ABSTRACT
Middle East Respiratory Syndrome coronavirus (MERS-CoV) emerged in 2012 has since resulted in sporadic cases, intra-familial transmission and major outbreaks in healthcare settings. The clinical picture of MERS-CoV includes asymptomatic infections, mild or moderately symptomatic cases and fatal disease. Transmissions of MERS-CoV within healthcare settings are facilitated by overcrowding, poor compliance with basic infection control measures, unrecognized infections, the superspreaders phenomenon and poor triage systems. The actual contributing factors to the spread of MERS-CoV are yet to be systematically studied, but data to date suggest viral, host and environmental factors play a major role. Here, we summarize the known factors for the diverse transmission of MERS-CoV.
KEYWORDS: MERS-CoV, Coronavirus, Transmission, Prevention, Health Care Facilities
Introduction
The Middle East Respiratory Syndrome (MERS) was initially recognized in 2012 and since then a total of 1621 cases have been reported from 26 countries, with a case fatality rate of 36% [1]. The disease has a wide range of clinical presentation and epidemiology [2–6]. Three main factors contribute to the transmission of MERS coronavirus (MERS-CoV): the virus, the host, and the environment. With regard to the patterns of transmission, three patterns have been recognized: sporadic transmission, limited intrafamilial transmission, and health-care-associated transmissions. Here, we review the available data regarding the diversity of transmission and summarize known risk factors for such transmission.
Clinical presentation
One of the reasons for the delayed diagnosis of MERS cases is the wide clinical spectrum of MERS, which ranges from asymptomatic to mild upper respiratory tract symptoms to acute fulminant pneumonia associated with multisystem failure and death [2–6]. The most common presentations among hospitalized MERS patients were fever, cough, shortness of breath, and clinical and radiological evidence of pneumonia [7–10]. Patients may also present with nonspecific symptoms such as fatigue, myalgia, fever, cough, headache, vomiting, and diarrhea [7–10]. Patients with severe disease develop respiratory failure secondary to acute lung injury, and may develop renal failure and coagulopathy [11–15]. The case fatality rate is related to the viral load, severity of the disease, and the presence of comorbidities [11–15]. In a recent study, multivariate analysis revealed predictors of mortality as: age >60 years (odds ratio [OR] 11.7), underlying illness (OR 5.19), lower Ct values where the odds of death increased 17% for each 1 point drop in Ct (OR 1.17) [16]. The risk of death in patients with MERS was significantly associated with age >65 years (9.3 times that of younger patients) and treatment for underlying diseases (7.8 times that of other patients) [17]. The majority (>95%) of the cases of MERS infection occur in adults and older adults with one or more comorbidities, and few cases occurred among children [18,19]. Comorbidities associated with MERS-CoV infection include: diabetes mellitus, renal failure, and hypertension, as shown in Table 1 [7,9,12,13,20].
Table 1.
A summary of comorbidities among MERS patients in different studies.
| Al-Tawfiq et al. [9] | Arabi et al. [12] | Asiri et al. [7] | Shalhoub et al. [15] | Assiri et al. [13] | Korean CDC [20] | Grand total | % of the total | |
|---|---|---|---|---|---|---|---|---|
| Number | 17 | 12 | 47 | 24 | 23 | 186 | 309 | |
| Diabetes mellitus | 13 | 8 | 32 | 15 | 17 | 52 | 137 | 71.0 |
| Cardiac disease | 11 | 7 | 13 | 0 | 9 | 42 | 82 | 42.5 |
| Chronic Renal failure | 5 | 23 | 10 | 12 | 9 | 59 | 30.6 | |
| Hemodialysis | 5 | 1 | 6 | 12 | 6.2 | |||
| Malignancy | 1 | 1 | 1 | 0 | 43 | 46 | 23.8 | |
| Hypertension | 6 | 6 | 16 | 18 | 46 | 23.8 |
The diagnosis of MERS-CoV infection is based on the detection of viral RNA in respiratory samples such as nasopharyngeal swabs, bronchoalveolar wash, or sputum using real-time reverse transcriptase polymerase chain reaction (RT-PCR) assays targeting upE and ORF1b regions of the MERS-CoV genome [7,21]. Currently, the detection of MERS-CoV in respiratory samples is considered the gold standard for the diagnosis of MERS in a clinical setting. Lower or deeper respiratory samples were more likely to be positive for MERS-CoV than upper respiratory samples and thus these samples are the preferred method of testing using real-time PCR [16,21]. Viral loads in lower respiratory samples were at least 100 times more compared to upper respiratory tract samples (5 × 106 vs. 1.9 × 104 cop/mL, respectively) [22]. Serology using immunofluorescence, serum neutralization, or protein microarray assays adds additional diagnostic methods for the detection of MERS-CoV antibodies, but most of these tests require a minimum of 3 weeks postinfection to reveal positive results [21,23–26].
