Introduction
Insulin-derived amyloidosis is defined as a subcutaneous amyloid mass at the site of insulin injections, in which the amyloid deposit shows a positive staining result with anti-insulin antibodies.1 Most patients present with a solitary mass. Nagase et al2 referred to this as the “insulin ball,” whereas others have referred to it as an amyloidoma.3 Insulin-derived amyloidosis is relatively unknown in the dermatologic literature. We report an unusual case of a patient who presented with 4 sites of insulin-derived subcutaneous amyloidosis.
Case report
A 61-year-old white man presented for evaluation of subcutaneous masses. He had a long-term history of type 1 diabetes, with onset at aged 23 years. His diabetes was first treated with standard doses of glargine, aspart, and lispro. He injected himself a total of 7 times a day (long-lasting insulin in his leg lumps and short-acting insulin in his left arm lump and right-sided abdomen lump). He also injected short-acting insulin into his buttocks. He noted lumps developing when he was approximately aged 51 years, first on his legs, then the left arm, and finally his abdomen. His glycosylated hemoglobin values were stable, between 6.0% and 6.3%. He did not believe the masses had enlarged much during the last 8 years, so he continued to inject into the masses. Injecting the lumps was more painful than doing so in other areas. During the past few years, he had been treated with glargine, degludec (the leg masses), and lispro.
On physical examination, he had 4 firm subcutaneous masses (Fig 1, A to C) ranging from 13 cm (left arm) to 8 cm (abdomen). Removal of tissue involved making small incisions over each mass, and the amyloid extruded as it would during the removal of lipomas. All 4 sites were sampled and shown to be insulin-derived amyloidosis. After removal of the amyloidomas on his left arm and abdomen, he noted improvement in his insulin dosing, with short-acting insulin requirements decreasing from 3 to 4 units to 1 unit injected into the same areas where the lumps were removed.
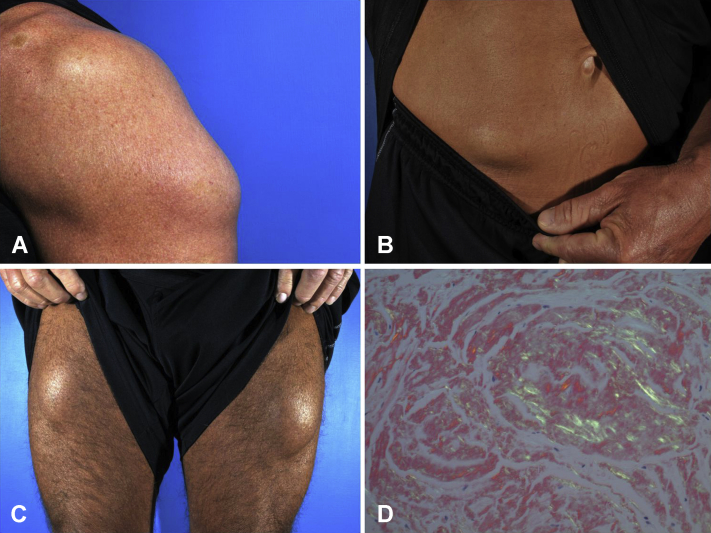
Fig 1.
A, Firm subcutaneous mass (13.0 cm) on palpation, left triceps region. B, Firm subcutaneous mass (8.0 cm) on palpation, right side of the lower abdomen. C, Bilateral firm palpable subcutaneous masses (10 cm each), proximal portion of the thighs. D, Polarized Congo red stain demonstrating apple-green–colored birefringence.
Histology result was remarkable for dense eosinophilic amorphous material in all 4 sites. Congo red stains showed high congophilia and on polarization demonstrated apple-green birefringence (Fig 1, D). Immunohistochemical staining for insulin showed dark deposits, confirming the presence of insulin and the diagnosis of insulin-derived amyloid.
