Abstract
One of the current limitations of retinal transplantation of stem cells as well as other cell types is the dispersion of cells from the injection site (including loss of cells into the vitreous chamber) and low survival after transplantation. Gelatin-hydroxyphenyl propionic acid (Gtn-HPA) conjugate is a biodegradable polymer that can undergo covalent cross-linking in situ, allowing for injection of incorporated cells through a small caliber needle followed by gel formation in vivo. We tested the hypothesis that Gtn-HPA hydrogel supports survival and integration of retinal progenitor cells (RPCs) post-transplantation. In vitro compatibility and in vivo graft survival were assessed by mixing an equal volume of Gtn-HPA conjugate and RPC suspension and triggering enzyme-mediated gelation, using minute amounts of horseradish peroxidase and peroxide. Immunocytochemistry showed >80% survival of cells and minimal apoptosis for cells incorporated into Gtn-HPA, equivalent to controls grown on fibronectin-coated flasks. RPCs undergoing mitosis were seen within the three-dimensional Gtn-HPA hydrogel, but the percentage of Ki-67-positive cells was lower compared with the monolayer controls. For in vivo studies, gel–cell mixture or cell suspension in saline was trans-sclerally injected into the left eye of female Long Evans rats immunosuppressed with cyclosporine A. Grafts survived at the 1 week time point of the study, with Gtn-HPA-delivered grafts showing less inflammatory response demonstrated by anti-leukocyte staining. More eyes in the gel–cell mixture group showed surviving cells in the subretinal space compared with saline-delivered controls, while the number of cells surviving per graft was not significantly different between the two groups. This work demonstrates an injectable in situ cross-linking hydrogel as a potential vehicle for stem cell delivery in the retina.
Keywords: graft survival, hydrogel, retina, stem cell therapy, tissue engineering
Introduction
Sub-retinal stem cell transplantation holds great promise for vision restoration in diseases such as age-related macular degeneration (ARMD) and retinitis pigmentosa (RP), which involve progressive damage to cells of the outer retina1,2. One of the current limitations to this therapeutic approach is the loss of cells during transplantation and subsequent low graft survival when delivered in saline buffer. For the past two decades, biodegradable polymers have been explored as vehicles to deliver retinal cells into the subretinal space. Previous experience with synthetic scaffolds such as poly(lactic-co-glycolic acid) (PLGA)3–5, poly(glycol sebacate) (PGS)6,7, and polycaprolactone (PCL)8, as well as protein- and carbohydrate-based materials9,10, resulted in improved retinal progenitor cell (RPC) post-transplant survival and differentiation to mature cells compared with a single cell suspension. However, the polymer scaffold/cell composite grafts there are solid materials that can result in more extensive retinal detachment than desirable.
In situ cross-linking polymers may provide a “middle ground” between solid scaffolds and saline injections. While many carbohydrate-, protein-, or synthetic-polymer-based hydrogels can be formulated as injectable carriers for cells11, few are able to be injected as liquids and then subsequently undergo covalent cross-linking in vivo to become solid gels (“sol–gel transition”)12–15. Injectable gelatin-hydroxyphenyl propionic acid (Gtn-HPA) hydrogel system is one example of in situ cross-linking hydrogel. This particular polymer utilizes a time-sensitive cross-linking reaction catalyzed by hydrogen peroxide (H2O2) and horseradish peroxidase (HRP)16–18. A homogenous gel–cell mixture is created when the HPA moieties of the polymer strand are cross-linked inside a co-suspension of Gtn-HPA conjugate and cells of interest. After transplantation, no gelatinous material is typically seen after 1–2 weeks, during which the polymer is degraded by host and donor cell enzymes19. Given Gtn-HPA’s compatibility with neural stem cells20, we aimed to investigate whether this particular polymer could improve subretinal graft survival as well. The presented study is the first pilot study, as far as we are aware, characterizing biocompatibility and transplantation of injectable gel/retinal cell mixtures containing in situ cross-linkers.
Materials and Methods
Cell Culture of Human RPCs and GFP+ Pig RPCs
Human RPCs (hRPCs), obtained as described previously 21, were thawed from cryovials and then maintained in passage in low oxygen conditions (5% O2, 5% CO2, 100% humidity, 37°C). The hRPCs were not transfected with green fluorescent protein (gfp), because the downstream cell viability assays involved fluorescein derivatives.
All culture flasks were coated with human fibronectin (Akron biotechnologies, Boca Raton, FL, USA) for 1–2 hours on culture-treated flasks (Denville Scientific, Holliston, MA, USA) at room temperature. hRPC medium was created by combining 20 ng/mL recombinant human (rh) epidermal growth factor (rhEGF), 10 ng/mL rh fibroblast growth factor-2 (rhFGF-2), 1% antibiotic/antimycotic (100× solution, Gibco, Waltham, MA, USA) and 1% L-glutamine (100× solution, Gibco) in Ultraculture media (Lonza, Basel, Switzerland), as previously published21. Cells were passaged to 80–90% confluence, with recombinant trypsin (Sigma-Aldrich, St. Louis, MO, USA) and defined trypsin inhibitor (Gibco). Cell numbers were counted using trypan blue stain and an automatic hemacytometer (Countess II, Thermo Fisher, Waltham, MA, USA). RPCs were replated at a density of 10,000 cells/cm2. Only cells between passage numbers 10–12 were used for in vitro assays.
For xenograft studies, green fluorescent protein-positive (GFP+) pig RPCs (pRPCs) from fetal pigs, transfected with a retroviral vector containing the gfp gene following a β-actin promoter, were thawed from cryovials and cultured as described previously 22. GFP+ pRPCs were chosen for easier detection of transplanted cells via immunostaining for GFP. Culture protocol was identical to hRPCs, with the same media on a fibrinogen-coated flask. Prior to transplantation, pRPCs were suspended in Hank’s balanced salt solution (HBSS) with N-acetylcysteine (NAC) at previously published concentrations, which was shown to enhance transplant survival due to antioxidant effects22.
