Abstract
Aim
We conducted a study to validate the MDASI-HN based nomogram, which is used to predict the acute toxicities in head and neck cancer patients undergoing radiation therapy with or without chemotherapy.
Background
Tolerance to radiation varies from patient to patient and also depends on various other factors like tumor volume, dose of radiation, chemotherapy. Predicting the toxicities allow us to identify potential candidates who are likely to have a higher toxicity and, in addition, evaluates the nomogram when done on an independent group of patients.
Materials and Methods
Sixty biopsy confirmed head and neck cancer patients undergoing radiation were the subjects of the study. The patients completed patient reported outcome instrument (PRO) MDASI-HN questionnaire at the beginning and at the fifth week of radiation. The baseline score obtained was used to obtain the predicted score using nomogram. The nomogram was also externally validated as per the TRIPOD guidelines.
Results
The mean baseline, predicted and score at the fifth week were 27.28 ± 11.04, 73.33 ± 15.51 and 82.62 ± 17.67, respectively, for all sub-sites. A positive, significant correlation (p < 0.01) between the predicted score and the score at the fifth week was seen across all sub sites such as Oral cavity (p = 0.05), Oropharynx (p = 0.02), Hypo pharynx (p = 0.02) and Larynx (p = 0.02).
Conclusion
The MDASI-HN questionnaire based nomogram is simple, easily doable and takes into consideration the initial symptoms as well the treatment details; thereby, it is able to predict the toxicities accurately.
Keywords: Head and neck cancer, Radiotherapy, Nomogram, Toxicity prediction, Mucositis, PRO
1. Introduction
Head and Neck Carcinomas (HNC) are one of the most common malignancies in India and account for 30% of cases as per ICMR data.1 About 60–80% of the patients present at an advanced stage as compared to 40% in developed countries.2 These patients are treated by a multimodality approach consisting of surgery, chemotherapy and radiation therapy. Treatment related factors such as the volume of radiation therapy field, tumor dose and dose per fraction, addition of chemotherapy and its intensity, technique of radiation therapy can cause significant acute toxicities in terms of mucositis and nutritional disturbances. The malnutrition prevalence rate of head and neck cancer patients as a result of these nutritional disturbances is as high as 74.2%.3 Approximately 20% of cancer patients do not succumb to the tumor itself, but as a consequence of malnutrition.4 The degree of toxicities vary depending on the patient characteristics, disease burden and treatment related factors like the technique of radiation therapy.
The standard of care for loco-regionally advanced head and neck cancers is based on the meta-analysis on the role of concurrent chemoradiation in head and neck cancers by Pignon et al. which showed an absolute overall survival benefit of 6.5% and 6.2% in terms of event free survival at 5 years.5 The use of IMRT has reduced the incidence of grade II and above xerostomia significantly (p < 0.0001) and thereby improved the quality of life.6 But there is no difference based on the technique in the incidence of mucositis which is the most common acute side effect. Due to mucositis, the patient’s quality of life is affected, hospital admission rates are higher, the use of total parenteral nutrition is increased and interruption of treatment is more frequent, all of which compromises tumor control.7
The development of an individual symptom management plan prior to the start of radiation therapy and estimation of potential toxicities with quantification of the patient symptom burden can help in better management. If these toxicities can be predicted at the start of the treatment, better prophylactic care can be instituted thereby reducing patient distress and suffering, minimizing treatment interruptions and need for feeding procedures.
Patient reported outcome (PRO) tools have provided additional prognostic information regarding the outcome of cancer treatment. Despite there being evidence supporting the prognostic importance of PRO, the data used to derive symptom prediction models have been based on clinical assessment of symptoms in patients. One such module which uses patient reported outcomes is MD Anderson Symptom Inventory-Head and Neck (MDASI-HN).
Sheu et al. used the MDASI-HN module for prediction of acute toxicities in head and neck patients.8 They showed that MDASI-HN module predicted symptom severity during treatment better than a model based on clinical variables and physician-rated patient performance status alone. The pre-treatment MDASI-HN symptom severity (p < 0.001), concurrent chemotherapy (p = 0.006), primary tumor site (p = 0.016), and receipt of definitive (rather than adjuvant) RT (p = 0.044) correlated with MDASI-HN symptom scores during week five.8 That model was used to construct a nomogram which included baseline MDASI score, site of the disease in head and neck region, intent of treatment, with or without concurrent chemotherapy.
