Abstract
The FilmArray Respiratory Panel (RP) (BioFire™ Diagnostics, Inc., Salt Lake City, UT, USA) is the first multiplex molecular panel cleared by the US FDA for the detection of both bacterial and viral respiratory pathogens in nasopharygeal swabs. The FilmArray RP targets 20 pathogens including 17 viruses and subtypes and three bacteria, and is performed with minimal sample manipulation. The FilmArray RP has a fully automated sample-to-answer workflow with a turn-around-time of approximately 1 h. The reported sensitivity and specificity of the assay ranges from 80 to 100 and 100%, respectively, with the sensitivity for the adenovirus as low as 46%. A new version of the FilmArray RP assay (version 1.7) with improved sensitivity for the adenovirus was released in 2013. The performance characteristics and simplified workflow have allowed its implementation in a wide range of laboratories. The FilmArray RP has changed the diagnostic landscape and will have a significant impact on the care of patients with respiratory tract infection.
Keywords: automation, multiplex PCR, rapid diagnosis, respiratory tract infection
Community-acquired respiratory tract infections occur with varied frequency throughout the year. These infections are among the most common reasons for healthcare visits and can be caused by both viral and bacterial pathogens [1]. Symptoms caused by viruses are non-specific and include sore throat, runny nose, watery eyes, cough, wheezing, shortness of breath, sputum production and nasal congestion. Additionally, these symptoms are indistinguishable from those caused by bacterial pathogens [2]. Mixed infections with two or more respiratory pathogens are not uncommon and might be underreported due to the limited sensitivity of methods currently used for diagnosis [3–5]. The identity of the pathogen causing symptoms is critical for rapid institution of adequate antiviral or antibiotic therapy as well as proper isolation of infected patients to prevent health-care associated infection, which can have devastating effects in an immunocompromised population [6,7]. Empirical, broad-spectrum therapy is often administered due to limitations of conventional diagnostic methods to provide timely results to guide clinician’s decision. A great illustration of the importance of specific diagnosis came with the emergence of the novel H1N1 influenza virus in 2009. Unlike the seasonal H1N1 influenza virus, the novel virus was sensitive to the antiviral drug oseltamivir (Tamiflu®), but resistant to amantadine and rimantadine (Symadine® and Flumadine®). Since respiratory symptoms of these two influenza viruses are similar, accurate and timely identification of the correct virus was critical for the administration of appropriate therapy [8].
In normal hosts, most respiratory viral infections are self-limited, although complications can occurs. In the last two decades, respiratory viruses have become increasingly recognized as a serious cause of morbidity and mortality in hematopoietic stem cell transplant (HSCT) and solid organ transplant (SOT) recipients [9–13]. As part of their treatment, patients undergoing HSCT and SOT become severely immunosuppressed, rendering them highly susceptible to infectious pathogens. Serious complications, which can be fatal, include viral pneumonia, lateral airflow obstruction syndrome, graft rejection and super-infection with bacterial and fungal pathogens [9,12]. Similarly, respiratory viruses can have a significant impact in young children and elderly patients resulting in frequent hospitalization and significant health costs [14,15].
The most common viruses causing respiratory illness include influenza virus type A, influenza virus type B, respiratory syncytial virus (RSV), parainfluenza viruses (PIV) 1, 2 and 3, human metapneumovirus (hMPV) and picornaviruses (rhinoviruses and enteroviruses) [7]. In children undergoing treatment for hematologic malignancies, infections due to influenza, parainfluenza parainfluenza viruses, and RSV are particularly common and have significant impact on oncologic care [16,17]. The clinical significance of other viruses such as bocavirus, human coronaviruses NL63, human coronaviruses HKU1, parainfluenza virus 4 and rhinovirus is less clear [18]. Respiratory tract infections caused by bacterial pathogens includes Streptococcus pneumoniae, Haemophilus influenzae, as well as atypical bacterial that are often difficult to culture in the laboratory including Chlamydophila pneumoniae and Mycoplasma pneumoniae [19].
