Abstract
Attempts to expand ex vivo the numbers of human hematopoietic stem cells (HSCs) without compromising their marrow repopulating capacity and their ability to establish multilineage hematopoiesis has been the subject of intense investigation. Although most such efforts have focused on cord blood HSCs, few have been applied to adult HSCs, a more clinically relevant HSC source for gene modification. To date, the strategies that have been used to expand adult HSCs have resulted in modest effects or HSCs with lineage bias and a limited ability to generate T cells in vivo. We previously reported that culturing umbilical cord blood CD34+ cells in serum‐free media supplemented with valproic acid (VPA), a histone deacetylase inhibitor, and a combination of cytokines led to the expansion of the numbers of fully functional HSCs. In the present study, we used this same approach to expand the numbers of adult human CD34+ cells isolated from mobilized peripheral blood and bone marrow. This approach resulted in a significant increase in the numbers of phenotypically defined HSCs (CD34+CD45RA‐CD90+D49f+). Cells incubated with VPA also exhibited increased aldehyde dehydrogenase activity and decreased mitochondrial membrane potential, each functional markers of HSCs. Grafts harvested from VPA‐treated cultures were able to engraft in immune‐deficient mice and, importantly, to generate cellular progeny belonging to each hematopoietic lineage in similar proportion to that observed with unmanipulated CD34+ cells. These data support the utility of VPA‐mediated ex vivo HSC expansion for gene modification of adult HSCs.
Keywords: adult bone marrow, ex vivo expansion, hematopoietic stem cells, histone deacetylase inhibitor, mobilized peripheral blood, valproic acid
Valproic acid (VPA)‐mediated ex vivo expansion of adult bone marrow and mobilized peripheral blood CD34+ cells resulted in a cellular product characterized by high viability, enrichment with CD34+CD45RA‐CD90+ cells, increased aldehyde dehydrogenase (ALDH) activity, robust multipotent clonogenic potential, and decreased mitochondrial potential. VPA‐expanded grafts were able to establish unbiased multilineage human hematopoietic‐cell chimerism in NSG mice at 16 weeks post‐transplantation.
Significance statement.
This study shows that the transplantation of ex vivo valproic acid (VPA)‐treated, adult mobilized peripheral blood and bone marrow hematopoietic stem cells (HSCs) into immune‐deficient mice led to nonbiased long‐term multilineage hematopoietic cell engraftment including T cells. These data support the use of VPA‐mediated ex vivo HSC expansion for future gene modification strategies.
1. INTRODUCTION
Human hematopoietic stem cells (HSCs) are rare cells, quiescent under steady‐state conditions, which express specific phenotypic markers and possess characteristic functional properties.1 There are several ontological sources of HSCs: fetal liver, fetal marrow, umbilical cord blood (UCB), and adult bone marrow (ABM) or cytokine mobilized peripheral blood (mPB). Each source of HSCs is defined by their own proliferative capacity and their ability to establish multilineage hematopoiesis in myeloablated hosts.2, 3, 4 Since the number of HSCs present in a single UCB collection is frequently insufficient to repopulate in a timely fashion adult patients with refractory hematological malignancies or genetic disorders involving blood cells, most ex vivo HSC expansion methods have focused on UCB rather than adult sources of HSCs.5 However, the biological differences that distinguish the different ontological sources of HSCs do not assure that conditions that were effective for UCB‐HSC expansion would be equally effective for the adult sources of HSCs. Several studies have indicated that UCB‐CD34+ cells expanded ex vivo possess a higher marrow repopulating potential as compared to adult CD34+ cells from either ABM of mPB.2, 3 In addition, Wang and coworkers reported that UCB contains a higher percentage of cells capable of repopulating NSG mice as compared to HSCs from adult sources.4 The ability to expand the numbers of adult HSCs and/or preserve their function would be potentially useful as a cellular platform for genetic modification strategies.6, 7
Our laboratory has utilized the histone deacetylase inhibitor (HDACI)‐valproic acid (VPA) to expand the numbers of UCB‐HSCs. Treatment of UCB‐CD34+ cells with VPA and cytokines for 7 days (after 16 hours of priming with cytokines only) resulted in a significant increase in the numbers of phenotypically defined HSCs. The expanded HSCs were capable of long‐term engraftment when assessed in vivo in immune‐deficient mice, and lacked malignant potential.8, 9 We recently reported the preclinical development of a UCB‐derived HSC product for allogeneic transplantation for patients with hematological malignancies,10 and a clinical trial is underway (NCT03885947). In the present study, we applied our UCB expansion approach to granulocyte colony‐stimulating factor (G‐CSF) mPB and ABM‐CD34+ cells from healthy adult donors in order to determine if this same strategy was an effective means of preserving HSCs function.
2. MATERIALS AND METHODS
2.1. Ex vivo cultures
Cryopreserved CD34+ cells from mPB or ABM from healthy adult donors were purchased from AllCells (Alameda, California) and stored in liquid nitrogen. The highly purified (90%‐98%) CD34+ cells were thawed and cultured in serum‐free medium Stemline II (Sigma‐Aldrich, St. Louis, Missouri) supplemented with 150 ng/mL human stem cell factor (SCF), 100 ng/mL human fms‐like tyrosine kinase receptor 3 (FLT‐3‐ligand), 100 ng/mL human thrombopoietin (TPO), and 50 ng/mL human interleukin 3 (IL3) (R&D Systems, Minneapolis, Minnesota). Cells were seeded at 3.3 × 104 cells/mL in 22.1 mm wells (12‐well plates) and incubated in a humidified incubator maintained at 37°C with 5% CO2. The cells were incubated with the cytokines alone for 16 hours (cytokine priming) followed by addition of VPA 1 mM (Sigma‐Aldrich), then incubated in the presence of VPA and cytokines for additional 7 days (Figure 1A). The viability, number of the total nucleated cells (TNC), CD34+ cells, and frequency of various HSC subpopulations were determined shortly after thawing of the primary cells (PCs) and at daily time points, in both VPA‐treated cultures and control cultures containing cytokines alone (control).
Figure 1.
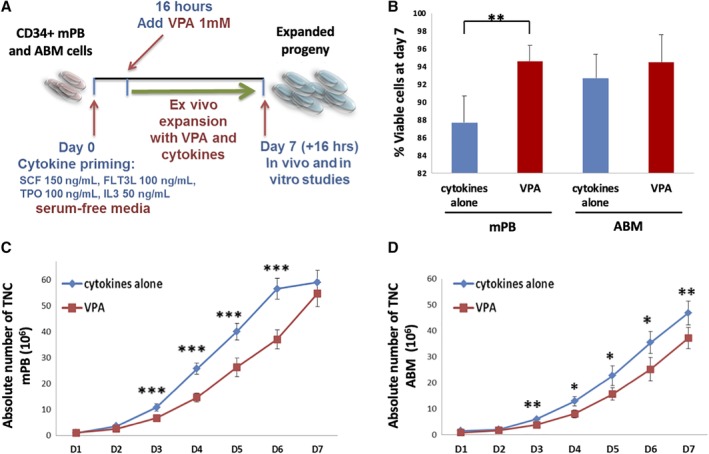
Human hematopoietic stem cells from adult sources proliferate and maintain a high viability in a culture supplemented with valproic acid (VPA) and cytokines. A, Schematic representation of the ex vivo expansion strategy. B, Cell viability after 7 days of culture of human mPB (n = 16) or adult bone marrow (ABM; n = 9)‐CD34+ cells with either cytokines alone or cytokines and VPA (preceded by 16 hours of exposure to only the cytokines). Error bars represent SD. C,D, Absolute numbers of total nucleated cells from mobilized peripheral blood (mPB; n = 16, panel C) or ABM (n = 9, panel D) throughout 7 days of culture with either cytokines alone or cytokines and VPA. Error bars represent SEM; *P < .05; **P < .01; ***P < .001; “n” represents the number of independent experiments
2.2. Cell counting and viability assay
A Cellometer Auto 2000 Cell Viability Counter (Nexcelom Bioscience, Lawrence, Massachusetts) was used for both cell counting and assessment of cell viability as per the manufacturer's instructions. Cell counting was validated using the trypan blue exclusion method in selected situations: after the thawing of cryopreserved specimens, at early‐day time points, and when outlier values were encountered. The fold expansion of a particular cell population was calculated by dividing the total number of nucleated cells recovered at day 7 by the input cell number expressing the identical phenotype.