Different serologic tests were used for the diagnosis of MERS-CoV infection, which included: plaque reduction neutralization tests (PRNTs), microneutralization (MN) test, MERS-CoV-spike pseudoparticle neutralization test (ppNT), and MERS-CoV S1-enzyme-linked immunosorbent assay (ELISA) antibody test [27]. These antibody detection tests (PRNT, MN, ppNT, and ELISA test) have an excellent sensitivity of 94–100% [27]. The ppNT does not require biosafety level (BSL)-3 containment [27–30]. In one study, serum dilutions causing plaque reductions of 90% (PRNT90) and 50% (PRNT50) showed that the use of PRNT50 detects few infections that were not detected by PRNT90 tests [25]. However, these tests were not used for diagnostic purposes, and the exact sensitivity and specificity of MERS-CoV antibody tests in clinical settings are not known.
Drivers for MERS-CoV transmission
Three main patterns of the transmission of MERS-CoV are well characterized. These include: sporadic transmission, intrafamilial transmission, and health-care-associated transmissions [31–38]. Of all the cases in Saudi Arabia, 45% were health-care-associated infections, 38% were primary cases, and 13% were among household contacts [39]. The source of the infection in the remaining 4% was not reported [39]. The dynamics of the transmission of MERS-CoV possibly involves small animals, such as bats, and dromedary camels of Africa, with the importation of camels into the Kingdom of Saudi Arabia resulting in subsequent zoonotic transmission. These sporadic cases then lead to intrafamilial transmission and outbreaks in the health-care settings (Figure 1).
Figure 1.
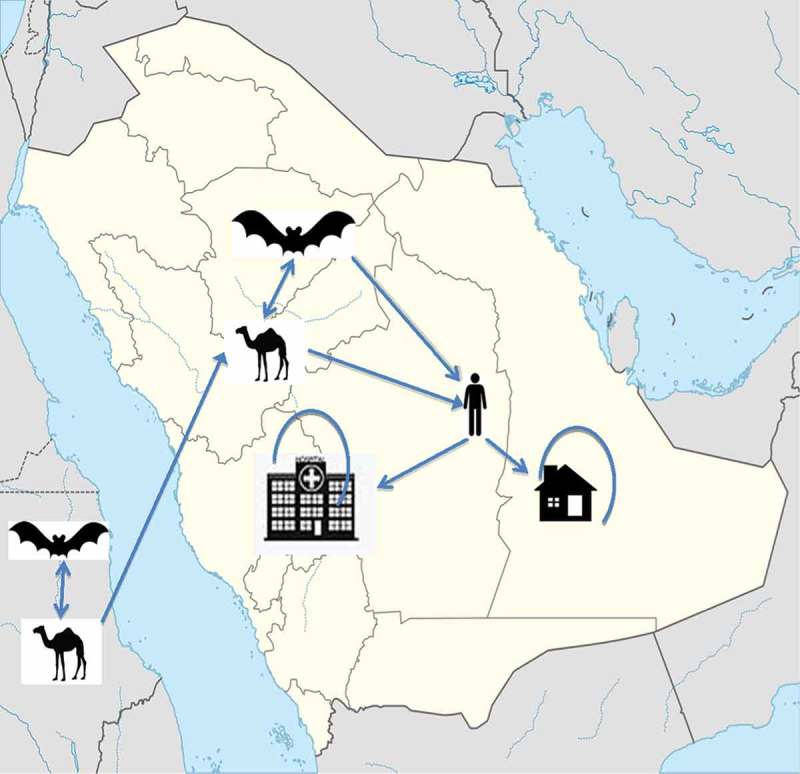
The dynamics of the transmissions of MERS-CoV involves possibly small animals such as bats, dromedary camels in Africa with the importation of camels into the Kingdom of Saudi Arabia with subsequent zoonotic transmission,. These sporadic cases then lead to intra-familial transmission (house) and outbreaks in the healthcare settings (hospital).
Animal to human transmission
Primary cases occurring in the community are thought to be linked to dromedary camels [40,41]. The evidence for dromedary camels as a potential source of infections relies on four different lines of evidence. These lines of evidence are: the high seroprevalence of MERS-CoV antibodies in dromedary camels, shepherds and abattoir, [42–45] as well as similarity in the genome sequences of MERS-CoV from dromedary and human specimens, [40,44–47] the isolation of MERS-CoV from camels, [48–50] and serological evidence that animal infection preceded the human infection in the Jeddah case [41,51]. The exact mode of transmission of MERS-CoV from dromedary camels to humans remains to be elucidated. In a recent case-control study, 30 primary cases were matched to 116 controls [52]. The answers of the questions for the primary cases were provided by family members in 57% of instances compared to 1% in the control group [52]. In a univariate analysis, the following risks were significant: direct physical contact with a dromedary camel during the last 6 months, household members frequently visited farms with dromedaries, household members had direct contact with dromedaries during the exposure period, dromedaries were present on farm, milked dromedaries while on farm, household members visited a farm with dromedaries during the exposure period, and any direct contact with a dromedary camel during the exposure period [52]. In a multivariate analysis, risks were associated with direct dromedary exposure in the preceding two weeks, diabetes mellitus, heart disease, and current tobacco smoking [52]. In June 2012, the investigation of the source of MERS-CoV around the house of the first patient in Bisha showed that a sample from a Taphozous perforatus bat (Egyptian tomb bat) had 100% identity to the human MERS-CoV cloned from the index case-patient [53]. There are no studies of bats in Saudi Arabia to further confirm this finding.
