Short abstract
Macrophages are presents in the tumor microenvironment and acquire different phenotypic and functional characteristics in response to microenvironmental signals. Macrophages can be differentiated into two phenotypes: M1 or pro-inflammatory (classically activated), and M2 or anti-inflammatory macrophage (alternatively activated). In response to the microenvironment, macrophages activate transcription factors as STAT1 and NF-κB-p65 for M1 macrophages or STAT3 and STAT6 for M2 macrophages; activation impacts on the profile of cytokine, chemokines and growth factors secreted by macrophages. We evaluated the effect of the supernatant of cervical-derived carcinoma cell lines HeLa, SiHa, and C-33A on the phosphorylation of transcriptional factors STAT1, NF-κB-p65, and STAT6, and their impact in the profile of secretion of cytokines and growth factors by macrophages derived from the U937 cell line. The results show that in macrophages, these supernatants induce a decrease in the phosphorylation of NF-κB-p65 and STAT1 in U937-macrophages accompanied by an increase in the secretion of IL-10, IL-6, MCP-1, and IL-8, as well as GM-CSF, G-CSF, PDGF-AA, PDGF-BB, and VEGF. Our results suggest that HeLa, SiHa, and C-33A cell lines down-regulate the activation of transcription factors characteristic of M1 macrophages (STAT1, NF-κB-p65) and induce the secretion of factors that favor tumor growth.
Keywords: Cervical cancer cell lines, cytokines, growth factors, macrophages, transcription factors
Introduction
The tumor microenvironment is key to understanding how it contributes significantly to cancer progression.1 Tumor cells are in direct contact with other cells, such as fibroblasts and endothelial and immune system cells.2 Various immune effector cells with anti-tumor functions, such as dendritic cells (DC), NK cells, macrophages, and CD8+ T lymphocytes, can be recruited to the tumor microenvironment. However, over time, via secretion of soluble molecules such as growth factors and cytokines by the tumor cells, as well as mechanisms of direct contact between tumor cells and the immune system, cells are able to educate immune cells in order to minimize or turn off their anti-tumor functions, which finally promotes tumor growth.3,4 Macrophages in the tumor microenvironment are called tumor-associated macrophages (TAM) and constitute a significant part of the leukocytic infiltrate in this microenvironment.5–8 The role of macrophages here is controversial; however, there is a tendency for these cells to be related with poor prognosis in several cancer types, including cervical cancer (CC).9,10 During tumor progression, circulating monocytes are recruited into the tumor in response to growth factors such as Colony-stimulating Factor-1 (CSF-1), Vascular Endothelial Growth Factor (VEGF), and Monocyte Chemotactic Protein-1 (CCL2/MCP-1), CCL-3, -4, -5, and -8 chemokines, as well as other molecules.11 Once they have migrated into the tumor mass, monocytes are differentiated into macrophages, which exhibit different phenotypical and functional characteristics in response to microenvironmental signals generated by tumor and stromal cells. In general, macrophages can be divided into two phenotypes with opposite functions.
Classically activated macrophages (M1 macrophages) can be induced by IFN-γ and microbial components such as LPS; these signals dictate a transcriptional response in macrophages that shapes the phenotype and function of these cells. M1 macrophages are controlled mostly by Signal Transducers and Activators of Transcription 1 (STAT1), as well as by NF-κB.12 In response to the activation of these factors a secretion high of pro-inflammatory cytokines such as IL-1, TNF-α, IL-12, IL- 6, IL-23, as well as Reactive Oxygen Species (ROS), and nitrogen intermediates are induced, which can induce death in cancer cells.7,13
In contrast, alternatively activated macrophages (M2 macrophages) can be induced by distinct stimuli, and are additionally classified as M2a, M2b, and M2c.7,14 M2 macrophage responses are triggered primarily through activation of STAT3 and STAT6; these macrophages are characterized by low secretion of pro-inflammatory cytokines, and instead, they express markers related with immune-suppressive phenotypes such as Arg-1, Ym1, FIZZ1, the mannose (CD206) and scavenger receptors (CD163), IL-10, and chemokines such as CCL-17, CCL-22, and CCL-24. M2 macrophages release a variety of growth factors, such as VEGF, Epidermal Growth Factor (EGF), and Fibroblast Growth Factor (FGF); factors that, in a tumor environment, provide a nutritional advantage for cancer cells.7,11,13 Cancer cells secrete various soluble molecules that can promote their growth and differentiation, and, at the same time, can modulate the immune response to create an optimal environment for the tumor. Among the cytokines and growth factors delivered by tumor cells that favor differentiation into M2 macrophages are IL-10, CCL chemokines, VEGF, and Platelet-Derived Growth Factor (PDGF), among others.13,15–17 CC remains one of the most common cancer types among women in developing regions.18 In nearly all the cases, CC is accompanied by persistent infection with some high-risk subtype of human papilloma viruses (HPV) such as HPV-16 or HPV-18. HPV can use several mechanisms to down-regulate the innate and cell-mediated immune response, allowing host immune evasion and persistent infection.19 In this sense, it has been observed that differentiation from monocyte to dendritic cells is hampered and that, instead, monocytes are differentiated toward M2 macrophages due to the production of PGE2 and IL-6 secreted by CC cell lines.20 We recently demonstrated that the supernatant of CC-derived cells induced the change from M1 to M2 macrophages.21 In this study, we evaluated the effect of supernatants from cervical-derived carcinoma cells on the phosphorylation of transcriptional factors STAT1, NF-kB-p65, and STAT6 and their impact in the secretion of cytokines and growth factors by macrophages derived from U937 cells.
Materials and methods
Cell culture
Cervical derived carcinoma cell lines HeLa (HPV-18-positive), SiHa (HPV-16-positive), and C-33A (HPV-negative) were kindly provided by Petra Boukamp PhD (DKFZH, Heidelberg, Germany). Human leukemic monocyte-lymphoma cell line U937 was obtained from the American Type Culture Collection (ATCC CRL- 1593.2TM). Cervical derived carcinoma cell lines were grown in DMEM containing 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin (all from GIBCO™ Invitrogen Corp., Carlsbad, CA, USA). U937 cells were grown in Roswell Park Memorial Institute (RPMI)-1640 culture medium supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin (all from GIBCO™ Invitrogen Corp.). These media will be referred to as DMEM-S and RPMI-S; respectively. All cell lines were incubated at 37°C in a humidified atmosphere with 5% of CO2.
