Graphical abstract
Keywords: Atomic absorption spectrometer, Cyprinus carpio, Heavy metals, Lesotho, Maqalika reservoir, Sediments
Highlights
-
•
As, Pb and Zn concentration levels in sediments and gills of Cyprinus carpio from Maqalika Reservoir were assessed..
-
•
Metals were present in both sediment and gills of Cyprinus carpio and increased in order of downstream>midstream>upstream.
-
•
Sediment played an important role in the uptake of metals by Cyprinus carpio as the major sink for metals in the reservoir.
-
•
As and Pb in gills of Cyprinus carpio exceeded FAO/WHO safety thresholds for human consumption.
-
•
Anthropogenic activities were the sources of metals.
Abstract
The determination of heavy metal contaminants in fish is very important in monitoring health risks to humans who consume them. This study assessed the concentration of heavy metals (As, Pb and Zn) in sediments and gills of Common Carp fish (Cyprinus carpio) from Maqalika Reservoir in Maseru, Lesotho and their potential health risks to such fish consumers. Sediment and Cyprinus carpio samples were collected from upstream, midstream and downstream sites of Maqalika Reservoir and examined for As, Pb and Zn using atomic absorption spectrometer. Potential health risks were based on comparing the derived metal concentrations in gills of Cyprinus carpio with the World Health Organization (WHO) permissible limits for human consumption. The mean concentration levels of Zn, As and Pb in sediment were in the order: 78.5; 2.34; and 0.29 mg/kg respectively. In the gills of Cyprinus carpio the mean concentration levels were in the order 7.85; 1.29; and 0.33 mg/kg for Zn, As and Pb respectively. The magnitude of concentration of Zn, As and Pb by location in the reservoir varied spatially in the order of downstream > midstream > upstream in both sediment and gills of Cyprinus carpio. Significant differences (p < 0.05) in metal concentrations between upstream and downstream sites of the reservoir were observed. The metals concentration in gills of Cyprinus carpio were lower than those in sediments, but positively and significantly correlated (P < 0.05). As and Pb concentration levels in the gills of Cyprinus carpio were higher than the WHO permissible limits recommended for fish consumption of 1 mg/kg and 0.2 mg/kg respectively, suggesting that residents could experience significant health risks from the intake of individual metals through fish consumption. Measures should be taken to reduce heavy metal concentrations in sediment and Cyprinus carpio exposure in the general population in order to minimize the risk of human health adverse effects.
1. Introduction
Heavy metals are recognized hazardous environmental pollutants with long environmental persistence and capable of bioaccumulation in aquatic ecosystems, posing significant health risk to humans through the food chain [[1], [2], [3], [4], [5]]. Among all foods, fish is the main source of heavy metal contaminants [4]. The Minamata catastrophe, caused by human consumption of methyl mercury contaminated-fish, a toxic and persistent organic mercury compound has increasingly emerged as a case example of health risk to human consumption of contaminated fish [2]. Increasingly, heavy metal contamination of fish is a significant health problem because of its accumulation and magnification through the food chain, causing several adverse effects to human health [4]. This has necessitated a critical need for studies that examine levels of heavy metals in fish to monitor its adverse effects on human health [3,5].
In third world cities, indiscriminate discharge of raw industrial effluents, unregulated urban agriculture and poor solid waste management contribute to increased concentrations of heavy metals in aquatic ecosystems, raising concerns because of its toxicological impacts on aquatic ecosystems and associated human health risks [6,7]. Water bodies and aquatic flora and fauna are the worst affected and this often renders these resources unsuitable for human consumption [[4], [5], [6], [7]]. It is increasingly reported [3,[5], [6], [7]] that heavy metal accumulation in urban aquatic ecosystems watersheds and sediments mirrors its bioaccumulation in fish from such reservoirs and thus merits regular monitoring to safeguard human health. Some studies [4,6,7] have confirmed that more than 99% of the heavy metals entering aquatic ecosystems are stored in sediments and subsequently transferred to humans through the food chain.