2.3. Phenotypic analyses
PCs or cultured cells expanded with cytokines in the presence or absence of VPA were stained with the anti‐human antibodies 7AAD‐PerCP‐Cy5, CD34‐APC, CD90‐FITC, CD49f‐PE, and CD45RA‐PE‐Cy7 or their isotype‐matched controls (BD Biosciences, San Jose, California) for 20 minutes in room temperature, washed and analyzed using the FACS‐Canto II (BD Biosciences). Analyses were performed with BD FACSDiva Software and FlowJo Software (BD Biosciences).
2.4. Aldehyde dehydrogenase (ALDH) assay
To identify cell populations with high ALDH activity, an Aldefluor kit (StemCell Technologies, Vancouver, Canada) was used according to the manufacturer's instructions. Cells (1 × 106 or 0.5 × 106) were suspended in assay buffer, then half of the cells were added to the Aldefluor substrate (test sample) and the remaining half was added to N,N‐diethylaminobenzaldehyde (DEAB) inhibitor (control sample). The test and control samples were incubated for 40 minutes at 37°C. Cells were subsequently stained with CD34‐APC and CD90‐PE mAbs or isotype‐matched controls for an additional 20 minutes. Cells were washed and analyzed by flow cytometry using a FACS‐Canto II (BD Biosciences).
2.5. Assessment of mitochondrial membrane potential
Mitochondrial membrane potential was measured using tetramethylrhodamine methyl ester (TMRM) (Invitrogen, Carlsbad, California) with or without the efflux pump inhibitor, verapamil. Cells were incubated for 20 minutes at 37°C with 200 nM TMRM. Following washes, the cells were stained with CD34‐APC and CD90‐FITC in PBS containing 7.5% BSA and 0.5% EDTA for 20 minutes at room temperature, then washed and analyzed immediately using a FACS‐Canto II (BD Biosciences). To rule out excess cell death or apoptosis that can bias TMRM analysis, cells from four additional donors were stained with 7AAD‐PerCP‐Cy5 and Annexin V‐FITC along with TMRM, CD34‐APC, and CD90‐APC‐Cy7 and analyzed as detailed.
2.6. Colony forming unit (CFU) assays
CFUs were generated by seeding cells into methylcellulose‐enriched media (MethoCult H4436, StemCell Technologies) supplemented with SCF, IL3, IL6, FLT3‐ligand, TPO, erythropoietin, and granulocyte monocyte colony‐stimulating factor (GM‐CSF). The mixture was plated in 35 mm culture dishes at densities of 100 and 500 cells per dish. Colonies were counted and classified according to their cellular composition after 14 days of incubation using an inverted microscope. The number of CFUs present in the cell product after culture was calculated as follows: (the average number of colonies enumerated in four dishes × total mononuclear cell number)/input mononuclear cell number. Total mononuclear cells were determined by multiplying the number of mononuclear cells per milliliter by the culture volume. Secondary plating was performed by plucking single CFU‐GEMM derived colonies at day 14 and suspending the cells in methylcellulose media supplemented with the above cytokine combination, in 35‐mm culture dishes. Colonies were counted and classified after 14 days of secondary culture.
2.7. In vivo marrow repopulating potential of ex vivo expanded CD34+ cells
Female immunodeficient NOD/SCID IL2Rγ‐null (NSG) mice (Jackson Laboratory, Bar Harbor, Maine) were maintained in a pathogen‐free environment and monitored at the Center for Comparative Medicine and Surgery at the ISMMS. All animal experiments were reviewed and approved by the institutional IACUC. Animals were randomly assigned to experimental groups. Mice were sublethally irradiated with 300 cGy (using a 137Cs‐irradiator) 18‐20 hours prior to transplantation. 2 × 105 PCs or their ex vivo generated progeny was transplanted into irradiated mice by a tail vein injection. Mice were administered antibiotics for 4 weeks postirradiation and sacrificed 16‐18 weeks post‐transplantation. PB was collected by cardiac puncture, spleens extracted, and bone marrow cells collected by flushing femurs and tibias with PBS containing 2% FBS. PB cells were lysed on ice with red blood cell lysis solution (Invitrogen) and washed in PBS with 2% FBS. The presence of human cells was assessed by flow cytometry in cell suspensions derived from PB, marrows, and spleens after labeling with human antibodies against CD45 pan‐hematopoietic marker or against lineage‐specific makers such as CD34, CD33, CD19, CD3, CD15, CD235a (Glycophorin A), and CD41. The presence of at least 0.1% cells in the marrow of each recipient mouse was considered as indicative of donor human hematopoietic cell engraftment.
2.8. Statistical analysis
Results are expressed as the mean ± SD or mean ± SEM of varying numbers of individual experiments. Statistical differences were evaluated using the Student's t test for comparisons between two groups, whereas two‐way ANOVA was used for comparisons between multiple groups. Statistical significance was defined as *P < .05; **P < .01; ***P < .001.
3. RESULTS
3.1. Addition of VPA to a combination of cytokines limits cell proliferation but maintains cell viability
CD34+ cells derived from either adult mPB or ABM were incubated in serum‐free media supplemented with the cytokines for 16 hours (cytokine priming) followed by addition of VPA (1 mM) and incubation for additional 7 days. Cells cultured with cytokines alone during the entire 16‐hour‐plus 7‐day period were used as control. A schema of the culture system employed in this study is illustrated in Figure 1A. This culture system has been previously optimized, using initially fresh8 and subsequently cryopreserved10 UCB, and has been evaluated using different chromatin modifying agents, of which VPA emerged as the most potent.8 The viability of mPB and ABM cells treated with VPA and cytokines for 7 days, each exceeded 94% (Figure 1B and Table 1). Cultures containing cytokines alone generated greater numbers of TNCs than cultures containing cytokines and VPA. The numbers of TNCs generated in VPA‐containing mPB cultures were 6.7‐fold (day 3) and 55‐fold (day 7) greater than the number of cells used to initiate the cultures (Figure 1C and Table 1). ABM‐derived CD34+ cells displayed a more delayed pattern of proliferation regardless of VPA treatment. Yet, there was a 3.8‐fold expansion of TNCs by day 3 and 37‐fold by day 7 in the cultures treated with VPA (Figure 1D and Table 2).
Table 1.