Supernatant of cervical derived carcinoma cell lines HeLa, SiHa, and C-33A
Cervical derived carcinoma cell lines HeLa, SiHa, and C-33A were grown in 75 cm2 flasks until reaching 80–90% confluence. Cells were harvested using trypsin-EDTA solution (GIBCO® Life Technologies Corp.). A total of 250,000 cells were plated into a 25 cm2 flask containing 6 ml of DMEM-S. Cultures were maintained at 37°C in a humidified atmosphere with 5% of CO2; after 5 d of incubation the supernatant was collected under sterile conditions,20 centrifuged at 400 g for 10 min, and filtered through a membrane (0.2 µm, Millipore Corp.). The supernatant was stored at –80°C until use.
Differentiation and activation of the U937 monocyte cell line toward the macrophage
For complete differentiation of U937 cells into macrophages, 1 × 106 U937 cells were plated into 12-well tissue culture plates containing 2 ml of RPMI-S at a final concentration of 200 nM of Phorbol Myristate Acetate (PMA) and incubated for 3 d.22 Adherent cells were washed three times with PBS. These cells will be referred from now as M0 macrophages.
Activation of the macrophages was achieved by incubating M0 macrophages previously differentiated in the presence of 100 ng/ml of LPS for 24 h.23 After this, the medium containing LPS was removed completely, and cells were washed with PBS. These cells are referred to as M1 macrophages.
Likewise, M0 macrophages were treated with 20 ng/ml of IL-10 (recombinant human IL-10, BioLegend, San Diego, CA, USA) for 24 h, after that the cells were washed. These cells are referred to as M2 macrophages.
All incubations were at 37°C in a humidified atmosphere with 5% of CO2.
Culture of macrophages in conditioned medium containing supernatants of cervical derived carcinoma cell lines
Previously obtained M0 and M1 macrophages were cultured in a total volume of 2 ml of RPMI-S containing 30% of the supernatant of HeLa, SiHa, or C-33A cell lines for 1 h; subsequently, the cells were harvested with Accutase (StemPro® Accutase®; Thermo Fisher Scientific, Waltham, MA, USA) and stained for assessment of the phosphorylation of transcription factors (STAT1, STAT6, and NF-κB-p65) by Flow Cytometry (FC).
For assessment of cytokines and growth factors, M0 macrophages were cultured in RPMI-S containing 30% of the supernatants of HeLa, SiHa, or C-33A cell lines or for 3 and 5 d at 37°C in a humidified atmosphere with 5% of CO2. We used M1 and M2 macrophages like experimental controls of this assays. Afterward, the supernatant was collected under sterile conditions, centrifuged at 400 g for 10 min, and stored at –80°C until determination of cytokines and growth factors.
Cytokine and growth factor quantification by flow cytometry
Bead-based multiplex assays were employed to quantify cytokines (LEGENDplex™ Human Inflammation Panel) and growth factors (LEGENDplex™ Human Growth Factor Panel; both kits from BioLegend, Inc., San Diego, CA, USA) in supernatants of cervical derived carcinoma cell lines and in the supernatant from macrophages incubated or not with tumor cell supernatant.
Briefly, 25 µl of thawed supernatants, diluted standard, and blanks were added into the corresponding tubes; 25 µl of pre-mixed beads and detection Abs were added to all of the tubes. Then, the tubes were incubated for 2 h at room temperature with shaking. After this, and without washing, 25 µl of StreptAvidin- PhycoErythrin (SA-PE) conjugate was added, and the tubes were incubated for 30 min and finally washed and suspended in 200 µl of wash buffer. Data were acquired in an Attune Acoustic Focusing Cytometer (Life Technologies, Carlsbad, CA, USA). A total of 4000 and 2000 events were acquired for inflammation panel or growth factor panel; respectively. The files were analyzed utilizing BioLegend LEGENDplex™ Data Analysis Software. Results represent the concentration expressed in pg/mL.
TGF-β1 and PGE-2 quantification in the supernatants of cervical derived carcinoma cell lines HeLa, SiHa, and C-33A
Identification of TGF-β1 and PGE-2 was performed by ELISA using TGF-β1 immunoassay and Prostaglandin E2 Assay Kits (both R&D Systems), respectively. The assay was made following the manufacturer’s instructions. The OD was determined using a microplate reader (SynergyTM HT Multi-Mode Microplate Reader; Bio Tek Instruments, Inc., Winooski, VT, USA) at 450 nm (wavelength correction at 540 nm). Results represent the concentration expressed in pg/mL.
Antibodies
Fluorochrome-labeled monoclonal primary Abs for staining were purchased from BD Biosciences (BD Biosciences, San Jose, CA, USA), and the following were utilized: PE anti-human STAT1-(N-terminal) (Clone 1/Stat1); PE anti-human STAT1 (pY701) (Clone 4a); PE anti-human STAT6 (Clone 23/Stat6); PE anti-human STAT6 (pY641) (Clone 18), and Alexa-Fluor 647 anti-human NF-κB-p65 (pS532) (Clone K10-895.12.50).
Assessment of transcription factors STAT1, STAT6, and NF-κB-p65 by FC
Expression transcription factors in M0 and M1 macrophages treated or not for 1 h with the supernatant of cervical-derived carcinoma cell lines HeLa, SiHa, or C-33A were assessed by FC analysis. Briefly, M0 or M1 macrophages in assay tubes were stained with LIVE/DEAD® viability stain (Life Technologies Corp.) for 30 min to discriminate between viable and non-viable cells. Afterward, cells were washed first with PBS and then with stain buffer (FBS) (BD Pharmingen, San Jose, CA, USA), re-suspended by vortexing in 250 µl of Fixation buffer (BD™ Phosflow) and immediately incubated for 20 min at 4°C. Fixed cells were washed with stain buffer and immediately permeabilized, by slowly adding and under vortex conditions, the cold Perm Buffer III (BD™ Phosflow). Cells were incubated on ice for 30 min and then washed with 2 ml of stain buffer. Cells were re-suspended in 100 µl of stain buffer and were stained with the corresponding Abs for 30 min at 4°C. Afterwards, the incubated cells were washed and re-suspended in stain buffer for analysis by FC. Attune Acoustic Focusing Cytometer (Life Technologies, Carlsbad, CA, USA) was utilized to acquire 20,000 events in the region of viable cells. Data were processed with FlowJo ver. 10.0.8 statistical software (Tree Star, Inc., Ashland, OR, USA). Results are reported as percentage of expression or as the geometric Mean Fluorescence Intensity (MFI).