Effects on human health of consuming contaminated fish account for the primary exposure of humans to toxic metals [[8], [9], [10], [11]]. The contaminants that have been bioaccumulated by the fish pose carcinogenic, genotoxic and other health risks to consumers [8,11]. For example, consumption of arsenic (As) laden fish has been linked to cancer of the skin, kidneys, bladder and lungs [8]. Consumption of fish contaminated with lead (Pb) above the World Health organization (WHO) permissible levels, has also been associated with nervous system damage, blood and brain disorders and developmental delays in children [8]. Owing to their toxicity, persistence and tendency to accumulate in water and sediments, heavy metals have been reported to cause mutation of inner organs, disturb immune reactions and reduce their adaptation and resistance to diseases in humans [12].
Maqalika Reservoir serves as a source of drinking water and fish for local residents of Maseru, the capital city of Lesotho [13]. Pollution caused by industrial effluent, municipal waste and agricultural inputs discharges is the major threat to the reservoir [13]. The sedimentation of the reservoir due to inputs from industrial wastes, sewage effluent, agricultural runoff and domestic wastes has been previously reported [13,14]. However, there has been no study on heavy metals in sediments and fish fauna of the reservoir consumed by local residents.
This study assessed the concentration levels of As, Pb and Zn in sediment and gills of Cyprinus carpio from Maqalika Resrvoir and their potential health risks to consumers of fish from the reservoir.
2. Methodology
2.1. Study area
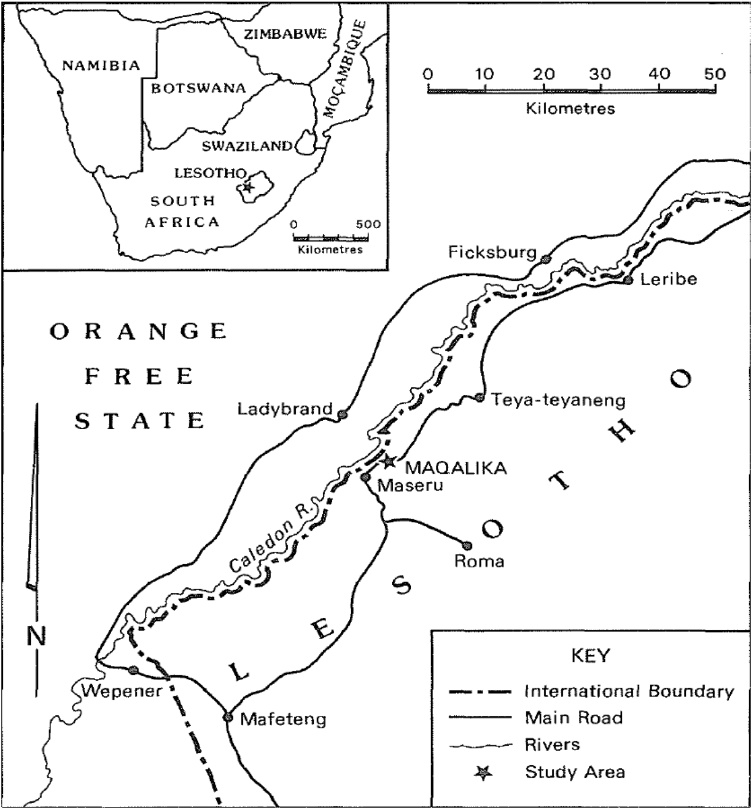
Maqalika Reservoir (longitude 27o30'38.91" and latitude -29˚18′44.58″) was constructed in 1983 as a primary source for water supply to the capital city of Maseru in Lesotho [13]. It is located on the Caledon River, north-east of Maseru (Fig. 1). The reservoir’s catchment area is approximately 44 km2, with a length of approximately 13 km and channel slope of 0.4 km/km [13]. Over the years, the reservoir has acted as a trap for sediment originating from the catchment area resulting in its storage capacity decreasing as sediments accumulate.
Fig. 1.
Location of Maqalika Reservoir.
Source: Rowntree et al. [14]
Maqalika Reservoir is located in one of the most human settled part of Maseru and receives many effluent discharges from industry and sewage from residential areas [14]. Residential settlements are unplanned and most houses are not connected to the sewerage reticulation system. As a result the catchment area is characterized by septic tanks that constantly leak, waste from informal car repair garages, illegal and legal waste dumping sites and sedimentation from run-off that is released untreated into the reservoir, resulting in high input of heavy metals.
A lot of fish from Maqalika Reservoir is consumed by residents living around the reservoir. Cyprinus carpio is the most common fish species harvested by fishermen. It is therefore important to clarify the status of heavy metal in fish from the reservoir in order to assess the health risks posed to residents.
