Abstract
The autonomic innervation of the uterus is involved in multiple pathophysiological processes in both humans and animals. Pathological conditions such as adenomyosis or inflammatory pelvic disease are usually accompanied by significant alterations in uterine innervation. In the current study, we focused on autonomic innervation of uterine fibroids, the identification of recently described interstitial cells, telocytes, and the possible interplay between these structures. In this work, uterine telocytes were identified by immunopositivity for c-kit, CD34, and PDGFRα. Nerves were revealed by immunolabeling for neuronal markers: protein gene product PGP 9.5, inducible nitric oxide synthase (iNOS), choline acetyltransferase (ChAT), and tyrosine hydroxylase (TH). The gross organization of myometrial tissue has been analyzed by routine histology. The results demonstrated that the density of iNOS and ChAT-immunopositive neurons in the uterine fibroids was higher than that in the control samples. The density of telocytes in the fibrosis foci was lower than that in the normal myometrium. Our results suggest that autonomic innervation and telocytes are involved in the microenvironment imbalance characteristic of uterine leiomyoma. Since NOS-positive nerves play an important role in oxidative stress modulation, they might lead to a decrease in the number of telocytes, which are crucial components in the pathogenesis of leiomyoma formation.
Keywords: leiomyoma, telocytes, protein gene product 9.5 (PGP 9.5), nitric oxide synthase (NOS), PDGFRα
Introduction
Uterine innervation is derived from two components: afferent (interoceptive type) and efferent (autonomic type)1. The uterine autonomic nerve fibers release noradrenaline (from sympathetic endings) and acetylcholine (from parasympathetic fibers). The characteristic local feature is that axonal endings are not in close contact with myocytes, but neurotransmitters are secreted in the perifascicular space2. The neurogenic component is invaluably important for uterine contractility and blood flow regulation. It plays an important role in the pathophysiological mechanisms of chronic pelvic pain and co-occurs in such diseases as endometriosis, adenomyosis, inflammatory pelvic disease and leiomyomata. Foci of adenomyosis are characterized by a decline up to a disappearance of nerve fibers around lesions, whereas uterine tissue samples from subjects with chronic pelvic pain are characterized by a proliferation of small-diameter nerve fibers, which can be asymmetric, throughout the myometrial stroma3.
Previous studies of the innervation of the mammalian female reproductive system have revealed the great importance of the autonomic nervous system, especially adrenergic fibers4,5. For the primary identification of both myelinated and unmyelinated nerve fibers in the uterus, the protein gene product 9.5 (PGP 9.5) has been widely used; for example, in tissue samples from women affected by endometriosis6 or uterine fibroids7. PGP 9.5 is a highly specific pan-neuronal marker used for the visualization of nerve cell bodies and fibers as well as their qualitative and quantitative assessment.
Adrenergic innervation is involved in myometrial contractility, blood flow, and endometrial secretory function8. The adrenergic contribution to uterine innervation was demonstrated by the presence of tyrosine hydroxylase (TH) immunoreactivity in myometrial tissue5,8. Several animal models have been used for observation of the sympathetic branch of the uterine autonomic nervous system; for example, rat, guinea pig, and equine models. TH-immunoreactive nerves were found in all regions of the equine uterus by Bae et al5. They were mostly located parallel to the muscle fibers and along blood vessels. Their density was higher in the myometrium than in the endometrium4. The same locational (myometrial) prevalence was found in the rat uterus. In its upper part, TH-immunoreactive nerves were observed in the longitudinal layer, whereas in the lower uterus, the circular layer was predominant9. Reproductive hormones also have an impact on sympathetic nerves. For instance, pregnancy-induced degeneration was reported in the adrenergic innervation of the guinea pig uterus10. In women with endometriosis, the prevalence of estrogens correlates with an increasing number of adrenergic nerves in myometrium and all endometrial layers (functional and basal)11.
The human and animal uterus also contains abundant nitric oxide-synthesizing nerves that could be either autonomic and/or sensory. They are undoubtedly involved in the pathophysiology of common gynecological pathologies, including uterine fibroids, due to their participation in inflammatory reactions and oxidative stress8,12,13.
Telocytes (TCs) are a newly discovered type of interstitial cell with unique morphology and functions, described in animals and humans. The TC has a small, oval-shaped cellular body, containing a nucleus surrounded by a small amount of cytoplasm14–18. TCs have a variable number of telopodes (Tps) (very long cellular extensions), which are probably the longest cellular prolongations in the human body. It is important to note that their form and amount could change during pregnancy or disease19,20. Ten morphological criteria named “the platinum standard” are currently used for their primary detection, which is always confirmed by immunohistochemical identification in tissues14,19. Making homo- and heterocellular contacts with myocytes, nerves, immune, and stem cells, these cells are involved in contractility and the immune response, and form a three-dimensional network that may function as a scaffold to define the correct organization of tissues and organs. TCs also take part in neurotransmission21–24.
Despite progress in current gynecology, uterine leiomyoma (UL) is still the most widespread pathology that affects women of reproductive age. UL arises from uterine smooth muscle tissue and is characterized by the production of excessive quantities of extracellular matrix25–27. The aim of our study was to examine the autonomic nervous system and identify TCs in normal myometrium and ULs in order to reveal diversity in a gross structural organization between normal and affected myometrial tissue.
Materials and Methods
Subjects
Fifteen consecutive patients with symptomatic UL were scheduled for elective surgery (laparoscopic hysterectomy) and selected for the study group (15 women, mean age 54.7 ± 12.3 years). Patients with UL had detectable tumors in the uterus during gynecological examination before the operation. They presented with mild, recurrent episodes of vaginal bleeding and pain. The control group consisted of 15 consecutive patients (15 women, mean age 55.2 ± 13.5 years) who underwent elective surgery for other reasons and had no pre- or intraoperative signs of uterine fibroids. Hysterectomy was performed according to the standard procedure. Post-surgery histological examination did not reveal any signs of UL. Samples of tissue from the foci of fibrosis and adjacent myometrium were taken for further observation from the study group. Samples of unaffected myometrium were also prepared from the control group. All patients were surgically treated at the Institute of Gynecology Jagiellonian University Medical College in 2018.
