Short abstract
Sepsis is a syndrome associated with excessive inflammation. Since mortality from sepsis remains high, more laboratory research is needed to provide insight into more effective ways to use novel, potentially more beneficial agents in sepsis. We investigated the ex vivo immunomodulatory effect of a novel polyclonal Ab preparation, trimodulin, containing IgM (∼23%), IgA (∼21%) and IgG (∼56%). Using whole blood and purified PBMCs from healthy volunteers and patients with sepsis, various ex vivo investigations upon endotoxin challenge and pre- and post-trimodulin treatment were performed. Endotoxin-induced TNF-α secretion was noticeably lower with than without trimodulin, implying attenuation of the hyper-responsive state. Trimodulin also lowered TLR2, TLR4, CD11b and CD64 detection on LPS/lipoteichoic acid-stimulated monocytes. These responses were observed in cells from healthy volunteers only shortly after ex vivo endotoxin stimulation and in whole blood from patients with early-stage sepsis. Furthermore, trimodulin markedly reduced lymphocyte proliferation and release of pro- and anti-inflammatory cytokines, but did not affect phagocytosis or oxidative-burst activities of endoxin-stimulated cells. Thus, trimodulin mitigated monocyte and lymphocyte hyperinflammatory responses early after endotoxin exposure. Determining whether early in vivo administration of trimodulin will elicit similar positive immunomodulatory effects and offer a clinical benefit warrants investigation.
Keywords: Cytokines, Ig, inflammatory response, monocytes, sepsis
Introduction
Sepsis and septic shock are life-threatening conditions caused by a dysfunctional host immune response to infection, which may lead to system-wide tissue damage, organ injuries and death.1,2 In sepsis, there are two recognised opposing responses:3–8 a dominant systemic hyper-inflammatory response, which is characterised by leukocyte activation, cytokine storm and tissue-wide damage; and counter-regulatory mechanisms, which start simultaneously. These mechanisms in turn may lead to down-regulation of effector-cell functions, including T-cell exhaustion, defective Ag presentation and immunosuppression. The resulting massive immune suppression is paralleled by high mortality due to unresolved septic foci and secondary infections.3–8
Both monocytes and macrophages play a major role in orchestrating these host immune responses, detecting and phagocytosing pathogens opsonised with complement and/or Ig.9 Monocytes and macrophages also activate T cells by presenting bacterial Ags on major histocompatibility complex class II molecules. In addition, monocytes contribute to the cytokine storm by producing pro-inflammatory ILs and TNF-α, mainly via bacterial endotoxin-induced TLR4 signalling. Prolonged and repeated pathogen stimulation triggers endotoxin tolerance in the monocyte lineage, finally releasing anti-inflammatory factors and adapting the response to an immunosuppressive phenotype.10 Blood lymphocyte dysfunction during sepsis has also been reported with significant lymphopenia, including decreased T lymphocytes and NK cells.11
Sepsis represents a major health-care problem worldwide, and novel adjunctive therapies are much needed. Ig preparations comprising polyvalent IgG molecules alone (IVIG) have not shown clear benefits.12 However, a preparation containing pentameric IgM, Pentaglobin® (∼12% IgM, ∼12% IgA and ∼76% IgG), reduced mortality amongst adults with sepsis.12,13 These findings may be related to the observed hypogammaglobulinemia and reduced IgM levels in early-stage sepsis and severe infections.14–16 Relevant mechanisms of action that may be deficient in sepsis, due to reduced IgM concentrations in severely infected patients, include agglutination and opsonisation activity against bacteria contributing to pathogen recognition and clearance.17–19 Furthermore, IgM has a higher neutralisation capacity for bacterial endotoxins and influences the complement activity cascade more efficiently than IgG alone.17,20 The pentameric nature of IgM renders it very efficient in these processes.21
A novel polyclonal Ab preparation, trimodulin, containing ∼23% IgM, ∼21% IgA and ∼56% IgG, is being developed. Safety and efficacy of trimodulin has already been investigated in a randomised placebo-controlled trial as an add-on therapy in patients with severe community-acquired pneumonia requiring invasive mechanical ventilation.22 Although no significant difference was found in ventilator-free days and mortality between the trimodulin and the placebo group (albumin), post hoc analyses supported improved outcome regarding mortality with trimodulin in subsets of patients with elevated C-reactive protein, reduced IgM concentrations or both. C-reactive protein is a marker of inflammation and increases in response to bacterial infections. Improved outcomes of trimodulin-treated patients with reduced IgM concentrations at baseline support the important functions of this Ig preparation.
In this study, we investigated the immunomodulatory activity of trimodulin to gain insight into the potential utility of this preparation as a novel therapeutic agent in severe infections. Ex vivo experiments on whole blood from patients with sepsis and PBMCs from healthy donors were performed, including the endotoxaemia second-hit model. The model was used to reflect the onset, early phases and late phases of sepsis. It is based on the knowledge that the first endotoxin hit leads to a strong secretion of TNF-α and, additionally, in monocytes and macrophages, to an increased production of the TLR4-negative regulator, IL-1-receptor-associated-kinase M (IRAK-M). IRAK-M inhibits the release of IRAK/IRAK-4 from the TLR4/MD2 complex, blocking downstream signalling in monocytes via NF-κB.23 Despite a second endotoxin challenge, blockage of this intracellular signalling pathway prevents the new induction of TNF-α, which is a key event in immune suppression.24
Here we show that early intervention with trimodulin impedes on pro- and anti-inflammatory functions of monocytes and lymphocytes induced by endotoxins and provide insight into the nature of the immunomodulating activity of this unique preparation.
Materials and methods
Ethics statement
The study was approved by the local institutional Ethics Committee, Medical University of Vienna, Vienna, Austria (approval number: EK Nr. 581/2008), and written informed consent was obtained from all patients or their next of kin. The study was conducted according to the ethical principles for medical research involving human patients described in the Declaration of Helsinki.
Reagents
The novel polyclonal Ig preparation, trimodulin (∼23% IgM, ∼21% IgA and ∼56% IgG), was kindly provided by Biotest AG (Dreieich, Germany). Purified Escherichia coli LPS 0111:B4, Staphylococcus aureus-derived lipoteichoic acid (LTA), ConA and recombinant IL-2 were obtained from Sigma-Aldrich (St. Louis, MO).
Fluorescently labelled Abs for flow cytometry were purchased from the following manufacturers: anti-human leukocyte Ag–DR isotype (HLA-DR G46-6-PE) and anti-TNF-α (mAB11) from BD Biosciences (San Jose, CA); anti-TLR2 (CD282-PE) and anti-TLR4 (HTA125-PE) from eBioscience (San Diego, CA); anti-cluster of differentiation 16 (CD16; 3G8-PE), anti-CD64 (22-PC5), anti-CD11b (Bear1-PC5), anti-CD11c (BU15-PC5), anti-CD3 (UCHT1-PC7), anti-CD4 (13B8.2-PC5) and anti-CD8 (SFCI21Thy2D3-ECD) from Beckman Coulter (Fullerton, CA); and anti-CD14 (My4; FITC, PC5 or PC7 conjugated) from Beckman Coulter (Indianapolis, IN).
Collection and cultivation of blood samples from patients with sepsis
Heparinised whole blood was collected from eligible adult patients with sepsis admitted to the intensive-care unit of the Departments of Surgery (n = 10) and Internal Medicine (n = 5), General Hospital of Vienna, Austria (Table 1). Sepsis was defined according to the International Sepsis Definitions Conference criteria.25,26 Blood samples were obtained within 24 h (early) of, or ≥ 5 d (late) after, a diagnosis of sepsis. Eligible patients had not received chemotherapy or immunotherapy 4 wk prior to blood sample collection. Heparinised whole blood was cultured undiluted in 2 ml pyrogen-free tubes (Eppendorf AG, Hamburg, Germany).
Table 1.
Blood samples collected from patients with sepsis upon admission to the intensive-care unit.
| Parameter | Characteristics | |
|---|---|---|
|
Sample |
Early (≤ 24 h; n = 7) |
Late (≥ 5 d; n = 8) |
| Age (yr)a | 56.4 ± 14.3 | 62.3 ± 13.7 |
| Male/female | 5/2 | 4/4 |
| Leukocytes (×109/L)a,b | 18.3±2.9 | 17.2±1.5 |
| Diagnosis at admission | ||
| Septic shock | 1 | 1 |
| Peritonitis | 0 | 3 |
| Pneumonia | 2 | 2 |
| Mediastinitis | 1 | 1 |
| Septic-wound infection | 2 | 1 |
| Unknown | 1 | 0 |
Mean ± SD.