Discussion
The first case of insulin-induced amyloidosis was reported in 1983, when Storkel et al4 identified insulin amyloid fibrils in a diabetic patient after continuous infusion of porcine insulin for 5 weeks. In 1988, Dische et al5 reported the first case of amyloid deposit at the injection site of a diabetic patient receiving porcine insulin. Since then, more than 75 cases have been reported in patients using a wide range of insulin forms, and the incidence is steadily increasing.6, 7, 8
Patients who develop insulin-derived amyloidosis tend to have poor glycemic control at diagnosis, with glycosylated hemoglobin values between 7.6% and 15.5% and durations of insulin treatment ranging from 4 to 60 years.8 The majority of reported patients (90%) had a single lump that on physical examination was firm to palpation, and only 2 cases reported a soft lump. Areas of involvement include the abdomen (70%), thigh (15%), arms (13%), breast (1%), and inguinal lymph node (1%).6, 7, 8 The breast location was observed in a single case when a patient shifted her injection site from the abdomen to the breast during pregnancy.6 The patient with the inguinal lymph node involvement injected into the corresponding thigh.9 Not all patients with insulin-derived amyloidosis had palpable masses. Detection of insulin amyloid deposits was found in 3.5% of reported patients who had blind abdominal fat aspirations to rule out systemic amyloidosis.8 This suggests that insulin-derived amyloidosis may be much more common than believed.
Hyperglycemia can occur if a patient injects his or her normal insulin bolus into an insulin-derived amyloidosis lump. Nagase et al10 showed that only 34% of injected insulin is absorbed from a site with amyloid deposits. As a result, these diabetic patients require higher doses of insulin to achieve adequate blood levels. Patients repeatedly inject into these lumps because they are easy to grasp and it often is less painful, although this was not confirmed in our case.2 To promote better control, they are instructed to inject in other areas, but this can result in hypoglycemia, so blood levels need to be carefully monitored and dosage adjusted.10
The mechanism by which amyloid deposits prevent the absorption of insulin is unclear. Nilsson8 suggested 3 possibilities: injected insulin may not be able to penetrate the deposit to reach the bloodstream, preformed amyloid fibrils can act as a seed and convert additional monomeric protein into amyloid fibrils, and insulin amyloid formation may be modulated by insulin-degrading enzyme, which could damage the injected insulin, making it biologically inactive.
The 3 main forms of primary cutaneous amyloidosis are lichen (or papular) amyloidosis (altered keratin), macular amyloidosis (altered keratin), and nodular amyloidosis (AL amyloid).6,11 The mechanism of insulin conversion into amyloid fibrils is not fully understood, and in vitro experiments suggest that multiple pathways exist.8 The dissociation of insulin hexamers and dimers into the monomeric form appears to be a key step because fibril formation is faster under conditions in which the monomer is the dominant species and is inhibited in the presence of covalent dimers. Additionally, both the A and B chains of insulin are amyloidogenic.8 The Nomenclature Committee of the International Society of Amyloidosis has designated this type of localized, iatrogenic, insulin-induced amyloid as AIns.12
Commercial insulin preparations are expected to be free of insulin fibrils and their seeds; however, human insulin has the highest aggregation propensity in vitro and is the most common form found in amyloid lumps.8 The insulin used in reported insulin-derived amyloidosis patients to date includes porcine insulin (2 patients), human insulin (8 patients), human insulin plus analog (8 patients), and analogs only (8 patients). Analogs included lispro, glargine, aspart, and detemir. To our knowledge, there have not been any cases reported for glulisine or degludec analogs.8 Our patient developed amyloidomas at sites injected with long-lasting and short-acting insulins.