Respiratory tract pathogens
Infulenza
Influenza viruses are enveloped, negative sense, single-stranded RNA (ssRNA) viruses belonging to the Orthomyxoviridae family. There are three types of influenza viruses: influenza virus type A, influenza virus type B and influenza virus type C. Types A and B are the most commonly isolated while type C is rare and often not included in routine diagnostic test. Influenza viruses type A are further sub-typed based on the sequence of two glycoproteins: hemagglutinin (H) and neuraminidase (N) [20]. Influenza viruses cause annual, seasonal epidemic characterized by an increase in the frequency of upper respiratory symptoms seen in the community between December and May in the northern hemisphere [21]. Although the infection usually remains limited to the upper respiratory tract in most patients, complications due to influenza viruses have been reported in HSCT and SOT patients, especially lung transplant recipients [18,22,23]. In one study, the frequency of pneumonia caused by influenza viruses was 30% with a fatality rate of 15% in HSCT recipients [12]. However, reports on frequency of complications has varied (7–44%) with fatality ranging from 15 to 28% in HSCT recipients [18]. In SOT, the complication rate varies based on the organ transplanted with lung transplants recipients being particularly at increased risk [22,23].
RSV
RSV is an enveloped, non-segmented, ssRNA virus belonging to the Paramyxoviridae family. The seasonality of RSV follows closely that of influenza viruses with increased incidence in the winter months in the northern hemisphere [24]. In transplant patients and young children, infection with RSV can result in serious complications [25,26]. As such, in most centers, detection of RSV pre-transplant results in a delay of SCT until patient clears the infection. In one study of HSCT recipients, 80–90% of patients with upper respiratory RSV infection developed pneumonia with 30–40% exhibiting symptoms within 7 days following upper respiratory tract symptoms [26]. In a large study of children under 5 years of age, the incidence of RSV infection was 18%. RSV infections occurred in previously healthy children with no pre-existing conditions. Risk factors were identified only for those under 2 year of age [25].
PIV
PIV are non-segmented, negative sense RNA viruses, structurally related to RSV and belong to the Paraxmyxoviridae family. There are four predominant PIV serotypes including: PIV-1, PIV-2, PIV-3 and PIV-4 [27]. Until recently, PIV-4 was rarely isolated since most diagnostic assays available did not include reagents for the detection of PIV-4 and so less information is available on this PIV subtype. In the northern hemisphere, PIV occurs year-round with PIV-1 occurring most frequently in the fall, PIV-2 occurring in the fall to early winter and PIV-3 occuring in the spring and summer months [24,27]. Infections caused by PIV are usually mild, ranging from asymptomatic shedding to the common cold, croup and bronchiolitis. Similar to RSV, PIV infection occurs frequently in children, with most children exposed to PIV-3 by the age of two and PIV-1 by the age of 5 [24]. The reported incidence of PIV in HSTC populations ranges from 0.2 to 18% with 13 to 43% progressing to lower respiratory tract infections (LRTIs) and 12 to 50% of these LRTI resulting in death with most infections caused by PIV-3 [18,28]. In lung transplant recipients, PIV LRTI have been associated with high rate of allograft rejection [29].
hMPV
hMPV is another member of the Paraxmyxoviridae family and is structurally related to RSV and PIV. It is a relatively newly described virus and has become increasingly recognized as a cause of significant respiratory illness in young children, older adults and immunocompromised patients [30,31]. The incidence of hMPV in HSCT patients ranges from 3 to 7% with complications and fatality rate similar to those of RSV [18,32]. Similar to other Paramyxoviridae viruses, hMPV can result in graft dysfunction and/or rejection in lung transplant recipients [33,34].
Adenoviruses
Adenoviruses are non-enveloped, double stranded DNA viruses belonging to the Adenoviridae family. Adenoviruses are divided into seven types (A–G) and 52 serotypes, with type B/C most commonly associated with respiratory illnesses [35]. Adenoviruses can remain dormant in lymphoid cells and may reactivate following immunosuppression [36]. Adenoviruses are present year-round and common in pediatric populations where they generally cause self-limited, mild infections [37]. The incidence of adenovirus in HSCT and SOT patients ranges from 3 to 29% and 5 to 10%, respectively and varies with patient age, type of transplant, the degree of immunosuppression, and the organ being transplanted [13,18,38].