Leukocyte counts upon admission to the intensive-care unit.
Purification and cultivation of PBMCs from healthy donors and patients with sepsis
Fresh blood was collected from apparently healthy adult donors into lithium–heparin-coated tubes (VACUETTE®; Greiner Bio-One International GmbH, Kremsmünster, Austria). Whole blood was either directly cultivated or PBMCs were isolated using Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden) according to standard protocols. Approximately 2 × 106 PBMCs (or 1 × 106 for proliferation assays) were seeded onto 24-well plates and cultured in RPMI 1640 medium plus GlutaMAX™-I (GIBCO®; Invitrogen, Carlsbad, CA) supplemented with 10% FCS (Linaris GmbH, Wertheim, Germany). Toxins and trimodulin were added as described for individual investigations below. The medium was carefully exchanged before the second addition of endotoxins.24 Generally, experiments (e.g. ELISA) were performed in duplicate per donor, or according to specifications in the manuals provided with the different assays (see below).
Ex vivo endotoxaemia second-hit model
The endotoxaemia LPS/LTA second-hit model (endotoxin-tolerance model) was based on methods published previously for LPS and was conducted as depicted in Figure 1.27 The model reflects the onset, early phases (first hit) and late phases of sepsis (second hit), based on the knowledge that the first endotoxin hit leads to a strong secretion of TNF-α, which is inhibited via blockage of NF-κB signalling and impedes response after a second toxin challenge.
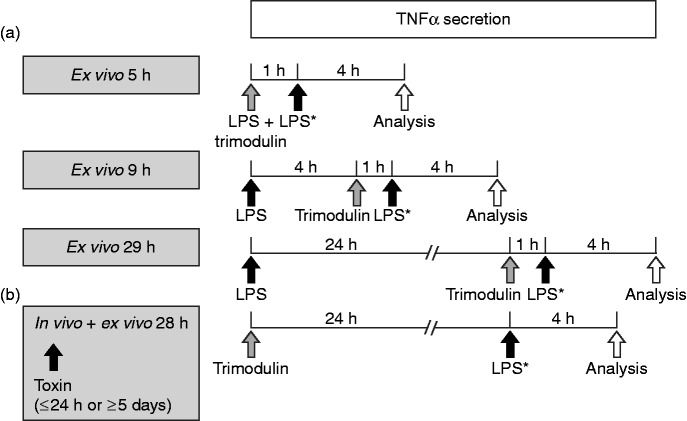
Figure 1.
The ex vivo endotoxaemia second hit model. (a) PBMCs or whole blood from healthy donors were primed with endotoxins (LPS: either 0.05 or 0.5 ng/ml LPS, or 1 or 10 µg/ml lipoteichoic acid (LTA) for 1, 4 or 24 h). Different trimodulin concentrations (0, 0.5 or 5 mg/ml) were added for 1 h either simultaneously or after 4 or 24 h of incubation with LPS/LTA. Medium was carefully removed, and cells were challenged with a second high endotoxin dose (LPS*: either 0.5 ng/ml LPS or 10 µg/ml LTA) for exactly 4 h prior to analysis of TNF-α in supernatants. (b) Blood cells from patients with sepsis were considered primed in vivo with bacterial endotoxins for either maximally 24 h (early) or minimally 5 d (late). Whole blood was obtained and treated directly with 0, 0.5 or 5 mg/ml trimodulin for 24 h before ex vivo challenge with a second high LPS* dose for exactly 4 h prior to analysis of TNF-α in plasma. Total endotoxin exposure time is indicated in the grey boxes.
The LPS concentrations inducing TNF-α responsiveness in PBMCs were determined in checkerboard experiments, and LTA concentrations were derived from literature.28 The trimodulin concentration used in these investigations did not exceed concentrations measured in patients upon administration.22 After treatment of PBMCs or whole blood, the supernatants or plasma, respectively, were collected and stored at –80°C until analysis.
Quantitative PCR analysis
PBMCs were treated according to the second-hit model, and RNA was isolated using the RNeasy Kit (Qiagen, Venlo, The Netherlands) according to the manufacturer’s instructions. A total of 400 ng RNA was reverse transcribed to cDNA using the ThermoScript RT-PCR System (Thermo Fisher Scientific, Waltham, MA). Quantitative (real-time) PCR of target cDNA was performed with the 7500 Fast Real-time PCR System (Thermo Fisher Scientific). All reagents and devices used for real-time PCR were obtained from Invitrogen. All primers were obtained from VBC Biotech (Vienna, Austria). Amplification of TLR2, TLR4 and TNF-α cDNA was normalised to amplification of 18S rRNA. Changes in transcript levels (ΔΔCt) were calculated by subtracting ΔCttreated control values (5 h LPS with or without trimodulin) from ΔCtuntreated sample values (no LPS or trimodulin) and fold change with 2ΔΔCt compared to non-treated cells.
Flow cytometry analysis
Prior to flow cytometric analysis of the cells, purified PBMCs from healthy donors were treated according to the one-hit protocol, as described in detail for this specific investigation below. After treatment, cells were harvested, and Ab incubation was performed on ice in the dark for 25 min. All cells were co-stained with anti-CD14 and washed twice with Dulbecco’s PBS without calcium and magnesium (DPBS–/–) containing 0.1% NaN3 and 1% FCS. To analyse various surface markers on monocytes, single samples with a total of 2500 CD14+ cells were analysed by flow cytometry (Beckman Coulter FC500 equipped with CXP software).
Determination of phagocytic activity
Whole blood from healthy donors and patients was treated according to the respective protocol, as shown below (phagocytosis experiments). Here, the second hit was not performed with purified LPS, but with fluorescently labelled E. coli cells. After pre-treatment with LPS (first hit), the isolated PBMCs from healthy donors were harvested, washed and incubated with non-opsonised FITC-labelled E. coli (second hit). Whole blood from patients was incubated directly with non-opsonised FITC-labelled E. coli cells (PHAGOTEST®; ORPEGEN Pharma, Heidelberg, Germany) or with endocytosis/acidification-sensitive pHrodo-conjugated E. coli bioparticles (pHrodo™ Red E. coli BioParticles™ Conjugate for Phagocytosis; Life Technologies, Carlsbad, CA).29 The percentage of phagocytic monocytes and phagocytic activity per cell were analysed by flow cytometry as recommended by the manufacturer.
Determination of oxidative-burst activity
Heparinised whole blood from healthy donors was treated for 4 h with LPS and subsequently stimulated for 1 h with various concentrations of trimodulin. Following the addition of non-opsonised, non-labelled E. coli (second hit), the percentage of phagocytic cells (granulocytes and monocytes) that produced reactive-oxygen metabolites and their mean fluorescence intensity were measured by flow cytometry (PHAGOBURST™; BD Biosciences, Heidelberg, Germany).
Proliferation assay
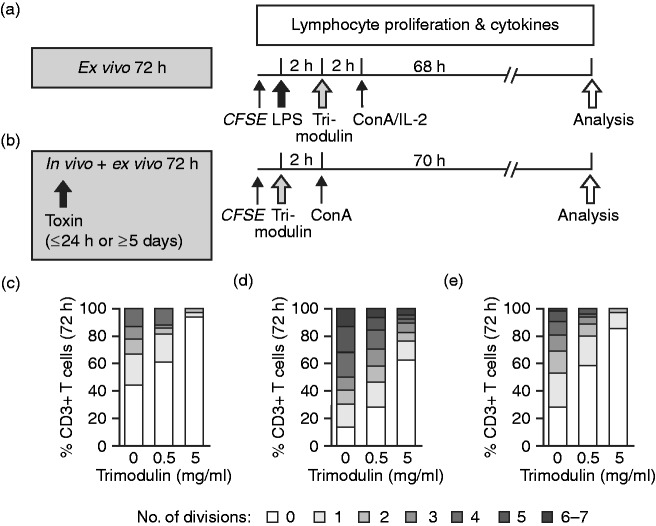
PBMCs from healthy donors and patients with sepsis were treated as described in detail for this specific investigation below. Cells were stained with 1 µM 5,6-carboxyfluorescein diacetate N-succinimidyl ester (CFSE; Sigma–Aldrich, Vienna, Austria) for 10 min at room temperature and washed with RPMI 1640 prior to 2 h stimulation with LPS (one hit, with healthy PBMCs only) and 2 h treatment with trimodulin. To induce proliferation of T lymphocytes in vitro, the cells were treated with ConA (10 µg/ml) and/or IL-2 (100 ng/ml).