2.2. Sample collection
Sediment and Cyprinus carpio samples were collected from three purposively selected sampling sites, upstream (S1), midstream (S2) and downstream (S3) of the Maqalika Reservoir. The locations were selected based on differing flow regime and hence sediment depositions.
Sediment samples were collected with a spade from the reservoir’s bottom. The samples were sealed in sterile plastic bags and transported to the laboratory for heavy metal concentration analysis.
Cyprinus carpio samples were purchased from fishermen at the Maqalika Reservoir. Three Cyprinus carpio caught at each of the designated sampling sites were selected for the study. The considered weights of each Cyprinus carpio were 400−500 g. The fish were washed with distilled water and packed in polyethylene bags and stored in ice box before being transported to the laboratory for heavy metal analysis.
2.3. Digestion of samples
Determination of heavy metals in collected samples was done using atomic absorption spectrophotometer. Sediment samples were first air dried; then stones and plant fragments removed by passing the samples through a 2 mm sieve. The sieved samples were later pounded into powder using a mortar and pestle and finally passed through a 500 μm sieve and stored in glass bottles.
Finely grounded one gramme of sediment samples were later digested with 10 ml of acid mixture of nitric acid (HNO3), sulphuric acid (H2SO4), and perchloric acid (HCLO4) in the ratio of 5:2:1 at 60 °C. Five drops of hydrochloric acid were added to allow complete digestion of sample.
The samples were transferred into sterile sample bottles, labelled and kept for digestion and analysis of heavy metals. After complete digestion, samples were stored in pre-cleaned polyethylene bottles until analysis using atomic absorption spectrophotometer.
Cyprinus carpio were dissected to remove gills using a stainless steel knife. The gills were placed in a clean petri dish and dried in an oven at 120 °C for 48 h at which time the weight was constant. The dried gills were crushed into powder in a mortar and then the samples were kept in polyethylene bottles for analysis.
One gramme portions of Cyprinus carpio gills were digested by means of a microwave digestion after addition of HNO3 (65%) and hydrogen peroxide (H2O2) (30%). The samples were transferred into sterile sample bottles, labelled and kept for digestion and analysis of heavy metals. The obtained results were expressed as mg/kg weight.
2.4. Data analysis
Statistical Package for Social Scientists (SPSS) software Version 18.0 was used to analyze the collected data. Descriptive statistics were used to summarize the data. Pearson’s correlation was used to determine the significance of the relationship between metal concentration in sediment and gills of Cyprinus carpio at significance level of 0.05. The measured metal concentration levels were expressed in mg/kg.
The human health risks associated with consumption of contaminated-fish was characterized using the FAO/WHO permissible guidelines. In this respect if the concentration levels were above the FAO/WHO guidelines they were considered a health risk to consumers of that fish.
3. Results
3.1. Heavy metal concentrations in sediment and Cyprinus carpio
The concentrations of As, Pb and Zn in sediment and gills of Cyprinus carpio are summarized in Table 1. Mean concentrations of Zn, As and Pb in sediment were 78.5, 2.34 and 0.29 mg/kg respectively. The order of accumulation of metals was: Zn > As > Pb. Relatively, the concentrations of metals in sediment spatially varied by site as: downstream > mid stream > upstream of the reservoir. The highest concentration was Zinc with values ranging from 72 to 85 mg/kg while the lowest levels were Pb in the range from 0.25 to 0.33 mg/kg. The downstream site showed significantly higher concentrations levels of the metals compared to the upstream site (p < 0.05).
Table 1.
Concentrations of heavy metals in sediments and gills of Cyprinus carpio in Maqalika Dam.
| Sampling site | Metals concentration levels in sediment samples (mg/kg) |
Metals concentration in gills of Cyprinus carpio samples(mg/kg) |
||||
|---|---|---|---|---|---|---|
| As | Pb | Zn | As | Pb | Zn | |
| S1 | 1.87 | 0.25 | 72 | 1.5 | 0.32 | 8.0 |
| S2 | 2.53 | 0.33 | 78 | 1.08 | 0.31 | 6.5 |
| S3 | 2.81 | 0.28 | 85 | 1.36 | 0.34 | 9.2 |
Heavy metals accumulations in the gills of Cyprinus carpio were in the ranges: Zn: 6.5–9.2 mg/kg; As: 1.08–1.5 mg/kg; Pb: 0.31-0.34 mg/kg. The mean levels were 7.5, 1.29 and 0.325 mg/kg for Zn, As and Pb respectively. The order of bioaccumulation of metals in gills of Cyprinus carpio was: Zn > As > Pb. A trend of concentration of metals by sampling sites was downstream > mid stream > upstream of the reservoir.