Culture of hRPCs in Gtn-HPA Hydrogel
Lyophilized Gtn-HPA was obtained from our collaborator at the Institute of Bioengineering and Nanotechnology in Singapore, where it was synthesized as described previously17. The make-up of the Gtn-HPA was as characterized previously17: 90% of amine groups in Gtn (8–14 kDa) were conjugated with HPA. A 2× solution of Gtn-HPA was prepared by dissolving 40 mg of Gtn-HPA in 1 mL PBS in a 37°C water bath. Subsequently, 100 µL of Gtn-HPA: PBS solution was mixed with an equal volume of hRPC cell suspension of various concentrations of cells at passage numbers 10–12, ranging from 2000 to 30,000 cells/mL of gel solution. For cross-linking, 0.1 U/mL HRP and 1.0 mM H2O2 were added to the mix in a dark room. The gel–cell liquid mixture was immediately pipetted into polystyrene 6-well or 12-well plates (Falcon, BD Biosciences, Franklin Lakes, NJ, USA) containing tissue culture-treated 15 mm diameter round plastic coverslips (Thermo Fisher) in each well. Then 25 µL of gel–cell mixture was used for 12-well plates, and 100 µL of gel–cell mixture was used for 6-well plates. A plastic low-attachment cell spreader (Costar, St. Louis, MO, USA) was used to minimize loss of gel–cell mixture during the spreading process. The spread mixture was allowed to gel for 5 minutes in a humidified incubator set at 37°C. Subsequently 4 mL of hRPC medium (see “2D Culture”) was added per well on top of the resulting thin (approximately 0.2 mm thick) hydrogel–cell layer, carefully without dislodging the sheet of hydrogel.
As a control, the same number of RPCs in 4 mL of hRPC medium was seeded onto 6-well or 12-well plates containing tissue culture-treated plastic coverslips coated with fibronectin, duplicating standard hRPC culture conditions. Cells were allowed to proliferate for 1–7 days, and medium was changed every 2–3 days.
Cell Viability Assay
The 6-well or 12-well plates containing hRPCs were taken for phase contrast microscopy for assessment of gross features. Then hRPC medium was replaced with a solution of 1 µL calcein-acetoxymethyl (Calcein-AM) and 2 µL ethidium homodier-1 (EthD-1) (Live/Dead Viability/Cytotoxicity kit; Invitrogen, Carlsbad, CA, USA) in 1 mL of hRPC medium for staining. Calcein-AM is a fluorescent dye that is activated by intracellular esterases that remove the acetomethoxy group, upon which the calcein dye is trapped inside the intact membrane of live cells. EthD-1 is a red/orange fluorescent dye which is impermeable to an intact cell membrane. Staining was performed for 5 minutes at 37°C in a humidified incubator. After staining, plastic coverslips (see “Culture of hRPCs in Gtn-HPA Hydrogel”) were collected from plates with gel–cell mixture or cell culture on fibronectin, and placed on an absorbent pad with the side with attached cells facing up. Coverslips were then washed once with HBSS. Meanwhile, 100 µL of polyvinyl alcohol (PVA)-DABCO® anti-fading mounting solution (Sigma-Aldrich) was applied to fresh glass slides. The washed coverslips were then flipped (the cell side now facing down) and placed over the drop of mounting solution on each glass slide. Each plastic coverslip was pressed gently to remove any air bubbles trapped inside the mounting solution between the glass slide and plastic coverslip. Because this procedure does not include a fixation step with paraformaldehyde (PFA), micrographs were taken immediately after staining in order to prevent loss of cell structure.
Cell Proliferation Assay
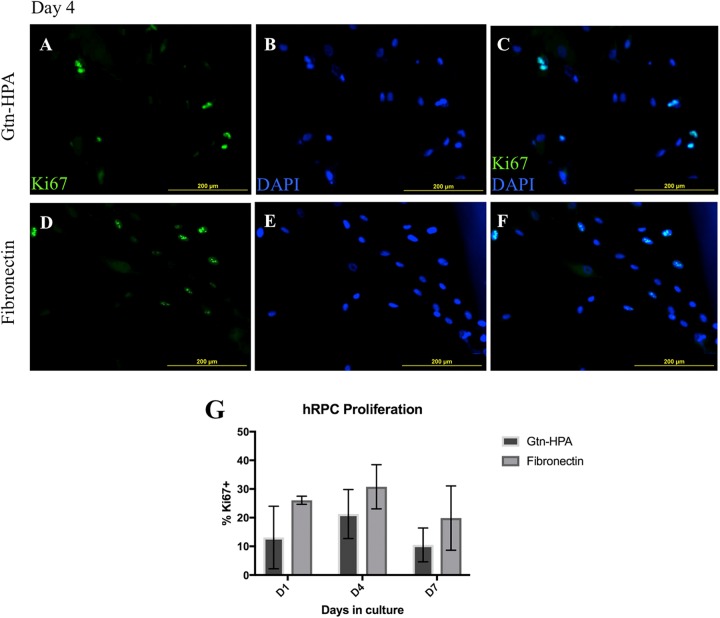
In order to evaluate whether hRPCs are not only viable but also able to proliferate, cultured hRPCs (2000 cells/µL) in Gtn-HPA hydrogel and on fibronectin were stained with Ki-67 at 96 h post-culture. Ki-67 localizes to outer surface of chromosomes in mitotic cells. Cells were counter-stained with DAPI to confirm nuclear localization of Ki-67+ signals (Fig. 2A–F). Ki-67 positive cells were defined as cells that show a clear heterochromatin-like pattern within the nucleus, at 10× magnification.
Fig. 2.