To analyse the number of completed T-lymphocyte divisions after 72 h (according to the CFSE instructions), cells were co-stained with anti-CD3, anti-CD4 and anti-CD8. PBMCs were washed with DPBS–/– containing 0.1% NaN3 and 1% FCS and then analysed by flow cytometry. Completed cycles of division were defined by the progressive halving of the CFSE fluorescence intensity after each cell division and were gated manually on lymphocyte subpopulations for quantification.
Cytokine analysis
To explore the influence of trimodulin on TNF-α expression, PBMCs or whole blood from healthy donors or whole blood samples from patients with sepsis were treated according to the second-hit model, and were analysed for TNF-α by colorimetric sandwich ELISA (Human TNF-α Quantikine ELISA kit; R&D Systems, Minneapolis, MN). In further experiments, PBMCs from healthy donors and patients with early-stage sepsis were treated according to the one-hit protocol used to measure proliferation. Cell culture supernatants from these cultures were collected and stored at –80°C. Cytokines (IFN-γ, IL-10, IL-17A and TNF-α) were measured in supernatants using a commercially available cytometric bead array (Human Th1/Th2/Th17 Kit; BD Biosciences, Heidelberg, Germany).
Statistical analysis
Normal distribution was assumed and confirmed by analyses of PBMCs or blood samples from more than five donors using the Kolmogorov–Smirnov test.30 To compare treated and untreated donor PBMCs, a paired parametric t-test was applied if n > 3. Associations between trimodulin concentrations and cytokine secretion or surface-marker expression were analysed using paired one-way ANOVA with Greenhouse–Geisser correction.31 Pearson’s chi-square test was used to determine if the observed number of lymphocyte generations after trimodulin treatment was significantly different from the untreated control. Associations between cytokine concentration and trimodulin were graphically represented by a scatterplot and assessed by Spearman’s correlation coefficient (rs). Statistical analysis was performed by MS Excel (Microsoft Corp., Redmond, WA), SPSS Statistics for Windows v17.0 (SPSS, Inc., Chicago, IL) and GraphPad Prism (GraphPad Software, Inc., San Diego, CA). All calculated P-values are two-sided, and significance was set at P < 0.05.
Results
Trimodulin attenuates TNF-α secretion by blood cells from healthy donors after repeated endotoxin doses at the early stage
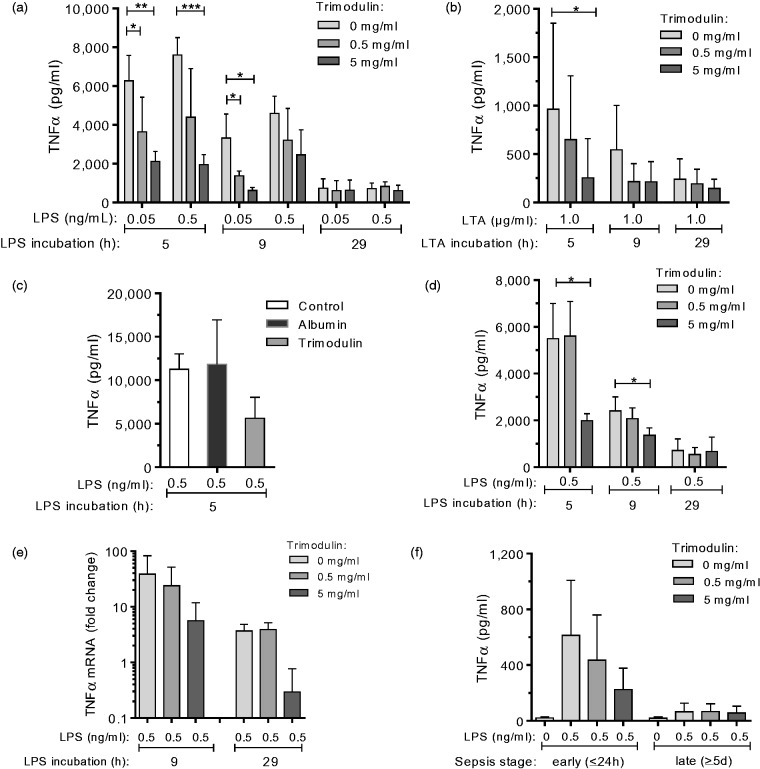
To explore the influence of trimodulin on expression of the pro-inflammatory cytokine TNF-α upon bacterial endotoxin stimulation, the ex vivo endotoxaemia second-hit model was used as depicted and described in detail in Figure 1. ELISA analysis of TNF-α secreted by PBMCs from healthy donors showed that its detection was dependent on LPS/LTA dose and incubation time (P ≤ 0.01, ANOVA; Figure 2a and b). The highest TNF-α levels (hyper-inflammatory stage) were observed after 5 h, and levels had clearly lowered after 29 h of endotoxin challenge (hypo-inflammatory stage). Trimodulin attenuated TNF-α levels (P ≤ 0.05, t-test) dose dependently and early during the first 5–9 h of endotoxin challenge (P ≤ 0.05, ANOVA). The effect on TNF-α secretion was not elicited by serum protein albumin if used in equimolar protein concentrations (Figure 2c). Albumin was used as a placebo control in the trimodulin clinical trial.22
Figure 2.
TNF-α secretion upon repeated endotoxin stimulation and trimodulin treatment. Purified PBMCs (a, n = 5; b, n = 7; c, n = 3) or whole blood (d, n = 5) from healthy donors were challenged with LPS (a, c and d) or LTA (b) and treated with trimodulin according to the second hit protocol shown in Figure 1(a). (c) Control experiment performed by simultaneous incubation of PBMCs (n = 3) with LPS, without (control) or with 5 mg/ml trimodulin, or with an equimolar concentration of albumin for 1 h, followed by a second LPS* hit for a total LPS incubation time of 5 h. (e) RT-PCR analysis showing fold change in TNF-α transcript levels in PBMCs (n = 3) from healthy donors upon treatment compared to non-treated cells (no LPS, no trimodulin). (f) Whole blood obtained from patients with sepsis in the early (n = 7) or late (n = 8) phase was treated with trimodulin and with 0.5 ng/ml LPS* or without endotoxins (control) as shown in Figure 1b. (a–d and f) The TNF-α concentration in PBMC supernatants or in plasma from whole blood was analysed by ELISA. Bars represent mean ± SD. Differences versus control (0 mg/ml trimodulin) are indicated with asterisks (*P ≤ 0.05, **P ≤ 0.01 and **P ≤ 0.005 using a parametric paired t-test). Paired one-way ANOVA showed strong correlations (a, P ≤ 0.001; b, P ≤ 0.05) between trimodulin dose (5 and 9 h) and TNF-α detection.
Similar results to those seen with purified PBMCs were obtained using whole blood samples from healthy donors (Figure 2d). This was important, since experiments with patients samples (below) could only be done with whole blood due to volume limitations.
RT-PCR analysis revealed that the regulation of TNF-α, by LPS and increasing concentrations of trimodulin, occurs on the transcriptional level (Figure 2e), confirming the observed reduction in the secretion of TNF-α (Figure 2a).
Trimodulin attenuates TNF-α secretion by blood cells from patients with early-stage sepsis after repeated endotoxin doses
The impact of trimodulin on TNF-α secretion by blood samples from healthy donors (Figure 2d) was compared to the reactivity of in vivo endotoxin-primed samples from patients with early- and late-stage sepsis. Whole blood was collected from eligible patients with sepsis. Baseline characteristics of patients with early (≤ 24 h; n = 7) and late (≥ 5 d; n = 8) sepsis are shown in Table 1.
The TNF-α level secreted by in vivo and ex vivo endotoxin-stimulated cells obtained during early-stage sepsis was attenuated (P = 0.0570, t-test) with 5 mg/ml trimodulin compared to cells not treated with trimodulin (Figure 2f). Cells from patients with sepsis obtained within 24 h after diagnosis were responsive to endotoxin and trimodulin.
During late-stage sepsis, TNF-α levels (16–154 pg/ml) were not altered by trimodulin treatment (16–132 pg/ml) and were more than eightfold lower when compared to early-stage sepsis (134–1238 pg/ml). During late-stage sepsis, cells were thus non-responsive to LPS and trimodulin. Furthermore, the TNF-α level with ex vivo repeatedly stimulated healthy PBMCs (Figure 2a and b) or whole blood (Figure 2d) was, after 29 h, similar to the TNF-α levels observed in whole blood samples from patients with late-stage sepsis (Figure 2f, late), supporting the underlying mechanism of the endotoxaemia model.