Statistical analysis
The data obtained are shown as means ± SD of the values of three independent experiments. Comparisons among groups were performed with Student t-test. Only values of P < 0.05 were considered significant.
Results
Secretion of MCP-1, IL-6, and IL-8 by cervical derived carcinoma cell lines HeLa, SiHa, and C-33A
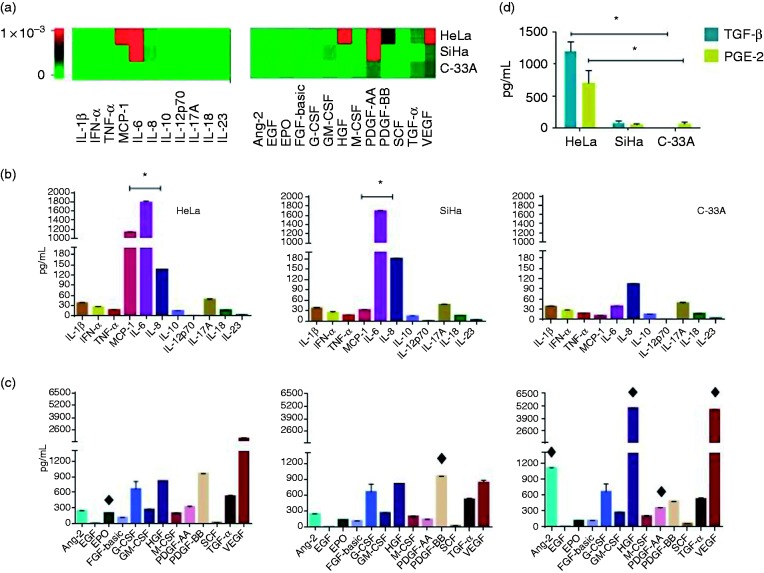
We determined in the supernatant of HeLa, SiHa (HPV-18 and -16-positive; respectively) and C-33A (HPV-negative) cell lines, the profile of secretion of cytokines and growth factors using bead-based multiplex kits. The concentrations of cytokines and growth factors for each supernatant are depicted in Figure 1A, where heatmaps are depicted representing changes in the profile of cytokines (left) and growth factors (right) secreted by HeLa, SiHa, and C-33A cervical derived carcinoma cell lines in their respective supernatants. Interestingly, MCP-1, IL-6, and IL-8 were secreted by these cancer cells (Figure 1B), HeLa and SiHa released higher amounts of these cytokines compared with C-33A (all comparisons with a significant difference of *p < 0.001).
Figure 1.
Secretion of cytokines, growth factors, TGF-β1 and PGE-2 by cervical derived carcinoma cell lines HeLa, SiHa, and C-33A. Cell lines were cultured for 5 d, and cytokines and growth factors were quantified using bead-based multiplex assays by flow cytometry. TGF-β1 and PGE-2 were determined by ELISA. (A) Heat maps for cytokines (left) and growth factors (right) generated by LEGENDplex™ Data Analysis software. Graphics illustrate the median ± SD of concentrations in pg/ml of cytokines (B), growth factors (C), and TGF-β1 and PGE-2 (D). *p < 0.001 to each comparison between HeLa, SiHa, and C-33A regarding cytokines; ♦p < 0.05 in HeLa, SiHa, and C-33A regarding growth factors; *p < 0.001 HeLa vs SiHa and C-33A regarding TGF-β1 and PGE-2.
Release of VEGF, PDGF-AA, PDGF-BB, HGF, EPO, Ang-2, G-CSF as well as TGF-β1 and PGE-2 by HeLa, SiHa, and C-33A cell lines
The concentrations of growth factors released by cervical derived carcinoma cell lines are presented in Figure 1C. In general, we observed that VEGF, PDGF-BB, HGF, Ang-2, and G-CSF were secreted in high amounts in all CC cells. It is important to highlight that EPO and PDGF-BB were found in high quantities in HeLa and SiHa supernatants in comparison with C-33A supernatant (♦p < 0.05).
On the other hand, in C-33A supernatant, were found HGF, VEGF, Ang-2, and PDGF-AA in elevated concentrations compared with the HeLa and SiHa supernatants (♦p < 0.05). In relation to TGF-β1 and PGE-2 secreted by cervical derived carcinoma cell lines, we found that HeLa is the primary cell line that produces these intermediaries in relation to SiHa that produces a smaller amount of these two factors or C-33A that only release PGE-2 in a low proportion (*p < 0.001) HeLa vs SiHa and C33-A.
Effect of HeLa, SiHa, and C-33A supernatants in cytokine production by macrophages
We also evaluated the effect of supernatant of cervical derived carcinoma cells on the secretion of cytokines by macrophages cultured in the presence of supernatant for 3 and 5 d with respect to M0 (only differentiated with PMA), M1 (differentiated with PMA and activated with LPS), and M2 macrophages (macrophages differentiated with PMA and incubated in the presence of IL-10).
