Transl Med. 2015;4(9):1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6(2):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun Y, Li A, Huang K, Luo R, Wang L, Liu Y. et al. Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen (Lond). 2013;2(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang YC, Li WC, Twu NF, Li HY, Lo WL, Chang YL, Lee YY, Lin CF, Shih YH, Chen MT. Induction of dental pulp-derived induced pluripotent stem cells in the absence of c-myc for differentiation into neuron-like cells. J Chin Med Assoc. 2014;77(12):618–625. [DOI] [PubMed] [Google Scholar]
- 33. Chen Y, Luo R, Xu Y, Cai X, Li W, Tan K, Huang J, Dai Y. Generation of systemic lupus erythematosus-specific induced pluripotent stem cells from urine. Rheumatol Int. 2013;33(8):2127–2134. [DOI] [PubMed] [Google Scholar]
- 34. Chen YJ, Liou YJ, Chang CM, Li HY, Chen CY, Twu NF, Yen MS, Chang YL, Peng CH, Chiou SH, Chen CP. et al. Reprogramming human endometrial fibroblast into induced pluripotent stem cells. Taiwan J Obstet Gynecol. 2012;51(1):35–42. [DOI] [PubMed] [Google Scholar]
- 35. Higgins CA, Itoh M, Inoue K, Richardson GD, Jahoda CA, Christiano AM. Reprogramming of human hair follicle dermal papilla cells into induced pluripotent stem cells. J Invest Dermatol. 2012;132(6):1725–1727. [DOI] [PubMed] [Google Scholar]
- 36. Kawano E, Toriumi T, Iguchi S, Suzuki D, Sato S, Honda M. Induction of neural crest cells from human dental pulp-derived induced pluripotent stem cells. Biomed Res. 2017;38(2):135–147. [DOI] [PubMed] [Google Scholar]
- 37. Kim Y, Rim YA, Yi H, Park N, Park SH, Ju JH. The generation of human induced pluripotent stem cells from blood cells: an efficient protocol using serial plating of reprogrammed cells by centrifugation. Stem Cells Int. 2016;2016:1329459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rossbach B, Hildebrand L, El-Ahmad L, Stachelscheid H, Reinke P, Kurtz A. Generation of a human induced pluripotent stem cell line from urinary cells of a healthy donor using an integration free vector. Stem Cell Res. 2016;16(2):314–317. [DOI] [PubMed] [Google Scholar]
- 39. Skrzypczyk A, Giri S, Bader A. Generation of induced pluripotent stem cell line from foreskin fibroblasts. Stem Cell Res. 2016;17(3):572–575. [DOI] [PubMed] [Google Scholar]
- 40. Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, Mostoslavsky G, Jaenisch R. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7(1):20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Veraitch O, Kobayashi T, Imaizumi Y, Akamatsu W, Sasaki T, Yamanaka S, Amagai M, Okano H, Ohyama M. Human induced pluripotent stem cell-derived ectodermal precursor cells contribute to hair follicle morphogenesis in vivo. J Invest Dermatol. 2013;133(6):1479–1488. [DOI] [PubMed] [Google Scholar]
- 42. Wang Y, Liu J, Tan X, Li G, Gao Y, Liu X, Zhang L, Li Y. Induced pluripotent stem cells from human hair follicle mesenchymal stem cells. Stem Cell Rev. 2013;9(4):451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang XB. Cellular reprogramming of human peripheral blood cells. Genomics Proteomics Bioinformatics. 2013;11(5):264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou J, Wang X, Zhang S, Gu Y, Yu L, Wu J, Gao T, Chen F. Generation and characterization of human cryptorchid-specific induced pluripotent stem cells from urine. Stem Cells Dev. 2013;22(5):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou T, Benda C, Duzinger S, Huang Y, Li X, Li Y, Guo X, Cao G, Chen S, Hao L, Chan YC. et al. Generation of induced pluripotent stem cells from urine. J Am Soc Nephrol. 2011;22(7):1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang J, Gu Q, Hao J, Bai D, Liu L, Zhao X, Liu Z, Wang L, Zhou Q. Generation of induced pluripotent stem cells with high efficiency from human umbilical cord blood mononuclear cells. Genomics Proteomics Bioinformatics. 2013;11(5):304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. [DOI] [PubMed] [Google Scholar]
- 48. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 49. Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321(5889):699–702. [DOI] [PubMed] [Google Scholar]
- 50. Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–106. [DOI] [PubMed] [Google Scholar]
- 51. Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with ips cells generated from autologous skin. Science. 2007;318(5858):1920–1923. [DOI] [PubMed] [Google Scholar]
- 52. Maherali N, Ahfeldt T, Rigamonti A, Utikal J, Cowan C, Hochedlinger K. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell. 2008;3(3):340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. [DOI] [PubMed] [Google Scholar]