In this prospective questionnaire based study, we tried to externally validate the nomogram developed using the MDASI-HN module, used it to predict the acute toxicities in head and neck cancer patients and correlated the toxicities at the fifth week of radiation.
2. Materials and methods
In this prospective, longitudinal questionnaire based study, sixty head and neck cancer patients undergoing definitive radiation therapy to a minimum dose of 60 Gray with or without chemotherapy at a tertiary care centre in India from November 2016 to March 2018 were studied.
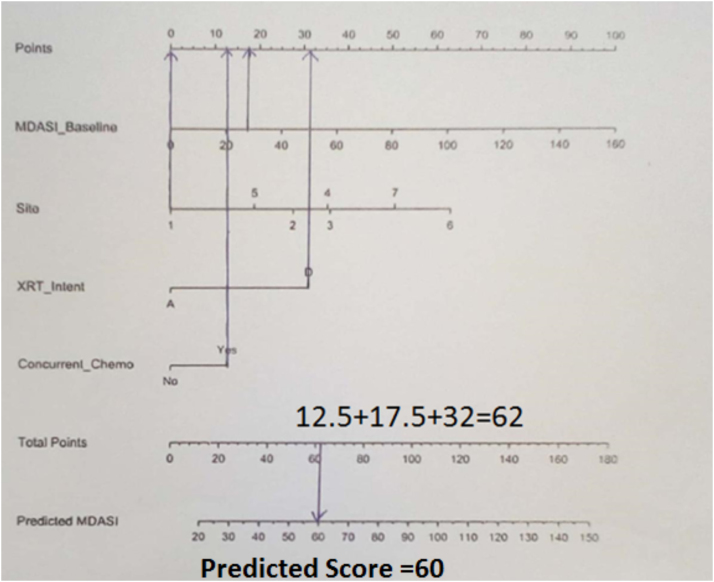
With the approval of the institutional ethics committee, the patients filled the MDASI-HN module before the start of treatment. This included 13 “core” symptoms common across all cancer types and nine “head and neck cancer‒specific” symptoms representing the disease as well as treatment.9 The patients graded their symptoms on a scale of 1–10 with one being not present and 10 being the worst. All the scores were added up to obtain the baseline score. This score was used to obtain the predicted score using nomogram (Fig. 1). Patients were treated to a dose of 60 Gy in 30 fractions in a post operative setting and 66–70 Gy in 33–35 fractions in a definitive setting using Three Dimensional Conformal Radiation Therapy (3DCRT) or Intensity Modulated Radiation Therapy (IMRT) technique with concurrent chemotherapy using weekly cisplatin or carboplatin to a dose of 40 mg per square metre and AUC 2, respectively. At the end of the fifth week, the patient was asked to fill the module again and the fifth week score was obtained. The predicted score was compared with the score at fifth week. We also used the TRIPOD checklist to externally validate the nomogram.9
Fig. 1.
A patient undergoing definitive concurrent chemoradiation for hypopharyngeal cancer with a baseline score of 27 would have a total of 62 points and a predicted score of 60.
2.1. Statistical analysis
A previous study conducted by Sheu et al.8 revealed that the mean and standard deviation of MDASI-HN score at base line and during treatment was 26.19 ± 1.7 and 72.36 ± 4.45, respectively. Based on the above findings of the study, with a power of 90% and an alpha error of 1%, the sample size for the study was found to be extremely small due to a large difference in the baseline and during treatment scores. Keeping in view a sub group analysis, it was proposed to include 60 patients for the study.
Data was analyzed using statistical software, SPSS Inc. Release 2009.PASW statistics for windows version 20.0, Chicago. All the quantitative variables were summarized using descriptive statistics such as mean and standard deviation. All qualitative variables, such as staging, gender etc., were summarized and presented using frequency and percentage. The scores of the fifth week and the predicted score were correlated using the Spearman correlation coefficient and the performance of the prediction model was validated using the same. A p value of less than 0.05 was considered as significant.
3. Results
The patient and tumor characteristics are as shown in Table 1. The mean age of patients was 55 years and 82% of them were between the age 41–70 years. Majority had hypopharyngeal malignancies constituting 33.3% of the patients, 26.6% had oral cavity malignancies and 21.6% of patients had oropharynx malignancies. About 45.6% of patients had stage IVA disease, 36.6% had III disease and 15% had stage II.
Table 1.