Ethical Approval
The study was conducted in accordance with the moral, ethical, regulatory, and scientific principles governing clinical research. All surgical samples were retrieved with the approval of the Jagiellonian University Bioethical Committee using procedures that conformed to the Declaration of Helsinki guidelines (protocol number – 122.6120.40.2016).
Tissue Processing
Fresh hysterectomy specimens were collected and rinsed thoroughly with phosphate buffered saline (PBS, 0.01 M, pH = 7.4), fixed in 4% phosphate-buffered paraformaldehyde, routinely processed and embedded in paraffin. Serial sections were cut and mounted on poly-L-lysine-coated glass slides.
Routine Histology
The sections were deparaffinized, rehydrated, and stained with either hematoxylin–eosin (H&E) to evaluate the gross tissue organization or Masson trichrome staining to detect collagen deposits.
Immunofluorescence
After deparaffinization and rehydration, the slides were incubated for 30 min in PBS with the appropriate normal serum and 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) at room temperature, followed by overnight incubation at 4°C in a solution of PBS with the appropriate normal serum containing a primary antibody (or a mixture of primary antibodies) and 0.3% Triton X-100. After 5 washes (10 min each) in PBS, the specimens were incubated for 1 h at room temperature with a secondary antibody (or a mixture of secondary antibodies) diluted in PBS with the appropriate normal serum and 0.3% Triton X-100. Finally, the slides were washed in two changes (10 min each) of PBS and cover-slipped with fluorescence mounting medium (Dako, Glostrup, Denmark). Label specimens were analyzed immediately. The following primary and secondary antisera were used (Table 1).
Table 1.
Type, Sources and Dilution of Antibodies.
| Antibody | Catalog number and company | Dilution |
|---|---|---|
| Primary antibodies | ||
| Polyclonal rabbit anti-PGP 9.5 | Z5116, Dako, Denmark | 1:200 |
| Polyclonal goat anti-NOS | sc-49055, Santa Cruz, USA | 1:100 |
| Monoclonal mouse anti-ChAT | sc-55557, Santa Cruz, USA | 1:100 |
| Monoclonal mouse anti-TH | AB318, Millipore, USA | 1:200 |
| Polyclonal rabbit anti-c-kit | A4502, Dako, Denmark | 1:100 |
| Monoclonal mouse anti-CD34 | M7165, Dako, Denmark | 1:100 |
| Polyclonal goat anti-PDGFR alpha | AF-307-NA, R&D Systems, USA | 1:100 |
| Monoclonal mouse anti-tryptase | M7052, Dako, Denmark | 1:100 |
| Secondary antibodies | ||
| Biotinylated goat anti-mouse | 115-065-146, Jackson ImmunoResearch, USA | 1:500 |
| Cy3-conjugated polyclonal goat anti-rabbit | 111-165-144, Jackson ImmunoResearch, USA | 1:500 |
| FITC-conjugated streptavidin | 016-010-084, Jackson ImmunoResearch, USA | 1:500 |
| FITC-conjugated polyclonal donkey anti-goat | J2609, Santa Cruz, USA | 1:40 |
| Alexa Fluor 488 Goat Anti-Mouse | 115-545-146, Jackson ImmunoResearch, USA | 1:400 |
| Alexa Fluor 594 Goat Anti-Rabbit | 111-585-144, Jackson ImmunoResearch, USA | 1:400 |
| Alexa Fluor 488 Rabbit Anti-Mouse | 315-545-045, Jackson ImmunoResearch, USA | 1:400 |
| Alexa Fluor 488 Goat Anti-Rabbit | 111-545-144, Jackson ImmunoResearch, USA | 1:400 |
| Alexa Fluor 594 Donkey Anti-Goat | 705-585-003, Jackson ImmunoResearch, USA | 1:400 |
Microscopic Examination
Slides were examined using an MN800FL epifluorescence microscope (OptaTech, Warszawa, Poland) equipped with a Jenoptik Progress C15Plus color camera (Figs 1, 2, 4) and Olympus DP74 digital CCD camera (Figs 3, 6, 7). In turn, immunofluorescence in CD34/PDGFRα-positive cells (Fig. 5) was detected and analyzed using the scanning confocal microscopy (FV1200, Olympus). Digital images were collected at either 200× or 400× magnification. The qualitative analysis of cells and nerve fibers was provided in 10 consecutive high-power fields of vision (400×) using the computer-based image analysis system Multiscan 18.03 software (CSS, Warsaw, Poland). All samples were assessed by two independent specialists (each blinded to the other) without any knowledge of the clinical parameters or other prognostic factors to avoid bias. The presence and distribution of the pan-neuronal marker PGP 9.5 immunoreactivity was evaluated to assess the uterine autonomic innervation. Nerve cells and nerve fibers were evaluated on the basis of their morphology. TH, choline acetyltransferase (ChAT), and inducible nitric oxide synthase (iNOS) immunoreactivity were evaluated to assess the presence and distribution of different populations and subtypes of autonomic nerves. The use of mast cell tryptase staining enabled c-kit-positive mast cells to be distinguished from c-kit-positive TCs. TCs were considered as cells that were c-kit positive and tryptase negative concurrently, with the characteristic morphology and distribution28–30 in tissue samples. In addition, cells positive for CD34 and PDGFRα with the characteristic morphology and localization were also recognized as TCs.
Figure 1.