Seasonal pattern of MERS-CoV transmission
MERS-CoV seems to have two seasons for the transmission: March–May and September–November [54–56]. The reason for the increased cases in April–May 2014 was the occurrence of the Jeddah outbreak, the associated increase in the number of tests performed, [57] and increased health-care-associated transmission[5,57]. So increased surveillance identified more cases, although the overall percentage of patients identified did not differ significantly between Jeddah (4.6%) and other cities (5.2%) in the same time period [57]. In addition, data showed that the seroepidemiology is low in Saudi Arabia with an overall prevalence of 0.15% of 10,007 screened individuals [24].
Any seasonal variation may reflect the risk of transmission of MERS-CoV between animals and humans, seasonal variation in the circulation of the virus in animals, and the natural reservoirs of MERS-CoV [56]. A study evaluating the seasonality of MERS-CoV transmission showed that MERS-CoV infections followed influenza A epidemics, and most of the influenza waves did not co-occur with the MERS-CoV waves [58]. It is important to study the factors leading to this seasonality of MERS-CoV, such as parturition of camels.
Intrafamilial transmission
The majority of reported MERS cases were secondary cases due to human-to-human transmission. The transmission occurred within family clusters, [2–4,25] community settings, [32,59] travel-associated transmission, [60–64] and health-care settings [4,9,20,57,65–71]. The transmission within any particular family is also variable and the factors leading to heterogeneity in transmission had not been elucidated. In an outbreak in Al-Madinah Al-Munawarah, 61% were health-care-associated, 28% were primary cases, and 11% were among family members [59]. Genomic testing of MERS-CoV is needed to accompany any cluster investigation to examine the relatedness of the cases to each other. An investigation of a family cluster in Hafr Al-Bain, Saudi Arabia, indicated that certain cases in the same family were not related to the cluster, but these cases were caused by community transmissions [72].
The evaluation of contacts of the first MERS case was extended to the patient’s family contacts, which included three wives, 10 sons, 11 daughters, 12 grandchildren, and one house maid; two Shepherds and 14 health-care workers [33]. None of the 53 contacts was MERS-CoV-positive by PCR [33].
Health-care-associated transmission
The largest data on contact investigation revealed that the percentage of positive cases was 2.5%, 1.12%, and 3.03% among hospital patients, health-care worker contacts, and family contacts, respectively [73]. Thus, the transmission of MERS-CoV among family members seems to be variable. Possible factors leading to heterogeneity in transmissions may include: genetic predisposition, severity of the disease, and other environmental factors.
It is clear that health-care-associated infections of MERS-CoV contribute significantly to the increased number of cases. Since 2012, there had been many large outbreaks in the health-care setting involving many facilities. Examples of these outbreaks include: Al-Hasa outbreak, Zarqa outbreak in Jordan, Jeddah, Taif, and the Republic of Korea [13,20,57,58,65,67,71]. In Al-Hasa, Saudi Arabia, 23 confirmed cases and 11 probable cases constituted the outbreak [13]. Further genotyping studies revealed multiple introduction of MERS-CoV into the hospital outbreak [74]. Between 17 February and 26 April 2014, a multiple health-care-associated MERS outbreak took place in Jeddah and involved 14 hospitals [5,57]. Of all the cases in that outbreak, 60% of the cases resulted from health-care-associated transmission [75]. Factors contributing to the transmissions and increased number of cases included: sensitive case definition, active search for cases, and contact tracing [57]. In 2015, a large MERS outbreak occurred in the Republic of Korea [76]. The outbreak was epidemiologically linked to a single patient who visited multiple countries in the Arabian Peninsula [76]. The outbreak resulted the quarantine of 17,000 contacts as of 20 July 2015 [77–80]. And the outbreak resulted in a total of 186 cases and 36 deaths in a short time [81].
Variability of intrahospital transmission of MERS-CoV
Variable intrahospital transmissions occurred among contacts of MERS patients. Among the contacts of the first case, none of the 48 health-care workers who had significant contact during the patient’s stay of 10 days in the private hospital in Jeddah were positive [12].
Factors contributing to health-care-associated transmissions
The described health-care-associated transmissions are driven by multiple factors. These factors include: late recognition, overcrowding, inadequate infection control precautions, prolonged viral shedding, and the occurrence of superspreading events [32]. The exact definition of a superspreader is not well defined. In one study of SARS, a ‘superspreading event’ was defined as transmission of SARS to at least eight contacts, [82] and others used the term to refer to individuals infecting unusually large numbers of secondary cases [83,84]. A superspreading event was recognized during the SARS outbreak when a flight attendant infected more than 100 patients in Singapore [85–87]. In the Al-Hasa outbreak, a single patient caused seven secondary cases; [13] thus, a superspreading event may have contributed to the outbreak. The index case in the Republic of Korea caused 27 secondary cases; one of these cases caused 24 tertiary cases while another patient caused 73 tertiary cases [80]. In the Republic of Korea outbreak, superspreaders contributed to the infection of 85, 28, 23, 11, and 6 from patients’ number 14, 1, 16, 76 and 15, respectively [20]. Another secondary patient resulted in 91 tertiary MERS cases, of which 39% were other patients in the emergency room, and 13% were health-care workers [88]. Table 2 provides a summary of possible superspreaders in different SARS and MERS outbreaks [8,13,20,59,72,88].