Trimodulin down-regulates mRNA expression of TLRs in monocytes after repeated LPS doses
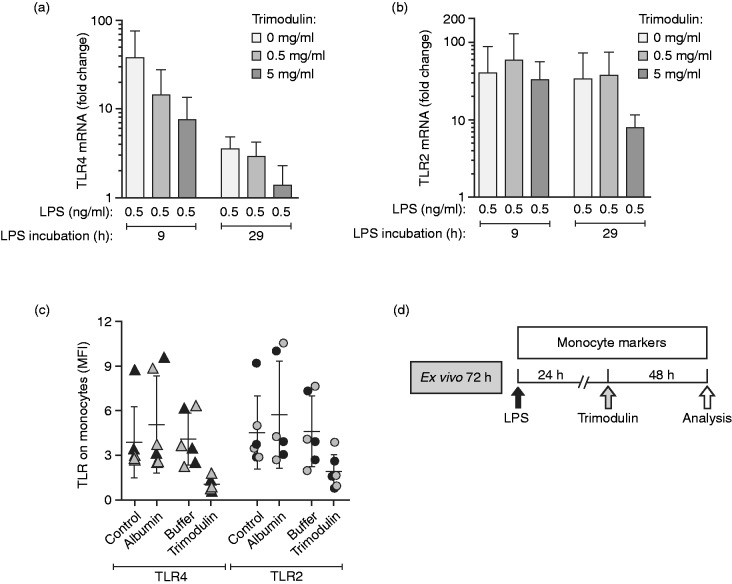
The second-hit experiments were further extended to gain more insight into the receptor-dependent regulation of TNF-α secretion by LPS and trimodulin treatment. RT-PCR analysis of TLR4 (LPS receptor, Figure 3a) and TLR2 (LTA receptor, Figure 3b) showed that 9 or 29 h of LPS stimulation alone caused a mean 32- and 3.6-fold increase in TLR4-mRNA and a 40- and 34-fold increase in TLR2-mRNA, respectively, compared to untreated cells. The LPS-induced up-regulation of TRL4-mRNA after 9 or 29 h was clearly less pronounced after trimodulin treatment (7.6- and 1.3-fold, respectively) and supports the endotoxin neutralisation activity of trimodulin. Down-regulation of TLR2 (at 29 h) and TLR4 revealed through RT-PCR was confirmed by flow cytometry; a decrease in cell-surface TLR2 and TLR4 was seen on monocytes in this second-hit model (Figure 3c).
Figure 3.
Regulation of TLR expression in monocytes. (a–c) PBMCs from healthy donors (n = 3) were treated according to the second-hit model depicted in Figure 1a for 9 or 29 h without endotoxin (control) or with 0.5 ng/ml LPS and LPS*, and with or without trimodulin. (a) RT-PCR analysis showing fold change in TLR4 mRNA and (b) TLR2 mRNA compared to untreated cells (no LPS, no trimodulin) and analysed after the second LPS* hit. (c) Detection of TLR2 and TLR4 on monocytes after 9 h (black symbols) or 29 h LPS/LPS* stimulation (grey symbols) including either 5 h albumin, formulation buffer or trimodulin treatment, or without further additives (control). (d) Single endotoxin dose (one hit) model representing treatment of early inflammation (24 h) with trimodulin. This model was used for experiments presented in Tables 2 and 3. PBMCs from healthy donors were stimulated with 0, 0.05 or 0.5 ng/ml LPS, or 0, 1 or 10 µg/ml LTA. After 24 h of LPS/LTA, different trimodulin concentrations (0, 0.5 or 5 mg/ml) were added for 48 h prior to analysis of cells by flow cytometry (total incubation time: 72 h). Bars in a–c represent mean (n = 3 ± SD).
Trimodulin attenuates TLR4 on monocytes if applied early after a single endotoxin dose
The effect of trimodulin on the monocyte phenotype, if applied in the early endotoxin-induced hyper-inflammatory phase, was investigated in more detail (Figure 3d). PBMCs from healthy donors were stimulated with or without a single endotoxin dose, followed by early treatment at 24 h with or without trimodulin for an extended period of 48 h. The expression of TLR2 and TLR4 on CD14+ monocytes upon LPS or LTA stimulation was assessed by flow cytometry (Tables 2 and 3). Upon endotoxin stimulation alone, surface expression of these TLRs was largely unchanged. However, TLR2 and TLR4 decreased markedly and dose dependently upon the addition of trimodulin. This effect was most evident with LPS stimulation. These data are in agreement with the TLR4- and TLR2-mRNA results shown in Figure 3a and b. Reduced TLR4 expression was not observed after treatment with the control protein, albumin (data not shown).
Table 2.
Detection of surface markers on CD14+ monocytes after LPS and trimodulin treatment.
| LPSa (ng/ml) | Trimodulinb (mg/ml) | TLR2c | TLR4c | CD64c | CD11bc | HLA-DRc |
|---|---|---|---|---|---|---|
| 0 | 5.0 ± 0.6 | 3.5 ± 1.6 | 7.5 ± 1.5 | 63.3 ± 8.3 | 204.4 ± 59.2 | |
| 0 | 0.5 | 2.8 ± 0.3*** | 1.6 ± 0.3* | 5.9 ± 2.8 | 58.2 ± 7.7 | 170.2 ± 40.3 |
| 5 | 1.8 ± 0.2*** | 1.5 ± 0.6* | 13.2 ± 5.5 | 50.3 ± 14.3 | 137.6 ± 22.4* | |
|
| ||||||
| 0 | 8.5 ± 3.8 | 5.1 ± 3.3 | 10.2 ± 4.2 | 70.2 ± 18.3 | 115.0 ± 43.6 | |
| 0.05 | 0.5 | 4.4 ± 2.0 | 1.6 ± 0.6* | 5.6 ± 1.6 | 71.5 ± 18.3 | 128.5 ± 32.6 |
| 5 | 2.8 ± 1.0* | 0.9 ± 0.3* | 11.1 ± 2.4 | 55.5 ± 14.8 | 102.6 ± 38.6 | |
|
| ||||||
| 0 | 8.2 ± 0.3 | 3.3 ± 1.6 | 46.4 ± 9.0 | 57.9 ± 12.7 | 42.5 ± 15.8 | |
| 0.5 | 0.5 | 5.0 ± 0.7*** | 1.4 ± 0.6* | 25.7 ± 3.1** | 58.5 ± 12.5 | 42.2 ± 16.6 |
| 5 | 3.4 ± 1.1*** | 0.9 ± 0.5* | 30.1 ± 4.0** | 38.0 ± 11.0* | 42.9 ± 15.5 | |
Total endotoxin treatment time (72 h) on healthy donor PBMCs.
Treatment 24 h after initiation of LPS stimulation.
Mean +/− SD values were obtained from five donors (values represent mean channel fluorescence intensity).
*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 showing the differences between trimodulin treated and untreated control (two-tailed t-test).
Bold numbers: increase or decrease is different with P ≤ 0.05 for LPS stimulation alone compared to unstimulated cells (two-tailed t-test).
Table 3.
Detection of surface markers on CD14+ monocytes after LTA and trimodulin treatment.
| LTAa (µg/ml) | Trimodulinb (mg/ml) | TLR2 (n = 6)c | TLR4 (n = 4)c | CD16 (n = 6)c | CD64 (n = 6)c | CD11b (n = 5)c | CD11c (n = 4)c | HLA-DR (n = 5)c |
|---|---|---|---|---|---|---|---|---|
| 0 | 4.9 ± 2.4 | 3.2 ± 2.0 | 50.0 ± 21.8 | 19.6 ± 4.7 | 142.5 ± 31.6 | 126.5 ± 18.6 | 193.6 ± 85.5 | |
| 0 | 0.5 | 3.3 ± 0.8 | 1.8 ± 0.5 | 57.8 ± 34.4 | 29.7 ± 12.2 | 136.1 ± 24.9 | 138.9 ± 8.3 | 142.4 ± 73.3 |
| 5 | 2.6 ± 1.2 | 1.9 ± 0.5 | 40.2 ± 15.6 | 46.1 ± 23.8* | 94.9 ± 20.6* | 123.5 ± 5.3 | 107.1 ± 44.0 | |
|
| ||||||||
| 0 | 4.7 ± 2.0 | 2.5 ± 0.5 | 67.4 ± 30.5 | 19.7 ± 7.4 | 127.9 ± 27.3 | 130.5 ± 17.4 | 143.8 ± 80.5 | |
| 1 | 0.5 | 3.1 ± 1.3 | 2.2 ± 0.9 | 53.8 ± 22.2 | 15.6 ± 6.1 | 115.6 ± 1 9.3 | 131.5 ± 3.8 | 130.4 ± 64.6 |
| 5 | 2.2 ± 1.0* | 1.4 ± 0.6* | 45.6 ± 5.5 | 32.2 ± 16.5 | 85.7 ± 10.0* | 104.4 ± 12.0* | 117.9 ± 44.2 | |
|
| ||||||||
| 0 | 7.1 ± 3.4 | 2.6 ± 1.6 | 128.9 ± 54.1 | 93.8 ± 41.8 | 55.3 ± 31.7 | 59.4 ± 5.3 | 24.6 ± 16.7 | |
| 10 | 0.5 | 4.6 ± 1.6 | 1.1 ± 0.5 | 81.6 ± 27.2 | 66.7 ± 29.7 | 67.4 ± 21.9 | 63.2 ± 8.9 | 26.7 ± 13.6 |
| 5 | 3.5 ± 1.3* | 0.9 ± 0.3 | 36.0 ± 5.2** | 54.2 ± 32.6 | 35.8 ± 12.9 | 49.4 ± 6.5 | 30.3 ± 17.6 | |
Total endotoxin treatment time (72 h) on healthy donor PBMCs.