Ex vivo expansion of mPB‐HSCs with VPA treatment versus treatment with cytokines alone (control) after 3 and 7 days of incubation (n = 16)
| Uncultured | Control | VPA | P value | Control | VPA | P value | |
|---|---|---|---|---|---|---|---|
| PCs | Day 3 | Day 3 | Day 7 | Day 7 | |||
| Cell viability (%) | 86.7 ± 6.5 | 95.5 ± 1.7 | 94.3 ± 3.8 | NS | 87.7 ± 8 | 94.6 ± 1.8 | <.01 |
| TNC fold expansion | 11 ± 5.2 | 6.7 ± 3.4 | NS | 59 ± 18 | 55 ± 20 | NS | |
| CD34+ (%) | 95.4 ± 2.7 | 57.2 ± 10 | 89.1 ± 4.6 | <.0001 | 8.5 ± 5 | 48.2 ± 15 | <.0001 |
| CD34+ fold expansion | 6.7 ± 4.3 | 6.3 ± 3.2 | NS | 5.5 ± 4.2 | 29 ± 16 | <.0001 | |
| CD34+CD45RA‐CD90+ (%) | 5.6 ± 3.8 | 3.3 ± 1.4 | 70.4 ± 6 | <.0001 | 0.2 ± 0.18 | 30.7 ± 15 | <.0001 |
| CD34+CD45RA‐CD90+ fold expansion | 7.1 ± 4.6 | 124 ± 70 | <.01 | 4 ± 3.3 | 807 ± 480 | <.01 | |
| CD34+CD45RA‐CD90+CD49f+ (%) | 0.16 ± 0.2 | 0.32 ± 0.3 | 24.5 ± 9.4 | <.001 | 0 | 10.9 ± 7.5 | <.001 |
| CD34+CD45RA‐CD90+CD49f+ fold expansion | 31 ± 12.5 | 1636 ± 740 | <.001 | 0 | 3758 ± 1150 | <.001 | |
| CD34+ALDH+ (%) | 3 ± 1.5 | 7.3 ± 3.5 | 35.1 ± 15 | <.05 | 13.8 ± 3.1 | 62 ± 9.2 | <.01 |
| CD34+ALDH+ fold expansion | 15 ± 6 | 40 ± 21 | NS | 11 ± 7 | 139 ± 35 | <.01 | |
| TMRM: MFI in CD34+ cells | 120 ± 15 | 698 ± 11 | 409 ± 51 | <.01 | 768 ± 33 | 438 ± 58 | <.01 |
| TMRM: MFI in CD34+CD90+ cells | 235 ± 10 | 1146 ± 30 | 481 ± 76 | <.01 | 960 ± 47 | 393 ± 151 | <.01 |
| Human chimerism in NSG mice (%) | 41 ± 10 | Not available | 0.5 ± 0.3 | 18 ± 10 | <.05 | ||
| Engrafted mice | 7/7 | 4/7 | 7/7 | ||||
Note: Values are mean ± SD.
Abbreviations: ALDH, aldehyde dehydrogenase; MFI, mean fluorescence intensity; mPB, mobilized peripheral blood; NS, not significant; PC, primary cells; TMRM, tetramethylrhodamine methyl ester; TNC, total nucleated cells; VPA, valproic acid.
Table 2.
Ex vivo expansion of ABM‐HSCs with VPA treatment versus treatment with cytokines alone (control) after 3 and 7 days of incubation (n = 9)
| Uncultured | Control | VPA | P value | Control | VPA | P value | |
|---|---|---|---|---|---|---|---|
| PCs | Day 3 | Day 3 | Day 7 | Day 7 | |||
| Cell viability (%) | 84.5 ± 3.5 | 95.3 ± 4.8 | 93.4 ± 2.4 | NS | 92.7 ± 2.7 | 94.5 ± 3 | NS |
| TNC fold expansion | 6 ± 1.3 | 3.8 ± 1.2 | <.01 | 47 ± 20 | 37 ± 17 | <.01 | |
| CD34+ (%) | 91.7 ± 5 | 34.7 ± 5.6 | 54.3 ± 6.2 | <.001 | 8.3 ± 3.1 | 42.1 ± 7 | <.0001 |
| CD34+ fold expansion | 2.3 ± 0.6 | 2.2 ± 0.8 | NS | 5 ± 2.1 | 20.7 ± 9 | <.0001 | |
| CD34+CD45RA‐D90+ (%) | 8 ± 5.3 | 4.6 ± 1.9 | 30 ± 7.2 | <.001 | 0.37 ± 0.3 | 22.7 ± 4.5 | <.0001 |
| CD34+CD45RA‐CD90+ fold expansion | 4.4 ± 3.2 | 16 ± 6.8 | <.05 | 3.9 ± 3 | 138 ± 29 | <.0001 | |
| CD34+CD45RA‐CD90+CD49f+ (%) | 1.6 ± 1.3 | 0.4 ± 0.3 | 10.7 ± 4.2 | <.01 | 0.17 ± 0.2 | 13 ± 4.4 | <.0001 |
| CD34+CD45RA‐CD90+CD49f+ fold expansion | 5.3 ± 3.5 | 89 ± 40 | NS | 4.8 ± 3 | 1573 ± 718 | <.01 | |
| Human chimerism in NSG mice (%) | 0.67 ± 0.3 | Not available | 0.12 ± 0.05 | 3.9 ± 0.8 | <.01 | ||
| Engrafting mice | 7/9 | 3/9 | 9/9 | ||||
Note: Values are mean ± SD.
Abbreviations: ABM, adult bone marrow; NS, not significant; PC, primary cells; TNC, total nucleated cells; VPA, valproic acid.
3.2. VPA expands phenotypically defined HSCs from both mPB and ABM
VPA‐treated cultures contained a higher percentage of CD34+ cells throughout the culture period and led to a greater expansion of the number of CD34+ cells as compared to control cultures containing cytokines alone (29 vs 5.5‐fold for mPB and 21 vs 5‐fold for ABM, P < .0001 for both) (Figures 2 and 3A,C; Tables 1 and 2). The CD34+ fraction was further assessed for CD45RA and co‐expression of CD90 and CD49f, which both mark long‐term repopulating HSCs (Figure S1).11, 12 Although only a small percentage of CD34+ cells from either uncultured PCs or control cultures expressed CD90 and CD49f (range 1%‐10%), up to 70% and 60% of VPA‐treated CD34+ cells expressed CD90 and CD49f, respectively, as early as 24‐48 hours after exposure to VPA (Figures 2 and 3B; Tables 1 and 2). CD34+ cells expressing CD90 and CD49f were negative for CD45RA (Figure S1). By day 7 of culture, cells expressing CD90 and CD49f decreased to 35% and 25%, respectively, in the VPA‐treated cultures, whereas expression of these markers was completely or almost completely lost in the control cultures from both HSC sources (Figures 2 and 3B). The early increase in the percentage of phenotypically defined HSCs occurred without a significant degree of cell proliferation (Figures 1C,D and 3D). The combined effect of cytokines and VPA led to a dramatic expansion of the phenotypically defined HSC subpopulations, resulting in a 807‐ and 3000‐fold increase in the numbers of CD34+CD45RA‐CD90+ and CD34+CD45RA‐CD90+CD49f+ cells, respectively, with mPB, and a 138‐ and 1507‐fold expansion of CD34+CD45RA‐CD90+ and CD34+CD45RA‐CD90+CD49f+ cells, respectively, with ABM (Figure 3E; Tables 1 and 2). This expansion of HSCs as defined by phenotype occurred solely in the VPA‐containing cultures (Figures 2 and 3D,E).
Figure 2.