Ki-67 staining of hRPCs as a marker of proliferation. (A–F) Representative photos of hRPCs cultured for 96 hours within Gtn-HPA (A–C) or on fibronectin (D–F). Ki-67 in green, DAPI in blue. Ki-67+ cells show clear heterochromatin-like pattern (bottom left). Ki-67+ signals are shown to have nuclear localization, from co-staining with DAPI. (G) Quantification of percentage population of Ki-67+ cells. Two-factor ANOVA did not show significant effect of culture conditions (Gtn-HPA vs. fibronectin) on percentage of Ki-67+ cell numbers after post hoc analysis (F(1, 12)=6.276, p=0.028; post hoc D1: p=0.213, D4: p=0.702, D7: p=0.467).
Immunocytochemistry was done per the following protocol. Plastic coverslips from 6-well or 12-well plates (see “Culture of hRPCs in Gtn-HPA Hydrogel”) were collected and washed once with HBSS similarly to when preparing for cell viability assay (see “Cell Viability Assay”). Coverslips were placed on glass slides and a hydrophobic marker was used to encircle the area. Then cells were fixed with BD perm/fix solution (BD Biosciences) for 10 minutes, checking under brightfield microscopy for preservation of cellular structure. Cells were washed with BD perm/wash solution (BD Biosciences) once and then was blocked with solution containing 10% goat serum, 1% BSA, 0.1% sodium citrates, 0.1% triton-X, and 0.1% tween-20 for 1 h. After washing once more, primary staining with anti-Ki-67 antibody (Supplementary Table 1) was done overnight in 1% BSA solution with the same concentration of triton-X and tween-20 surfactants without goat serum or sodium citrate. Secondary staining was performed the next day for 4 h after washing twice with BD perm/wash solution. Starting concentrations of primary and secondary antibodies were 1:200, but dosages were adjusted for each antibody (supplementary data). Then 1 µg/mL DAPI solution was used for nuclear staining. Coverslips were washed twice with PBS and flipped (the cell side now facing down) on top of 25 µL droplot of PVA-DABCO® mounting solution (Sigma-Aldrich) on glass slides.
Cell Apoptosis Assay
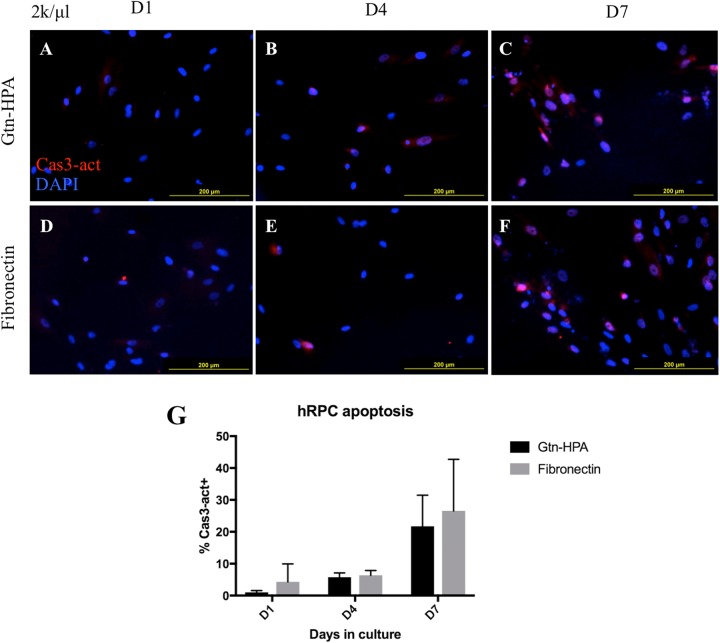
In order to evaluate whether hRPCs are resistant to oxidative stress from H2O2, we stained hRPC cultures in Gtn-HPA hydrogel or on fibronectin (2000 cells/µL) with antibodies against activated caspase-3, which is a highly sensitive marker of cells committed to apoptosis. Slides were counter-stained with DAPI to confirm intracellular localization of caspase-3. Immunocytochemistry protocol was identical to that used for the cell proliferation assay (see “Cell Proliferation Assay”) except the antibodies used.
Transplantation of GFP+ pRPCs in Rats
For transplantation, Long Evans rats were immunosuppressed with 10 mg/kg/day of cyclosporine A for at least 14 days. Anesthesia and analgesic postoperative care followed the AALAS core facility guidelines at the Schepens Eye Research Institute. Rats were sedated first with 2–4% isoflurane by inhalation in an induction chamber, followed by one intraperitoneal injection of 40–80 mg/kg ketamine and 10 mg/kg xylazine for anesthesia. A drop of topical ophthalmic anesthetic (0.5% proparacaine) was applied before the surgery and reapplied once every 15 minutes until the procedure ended. Either a single cell suspension of GFP+ pRPCs in liquid vehicle (HBSS-NAC) or a 1:1 mixture of the same pRPC suspension and Gtn-HPA with 0.1 U/mL HRP and 1.0 mM H2O2 (see “Culture of hRPCs in Gtn-HPA”) was prepared, to a final concentration of 40,000 cells in 4 µL liquid vehicle. The solution was loaded to a glass micropipette, which was connected with rubber tubing to a 26 s gauge Hamilton syringe filled with phosphate-buffered saline. An equal number of rats were injected with pRPC-containing hydrogel and pRPCs in HBSS-NAC liquid vehicle, in the left eye only. Injection was performed under a dissection microscope, and formation of a subretinal bleb was checked for successful injection. A triple antibiotic (Neo/Poly/Bac) ointment was applied to the eye post-procedure. Experimental rats were checked for any signs of weight loss or distress, and were maintained on cyclosporine until sample collection.
Immunohistochemistry
Rats were euthanized with CO2 at 168 hours after subretinal injection. Eyes were dissected and fixed with 4% paraformaldehyde, followed by sequential infiltration in 10% and 30% sucrose overnight. Eyes were embedded and frozen in optimal cutting temperature (OCT) compound. Horizontal 1:4 serial sections (10–15 µm thick) were made with cryostat. Slides were stained with anti-GFP and anti-CD45 as well as 1 µg/mL DAPI. The staining process was identical to immunocytochemistry of cultured cells (see ”Immunocytochemistry”), but concentrations of primary and secondary antibodies were adjusted for each antibody (supplementary data).