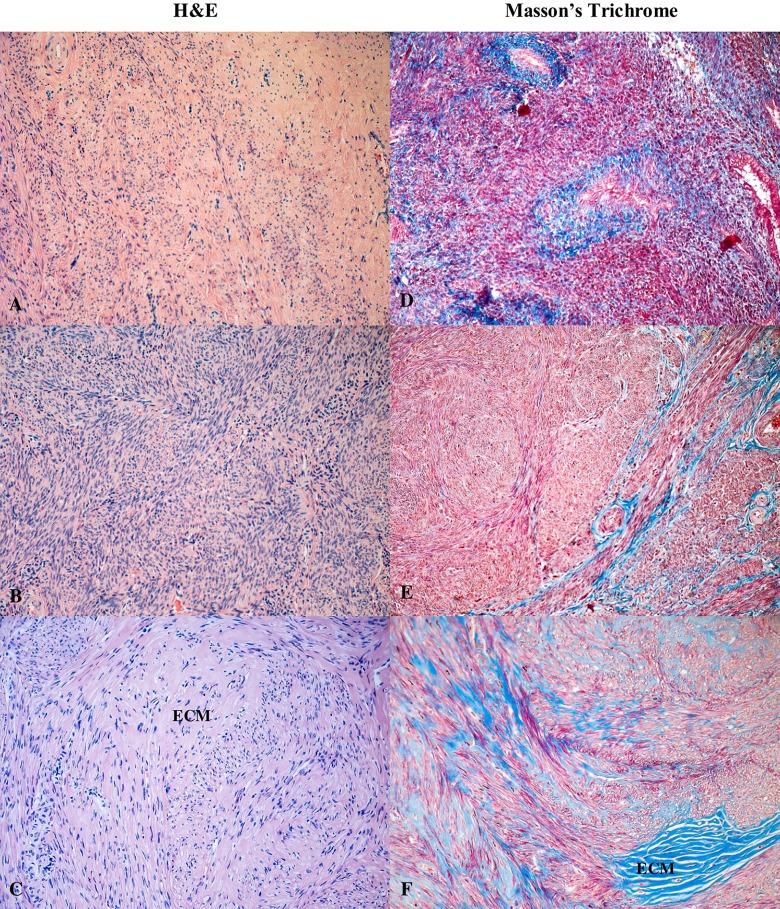
Hematoxylin–eosin and Masson’s trichrome stained sections of human myometrium. The myometrium sections from the control group (A, D) compared with the foci of leiomyoma (C, E) and adjacent myometrium from the same uterus (B, F). With Masson’s trichrome staining, collagen deposits were blue and muscle fibers were red. Fragments of disordered smooth muscle cells were separated by abundant extracellular matrix. Total magnification: × 200.
Figure 2.
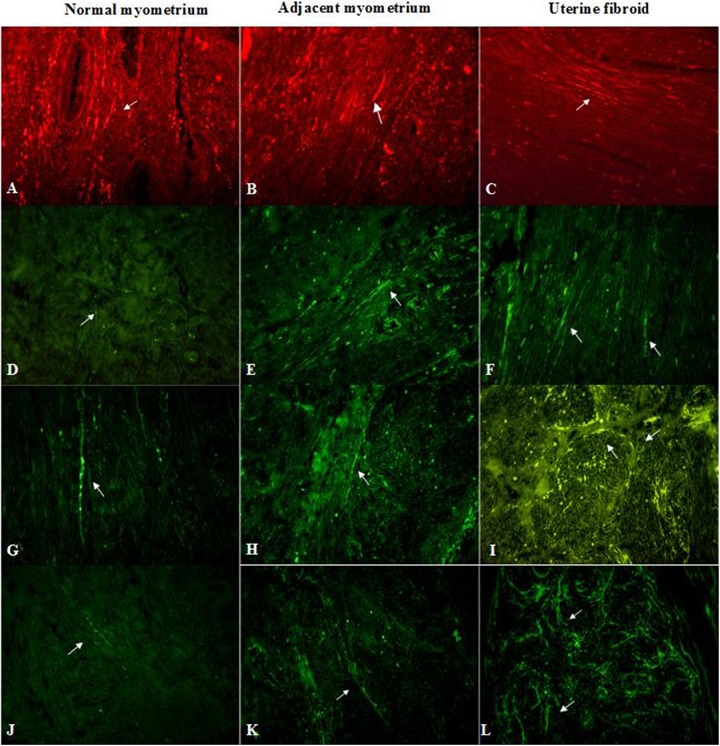
Myometrial samples stained for PGP 9.5 (red, Cy3) – A, B, C; TH (green, FITC) – D, E, F; ChAT (green, FITC) – G, H, I; and NOS (green, FITC) – J, K, L in an unaffected uterus (A, D, G, J), the foci of leiomyoma (C, F, I, L) and adjacent myometrium (B, E, H, K). Arrows indicate immunopositive nerve fibers in all samples. Total magnification: × 400.
Figure 4.
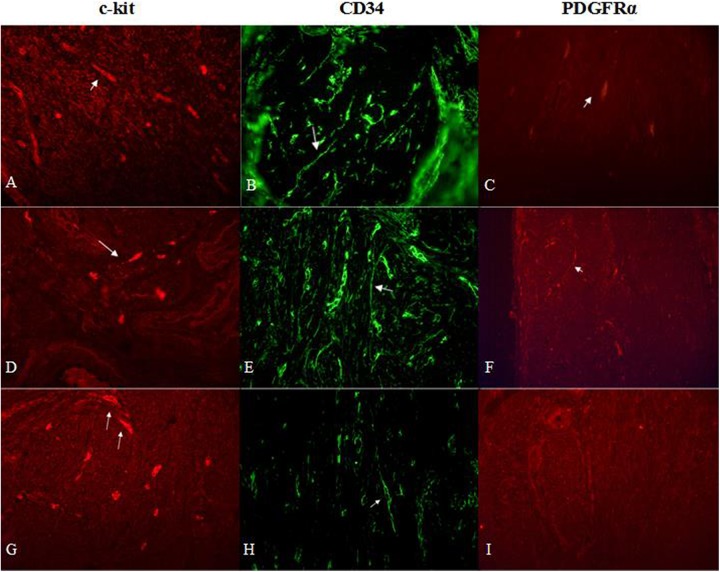
Myometrial samples stained for c-kit (red, Alexa Fluor 594), CD34 (green, Alexa Fluor 488) and PDGFRα (red, Alexa Fluor 594) in an unaffected uterus (A, B, C) and affected by leiomyoma (focus of fibroid (G, H, I) and adjacent myometrium (D, E, F)). Arrows indicate telocytes in all samples. Total magnification: × 400.
Figure 3.