Treatment 24 h after initiation of LTA stimulation.
Mean +/− SD values were obtained from four to six donors (values represent mean cell fluorescence).
*P ≤ 0.05 and **P ≤ 0.01 statistically different compared to trimodulin untreated control (two-tailed t-test).
Bold numbers: increase or decrease is different with P ≤ 0.05 for LTA stimulation alone compared to unstimulated cells (two-tailed t-test).LTA: lipoteichoic acid.
Trimodulin modulates surface markers on monocytes if applied early after a single endotoxin dose
Fcγ-RI–III expressed on monocytes mediate Ab-dependent cellular cytotoxicity and phagocytosis activities. Their surface expression was investigated in the early inflammatory model according to Figure 3d. The level of expression of CD64 (Fcγ-RI, a high-affinity receptor for IgG1 and IgG3) on PBMCs from healthy donors markedly increased five- to sixfold (P ≤ 0.01) upon strong LPS/LTA stimulation alone (0.5 ng/ml LPS or 10 µg/ml LTA) or trimodulin treatment alone. Interestingly, only high expression levels of CD64 on monocytes due to strong endotoxin induction on monocytes were counterbalanced by trimodulin treatment (LPS; P ≤ 0.01; Tables 2 and 3). Similarly, the increase in expression of CD16 (Fcγ-RIII) by LTA was reduced markedly by trimodulin (P ≤ 0.01).
Expression of complement receptors on endotoxin-activated monocytes was analysed. Levels of both CD11b and CD11c were decreased upon endotoxin stimulation alone, which was further reduced by trimodulin (P ≤ 0.05; Tables 2 and 3).
Trimodulin markedly reduced HLA-DR expression on CD14+ monocytes in a dose-dependent manner in the absence of endotoxins (P ≤ 0.05; Tables 2 and 3). No additive down-regulation was observed with trimodulin over that seen with LPS/LTA activation. Monocytes from patients with early and late stage sepsis showed a slight dose-dependent increase in HLA-DR expression upon LPS treatment, which was not significant (data not shown). However, more detailed experiments are required to verify these results further.
No trimodulin-mediated alterations in expression levels of CD32b, CD35 and CD71 were observed (data not shown).
Antibacterial functions of endotoxin-activated monocytes and granulocytes are not affected by trimodulin
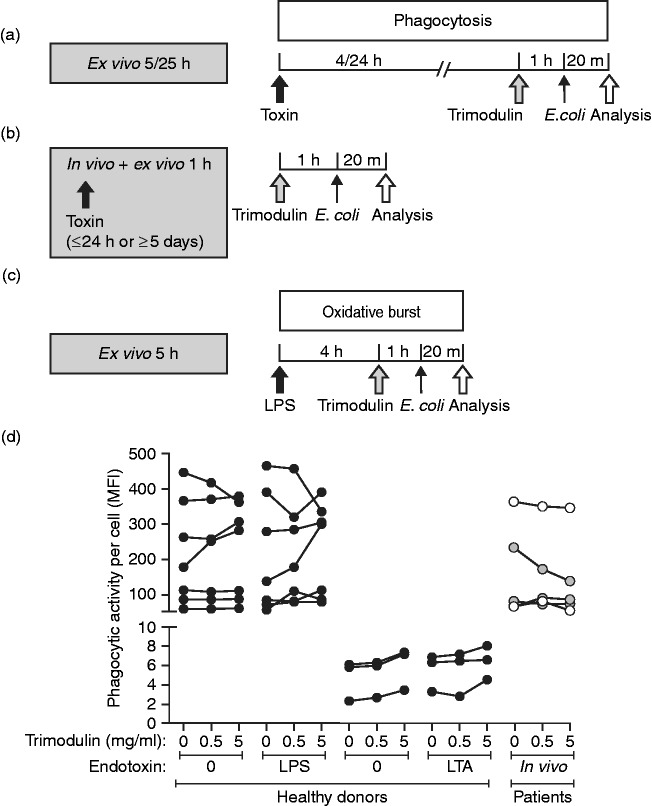
The effect of trimodulin on the phagocytic activity of monocytes before and after LPS/LTA stimulation was assessed. In vitro phagocytosis occurs quickly after opsonisation, and the treatment protocol was adjusted accordingly (Figure 4a). Whole blood from healthy donors was treated 24 h ex vivo with LPS or LTA, followed by 1 h of treatment with trimodulin (early inflammation model; Table 4). Whole blood samples from patients with sepsis were also treated with trimodulin for 1 h, but without additional ex vivo LPS or LTA pre-stimulation (Figure 4b and d). LPS, LTA and trimodulin did not have an overall reducing effect on the phagocytic activity of monocytes obtained from healthy donors or patients with sepsis (Figure 4d). Phagocytosis was, for most donors and patients, largely unchanged (Figure 4d).
Figure 4.
Phagocytosis and oxidative-burst activity in monocytes and granulocytes. (a) Whole blood of healthy donors was treated with 0, 0.05 or 0.5 ng/ml LPS or 0, 1 or 10 µg/ml LTA. After 4 or 24 h of endotoxin stimulation, different trimodulin concentrations (0, 0.5 or 5 mg/ml) were added for 1 h. (b) Whole blood of patients was treated with trimodulin without ex vivo LPS/LTA stimulation. (a and b) Cells were harvested and incubated with 20 µl FITC-(LPS) or pHrodo-conjugated E. coli cells (LTA) for 20 min before analysis in flow cytometry. (c) Whole blood of healthy donors was stimulated with 0, 0.05 or 0.5 ng/ml LPS for 4 h, and then treated with or without trimodulin (5.0 mg/ml) for 1 h before the addition of 20 µl non-labelled E. coli. After 20 min of staining, cells were washed and analysed by flow cytometry. (d) Before–after plot of phagocytic activity measured in monocytes from seven or three healthy donors (black circles), three patients with early stage sepsis (grey circles) and two patients with late-stage sepsis (open circles) without or with trimodulin treatment. Results are shown for FITC-labelled E. coli with or without 0.5 ng/ml LPS for 24 h, or for pHrodo-conjugated E. coli without or with 10 µg/ml LTA for 4 h. A paired parametric t-test revealed no difference between untreated and 0.5 mg/ml trimodulin-treated cells from healthy donors without LPS (P = 0.6527) or with LPS (P = 0.6205), nor for cells from patients with sepsis (P=0.3167).
Table 4.