- 54. Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2(2):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27(3):543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chang CW, Lai YS, Pawlik KM, Liu K, Sun CW, Li C, Schoeb TR, Townes TM. Polycistronic lentiviral vector for “hit and run” reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27(5):1042–1049. [DOI] [PubMed] [Google Scholar]
- 57. Mali P, Ye Z, Hommond HH, Yu X, Lin J, Chen G, Zou J, Cheng L. Improved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells. 2008;26(8):1998–2005. [DOI] [PubMed] [Google Scholar]
- 58. Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O. et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136(5):964–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee J, Kim Y, Yi H, Diecke S, Kim J, Jung H, Rim YA, Jung SM, Kim M, Kim YG, Park SH. et al. Generation of disease-specific induced pluripotent stem cells from patients with rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2014;16(1):R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Malik N, Rao MS. A review of the methods for human iPSC derivation. Methods Mol Biol. 2013;997:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodriguez-Piza I, Vassena R, Raya A, Boue S, Barrero MJ, Corbella BA, Torrabadella M. et al. Generation of induced pluripotent stem cells from human cord blood using oct4 and sox2. Cell Stem Cell. 2009;5(4):353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Giorgetti A, Montserrat N, Rodriguez-Piza I, Azqueta C, Veiga A, Izpisua Belmonte JC. Generation of induced pluripotent stem cells from human cord blood cells with only two factors: Oct4 and sox2. Nat Protoc. 2010;5(4):811–820. [DOI] [PubMed] [Google Scholar]
- 63. Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, Nakata H, Tohyama S, Hashimoto H, Kodaira M, Okada Y. et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating t cells. Cell Stem Cell. 2010;7(1):11–14. [DOI] [PubMed] [Google Scholar]
- 65. Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, Goshima N, Yamanaka S. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31(3):458–466. [DOI] [PubMed] [Google Scholar]
- 66. Toh WS, Yang Z, Liu H, Heng BC, Lee EH, Cao T. Effects of culture conditions and bone morphogenetic protein 2 on extent of chondrogenesis from human embryonic stem cells. Stem Cells. 2007;25(4):950–960. [DOI] [PubMed] [Google Scholar]
- 67. Yamashita A, Nishikawa S, Rancourt DE. Identification of five developmental processes during chondrogenic differentiation of embryonic stem cells. Plos One. 2010;5(6):e10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gong G, Ferrari D, Dealy CN, Kosher RA. Direct and progressive differentiation of human embryonic stem cells into the chondrogenic lineage. J Cell Physiol. 2010;224(3):664–671. [DOI] [PubMed] [Google Scholar]
- 69. Craft AM, Rockel JS, Nartiss Y, Kandel RA, Alman BA, Keller GM. Generation of articular chondrocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33(6):638–645. [DOI] [PubMed] [Google Scholar]
- 70. Zhu Y, Wu X, Liang Y, Gu H, Song K, Zou X, Zhou G. Repair of cartilage defects in osteoarthritis rats with induced pluripotent stem cell derived chondrocytes. BMC Biotechnol. 2016;16(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yamashita A, Liu S, Woltjen K, Thomas B, Meng G, Hotta A, Takahashi K, Ellis J, Yamanaka S, Rancourt DE. Cartilage tissue engineering identifies abnormal human induced pluripotent stem cells. Sci Rep. 2013;3:1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guzzo RM, Scanlon V, Sanjay A, Xu RH, Drissi H. Establishment of human cell type-specific ips cells with enhanced chondrogenic potential. Stem Cell Rev. 2014;10(6):820–829. [DOI] [PubMed] [Google Scholar]
- 73. Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, Guilak F. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109(47):19172–19177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yamashita A, Morioka M, Yahara Y, Okada M, Kobayashi T, Kuriyama S, Matsuda S, Tsumaki N. Generation of scaffoldless hyaline cartilaginous tissue from human ipscs. Stem Cell Reports. 2015;4(3):404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Koyama N, Miura M, Nakao K, Kondo E, Fujii T, Taura D, Kanamoto N, Sone M, Yasoda A, Arai H, Bessho K. et al. Human induced pluripotent stem cells differentiated into chondrogenic lineage via generation of mesenchymal progenitor cells. Stem Cells Dev. 2013;22(1):102–113. [DOI] [PubMed] [Google Scholar]
- 76. Nam Y, Rim YA, Ju JH. Chondrogenic pellet formation from cord blood-derived induced pluripotent stem cells. J Vis Exp. 2017;12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rim YA, Nam Y, Park N, Lee J, Park SH, Ju JH. . Repair potential of nonsurgically delivered induced pluripotent stem cell-derived chondrocytes in a rat osteochondral defect model. J Tissue Eng Regen Med. 2018;12(8):1843–1855. [DOI] [PubMed] [Google Scholar]