Short abstract
Since the first description of dendritic cells by Steinman and Cohn in 1973, this important cell type has gained increasing attention. Over 4000 papers have been published on this topic annually during the last few years. At the beginning, dendritic cells were recognized for their immune stimulatory properties and their importance in initiating an adaptive immune response. Later, it was found that dendritic cells do not only initiate but also regulate immune responses. This attribute makes the so-called regulatory dendritic cells highly important for the prevention of exaggerated immune responses. Immune cells make contact with different Ags every day and must be tightly controlled to prevent excessive inflammation and subsequent organ destruction, particularly in organs such as the gut and lungs. Here, we give a brief overview of our current knowledge on how immune responses are controlled by dendritic cells, highlighting how they are involved in the induction of peripheral tolerance. We focus on what is known about these processes in the lung, with a closer look at their role in the induction and control of diseases such as bronchial asthma, chronic obstructive pulmonary disease and lung infections. Finally, we summarize some current approaches to modulate the behavior of dendritic cells that may hopefully lead to future therapeutics to control exaggerated immune responses.
Keywords: Asthma, COPD, tolerogenic, dendritic cell, anergy
The role of dendritic cells in innate and adaptive immunity
Dendritic cells (DCs) were first described by Steinman and Cohn in 1973 as a distinct population of immune cells in the spleen of mice.1,2 This cell type was subsequently identified in all lymphoid tissues and many other organs, and is particularly detectable in the epithelium.3 DCs are a highly specialized cell population able to present peptides bound to the proteins of the MHC on their surface.4 Proteins from the cytosolic compartment are cleaved via the proteasome, transported to the lumen of the endoplasmic reticulum and bound to MHC class I molecules, the MHC I peptide complex is then transported to the cell surface. Proteins from the environment surrounding the cell are continuously engulfed by pinocytosis or when opsonized by Abs, or complement can be taken up by receptor-mediated endocytosis later being cleaved to generate peptides in the endosomal compartment.5 Subsequently, these peptides are loaded on MHC class II molecules and transported to the cell surface. Although peptides from exogenous proteins are predominantly displayed on MHC II, they can also be presented on MHC I via cross-presentation.6 Similarly, proteins from the extracellular compartment can enter the endoplasmic reticulum to be presented on MHC I molecules. Therefore, a portion of the proteins engulfed are transported from the phagosomes to the cytosol via retro-translocation. In contrast, proteins from the cytosol can be brought to the endosomal compartment via autophagy and, in this way, introduced into the MHC II presentation pathway. Cross-presentation is important not only for the induction of antiviral and antitumor immunity, but also in peripheral tolerance.7
In their role as professional APCs, DCs are the most important immune cells for the activation of T lymphocytes.8 This is particularly clear due to the recent advances in immunological tools such as transgenic mice.9 However, in their bridging role between innate and adaptive immunity, DCs do not only play a role in the protection against pathogens, but also in the induction and the perpetuation of diseases such as asthma.10 The activation status of DCs decides the activation or inhibition of a T cell. Activated DCs differ from resting cells in their shape and the expression of specific membrane receptors. The expression, for instance, of CD40, CD80, CD83, CD86 and CD252 (OX40 ligand), the so-called costimulatory molecules, were all shown to have an impact on the activation of T lymphocytes.4,11–13 All these membrane receptors are expressed at low-density under steady state conditions but become up-regulated after DC activation. This gives the DCs the license to activate naive T lymphocytes, given that the MHC-presented peptides are recognized by the specific T-cell receptor.14
Different exogenous signals can trigger the productive activation of DCs after binding to their PRRs. The large group of molecules belonging to the PAMPs provide the ligands for the PRRs.15 The PRRs can be divided into the Toll-, NOD- and retinoic acid (RA)-inducible gene I-like receptors predominantly recognizing bacterial and viral PAMPs and the C-type lectin receptors (CLR) detecting glycostructures from fungi and from bacteria. In addition to these pathogen-derived signals, endogenous ‘danger signals’ coming from dying cells and necrotic tissues can further lead to the activation of DCs.16,17 Later, these danger signals were called a damage-associated molecular pattern and shown to activate the inflammasome leading to maturation of strong pro-inflammatory signals such as IL-1β and IL-18.18 The production of cytokines plays an important role not only in the regulation of the induced T-cell response regarding T-cell polarization into subpopulations, but also in preventing exaggerated T-cell responses.
The phenotype of different DC subsets of the lung
Conventional DCs (cDCs), plasmacytoid DCs (pDCs) and macrophages of the tissues are derived from Lin−cKitintCD115+Flt3+ common progenitors.19 These cells are generated in the bone marrow, from where they start to populate the lymphatic tissues and different organs. Mouse cDCs are characterized by their high expression of CD11c, whereas mouse pDCs only express low levels of CD11c but do express additional characteristic markers, such as CD45R (B220) and CD317 (PDCA1). Many cDCs are found in the mucosa of the conducting airways.20 They protrude their pseudopodia between the epithelial cells to capture Ag from the airway lumen. In addition to CD11c, these cDCs partially express the integrin CD103, which is a specific marker for a subset of lung cDCs (cDC1). The other subset of cDCs express CD11b in addition to the CD11c marker (cDC2). When a trigger of airway inflammation is encountered, another population of cells comes into play, the inflammatory DCs. They are generated from Ly6C+ blood monocytes under the influence of a granulocyte macrophage colony stimulating factor. These inflammatory CD11c+ and CD11b+ DCs retain the monocytic marker Ly6c.21 This cell type shows many similarities to the widely used DCs generated in vitro that can be utilized, for instance, to sensitize mice via their airways.22 The pDCs can also be detected in the conducting airways, although in a lower density, and they have also been described as present in lung parenchyma.23 Human DCs can generally be divided in similar subgroups to their murine counterparts.24 However, there are additional markers used for the characterization of human DCs in addition to markers such as CD11c, also common for human DCs. The different subsets of human lung cDCs express CD141 (BDCA3) on cDC1 cells and CD1c (BDCA1) on cDC2 cells, respectively.25 Human lung pDCs express CD303 (BDCA2) and CD304 (BDCA4).