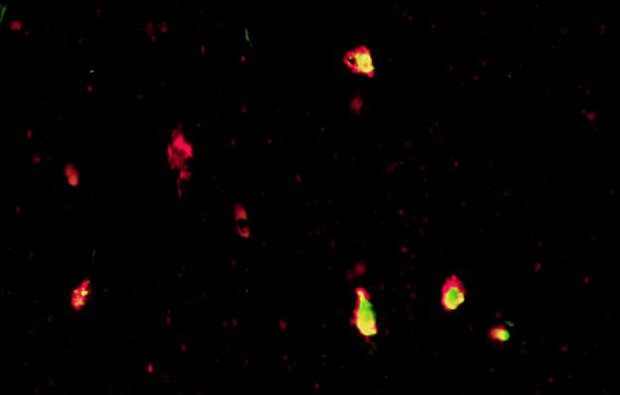
Tissue sample from the foci of leiomyoma stained for c-kit (red, Alexa Fluor 594) and tryptase (green, Alexa Fluor 488). C-kit-positive/tryptase-negative cells have red color and presented by telocytes, while c-kit-positive/tryptase-positive mast cells are yellow because of combination of both colors. Total magnification: × 400.
Figure 6.
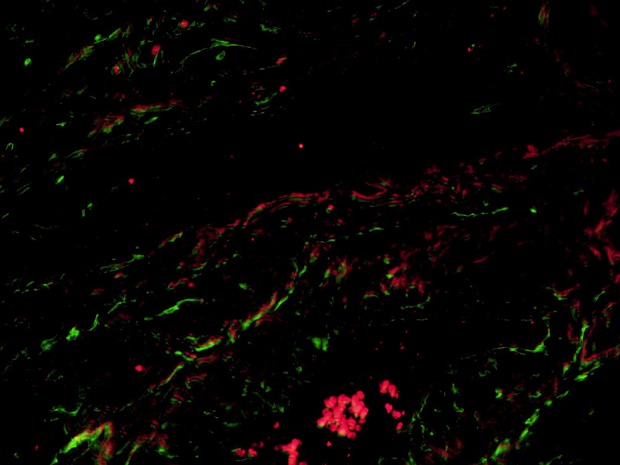
Double immunolabeling of uterine fibroid’s tissue for CD34 (green, Alexa Fluor 488) and iNOS (red, Alexa Fluor 594). Nerves are presented as red filaments accompanied by green structures (telocytes and blood vessels) in the longitudinal direction. Total magnification: × 400.
Figure 7.
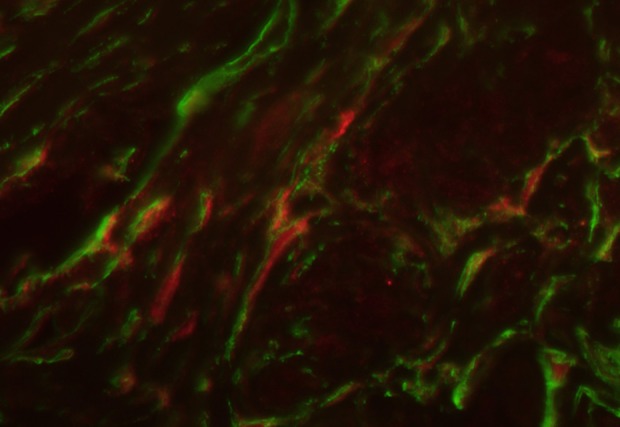
Myometrial tissue sample stained for CD34 (green, Alexa Fluor 488) and PGP 9.5 (red, Alexa Fluor 594). Nerve fibers (red network) are crossed by telocytes (marked by green) throughout or/and located in their vicinity. Total magnification: × 400.
Figure 5.
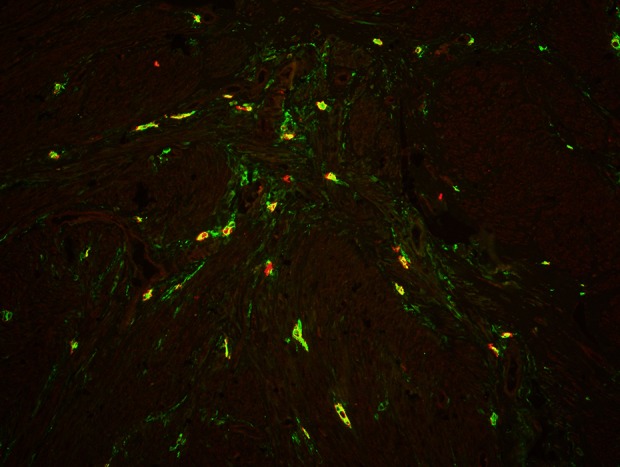
Sample of leiomyoma stained for PDGFR alpha (red, Alexa Fluor 594) and CD 34 (green, Alexa Fluor 488). Double-immunopositive cells with elongated bodies located between muscle fibers and close to blood vessels are identified as telocytes. Laser scanning confocal microscopy FV1200 (Olympus). Total magnification: × 400.
Results
Light microscopy of uterine fibroids, adjacent myometrium, and normal myometrium using Masson’s trichrome staining for collagen revealed collagen to be abundant in the fibroid tissue, while the myometrium had sparse, well-aligned collagen bundles adjacent to smooth muscle cells (Fig. 1). Hematoxylin and eosin staining demonstrated that myomas were mainly composed of smooth muscle cells and fibrous connective tissue. Smooth muscle cells were uniformly sized and spindle shaped with rhabditiform nuclei. Cells were arranged in a swirl-type pattern.
In the foci of fibroids, nerve fibers immunoreactive for PGP 9.5 were particularly parallel to each other and formed bundles around myometrial nodules. Most of them were placed in the fibroid pseudocapsule (Fig. 2, images A, B, and C). However, single neurons and plexuses of nerve fibers were observed inside leiomyomata. Normal myometrium had individual nerve fibers positive for PGP 9.5. The surrounding myometrium from myomatous uteri showed the presence of nerve fibers immunopositive for PGP 9.5 whereas its number was lower than that in the foci of myoma but higher than in the normal myometrium.
Nerves with immunopositivity for TH were detected in the foci of leiomyoma, adjacent myometrium and normal myometrium (Fig. 2, images D, E and F). In unaffected myometrium single, thin fibers, without strict orientation in space manifested themselves as TH-positive neurons. In contrast, in fibroids, spindle-shaped nerve fibers were densely located parallel to the muscular bundles. In samples of adjacent myometrial tissue, its orientation had less strict character—individual fibers repeated the eccentric lines framing the muscle fibers.