Phagocytosis and oxidative-burst activity of immune cells after endotoxin and trimodulin treatment.
| Trimodulin (mg/ml) | Phagocytosisa,b |
Oxidative bursta,c |
||||||
|---|---|---|---|---|---|---|---|---|
| 24 h LTA+1 h trimodulin
(pHrodo-conjugated E.
coli) |
24 h LPS+1 h trimodulin
(FITC-labelled E. coli) |
Reactive oxidant-positive
monocytes |
Enzymatic activity |
|||||
| LTA (µg/ml) | % of cells (n=3)d | LPS (ng/ml) | % of cells (n=7) | LPS (ng/ml) | % of cells (n=5) | Monocytes (n=5) | Granulocytes (n=5) | |
| 0 | 0 | 7.7 ± 2.1 | 0 | 92.0 ± 4.8 | 0 | 45.9 ± 14.6 | 2.3 ± 1.4 | 3.8 ± 1.5 |
| 0.5 | 0 | 7.5 ± 2.3 | 0 | 91.6 ± 5.4 | 0 | n.d. | n.d. | n.d. |
| 5 | 0 | 6.5 ± 3.0 | 0 | 90.8 ± 4.6 | 0 | 65.0 ± 11.6 | 3.1 ± 1.2 | 7.3 ± 1.7* |
| 0 | 1 | 7.0 ± 2.0 | 0.05 | 88.5 ± 6.2 | 0.05 | 56.2 ± 9.4 | 2.6 ± 1.3 | 5.3 ± 0.7 |
| 0.5 | 1 | 7.3 ± 2.2 | 0.05 | 89.8 ± 7.5 | 0.05 | n.d. | n.d. | n.d. |
| 5 | 1 | 6.6 ± 2.9 | 0.05 | 88.0 ± 7.4 | 0.05 | 60.2 ± 10.5 | 3.5 ± 1.1 | 6.6 ± 0.7 |
| 0 | 10 | 7.6 ± 1.2 | 0.5 | 91.0 ± 9.3 | 0.5 | 63.6 ± 12.4 | 2.8 ± 1.3 | 5.4 ± 1.1 |
| 0.5 | 10 | 7.7 ± 1.1 | 0.5 | 91.3 ± 9.5 | 0.5 | n.d. | n.d. | n.d. |
| 5 | 10 | 7.0 ± 1.8 | 0.5 | 91.6 ± 6.8 | 0.5 | 70.8 ± 10.6 | 3.8 ± 1.7 | 7.5 ± 2.1 |
Activities measured in whole blood samples.
Phagocytosis measured according to Figure 4a.
Oxidative burst after 24 h of LPS stimulation of whole blood and 1 h of trimodulin treatment (Figure 4c).
Mean and SD values obtained from a number (n) of donors as indicated.
*One condition comparison between untreated and trimodulin-treated cells resulted in P ≤ 0.05 (t-test). All data are mean (SD) unless otherwise specified.
n.d.: not determined.
To study oxidative-burst activity in monocytes and granulocytes, whole blood of healthy donors was treated as shown in Figure 4c. A slight, but not significant, increase in oxidative activity upon trimodulin treatment alone was observed for both monocytes and granulocytes (Table 4). The results imply that the antibacterial functions of the monocytes and granulocytes were not negatively influenced by endotoxins and trimodulin treatment.
Trimodulin impedes LPS-driven T lymphocyte proliferation
During sepsis high endotoxin concentrations induce a cytokine storm, which leads to accelerated polyclonal T cell proliferation in the acute phase and to T-cell exhaustion and immune suppression at the late stage.6 The influence of trimodulin on expansion of CFSE-stained T lymphocytes after a single LPS hit from healthy donors and patients was investigated (Figure 5a and b). After 72 h in culture, trimodulin dose dependently reduced the number of cell divisions completed by LPS-stimulated CD3+ T lymphocytes from healthy donors (P ≤ 0.01). The percentage of CD3+-resting T lymphocytes in cultures pre-stimulated with LPS increased from 44% to 61% and 94% upon the addition of 0.5 and 5 mg/ml trimodulin, respectively (Figure 5c). Upon LPS/ConA/IL-2 stimulation, about 10% of CD3+ T lymphocytes completed up to four cell divisions in 72 h; by contrast, >95% of trimodulin-treated cells did not divide more than once. Similar results were obtained with the CD3+/CD4+ and CD3+/CD8+ T lymphocyte subpopulations.
Figure 5.
Proliferation analysis of T lymphocytes. (a) PBMCs from healthy donors (n = 3) were stained with CFSE before stimulation with 0.5 ng/ml LPS for 2 h and treatment with 0, 0.5 or 5 mg/ml trimodulin for 2 h. Subsequently, IL-2 (100 ng/ml) and/or ConA (10 µg/ml) were added to induce proliferation of T cells. (b) PBMCs from patients with early (n = 3) or late (n = 3) sepsis were treated only with trimodulin and ConA at the same concentrations as in (a). (c–e) After a total ex vivo incubation time of 72 h, (c) healthy donor PBMCs or PBMCs from patients with (d) early or (e) late-stage sepsis were analysed by flow cytometry to determine the percentage of CD3+ T lymphocytes that had divided up to seven times. Chi-square test, P<0.0001.
Interestingly, upon treatment with an increasing trimodulin dose (0, 0.5 or 5 mg/ml), similar ex vivo enrichments in the percentages of CD3+-resting T lymphocytes were observed with PBMCs from patients in the early (n = 3; from 12% to 27% and 61%; Figure 5d) and late (n = 3; from 27% to 59% and 84%) phases of sepsis (Figure 5e). Untreated cells from late-stage sepsis showed a twofold increase in resting cells compared to cells from patients with early-stage sepsis, which might in part reflect exhaustion occurring during late-stage sepsis.
Trimodulin dampens endotoxin-induced cytokine secretion
The early phase of sepsis is characterised by an initial intense inflammatory response involving both pro- and anti-inflammatory cytokines (cytokine storm) secreted by activated monocytes and expanding T lymphocytes. Since trimodulin modulates monocyte and lymphocyte functions, cytokine patterns were analysed in the cell-culture supernatants of PBMCs cultivated according to Figure 5a and b.
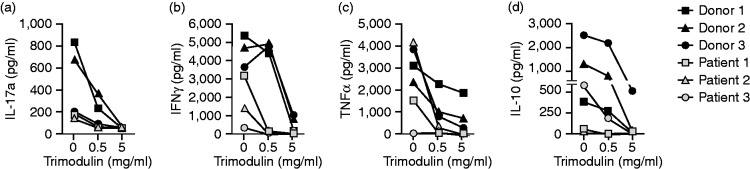
In PBMCs from healthy donors pre-stimulated with LPS ex vivo, a noteworthy negative correlation was observed between trimodulin dose and levels of the pro-inflammatory cytokines IL-17a (rs = –0.843, P = 0.004) and IFN-γ (rs = –0.685, P = 0.042), and also in this experimental setting for TNF-α (rs = –0.843, P = 0.004; Figure 6a–c). Additionally, a clear (72 h; rs = –0.632, P=0.068) and relevant (120 h; rs = –0.791, P=0.011; data not shown) reduction was detected in the anti-inflammatory cytokine, IL-10, with trimodulin treatment (Figure 6d), in contrast to IVIG, which was shown to increase IL-10.32 Levels for IL-4 and IL-6 were below and above detection thresholds, respectively (data not shown).
Figure 6.
Cytokines expressed by PBMCs. Plots showing bead-array results of cytokine levels detected in supernatants of PBMC cultures from healthy donors and patients with early-stage sepsis. PBMCs were treated as shown in Figure 5a and b with 0.5 ng/ml LPS and increasing trimodulin concentrations (grey symbols: healthy donors; n = 3) or after trimodulin treatment alone (black symbols: patients with early stage sepsis; n = 3).
Similarly, ex vivo cytokine release of PBMCs from patients with early-stage sepsis showed a negative correlation between trimodulin dose and IL-17a (rs = −0.896, P=0.001), TNF-α (rs = –0.798, P = 0.010), IFN-γ (rs = –0.908, P=0.001) and IL-10 (rs = –0.751, P=0.020). The levels of the cytokines analysed were lower in PBMCs from patients with sepsis than in PBMCs from healthy donors. Furthermore, one patient did not show the cytokine storm, possibly because they were in an early septic stage (low LPS blood level) or already in an immune-suppressed stage.
Discussion
In this study, we present new insights into the underlying immunomodulatory mechanisms of trimodulin on the reactivity of cells in purified PBMCs and whole blood samples, with reference to sepsis. A translational approach, such as that employed here, is essential for the careful evaluation of biological plausibility and mode-of-action elucidation for this investigational immunomodulatory therapy. In addition, results from such studies may help to identify strategies for determining which patients are most likely to benefit from trimodulin.
Our results provide insights into the mechanism of action of trimodulin. First, trimodulin was shown to down-regulate endotoxin-induced secretion of pro- and anti-inflammatory cytokines, including TNF-α, IL-17a, IFN-γ and IL-10 (Figures 2 and 5). In contrast to IVIG, IL-10 was down-regulated by trimodulin.33 Second, down-regulation of the complement receptors, CD11b (LPS and LTA) and CD11c (LTA), by high endotoxin concentrations was further enhanced by trimodulin (Tables 2 and 3). CD11b and CD11c mediate inflammation by regulating leukocyte adhesion and migration, phagocytosis, cytotoxicity and chemotaxis, and are able to bind the inactivated complement component, iC3b. Early trimodulin treatment thus seems to attenuate such pro-inflammatory responses. As shown previously, the IgM containing Ig preparation Pentaglobin® prevented complement deposition more effectively compared with IVIG.34 For other sepsis studies, early intervention with Pentaglobin® was also important for improved efficacy.35–37
Furthermore, cell-surface markers on monocytes, including CD64, CD16, TLR2 and TLR4, were induced with high endotoxin concentrations, but were counterbalanced by trimodulin (Tables 2 and 3). Our analysis showed that the regulation of TLRs by endotoxins and trimodulin occurred at the mRNA level, resulting in up- and down-regulation, respectively, of TLR2 and TLR4 proteins on the monocyte surface (Figure 3).