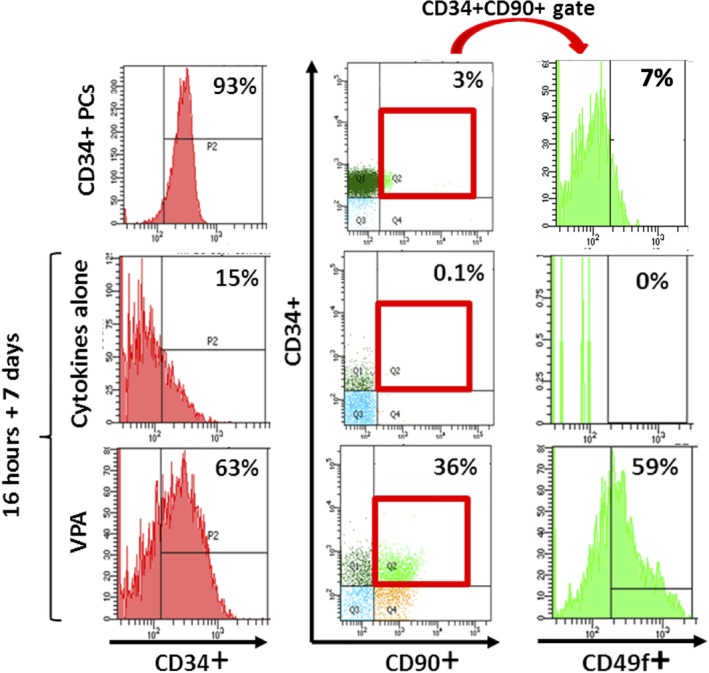
Valproic acid (VPA) induces a primitive stem cell phenotype. Representative phenotypic analysis of CD34, CD90, and CD49f expression in primary mobilized peripheral blood (mPB)‐CD34+ cells (PCs) and in the expanded mPB grafts after 7 days of ex vivo culture with either cytokines alone or cytokines and VPA. Note: CD34+CD90+ cells gated for expression of CD49f were CD45RA‐ (not shown)
Figure 3.
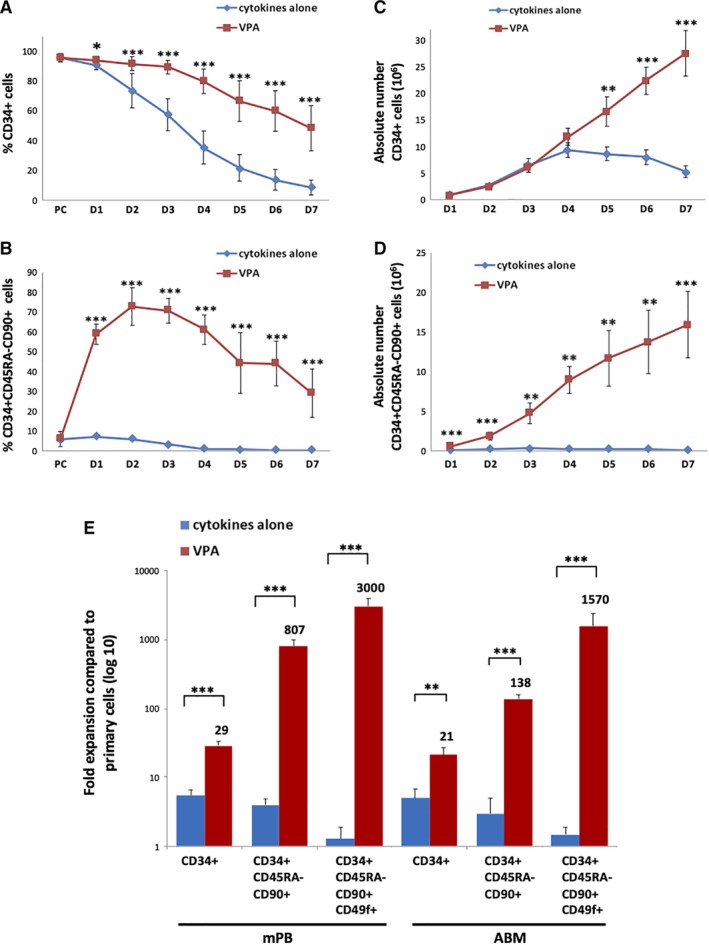
The combination of valproic acid (VPA) and cytokines drives ex vivo expansion of phenotypically defined hematopoietic stem cells (HSCs). A,B, Percentage of mobilized peripheral blood (mPB)‐CD34+ (A) and CD34+CD45RA‐CD90+ (B) cells throughout 7 days of culture in the presence of either cytokines alone or cytokines and VPA (mean ± SD, n = 16). C,D, Absolute numbers of mPB‐CD34+ (C) and CD34+CD45RA‐CD90+ (D) cells throughout 7 days of culture (mean ± SEM, n = 16). E, Summary of the fold expansion of phenotypically defined HSC populations from both mPB (n = 16) and adult bone marrow (ABM; n = 9) cultures, on day 7 (mean ± SEM). *P < .05; **P < .01; ***P < .001
3.3. VPA treatment preserves functional characteristics of HSC activity
Since the phenotype of cells expanded ex vivo does not always correlate with function, we used aldehyde dehydrogenase (ALDH) activity as a functional marker of HSCs.13, 14 The CD34+ population within VPA‐expanded mPB cultures displayed significantly greater numbers of cells with detectable ALDH activity as compared to uncultured CD34+ cells and CD34+ cells within control cultures, after both 3 and 7 days of incubation (Figure 4A and Figure S2A; Table 1). Moreover, VPA treatment led to a 140‐fold expansion in the absolute number of CD34+ALDH+ cells by day 7, vs 11‐fold under control conditions (P < .01) (Figure 4B).
Figure 4.
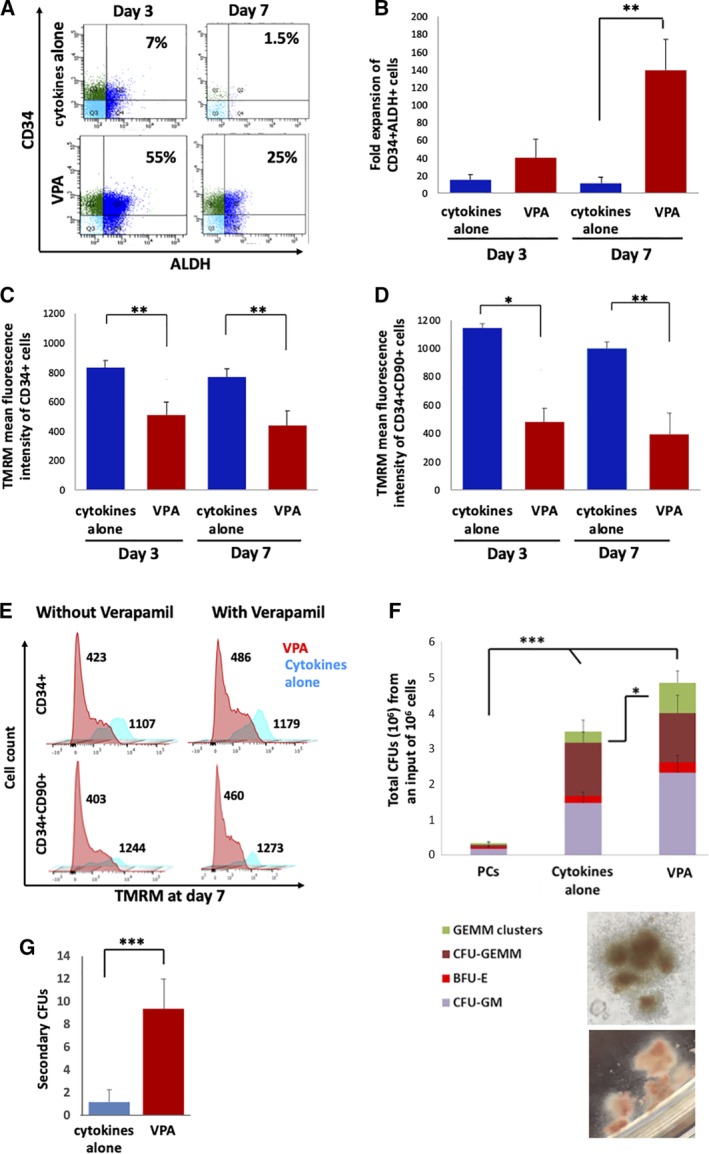
Valproic acid (VPA) expanded cells possess stem cell characteristics in functional studies. A, Representative flow cytometric analysis of CD34 expression and aldehyde dehydrogenase (ALDH) activity in mobilized peripheral blood (mPB) cultures treated with cytokines with or without VPA, on days 3 and 7 of the ex vivo culture. B, Expansion of the numbers of CD34+ALDH+ cells in cultures treated with cytokines with or without VPA for 3 and 7 days (n = 5). C,D, Mitochondrial membrane potential of CD34+ (C) and CD34+CD90+ cells (D) stained with TMRM in mPB cultures treated as indicated (n = 5). Y‐axis represents the mean TMRM fluorescence intensity (MFI). E, Representative flow cytometry plots indicating mitochondrial potential of CD34+ and CD34+CD90+ cells stained with TMRM in a single mPB culture treated as indicated for 7 days in the presence or absence of the efflux pump inhibitor, verapamil. Numbers represent TMRM fluorescence intensity. F, Total colony forming unit (CFU) and distribution of CFU subtypes in mPB‐PCs and after 7 days of culture in the indicated culture condition, including quantification of GEMM clusters (green bars) (n = 10); bottom panels show microphotographs of GEMM clusters, typical of VPA‐treated cultures (magnification ×100). G, Number of secondary CFUs from each single CFU‐GEMM derived from either VPA‐treated or cytokines alone grafts (n = 10). Error bars represent SD; *P < .05; **P < .01; ***P < .001
Functional HSCs rely heavily on glycolysis for energy production and generate low levels of reactive oxygen species (ROS).15, 16, 17, 18, 19, 20 We have previously shown that VPA‐expanded UCB‐CD34+ cells exhibit a remodeled primitive mitochondrial network characterized by a low mitochondrial membrane potential, mass, and ROS generation.21 We used a mitochondrial membrane potential dye, TMRM, as an indicator of mitochondrial activity in cultures of adult HSCs. The mitochondrial membrane potential of both CD34+ and CD34+CD90+ cells was significantly reduced in the VPA‐treated mPB and ABM cultures as compared to control cultures (Figure 4C‐E; Figure S2B). The decrease in mitochondrial membrane potential observed within VPA‐treated cultures was not a result of cationic dye efflux or a higher number of cells undergoing apoptosis within these cultures, as confirmed by the use of the efflux‐pump inhibitor, verapamil, and by 7AAD and Annexin V staining, respectively (Figure 4E; Figure S2B‐D).
3.4. VPA treatment increases the numbers of assayable hematopoietic progenitor cells