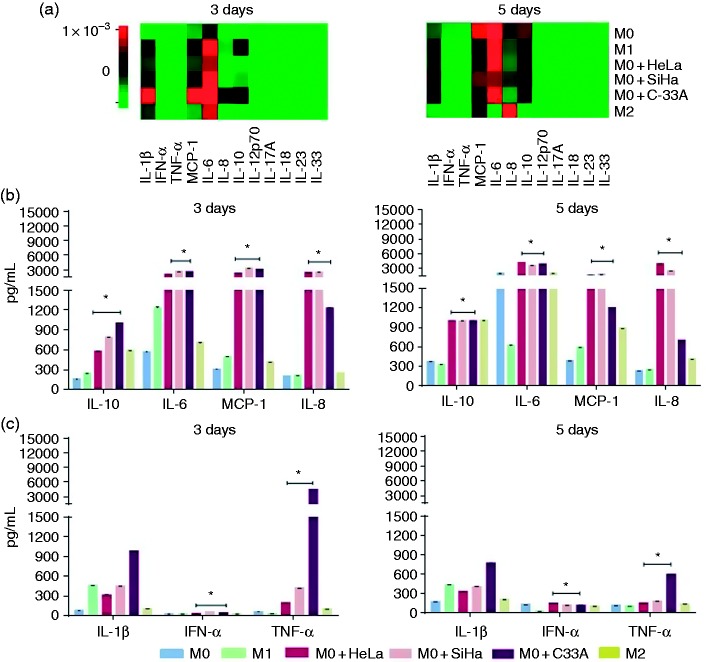
Cytokines were measured in supernatants of macrophages by using the bead-based multiplex assay. The heatmaps in Figure 2A provide evidence about the differential amounts of cytokines secreted by macrophages incubated or not with supernatants for 3 and 5 d; respectively. Figure 2B shows the increase in the secretion of IL-10, IL-6, MCP-1 and IL-8 cytokines at 3 and 5 d compared with M0 and M1 macrophages (*p < 0.01). Another interesting fact was the increase in the secretion of TNF-α and IL-1β by macrophages cultivated with supernatant of C-33A cervical carcinoma cell line in comparison with all experimental groups in both 3 and 5 d (*p < 0.01; Figure 2C). Finally, macrophages increased the secretion of IFN-α in the presence of supernatants of HeLa, SiHa, and C-33A in comparison to M0, M1, and M2 macrophages (*p < 0.01; Figure 2C).
Figure 2.
HeLa, SiHa, and C-33A supernatants induced the secretion of IL-10, IL-6, MCP-1, and IL-8 by M2 macrophages compared with M0 and M1 macrophages. Macrophages were obtained by differentiation of the U937 cell line with PMA (M0 macrophages), stimulation with LPS (M1 macrophages), treatment with IL-10 (M2 macrophages), or cultivation in the presence of 30% of HeLa, SiHa, or C-33A for 3 and 5 d (see Materials and methods). The supernatant was collected again, and cytokines were quantified using bead-based multiplex assay by flow cytometry. (A) Heatmaps generated by LEGENDplex™ Data Analysis software, representing the changes in the concentrations of cytokines on d 3 (left) and 5 (right) in each experimental group. (B) and (C) More representative cytokines are shown, based on the corresponding analysis for macrophages cultured in the presence or not of HeLa, SiHa, and C-33A supernatants for 3 and 5 d; respectively. The results of each experimental condition were obtained from assays conducted in triplicate and are represented as the mean ± SD of the concentrations in pg/ml. *p < 0.01 in HeLa, SiHa, and C-33A vs M0 and M1.
Induction of growth factors in macrophages treated with the supernatant of HeLa, SiHa, and C-33A cell lines
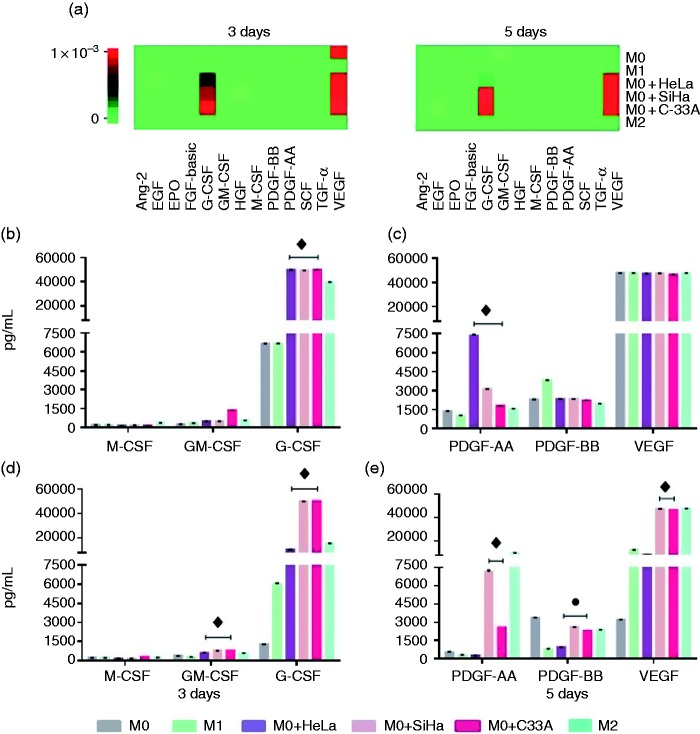
It has been reported that macrophages associated with tumors possess the ability to release growth factors that can contribute to tumor growth. To evaluate the effect of the supernatant of cervical derived carcinoma cell lines on the secretion of growth factors by macrophages, supernatants from macrophages cultured under the conditions described previously were recollected and analyzed by FC based on a bead-based multiplex assay. In Figure 3A, the heatmaps are shown for growth factors secreted by macrophages at 3 (left) and 5 d (right) of culture for the different experimental groups, in general, it can be observed that macrophages incubated with the different supernatants secreted G-CSF and VEGF.
Figure 3.
Secretion of VEGF, PDGF, GM-CSF, and G-CSF by macrophages is increased by the addition of HeLa, SiHa, and C-33A supernatants. Macrophages were obtained by differentiation of the U937 cell line with PMA (M0 macrophages), stimulation with LPS (M1 macrophages) treatment with IL-10 (M2 macrophages), or incubation in the presence of 30% of HeLa, SiHa, or C-33A for 3 and 5 d (see Materials and methods). Growth factors were quantified using bead-based multiplex assay by flow cytometry. (a) Heatmaps generated by LEGENDplex™ Data Analysis software, representing changes in the concentrations of growth factors at d 3 and 5 for each experimental group. (b)–(e) The most representative growth factors were graphed based on the corresponding analyses for different macrophages. Results of each experimental condition were obtained from assays conducted in triplicate and are represented as the mean ± SD of concentrations in pg/ml. ♦p < 0.05 HeLa, SiHa, and C-33A vs M0 and M1; ● p < 0.05 HeLa, SiHa, and C-33 vs M1; ♦ p < 0.05 SiHa and C-33A vs M0 and M1.