Rhinoviruses
Rhinoviruses are small, ssRNA viruses of the Picornaviridae family. Rhinoviruses are a diverse group of viruses with more than 100 serotypes identified to date [39]. Rhinoviruses are the most common cause of the ‘common cold’ and can be isolated throughout the year with a peak incidence in September [40]. In healthy individuals, rhinovirus infections are usually self-limited with symptoms lasting between 7–14 days [41]. Several reports have linked rhinoviruses with more severe presentations including otitis media, bronchiolitis and exacerbation of asthma in children [41]. In immunocompromised hosts, severe complications including pneumonia, can occur. In one recent study, the severity of rhinovirus infection in immunocompromised patients was similar to that of the 2009 H1N1 pandemic influenza A [42]. With the use of current diagnostic molecular methods, information of the frequency and severity of infections caused by rhinoviruses will become available.
Coronaviruses
Coronaviruses (CoV) are the largest RNA viruses and belong to the Coronaviridae family along with toroviruses [43]. CoV are divided into three groups based on sequence homology with group 1 including CoV-229E and CoV-NL63, group 2 including CoV-OC43 and CoV-HKU1 and group 3 which does not include any human CoVs. CoV are responsible for a wide range of upper respiratory tract infections (URTI) but are associated mainly with the ‘common cold’ [43]. Only a limited number of reports on CoV infections in immunocompromised hosts are available. In one study, asymptomatic shedding of CoV was detected in the first 100 days following allogeneic HSCT [44]. In other reports, LIRTs, with fatal outcomes have been reported in patients following SCT [45,46].
Bacterial pathogens
Bacterial pathogens included in the respiratory panel (RP) include Bordetella pertussis, Mycoplasma pneumoniae and Chlamydophila pneumoniae. All three organisms can cause both upper and lower respiratory tract infections including acute sinusitis, bronchitis and pneumonia [47]. B. pertussis causes pertussis or whooping cough primarily in non-vaccinated children and in adults, the infection is associated with a persistent cough of up to 2 weeks [48]. Atypical organisms, including M. pneumoniae and C. pneumoniae, account for about one third of cases of bacterial pneumonia [19,49]. Unlike other common bacteria associated with these symptoms, B. pertussis, M. pneumoniae and C. pneumoniae do not grow readily on media used for routine bacterial culture and therefore require a high level of suspicion from the clinician to inform the laboratory and request specific diagnostic tests. In one study, persistent cough of at least 2 weeks was observed in children infected with one or more of these three organisms underscoring the need for sensitive and specific diagnosis to guide therapy [50].
Diagnostic assays for respiratory pathogens
Conventional methods used to diagnose respiratory viral infections include rapid antigen tests, direct fluorescent antibody assays, shell vials and viral culture [51]. Each of these methods has advantages and disadvantages with sensitivities and specificities ranging from 44 to 99% and 74 to 100%, respectively requiring additional testing of negative specimens. In addition, these assays can be subjective, require expertise for interpretation of cytopathic effect, have a limited range of detection and a turn-around-time as long as 14 days [51].
Molecular assays, based on PCR, are rapid and sensitive compared to conventional methods. Until recently, individual laboratories with the appropriate expertise, developed and implemented their own real-time PCR assays for each of the most common respiratory viruses [31,52–54]. Several commercial assays, for both research-use only (RUO) and those cleared by the FDA (Table 1) are now widely available, facilitating the implementation of these technologies in diagnostic microbiology laboratory.
Table 1.
FDA cleared molecular diagnostic assays for respiratory pathogens†.