Showing patient, tumor and treatment characteristics.
| Total no. of patients (60) | Number of patients (%) | |
|---|---|---|
| Sex: | Male | 45 (75) |
| Female | 15 (25) | |
| Age: (Mean 55 years) | 31–40 | 7 (11.66) |
| 41–50 | 17 (28.33) | |
| 51–60 | 15 (25) | |
| 61–70 | 16 (26.66) | |
| 71–80 | 4 (6.66) | |
| 81–94 | 1 (1.66) | |
| Sub-sites: | Oral cavity | 16 (26.66) |
| Oropharynx | 13 (21.66) | |
| Hypopharynx | 20 (33.33) | |
| Larynx | 9 (15) | |
| Nasal Cavity | 1 (1.66) | |
| PNS | 1 (1.66) | |
| Stage: | II | 9 (15) |
| III | 22 (36.66) | |
| IVA | 28 (46.66) | |
| IV B | 1 (1.66) | |
| Comorbidities | Yes | 47 (78.33) |
| No | 13 (21.66) | |
| Surgery | Yes | 20 (33.33) |
| No | 40 (66.66) | |
| Technique | IMRT | 50 (83.33) |
| 3DCRT | 10 (16.66) | |
| Chemo drug | No chemo | 14 (22.33) |
| Cisplatin | 41 (68.33) | |
| Carboplatin | 2 (3.33) | |
| Cis + 5Fu | 1 (1.66) | |
| Cis + carbo | 2 (3.33) | |
| No of chemo cycles (46) | 2 cycles | 2 (3.33) |
| 3 cycles | 5 (8.33) | |
| 4 cycles | 14 (23.33) | |
| 5 cycles | 25 (41.66) | |
Ten patients were diabetic, two patients were hypertensive, one was both and 33% of the patients had undergone surgery. Radiation therapy was delivered using the IMRT technique in 83.3% of the patients whereas 16.6% of the patients underwent radiation therapy by the 3DCRT technique. In our study, 78% of the patients received concurrent chemotherapy, of whom 68% received cisplatin chemotherapy and 3% received carboplatin chemotherapy. Of the patients who received concurrent chemotherapy, 41.6% had completed five cycles of chemotherapy and 23.3% had completed four cycles of chemotherapy.
The mean baseline, predicted score and week five scores are shown in Table 2. The mean baseline score, the predicted score at the beginning was 27.28 ± 11.04, 73.33 ± 15.51 and the score at the fifth week was 82.62 ± 17.67, respectively for all the sub-sites. A positive, significant correlation (rs = .629, p < 0.01) between the predicted score and the score at the fifth week was seen across all sub-sites, such as Oral cavity (p = 0.05), Oropharynx (p = 0.02), Hypopharynx (p = 0.02), Larynx (p = 0.02) with a 95% CI of 0.629 ± 0.186 for the correlation between the mean predicted score and the mean score at the fifth week.
Table 2.
Showing the mean baseline and mean fifth week score obtained using the MDASI-HN questionnaire and mean predicted score obtained using MDASI-HN based nomogram.
| Diagnosis | Baseline score | Predicted score | Score at 5th wk | p value (<0.05 was significant) | |
|---|---|---|---|---|---|
| Oral cavity | Mean | 24.56 | 79.21 | 88.81 | 0.05 |
| N | 16 | 16 | 16 | ||
| Std. deviation | 8.786 | 13.964 | 17.509 | ||
| Oropharynx | Mean | 30.08 | 85.38 | 87.92 | 0.02 |
| N | 13 | 13 | 13 | ||
| Std. deviation | 14.338 | 9.233 | 19.543 | ||
| Hypopharynx | Mean | 27.90 | 66.25 | 77.85 | 0.02 |
| N | 20 | 20 | 20 | ||
| Std. deviation | 10.457 | 14.804 | 16.161 | ||
| Larynx | Mean | 26.00 | 62.22 | 78.44 | 0.02 |
| N | 9 | 9 | 9 | ||
| Std. deviation | 12.298 | 13.255 | 15.852 | ||
| Nasal cavity | Mean | 27.00 | 67.50 | 71.00 | |
| N | 1 | 1 | 1 | ||
| Std. deviation | . | . | . | ||
| PNS | Mean | 34.00 | 67.50 | 59.00 | |
| N | 1 | 1 | 1 | ||
| Std. deviation | . | . | . | ||
| Total | Mean | 27.28 | 73.33 | 82.62 | |
| N | 60 | 60 | 60 | ||
| Std. deviation | 11.047 | 15.516 | 17.678 | ||
Among various sub-sites, mean baseline score was the highest for oropharyngeal cancer (30.08), but at the fifth week the score was the highest for oral cavity cancer patients (88.81).