Metal concentrations in sediment samples were high on slow flowing site downstream of the reservoir. Analysis of heavy metal levels in gills of Cyprinus carpio also showed higher values downstream of the reservoir compared to upstream. This may be due to the low flow velocity of water downstream of the reservoir and deposition of suspended sediments containing heavy metals.
The concentration of As and Pb found in the gills of Cyprinus carpio samples were above the FAO/WHO [15] permissible limits of 1mg/kg and 0.3 mg/kg respectively, suggesting potential health risks to consumers of fish in Maqalika Reservoir. The concentrations of As and Pb in the study ranged from 1.08 to 1.5 mg/kg and 0.31and 0.34 mg/kg respectively. The Zn concentrations in the range of 6.5–9.2 mg/kg were below the FAO/WHO [15] limit of 20 mg/kg.
3.2. Spatial variation of heavy metals in sediment and gills of Cyprinus carpio
The concentrations of As, Pb and Zn varied significantly from upstream to downstream sites in both sediments and gills of Cyprinus carpio (p < 0.05). Downstream location showed the highest heavy metal concentrations. The highest heavy metal concentrations were found in the sediment samples compared to those in gills of Cyprinus carpio.
All the metal concentrations in gills of Cyprinus carpio were lower than those in sediment. However, the differences in heavy metals concentration between sediments and gills of Cyprinus carpio were significant, but positively correlated (p < 0.05).
4. Discussion
The results of this study may be considered an important warning signal to humans consuming fish from Maqalika Reservoir. The results are in agreement with findings from other studies elsewhere that identify concentration of heavy metals in sediment and fish [16,17]. Arizhibowa [16], for example, found high concentration levels of heavy metals in sediment and fish in Lake Chivero in Harare, Zimbabwe. Similar results of high concentrations of heavy metals were recorded by Ogendi [17] in reservoir near Naivasha town in Kenya, suggesting that organisms living in it can take up the pollutants from the sediments. The possible reason for the detection of heavy metals in sediment and gills of Cyprinus carpio could be because of anthropogenic activities in the reservoir’s watershed, including agricultural activities, discharge of untreated sewage and uncontrolled waste from informal industries.
Sediments play an important role on the quality of aquatic ecosystems as reservoirs where they can either be a sink or a source of contaminants. The concentrations of metals in sediment and gills of Cyprinus carpio samples were significant and positively correlated (p < 0.05). This suggests that the bioaccumulation of metals in the gills of Cyprinus carpio could be linked to those in sediment. There are agreements with these results in several studies [[18], [19], [20]] that have reported that sediments act as sources and sinks for heavy metals and are in direct contact with fish. According to Tekin-Özan and Aktan [18] sediment may represent long-term sources of contamination to higher trophic levels.
To asses public health risks to fish consumers, metal levels in gills of Cyprinus carpio were compared to the WHO [15] permissible limits for human consumption. As and Pb concentrations were found above the WHO [15] permissible limits of of 1 mg/kg and 0.2 mg/kg respectively, suggesting that local residents could experience significant health risks from the intake of these metals through fish consumption. According to Rajeshkumar and Li [21] the gill is an important site for heavy metals entry into the fish body. Even though gills are seldom consumed, they are good bio-monitors of metals present in the surrounding environment [21]. Pb is absorbed into the fish’s bloodstream and accumulates in body tissues, bones, gills, kidneys, liver and scales [22, 23]. It enters the human body through fish consumption and is responsible for reduced cognitive development and intellectual performance in children and increased blood pressure and cardio vascular disease in adults [24, 25,26]. As is a major risk factor for vascular disorders, leucopenia, anemia, black foot disease skin and lung cancer [11]. On the basis of As and Pb concentration levels the fish in Maqalika Reservoir were found unsafe for consumption and pose a threat to health of fish consumers.