A network of nerve bundles and fibers with ChAT-immunoreactivity was found in the uterine fibroids. They formed a net structure inside the myoma and were distributed around the perimeter of collagen deposits. Conversely, in the normal myometrium, single nerves were parallel to muscle fibers. Fewer ChAT-positive nerve fibers, which are in a longitudinal direction, were revealed in unaffected tissue (Fig. 2, images G, H and I).
Nerves immunoreactive for iNOS were found throughout all samples. Numerous iNOS-immunoreactive nerve bundles and fibers were found in the uterine myoma. They were distributed around vessels and muscle bundles, repeating its nodular structure. The density of immunoreactivity for iNOS was greater in the uterine fibroids than in the adjacent myometrium. In the normal myometrium from the control group, few linear nerves were observed between muscle layers (Fig. 2, images J, K and L).
Double immunolabeling for c-kit and tryptase was performed for the identification of mast cells. C-kit and tryptase double-positive mast cells were generally round or oval shaped, with a centrally located nucleus. The c-kit-positive/mast cell tryptase-negative cells were considered TCs (Fig. 3).
The CD34-positive cells had an elongated oval-shaped cellular body (Fig. 4). They were located among the intertwined myometrial fibers and in close vicinity to blood vessels. The general pattern of their localization resembled parallel eccentric lines. However, in some regions their localization reflected the direction of smooth muscle bundles. PDGFRα-positive cells were found throughout all samples. PDGFRα-positive cells were mostly present in normal myometrium close to blood vessels, whereas some single cells were separately observed in leiomyomas and the adjacent space (Fig. 5).
Double immunostaining for such neuronal markers as iNOS and PGP 9.5 combined with a telocyte marker CD34 revealed double-immunolabeled cells (Figs 6, 7).
Discussion
In this study, autonomic innervation combined with the detection of TCs was observed in normal myometrium and in leiomyomata. We expect that the interaction between TCs and nerves might be significant for the pathophysiological mechanisms of uterine fibroids, and thus we examined disease-affected and unaffected specimens of myometrial segments from patients with UL. In particular, we identified TCs by CD34 and PDGFRα immunolabeling. We also used CD34/c-kit and c-kit/tryptase double immunolabeling to clearly distinguish TCs from mast cells, respectively.
The density of TCs and its possible interplay with nerves have been described in a variety of muscular organs (for example, intestine, uterus, heart, Fallopian tubes). For instance, the human appendix has more TCs than do the other parts of the digestive system, which is explained by its complex innervation20. The myenteric plexus of the human appendix consists of several distinct networks, localized between and within the circular and longitudinal muscle layers. Hanani noted this morphological feature as unique in comparison with other parts of the intestine31. According to the functional classification, enteric motor neurons are either inhibitory (e.g., NOS-positive neurons) or excitatory (e.g., ChAT-positive neurons)32. They both play an important role in the regulation of vascular tone and myometrial contractility as well. In Crohn’s disease, for instance, the gut dysmotility is accompanied by a decrease in and disappearance of TCs30. The cholinergic excitatory output is suppressed in animal models of Crohn’s colitis and ulcerative colitis33. Despite the decrease in TCs in UL, the density of iNOS and ChAT-positive neurons increased. We hypothesize that changes in the number of TCs lead to local myometrial misbalance.
UL usually has a highly vascularized pseudocapsule formed by compressed myometrium. Its architecture is similar to unaffected myometrium and contains nerve fibers and neuropeptides. The normal myometrium and pseudocapsule of fibroids exhibited similar immunoreactivity for PGP 9.534–36. Díaz-Flores et al. stressed that TCs are present in neuromuscular spindles, mostly located in the inner and outermost layer of the capsule37. A pronounced tendency has been revealed by Zhang et al. during observation of patients with endometriosis, adenomyosis, and uterine fibroids accompanied by pain or without it38. Women with pain symptoms have PGP 9.5-immunoactive nerve fibers in the functional layer of the endometrium, while those without pain do not. Thus, PGP 9.5-immunoreactive nerves might be involved in mechanisms of pain generation in common gynecological diseases38–41. Our results showed the prevalence of PGP 9.5-positive nerves in the pseudocapsule.
Adrenoceptors might also be involved in leiomyoma growth. Receptors with noradrenaline as a neuromediator are divided in two groups with regional domination. Most α1-adrenergic receptors are present in circular bundles of the uterus (cervico-isthmic area), whereas α2-adrenergic receptors are common in the body and have three subtypes (A, B, and C). Likewise, β2 adrenergic receptors are located in the uterine body. Generally, α-adrenergic receptors are involved in contraction, while β-adrenoreceptors play a role in tocolysis. However, the role of α2-, β1-, and β3-receptors in myometrial contractility is not clear2. Controversial data encourage attention to the dynamics of adrenergic receptors in uterine fibroids and their possible involvement in pathogenesis. Lee et al. described the expression of β-adrenergic receptor subtypes at different levels of UL cells and adjacent myometrium42. The distribution of β1-adrenergic receptor expression was the same in the two cell types, while β2-adrenergic receptors were more highly expressed in UL than in normal myometrium. No difference in β3-adrenergic receptor expression was found. These authors found that c-fos induction by Scutellaria barbata D. Don in uterine fibroid cells led to a regression of leiomyoma. At the same time, Adolfsson et al. found that the α2/β2-adrenoceptor ratio was increased in leiomyoma, due to a significant decrease in β2-adrenoceptor expression43.
Uterine autonomic innervation is influenced by hormonal regulation. Estrogens enhance the growth of UL and depress the development of uterine innervation (especially the sympathetic branch of the autonomic nervous system)44–46. TCs also express estrogen and progesterone receptors, which are specific for their localization. These cells might function as affecters and/or effectors in the pathogenesis of UL. Gevaert et al. have already described their possible role in signal transduction between the urothelium and the underlying nerve endings and stressed the role of the regional expression of hormonal receptors in upper lamina propria TCs47.