To further evaluate the function of HSCs generated in VPA‐treated mPB‐CD34+ cell cultures, the resulted cells were tested for their ability to generate CFUs. VPA‐treated cells generated a 16‐fold greater number of total CFUs, as compared to PCs and slightly higher than that generated by cells from the control cultures. Similarly, the fraction of assayable CFU‐GEMM derived colonies was comparable between the VPA‐treated and control cultures, but was significantly higher than those detected in PCs. Interestingly, among the CFU‐GEMM generated by the VPA‐treated cultures, a significantly greater number of large colonies with multiple foci, herein referred to as CFU‐GEMM clusters, were observed as compared to both PCs and control cultures (Figure 4F). Identical observations were detected in cultures initiated with ABM‐derived cultures (Table S1), suggesting that the cells generated in the presence of VPA have greater multipotent potential. To further support HSC activity within VPA‐treated cultures, single CFU‐GEMM colonies from control and VPA cultures from two mPB donors were plucked and replated. CFU‐GEMMs from VPA‐treated cultures generated a significantly higher number of secondary CFUs (Figure 4G).
3.5. Grafts from VPA‐treated cultures are capable of multilineage engraftment in NSG mice
To evaluate the functional behavior of the expanded HSCs in vivo, we transplanted the progeny of 2 × 105 mPB‐ or ABM‐CD34+ cells cultured with or without VPA, into sublethally irradiated NSG mice. Transplantation of 2 × 105 mPB‐ or ABM‐CD34+ unmanipulated PCs was used as additional control. The degree of human cell chimerism in the bone marrows of the NSG mice was determined after 16 weeks by quantifying the degree of human CD45+ cell chimerism. Although mice transplanted with grafts generated in the presence of cytokines alone (control cultures) had limited or no human cell chimerism (range 0%‐1.4%), all mice transplanted with VPA‐expanded mPB grafts engrafted and achieved up to 50% human chimerism (range 2%‐50%) (Figure 5A; Table 1). Importantly, human cells belonging to both myeloid and lymphoid lineages were observed with a lineage distribution similar to that observed with PCs (CD34+, CD33+, CD15+, CD41a, glycophorin A+, CD19+, and CD3+ cells) in the marrows (Figure 5C), spleens (Figure 5D) and peripheral blood (not shown) of the mice transplanted with the VPA‐expanded cells. B‐cell engraftment was greater in the bone marrow irrespective of the source of transplanted cells, whereas the degree of T‐cell engraftment exceeded the B‐cell engraftment in the spleen especially with the VPA‐treated grafts. In xenotransplantation studies of the ABM‐derived grafts, all mice transplanted with VPA‐treated grafts engrafted displaying an average 3.5% human chimerism, whereas mice transplanted with grafts from control cultures had little or no human cell chimerism (average 0.15%, P < .001) (Figure 5B; Table 2). Moreover, the average human chimerism in grafts from VPA‐treated cultures exceeded that of the unmanipulated PCs (3.5% vs 0.8%, P < .001). Human cells detected in these mice belonged to both myeloid and lymphoid lineages without a significant degree of lineage bias as compared to that observed with PCs (Figure 5E).
Figure 5.
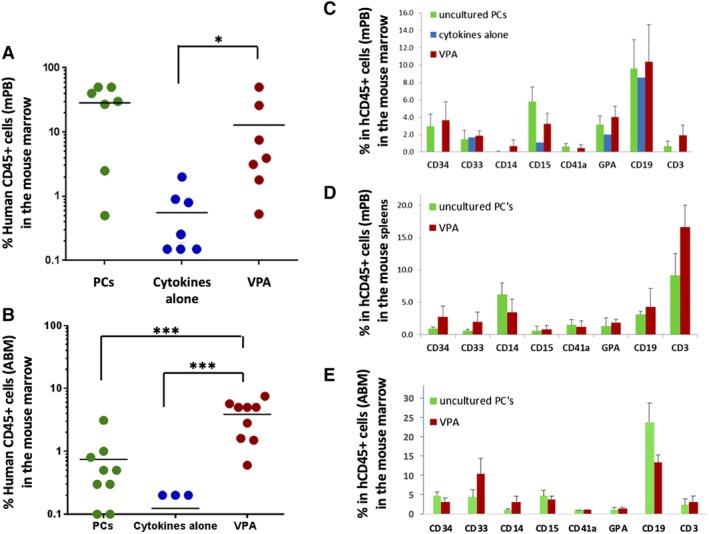
Valproic acid (VPA)‐treated grafts maintain long‐term multilineage human cell chimerism in vivo. A,B, Human CD45 cell engraftment in the marrows of NSG mice harvested at 16 weeks post‐transplantation with grafts from PCs, VPA and control cultures, initiated from mobilized peripheral blood (mPB; n = 7, panel A) and adult bone marrow (ABM; n = 9, panel B). Note: six of nine mice transplanted with grafts from control ABM cultures did not show any human cell chimerism, and hence cannot be displayed on a logarithmic scale. C‐E, Lineage analysis of the long‐term engrafting human cells in the marrows (n = 7, panel C) and spleens (n = 5, panel D) of mice transplanted with mPB‐derived grafts, and in the marrows of mice transplanted with ABM‐derived grafts (n = 9, panel E). Error bars represent SD; *P < .05; ***P < .001
3.6. Effect of VPA on HSCs from adult sources is comparable to that observed with UCB
To evaluate the effects triggered by VPA toward different ontogenic sources of HSCs, we collected published8, 10, 21 and unpublished data from studies performed in our laboratory with UCB‐CD34+ cells and compared it to our findings with ABM and mPB (Table S1). The fold expansion of TNCs and of the various subpopulations studied was greatest with UCB as compared to mPB; the expansion of mPB exceeded that observed with ABM. However, the percentages of phenotypically defined HSC subpopulations out of the total number of viable cells in the cultures were remarkably comparable. The frequency of CD34+ALDH+ cells, the degree of reduction in mitochondrial membrane potential, and the increase in the numbers of CFU‐GEMM‐clusters were also comparable, as was the consistent human cell engraftment achieved 16 weeks following transplantation into NSG mice (albeit with a wide range of the degree of human cell chimerism).