We observed that, on d 3 of culture the concentration of G-CSF was increased in HeLa, SiHa, and C-33A groups in comparison with M0 and M1 (Figure 3B); in addition, the amount of G-CSF, as well as GM-CSF, were increased by macrophages in the presence of HeLa, SiHa, and C-33A supernatants vs. M0 and M1 groups at d 5 of culture (Figure 3C; ♦p < 0.05). On the other hand, at d 3, as shown in Figure 3C, the secretion of PDGF-AA was induced in macrophages by the effect of the three supernatants in comparison with M0 and M1 macrophages (♦p < 0.05). At 5 d of culture of the macrophages in their different conditions, we observed an increase of PDGF-BB secretion by effect of HeLa, SiHa, and C-33A supernatants in comparison with M1 (●p < 0.05), Figure 3E, while SiHa and C-33A increased the release of PDGF-AA and VEGF compared with M0 and M1 macrophages (♦p < 0.05).
Hela, SiHa, and C-33A supernatants induce a decrease in the phosphorylation of STAT1 and NF-κB-p65
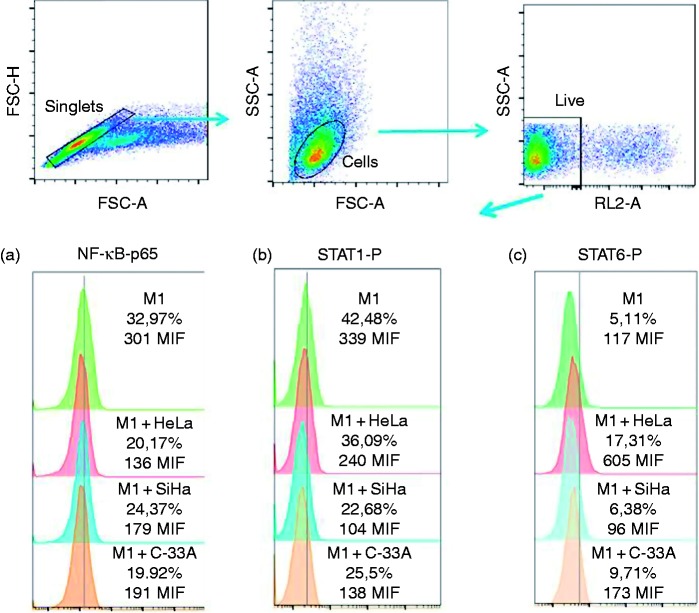
Macrophage responds to different stimuli of the microenvironment that affects the profile of phosphorylation of its transcriptional factors, which will determine the phenotype of secretion and expression of markers corresponding to each phenotype. We evaluated by FC the phosphorylation of NF-κB-p65, STAT1, and STAT6. Our results are presented in Figure 4. We observed that the supernatants of HeLa and C-33A induced a significant decrease in the phosphorylation of NF-κB-p65 in M1 macrophages. Interestingly, HeLa, SiHa, and C-33A supernatants induced a significant decrease in phosphorylated STAT1 in M1 macrophages. Finally, we observed that there was a tendency toward the increase of the percentage of expression of STAT6-P due to the effect of supernatants in macrophages, especially in the case of the HeLa supernatant.
Figure 4.
Phosphorylation of STAT1 (Y701) and NF-κB-p65 is decreased in macrophages due to HeLa, SiHa, and C-33A supernatants. Macrophages were obtained by differentiation of the U937 cell line with PMA (M0 macrophages), stimulation with LPS (M1 macrophages), cultured in the presence or not of 30% of HeLa, SiHa, or C-33A for 1 h (see Materials and methods), and harvested and processed by intracellular staining for measuring the expression of NF-κB-p65, STAT1(Y701), and STAT6 (Y641). Results are shown as % and MFI. Representative flow cytometric graphs of three independent experiments performed in triplicate are shown.
Supernatants of HeLa, SiHa, and C-33A did not induce any effect in these transcription factors in M0 macrophages (data not shown).
Discussion
In response to the tumor microenvironment, macrophages can acquire an immunosuppressive phenotype that promotes growth, cell invasion, neovascularization, and metastases. Our data are derived from macrophages exposed to a microenvironment generated by soluble factors secreted by CC cells lines. First, as a result of the characterization of the tumor cell supernatant, it was interesting to observe that HeLa and SiHa produce a higher concentration of IL-8, MCP-1, and IL-6. These factors could be related to the presence of high-risk HPV, such as HPV-18 and -16, in HeLa and SiHa cell lines, respectively. Expression of IL-8 and MCP-1 at the mRNA and protein levels has been reported in other tumor cells, such as human esophageal carcinoma cell lines, in breast and prostate cancer, in which these have been related positively with the recruitment of immunosuppressive macrophages and angiogenesis.24–28 IL-8 is a factor related to tissue injury, fibrosis, and angiogenesis, and also it is considered a growth factor for tumor cells.29 Additionally, IL-8 has been found in higher concentrations in serum of patients with prostate cancer, hepatocellular carcinoma, and in those patients positive for HPV infection in comparison with healthy controls.30–33 MCP-1 is expressed in various types of cancer, and, in turn, its expression in tumor cells is significantly correlated with the infiltration of TAM in the tumor site.34,35 Production of IL-6 has been reported for other human cell lines of liver and prostate carcinoma, among others.36–38 IL-6 also has been reported in CC in in vitro studies and dysplastic lesions, especially in epithelial cells of high-grade CIN.38 We previously reported the secretion of IL-6 by CC cell lines, and Heusinkveld et al. demonstrated that IL-6 and PGE-2 secreted by CC cell lines were key factors for inducing M2 macrophages with the suppressor phenotype.20,21 Underlining the role of this cytokine in macrophage polarization, it has been found in a model of insulin resistance that IL-6 promotes the M2 phenotype in macrophages, and Chastain et al. demonstrated recently that the treatment of RAW cell macrophages with IL-6 up-regulated the expression of characteristic markers of M2 macrophages, like CD206 and IL-4.39,40 It is important to stress that we observed a high secretion of TGF-β1 and PGE-2 by the HeLa carcinoma derived cell line. TGF-β1 can regulate innate and acquired immunity by inducing regulatory T cells, which can suppress T effector cells. This cytokine is able to change NKT cells and macrophages to the regulatory phenotype. In addition to the impact on regulation of the immune system, TGF-β1 and IL-6 act as promoters of Epithelium Mesenchymal Transition (EMT).41 Taken together, HeLa and SiHa have a secretion profile of cytokine and chemotactic factors that collaborates with establishment and growth of the tumor. Also, its secreted factors cooperate in the infiltration of immune cells as monocytes that upon arrival at the tumor niche are influenced by a microenvironment dominated by cytokines like IL-6, TGF-β1, and PGE-2, which promote a