Nitric oxide (NO) is produced in neurons from L-arginine by the action of the enzyme nitric oxide synthase (NOS). This process usually passes upon stimulation by pro-inflammatory cytokines (Interleukin-1, tumor necrosis factor α, and interferon γ)48. NO is a potent dilatator of smooth muscle. In the uterus, myometrium contains NO-synthesizing nerves that could be autonomic and/or sensory. Some NOS-positive nerves in the uterus are parasympathetic and originate from neurons in the pelvic paracervical ganglia, and some are sensory and originate from neurons in thoracic, lumbar, and sacral dorsal root ganglia49,50. Papka et al. did not report any NOS-positive sympathetic nerves in the uterus. These authors described that in parasympathetic neurons, NOS-immunoreactivity coexists with acetylcholinesterase immunoreactivity in sensory nerves49. TH-positive neurons of the paracervical ganglia do not contain NOS-reactivity but some of them are apposed by NOS-varicosities50. iNOS expression is higher in leiomyoma than in normal myometrial cells7,51. Moreover, the uterus with leiomyoma or adenomyosis exhibited a higher expression of endothelial NOS, especially in cases associated with symptoms (menorrhagia and dysmenorrhea)12. This result illustrates that NOS may be involved in the pathomechanisms of invasion and excess growth of myometrium, similar to the process of vessel formation. Increased iNOS activity may decrease the tubal ciliary beat frequency and oviductal smooth muscle activity, and consequently could lead to tubal factor infertility48–52. On the other hand, in the uterus, NO plays a key role in mechanisms of uterine cyclicity53, decidualization54, and implantation55. Balance in the iNOS/NO system is essential for successful early implantation, pregnancy, and labor. In animal models, uterine TCs activated peritoneal macrophages and stimulated production of iNOS48. Consequently, TCs should be involved in all main pathomechanisms of uterine and tubal reproductive function. Double immunostaining for neuronal markers such as iNOS and PGP 9.5 combined with a TC marker CD34 in our specimens confirmed/demonstrated its interaction. Mostly cells that we considered as TCs were in parallel to nerve fibers in the myometrial tissue. We assume that partially CD34 immunopositive structures may represent the myometrial vessels. However, some of them were identified as TCs as well, that commonly form a network with smooth muscle bundles as well as nerve fibers.
Endometrial stem cells in culture differentiate into high-efficiency cholinergic neurons after stimulation with nerve growth factor and basic fibroblast growth factor. Moreover, ChAT activity increases56. Interaction between these growth factors and TCs is unclear, but TCs have immunopositivity for several growth factors, including vascular endothelial growth factor and plated-derived growth factor receptor alpha and beta, which merit further investigation in this area.
The alterations in the presence and location of adrenergic and cholinergic innervation in the human myomatous uterus indicate an important role for neural factors in the pathogenesis of the disease. The location of TCs, their immunopositivity to hormonal receptors, and their ability to induce NOS production give enough reasons to suggest their essential role in the regulation of myometrial proliferation. The close vicinity of TCs with nerve endings demonstrates the unique involvement of these cells in neuronal regulation in the uterus; however, the role of the cell–cell interaction with nerve fibers needs greater explanation. Further observation of TCs in the context of innervation in the healthy and myomatous uterus is needed as well.
Impact Statement
The current research has scientific value because of its primacy. There are no previous descriptions of the interplay between telocytes and autonomic innervation in leiomyomata. This study integrates modern knowledge of the pathological mechanisms of one of the oldest gynecological diseases, uterine leiomyoma. The presence of TCs in the foci of uterine fibroids and changes in density correlate with the myometrial structure. On the other hand, the difference in adrenergic and cholinergic innervation between affected and unaffected myometrium demonstrates the importance of the neuronal component in fibroid development. The correlation of those components brings new features to the pathogenesis of leiomyoma.
Acknowledgments
The authors would like to extend special thanks to Dr. Mariusz Gajda for his excellent knowledge and technical support of image analysis.
Footnotes
Author Contribution: Veronika Aleksandrovych, Krzysztof Gil: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; study supervision; final approval of the manuscript. Magdalena Kurnik-Łucka; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; final approval of the manuscript. Tomasz Bereza: collection of data; critical revision of the manuscript for important intellectual content; final approval of the manuscript Magdalena Białas: histology; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; final approval of the manuscript. Dragos Cretoiu: critical revision of the manuscript for important intellectual content; final approval of the manuscript. Artur Pasternak: searching bibliographic databases; editing and revising of the manuscript; analysis and interpretation of data; final acceptance of the manuscript. Jerzy A. Walocha: critical revision of the manuscript for important intellectual content; final approval of the manuscript.
Ethical Approval: The study was conducted in accordance with the moral, ethical, regulatory and scientific principles governing clinical research. All surgical samples were retrieved with the approval of the Jagiellonian University Bioethical Committee using procedures that conformed to the Declaration of Helsinki guidelines (protocol number – 122.6120.40.2016).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Jagiellonian University Bioethical Committee (protocol number – 122.6120.40.2016) approved protocols.