4. DISCUSSION
The expansion or maintenance of the numbers of functional human HSCs following ex vivo culture can be utilized as a platform to improve outcomes of HSC‐based therapies including transplantation and gene modification. Although numerous expansion strategies have been described for UCB‐HSCs, several of which have advanced to clinical trials,5, 8, 20 the evaluation of such expansion strategies with HSCs from adult sources remains limited. The present study reports the application of an ex vivo HSC expansion strategy to adult HSCs from both clinically relevant sources (mPB and ABM) and importantly confirms long‐term unbiased hematopoietic reconstitution in vivo including myeloid and lymphoid lineages.
Although the ex vivo expansion of adult HSCs has proven difficult,2, 3, 4 some progress has been made. Milhem et al treated ABM‐CD34+ cells with a combination of two epigenetic modifiers, the DNA methyltransferase inhibitor, 2‐deoxy 5‐azacitidine (5azaD), and the HDACI, trichostatin A (TSA), in a 9‐day serum‐containing culture system. A modest 2.5‐fold expansion of the number of CD34+CD90+ cells was observed with a 1.5‐fold expansion of primitive CFUs.22 More recently, Saraf et al used a similar strategy to evaluate the effect of 5azaD and TSA on mPB CD34+ cells. A 3.5‐fold expansion of CD34+CD90+ cells and 10.1‐fold expansion of primitive CFUs were observed. Importantly, this expansion was accompanied by global hypomethylation that corresponded with increased transcript levels of several genes implicated in HSC self‐renewal, supporting a role for epigenetic modification in the regulation of adult HSC fate.23 5aza/TSA‐expanded ABM and mPB cells were able to establish human hematopoietic cell chimerism in NSG mice 8 weeks post‐transplantation.22, 23 This 8‐week period was, however, insufficient to allow for the evaluation of the long‐term repopulating capacity of the expanded graft. The limited success reported by Milhem et al and Saraf et al might be due to the inclusion of serum in their culture system, which is known to impair HSC expansion.8 Similarly, Boitano et al used StemRegenin1 (SR1), an aryl hydrocarbon receptor antagonist, to promote the ex vivo expansion of CD34+ cells from both UCB and mPB, however in vivo engraftment data were not provided for the mPB‐CD34+ cells.24 A subsequent study reported the expansion of genetically modified mPB and ABM‐HSCs with SR1, however, the degree of human cell engraftment in NSG mice was modest and lineage analyses were not provided.25 Studies performed by other laboratories have shown multilineage hematopoietic cell engraftment but with limited generation of B and T cells following the transplantation of SR1‐expanded mPB grafts26 and limited generation of T cells following expansion of mPB‐CD34+ cells with two other aryl hydrocarbon receptor antagonists.27 Furthermore, Psatha et al evaluated the ability of SR1, as well as a p38‐MAPK14 inhibitor and the pyrimidoindole derivative, UM171, alone or in combination, to expand UCB‐ and mPB‐CD34+ cells. Each of these agents led to the expansion of phenotypically defined HSCs that were capable of human hematopoietic cell engraftment following transplantation into NSG mice. However, lineage biases were observed and the degree of T‐cell chimerism was not reported.28 Our study demonstrates that a VPA‐based expansion strategy results in a significant expansion of HSCs from both clinically relevant adult sources as assessed using phenotypic and in vitro functional studies, with retention of long‐term multilineage engraftment potential in vivo including T cells. The ability of VPA‐treated HSCs to generate T cells is critical if such graft were to be used as allogeneic grafts or autologous grafts for genetic modification for patients with inherited and acquired immune deficiency disorders including HIV infections.
Significant support for the expansion or at the least maintenance of the numbers of functional HSCs within the VPA‐containing cultures is provided by in vitro functional studies provided here. VPA‐treated CD34+ cells exhibited a significantly greater number of cells with detectable ALDH activity, an established marker of long‐term repopulating HSCs,13, 14 as compared to CD34+ cells within control cultures and PCs. A 140‐fold expansion of the number of CD34+ALDH+ cells further supports the ability of VPA to expand functional HSCs. Recently, the cellular metabolic state has emerged as a reliable determinant of stem cell activity.15 Low mitochondrial activity has been shown to correlate with long‐term repopulating capacity and mark self‐renewing HSCs.16, 17, 18, 19, 20 We have previously shown in UCB‐HSCs that VPA treatment leads to a decrease in mitochondrial mass (as assayed by both Mitogreen dye and the ratio of mitochondrial to nuclear DNA), membrane potential (as assayed by TMRM), oxygen consumption, and production of ROS.21 We used the TMRM assay (in both the presence and absence of verapamil) to functionally evaluate of the effects of VPA on adult HSCs, and the findings were comparable under these experimental conditions. The significant reduction in mitochondrial membrane potential observed in CD34+ cells generated in VPA‐treated cultures as compared to control cultures suggests that these cells possess properties that resemble primitive HSCs. CFU assays are used to assess progenitor cell activity, however their reliability as surrogates for HSC activity is unclear.1 The expansion of the number of total CFUs including CFU‐GEMM in both the VPA and control cultures confirmed the presence of multipotential progenitors in both types of cultures. However, the observation of a two‐ to threefold increase in the number of clusters of multilineage colonies may reflect the presence of multipotent progenitors with a greater proliferative potential in the VPA‐treated cultures. The ability of progenitor cells assayed from VPA‐treated cultures to generate both primary and secondary hematopoietic colonies further supports the presence of HSC‐like activity.