From a public health perspective, even though the gills of Cyprinus carpio where concentration levels of As and Pb were above FAO/WHO recommended limits, they represent good bio-monitor of metals present in the surrounding environment, which reflect potential risk to humans consuming the fish [21,23]. Thus, a more cautious viewpoint should be assumed and further research focusing on edible parts of the fish be done. Preventive measures should also be taken to reduce heavy metal pollution in Maqalika Reservoir’s catchment area. Further research, monitoring and policing of disposal of untreated industrial and municipal wastes in the reservoir’s catchment area need to be conducted to make appropriate calculations of associated risks.
5. Conclusion
This study detected heavy metal in sediment and gills of Cyprinus carpio from Maqalika Reservoir. The heavy metal showed spatial variation in concentration between upstream and downstream sites of the reservoir. The metal concentration in both sediment and gills of Cyprinus carpio could be attributed to discharge of untreated sewage and uncontrolled waste as well as agricultural activities in the reservoir’s catchment. As and Pb levels in gills of Cyprinus carpio exceeded the permissible limit recommended for fish consumption by FAO/WHO, suggesting potential danger to human consuming Cyprinus carpio from Maqalika Reservoir. In view of potential risk of heavy metals, regular monitoring and implementation of strict environmental laws to avoid concentration of heavy metals in Maqalika Reservoir are recommended.
Author statement
Patrick Gwimbi: Conception and design of the paper, data analysis and interpretation, drafting the article, critically revising the article, final approval of the version to be published.
Ts’alikoe Kotelo: Contribution to acquisition of data and proof reading.
Masepele Jenette Selimo: Contribution to acquisition of data and proof reading.
Conflict of interest
There is no known conflict of interest associated with this publication.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.03.005.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Tang W., Shan B., Zhang H., Mao Z. Heavy metal sources and associated risk in response to agricultural intensification in the estuarine sediments of Chaohu Lake Valley, East China. J. Hazard. Mater. 2010;176:945–951. doi: 10.1016/j.jhazmat.2009.11.131. [DOI] [PubMed] [Google Scholar]
- 2.Tsuda T., Yorifuji T., Takao S., Miryai M., Babazono A. Minamata Disease: Catastrophic Poisoning Due to a Failed Public Health Response. J. Public Health Policy. 2009;30(No. 1):54–67. doi: 10.1057/jphp.2008.30. [DOI] [PubMed] [Google Scholar]
- 3.Klake R.K., Nartey V.K., Doamekpor L.K., Edor K.A. Correlation between heavy metals in fish and sediment in Sakumo and kpeshie lagoons, Ghana. J. Environ. Prot. (Irvine, Calif) 2012;3:1070–1077. [Google Scholar]
- 4.Chowdhury R., Ramond A., O’Keeffe L.M., Shahzad S., Kunutsor S.K., Muka T., Gregson J., Willeit P., Warnakula S., Khan H., Chowdhury S., Gobin R., Franco O.H., Angelantonio E.D. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:3310. doi: 10.1136/bmj.k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alturiqi A.S., Albedair L.A. Evaluation of some heavy metals in certain fish, meat and meat products in Saudi Arabian markets. Egypt. J. Aquat. Res. 2012;38:45–49. [Google Scholar]
- 6.Ujah I., Okeke D., Okpashi V.E. Determination of heavy metals in fish tissues, water and sediment from the Onitsha segment of the river niger Anambra State Nigeria. J. Environ. Anal. Toxicol. 2017;7:5. [Google Scholar]
- 7.Shen F., Mao L., Sun R., Du J., Tan Z., M Ding. Contamination evaluation and source identification of heavy metals in the sediments from the Lishui River Watershed, Southern China. Int. J. Environ. Res. Public Health. 2019;16:336. doi: 10.3390/ijerph16030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousafza A.M., Ullah F., Bali F., Raziq S., Riaz M., Khan K., Sthanadar I.A., Shaheen B., Shaheen M., Ahmad H. Bioaccumulation of some heavy metals: analysis and comparison of Cyprinus carpio and Labeo rohita from Sardaryab, khyber pakhtunkhwa. BioMed Res. Int. 2017;5 doi: 10.1155/2017/5801432. Article ID 5801432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eimers R.D., Evans R.D., Welbourn P.M. Cadmium accumulation in the freshwater isopod Asellus racovitzai: the relative importance of solute and particulate sources at trace concentrations. Environ. Pollut. 2001;111:247–258. doi: 10.1016/s0269-7491(00)00066-x. [DOI] [PubMed] [Google Scholar]