Induction of peripheral tolerance by DCs
There are distinct immunological mechanisms responsible for the induction of tolerance of T lymphocytes to harmless Ags. Central tolerance is induced in the thymus during the interaction of maturing T cells with specialized thymic epithelial cells and DCs presenting a huge repertoire of self-Ags. Even tissue-restricted Ags are produced under the influence of the transcription factor, Auto Immune Regulator. However, it is known that some potentially autoreactive T lymphocytes leave the thymus, because not every possible auto-Ag is presented.26 Induction of peripheral tolerance comes into play to prevent the activation of these potentially autoreactive T lymphocytes. Three distinct mechanisms of peripheral tolerance induction by DCs are known: activation of regulatory T cells (Treg), induction of anergy in Ag-specific T cells and negative feedback regulation.27
Negative feedback regulation
Negative feedback regulation in DCs means extracellular stimulation addressed to DCs, which leads to a tolerogenic DC phenotype (Figure 1a). An example of this feedback regulation is the action of IL-10 on DCs. The source of this anti-inflammatory cytokine could be, for example Treg, known to be good producers of IL-10, which may act in a paracrine manner on the DCs, arresting the latter in an immature state with low expression of costimulatory molecules. Another possibility is that the IL-10 released acts in an autocrine manner after being induced, for instance, by activation of the CLR, for example, DC-specific ICAM3-grabbing non-integrin (DC-SIGN).28 This mechanism is thought to be involved in the dormancy of mycobacteria infection, since polysaccharides of the cell envelope of, for example, Mycobacterium tuberculosis, are known to activate DC-SIGN and, therefore lead possibly to anergy induction in T-helper cells. Another example of such a negative feedback is the direct cell contact-dependent interaction of DCs with Treg. This interaction was shown to be extraordinarily strong and may lead to down-regulation of costimulatory molecules on DCs.29,30 This negative feedback given by Treg can lead to enhanced anergy induction by DCs after interaction with an Ag-specific T cell. It has been shown more recently that DCs also respond to the cytokines of the WNT family. Canonical signaling via β-catenin is able to down-regulate the NFκB pathway and may, therefore, block DC activation by PAMPs or cytokines, leaving the DC in a tolerogenic state.31 Similarly, signaling through DC receptors with intracellular immunoreceptor tyrosine-based inhibitory motif domains (results in the inhibition of the NFκB pathway and results, therefore, in negative feedback regulation.32
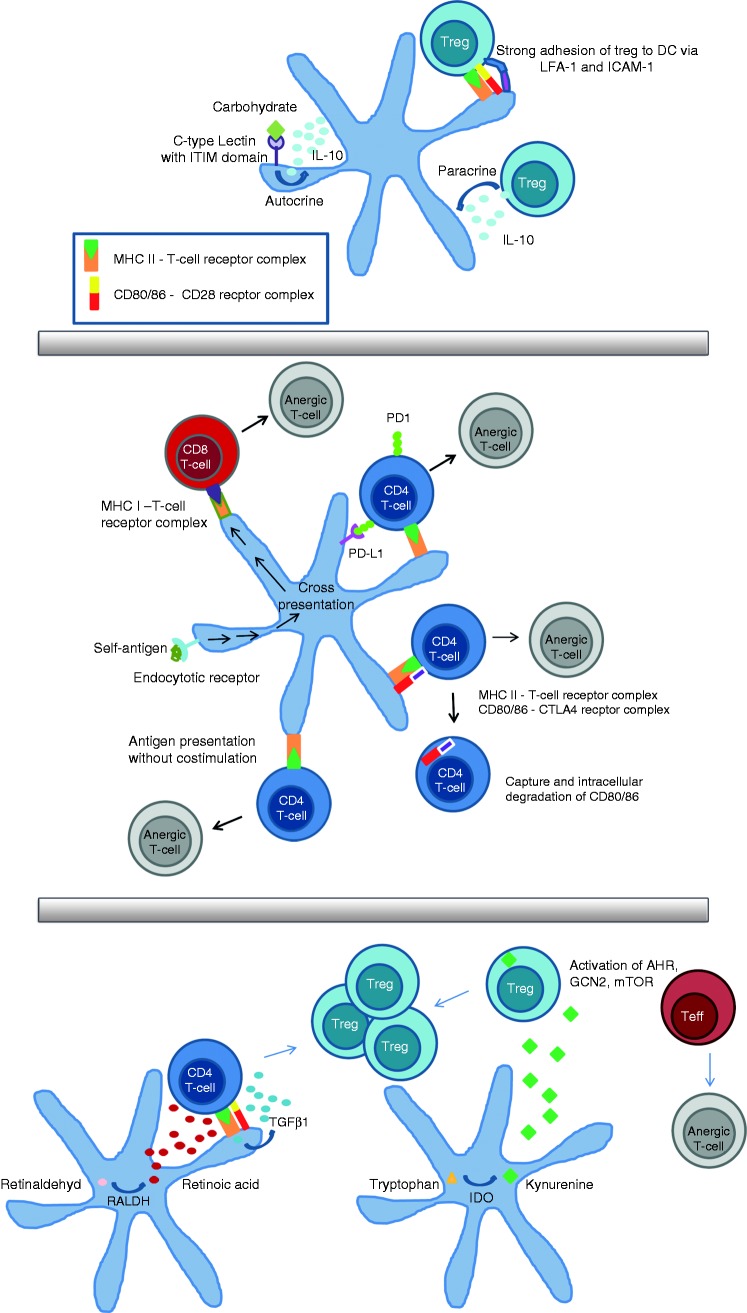
Figure 1.
Negative feedback regulation (a): IL-10 is secreted in a paracrine manner by regulatory T cell (Treg) and by dendritic cells (DCs) in an autocrine manner upon activation of DC-specific ICAM3-grabbing non-integrin (DC-SIGN), which is a carbohydrate receptor containing an immunoreceptor tyrosine-based inhibitory motif domain. The Treg can adhere very strongly to DCs via LFA-1 and ICAM-1. These mechanisms result in the down-regulation of costimulatory molecules on DCs. Induction of anergy (b): the longest known way to induce anergy is via Ag presentation in the absence of costimulation. This way is known in T-helper cells and via cross-presentation in cytotoxic T cells. Anergy induction is enhanced by the interaction of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) with B7 molecules on DCs or the binding of PD1 to PD-L1. Induction of Treg (c): CD103-positive DCs secrete TGFβ1 and retinoic acid (RA), which leads to the induction of Treg in the absence of IL-6. Activity of indoleamine 2,3-dioxygenase (IDO) in DC catalysis the production of kynurenine, which may bind to the aryl hydrocarbon receptor (AHR) in T cells. Without a ligand, the AHR transcription factor is localized in the cytoplasm in complex with other proteins. Upon ligand binding, its conformation changes and a translocation into the nucleus takes place, where AHR can activate gene transcription and the activation of Treg. The amino acid sensors mammalian target of rapamycin (mTOR) and general control nonderepressible 2 (GCN2) kinase are influenced by tryptophan deficiency as a result of IDO activity. This contributes, on the one hand, to anergy and suppression of effector cells and, on the other hand, to a hyperactivation of Treg.