A growing body of evidence supports the role of chromatin modifying agents,22, 23, 29, 30 and specifically VPA in epigenetic reprogramming of human HSCs during ex vivo culture. Treatment of UCB as well as adult HCSs with VPA was associated with increased histone H4 acetylation at regulatory sites of genes implicated in HSC activity and self‐renewal including HOXB4, AC133, CXCR4, GATA2, EZH2, and BMI1.31, 32, 33 The inhibition of HDAC3, a target of VPA, recapitulated the VPA effects on HSC ex vivo expansion and resulted in a dramatic expansion of CD34+ cell numbers.34 We have previously shown that VPA treatment of UCB‐CD34+ upregulates the pluripotency genes SOX2, OCT4, and NANOG and their downstream targets.8 Recently, we confirmed that the cellular reprogramming in UCB‐CD34+ cells occurs shortly (24‐48 hours) after exposure to VPA and is accompanied not only by phenotypic and transcriptomic changes but also by remodeling of the mitochondrial profile and suppression of ROS.21 In this study, the early “phenotypic switch” and the changes in ALDH activity and mitochondrial membrane potential induced by VPA were highly comparable to our observations in UCB8, 10, 21 and are suggestive of cellular reprogramming in CD34+ cells from adult sources. Few studies directly compared ex vivo culture of HSCs from different ontogenic sources.2, 3, 4, 31 In the present study, we demonstrate that VPA exerts similar effects on mPB and ABM‐CD34+ cells with those observed in ex vivo expansion of UCB‐CD34+cells. These similarities suggest that common biological effects are achieved with VPA irrespective of whether the primary CD34+ cells were derived from adult tissues or UCB. Our findings are corroborated by a recent study that identified VPA as a significant enhancer of the number phenotypic and functional HSCs in a small molecule screen using a zebrafish embryo model.35 This study also indicated that VPA increased human mPB‐CD34+ cell adhesion to mesenchymal stromal cells, thus providing another putative mechanism for VPA's ability to positively affect the HSC function.
Despite an inconsistent correlation between phenotype and function in studies evaluating the ex vivo expansion of human HSCs,28 several studies have shown a strong association between the CD34+CD45RA‐CD90+ phenotype, and specifically CD90 (Thy1) expression, and the long‐term repopulating capacity in vivo.36, 37 In the present study, VPA treatment led to a robust generation of a CD34+CD45RA‐CD90+ cell population that correlated with HSC activity in functional studies and long‐term repopulating capacity in vivo. In contrast, the lack of generation of phenotypically defined HSC subpopulations observed with cytokines alone control cultures correlated with poor engraftment outcomes under these culture conditions. Since the VPA‐treated grafts retained the ability to engraft and support multilineage hematopoietic reconstitution, 16 weeks after their transplantation, we conclude that this cell product contains fully functional HSCs. To confirm that such a strategy led to an actual expansion of fully functional HSCs would require serial transplantation studies as well as HSC quantitation using limited dilution analyses that were beyond the scope of these studies. Nevertheless, the preservation of HSC function after a 16 hour‐ and 7‐day cytokine containing culture, including multilineage hematopoietic reconstitution in vivo, is of key importance to the potential application of this culture strategy to gene modification protocols. Since the genetic modification of autologous HSCs requires cytokine stimulation, which often results in HSC differentiation with a significant loss of gene‐modified cells with long‐term repopulating capacity, we hypothesize that adding VPA to such cultures could avoid HSC exhaustion and allow for increased efficiency of the transduction process. This hypothesis is supported by a recently published study demonstrating increased efficiency of lentiviral transduction and elimination of lentiviral‐induced myeloid bias by VPA treatment of human UCB‐CD34+ cells.38
5. CONCLUSION
We have shown that VPA is capable of promoting the ex vivo expansion of the numbers of phenotypically and functionally defined adult mPB and ABM HSCs, with maintenance of their in vivo reconstitution potential. These VPA‐expanded grafts when transplanted into immune‐deficient mice were able to give rise to human cells belonging to all lineages, including T cells. The effects of VPA were evident as early as after 3 days of culture, a time point relevant to gene modification protocols. These results strongly support that a VPA‐based strategy might prove useful for genetic modification of human adult HSCs.
CONFLICT OF INTEREST
The authors indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
E.Z., L.P.: conception and design, assembly of data, analysis and interpretation, manuscript writing; M.D., A.P.: assembly of data, analysis and interpretation, manuscript writing; C.I.R.: analysis and interpretation, manuscript writing; R.H.: study supervision, conception and design, assembly of data, analysis and interpretation, manuscript writing.
Supporting information
Supplementary Figure 1 Flow cytometric gating strategy used for the phenotypical analysis of ex vivo expansion of mPB and ABM‐CD34+ cells. Schema of the gating strategy used in our studies (top panels) and flow cytometry plots from a representative mPB culture showing the corresponding phenotypical analysis of unmanipulated primary cells at day 0 (middle panels) and VPA expanded cells after 16 hours and 7 days of culture (bottom panels).
Supplementary Figure 2 VPA‐expanded cells possess stem cell characteristics in functional studies. (A) Representative flow cytometric analysis of ALDH activity in mPB cultures treated with cytokines in the presence or absence of VPA for 7 days. Cultured cells treated with DEAB inhibitor were used as control. (B) Representative flow cytometry plots indicating mitochondrial potential of CD34+ and CD34 + CD90+ cells stained with TMRM in a single ABM culture treated as indicated for 7 days in the presence or absence of the efflux pump inhibitor, verapamil. Numbers represent TMRM fluorescence intensity. (C) Representative flow cytometric analysis of Annexin V and 7AAD staining of VPA expanded cells in an mPB culture at day 7. (D) Percent viable cells that are negative for both Annexin V and 7AAD in both mPB (n = 2) and ABM donors (n = 2). “Cyt” denotes the cytokines alone condition.
Supplementary Table 1 Comparison of VPA‐mediated ex vivo expansion of HSCs from three clinically relevant sources: ABM, mPB and UCB
ACKNOWLEDGMENT
The authors would like to thank Goar Mosoyan and Joe Tripodi for their generous technical assistance.
Zimran E, Papa L, Djedaini M, Patel A, Iancu‐Rubin C, Hoffman R. Expansion and preservation of the functional activity of adult hematopoietic stem cells cultured ex vivo with a histone deacetylase inhibitor. STEM CELLS Transl Med. 2020;9:531–542. 10.1002/sctm.19-0199
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available within the paper or can be obtained from the corresponding author upon request.