Table 2.
A summary of different outbreaks showing possible superspreading events in MERS-CoV infection.
Many theories have been given to explain the occurrence of superspreading events and cited factors include the virus characteristics, the host, the environment, and cultural and travel-related behaviors. Other contributing factors include: prolonged duration of exposure, the practice of seeking care at multiple health-care facilities, frequent interhospital transfer, and large numbers of contacts [20]. Additional factors include: multi-bedded hospital rooms, crowded hospital rooms, and aerosol-generating procedures [20].
Additional influencing factors include: viral mutation, duration of contact with an infectious host and routes of transmission of infections, genetic susceptibility, and underlying comorbidities [89]. A higher viral load, more environmental contacts, more interpersonal contacts, and complex network of interactions made by individuals may also play further roles in superspreading events.
Although initial studies showed that no significant mutation was detected among MERS-CoV isolates, [57,90,91] complete genome analysis of MERS-CoV showed genetic recombination events between group 3 and group 5 of clade B [92]. The significance of this recombination in the transmissibility is not known [92]. Many patients with MERS have underlying comorbidities [7]. In one study, 96% had underlying comorbid medical disorders, including diabetes (68%), hypertension (34%), chronic cardiac disease (28%), and chronic renal disease (49%) [7]. The viral shedding characteristics in those with comorbid diseases might be different from healthy individuals. After 12 days from the initial positive samples, 30% of contacts and 76% of cases were still positive for MERS-CoV by PCR [89]. A health-care worker shed MERS-CoV for about 42 days after initial sample [93].
Environmental persistence of the MERS-CoV virus was recently investigated. In one study, it was found that MERS-CoV survives well on surfaces and in the air and that the virus is more stable at low temperature and low humidity conditions [94]. Most of the touchable environments in rooms where MERS-CoV patients were cared for were contaminated, and viable virus could be isolated in three of the four enrolled patients on days 18–27 after symptom onset [95]. Specimens from bed controller and thermometer were PCR-positive until the fifth day from the last positive PCR of the patient’s respiratory specimen [95]. Environmental factors contributing to a superspreading event include air recirculation, as occurred in the SARS outbreak at the Hotel Metropole, Amoy Gardens housing complex, and a flight between China and Canada [96]. The contribution of air recirculation to the spread of MERS-CoV is yet to be investigated.
The custom and behavior of the affected population may also contribute to a superspreading event, and these behaviors include: ‘doctor shopping’, traditional ways of greetings, hugging and kissing. In the Republic of Korea outbreak, the presence of many visitors and family members staying with patients contributed to the superspreading event [85]. Hospital environment may also contribute to the spread of the virus, and these include overcrowding, multi-bedded rooms, and inadequate environmental cleaning.
Asymptomatic individuals and prolonged viral shedding
It is estimated that between one-fourth and one-fifth of laboratory-confirmed MERS cases are asymptomatic. The exact contribution of asymptomatic individuals and those with prolonged viral shedding to the epidemiology and transmission of MERS-CoV are not well characterized. A recent survey of 225 patients with laboratory-confirmed MERS found that 64 (25.1%) were reported as asymptomatic at time of specimen collection; however, when 33 (52%) of those patients were interviewed, 26 (79%) reported at least one symptom that was consistent with a viral respiratory illness [5,97]. It is estimated that between 20% and 25% of laboratory-confirmed MERS cases are asymptomatic. Prolonged viral shedding of patients and asymptomatic contacts pose potential, important challenges for infection control [25]. MERS-CoV was detected by RT-PCR for 18–24 days from the respiratory tract secretions [98]. Asymptomatic individuals were also described to harbor MERS-CoV by RT-PCR [21]. Asymptomatic health-care workers shed MERS-CoV for 42 days from 24 April to 12 June 2014 [93].
Expert commentary
Since the emergence of MERS-CoV in 2012, the virus caused sporadic cases, intrafamilial transmission, and major outbreaks in health-care settings. In particular, the transmission within health-care settings is of major concern due to the potential to cause large outbreaks even outside the Arabian Peninsula. This is exemplified by the large outbreak in the Republic of Korea. Transmissions of MERS-CoV within health-care settings are facilitated by overcrowding, poor compliance with basic infection control measures, unrecognized infections, the superspreader phenomenon, and poor triage systems. The actual contributing factors to the spread of MERS-CoV are yet to be systematically studied, but data to date suggest that viral, host, and environmental factors play a major role. Understanding these factors and the contribution of each factor to the superspreading events would further enhance the control measures of MERS-CoV transmission. The epidemiology of the disease was studied in many aspects and showed propensity for the older and those with underlying medical conditions. There is yet an unexplained low rate of involvement of children. The clinical picture of MERS had been further elucidated to include asymptomatic infections, mild or moderately symptomatic cases, and fatal disease. This heterogeneity in the clinical presentation is similar to other known infectious diseases and might be related to the immune response. MERS-CoV is present in the lower respiratory tract system for a prolonged period of time and at higher concentrations than those of the upper respiratory tract, the urine, and the stool. This finding puts the MERS-CoV dynamics in the same clinical presentation as SARS. The finding also has an important role for the prospect of control in health-care settings.