| Name | Technology | Tests | Targets | Run time(min) | Maximum run size | Random access? | Specimen types |
|---|---|---|---|---|---|---|---|
| Focus Diagnostics | Real-time PCR | Simplexa Flu A/B & RSV Direct |
FluA, FluB and RSV |
50 | 8 | No | NPS |
| IQuum | Real-time PCR | Liat Influenza A, B | FluA and FluB | 20 | 1 | Yes | NPS |
| Cepheid | Real-time PCR | Xpert Flu | FluA, FluA H1 2009 and FluB |
∼65 | 1 | Yes | NPS, NW, NA |
| BioFire Diagnostics, Inc. | Real-time PCR | Respiratory Panel |
FluA, FluA H1, H1 2009, H3, FluB, RSV, PIV 1-4, CoV OC43, HKU1, NL63 and 229E, hMPV, AdV, rhinovirus/enterovirus, B. pertussis, M. pneumoniae, C. chlamydophilae |
60 | 1 | Yes | NPS |
| Nanosphere Verigene, Inc. | RT-PCR, multiplex gold nanoparticle probes |
Respiratory virus plus test |
FluA, FluA H1, H1 2009, H3, FluB, RSV A, RSV B |
<2.5 h | 1 | Yes | NPS |
| Gen-Probe Prodesse, Inc. | Real-time PCR |
ProFlu+ ProFAST+ ProParaflu+ Pro hMPV+ ProAdeno+ |
FluA, FluB, RSV FluA H1, H3, 2009 H1 PIV 1–3 hMPV adenovirus |
4–5 h | Varies† | No | NPS |
| Focus Diagnostics | Real-time PCR |
Simplexa Flu A/B & RSV |
FluA, FluB, RSV | 4–5 h | Up to 96 | No | NPS |
| Quidel Corporation | Real-time PCR |
Influenza A + B hMPV and RSV |
FluA, FluB hMPV and RSV |
4–5 h | Varies‡ | No | NS, NPS |
| Luminex Molecular Diagnostics, Inc. | PCR, beads hybridization and flow cytometry |
xTAG RVP | FluA, FluA H1, H3, FluB, RSV A, RSV B, PIV 1–3, hMPV, AdV, rhinovirus |
6–8 h | Up to 96 | No | NPS |
| Luminex Molecular Diagnostics, Inc. | PCR, beads hybridization and flow cytometry |
xTAG RVP Fast | FluA, FluA H1, H3, FluB, RSV, hMPV, AdV, rhinovirus |
5–6 h | Up to 96 | No | NPS |
| GenMark Diagnostic | PCR, hybridization, electric current |
eSensor RVP | FluA, FluA H1, H3, FluB, RSV A, RSV B, PIV 1–3, hMPV, AdV C, AdV B/E, rhinovirus |
∼6 h | Varies‡ | No | NPS |
†As of 24 June 2013.
‡Depends on size of instrument available: Smartcycler/ABI Fast Dx (up to 96); GenMark Dx: 1 tower/8 tests, up to three towers/PC.
Adv: Adenovirus; Co: Coronavirus; Flu: Influenza; hMPV: Human metapneumovirus; NPS: Nasopharyngeal swabs; NS: Nasal swabs; NW: Nasal washes; RSV: Respiratory syncytial virus.
Until recently, most FDA-approved molecular assays targeted only the most commonly isolated viruses, including influenza A and B viruses and RSV, either as singleplex or duplex assay. Although this method has the benefit of targeted approach to diagnosis of respiratory infections, it is limited to only the most common viruses, requires clinicians to order multiple tests and does not always permit the detection of co-infecting pathogens. An alternative approach was the development of broadly multiplexed assay that allow simultaneous detection of a wider range of possible pathogens (5 or greater) in one single tube [4,55–59].
In 2008, the FDA cleared the first broadly multiplexed molecular assay, the xTAG® respiratory viral panel (RVP) (Luminex Molecular Diagnostics, Toronto, ON, Canada). The xTAG RVP detects 12 viruses and subtypes including: RSV A, RSV B, influenza virus A, influenza virus A H1 subtype, influenza virus A H3 subtype, influenza virus B, PIV 1, 2, 3, MPV, adenovirus and enterovirus/rhinovirus. The xTAG RVP includes five steps: nucleic acid extraction, multiplex RT-PCR, multiplex target-specific primer extension, hybridization; and detection of the amplified target on a Luminex 100/200 platform [60].
A second version of the assay, the xTAG® Respiratory Viral Panel Fast (RVP FAST), was approved in 2011 and detects RSV, influenza virus A (H1 subtype, H3 subtype and untypeable), influenza virus B, MPV, adenovirus and rhinovirus. The assay was simplified to reduce hands on time, workflow and turn-around-time from approximately 8 to 6 h. A study comparing the xTAG RVP to a pre-market, RUO version of the xTAG RVP FAST reported a reduced sensitivity of the xTAG RVP FAST (88.6 and 77.5%), especially for influenza B virus, parainfluenza virus Type 2 and HCoV 229E, with the last two targets not included in the FDA cleared version of the assay [61].