REFERENCES
- 1. Dolatov S, Notta F, Laurenti E, et al. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10(2):120‐137. [DOI] [PubMed] [Google Scholar]
- 2. Rosler ES, Brandt JE, Chute J, et al. An in vivo competitive repopulation assay for various sources of human hematopoitic stem cells. Blood. 2000;96(10):3414‐3421. [PubMed] [Google Scholar]
- 3. Tanavde VM, Malehorn MT, Lumkul R, et al. Human stem‐progenitor cells from neonatal cord blood have greater hematopoietic expansion capacity than those from mobilized adult blood. Exp Hematol. 2002;30:816‐823. [DOI] [PubMed] [Google Scholar]
- 4. Wang JCY, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID‐repopulating assay. Blood. 1997;89(11):3919‐3924. [PubMed] [Google Scholar]
- 5. Baron F, Ruggeri A, Nagler A. Methods of ex vivo expansion of human cord blood cells: challenges, successes and clinical implications. Exp Rev Hematol. 2016;9(3):297‐314. [DOI] [PubMed] [Google Scholar]
- 6. Psatha N, Karponi G, Yannaki E. Optimizing autologous cell grafts to improve stem cell gene therapy. Exp Hematol. 2016;44:528‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu KR, Natanson H, Dunbar CE. Gene editing of human hematopoietic stem and progenitor cells: promise and potential hurdles. Human Gene Ther. 2016;107:729‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaurasia P, Gajzer DC, Schaniel C, et al. Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest. 2014;124(6):2378‐2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iancu‐Rubin C, Hoffman R. Role of epigenetic reprogramming in hematopoietic stem cell function. Curr Opin Hematol. 2015;22:279‐285. [DOI] [PubMed] [Google Scholar]
- 10. Iancu‐Rubin C, Fong H, Mosoyan G, et al. Preclinical development of a cord blood (CB)‐derived hematopoietic stem cell (HSC) product for allogeneic transplantation in patients with hematological malignancies. Blood. 2016;128:818. [Google Scholar]
- 11. Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Notta F, Doulatov S, Laurenti E, et al. Isolation of single human hematopoietic stem cells capable of long‐term multiliniage engraftment. Science. 2011;333:218‐221. [DOI] [PubMed] [Google Scholar]
- 13. Hess DA, Meyerrose TE, Wirthlin L, et al. Functional characterization of highly purified human hematopoietic human repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104(6):1648‐1655. [DOI] [PubMed] [Google Scholar]
- 14. Lioznov MV, Freiberger P, Kroger N, et al. Aldehyde dehydrogenase activity as a marker for the quality of hematopoietic stem cell transplants. Bone Marrow Transplant. 2005;35(9):909‐914. [DOI] [PubMed] [Google Scholar]
- 15. Kohli L, Passegue E. Surviving change: the metabolic journey of hematopoietic stem cells. Trends Cell Biol. 2014;24(8):479‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vannini N, Girotra M, Naveiras O, et al. Specification of haematopoieitc stem cell fate via modulation of mitochondrial activity. Nat Commun. 2016;7:13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Romero‐Moya D, Bueno C, Montes R, et al. Cord blood‐derived CD34+ hematopoietic cells with low mitochondrial mass are enriched in hematopoietic repopulating stem cell function. Haematologica. 2013;98(7):1022‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simsek T, Kocaba F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7(3):380‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papa L, Djedaini M, Hoffman R. Mitochondrial role in stemness and differentiation of hematopoietic stem cells. Stem Cells Int. 2019; 10.1155/2019/4067162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papa L, Djedaini M, Hoffman R. Ex vivo HSC expansion challenges the paradigm of unidirectional human hematopoiesis. Ann N Y Acad Sci. 2019. 10.1111/nyas.14133 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papa L, Zimran E, Djedaini M, et al. Ex vivo human HSC expansion requires coordination of cellular reprogramming with mitochondrial remodeling and p53 activation. Blood Adv. 2018;2(20):2766‐2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Milhem M, Mahmud N, Lavelle D, et al. Modification of hematopoietic stem cell fate by 5aza2'deoxycytidine and trichostatin A. Blood. 2014;103(11):4102‐4110. [DOI] [PubMed] [Google Scholar]
- 23. Saraf S, Araki H, Petro B, et al. Ex vivo expansion of human mobilized peripheral blood stem cells using epigenetic modifiers. Transfusion. 2015;55:864‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoban MD, Goncalves KA, Proctor JL, et al. Aryl hydrocarbon receptor antagonists expand adult hematopoietic stem cells from mobilized peripheral blood and bone marrow and increase the dose of CRISPR/Cas9 gene‐edited NSG‐repopulating cells. Blood. 2017;130:3341. [Google Scholar]
- 26. Gu A, Torres‐Coronado M, Tran CA, et al. Engraftment and lineage potential of adult hematopoietic stem and progenitor cells is compromised following short‐term culture in the presencce of an aryl hydrocarbon receptor antagonist. Hum Gene Ther Methods. 2014;25(4):221‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carlin SM, Ma DD, Moore JJ. T‐cell potential of human adult and cord blood hemopoietic stem cells expanded with the use of aryl hydrocarbon receptor antagonists. Cytotherapy. 2013;15(2):224‐230. [DOI] [PubMed] [Google Scholar]
- 28. Psatha N, Georgolopoulos G, Phelps S, et al. Brief report: a differential transcriptomic profile of ex vivo expanded adult human hematopoietic stem cells empowers them for engraftment better than their surface phenotype. Stem Cell Trans. 2017;6(10):1852‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Araki H, Yoshinaga K, Boccuni P, et al. Chromatin‐modifying agents permit human hematopoietic stem cells to undergo multiple cell divisions while retaining their repopulating potential. Blood. 2007;109(8):3570‐3578. [DOI] [PubMed] [Google Scholar]
- 30. Araki H, Baluchamy S, Yoshinaga K, et al. Cord blood stem cell expansion is permissive to epigenetic regulation and environmental cues. Exp Hematol. 2009;37:1084‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Felice L, Tatarelli C, Mascolo MG, et al. Histone deacetylase inhibitor valproic acid enhances the cytokine‐induced expansion of human hematopoietic stem cells. Cancer Res. 2005;65(4):1505‐1513. [DOI] [PubMed] [Google Scholar]
- 32. Gul H, Marquez‐Curtis LA, Jahroudi N, Lo J, Turner AR, Janowska‐Wieszorek A. Valproic acid increases CXCR4 expression in hematopoietic stem/progenitor cells by chromatin remodeling. Stem Cells Dev. 2009;18(6):831‐838. [DOI] [PubMed] [Google Scholar]
- 33. Mahmud N, Petro B, Baluchamy S, et al. Differential effects of epigenetic modifiers on the expansion and maintenance of human cord blood stem/progenitor cells. Biol Blood Marrow Transplant. 2014;20:480‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elizade C, Fernandez‐Rueda J, Salcedo JM, et al. Histone deacetylase 3 modulates the expansion of human hematopoietic stem cells. Stem Cells Dev. 2012;21(4):2581‐2591. [DOI] [PubMed] [Google Scholar]
- 35. Arulmozhivarman G, Krater M, Wobus M, et al. Zebrafish in‐vivo screening for compounds amplifying hematopoietic stem and progenitor cells: preclinical validation in human CD34+ stem and progenitor cells. Sci Rep. 2017;7(1):12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Radtke S, Adair JE, Giese MA, et al. A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates. Sci Trans Med. 2017;9(414):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Danet GH, Lee HW, Luongo JL, Simon MC, Bonnet DA. Dissociation between stem cell phenotype and NOD/SCID repopulating activity in human peripheral blood CD34+ cells after ex vivo expansion. Exp Hematol. 2001;29:1465‐1473. [DOI] [PubMed] [Google Scholar]
- 38. Moussy A, Papili Gao N, Corre G, et al. Constraints on human CD34+ cell fate due to lentiviral vectors can be relieved by valproic acid. Hum Gene Ther. 2019;30(8):1023–1034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Flow cytometric gating strategy used for the phenotypical analysis of ex vivo expansion of mPB and ABM‐CD34+ cells. Schema of the gating strategy used in our studies (top panels) and flow cytometry plots from a representative mPB culture showing the corresponding phenotypical analysis of unmanipulated primary cells at day 0 (middle panels) and VPA expanded cells after 16 hours and 7 days of culture (bottom panels).
Supplementary Figure 2 VPA‐expanded cells possess stem cell characteristics in functional studies. (A) Representative flow cytometric analysis of ALDH activity in mPB cultures treated with cytokines in the presence or absence of VPA for 7 days. Cultured cells treated with DEAB inhibitor were used as control. (B) Representative flow cytometry plots indicating mitochondrial potential of CD34+ and CD34 + CD90+ cells stained with TMRM in a single ABM culture treated as indicated for 7 days in the presence or absence of the efflux pump inhibitor, verapamil. Numbers represent TMRM fluorescence intensity. (C) Representative flow cytometric analysis of Annexin V and 7AAD staining of VPA expanded cells in an mPB culture at day 7. (D) Percent viable cells that are negative for both Annexin V and 7AAD in both mPB (n = 2) and ABM donors (n = 2). “Cyt” denotes the cytokines alone condition.
Supplementary Table 1 Comparison of VPA‐mediated ex vivo expansion of HSCs from three clinically relevant sources: ABM, mPB and UCB
Data Availability Statement
The data that support the findings of this study are available within the paper or can be obtained from the corresponding author upon request.