- 10.Rauf A., Javed M., Ubaidullah M. Heavy metal levels in three major Carps (Catla Catla, Labeo Rohita And Cirrhina Mrigala) from the River Ravi, Pakistan. Pakistan Vet. J. 2009;29(1):24–26. [Google Scholar]
- 11.Janadeleh H., Jahangiri S. Risk assessment and heavy metal contamination in fish (Otolithes ruber) and sediments in Persian Gulf. J. Commun. Health Res. 2016;5(3):169–181. [Google Scholar]
- 12.Castro-González M.I., Méndez-Armenta M. Heavy metals: implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008;26:263–271. doi: 10.1016/j.etap.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Letsie M. Durban Institute of Technology; 2005. Utilization of Maqalika Reservoir As a Source of Potable Water for Maseru City in Lesotho. MSc. Thesis. [Google Scholar]
- 14.K.M. Rowntree, M. M. Ntsaba, A. V. B. Weaver, Erosion in a Peri-Urban Catchment, Maseru, Lesotho. Proceedings of the Vienna Symposium, August 1991, IAHS Publ. no. 203, 199.
- 15.FAO/WHO, Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF. Food Standards Programme, Codex Committee on Contaminants in Foods, Fifth Session (2011), The Hague, Netherlands, March 21 - 25.
- 16.Arizhibowa F. University of Zimbabwe; 2011. Human Health Risk Assessment of Heavy Metal Bioaccumulation in Nile Tilapia Oreochromis niloticus From Lake Chivero, Zimbabwe. MSc. Thesis. Tropical Hydrobiology and Fisheries. [Google Scholar]
- 17.Ogendi G.M., Maina G.M., Mbuthia J.W., Koech H.K., Ratemo C.M., Koskey J.C. Heavy metal concentrations in water, sediments and common carp (Cyprinus carpio) fish species from Lake Naivasha, Kenya. J. Environ. Earth Sci. 2014;6(8):416–423. ISSN: 2041-0484; e-ISSN: 2041-0492. [Google Scholar]
- 18.Tekin-Özan S., Aktan N. Relationship of heavy metals in water, sediment and tissues with total length, weight and seasons of Cyprinus carpio L., 1758 from Işikli Lake (Turkey) Pakistan J. Zool. 2012;44(5):1405–1416. [Google Scholar]
- 19.Sreedhara B.M., Nayaka Ramakrishna S., Jayaprakash J., Delvi M.R. Impact of heavy metals on water, fish (Cyprinus carpio) and sediments from a water tank at Tumkur, India. Int. J. Oceanogr. Hydrobiol. 2009 Vol. XXXVIII, No.2. [Google Scholar]
- 20.Sojka M., Jaskuła J., Siepak M. Heavy metals in bottom sediments of reservoirs in the Lowland Area ofWestern Poland: concentrations,Distribution, sources and ecological risk. Water. 2019;11:56. [Google Scholar]
- 21.Rajeshkumar S., Li X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018;5:288–295. doi: 10.1016/j.toxrep.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreji J., I Stranai, Massanyi P., Valent M. Accumulation of some metals in muscles of five fish species from Lower Nitra River. J. Environ. Sci. Health A. 2006;41:2607–2622. doi: 10.1080/10934520600928003. [DOI] [PubMed] [Google Scholar]
- 23.Ullah A.K.M.A., Maksud M.A., Khan S.R., Lutfa L.N., Quraishi S.B. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 2017;4:574–579. doi: 10.1016/j.toxrep.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajeshkumar S., Li X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018;5:288–295. doi: 10.1016/j.toxrep.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atobatele O.E., Olutona G.O. Distribution of three non-essential trace metals (Cadmium, Mercuryand Lead) in the organs of fish from Aiba Reservoir, Iwo, Nigeria. Toxicol. Rep. 2015;2:896–903. doi: 10.1016/j.toxrep.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alturiqi A.S., Albedair L.A. Evaluation of some heavy metals in certain fish, meat and meat products in Saudi Arabian markets. Egypt. J. Aquat. Res. 2012;38:45–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.