The differential diagnosis for patients with insulin-injection-site masses includes the possibility of lipohypertrophy, which has many symptoms in common with insulin-derived amyloidosis. The distinction between the 2 may not be possible on clinical grounds, but insulin-derived amyloidosis masses tend to be firmer on palpation.1 Indeed, many cases of insulin-associated subcutaneous masses are presumed to be lipohypertrophy and further investigation may not occur. As with insulin-derived amyloidosis, patients with lipohypertrophy have reduced insulin efficacy, although less markedly than with injection into amyloid.2 In general, unlike insulin-derived amyloidosis, insulin-associated lipohypertrophy masses tend to shrink once the insulin injections are discontinued at the site.1,8
Biopsy, aspiration, and surgical excision have been used to obtain tissue to provide a diagnosis and potential treatment. Recent literature suggests that computed tomographic and magnetic resonance imaging scans may assist in differentiating between insulin-derived amyloidosis and lipohypertrophy.8 Mayhew et al6 used ultrasonography to define the lesion in the right breast of their patient and performed guided biopsies. Hagiwara et al13 also used ultrasonography to define the extent of involvement in a surgical excision case. We chose an extrusion technique much like that used in treating large lipomas, resulting in major debulking and near resolution of the amyloidoma. Frequent, diffuse rotation of injection sites should be emphasized to prevent the development of an amyloidoma.
We now have a new addition to the list of causes of primary cutaneous amyloidosis: drug-induced amyloidosis. Enfuvirtide, an antiretroviral drug used to treat patients with HIV infection, has recently been shown to induce amyloid deposits (AEnf amyloid) at injection sites, just like insulin, and there will likely be more subcutaneously administered protein and peptide drugs to follow.9,12
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Shiba M., Kitazawa T. Progressive insulin-derived amyloidosis in a patient with type 2 diabetes. Case Rep Plast Surg Hand Surg. 2016;3:73–76. doi: 10.1080/23320885.2016.1247650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagase T., Iwaya K., Iwaki Y. The insulin ball. Lancet. 2009;373:184. doi: 10.1016/S0140-6736(09)60041-6. [DOI] [PubMed] [Google Scholar]
- 3.Grunes D., Rapkiewicz A., Simsir A. Amyloidoma secondary to insulin injection: cytologic diagnosis and pitfalls. Cytojournal. 2015;12:15. doi: 10.4103/1742-6413.161602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storkel S., Schneider H.M., Muntefering H. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108–111. [PubMed] [Google Scholar]
- 5.Dische F.E., Wernstedt C., Westermark P. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158–161. doi: 10.1007/BF00276849. [DOI] [PubMed] [Google Scholar]
- 6.Mayhew J.M., Alan T., Kalidindi V., Gandamihardja T.A.K. Isolated insulin-derived amyloidoma of the breast. BMJ Case Rep. 2017:1–3. doi: 10.1136/bcr-2017-219491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonsdale-Eccles A.A., Gonda P., Gilbertson J.A., Haworth A.E. Localized cutaneous amyloid at an insulin injection site. Clin Exp Dermatol. 2009;34:e1027–e1028. doi: 10.1111/j.1365-2230.2009.03711.x. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson M.R. Insulin amyloid at injection sites of patients with diabetes. Amyloid. 2016;23:139–147. doi: 10.1080/13506129.2016.1179183. [DOI] [PubMed] [Google Scholar]
- 9.D’Souza A., Theis J.D., Vrana J.A., Dogan A. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71–75. doi: 10.3109/13506129.2013.876984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagase T., Iwaya K., Iwaki Y. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450–454. doi: 10.1016/j.amjmed.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Touart D., Sau P. Cutaneous deposition disease: part I. J Am Acad Dermatol. 1998;39:149–171. doi: 10.1016/s0190-9622(98)70069-6. [DOI] [PubMed] [Google Scholar]
- 12.Benson M.D., Buxbaum J.N., Eisenberg D.S. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 2018;25:215–219. doi: 10.1080/13506129.2018.1549825. [DOI] [PubMed] [Google Scholar]
- 13.Hagiwara S., Taneda S., Fukumoto T. Localized subcutaneous insulin-derived amyloidosis excised after evaluation using ultrasonography in a patient with type 2 diabetes mellitus. Case Rep Endocrinol. 2017;2017 doi: 10.1155/2017/3985214. 3985214. [DOI] [PMC free article] [PubMed] [Google Scholar]