Five-year view
In the following years and months to come, it is important to fill the knowledge gap in our understanding of the epidemiology and transmission of MERS-CoV. It is important to elucidate other intermediate and natural hosts of MERS-CoV. Understanding the factors leading to the amplification of transmission in health-care setting would provide further tools for the control of the virus spread. Understanding the heterogeneity in the transmission and clinical presentation may also aid in the overall control measures. Understanding the risk factors for the virus transmission within the community and the hospital settings would enhance the ability to control the transmission of MERS-CoV. The best strategies to prevent the transmission within health-care facilities are to further enhance the infection control measures and develop a sustained system for the triage of patients presenting with respiratory symptoms.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Key issues
MERS-CoV causes sporadic cases, intrafamilial transmission, and nosocomial infections.
MERS clinical presentation ranges from mild or asymptomatic cases to severe and fatal disease.
Transmission of MERS-CoV within health-care settings is facilitated by overcrowding, poor compliance with basic infection control measures, unrecognized infections, the superspreader phenomenon, and poor triage systems.
Contributing factors for superspreading phenomena include viral, host, and environmental factors.
The virus is present in the lower respiratory tract system for prolonged period of time and at higher concentrations than the upper respiratory tract, the urine, and the stool.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.World Health Organization http://www.who.int/emergencies/mers-cov/en/ Available from:
- 2.Memish ZA, Zumla AI, Al-Hakeem RF, et al. Family cluster of middle east respiratory syndrome coronavirus infections. N Engl J Med. 2013;368(26):2487–2494. [DOI] [PubMed] [Google Scholar]
- 3.Omrani AS, Matin MA, Haddad Q, et al. A family cluster of middle east respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17(9):e668–e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abroug F, Slim A, Ouanes-Besbes L, et al. Family cluster of middle east respiratory syndrome coronavirus infections, Tunisia, 2013. Emerg Infect Dis. 2014;20(9):1527–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oboho IK, Tomczyk SM, Al-Asmari AM, et al. 2014 MERS-CoV outbreak in Jeddah–a link to health care facilities. N Engl J Med. 2015;372(9):846–854.• An interesting study of one of the largest outbreak in Jeddah, Saudi Arabia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalid M, Khan B, Al Rabiah F, et al. Middle eastern respiratory syndrome corona virus (MERS CoV): case reports from a tertiary care hospital in Saudi Arabia. Ann Saudi Med. 2014;34(5):396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of middle east respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. The Lancet Infectious Diseases. 2013;13:752–761. •• First study describing the clinical features of MERS cases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with middle east respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Tawfiq JA, Hinedi K, Ghandour J, et al. Middle east respiratory syndrome-coronavirus (MERS-CoV): a case-control study of hospitalized patients. Clin Infect Dis. 2014;59(2):160–165. •• A case control study of hospitalized patients with Severe Acute Respiratory Illness (SARI). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagbo SF, Skakni L, Chu DK, et al. Molecular epidemiology of hospital outbreak of middle east respiratory syndrome, Riyadh, Saudi Arabia, 2014. Emerg Infect Dis. 2015;21(11):1981–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Hameed F, Wahla AS, Siddiqui S, et al. Characteristics and outcomes of middle east respiratory syndrome coronavirus patients admitted to an intensive care unit in Jeddah, Saudi Arabia. J Intensive Care Med. 2015. April 9 pii: 0885066615579858. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389–397. •• Outcome of critical MERS cases admitted to the intensive care unit. [DOI] [PubMed] [Google Scholar]
- 13.Assiri A, McGeer A, Perl TM, et al. Hospital outbreak of middle east respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. • First description of Hospital Outbreak of MERS cases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe middle east respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14(11):1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-alpha2a or IFN-beta1a in combination with ribavirin to treat middle east respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70(7):2129–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feikin DR, Alraddadi B, Qutub M, et al. Association of higher MERS-CoV virus load with severe disease and death, Saudi Arabia, 2014. Emerg Infect Dis. 2015;21(11):2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizumoto K, Endo A, Chowell G, et al. Real-time characterization of risks of death associated with the middle east respiratory syndrome (MERS) in the Republic of Korea, 2015. BMC Med. 2015; 13:228. doi: 10.1186/s12916-015-0468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Memish ZA, Al-Tawfiq JA, Assiri A, et al. Middle east respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J. 2014;33(9):904–906. [DOI] [PubMed] [Google Scholar]