Another recently cleared multiplexed RVP is the eSensor RVP (GenMark Diagnostics Inc., Carlsbad, CA, USA). The eSensor RVP can detect influenza virus A, influenza virus A H1, influenza virus A H3, influenza virus A 2009 H1N1 influenza B, RSV subtype A, RSV subtype B, PIV 1, PIV 2, PIV 3, HMPV, human rhinovirus, adenovirus species B/E and adenovirus species C.
Two studies have evaluated the performance of the eSensor RVP compared to other multiplex respiratory panels including the FilmArray RP, the xTAG RVP and the xTAG RVP FAST and to laboratory developed tests [58,59]. The sensitivity and specificity of the eSensor RVP varied from 90 to 100% and 99 to 100% depending on the viral target.
The FilmArray RP
The FilmArray RP assay is the first and only FDA-cleared assay for the qualitative detection of nucleic acid targets from both viruses and bacteria in nasopharyngeal swab specimens. The FilmArray RP was initially FDA-cleared for the detection of 15 viruses including the same viruses as the xTAG RVP plus CoV NL63 and HKU1 and PIV-4. In 2012, five more targets were cleared including CoV 229E and OC43, and three bacterial targets B. pertussis, C. pneumoniae and M. pneumoniae (version 1.6). To increase the detection rate for adenovirus, a second assay for the virus was added to the RP and this was FDA cleared in 2013 (version 1.7). The assay is performed on the FilmArray instrument and can be completed in approximately 1 h.
The FilmArray instrument
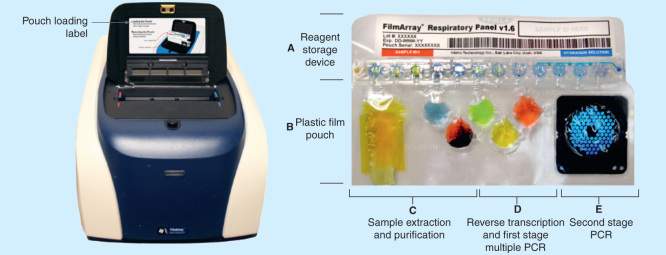
The FilmArray is an integrated platform that combines automated sample preparation, total nucleic acid extraction with nested, multiplex PCR and reverse transcriptase PCR and automated detection of amplified targets [62]. The instrument has a small footprint (39.1 × 25. 4 × 16.3 cm) and contains pneumatic and electrical components to move reagents through the pouch and perform the functions described. Nested PCR is effected using two Peltier devices, and melt curves are detected using a blue LED light source and a charge couple device (CCD) camera (Figure 1) [62,63].
Figure 1.
The FilmArray instrument and pouch.
Reproduced with permission from [63] © BioFire Diagnostics (2010).
Nucleic acids are released from cells by the combined action of denaturing buffers, ceramic beads and a bead beater. Once lysed, cells are transferred to a blister containing magnetic beads which binds the released nucleic acids. Following application of a magnet and several washes, the nucleic acids are eluted and moved to PCR sites for amplification.
Once purified, the DNA/RNA extract is mixed with the first stage multiplexed PCR reagents, including a set of outer primers, and temperature cycling occurs on the first Peltier device. A reverse transcription PCR step is performed prior to the first PCR cycle. Amplification of target nucleic acids is then accomplished using the principle of nested PCR which uses a two stages approach to increase the sensitivity and specificity of the PCR reaction [64].
The resulting mixture is diluted and moved to the second stage PCR where the amplified products from the first stage PCR are used as templates and further amplified using a second set of inner primers with cycling occurring on the second Peltier device. During the second stage PCR, LCGreen Plus, a fluorescent, DNA intercalating dye, is incorporated into the DNA as it is amplified.