4. Discussion
The fact that the tolerance to radiation varies widely from one patient to another means it is dependent on many factors. There are different ways of assessing tolerance to radiation. Most commonly followed are weekly monitoring of symptoms, mucositis assessment, weight monitoring. All these will help us identify the side effects early and treat them. However, if they could be predicted before treatment is instituted, we should be able to monitor high risk groups more closely and provide tailored superior supportive care.
Factors which help us to predict are higher tumor burden comorbidities, poor general condition, post-operative status and addition of chemotherapy. Nomogram is a statistical tool that has the ability to take into account numerous variables to predict an outcome of interest for an individual patient. They are a graphical representation of multiple factors implemented in various clinical settings, and can serve as a “look-up” tool to come to an individualized end-point-specific scores, like toxicity or mortality from a given patient’s clinical profile.8 Once the patients are categorized, it would be easier to monitor and tailor the treatment of the expected sequelae.
There are various nomograms available incorporating many of the above factors. Since the dose, fractionation and technology of radiation are the same, the factors that are different are patient related and, hence, patient reported outcome (PRO) tools have provided additional prognostic information. There are only a few studies conducted using nomogram based on the MD Anderson Symptom Inventory-Head and Neck (MDASI-HN), Functional Assessment of Cancer Therapy-Head and Neck (FACT-HN) available in the literature.
The MDASI-HN is a brief, psychometrically validated patient-reported multisymptom assessment questionnaire which takes into account general symptoms of any cancer as well as those particular to head and neck.10 MDASI-HN is one of the first modules which assessed patient’s symptoms during radiation therapy. Rosenthal et al. compared the MDASI-HN and FACT-HN questionnaires; he concluded that both the MDASI-HN and FACT-HN modules can predict the mucositis scores but the MDASI-HN was more closely associated with the severity of radiation-induced mucositis than the FACT-HN.11 It also allows a complete assessment of the patient as it takes into account not only symptoms but other factors related to treatment, like addition of chemotherapy with radiotherapy. Due to the inherent advantages of MDASI-HN over other QOL questionnaires, we used the MDASI-HN questionnaire in our study.
In a prospective, questionnaire based study done by Sheu et al. using the MDASI-HN questionnaire in 264 patients pre-treatment variables were correlated with MDASI-HN symptom scores during therapy with multivariate modelling and then correlated with composite MDASI-HN score during week five of therapy.8 They found that a multivariate model incorporating pre-treatment PROs better predicted MDASI-HN symptom scores during treatment than did a model based on clinical variables and physician-rated patient performance status alone. They further added that pre-treatment MDASI-HN symptom severity (p < 0.001), concurrent chemotherapy (p = 0.006), primary tumor site (p = 0.016), and receipt of definitive (rather than adjuvant) RT (p = 0.044) correlated with MDASI-HN symptom scores during week five. This was used to construct a nomogram. We observed a significant correlation (rs = .629, p < 0.01) between the predicted score and the score at the fifth week using the same nomogram implying that this could be used to predict the side effects during treatment.
Ours is one of the very few studies which used the MDASI-HN based nomogram. In our study, 33.3% of the cases were hypopharyngeal malignancies forming the majority whereas Sheu et al. had majority of oropharyngeal malignancies. The mean score at the first fifth week in our study was 27.28 ± 11.04 and 82.62 ± 17.67, respectively. The mean first week score was similar at 26.19 ± 1.77 in the study by Sheu et al. but slightly lower at the fifth week at 72.36 ± 4.45 compared to our study. In our study, 66.6% of the patients received cisplatin chemotherapy, 3.3% of the patients received carboplatin and cisplatin plus 5-FU but Sheu et al. used cetuximab in 13% of patients when compared to none in ours. The MDASI nomogram takes into account the tumor characteristics, treatment modality, host factors as well as the patient related symptoms. Hence, this would be a good tool to assess the patients during chemoradiation.
An advantage with the use of nomograms is that many important factors that influence the morbidity of the treatment as well as prognosis of the disease can be incorporated. In the MDASI-HN nomogram that we used in our study, the factors incorporated included the baseline MDASI score, site, intent of treatment, usage of chemotherapy. It is comprehensive as it includes not only the treatment given but also incorporates the baseline score based on the symptoms experienced by the patient which, in turn, could be a reflection of the disease status. Most of the nomograms used in head and neck cancers have predicted local control or survival, our study is one of the few studies that have predicted the toxicities which have an important effect on compliance with treatment which, in turn, can have an effect on long term outcome.