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Veronika Aleksandrovych  https://orcid.org/0000-0001-9850-6269
https://orcid.org/0000-0001-9850-6269
Dragos Cretoiu  https://orcid.org/0000-0003-3214-030X
https://orcid.org/0000-0003-3214-030X
References
- 1. Freeman A. The distribution of the nerves to the adult human uterus. Am J Clin Pathol. 1946;16:117–123. [DOI] [PubMed] [Google Scholar]
- 2. Adrian A, Dun E, Tica V, Cojocaru V, Tica OS, Berceanu S. The autonomic innervation of the uterus: a short review on pharmacological aspects. Gineco.Ro. 2011;7(24):86–91. [Google Scholar]
- 3. Quinn MJ, Kirk N. Differences in uterine innervation at hysterectomy. Am J Obstet Gynecol. 2002;187(6):1515–1519. [DOI] [PubMed] [Google Scholar]
- 4. Alm P, Lundberg LM. Co-existence and origin of peptidergic and adrenergic nerves in the guinea pig uterus. Retrograde tracing and immunocytochemistry, effects of chemical sympathectomy, capsalcin treatment and pregnancy. Cell Tissue Res. 1998;254(3):517–530. [DOI] [PubMed] [Google Scholar]
- 5. Bae SE, Corcoran BM, Watson ED. Immunohistochemical study of the distribution of adrenergic and peptidergic innervation in the equine uterus and the cervix. Reproduction. 2001;122(2):275–282. [DOI] [PubMed] [Google Scholar]
- 6. Miller EJ, Fraser IS. The importance of pelvic nerve fibers in endometriosis. Womens Health (Lond). 2015;11(5):611–618. [DOI] [PubMed] [Google Scholar]
- 7. Joo BS, Park MJ, Kim CW, Lee KS, Joo JK. Differential expression of visfatin, leptin, stromal cell derived factor-1α, endothelial nitric oxide synthase, and vascular endothelial growth factor in human leiomyomas. Gynecol Endocrinol. 2017;33(4):306–310. [DOI] [PubMed] [Google Scholar]
- 8. Wrobel KH, Kujat R. The bovine tubouterine junction: general innervation pattern and distribution of adrenergic, cholinergic, and peptidergic nerve fibres. Cell Tissue Res. 1993;274(3):493–501. [DOI] [PubMed] [Google Scholar]
- 9. Houdeau E, Rousseau A, Meusnier C, Prud’Homme MJ, Rousseau JP. Sympathetic innervation of the upper and lower regions of the uterus and cervix in the rat have different origins and routes. J Comp Neurol. 1998;399(3):403–412. [PubMed] [Google Scholar]
- 10. Lundberg LM, Alm P, Wharton J, Polak JM. Protein gene product 9.5 (PGP 9.5). A new neuronal marker visualizing the whole uterine innervation and pregnancy-induced and developmental changes in the guinea pig. Histochemistry. 1988;90(1):9–17. [DOI] [PubMed] [Google Scholar]
- 11. Tokushige N, Markham R, Russell P, Fraser IS. Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertil Steril. 2007;88(4):795–803. [DOI] [PubMed] [Google Scholar]
- 12. Oh NJ, Ryu KY, Jung CN, Yi SY, Kim SR. Expression of endothelial nitric oxide synthase in the uterus of patients with leiomyoma or adenomyosis. J Obstet Gynaecol Res. 2013;39(2):536–542. [DOI] [PubMed] [Google Scholar]
- 13. Liu JJ, Duan H, Wang S. [Expression of nitric oxide in uterine junctional zone of patients with adenomyosis]. Zhonghua Fu Chan Ke Za Zhi. 2013;48(7):504–507. [PubMed] [Google Scholar]
- 14. Popescu LM, Faussone-Pellegrini MS. TELOCYTES — a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14(4):729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cretoiu SM, Cretoiu D, Marin A, Radu BM, Popescu LM. Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium. Reproduction. 2013;145(4):357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cretoiu SM, Popescu LM. Telocytes revisited. Biomol Concepts. 2014;5(5):353–369. [DOI] [PubMed] [Google Scholar]
- 17. Aleksandrovych V, Walocha JA, Gil K. Telocytes in female reproductive system (human and animal). J Cell Mol Med. 2016;20(6):994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cretoiu SM. Immunohistochemistry of telocytes in the uterus and fallopian tubes. Adv Exp Med Biol. 2016;913:335–357. [DOI] [PubMed] [Google Scholar]
- 19. Popescu LM, Ciontea SM, Cretoiu D. Interstitial Cajal-like cells in human uterus and fallopian tube. Ann N Y Acad Sci. 2007;1101:139–165. [DOI] [PubMed] [Google Scholar]
- 20. Díaz-Flores L, Gutiérrez R, Díaz-Flores LJR, Goméz MG, Sáez FJ, Madrid JF. Behaviour of telocytes during physiopathological activation. Semin Cell Dev Biol. 2016;55:50–61. [DOI] [PubMed] [Google Scholar]
- 21. Banciu DD, Banciu A, Radu BM. Electrophysiological features of telocytes. Adv Exp Med Biol. 2016;913:287–302. [DOI] [PubMed] [Google Scholar]
- 22. Faussone-Pellegrini MS, Gherghiceanu M. Telocyte’s contacts. Semin Cell Dev Biol. 2016;55:3–8. [DOI] [PubMed] [Google Scholar]
- 23. Xu T, Lu S, Zhang H. Transmission electron microscope evidence of telocytes in canine dura mater. J Cell Mol Med. 2016;20(1):188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aleksandrovych V, Pasternak A, Basta P, Sajewicz M, Walocha JA, Gil K. Telocytes: facts, speculations and myths (Review article). Folia Med Cracov. 2017;57(1):5–22. [PubMed] [Google Scholar]
- 25. Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344–1355. [DOI] [PubMed] [Google Scholar]
- 26. Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646–1655. [DOI] [PubMed] [Google Scholar]
- 27. Aleksandrovych V, Bereza T, Sajewicz M, Walocha JA, Gil K. Uterine fibroid: common features of widespread tumor (Review article). Folia Med Cracov. 2015;55(1):61–75. [PubMed] [Google Scholar]
- 28. Richter M, Kostin S. The failing human heart is characterized by decreased numbers of telocytes as result of apoptosis and altered extracellular matrix composition. J Cell Mol Med. 2015;19(11):2597–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matyja A, Gil K, Pasternak A, Sztefko K, Gajda M, Tomaszewski KA, Matyja M, Walocha JA, Kulig J, Thor P. Telocytes: new insight into the pathogenesis of gallstone disease. J Cell Mol Med. 2013;17(6):734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Milia AF, Ruffo M, Manetti M, Rosa I, Conte D, Fazi M, Messerini L, Ibba-Manneschi L. Telocytes in Crohn’s disease. J Cell Mol Med. 2013;17(12):525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hanani M. Multiple myenteric networks in the human appendix. Auton Neurosci. 2004;110(1):49–54. [DOI] [PubMed] [Google Scholar]