- 19.Khuri-Bulos N, Payne DC, Lu X, et al. Middle east respiratory syndrome coronavirus not detected in children hospitalized with acute respiratory illness in Amman, Jordan, March 2010 to September 2012. Clin Microbiol Infect. 2014;20(7):678–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korea Centers for Disease Control and Prevention Middle east respiratory syndrome coronavirus outbreak in the Republic of Korea, 2015. Osong Public Health Res Perspect. 2015;6(4):269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with middle east respiratory syndrome. J Infect Dis. 2014;210(10):1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corman VM, Albarrak AM, Omrani AS, et al. Viral shedding and antibody response in 37 patients with MERS-coronavirus infection. Clin Infect Dis. 2016. February 15;62(4):477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchholz U, Muller MA, Nitsche A, et al. Contact investigation of a case of human novel coronavirus infection treated in a German hospital, October–November 2012. Euro Surveill. 2013;18(8). pii: 20406. [PubMed] [Google Scholar]
- 24.Müller MA, Meyer B, Corman VM, et al. Presence of middle east respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15(5):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drosten C, Meyer B, Müller MA, et al. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371(9):828–835. [DOI] [PubMed] [Google Scholar]
- 26.Reusken CB, Farag EA, Haagmans BL, et al. Occupational exposure to dromedaries and risk for MERS-CoV infection, Qatar, 2013–2014. Emerg Infect Dis. 2015;21(8):1422–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SW, Perera RA, Choe PG, et al. Comparison of serological assays in human middle east respiratory syndrome (MERS)-coronavirus infection. Euro Surveill. 2015;20(41). doi: 10.2807/1560-7917.ES.2015.20.41.30042. [DOI] [PubMed] [Google Scholar]
- 28.Perera RA, Wang P, Gomaa MR, et al, et al. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralization assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 2013;18(36):20574. doi: 10.2807/1560-7917.ES2013.18.36.20574. [DOI] [PubMed] [Google Scholar]
- 29.Hemida MG, Perera RA, Al Jassim RA, et al, et al. Seroepidemiology of middle east respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveill. 2014;19(23):20828. doi: 10.2807/1560-7917.ES2014.19.23.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemida MG, Al-Naeem A, Perera RA, et al. Lack of middle east respiratory syndrome coronavirus transmission from infected camels. Emerg Infect Dis. 2015;21(4):699–701. doi: 10.3201/eid2104.141949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Tawfiq JA, Memish ZA.. Managing MERS-CoV in the healthcare setting. Hosp Pract. 1995;43(3):158–163. [DOI] [PubMed] [Google Scholar]
- 32.Al-Tawfiq JA, Perl TM. Middle east respiratory syndrome coronavirus in healthcare settings. Curr Opin Infect Dis. 2015;28(4):392–396. [DOI] [PubMed] [Google Scholar]
- 33.Al-Tawfiq JA, Memish ZA. An update on middle east respiratory syndrome: 2 years later. Expert Rev Respir Med. 2015;9(3):327–335. [DOI] [PubMed] [Google Scholar]
- 34.Memish ZA, Al-Tawfiq JA. Middle east respiratory syndrome coronavirus infection control: the missing piece? Am J Infect Control. 2014;42(12):1258–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Tawfiq JA, Memish ZA. Middle east respiratory syndrome coronavirus: transmission and phylogenetic evolution. Trends Microbiol. 2014;22(10):573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Tawfiq JA, Zumla A, Memish ZA. Coronaviruses: severe acute respiratory syndrome coronavirus and middle east respiratory syndrome coronavirus in travelers. Curr Opin Infect Dis. 2014;27(5):411–417. [DOI] [PubMed] [Google Scholar]
- 37.Al-Tawfiq JA, Memish ZA. Emerging respiratory viral infections: MERS-CoV and influenza. Lancet Respir Med. 2014;2(1):23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Tawfiq JA. Middle east respiratory syndrome-coronavirus infection: an overview. J Infect Public Health. 2013;6(5):319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saudi Ministry of Health http://www.moh.gov.sa/en/CCC/PressReleases/Pages/Statistics-2015-11-05-001.aspx Available from:
- 40.Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle east respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14(2):140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Memish ZA, Cotten M, Meyer B, et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20(6):1012–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemida MG, Perera RA, Wang P, et al. Middle east respiratory syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. 2013;18:20659. [DOI] [PubMed] [Google Scholar]
- 43.Reusken CB, Ababneh M, Raj VS, et al. Middle east respiratory syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveill. 2013;18:20662. [DOI] [PubMed] [Google Scholar]
- 44.Meyer B, Müller MA, Corman VM, et al. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis. 2014;20:552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemida MG, Elmoslemany A, Al-Hizab F, et al. Dromedary camels and the transmission of middle east respiratory syndrome coronavirus (MERS-CoV). Transbound Emerg Dis. 2015. August 10. doi: 10.1111/tbed.12401 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Briese T, Mishra N, Jain K, et al. Middle east respiratory syndrome coronavirus quasispecies that include homologues of human isolates revealed through whole-genome analysis and virus cultured from dromedary camels in Saudi Arabia. MBio. 2014;5:e01146–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowotny N, Kolodziejek J. Middle east respiratory syndrome coronavirus (MERS-CoV) in dromedary camels, Oman, 2013. Euro Surveill. 2014;19:20781. [DOI] [PubMed] [Google Scholar]