Induction of anergy
Induction of anergy or clonal deletion of specific T cells is a second mechanism by which DCs can establish peripheral tolerance (Figure 1b). One well-known pathway to induce anergy in specific T cells is the presentation of Ag in the absence of costimulation; this was shown for T-helper cells33 and for cytotoxic T cells.34 The constant uptake of auto-Ags from the surrounding tissues by DCs is important for anergy induction. This mechanism has already been exploited by delivering Ag via targeting endocytic receptors, such as CD205 (Dec205).35 Cross-presentation of engulfed Ags via MHC I is an essential mechanism for tolerizing cytotoxic T cells.36 As has already been mentioned, anergy induction is observed when Ag is presented in the absence of costimulation, i.e. the lack of expression of CD80/86 and cytokines. Anergy induction by DCs can also be facilitated when DCs express certain molecules, such as IL-10, discussed above, or the membrane receptor PD-L1, which interacts with PD1 on the T cell.37 The interaction between PD1 and its ligand on DCs is such an important mechanism in anergy induction that there have already been several clinical studies showing promising results in the reactivation of the immune response against tumor cells in cancer patients by blocking this interaction and, therefore, preventing the anergy of tumor-Ag-specific T cells.38 Another example of such a negative regulation is the interaction of DCs with T cells expressing cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).39 CTLA-4 binds to CD80/86 with a greater affinity than CD28, leading to the down-regulation of T-cell activity. CTLA-4 can capture its ligands, CD80 or CD86, from cells presenting Ag by a process of trans-endocytosis. The costimulatory molecules are degraded in the CTLA-4-expressing cells. By this depletion, CTLA-4 acts as an effector molecule to inhibit CD28 costimulation.40 Additionally, CTLA-4 induces the production of indoleamine 2,3-dioxygenase (IDO) in DCs. The role of IDO in tolerance induction is discussed in the following chapter.
Induction of Treg
The Treg are a highly important group of lymphocytes that protect the body from autoimmune responses. The essential role of these cells becomes clear in mouse models where Treg were depleted. Fatal autoimmunity was observed in this model even when the cells were depleted later in life.41 In addition to naturally occurring Treg that are already preformed in the thymus, inducible Treg are induced by DCs with TGFβ1 in the absence of IL-6, leading to the expression of the transcription factor Foxp3 (Figure 1c). Interestingly, the activation of inducible Treg also relies on conventional signals for T-cell activation, such as MHC II molecules and costimulation via CD80/86.42,43 The cDCs expressing CD103 are a source of TGFβ1 and known to be potent inducers of Treg in models of oral tolerance induction.44 The RA produced by the enzyme retinaldehyde dehydrogenases expressed by these cDCs stabilizes the Treg lineage. However, it seems to be important that RA signaling is in context with TGFβ signaling, otherwise RA can also have pro-inflammatory roles.45 Human CD1c+ DCs were also shown to be capable of producing RA under the influence of vitamin D3.46 Another molecule involved in the peripheral tolerance induction by DCs is the enzyme IDO. The importance of IDO for peripheral tolerance was first identified due to its important role in the protection of the fetus against attacks from the immune system.47 IDO acts through its enzymatic activity; it catalyzes the production of kynurenine from tryptophan. Kynurenine is a natural ligand of the aryl hydrocarbon receptor (AHR) and the consumption of tryptophan by IDO activates the amino acid sensors general control nonderepressible 2 and mammalian target of rapamycin. IDO activity facilitates the suppression and anergy of effector cells, on the one hand, and boosts the activity of Treg cells existing already, on the other hand.48
Regulation of inflammation by DCs in asthma
The DCs play an essential role in the induction and regulation of the asthmatic response. After the first contact with the allergen, they transport these to the bronchial lymph nodes and activate allergen-specific T cells. When DCs are eliminated by in vivo depletion of all CD11c-expressing cells during the sensitization phase in a mouse model of asthma, the characteristic features of asthma are abrogated.49 Meanwhile, the cells and processes involved in allergic sensitization via the airways are understood in more detail. After contact with allergen, the airway epithelium is exposed to adjuvants, such as proteases, PAMP and pollen-associated lipid mediators, leading to the release of chemokines and the alarmins IL-25, IL-33 and Thymic stromal lymphopoietin, resulting in the recruitment of innate lymphoid cells and DCs, such as cDC2 cells expressing CD11b.50 This highly migratory DC population transports the allergens to the lymph nodes to induce an allergen-specific Th2 response. Although the precise phenotype these migratory cells must achieve to elicit Th2 immunity in the secondary lymphatics has yet to be described, some parameters have already been revealed to be relevant. The expression, for instance, of OX40L on the surface of DCs due to the contact with TSLP was shown to be relevant.51 Moreover, it was believed that the expression of Jagged-2 on DCs is important in delivering signals via Notch receptors on T cells resulting in Th2 differentiation. However, this model has been challenged recently, because it was shown that the expression of Jagged-1/2 is dispensable for the induction of a Th2 response.52 IL-6 was shown to be significant regarding soluble factors that are important for Th2 induction and are released by DCs.53,54
The skin, gut and lungs are the most important contact zones between environmental Ags, such as allergens, and the immune system. Therefore, it is thought the healthy human contact with harmless Ags does not lead to an allergic sensitization. Indeed, it was shown that contact with a foreign Ag via the airways without an adjuvant leads to tolerance induction.55 This peripheral tolerance induction is mediated by transient production of IL-10 by DCs, leading to the generation of IL-10-producing Ag-specific Treg. The regulatory DCs described in the study of Akbari et al. were likely to be cDCs, because of their high expression of MHC II and Dec205. Newer studies have shown that induction of Treg to inhaled Ags is attributable to CD103+ cDCs. These cDCs up-regulate RALDH upon contact with the Ags and start to produce RA, which, in turn, leads to the generation of Treg-expressing Foxp3.56 Interestingly, the transcription factor Foxp3, known for its importance in determining the fate of Treg, has also been shown to be expressed by tolerogenic DCs of the lung.57 Because Foxp3 is known to bind to the promotor of TGFβ, these cells are good TGFβ producers, thereby inhibiting T-cell activation.