Cell Counting and Fluorescence Microscopy
For immunocytochemistry slides of cultured cells, four to five high quality photos at 10× were taken per each well under a fluorescence microscope. Fields were chosen by dividing the coverslip in four quadrants and taking one random photo per each quadrant. Calcein-AM or FITC-stained slides were seen under a fluorescein band-pass filter (420–620 nm), whereas the EthD-1 or Cy3-stained slides were seen under a Texas red filter (500–700 nm). Cell populations per photo were counted manually.
For quantification of cell viability, percentage viability was chosen over absolute number of cells due to the challenges of cell culture with Gtn-HPA hydrogel, specifically the loss of cell numbers during the gel spreading process onto coverslip containing 12-well plates (see “Culture of hRPCs in Gtn-HPA Hydrogel”). Viability was defined as (# of viable cells)/(# of non-viable cells + # of viable cells) in each micrograph taken at 10× magnification. Proliferating cell numbers were quantified as (# of Ki-67+ cells)/(# of DAPI+ bodies) ×100 in each photo taken at 10× magnification. Apoptotic cell numbers were defined as (# of Cas-3 active+ cells)/(# of DAPI+ bodies) ×100 in each micrograph taken at 10× magnification.
For immunohistochemistry slides of transplanted pRPCs, slides were surveyed for GFP+ cells at 10× and pictures were taken at 20× magnification. All available sections (approximately 400 total sections, 5–10 sections on each glass slide) from each specimen were scrutinized for any GFP+ signal, and five consecutive sections with the most numerous GFP+ cell count were chosen for grading. Post-transplant cell survival was graded with a scale of 0–3, based on the number of GFP+ cells per each 10 µm thick eye section. A grade of 3 was given for >100 cells per section, a grade of 2 for 10–100 cells, a grade of 1 for 1–10 cells, and a grade of 0 for no GFP+ cells. The total score was calculated by averaging the grades from five consecutive slides.
Statistical Analysis
All statistical data was presented as mean ± standard deviation. Two-tailed unmatched Student’s t-test, two-way analysis of variance (ANOVA) and post hoc multiple comparisons testing for two-way ANOVA (Tukey’s method) were done with the Prism 7 software. p<0.05 was considered significant in all tests.
Results
Cell Viability of hRPCs in Gtn-HPA Hydrogel
Viability of cells exposed to the Gtn-HPA covalent cross-linking process and grown within the three-dimensional gel was compared with cells grown on two-dimensional fibronectin-coated coverslips, at days 1, 4, and 7 in culture (Fig. 1A–F). Qualitatively, cells in both groups had similar confluences and morphology until day 4. Both groups reached near 100% confluence by day 4. Elongated and polar morphology was more noticeable by day 4, compared with day 1 in both groups. By day 7, RPCs in Gtn-HPA were present in sparser clusters at a lower confluence, whereas those cultured on fibronectin continued to have near 100% confluence with a greater number of nonviable cells as evidenced by red/orange-stained small cellular bodies. These small bodies were confirmed to be cells by concurrent Hoechst 33342 nuclear staining.
Fig. 1.
Calcein-AM and EthD -1 (LIVE/DEAD) staining of hRPCs. (A–C) Representative fluorescence photos of cells (seeding density 2000 cells/μL) cultured within the Gtn-HPA gel for 1, 4, and 7 days. Very few nonviable cells (red, highlighted with white arrows) are seen. (D–F) Photos of cells cultured on fibronectin-coated surfaces for 1, 4, and 7 days. (G) Percentage viability of cells in Gtn-HPA versus on fibronectin. Two-factor ANOVA did not show significant effect of culture conditions on percentage viability (F(1, 12)=1.183, p=0.298). (H) Percentage viability of cells in Gtn-HPA when seeded with varying cell concentrations (N=2–3). Cells were >60% viable up until concentrations of 8000 cells/μL when cultured for 7 days without passage.
When the percentage viability of cells grown inside Gtn-HPA hydrogel and on fibronectin were compared, two-tailed unmatched t-test (N=3) showed no significant difference between the two groups, at days 1, 4, and 7 (Fig. 1G, all groups p>0.1). Two-factor ANOVA showed statistical significance only with days in culture (D1 vs. D4 vs. D7) as a factor (F(2, 12)=6.567, p=0.012), whereas culture conditions (hydrogel vs. fibronectin) as a factor did not demonstrate statistical significance (F(1, 12)=1.183, p=0.298; see Supplementary Table 2). There was interaction between days in culture and culture conditions as factors (F(2, 12)=5.067, p=0.0254). Post hoc multiple comparison test showed changing effect of days in culture, depending on the culture condition (Hydrogel- D1 vs. D4: p<0.05, D1 vs. D7 and D4 vs. D7: p>0.05; Fibronectin- D1 vs. D4 and D1 vs. D7: p>0.05, D4 vs. D7: p<0.05).
In order to find optimal cell seeding densities in hydrogel in preparation for transplantation, we explored in vitro viability of hRPCs with varying concentrations from 2000 to 30,000 cells/µL in Gtn-HPA. Cells were >60% viable until D7 at seeding densities up to 8000 cells/µL (Fig. 1H). Two-factor ANOVA showed statistical significance of different cell concentrations on % viability (F(4, 18)=22.42, p<0.0001), as well as days in culture (F(2, 18)=3.969, p=0.0373; see Supplementary Table 3). There was no signification interaction between cell seeding concentration and days in culture (F(8, 18)=1.834, p=0.1358). Post hoc multiple comparisons test did not show any significant effect of days in culture (D1 vs. D4 vs. D7) given the same cell seeding density, so only the cell concentration effect is likely meaningful.