- 48.Chu DK, Poon LL, Gomaa MM, et al. MERS coronaviruses in dromedary camels, Egypt. Emerg Infect Dis. 2014;20:1049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemida MG, Chu DK, Poon LL, et al. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis. 2014;20:1231–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raj VS, Farag EA, Reusken CB, et al. Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014. Emerg Infect Dis. 2014;20:1339–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azhar EI, El-Kafrawy SA, Farraj SA, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370:2499–2505. [DOI] [PubMed] [Google Scholar]
- 52.Alraddadi BM, Watson JT, Almarashi A, et al. Risk factors for primary middle east respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22(1):49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Memish ZA, Mishra N, Olival KJ, et al. Middle east respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19(11):1819–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MERS-Coronavirus Molecular Epidemiology and Genetic Analysis – Origin and Evolution http://epidemic.bio.ed.ac.uk Available from:
- 55.World Health Organization http://www.who.int/csr/disease/coronavirus_infections/MERS_CoV_RA_20140424.pdf Available from:
- 56.World Health Organization http://www.who.int/csr/disease/coronavirus_infections/mers-5-february-2015.pdf Available from:
- 57.Drosten C, Muth D, Corman VM, et al. An observational, laboratory-based study of outbreaks of middle east respiratory syndrome coronavirus in Jeddah and Riyadh, kingdom of Saudi Arabia, 2014. Clin Infect Dis. 2015;60(3):369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hijawi B, Abdallat M, Sayaydeh A, et al. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J. 2013;19(Suppl 1):S12–S18. [PubMed] [Google Scholar]
- 59.Memish ZA, Al-Tawfiq JA, Alhakeem RF, et al. Middle east respiratory syndrome coronavirus (MERS-CoV): A cluster analysis with implications for global management of suspected cases. Travel Med Infect Dis. 2015;13(4):311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraaij-Dirkzwager M, Timen A, Dirksen K, et al, et al. Middle east respiratory syndrome coronavirus (MERS-CoV) infections in two returning travellers in the Netherlands, May 2014. Euro Surveill. 2014;19(23). pii: 20829. [DOI] [PubMed] [Google Scholar]
- 61.Fanoy EB, Van Der Sande MA, Kraaij-Dirkzwager M, et al. Travel-related MERS-CoV cases: an assessment of exposures and risk factors in a group of Dutch travellers returning from the Kingdom of Saudi Arabia, May 2014. Emerg Themes Epidemiol. 2014;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.ProMED-mail MERS-CoV – eastern Mediterranean (73): Saudi Arabia, Algeria, Jordan, WHO, RFI. Archive Number: 20140601.2512766. Published Date: 2014-06-0119:27:25
- 63.ProMED-mail MERS-CoV – eastern Mediterranean (80): S Arabia, Iran, Algeria, Tunisia. Archive Number: 20140612.2534478. Published Date: 2014-06-12 10:00:39
- 64.ProMED-mail MERS-CoV (01): Bangladesh, KSA, Algeria, UAE, Iran, WHO, RFI. Archive Number: 20140616.2541707. Published Date: 2014-06-16 15:12:09
- 65.Lee SS, Wong NS. Probable transmission chains of middle east respiratory syndrome coronavirus and the multiple generations of secondary infection in South Korea. Int J Infect Dis. 2015;38:65–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ki M. 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health. 2015;37:e2015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park HY, Lee EJ, Ryu YW, et al. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to June 2015. Eurosurveillance. 2015;20:21169. [DOI] [PubMed] [Google Scholar]
- 68.Petersen E, Pollack MM, Madoff LC. Health-care associate transmission of middle east respiratory syndrome corona virus, MERS-CoV, in the Kingdom of Saudi Arabia. Int J Infect Dis. 2014;29:299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hall AJ, Tokars JI, Badreddine SA, et al. Health care worker contact with MERS patient, Saudi Arabia. Emerg Infect Dis. 2014;20(12):2148–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Abdallat MM, Payne DC, Alqasrawi S, et al. Hospital-associated outbreak of middle east respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59(9):1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Assiri A, Abedi GR, Bin Saeed AA, et al, et al. Multifacility outbreak of middle east respiratory syndrome in Taif, Saudi Arabia. Emerg Infect Dis. 2016;22:32–40. doi: 10.3201/eid2201.151370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Memish ZA, Cotten M, Watson SJ, et al. Community case clusters of middle east respiratory syndrome coronavirus in Hafr Al-Batin, Kingdom of Saudi Arabia: a descriptive genomic study. Int J Infect Dis. 2014;23:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Screening for middle east respiratory syndrome coronavirus infection in hospital patients and their healthcare worker and family contacts: a prospective descriptive study. Clin Microbiol Infect. 2014;20(5):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cotten M, Watson SJ, Kellam P, et al, et al. Transmission and evolution of the middle east respiratory syndrome coronavirus in Saudi Arabia: a descriptive genomic study. Lancet. 2013;382(9909):1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.World Health Organization Middle east respiratory syndrome coronavirus (MERS-CoV) summary and literature update as of 9 May 2014. Available from: www.who.int/csr/disease/coronavirus_infections/MERS_CoV_Update_09_May_2014.pdf?ua=1