In addition to these reports indicating that cDCs may induce tolerance under certain conditions, pDCs were also shown to play an important role in the induction of tolerance against harmless Ags by transporting them to the regional lymph nodes.58 In the case of oral tolerance induction towards gastrointestinal Ags, they play a pivotal role by the induction of Treg.59 The pDCs were also shown to be essential in the induction of tolerance to harmless Ags in the lungs under steady-state conditions and to be of merit in suppressing ongoing allergic sensitization when transferred adoptively.60 This discovery reveals pDCs are a highly relevant target to be exploited for the control of allergic inflammation. There are first reports showing that the attraction of pDCs by treating mice with Flt3L resulted in down-regulation of the allergic immune response.61 However, one must be very careful when manipulating the Janus-faced DC network, because there have been recent reports showing that pDCs may also be involved in the exacerbation of allergic asthma.62
Regulation of inflammation by DCs in chronic obstructive pulmonary disease
The role of DCs in chronic obstructive pulmonary disease (COPD) pathogenesis is more obscure than their role in asthma. One reason for this might be the undefined nature of Ags involved in COPD immune response. By contrast, the Ags (allergens) playing a role in asthma are well defined and can be used as a tool to study the interaction of DCs with specific T cells. Interestingly, newer studies have shown that autoimmunity consisting of both autoreactive T cells and B cells are involved in the pathogenesis of COPD.63,64 Although the precise contribution of this maladaptive autoimmune response in COPD has yet to be revealed, it shows that at some time during disease development, auto-Ags have to be presented by DCs to activate autoreactive T cells. Nevertheless, it is known that cigarette smoke, the leading cause for the development of COPD, attracts cDCs into the human lung.65 The infiltrating DCs have an activated phenotype with the expression of costimulatory molecules, such as CD83.66
Because cigarette smoke is a source of high amounts of ligands for the AHR,67 one would think there must be a continuous stimulation of the AHR due to smoking leading to the induction of tolerogenic DCs.68 Indeed, there are reports showing that deletion of the AHR in mice resulted in enhanced inflammation upon smoke exposure.69 However, it is still unclear whether the increased inflammation depends on a dysfunction of regulatory DCs. Intriguingly, a subset of human lung cDCs, the cDC2 expressing CD1c, was demonstrated to exhibit enhanced tolerogenic activity in COPD patients.70 These cDC2 expressed tolerogenic surface molecules, such as PD-L1, and were potent inducers of Treg cells exerting their immune regulating activity via production of IL-10 and TGFβ1. From this study, the question arose of whether this regulatory activity may predispose COPD patients to respiratory infections leading to exacerbation of the disease. However, pDCs were also discussed as having an immune suppressive role due to smoking.71 In this report, Sorrentino et al. showed that murine cDCs exposed to cigarette smoke attract pDCs to the lung that subsequently suppress the immune response to Chlamydophila pneumoniae, presumably involving an IDO-mediated mechanism. A similar accumulation of pDCs was found in lung bronchial-associated lymphoid tissue in COPD patients.72 In contrast to the report in the murine model system, it was not investigated whether the accumulating pDCs have immune-suppressive properties. However, treatment of human pDCs with cigarette smoke extract renders the cells reluctant to activation with agonists of TLR7 or TLR9, suggesting they become unresponsive to the virus. Such a state of enhanced susceptibility to virus infection with influenza and respiratory syncytial virus is known to be one of the major problems in advanced COPD, often leading to the exacerbation of the disease.
Regulation of the immune response by DCs during respiratory infection
The most obvious and important role of DCs in the lungs is protection against respiratory infections by bridging innate and adaptive immunity to mount a robust adaptive immune response towards pathogens. Following influenza infection, cDC1 migrate from their intraepithelial origin to the draining mediastinal lymph nodes, where they can activate cytotoxic T lymphocytes.73 The cDC2 are attracted and activated by alarmins secreted by airway epithelial cells that were stimulated with PAMPs. These cells are also involved in the activation of T cells, but they are thought to activate predominantly T-helper cells via the presentation of virus Ag on MHC II.74 The pDCs are important cells particularly in defense against a virus. They are known for their high capacity to produce type 1 IFN, but they are also able to activate T lymphocytes via Ag presentation. It was shown recently that pDCs must be directly infected by influenza A to induce highly effective cytotoxic T-cell response.75 By contrast, pDCs stimulated with respiratory syncytial virus (RSV) do not directly activate T cells.76 They seem to support the immune response against RSV predominantly by producing type 1 IFN. The importance of this for the control of the RSV infection was shown in mice by in vivo depletion of pDCs. In pDC-depleted mice, there was reduced viral clearance and enhanced RSV-mediated pathology.77 The most severe RSV infections causing a bronchiolitis often requiring hospitalization are found in neonates. One reason for this might be that their immune system is still developing and the pDC compartment was shown to be immature. It was shown in neonatal mice that RSV infection leads to inefficient pDC activation, with an inadequate type 1 IFN response leading to problems in the control of virus infection. By contrast, the pDCs of adult mice were infected efficiently, consequently releasing IFN-α.78 As discussed earlier, pDCs may be involved in protection against de novo sensitization to allergens. Because sensitization is thought to occur predominantly during early infancy, it is interesting to speculate that this is due to the immature state of the pDCs. Intriguingly, Tsuchida et al. showed that the RSV infection of adult mice subverts pDCs allergy-protective activity, leading to aggravated asthma, as shown earlier.79 