suppressor phenotype. PDGF-BB is an essential component of the angiogenic process since it is involved in the maturation of the vessel inside the tumor microenvironment.42 VEGF is considered one of the more potent angiogenic factors; PDGF and VEGF were found in the supernatants of C-33A, HeLa, and SiHa. Their co-expression has been observed in breast cancer as well as in other cancer types.43 Additionally, it has been reported that the binding of PDGF to its receptor, and subsequent activation of the signaling pathway, induces VEGF secretion in ovarian epithelial carcinoma.44 In breast cancer and pancreatic cell lines, both factors are considered mitogenic in a paracrine or autocrine loop resulting in tumor growth and, specifically for PDGF, its involvement has been suggested in EMT that leads to tumor growth, angiogenesis, invasion, and metastasis in solid tumors such as CC.45–49 C33-A, despite being a cell line negative to HPV infection, was the main cell line secreting a significant amount of HGF and Ang-2, in addition to PDGF-AA and VEGF. The presence of HGF has also been reported in ovarian cancer cells, where it was associated with the mesothelial–mesenchymal transition, one of the key mechanisms in CC, as well as the invasion of mesothelial cells.50 The HGF/MET complex induced the expression of VEGF and synergized with the VEGF receptor (VEGFR), favoring angiogenesis and lymph-angiogenesis increasing tumor malignancy.51 The importance of Ang-2 lies in its potential to cooperate with the tumorigenic process related to invasiveness since it is co-overexpressed with some MMP, and is involved in lymph-angiogenesis and angiogenesis acting synergistically with the effects mediated by VEGF.52,53 The specific nature of each CC cell can determine a characteristic pattern of concentration of the different cytokines, TGF-β1, PGE-2, and growth factors measured. Practically all factors secreted significantly by the cell lines are involved in autocrine signaling to promote their growth and proliferation. These factors are closely related to tumorigenic processes such as angiogenesis, and lymph-angiogenesis. The improvement of the invasive capacity towards surrounding tissues establishes new tumor niches and immunosuppression.
Here, the effect of supernatants from CC cells on the phosphorylation of transcriptional factors and their effect on U937 macrophages regarding their secretion profile of cytokines and growth factors was evaluated. This cell line has been a widely used model to elucidate a variety of biological mechanisms related to monocyte and macrophage functions.54–56 In this sense, it is important to stress that the supernatants from CC HeLa, SiHa, and C-33A cells induced in macrophages an increase of the secretion of IL-10, IL-6, MCP-1 and IL-8, as well as of GM-CSF, G-CSF, PDGF-AA, PDGF-BB, and VEGF. Moreover, it is important to mention that IL-10 is an anti-inflammatory and immunosuppressive cytokine that can suppress the cytotoxic activity of T and NK cells, down-regulate Ag presentation, and prevent the maturation of DC in situ, favoring macrophage differentiation.57–60 IL-6 directly effects angiogenesis since this contributes to vascularization in ovarian cancer, but also can induce the release of VEGF from CC and glioblastoma cells and other inflammatory cytokines with angiogenic power, such as IL-8.61–63 Angiogenesis represents an essential process for the successful establishment and growth of tumor cells. Moreover, angiogenic factors as VEGF and IL-8 were released by macrophages treated with the supernatants of HeLa, SiHa, and C-33A; these could be factors induced in response to IL-6. In addition to angiogenesis, IL-8 favors the infiltration of monocytes and macrophages.64 In a model of human skin carcinoma utilizing the HaCaT cell line, IL-6 also regulated MCP-1 and GM-CSF, which promoted the proliferation and invasiveness of a benign skin tumor to an invasive and well vascularized by the increase of IL-8 and VEGF.63
MCP-1 expression and macrophage infiltration have been correlated with poor prognosis and metastasis in human breast cancer.25,65 Any augmentation of secretion of MCP-1 by macrophages in response to supernatants of HeLa, SiHa, and C-33A cells could result in a poorly immunogenic microenvironment where tumor cells can grow successfully. The increased release of GM-CSF and G-CSF by macrophages treated with supernatants could favor the growth and establishment of these cells with immunosuppressive activity through an autocrine response since G-CSF could act in synergy with IL-6 via STAT3, favoring the M2 phenotype in the macrophage.
Regarding PDGF, it has been shown that a higher concentration of PDGF in TAM is directly related to tumor growth in the healthy lung tissue surrounding the periphery of the tumor.66 Moreover, in breast cancer, PDGF-BB released by TAM can activate fibroblasts and osteoclasts in order to support cancer stem-cell self-renewal.67
HeLa, SiHa, and C-33A supernatants were able to induce an essential decrease in STAT1 phosphorylation as well as of NF-κB-p65 in activated M1 macrophages. Dys-regulation of NF-κB and STAT1 could comprise a mechanism through which an anti-tumor response is reduced by macrophages, because many cytokines and molecules with antitumor activity are secreted in response to the activation of NF-κB and STAT1. Likewise, we observed that the supernatant of HeLa cells induced phosphorylation in STAT6. Taken together, all of this suggested that HeLa, SiHa, and C-33A induce several effects in the macrophage, the main one being disruption of STAT1 phosphorylation, which is an essential pathway for the macrophage to perform anti-tumor and immune responses.