- 76.WHO Middle east respiratory syndrome coronavirus (MERS-CoV) – Republic of Korea. Available from: http://www.who.int/csr/don/30-may-2015-mers-korea/en/
- 77.FluTrackers South Korea coronavirus MERS case list – including imported and exported cases. Available from: https://flutrackers.com/forum/forum/novel-coronavirus-ncov-mers-2012-2014/novel-coronavirus-who-chp-wpro-ecdc-oie-fao-moa-reports-and-updates/south-korea-coronavirus/732065-south-korea-coronavirus-mers-case-list-including-imported-and-exported-cases
- 78.World Health Organization Middle east respiratory syndrome coronavirus (MERS-CoV): summary and risk assessment of current situation in the Republic of Korea and China – as of 19 June 2015. Available from: http://www.who.int/emergencies/mers-cov/mers-cov-republic-of-korea-and-china-risk-assessment-19-june-2015.pdf?ua=1
- 79.World health organization Middle east respiratory syndrome coronavirus (MERS-CoV). MERS-CoV in Republic of Korea at a glance. Available from: http://www.wpro.who.int/outbreaks_emergencies/wpro_coronavirus/en/
- 80.Cowling BJ, Park M, Fang VJ, et al. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. 2015;20(25):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsieh Y-H. 2015 middle east respiratory syndrome coronavirus (MERS-CoV) nosocomial outbreak in South Korea: insights from modeling. PeerJ. 2015;3:e1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen Z, Ning F, Zhou W, et al. Superspreading SARS events, Beijing, 2003. Emerg Infect Dis. 2004;10(2):256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lloyd-Smith JO, Schreiber SJ, Kopp PE, et al. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438(7066):355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.James A, Pitchford JW, Plank MJ. An event-based model of superspreading in epidemics. Proc Biol Sci. 2007;274(1610):741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gopinath S, Lichtman JS, Bouley DM, et al. Role of disease-associated tolerance in infectious superspreaders. Proc Natl Acad Sci USA. 2014;111:15780–15785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leavy O. Infectious disease: the tolerance of superspreaders. Nat Rev Immunol. 2014;14(12):776–777. [DOI] [PubMed] [Google Scholar]
- 87.WHO (World Health Organization) Severe acute respiratory syndrome (SARS): status of the outbreak and lessons for the immediate future. Report; Geneva 2003. [cited 2003 May20]. Available from: http://www.who.int/csr/media/sars_wha.pdf
- 88.Oh M-D, Choe PG, Oh HS, et al. Middle east respiratory syndrome coronavirus superspreading event involving 81 persons, Korea 2015. J Korean Med Sci. 2015;30(11):1701–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Memish ZA, Assiri AM, Al-Tawfiq JA. Middle east respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014;29:307–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim YJ, Cho YJ, Kim DW, et al. Complete genome sequence of middle east respiratory syndrome coronavirus KOR/KNIH/002_05_2015, isolated in South Korea. Genome Announc. 2015;3. doi: 10.1128/genomeA.00787-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu R, Wang Y, Wang W, et al, et al. Complete genome sequence of middle east respiratory syndrome coronavirus (MERS-CoV) from the first imported MERS-CoV case in China. Genome Announc. 2015;3:e00818-15. doi: 10.1128/genomeA.00818-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Liu D, Shi W, et al. Origin and possible genetic recombination of the middle east respiratory syndrome coronavirus from the first imported case in China: phylogenetics and coalescence analysis. MBio. 2015;6:e01280–15. doi: 10.1128/mBio.01280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al-Gethamy M, Corman VM, Hussain R, et al. A case of long-term excretion and subclinical infection with middle east respiratory syndrome coronavirus in a healthcare worker. Clin Infect Dis. 2015;60(6):973–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Doremalen N, Bushmaker T, Munster VJ. Stability of middle east respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18(38):20590 pii: 20590. [DOI] [PubMed] [Google Scholar]
- 95.Bin Seo Y, Heo JY, Song MS, et al. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin Infect Dis. 2015. December 17 pii: civ1020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wong G, Liu W, Liu Y, et al. MERS, SARS, and Ebola: the role of super-spreaders in infectious disease. Cell Host Microbe. 2015;18(4):398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wortmann GW. Middle east respiratory syndrome: SARS redux? Cleve Clin J Med. 2015;82(9):584–588. [DOI] [PubMed] [Google Scholar]
- 98.Spanakis N, Tsiodras S, Haagmans BL, et al. Virological and serological analysis of a recent middle east respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int J Antimicrob Agents. 2014;44(6):528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]