- 32. Kurnik M, Gil K, Gajda M, Thor P, Bugajski A. Neuropathic alterations of the myenteric plexus neurons following subacute intraperitoneal administration of salsolinol. Folia Histochem Cytobiol. 2015;53(1):49–61. [DOI] [PubMed] [Google Scholar]
- 33. Winston JH, Li Q, Sarna SK. Paradoxical regulation of ChAT and nNOS expression in animal models of Crohn’s colitis and ulcerative colitis. Am J Physiol Gastrointest Liver Physiol. 2013;305(4):G295–G302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malvasi A, Cavallotti C, Nicolardi G, Pellegrino M, Dell’Edera D, Vergara D, Kumakiri J, Greco M, Tinelli A. NT, NPY and PGP 9.5 presence in myomeytrium and in fibroid pseudocapsule and their possible impact on muscular physiology. Gynecol Endocrinol. 2013;29(2):177–181. [DOI] [PubMed] [Google Scholar]
- 35. Tinelli A, Malvasi A, Hurst BS, Tsin DA, Davila F, Dominguez G, Dell’edera D, Cavallotti C, Negro R, Gustapane S, Teigland CM. et al. Surgical management of neurovascular bundle in uterine fibroid pseudocapsule. JSLS. 2012;16(1):119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun Y, Zhu L, Huang X, Zhou CX. Immunohistochemical localization of nerve fibers in the pseudocapsule of fibroids. Eur J Histochem. 2014;58(2):2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Díaz-Flores L, Gutiérrez R, Sáez FJ, Díaz-Flores LJR, Madrid JF. Telocytes in neuromuscular spindles. J Cell Mol Med. 2013;17(4):457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil Steril. 2009;92(5):1799–1801. [DOI] [PubMed] [Google Scholar]
- 39. Brauer MM. Plasticity in uterine innervation: state of the art. Curr Protein Pept Sci. 2016;18(2):108–119. [DOI] [PubMed] [Google Scholar]
- 40. Cramer SF, Heller DS. Postablation neuroma of the myometrium-a report of 5 cases. Hum Pathol. 2017;67:211–216. [DOI] [PubMed] [Google Scholar]
- 41. Thorbert G, Alm P, Björklund AB, Owman C, Sjöberg NO. Adrenergic innervation of the human uterus. Disappearance of the transmitter and transmitter-forming enzymes during pregnancy. Am J Obstet Gynecol. 1979;135(2):223–226. [DOI] [PubMed] [Google Scholar]
- 42. Lee TK, Cho HL, Kim DI, Lee YC, Kim CH, Scutellaria barbata D. Don induces c-fos gene expression in human uterine leiomyomal cells by activating beta2-adrenergic receptors. Int J Gynecol Cancer. 2004;14(3):526–531. [DOI] [PubMed] [Google Scholar]
- 43. Adolfsson PI, Haug I, Berg G, Svensson SP. Changes in beta(2)-adrenoceptor expression and in adenylyl cyclase and phosphodiesterase activity in human uterine leiomyomas. Mol Hum Reprod. 2000;6(9):835–842. [DOI] [PubMed] [Google Scholar]
- 44. Richeria A, Viettro L, Chavey-Genaroa R, Burnstock G, Cowen T, Brauer MM. Effects of infantile/prepurtal chronic estrogen treatment and chemical sympathectomy with guanethidine on developing cholinergic nerves of the rat uterus. J Histochem Cytochem. 2002;50(6):839–850. [DOI] [PubMed] [Google Scholar]
- 45. Mónica Brauer M, Smith PG. Estrogen and female reproductive tract innervation: cellular and molecular mechanisms of autonomic neuroplasticity. Auton Neurosci. 2015;187:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martínez GF, Bianchimano P, Brauer MM. Estrogen-induced collagen reorientation correlates with sympathetic denervation of the rat myometrium. Auton Neurosci. 2016;201:32–39. [DOI] [PubMed] [Google Scholar]
- 47. Gevaert T, De Vos R, Van Der Aa F, Joniau S, van den Oord J, Roskams T, De Ridder D. Identification of telocytes in the upper lamina propria of the human urinary tract. J Cell Mol Med. 2012;16(9):2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chi C, Jiang XJ, Su L, Shen ZJ, Yang XJ. In vitro morphology, viability and cytokine secretion of uterine telocyte-activated mouse peritoneal macrophages. J Cell Mol Med. 2015;19(12):2741–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Papka RE, McNeill DL, Thompson D, Schmidt HH. Nitric oxide nerves in the uterus are parasympathetic, sensory, and contain neuropeptides. Cell Tissue Res. 1995;279(2):339–349. [DOI] [PubMed] [Google Scholar]
- 50. Papka RE, Traurig HH, Schemann M, Collins J, Copelin T, Wilson K. Cholinergic neurons of the pelvic autonomic ganglia and uterus of the female rat: distribution of axons and presence of muscarinic receptors. Cell Tissue Res. 1999;296(2):293–305. [DOI] [PubMed] [Google Scholar]
- 51. Fletcher NM, Abusamaan MS, Memaj I, Saed MG, Al-Hendy A, Diamond MP, Saed GM. Oxidative stress: a key regulator of leiomyoma cell survival. Fertil Steril. 2017;107(6):1387–1394. [DOI] [PubMed] [Google Scholar]
- 52. Martinez SP, Viggiano M, Franchi AM, Herrero MB, Ortiz ME, Gimeno MF, Villalón M. Effect of nitric oxide synthase inhibitors on ovum transport and oviductal smooth muscle activity in the rat oviduct. J Reprod Fertil. 2000;118(1):111–117. [DOI] [PubMed] [Google Scholar]
- 53. Huang J, Roby KF, Pace JL, Russell SW, Hunt JS. Cellular localization and hormonal regulation of inducible nitric oxide synthase in cycling mouse uterus. J Leukocyte Biol. 1995;57(1):27–35. [DOI] [PubMed] [Google Scholar]
- 54. Spencer F, Chi L, Zhu MX. Antiproliferative effects of inducible nitric oxide synthase inhibition on decidualization in pseudopregnant rats. Exp Biol Med. 1998;218(1):45–50. [DOI] [PubMed] [Google Scholar]
- 55. Khorram O. Nitric oxide and its role in blastocyst implantation. Rev Endocr Metab Dis. 2002;3(2):145–149. [DOI] [PubMed] [Google Scholar]
- 56. Noureddini M, Verdi J, Mortazavi-Tabatabaei SA, Sharif S, Azimi A, Keyhanvar P, Shoae-Hassani A. Human endometrial stem cell neurogenesis in response to NGF and bFGF. Cell Biol Int. 2012;36(10):961–966. [DOI] [PubMed] [Google Scholar]