Conclusions
The CC cell lines HeLa, SiHa, and C-33A release cytokines and growth factors that can de-regulate immune responses and also induce growth, immunosuppression, and angiogenic pathway activation. HeLa, SiHa, and C-33A supernatants induced a decrease in STAT1 and p65 phosphorylation in macrophages. Likewise, the supernatants induced the secretion of growth factors, some with angiogenic potential, providing advantages for successful tumor growth.
Acknowledgements
K. Sánchez-Reyes is grateful for scholarships obtained from CONACYT 442856 and IMSS 991429817.
Declaration of conflicting interests
The author(s) declare to have no potential conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant (FIS/IMSS/PROT/G15/1469) from the Instituto Mexicano del Seguro Social (IMSS).
References
- 1.Tsai M-J, Chang W-A, Huang M-S, et al. Tumor microenvironment: A new treatment target for cancer. ISRN Biochem 2014; 2014: 351959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solinas G, Germano G, Mantovani A, et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009; 86: 1065–1073. [DOI] [PubMed] [Google Scholar]
- 3.Edin S, Wikberg ML, Dahlin AM, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. 2012; PLoS One 2012; 7: e47045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiteside T. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008; 27: 5904–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4: 71–78. [DOI] [PubMed] [Google Scholar]
- 6.Mallmann MR, Schmidt SV, Schultze JL. Macrophages in human cancer: Current and future aspects. Atlas Genet Cytogenet Oncol Haematol 2012; 10.4267/2042/48157.
- 7.Hao NB, Lü MH , Fan YH, et al. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012. ; 2012: 948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006; 124: 263–266. [DOI] [PubMed] [Google Scholar]
- 9.Leek RD, Lewis CE, Whitehouse R, et al. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 1996; 56: 4625–4629. [PubMed] [Google Scholar]
- 10.Ding H, Cai J, Mao M, et al. Tumor‐associated macrophages induce lymphangiogenesis in cervical cancer via interaction with tumor cells. APMIS 2014; 122: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 11.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006; 66: 605–612. [DOI] [PubMed] [Google Scholar]
- 12.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32: 593–604. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, He Y, Sun X, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res 2014; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime Rep 2014; 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong L-Y, Wu AS, Doucette T, et al. Intratumoral mediated immunosuppression is prognostic in genetically engineered murine models of glioma and correlates to immunotherapeutic responses. Clin Cancer Res 2010; 16: 5722–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23: 549–555. [DOI] [PubMed] [Google Scholar]
- 17.Green CE, Liu T, Montel V, et al. Chemoattractant signaling between tumor cells and macrophages regulates cancer cell migration, metastasis and neovascularization. 2009; 4(8): e6713. [DOI] [PMC free article] [PubMed]
- 18.IAfRo Cancer: GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. World Health Organization. https://publications.iarc.fr/Databases/Iarc-Cancerbases/GLOBOCAN-2012-Estimated-Cancer-Incidence-Mortality-And-Prevalence-Worldwide-In-2012-V1.0-2012 (Accessed September 2014).
- 19.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol 2009; 10: 321–322. [DOI] [PubMed] [Google Scholar]
- 20.Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, et al. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol 2011; 187: 1157–1165. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez-Reyes K, Bravo-Cuellar A, Hernández-Flores G, et al. Cervical cancer cell supernatants induce a phenotypic switch from U937-derived macrophage-activated M1 state into M2-like suppressor phenotype with change in Toll-like receptor profile. BioMed Res Int 2014; 2014: 683068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daigneault M, Preston JA, Marriott HM, et al. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One 2010; 5: e8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreskin SC, Thomas GW, Dale SN, et al. Isoforms of Jun kinase are differentially expressed and activated in human monocyte/macrophage (THP-1) cells. J Immunol 2001; 166: 5646–5653. [DOI] [PubMed] [Google Scholar]
- 24.Ohta M, Kitadai Y, Tanaka S, et al. Monocyte chemoattractant protein‐1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int J Cancer 2002; 102: 220–224. [DOI] [PubMed] [Google Scholar]
- 25.Ueno T, Toi M, Saji H, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 2000; 6: 3282–3289. [PubMed] [Google Scholar]
- 26.Lu Y, Cai Z, Galson DL, et al. Monocyte chemotactic protein‐1 (MCP‐1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate 2006; 66: 1311–1318. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto J, Sakaguchi H, Aoki I, et al. Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res 2000; 60: 2632–2635. [PubMed] [Google Scholar]
- 28.Freund A, Chauveau C, Brouillet J-P, et al. IL-8 expression and its possible relationship with estrogen- receptor-negative status of breast cancer cells. Oncogene 2003; 22: 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo RC, Garcia CC, Teixeira MM, et al. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol 2014; 10: 593–619. [DOI] [PubMed] [Google Scholar]
- 30.Okada K, Shimizu Y, Tsukishiro T, et al. Serum interleukin-8 levels in patients with hepatocellular carcinoma. Int Hepatol Commun 1994; 2: 178–182. [Google Scholar]
- 31.Kemp TJ, Hildesheim A, García-Piñeres A, et al. Elevated systemic levels of inflammatory cytokines in older women with persistent cervical human papillomavirus infection. Cancer Epidemiol Biomarkers Prev 2010; 19: 1954–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spear GT, Sha B, Saarloos MN, et al. Chemokines are present in the genital tract of HIV-seropositive and HIV-seronegative women: correlation with other immune mediators. JAIDS 1998; 18: 454–459. [DOI] [PubMed] [Google Scholar]
- 33.Moore BB, Arenberg DA, Stoy K, et al. Distinct CXC chemokines mediate tumorigenicity of prostate cancer cells. Am J Pathol 1999; 154: 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato K, Kuratsu J-I, Takeshima H, et al. Expression of monocyte chemoattractant protein-1 in meningioma. J Neurosurg 1995; 82: 874–878. [DOI] [PubMed] [Google Scholar]