Ki-67 Staining of Proliferating hRPCs in Gtn-HPA Hydrogel
Ki-67+ cells were seen at days 1, 4, and 7 post-culture in both Gtn-HPA hydrogel and fibronectin groups, at various numbers (Fig. 2A–F). Two-factor ANOVA (Fig. 2G) revealed decrease in the percentage of Ki67-positive cells in the Gtn-HPA hydrogel compared with monolayer culture (F(1, 12)=6.276, p=0.028), but no meaningful effect of days in culture (F(2,12)=3.376, p=0.0687; see Supplementary Table 4). There was no interaction between culture conditions and days in culture (F(2,12)=0.216, p=0.8087). Post hoc multiple comparisons did not show significant effect of culture conditions given each day in culture group (Hydrogel vs. fibronectin, D1: p=0.2130, D4: p=0.7016, D7: p=0.4673).
Caspase-3 Staining of Apoptotic hRPCs in Gtn-HPA Hydrogel
Caspase-3 activation was shown to be minimal by day 4 in both Gtn-HPA hydrogel and fibronectin groups, whereas by day 7 many cells showed bright nuclear staining of caspase-3 (Fig. 3A–F). Two-factor ANOVA did not demonstrate culture conditions (Gtn-HPA hydrogel vs. fibronectin) as a significant factor influencing %Cas3+ population (F(1, 16)=0.914, p=0.353; Fig. 3G, Supplementary Table 5). Only the effects of days in culture were shown to be significant (F(2, 16)=17.01, p<0.0001), which was confirmed by post hoc test to be consistent between culture groups (D1 vs. D4, Gtn: p=0.624, Fib: p=0.916; D1 vs. D7, Gtn: p<0.005, Fib: p<0.005; D4 vs. D7, Gtn: p<0.05, Fib: p<0.01). There was no interaction between days in culture and culture conditions (F(2,16)=0.1604, p=0.8531).
Fig. 3.
Activated Caspase-3 staining of hRPCs as a marker of apoptosis. (A–F) Representative photos of cells grown in a thin layer of Gtn-HPA or on fibronectin-coated coverslips, at days 1, 4, and 7 post-culture. Red = Caspase-3, Blue = DAPI. Scant intracellular staining with activated Caspase-3 is seen by day 4 in both groups (B and E), whereas by day 7 a large proportion of cells show nuclear localization of activated Caspase-3 (C and F). (G) Quantification of percentage activated Caspase-3+ cells. Two-factor ANOVA did not show significant effect of culture conditions on apoptotic cell populations (F(1, 16)=0.914, p=0.353).
Post-Transplant Survival of GFP+ pRPCs
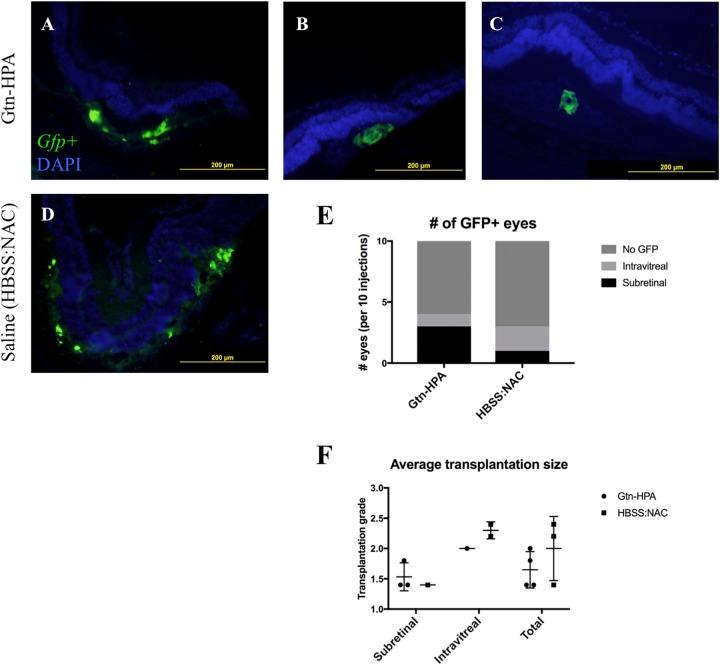
We evaluated subretinal transplantation using Gtn-HPA hydrogel as a vehicle to deliver GFP+ RPCs, compared with that using a HBSS-NAC saline solution (see “Transplantation of GFP+ pRPCs in Rats”). Overall transplantation success rate, defined by percentage of eyes with positive GFP+ cells under fluorescence microscopy, was 35%. GFP+ signal was seen in both hydrogel and liquid vehicle groups, and green signals were localized together with DAPI counter-staining (Fig. 4A–D). In the Gtn-HPA group, 3 out of 10 specimens showed subretinal GFP+ cells, whereas in the saline group only 1 out of 10 GFP+ specimens had subretinal GFP+ cells (Fig. 4E). A number of eyes showed GFP+ signals in the intravitreal space, although the eyes were checked for subretinal “bleb” formation during surgery. No GFP signal was seen with test eyes injected with empty hydrogel without cells. There was no crossover of GFP signal to other fluorescence channels, ruling out retinal pigment epithelium (RPE) autofluorescence as a source of the GFP+ findings.
Fig. 4.
Transplantation of GFP+ pRPCs in immunosuppressed Long Evans rats. Specimens were obtained 168 hours after transplant. (A–C) Examples of subretinally transplanted GFP+ cells with Gtn-HPA. Top of image = Vitreous side, Bottom = Choroidal side. GFP+ cells are below the outer nuclear layer (DAPI positive). (D) Example of subretinally transplanted GFP+ cells in saline control (HBSS-NAC). Looser distribution of GFP signal compared with A–C. No migration or integration into inner layers of retina seen with Gtn-HPA nor saline control. (E) Number of injected eyes with GFP+ findings, per 10 injections. Gtn-HPA showed more subretinally remaining transplants. A number of eyes showed GFP+ cells intravitreally in both groups, despite a confirmed subretinal bleb formation during transplantation. (F) Average transplantation grade of GFP+ eyes, calculated as average of five consecutive sections with the best grade (grade 3: >100 cells, grade 2: 10-100 cells, grade 1: 1–10 cells, grade 0: 0 cells per section). No significant difference seen between Gtn-HPA and HBSS-NAC groups.