Similar to the RSV, mouse, pneumovirus (PVM) belongs to the group of Paramyxoviridae. Infection of mice with pneumovirus once more reveals the importance of pDCs in prevention of severe bronchiolitis.80 Although they are not directly anticipating virus elimination, they prevent excessive inflammation by Treg induction via semaphorin 4a. What was shown to be beneficial in the prevention of exaggerated inflammation due to infection with virus was also found to contribute to the pathogenesis of the fungal infection mediated by Paracoccidioides brasiliensis.81 In this report, the authors demonstrated that elimination of pDCs resulted in decreased Treg numbers and an enhanced immune response against the fungus, leading to a reduced microbial burden. Later, the same authors showed the activation of IDO leading to the formation of AHR ligands is involved in the activation of Treg that suppresses the anti-fungal Th17 immune response.82 Similarly, it was shown in tuberculosis that the activity of Treg contributes to the persistence of M. tuberculosis in the host lung of patients who are extensively drug resistant.83 The exact pathways leading to activation of these Treg are still unknown, although there are some reports showing that cell wall compounds of mycobacteria may modulate the behavior of DCs in favor of Treg induction. Geitjenbeek et al. showed that mannosylated lipoarabinomannans from the cell envelope of mycobacteria bind to the CLR DC-SIGN on monocyte-derived DCs leading to enhanced IL-10 production by the DCs.84 The authors provide further evidence that this leads to negative feedback inhibition preventing the activation of DCs. In contrast, it is conceivable that the DCs producing IL-10 will contribute to Treg differentiation, because it is known that IL-10 is able to potentiate Treg formation.85 Later, it was shown that DC-SIGN-modulated IL-10 production may be involved in the down-regulation of the immune response against several different pathogens.86
‘Therapeutic’ induction of tolerogenic DCs
The first efforts to exploit DCs for clinical purposes came from trials to induce a tumor-specific immune response. However, the predominant proportion (> 90%) of clinical trials applying DCs is made to enhance the anti-tumor response. Due to the awareness that DCs not only induce but also suppress T-cell responses, new efforts were made to manipulate DCs in such a way that they induce tolerance. Tolerogenic DCs are already in use in some clinical trials to treat autoimmune disease, such as multiple sclerosis, type 1 diabetes, rheumatoid arthritis or to prevent organ rejection after transplantation. Several substances were shown to have the property to induce DCs with a tolerogenic behavior. For instance, dexamethasone, a drug that has been used for many years to control inflammation, was shown to activate tolerogenic DCs.87 Treatment of human DCs with this anti-inflammatory drug leads to cells in a semi-mature state that express high levels of PD-L1. Cultivation of DCs treated with dexamethasone alongside T cells leads to the activation of T cells producing IL-10. Similarly, DCs that were treated with rapamycin are able to induce Treg responses.88 Here, the authors showed that rapamycin-treated DCs that were loaded with allo-Ags led to the longtime survival of skin allografts in rats. In addition to small-molecule drugs that were used for the induction of tolerogenic DCs, there are studies revealing a pronounced activity of distinct cytokines in the induction of tolerogenic DCs. Both IL-10 and TGFβ1 in particular are under extensive investigation. Different protocols were described to differentiate tolerogenic DCs under the influence of IL-10 with or without TGFβ1.89 The DCs treated with IL-10 have shown their immune regulatory behavior in several animal models and it is believed they can also be of benefit for the treatment of patients.90 However, the stimulation of DCs with PRR or CLR ligands to generate cells that produce IL-10 on their own could be even more efficient in generating tolerogenic DCs to regulate immune responses. The advantage of this would be that these cells, on the one hand, lead to the activation of Treg and, on the other hand, arrest themselves in an immature state via negative feedback regulation. Some substances have already been shown to act in that way via activation of TLRs or CLR.91–94 The stimulation particularly of DCs via CLR seems to be effective in generating tolerogenic DCs.84,95,96 Treatment with substances aiming to induce tolerogenic DCs do not necessarily occur ex vivo and in vivo induction of tolerogenic DCs is possible, for example, by the targeting of Ag to DC receptors such as DEC205.97 Several examples for the manipulation of DCs to induce tolerance are given in Table 1.
Table 1.
Examples of substances known from the literature to induce tolerogenic DCs and their mode of action. Most experiments are performed in mouse models of allergic disease.
| Substances for stimulation of DCs | Disease | Mode of action | Reference |
|---|---|---|---|
| Arabinogalactan | Murine asthma model | IL-10 production by DCs | 98 |
| Cowshed dust extract | Murine asthma model | IL-10 production by DCs | 92 |
| Bacterial lipopeptides | Murine asthma model | IL-10 production by DCs, IRAK-M induction, arrest in immature state | 91 |
| Immune complexes Ag and sialylated IgG | Delayed type hypersensitivity (mouse model) | Arrest in immature state, inhibition of IL-12/23 and IL-6 release | 99 |
| Lactobacillus reuteri and Lactobacillus casei | In vitro studies with human cells | DC-SIGN ligation activation of IL-10 producing Treg | 100 |
| Lactococcus lactis | Murine asthma model | TLR13 activation | 94 |
| Complement (C5a) | Murine asthma model | Attraction of pDCs? | 101 , 102 |
| Staphylococcal enterotoxin B | Intestinal allergic inflammation | Foxp3 induction in DCs release of TGFβ | 57 |
| Fungal β-glucan | Autoimmune diabetes | Dectin-1 ligation, induction of Treg | 95 |
| IL-10 | Allergic sensitization (house dust mite) | In vitro induction of Treg | 103 |
| Flagellin | Allergic sensitization (birch pollen) | IL-10 production of DCs via a mTOR pathway | 93 |
| Cisplatin | In vitro studies with murine cells | IL-10 production of DCs via MAPK pathway | 104 |
DC: dendritic cell; pDC: plasmacytoid DC; mTOR: mammalian target of rapamycin; DC-SIGN: DC-specific ICAM3-grabbing non-integrin; Treg: regulatory T cell.