- 35.Deshmane SL, Kremlev S, Amini S, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuguchi T, Okamura S, Kawasaki C, et al. Production of interleukin 6 from human liver cell lines: production of interleukin 6 is not concurrent with the production of α-fetoprotein. Cancer Res 1990; 50: 7457–7459. [PubMed] [Google Scholar]
- 37.Siegall CB, Schwab G, Nordan RP, et al. Expression of the interleukin 6 receptor and interleukin 6 in prostate carcinoma cells. Cancer Res 1990; 50: 7786–7788. [PubMed] [Google Scholar]
- 38.Hess S, Smola H, de Silva US, et al. Loss of IL-6 receptor expression in cervical carcinoma cells inhibits autocrine IL-6 stimulation: abrogation of constitutive monocyte chemoattractant protein-1 production. J Immunol 2000; 165: 1939–1948. [DOI] [PubMed] [Google Scholar]
- 39.Mauer J, Chaurasia B, Goldau J, et al. Interleukin-6 signaling promotes alternative macrophage activation to limit obesity-associated insulin resistance and endotoxemia. Nature Immunol 2014; 15: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luckett-Chastain L, Calhoun K, Schartz T, et al. IL-6 influences the balance between M1 and M2 macrophages in a mouse model of irritant contact dermatitis. J Immunol 2016; 196: 17.26685314 [Google Scholar]
- 41.Taja-Chayeb L, Chavez-Blanco A, Martínez-Tlahuel J, et al. Expression of platelet derived growth factor family members and the potential role of imatinib mesylate for cervical cancer . Cancer Cell Int 2006; 6: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue Y, Lim S, Yang Y, et al. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med 2012; 18: 100. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Liu C, Qiu L, et al. Overexpression of both platelet- derived growth factor-BB and vascular endothelial growth factor-C and its association with lymphangiogenesis in primary human non-small cell lung cancer. Diagn Pathol 2014; 9: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matei D, Kelich S, Cao L, et al. PDGF BB induces VEGF secretion in ovarian cancer. Cancer Biol Ther 2007; 6: 1951–1959. [DOI] [PubMed] [Google Scholar]
- 45.Bronzert D, Pantazis P, Antoniades H, et al. Synthesis and secretion of platelet-derived growth factor by human breast cancer cell lines. Proc Natl Acad Sci USA 1987; 84: 5763–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008; 22: 1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Von Marschall Z, Cramer T, Höcker M, et al. De novo expression of vascular endothelial growth factor in human pancreatic cancer: evidence for an autocrine mitogenic loop. Gastroenterology 2000; 119: 1358–1372. [DOI] [PubMed] [Google Scholar]
- 48.Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res 1993; 53: 4727–4735. [PubMed] [Google Scholar]
- 49.Takahashi Y, Kitadai Y, Bucana CD, et al. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995; 55: 3964–3968. [PubMed] [Google Scholar]
- 50.Nakamura M, Ono YJ, Kanemura M, et al. Hepatocyte growth factor secreted by ovarian cancer cells stimulates peritoneal implantation via the mesothelial–mesenchymal transition of the peritoneum. Gynecol Oncol 2015; 139: 345–354. [DOI] [PubMed] [Google Scholar]
- 51.Sulpice E, Ding S, Muscatelli‐Groux B, et al. Cross‐talk between the VEGF‐A and HGF signalling pathways in endothelial cells. Biol Cell 2009; 101: 525–539. [DOI] [PubMed] [Google Scholar]
- 52.Hu B, Guo P, Fang Q, et al. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci USA 2003; 100: 8904–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu B, Cheng S-Y. Angiopoietin-2: development of inhibitors for cancer therapy. Curr Oncol Rep 2009; 11: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Passmore J, Lukey P, Ress S. The human macrophage cell line U937 as an in vitro model for selective evaluation of mycobacterial antigen‐specific cytotoxic T‐cell function. Immunology 2001; 102: 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L, Zubik L, Collins FW, et al. The antiatherogenic potential of oat phenolic compounds. Atherosclerosis 2004; 175: 39–49. [DOI] [PubMed] [Google Scholar]
- 56.Baek Y-S, Haas S, Hackstein H, et al. Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol 2009; 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guruvayoorappan C. Tumor versus tumor-associated macrophages: how hot is the link? Integr Cancer Ther 2008; 7: 90–95. [DOI] [PubMed] [Google Scholar]
- 58.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via down-regulation of class II major histocompatibility complex expression. J Exp Med 1991; 174: 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koppelman B, Neefjes JJ, de Vries JE, et al. Interleukin-10 down- regulates MHC class II αβ peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity 1997; 7: 861–871. [DOI] [PubMed] [Google Scholar]
- 60.Qin Z, Noffz G, Mohaupt M, et al. Interleukin-10 prevents dendritic cell accumulation and vaccination with granulocyte-macrophage colony- stimulating factor gene-modified tumor cells. J Immunol 1997; 159: 770–776. [PubMed] [Google Scholar]
- 61.Cohen T, Nahari D, Cerem LW, et al. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 1996; 271: 736–741. [DOI] [PubMed] [Google Scholar]
- 62.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res 2005; 65: 10794–10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lederle W, Depner S, Schnur S, et al. IL‐6 promotes malignant growth of skin SCCs by regulating a network of autocrine and paracrine cytokines. Int J Cancer 2011; 128: 2803–2814. [DOI] [PubMed] [Google Scholar]
- 64.Dirkx AE, oude Egbrink MG, Wagstaff J, et al. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukocyte Biol 2006; 80: 1183–1196. [DOI] [PubMed] [Google Scholar]
- 65.Valković T, Lučin K, Krstulja M, et al. Expression of monocyte chemotactic protein-1 in human invasive ductal breast cancer. Pathol Res Pract 1998; 194: 335–340. [DOI] [PubMed] [Google Scholar]
- 66.Vignaud J-M, Marie B, Klein N, et al. The role of platelet-derived growth factor production by tumor- associated macrophages in tumor stroma formation in lung cancer. Cancer Res 1994; 54: 5455–5463. [PubMed] [Google Scholar]
- 67.Okuda H, Kobayashi A, Xia B, et al. Hyaluronan synthase HAS2 promotes tumor progression in bone by stimulating the interaction of breast cancer stem-like cells with macrophages and stromal cells. Cancer Res 2012; 72: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]