The distribution of cells within the subretinal space was more widespread in the HBSS-NAC group, compared with cells found in tighter clusters in the Gtn-HPA hydrogel group. (Fig. 4A–D) Interestingly, the injection site contained considerably more DAPI-positive bodies compared with GFP+ cells, and GFP+ signals were only present in the center of the cluster. The size and shape of these GFP- cells were equivalent to GFP+ cells in the same cluster; however, only GFP+ cells were used for quantification of graft size.
Quantification of GFP+ cell population showed roughly equivalent numbers between the hydrogel and liquid vehicle groups (Fig. 4F), when taken as a whole (p=0.3). The sections from successful subretinal transplants showed less than 100 cells per section (grades 1–2, see “Cell Counting and Fluorescent Microscopy”), in both Gtn-HPA and HBSS-NAC groups. In eyes with intravitreal GFP+ cells, a few sections showed more than 100 cells per section (grade 3), resulting in a higher average grade in intravitreally injected eyes compared with subretinally injected eyes. Again, there was little difference in graft size between Gtn-HPA hydrogel and HBSS-NAC groups.
Cells in the Gtn-HPA hydrogel group showed a large and persistent detachment that was seen throughout different samples. No migration into the photoreceptor layer or into other layers of the retina was observed in both groups. No synaptic projections into other layers were observed at higher magnifications.
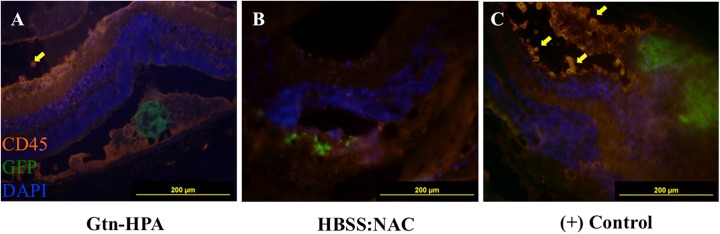
Anti-Leukocyte Staining of pRPC Transplants
Cells with either confirmed subretinal or intravitreal GFP+ transplant grafts were stained with anti-rat CD45, a pan-leukocytic marker. Subretinal grafts delivered by Gtn-HPA hydrogel (Fig. 5A) and HBSS-NAC (Fig. 5B) both showed no visible CD45+ cell infiltration in the subretinal space compared with controls (Fig. 5C). Fluorescence levels in the subretinal space under the tetramethylrhodamine (TRITC) filter in these groups were equivalent to that of nearby RPE, a prominent source of autofluorescence, especially in nocturnal animals. Scant leukocytes and CD45+ endothelial cells were seen in retinal layers, in both Gtn-HPA hydrogel and HBSS-NAC grafts.
Fig. 5.
Anti-CD45 staining of GFP+ pRPC transplants. RPE/Choroid facing bottom, vitreous body facing up. (A) Subretinal transplant in Gtn-HPA with no CD45+ cells in subretinal space. Minimal CD45+ cells seen in intravitreal space (yellow arrow), with evidenced by bright orange cell surface staining. (B) Subretinal transplant in HBSS-NAC (liquid vector) no CD45+ cells in subretinal space. (C) One intravitreal graft (positive control) showing infiltrating CD45+ cells (yellow arrows) in the ganglion cell/inner plexiform layer, with disruption of retinal architecture.
A number of intravitreal grafts, regardless of type of vehicle (hydrogel or liquid saline), showed infiltrating rat CD45+ cells in the intravitreal space as well as inner layers of the retina, including the ganglion cell layer and inner plexiform layer (Fig. 5C). Significant disruption and tearing of retinal architecture were notable for these sections. Infiltrating cells were smaller in diameter compared with the grafted cells, and fluorescent signals were visible only in the TRITC channel. These infiltrating cells were seldom seen in subretinal transplants in both Gtn-HPA hydrogel and HBSS-NAC groups.
Discussion
RPCs cultured in Gtn-HPA hydrogel showed equivalent survivability, proliferation and apoptosis to RPCs seeded on the optimal control of fibronectin-coated flasks. Optimal cell concentration for best viability in Gtn-HPA was 2000–8000 cells/µL. A cell delivered in Gtn-HPA showed a greater chance of cell survival in the subretinal space compared with saline controls, with very little evidence of immune rejection.
Previously, Lim et al. characterized the concentration of HRP for sufficient breakdown of H2O2 for NSCs survival20. Percentage viability of NSCs in Lim et al.’s study remained in the range of 60–80% despite oxidative stress from hydrogen peroxide, so we adapted this number as our minimum criteria to determine whether Gtn-HPA hydrogel is compatible with hRPCs as well. Viability of hRPCs were consistently above this number, and lower survival and higher apoptosis by day 7 in culture was deemed to be a part of a natural process in cell culture.
From our previous experience, hRPCs isolated from fresh fetal retina typically had excellent survival even after repeated freezing and thawing, and optimal 2D culture conditions using recombinant epidermal and fibroblast growth factors and low-oxygen incubators have repeatedly shown maintenance of viability and multipotency/stem-ness8,21–23. Despite good viability results, the proliferative potential of hRPCs seemed to be lowered by Gtn-HPA hydrogel overall (although not significant). Further experiments may be necessary to determine if altering the stiffness of Gtn-HPA hydrogel can overcome challenges of cell culture in hydrogel. Alternatively, growth factors can be mixed into the hydrogel in future studies: these growth factors can be those used in hRPC culture (EGF, FGF) or those that could assist in rod or cone differentiation (retinoic acid, Notch pathway inhibitors24).