Conclusions
Our knowledge about the circumstances under which tolerogenic DCs differentiate have increased continuously over the last 15 yr. This is an important development because DCs are already in use in clinical studies for the treatment of cancer. Knowing the precise parameters involved in the differentiation of tolerogenic DCs will avoid trials under risk of inducing Treg against tumor Ags. Exemplarily, DCs treated with the Dectin-1 ligand β-glucan were successfully used to induce tolerance against auto-Ags.95 Confusingly, DCs treated with β-glucan were also shown to initiate an anti-tumor immune response.105 Thus, much more research is needed to delineate all the parameters distinguishing between activating versus tolerogenic DCs. Once the best way to induce stable tolerogenic DCs is deciphered, the ground is prepared for the development of new treatment opportunities for asthma bronchiale and other chronic inflammatory airway disorders. Moreover, the extended knowledge will enable us to perform meaningful trials to modulate the tolerogenic behavior of DCs in vivo, aiming to break the tolerance against pathogens hiding in the cells of infected patients.
Acknowledgements
We would like to thank Philip Saunders (Language Support Services, Berlin, Germany) for proofreading the manuscript.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution . J Exp Med 1973; 137(5):1142–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro . J Exp Med 1974; 139(2):380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity 2008; 29(3):325–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392(6673):245–522. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Roche PA. Macropinocytosis in phagocytes: Regulation of MHC class-II-restricted antigen presentation in dendritic cells. Front Physiol 2015; 6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol 2013; 31:443–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinman RM. Decisions about dendritic cells: Past, present, and future. Annu Rev Immunol 2012; 30:1–22. [DOI] [PubMed] [Google Scholar]
- 8.Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol 2002; 14(4):432–436. [DOI] [PubMed] [Google Scholar]
- 9.Bar-On L, Jung S. Defining dendritic cells by conditional and constitutive cell ablation. Immunol Rev 2010; 234(1):76–89. [DOI] [PubMed] [Google Scholar]
- 10.Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet 2010; 376(9743):835–843. [DOI] [PubMed] [Google Scholar]
- 11.Aerts-Toegaert C, Heirman C, Tuyaerts S, et al. CD83 expression on dendritic cells and T cells: Correlation with effective immune responses. Eur J Immunol 2007; 37(3):686–695. [DOI] [PubMed] [Google Scholar]
- 12.Willoughby J, Griffiths J, Tews I, et al. OX40: Structure and function - What questions remain? Mol Immunol 2017; 83:13–22. [DOI] [PubMed] [Google Scholar]
- 13.Schoenberger SP, Toes RE, van der Voort EI, et al. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998; 393(6684):480–483. [DOI] [PubMed] [Google Scholar]
- 14.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol 2009; 27:591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janeway CA, Medzhitov R. Innate immune recognition . Annu Rev Immunol 2002; 20:197–216. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003; 425(6957):516–521. [DOI] [PubMed] [Google Scholar]
- 17.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol 2001; 13(1):114–119. [DOI] [PubMed] [Google Scholar]
- 18.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol 2009; 27:229–265. [DOI] [PubMed] [Google Scholar]
- 19.Onai N, Obata-Onai A, Schmid MA, et al. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol 2007; 8(11):1207–1216. [DOI] [PubMed] [Google Scholar]
- 20.Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: From protection to immunopathology. Annu Rev Immunol 2012; 30:243–270. [DOI] [PubMed] [Google Scholar]
- 21.Plantinga M, Guilliams M, Vanheerswynghels M, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013; 38(2):322–335. [DOI] [PubMed] [Google Scholar]
- 22.Peters M, Dudziak K, Stiehm M, et al. T-cell polarization depends on concentration of the danger signal used to activate dendritic cells. Immunol Cell Biol 2010; 88(5):537–544. [DOI] [PubMed] [Google Scholar]
- 23.von Garnier C, Filgueira L, Wikstrom M, et al. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol 2005; 175(3):1609–1618. [DOI] [PubMed] [Google Scholar]
- 24.Guilliams M, Ginhoux F, Jakubzick C, et al. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat Rev Immunol 2014; 14(8):571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granot T, Senda T, Carpenter DJ, et al. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity 2017; 46(3):504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh J, Shin J-S. The role of dendritic cells in central tolerance. Immune Netw 2015; 15(3):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer CT, Berod L, Sparwasser T. Layers of dendritic cell-mediated T cell tolerance, their regulation and the prevention of autoimmunity. Front Immunol 2012; 3:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Kooyk Y, Geijtenbeek TBH. DC-SIGN: Escape mechanism for pathogens. Nat Rev Immunol 2003. [cited 2018 Mar 7]; 3(9):697–709. [DOI] [PubMed]
- 29.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol 2000; 30(6):1538–53. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Ganguly A, Mucsi AD, et al. Strong adhesion by regulatory T cells induces dendritic cell cytoskeletal polarization and contact-dependent lethargy. J Exp Med 2017; 214(2):327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma B, Hottiger MO. Crosstalk between Wnt/β-catenin and NF-κb signaling pathway during inflammation. Front Immunol 2016; 7:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsch I, Janovec V, Stranska R, et al. Cross-talk between inhibitory immunoreceptor tyrosine-based activation motif-signaling and toll-like receptor pathways in macrophages and dendritic cells. Front Immunol 2017; 8:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 2001; 194(6):769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Probst HC, Lagnel J, Kollias G, et al. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity 2003; 18(5):713–720. [DOI] [PubMed] [Google Scholar]
- 35.Bonifaz L, Bonnyay D, Mahnke K, et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 2002; 196(12):1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luckashenak N, Schroeder S, Endt K, et al. Constitutive cross-presentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo. Immunity 2008; 28(4):521–532. [DOI] [PubMed] [Google Scholar]