In our study, approximately two thirds of the patients received concurrent chemotherapy which could be the probable reason for a higher fifth week score. Our study did not include patients with nasopharyngeal, early glottic tumors, thyroid, skin in which the target volumes can vary as compared to other sub-sites in head and neck. But study conducted to Sheu et al. included patients from all sub-sites of head and neck.8 We excluded these sites as the philosophy of treatment of these sites is completely different from the rest of the head and neck cancers.
We observed that baseline and predicted score was the highest for oropharyngeal cancer whereas the score at the fifth week was the highest for oral cavity cancer. Probable reason could be that radiation was used in an adjuvant setting in oral cancer and the postoperative morbidity could have added to the symptoms.
The strengths of our study are that it is a prospective study and ours is the only one which has been carried out on the Indian population where almost 60–70 percent of patients present with locally advanced disease, thereby prediction of acute toxicities can help in carrying out prophylactic measures to improve the tolerance of patients during the course of radiation therapy. The questionnaire was also translated into our regional languages so the patients were able to comprehend and relate with the symptoms better which, in turn, helped in more accurate prediction. Although it is one of the few studies in literature, its limitation is the low number of patients; however, this nomogram when used on a similar but independent group of patients from a different country showed similar results.
5. Conclusion
The prediction of acute toxicities in head and neck cancer patients undergoing concurrent chemoradiation helps in giving personalized care to each patient. The MDASI-HN questionnaire based nomogram is simple, easily doable and takes into consideration the initial symptoms as well the treatment details; thereby it is able to predict the toxicities accurately. It can be used as a tool during initial work up of the patient which helps carry out prophylactic measures in patients with a higher score, thereby overall treatment time will be as planned which translates to better long term outcomes.
Conflict of interest
None declared.
Financial disclosure
None declared.
Acknowledgement
Nil.
Contributor Information
Nishant Vidyasagar, Email: nishvidsar8391@gmail.com.
Janaki Manur Gururajachar, Email: drjanakimg@gmail.com.
References
- 1.Mehrotra R., Singh M., Gupta R.K. Trends of prevalence and pathological spectrum of head and neck cancers in North India. Indian J Cancer. 2005;42:89–93. doi: 10.4103/0019-509x.16698. [DOI] [PubMed] [Google Scholar]
- 2.Sarin R. Indian national cancer control programme: Setting sight on shifting targets. J Cancer Res Ther Oncol. 2005;1:240–248. doi: 10.4103/0973-1482.19603. [DOI] [PubMed] [Google Scholar]
- 3.Kang W.X., Li W., Huang S.G. Effects of nutritional intervention in head and neck cancer patients undergoing radiotherapy: A prospective randomized clinical trial. MolClinOncol. 2016;5(3):279–282. doi: 10.3892/mco.2016.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ottery F.D. Rethinking nutritional support of the cancer patient: The new field of nutritional oncology. Semin Oncol. 1994;21:770–778. [PubMed] [Google Scholar]
- 5.Pignon J.P., Maitre A., Maillard E. Meta Analysis of chemotherapy in head and neck cancer (MACH – NC): An update of 93 randomized controlled trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Nutting C.M., Morden J.P., Harrington K.J. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomized controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trotti A., Bellm L.A., Epstein J.B. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol. 2003;66(3):253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 8.Sheu T., Fuller C.D., Mendoza T.R. Nomogram for predicting symptom severity during radiation therapy for head and neck cancer. Otolaryngol Head Neck Surg. 2014;151(4):619–626. doi: 10.1177/0194599814545746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moons K.G., Altman D.G., Reitsma J.B. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann Intern Med. 2015;162:W1–73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal D.I., Mendoza T.R., Chambers M.S. Measuring head and neck cancer symptom burden: The development and validation of the M. D. Anderson symptom inventory, head and neck module. Head Neck. 2007;29:923–931. doi: 10.1002/hed.20602. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal D.I., Mendoza T.R., Chambers M.S. The M. D. Anderson symptom inventory-head and neck module, a patient-reported outcome instrument, accurately predicts the severity of radiation-induced mucositis. Int J RadiatOncol Biol Phys. 2008;72(Dec (5)):1355–1361. doi: 10.1016/j.ijrobp.2008.02.072. [DOI] [PubMed] [Google Scholar]