When the combination of cells and Gtn-HPA hydrogel that provided the maximum viability was transplanted, the benefit of using Gtn-HPA hydrogel over saline control was more in localization of RPCs (% grafts remaining in subretinal space) than an increase in absolute number of GFP+ cells in each graft. RPCs in the hydrogel group were clustered in the transplantation, which may evidence that Gtn-HPA hydrogel traps transplanted RPCs for at least 7 days until the animals were sacrificed.
Gtn-HPA/RPC subretinal transplants showed a persistent retinal detachment after 168 hours post-transplantation, despite the lack of visible hydrogel-like material at that time. Unlike previous works with PLGA4, PGS6, and PCL8 in which a thin sheet was inserted into the retina, cells within Gtn-HPA hydrogel formed a dome-shaped graft resembling the initial transplantation “bleb.” Because these grafts were typically several cell-diameters thick, the large graft size may have caused complications with healing and reattachment. For future studies, it may be necessary to modify the hydrogel such that it biodegrades faster, e.g. in a few days, to aid reattachment of the retina.
The study was complicated by a number of eyes showing RPC grafts transplanted in the intravitreal space in both experimental and control groups. Previous experience with hyaluronic acid as an injectable but non-in situ cross-linking hydrogel vehicle similarly showed a majority of grafted eyes showing subretinal localization of RPCs, but also a number of eyes with RPCs found along the ganglion cell layer at 3 weeks post-transplant11. Given that this finding is often seen in injectable hydrogels but not as commonly seen with lyophilized and cell-seeded scaffolds, there was most likely leakage of the gel/cell draft into the intravitreal space during when the hydrogel was yet to fully gelatinize. It is unclear when this leakage happens – during transplantation, aided by the pressure of injection, or over a few days following transplantation – or how quickly it occurs. Future studies focusing on short and long term follow-up via optical coherence tomography or explant can be instrumental for further characterization of subretinal hydrogel transplants using injectable hydrogel. Increasing the concentration of HRP to hasten the cross-linking reaction can be considered as well.
Despite immune rejection being a common concern for xenograft studies, leukocytic staining was minimal under the minimal immunosuppression the animals received. Our results reinforce the notion that gelatin-based hydrogels are generally well-tolerated in vivo25. This is in contrast with other protein-based materials. For example, collagen-based hydrogels are more known to cause significant immune rejection (despite gelatin being denatured collage), and collagen in retinal research has been more relevant as an inducer of choroidal neovascularization in animal models, rather than as vehicles for cell delivery26,27. Therefore, gelatin-based in situ cross-linking hydrogels merit further investigation as valuable materials for retinal tissue engineering. Using this system to delivery cells in diseased rat models, such as rho-/- mice (which may further complicate cell survival6), deserves further exploration as well.
In summary, the present study demonstrates compatibility of Gtn-HPA hydrogel with RPCs, in regards to cell survival and proliferation, and minimal apoptosis equivalent to optimized cell culture conditions. In situ gelation of Gtn-HPA conjugate may increase likelihood of the transplanted RPCs remaining in subretinal space. Gtn-HPA hydrogel system shows promise as an injectable and biodegradable polymer vehicle for subretinal stem cell transplantation.
Supplemental Material
Supplementary_Data for In Situ Cross-linking Hydrogel as a Vehicle for Retinal Progenitor Cell Transplantation by Jeayoung Park, Petr Baranov, Aybike Aydin, Hany Abdelgawad, Deepti Singh, Wanting Niu, Motoichi Kurisawa, Myron Spector and Michael J. Young in Cell Transplantation
Footnotes
Ethical Approval: This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Schepens Eye Research Institute at Massachusetts Eye & Ear.
Statement of Human and Animal Rights: All of the experimental procedures involving animals were conducted in accordance with the IACUC guidelines of the Schepens Eye Research Institute at Massachusetts Eye & Ear.
Statement of Informed Consent: There are no human subjects in this articles and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Falkner-Radler CI, Krebs I, Glittenberg C, Povazay B, Drexler W, Graf A, Binder S. Human retinal pigment epithelium (RPE) transplantation: outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. Br J Ophthalmol. 2011;95(3):370–375. [DOI] [PubMed] [Google Scholar]
- 2. van Zeeburg EJ, Cereda MG, Amarakoon S, van Meurs JC. Prospective, randomized intervention study comparing retinal pigment epithelium-choroid graft surgery and Anti-VEGF therapy in patients with exudative age-related macular degeneration. Ophthalmologica. 2015;233(3-4):134–145. [DOI] [PubMed] [Google Scholar]
- 3. Schugens CMV, Grandfils C. Polylactidemacroporousbio- degradable implants for cell transplantation. II. Preparation of poly-lactide foams by liquid-liquid phase separation. J Biomed Mater Res. 1996;30(4):449–461. [DOI] [PubMed] [Google Scholar]
- 4. Tomita M, Lavik E, Klassen H, Zahir T, Langer R, Young MJ. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells. 2005;23(10):1579–1588. [DOI] [PubMed] [Google Scholar]
- 5. Lavik EB, Klassen H, Warfvinge K, Langer R, Young MJ. Fabrication of degradable polymer scaffolds to direct the integration and differentiation of retinal progenitors. Biomaterials. 2005;26(16):3187–3196. [DOI] [PubMed] [Google Scholar]