Positive reactions are determined based on DNA melting curve analysis of amplified product. Each target is run in triplicate, in three separate wells of the microarray. The melt curve of each individual replicate for each target is measured following amplification and a positive reaction is determined if the melt curve shape and peak falls within pre-established ranges.
Control of the instrument and interpretation of the results are done automatically by the FilmArray software [62,63].
The pouch
All reagents necessary to perform the assay are contained in the vacuum sealed FilmArray pouch. The lyophilized reagents, which are distributed in 12 separate reservoirs, are rehydrated with a buffer solution just prior to adding the specimen. Additional reagents are contained in three of the pouch’s six blisters, including ceramic beads for lysis, magnetic beads for DNA/RNA purification and the oligonucleotides for the first stage PCR. The blisters are connected to channels which are used to move the liquid reaction from one blister to another. The last section of the pouch is the ‘solid-phase array’, a set of 102 wells with each well containing primers for the second stage PCR (Figure 1) [62,63].
The FilmArray RP
The FilmArray RP targets 20 viruses and bacteria including Influenza virus type A (including subtypes H1N1, H3N2 and the 2009-H1N1), influenza virus type B, RSV, adenovirus, hMPV, rhinoviruses/enteroviruses, CoV HKU1, NL63, 229E, OC43, PIV-1, PIV-2, PIV-3, PIV-4, B. pertussis, C. pneumoniae and M. pneumoniae. In addition, the assay contains two target controls, a RNA target from Schizosaccharomyces pombe, which controls the entire process from extraction to DNA melt analysis, and a DNA target control, which is included in the array well and controls for the second stage PCR. Each target has at least three replicate assays and the final interpretation is determined from the number of replicates that are positive. The FilmArray RP has multiple targets for some of the pathogens detected (e.g., influenza virus type A has two pan-influenza targets and one HA specific target).
Diagnostic performance characteristics
Published reports on the performance of the FilmArray RP are summarized in Table 2. The FilmArray RP has been evaluated against other FDA cleared molecular assays [59,65–67]. Most of the published reports evaluated the pre-market version of the FilmArray RP on samples from pediatric patients where the prevalence of respiratory viruses is expected to be higher. Overall, the FilmArray RP has a sensitivity and specificity >80%. However, the reported sensitivity of the first version of FilmArray RP for adenovirus is around 50%. As noted above, the manufacturer has released a new version of the FilmArray RP with improved adenovirus detection.
Table 2.
Clinical sensitivity and specificity of the biofire filmarray respiratory panel.
| Study (year) | FA RP version | n | Patient population | Comparator assay | Discordant analysis | Sensitivity (%) | Specificity (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Rand et al. (2011) | Premarket RUO | 200 | Adult and pediatrics | xTAG RVP | LDT | 90–100 | 100 | [67] |
| Babady et al. (2012) | Premarket RUO | 358 | Pediatrics | xTAG RVP Fast | ResPlex II v2.0 | 91.6–100 | 98.2–100 | [65] |
| Popowitch et al. (2013) | FDA cleared version 1 | 300 | Adult and pediatrics | xTAG RVP xTAG RVP Fast eSensor GenMark |
LDT | 57.1–100 | 100 | [59] |
| Loeffelholz et al.(2011) | Premarket RUO | 192 | Pediatrics | Prodesse assays | LDT | 54.5–100 | 98.4-100 | [66] |
| Hayden et al. (2012) | Premarket RUO | 176 | Pediatrics | ResPlex II v2.0 | ND‡ | ND‡ | ND‡ | [57] |
| Pierce et al. (2012) | Premarket RUO | 215 | Pediatrics | LDT | Repeat testing | 46–100 | 50–100 | [68] |
| Hammond et al. (2012) | Premarket RUO | 90 | Adults | Conventional methods | BioFire in-house singleplex assay | 100 | 94 | [70] |
†LDT: Laboratory-Developed tests.‡ND: Not done or not determined (discordant results were not further analyzed).FA RP: FilmArray respiratory panel; RUO: Research use only.