For CD64 (and CD16), up-regulation was observed upon LPS and LTA treatment alone, and the same was true for monocytes treated with trimodulin alone (Tables 2 and 3). Although trimodulin treatment alone caused up-regulation of CD64, and oxidative burst was nearly doubled, the combination of endotoxin and trimodulin did not result in an additive effect, but resulted in counterbalancing the expression level of CD16 and CD64. The phagocytic and oxidative-burst activities of the monocytes were not related to CD16/CD64 expression. The activities were largely unchanged upon endotoxin and trimodulin treatment, indicating that these important functions are not negatively influenced by trimodulin (Table 4 and Figure 4d). This finding is in agreement with an in vivo study showing no change in oxidative-burst activity of polymorphonuclear leukocytes after trimodulin treatment.38 Trimodulin appears to reduce the disproportionate expression of the CD64 receptor, which might prevent excessive release of pro-inflammatory cytokines induced by this receptor (Figures 2 and 5).39 Thus, our ex vivo experiments demonstrated that early intervention with trimodulin impedes various important pro-inflammatory functions of monocytes and lymphocytes induced by endotoxins.
It was postulated that extended LPS/LTA stimulation leads to an increase in IRAK-M inhibitory protein, resulting in the steady suppression of TNF-α during the late stages of sepsis.40,41 Trimodulin might prevent such immune suppression, since it prevents the initial hyperstimulation by endotoxins. IgG-based IVIG and IgM-enriched Pentaglobin® contain inhibitory or neutralising Abs against super Ag exotoxins released by Staphylococci and Streptococci.15,42–47 In contrast to IVIG, the IgM-enriched preparation Pentaglobin® contains superior concentrations of neutralising Abs against E. coli, Klebsiella species and Pseudomonas aeruginosa LPS.46,48,49 In accordance, recent publications have reported a benefit with Pentaglobin® in endotoxin-mediated diseases such as toxic shock syndrome and in severe infections with multidrug-resistant pathogens.18,50–52
In addition to its effects on monocytes, trimodulin was found to impede T-cell proliferation, which may counteract the sepsis-induced phenomenon of immune anergy in vivo (Figure 5). This impediment may be caused indirectly by the decrease in the cytokine storm induced by endotoxin-activated monocytes (TNF-α) and lymphocytes (IL-17a and IFN-γ) in the presence of trimodulin (Figure 6). Upon infection in the early phase, IFN-γ and IL-6 are produced by naïve T cells activating the differentiation and proliferation of Th1 and Th17 cells while inhibiting Th2 proliferation. The following counter-regulatory, anti-inflammatory state, characterised by a reduction in IFN-γ and IL-17a, might be co-responsible for the observed shift from Th1/Th17 to the Th2/Treg phenotype associated with poor prognosis in patients with sepsis. Trimodulin, if applied early, may prevent this cytokine storm and possibly the shift in T-cell phenotypes. Similarly for IVIG, it has been postulated that the modulation of T-cell populations and functions observed in treated patients was the indirect consequence of a direct effect of IVIG on accessory cells.53–55 Again, this suggests a window of opportunity for trimodulin during early-stage sepsis. Importantly, if trimodulin was added to cells during late-stage sepsis, suppression of proliferation was even stronger than observed during early sepsis. The administration of trimodulin at a time point that is too late may be immunosuppressive and may not support patient cure. Previous variable timing of therapy thus may also explain the inconsistent efficacy results of IVIG and Pentaglobin® in sepsis studies.12
In addition to modulating endotoxin-induced responses due to endotoxin-neutralising Abs, this study shows that trimodulin has additional immunomodulatory activity. A direct anti-inflammatory effect of trimodulin was observed by a dose-dependent reduction in cytokines (TNF-α, IL-17a and IFN-γ; data not shown) and by the down-regulation of TLR2/4, HLA-DR and CD64 on monocytes in the absence of endotoxins (Tables 2 and 3). This direct anti-inflammatory effect of Ig preparations is in accordance with previous studies with IVIG showing the dampening effect on TNF-α, for example in rheumatoid arthritis and Kawasaki disease.56
There are limitations of this model and study data in that the potential of trimodulin to modulate cellular processes ex vivo is not evidence of in vivo activity in patients. Nonetheless, these results are supported by clinical findings showing that the early application of IgM-enriched Ig preparation was associated with a survival benefit.36,57–59 The present study has revealed a possible rationale for this observation in that the immunoregulatory effect seen with trimodulin is determined by the strength and duration of endotoxin stimulation: strong, short-term stimulation, either ex vivo or in vivo, resulted in a greater dampening effect by trimodulin but, importantly, without completely blocking the immune response. After extended and repeated endotoxin challenge or in the late phase of sepsis, cytokine secretion by hyporeactive monocytes was not affected by trimodulin.
We hypothesise that targeting ‘upstream’ mediators preventing early induction of the hyper-inflammatory stage may reduce the risk of driving the suppression of ‘downstream’ mediators in the following phase, resulting in immune suppression. Our findings offer a mechanistic rationale for Berlot’s thesis that we should not miss ‘the window of opportunity in the first days that follow sepsis diagnosis’.35,36,57
Acknowledgements
This study was sponsored by Biotest AG (Dreieich, Germany). Professional editorial assistance was provided by Fiona Boswell, at Caudex and was funded by Biotest AG (Dreieich, Germany).
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: AS received a fee based on lectures for Biotest AG.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by Biotest AG (Dreieich, Germany).
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ 2016; 353: i1585. [DOI] [PubMed] [Google Scholar]
- 3.Jaffer U, Wade RG, Gourlay T. Cytokines in the systemic inflammatory response syndrome: a review. HSR Proc Intensive Care Cardiovasc Anesth 2010; 2: 161–175. [PMC free article] [PubMed] [Google Scholar]
- 4.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011; 306: 2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost 2009; 101: 36–47. [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013; 13: 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol 2018; 14: 121–137. [DOI] [PubMed] [Google Scholar]
- 8.Tamayo E, Fernandez A, Almansa R, et al. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur Cytokine Netw 2011; 22: 82–87. [DOI] [PubMed] [Google Scholar]
- 9.Santoni G, Morelli MB, Amantini C, et al. ‘ Immuno-transient receptor potential ion channels’: the role in monocyte- and macrophage-mediated inflammatory responses. Front Immunol 2018; 9 : 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 2009; 30: 475–487. [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003; 348: 138–150. [DOI] [PubMed] [Google Scholar]
- 12.Alejandria MM, Lansang MA, Dans LF, et al. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev 2013; CD001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreymann KG, De Heer G, Nierhaus A, et al. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med 2007; 35: 2677–2685. [PubMed] [Google Scholar]
- 14.Justel M, Socias L, Almansa R, et al. IgM levels in plasma predict outcome in severe pandemic influenza. J Clin Virol 2013; 58: 564–567. [DOI] [PubMed] [Google Scholar]
- 15.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 2010; 10: 778–786. [DOI] [PubMed] [Google Scholar]
- 16.Esen F, Tugrul S. IgM-enriched immunoglobulins in sepsis In: Vincent JL. (ed) Yearbook of intensive care and emergency medicine. New York: Springer-Verlag, 2009, pp.102–110. [Google Scholar]
- 17.Langereis JD, Henriet SS, Kuipers S, et al. IgM augments complement bactericidal activity with serum from a patient with a novel CD79a mutation. J Clin Immunol 2018; 38: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossmann FS, Kropec A, Laverde D, et al. In vitro and in vivo activity of hyperimmune globulin preparations against multiresistant nosocomial pathogens. Infection 2015; 43: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stehr SN, Knels L, Weissflog C, et al. Effects of IGM-enriched solution on polymorphonuclear neutrophil function, bacterial clearance, and lung histology in endotoxemia. Shock 2008; 29: 167–172. [DOI] [PubMed] [Google Scholar]