- 6. Redenti S, Neeley WL, Rompani S, Saigal S, Yang J, Klassen H, Langer R, Young MJ. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials. 2009;30(20):3405–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20(6):602–606. [DOI] [PubMed] [Google Scholar]
- 8. Yao J, Ko CW, Baranov PY, Regatieri CV, Redenti S, Tucker BA, Mighty J, Tao SL, Young MJ. Enhanced differentiation and delivery of mouse retinal progenitor cells using a micropatterned biodegradable thin-film polycaprolactone scaffold. Tissue Eng Part A. 2015;21(7-8):1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kundu J, Michaelson A, Baranov P, Chiumiento M, Nigl T, Young MJ, Carrier RL. Interphotoreceptor matrix based biomaterial: Impact on human retinal progenitor cell attachment and differentiation. J Biomed Mater Res B Appl Biomater. 2018;106(2):891–899. [DOI] [PubMed] [Google Scholar]
- 10. Singh D, Wang SB, Xia T, Tainsh L, Ghiassi-Nejad M, Xu T, Peng S, Adelman RA, Rizzolo LJ. A biodegradable scaffold enhances differentiation of embryonic stem cells into a thick sheet of retinal cells. Biomaterials. 2018;154:158–168. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Wang R, Zarembinski TI, Doty N, Jiang C, Regatieri C, Zhang X, Young MJ. The application of hyaluronic acid hydrogels to retinal progenitor cell transplantation. Tissue Eng Part A. 2013;19(1-2):135–142. [DOI] [PubMed] [Google Scholar]
- 12. Kang Derwent JJ, Mieler WF. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans Am Ophthalmol Soc. 2008;106:206–213. [PMC free article] [PubMed] [Google Scholar]
- 13. Rauck BM, Friberg TR, Medina Mendez CA, Park D, Shah V, Bilonick RA, Wang Y. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Invest Ophthalmol Vis Sci. 2014;55(1):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu CC, Chaw JR, Chen CF, Liu HW. Controlled release bevacizumab in thermoresponsive hydrogel found to inhibit angiogenesis. Biomed Mater Eng. 2014;24(6):1941–1950. [DOI] [PubMed] [Google Scholar]
- 15. Turturro SB, Guthrie MJ, Appel AA, Drapala PW, Brey EM, Perez-Luna VH, Mieler WF, Kang-Mieler JJ. The effects of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials. 2011;32(14):3620–3626. [DOI] [PubMed] [Google Scholar]
- 16. Hu M, Deng R, Schumacher KM, Kurisawa M, Ye H, Purnamawati K, Ying JY. Hydrodynamic spinning of hydrogel fibers. Biomaterials. 2010;31(5):863–869. [DOI] [PubMed] [Google Scholar]
- 17. Hu M, Kurisawa M, Deng R, Teo CM, Schumacher A, Thong YX, Wang L, Schumacher KM, Ying JY. Cell immobilization in gelatin-hydroxyphenylpropionic acid hydrogel fibers. Biomaterials. 2009;30(21):3523–3531. [DOI] [PubMed] [Google Scholar]
- 18. Wang LS, Boulaire J, Chan PP, Chung JE, Kurisawa M. The role of stiffness of gelatin-hydroxyphenylpropionic acid hydrogels formed by enzyme-mediated crosslinking on the differentiation of human mesenchymal stem cell. Biomaterials. 2010;31(33):8608–8616. [DOI] [PubMed] [Google Scholar]
- 19. Sakai S, Hirose K, Taguchi K, Ogushi Y, Kawakami K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials. 2009;30(20):3371–3377. [DOI] [PubMed] [Google Scholar]
- 20. Lim TC, Toh WS, Wang LS, Kurisawa M, Spector M. The effect of injectable gelatin-hydroxyphenylpropionic acid hydrogel matrices on the proliferation, migration, differentiation and oxidative stress resistance of adult neural stem cells. Biomaterials. 2012;33(12):3446–3455. [DOI] [PubMed] [Google Scholar]
- 21. Baranov PY, Tucker BA, Young MJ. Low-oxygen culture conditions extend the multipotent properties of human retinal progenitor cells. Tissue Eng Part A. 2014;20(9-10):1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abud M, Baranov P, Hicks C, Patel S, Lieppman B, Regatieri C, Sinden J, Isaac D, Avila M, Young M. The effect of transient local anti-inflammatory treatment on the survival of pig retinal progenitor cell allotransplants. Transl Vis Sci Technol. 2015;4(5):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo J, Baranov P, Patel S, Ouyang H, Quach J, Wu F, Qiu A, Luo H, Hicks C, Zeng J, Zhu J. et al. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014;289(10):6362–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kruczek K, Gonzalez-Cordero A, Goh D, Naeem A, Jonikas M, Blackford SJI, Kloc M, Duran Y, Georgiadis A, Sampson RD, Maswood RN. et al. Differentiation and transplantation of embryonic stem cell-derived cone photoreceptors into a mouse model of end-stage retinal degeneration. Stem Cell Reports. 2017;8(6):1659–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Celikkin N, Rinoldi C, Costantini M, Trombetta M, Rainer A, Swieszkowski W. Naturally derived proteins and glycosaminoglycan scaffolds for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2017;78:1277–1299. [DOI] [PubMed] [Google Scholar]
- 26. Shen D, Wen R, Tuo J, Bojanowski CM, Chan C-C. Neovascularization induced by subretinal injection of matrigel in CCL2/MCP-1-deficient mice. Ophthalmic Res. 2006;38(2):71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao J, Zhao L, Li Y, Liu Y, Xiao W, Song Y, Luo L, Huang D, Yancopoulos GD, Wiegand SJ, Wen R. A subretinal matrigel rat Choroidal Neovascularization (CNV) model and inhibition of CNV and associated inflammation and fibrosis by VEGF trap. Invest Ophthalmol Vis Sci. 2010;51(11):6009–6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Data for In Situ Cross-linking Hydrogel as a Vehicle for Retinal Progenitor Cell Transplantation by Jeayoung Park, Petr Baranov, Aybike Aydin, Hany Abdelgawad, Deepti Singh, Wanting Niu, Motoichi Kurisawa, Myron Spector and Michael J. Young in Cell Transplantation