- 37.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol 2007; 19(3):309–314. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3–potential mechanisms of action. Nat Rev Immunol 2015; 15(1):45–56. [DOI] [PubMed] [Google Scholar]
- 39.Slavik JM, Hutchcroft JE, Bierer BE. CD28/CTLA-4 and CD80/CD86 families: Signaling and function. Immunol Res 1999; 19(1):1–24. [DOI] [PubMed] [Google Scholar]
- 40.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 2011; 332(6029):600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 2007; 8(2):191–197. [DOI] [PubMed] [Google Scholar]
- 42.Darrasse-Jèze G, Deroubaix S, Mouquet H, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med 2009; 206(9):1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000; 12(4):431–440. [DOI] [PubMed] [Google Scholar]
- 44.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 2007; 204(8):1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erkelens MN, Mebius RE. Retinoic Acid and Immune Homeostasis: A Balancing Act. Trends Immunol 2017; 38(3):168–180. [DOI] [PubMed] [Google Scholar]
- 46.Sato T, Kitawaki T, Fujita H, et al. Human CD1c+ myeloid dendritic cells acquire a high level of retinoic acid-producing capacity in response to vitamin D3. J Immunol 2013; 191(6):3152–3160. [DOI] [PubMed] [Google Scholar]
- 47.Munn DH. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281(5380):1191–1193. [DOI] [PubMed] [Google Scholar]
- 48.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 2013; 34(3):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Rijt LS, Jung S, Kleinjan A, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 2005; 201(6):981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Q, Ho AWS, Schlitzer A, et al. GM-CSF-licensed CD11b+ lung dendritic cells orchestrate Th2 immunity to Blomia tropicalis. J Immunol 2014; 193(2):496–509. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y-J. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol 2007; 120(2):238–244; quiz 245-6. [DOI] [PubMed] [Google Scholar]
- 52.Tindemans I, Lukkes M, de Bruijn MJW, et al. Notch signaling in T cells is essential for allergic airway inflammation, but expression of the Notch ligands Jagged 1 and Jagged 2 on dendritic cells is dispensable. J Allergy Clin Immunol 2017; 140(4):1079–1089. [DOI] [PubMed] [Google Scholar]
- 53.Lin Y-L, Chen S-H, Wang J-Y. Critical role of IL-6 in dendritic cell-induced allergic inflammation of asthma. J Mol Med 2016; 94(1):51–59. [DOI] [PubMed] [Google Scholar]
- 54.Krishnamoorthy N, Oriss TB, Paglia M, et al. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat Med 2008; 14(5):565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol 2001; 2(8):725–731. [DOI] [PubMed] [Google Scholar]
- 56.Khare A, Krishnamoorthy N, Oriss TB, et al. Cutting edge: Inhaled antigen upregulates retinaldehyde dehydrogenase in lung CD103+ but not plasmacytoid dendritic cells to induce Foxp3 de novo in CD4+ T cells and promote airway tolerance. J Immunol 2013; 191(1):25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X-Y, Xu L-Z, Luo X-Q, et al. Forkhead box protein-3 (Foxp3)-producing dendritic cells suppress allergic response. Allergy 2017; 72(6):908–917. [DOI] [PubMed] [Google Scholar]
- 58.Kohli K, Janssen A, Förster R. Plasmacytoid dendritic cells induce tolerance predominantly by cargoing antigen to lymph nodes. Eur J Immunol 2016; 46(11):2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uto T, Takagi H, Fukaya T, et al. Critical role of plasmacytoid dendritic cells in induction of oral tolerance. J Allergy Clin Immunol 2018. [DOI] [PubMed] [Google Scholar]
- 60.de Heer HJ, Hammad H, Soullié T, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med 2004; 200(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kool M, van Nimwegen M, Willart MAM, et al. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J Immunol 2009; 183(2):1074–1082. [DOI] [PubMed] [Google Scholar]
- 62.Chairakaki A-D, Saridaki M-I, Pyrillou K, et al. Plasmacytoid dendritic cells drive acute asthma exacerbations. J Allergy Clin Immunol 2017. [DOI] [PubMed] [Google Scholar]
- 63.Kheradmand F, Shan M, Xu C, et al. Autoimmunity in chronic obstructive pulmonary disease: Clinical and experimental evidence. Expert Rev Clin Immunol 2012; 8(3):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wen L, Krauss-Etschmann S, Petersen F, et al. Autoantibodies in chronic obstructive pulmonary disease. Front Immunol 2018; 9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lommatzsch M, Bratke K, Knappe T, et al. Acute effects of tobacco smoke on human airway dendritic cells in vivo. Eur Respir J 2010; 35(5):1130–1136. [DOI] [PubMed] [Google Scholar]
- 66.Vassallo R, Walters PR, Lamont J, et al. Cigarette smoke promotes dendritic cell accumulation in COPD; a Lung Tissue Research Consortium study. Respir Res 2010; 11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Souza AR, Zago M, Eidelman DH, et al. Aryl hydrocarbon receptor (AhR) attenuation of subchronic cigarette smoke-induced pulmonary neutrophilia is associated with retention of nuclear RelB and suppression of intercellular adhesion molecule-1 (ICAM-1). Toxicol Sci 2014; 140(1):204–223. [DOI] [PubMed] [Google Scholar]
- 68.Takenaka MC, Quintana FJ. Tolerogenic dendritic cells. Semin Immunopathol 2017; 39(2):113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thatcher TH, Maggirwar SB, Baglole CJ, et al. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol 2007; 170(3):855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsoumakidou M, Tousa S, Semitekolou M, et al. Tolerogenic signaling by pulmonary CD1c+ dendritic cells induces regulatory T cells in patients with chronic obstructive pulmonary disease by IL-27/IL-10/inducible costimulator ligand. J Allergy Clin Immunol 2014; 134(4):944–954.e8. [DOI] [PubMed] [Google Scholar]
- 71.Sorrentino R, Gray P, Chen S, et al. Plasmacytoid dendritic cells prevent cigarette smoke and Chlamydophila pneumoniae-induced Th2 inflammatory responses. Am J Respir Cell Mol Biol 2010; 43(4):422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Pottelberge GR, Bracke KR, van den Broeck S, et al. Plasmacytoid dendritic cells in pulmonary lymphoid follicles of patients with COPD. Eur Respir J 2010; 36(4):781–791. [DOI] [PubMed] [Google Scholar]