- 20.Lissner R, Struff WG, Autenrieth IB, et al. Efficacy and potential clinical applications of Pentaglobin, an IgM-enriched immunoglobulin concentrate suitable for intravenous infusion. Eur J Surg Suppl 1999; 17–25. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder HW JrandCavacini L.. Structure and function of immunoglobulins. J Allergy Clin Immunol 2010; 125: S41–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welte T, Dellinger RP, Ebelt H, et al. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: a randomized, placebo-controlled, double-blind, multicenter, Phase II trial (CIGMA study). Intensive Care Med 2018; 44: 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi K, Hernandez LD, Galán JE, et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002; 110: 191–202. [DOI] [PubMed] [Google Scholar]
- 24.López-Collazo E, Del Fresno C. Pathophysiology of endotoxin tolerance: mechanisms and clinical consequences. Crit Care 2013; 17: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003; 29: 530–538. [DOI] [PubMed] [Google Scholar]
- 26.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 27.Petricevic B, Wessner B, Sachet M, et al. CL097, a TLR7/8 ligand, inhibits TLR-4–dependent activation of IRAK-M and BCL-3 expression. Shock 2009; 32: 484–490. [DOI] [PubMed] [Google Scholar]
- 28.Sadeghi K, Wisgrill L, Wessely I, et al. GM-CSF down-regulates TLR expression via the transcription factor PU.1 in human monocytes. PLoS One 2016; 11: e0162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirt W, Nebe T, Birr C. [Phagotest and Bursttest (Phagoburst), test kits for study of phagocyte functions]. Wien Klin Wochenschr 1994; 106: 250–252. [PubMed] [Google Scholar]
- 30.Justel A, Peña D, Zamar R. A multivariate Kolmogorov-Smirnov test of goodness of fit. Stat Probab Lett 1997; 35: 251–259. [Google Scholar]
- 31.Abdi H. The Greenhouse–Geisser correction In: Salkind N. (ed) Encyclopedia of research design. Thousand Oaks, CA: SAGE, 2010, pp.544–548. [Google Scholar]
- 32.Kessel A, Ammuri H, Peri R, et al. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J Immunol 2007; 179: 5571–5575. [DOI] [PubMed] [Google Scholar]
- 33.Kozicky LK, Zhao ZY, Menzies SC, et al. Intravenous immunoglobulin skews macrophages to an anti-inflammatory, IL-10-producing activation state. J Leukoc Biol 2015; 98: 983–994. [DOI] [PubMed] [Google Scholar]
- 34.Rieben R, Roos A, Muizert Y, et al. Immunoglobulin M-enriched human intravenous immunoglobulin prevents complement activation in vitro and in vivo in a rat model of acute inflammation. Blood 1999; 93: 942–951. [PubMed] [Google Scholar]
- 35.Berlot G, Vassallo MC, Busetto N, et al. Relationship between the timing of administration of IgM and IgA enriched immunoglobulins in patients with severe sepsis and septic shock and the outcome: a retrospective analysis. J Crit Care 2012; 27: 167–171. [DOI] [PubMed] [Google Scholar]
- 36.Cavazzuti I, Serafini G, Busani S, et al. Early therapy with IgM-enriched polyclonal immunoglobulin in patients with septic shock. Intensive Care Med 2014; 40: 1888–1896. [DOI] [PubMed] [Google Scholar]
- 37.Berlot G, Vassallo CM, Busetto N, et al. Effects of the timing of administration of IgM- and IgA-enriched intravenous polyclonal immunoglobulins on the outcome of septic shock patients. Ann Intensive Care 2018; 8: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shmygalev S, Damm M, Knels L, et al. IgM-enriched solution BT086 improves host defense capacity and energy store preservation in a rabbit model of endotoxemia. Acta Anaesthesiol Scand 2016; 60: 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van der Meer W, Pickkers P, Scott CS, et al. Hematological indices, inflammatory markers and neutrophil CD64 expression: comparative trends during experimental human endotoxemia. J Endotoxin Res 2007; 13: 94–100. [DOI] [PubMed] [Google Scholar]
- 40.Wiersinga WJ, van’t Veer C, van den Pangaart PS, et al. Immunosuppression associated with interleukin-1R-associated-kinase-M upregulation predicts mortality in Gram-negative sepsis (melioidosis). Crit Care Med 2009; 37: 569–576. [DOI] [PubMed] [Google Scholar]
- 41.Hubbard LL, Moore BB. IRAK-M regulation and function in host defense and immune homeostasis. Infect Dis Rep 2010; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toungouz M, Denys CH, De Groote D, et al. In vitro inhibition of tumour necrosis factor-alpha and interleukin-6 production by intravenous immunoglobulins. Br J Haematol 1995; 89: 698–703. [DOI] [PubMed] [Google Scholar]
- 43.Darvill A, Bergmann C, Cervone F, et al. Oligosaccharins involved in plant growth and host-pathogen interactions. Biochem Soc Symp 1994; 60: 89–94. [PubMed] [Google Scholar]
- 44.Takei S, Arora YK, Walker SM. Intravenous immunoglobulin contains specific Abs inhibitory to activation of T cells by staphylococcal toxin superantigens [see comment]. J Clin Invest 1993; 91: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norrby-Teglund A, Basma H, Andersson J, et al. Varying titers of neutralizing Abs to streptococcal superantigens in different preparations of normal polyspecific immunoglobulin G: implications for therapeutic efficacy. Clin Infect Dis 1998; 26: 631–638. [DOI] [PubMed] [Google Scholar]
- 46.Oesser S, Schulze C, Seifert J. Protective capacity of a IgM/IgA-enriched polyclonal immunoglobulin-G preparation in endotoxemia. Res Exp Med (Berl) 1999; 198: 325–339. [DOI] [PubMed] [Google Scholar]
- 47.Kato K, Sakamoto T, Ito K. Gamma-globulin inhibits superantigen-induced lymphocyte proliferation and cytokine production. Allergol Int 2007; 56: 439–444. [DOI] [PubMed] [Google Scholar]
- 48.Trautmann M, Held TK, Susa M, et al. Bacterial lipopolysaccharide (LPS)-specific antibodies in commercial human immunoglobulin preparations: superior antibody content of an IgM-enriched product. Clin Exp Immunol 1998; 111: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garbett ND, Munro CS, Cole PJ. Opsonic activity of a new intravenous immunoglobulin preparation: pentaglobin compared with sandoglobulin. Clin Exp Immunol 1989; 76: 8–12. [PMC free article] [PubMed] [Google Scholar]
- 50.Esposito S, De Simone G, Boccia G, et al. Sepsis and septic shock: new definitions, new diagnostic and therapeutic approaches. J Glob Antimicrob Resist 2017; 10: 204–212. [DOI] [PubMed] [Google Scholar]
- 51.Giamarellos-Bourboulis EJ, Tziolos N, Routsi C, et al. Improving outcomes of severe infections by multidrug-resistant pathogens with polyclonal IgM-enriched immunoglobulins. Clin Microbiol Infect 2016; 22: 499–506. [DOI] [PubMed] [Google Scholar]
- 52.Wilkins AL, Steer AC, Smeesters PR, et al. Toxic shock syndrome – the seven Rs of management and treatment. J Infect 2017; 74:S147–S152. [DOI] [PubMed] [Google Scholar]
- 53.Van Schaik IN, Lundkvist I, Vermeulen M, et al. Polyvalent immunoglobulin for intravenous use interferes with cell proliferation in vitro. J Clin Immunol 1992; 12: 325–334. [DOI] [PubMed] [Google Scholar]
- 54.Amran D, Renz H, Lack G, et al. Suppression of cytokine-dependent human T-cell proliferation by intravenous immunoglobulin. Clin Immunol Immunopathol 1994; 73: 180–186. [DOI] [PubMed] [Google Scholar]
- 55.Padet L, Bazin R. IVIg prevents the in vitro activation of T cells by neutralizing the T cell activators. Immunol Lett 2013; 150: 54–60. [DOI] [PubMed] [Google Scholar]
- 56.Ballow M. Mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory diseases. J Allergy Clin Immunol 1997; 100: 151–157. [DOI] [PubMed] [Google Scholar]
- 57.Shankar-Hari M, Spencer J, Sewell WA, et al. Bench-to-bedside review: immunoglobulin therapy for sepsis – biological plausibility from a critical care perspective. Crit Care 2012; 16: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Busani S, Serafini G, Mantovani E, et al. Mortality in patients with septic shock by multidrug resistant bacteria: risk factors and impact of sepsis treatments. J Intensive Care Med 2019; 34: 48–54. [DOI] [PubMed] [Google Scholar]
- 59.Wieczorek A, Gaszyński T. Successful treatment of septic shock with the use of IgM enriched immunoglobulin solution and antibiotic policy. Austin J Emerg Crit Care Med 2015; 2: 1014. [Google Scholar]