- 73.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS ONE 2009; 4(1):e4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim TH, Lee HK. Differential roles of lung dendritic cell subsets against respiratory virus infection. Immune Netw 2014; 14(3):128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hemann EA, Sjaastad LE, Langlois RA, et al. Plasmacytoid dendritic cells require direct infection to sustain the pulmonary influenza a virus-specific CD8 T cell response. J Virol 2015; 90(6):2830–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boogaard I, van Oosten M, van Rijt LS, et al. Respiratory syncytial virus differentially activates murine myeloid and plasmacytoid dendritic cells. Immunology 2007; 122(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med 2006; 203(5):1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsuchida T, Matsuse H, Fukahori S, et al. Effect of respiratory syncytial virus infection on plasmacytoid dendritic cell regulation of allergic airway inflammation. Int Arch Allergy Immunol 2012; 157(1):21–30. [DOI] [PubMed] [Google Scholar]
- 79.Schwarze J, Hamelmann E, Bradley KL, et al. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest 1997; 100(1):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lynch JP, Werder RB, Loh Z, et al. Plasmacytoid dendritic cells protect from viral bronchiolitis and asthma through semaphorin 4a-mediated Treg expansion. J Exp Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Araújo EF, de, Medeiros DH, Galdino NAdL, et al. Tolerogenic plasmacytoid dendritic cells control paracoccidioides brasiliensis infection by inducting regulatory T Cells in an IDO-dependent manner. PLoS Pathog 2016; 12(12):e1006115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Araújo EF, Feriotti C, Galdino NAdL, et al. The IDO-AhR axis controls Th17/Treg immunity in a pulmonary model of fungal infection. Front Immunol 2017; 8:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davids M, Pooran AS, Pietersen E, et al. Regulatory T cells subvert mycobacterial containment in patients failing extensively drug-resistant TB treatment. Am J Respir Crit Care Med 2018. [DOI] [PubMed] [Google Scholar]
- 84.Geijtenbeek TBH, van Vliet SJ, Koppel EA, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med 2003; 197(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsu P, Santner-Nanan B, Hu M, et al. IL-10 potentiates differentiation of human induced regulatory T cells via STAT3 and Foxo1. J Immunol 2015; 195(8):3665–3674. [DOI] [PubMed] [Google Scholar]
- 86.Gringhuis SI, den Dunnen J, Litjens M, et al. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 2007; 26(5):605–616. [DOI] [PubMed] [Google Scholar]
- 87.Unger WWJ, Laban S, Kleijwegt FS, et al. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD-L1. Eur J Immunol 2009; 39(11):3147–3159. [DOI] [PubMed] [Google Scholar]
- 88.Horibe EK, Sacks J, Unadkat J, et al. Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion. Transpl Immunol 2008; 18(4):307–318. [DOI] [PubMed] [Google Scholar]
- 89.Domogalla MP, Rostan PV, Raker VK, et al. Tolerance through education: How tolerogenic dendritic cells shape immunity. Front Immunol 2017; 8:1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boks MA, Kager-Groenland JR, Haasjes MSP, et al. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction: A comparative study of human clinical-applicable DC. Clin Immunol 2012; 142(3):332–342. [DOI] [PubMed] [Google Scholar]
- 91.Stiehm M, Peters K, Wiesmüller K-H, et al. A novel synthetic lipopeptide is allergy-protective by the induction of LPS-tolerance. Clin Exp Allergy 2013; 43(7):785–797. [DOI] [PubMed] [Google Scholar]
- 92.Gorelik L, Kauth M, Gehlhar K, et al. Modulation of dendritic cell function by cowshed dust extract. Innate Immun 2008; 14(6):345–355. [DOI] [PubMed] [Google Scholar]
- 93.Schülke S, Fiedler A-H, Junker A-C, et al. Critical role of mammalian target of rapamycin for IL-10 dendritic cell induction by a flagellin A conjugate in preventing allergic sensitization. J Allergy Clin Immunol 2017. [DOI] [PubMed] [Google Scholar]
- 94.Stein K, Brand S, Jenckel A, et al. Endosomal recognition of Lactococcus lactis G121 and its RNA by dendritic cells is key to its allergy-protective effects. J Allergy Clin Immunol 2017; 139(2):667–678.e5. [DOI] [PubMed] [Google Scholar]
- 95.Karumuthil-Melethil S, Gudi R, Johnson BM, et al. Fungal β-glucan, a Dectin-1 ligand, promotes protection from type 1 diabetes by inducing regulatory innate immune response. J Immunol 2014; 193(7):3308–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters M, Guidato PM, Peters K, et al. Allergy-protective arabinogalactan modulates human dendritic cells via C-type lectins and inhibition of NF-κB. J Immunol 2016; 196(4):1626–1635. [DOI] [PubMed] [Google Scholar]
- 97.Maaske A, Devos FC, Niezold T, et al. Mucosal expression of DEC-205 targeted allergen alleviates an asthmatic phenotype in mice. J Control Release 2016; 237:14–22. [DOI] [PubMed] [Google Scholar]
- 98.Peters M, Kauth M, Scherner O, et al. Arabinogalactan isolated from cowshed dust extract protects mice from allergic airway inflammation and sensitization. J Allergy Clin Immunol 2010; 126(3):648–656.e1-4. [DOI] [PubMed] [Google Scholar]
- 99.Oefner CM, Winkler A, Hess C, et al. Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs. J Allergy Clin Immunol 2012; 129(6):1647–1655.e13. [DOI] [PubMed] [Google Scholar]
- 100.Smits HH, Engering A, van der Kleij D, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol 2005; 115(6):1260–1267. [DOI] [PubMed] [Google Scholar]
- 101.Gutzmer R, Köther B, Zwirner J, et al. Human plasmacytoid dendritic cells express receptors for anaphylatoxins C3a and C5a and are chemoattracted to C3a and C5a. J Invest Dermatol 2006; 126(11):2422–2429. [DOI] [PubMed] [Google Scholar]
- 102.Stiehm M, Bufe A, Peters M. Proteolytic activity in cowshed dust extracts induces C5a release in murine bronchoalveolar lavage fluids which may account for its protective properties in allergic airway inflammation. Thorax 2013; 68(1):31–38. [DOI] [PubMed] [Google Scholar]
- 103.Pacciani V, Gregori S, Chini L, et al. Induction of anergic allergen-specific suppressor T cells using tolerogenic dendritic cells derived from children with allergies to house dust mites. J Allergy Clin Immunol 2010; 125(3):727–736. [DOI] [PubMed] [Google Scholar]
- 104.Kim WS, Kim H, Kwon KW, et al. Cisplatin induces tolerogenic dendritic cells in response to TLR agonists via the abundant production of IL-10, thereby promoting Th2- and Tr1-biased T-cell immunity. Oncotarget 2016; 7(23):33765–33782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ning Y, Xu D, Zhang X, et al. β-glucan restores tumor-educated dendritic cell maturation to enhance antitumor immune responses. Int J Cancer 2016; 138(11):2713–2723. [DOI] [PubMed] [Google Scholar]