Rand et al. compared the performance of the FilmArray RP and xTAG RVP on 200 specimens from both adult and pediatric patients, previously tested by viral culture and antigen testing [67]. The complete agreement between the two tests was excellent at 91.5% with more viruses detected by the FilmArray RP (160 viruses by FilmArray RP versus 149 viruses by xTAG RVP). Notably, the FilmArray RP had greater sensitivity for RSV than the xTAG RVP (100 vs 82.2%). Loeffelholz and colleagues compared the FilmArray RP to the series of FDA cleared Prodesse (GenProbe) Real-time PCR assays including the ProFlu+, ProFAST+, ProParaflu+, ProhMPV+ and ProAdeno+ [66]. The FilmArray RP and Prodesse assays showed good overall agreement (94.3%) when tested on 192 specimens from pediatric patients, with the FilmArray RP being more sensitive for PIV-1 and -3, detecting an additional two PIV-1 and three PIV-3 not detected by the ProParaflu + assay. The ProAdeno+ was more sensitive for adenoviruses than the FilmArray RP, detecting a total of 11 adenoviruses compared to five adenovirus detected by the FilmArray RP. Additionally, the authors compared the FilmArray RP to LDT for targets not included in the Prodesse assays with the sensitivity ranging from 50 to 100% although the number of positive specimens tested was small (n = 1 to 8), except for rhinovirus/enterovirus (n = 118) targets. We compared the performance of the FilmArray RP to the pre-market version of the xTAG RVP FAST on 358 respiratory specimens from pediatric patients [65]. In our report, the FilmArray RP was overall more sensitive than the xTAG RVP FAST for most viruses common to both assays, especially for RSV (100 vs 60%) and FluB (100 vs 50%). However, the xTAG RVP FAST was more sensitive than the FilmArray RP for PIV-4 (100 vs 33.3%) and rhinoviruses (97.6 vs 91.6%).
In a recent study, the FilmArray RP was evaluated along other multiplexed FDA-cleared molecular assays including the xTAG RVP, the xTAG RVP FAST and the GenMark eSensor [59]. The FilmArray RP had an overall sensitivity of 84.5% with sensitivity varying from 92–100% for influenza virus A/H3, MPV, PIV-1, PIV-2, PIV-3 and RSV B and sensitivity varying from 73–83% for influenza virus A H1/2009, influenza virus, RSV A and rhinoviruses/enteroviruses. Of note, the sensitivity of the FilmArray RP and the xTAG RVP for RSV was similar [59]. The sensitivity of the FilmArray RP for adenoviruses was especially low at 57% as reported in other studies [66,68].
Other studies have evaluated the FilmArray RP against conventional methods, laboratory-developed assays and RUO assays [57,68–70]. In one study done using mock specimens prepared by combining previously positive nasal washes, nasal swabs, bronchoalveolar lavage fluids, sputum and tracheal aspirates, the FilmArray RP detected 90% of viruses identified by the LDT assays with discordant results mainly occurring at low viral loads [69]. The FilmArray RP was also evaluated against the Resplex II Panel v2.0 (Qiagen) and the two tests had an overall agreement of 83.8% with the FilmArray RP detecting about 10% more positive specimens and 21% more viruses than the Resplex assays [57]. Pierce et al. reported an overall agreement between the FilmArray RP and their LDT real-time PCRs of 98.6% with the FilmArray RP adenovirus assay being less sensitive than the LDT adenovirus real-time PCR, missing 13/24 adenovirus positive specimens. The FilmArray RP was unable to specifically detect adenovirus serotypes 6 and 41 at any viral concentrations and serotypes 2, 20, 35 and 37 at low viral concentrations [68].
According to the manufacturer’s product insert, the low sensitivity of the FilmArray RP for adenovirus specifically relates to adenovirus C, serotypes 2 and 6 as confirmed in Pierce et al. study, and recommended testing of negative specimens using alternative methods to detect adenoviruses when suspected (FilmArray RP PI v1.6). In April 2013, a new version of the FilmArray RP assay (version 1.7) was released. The FilmArray RP v1.7 was designed to enhance the detection of adenoviruses by adding a second adenovirus assay to the panel. No peer-reviewed published reports are yet available on the performance of the improved assay for adenovirus. Abstracts presented at the 29th clinical virology symposium showed increased sensitivity of the FilmArray RP v1.7 when compared to FilmArray RP v1.6 (81.6 vs 23.8%) but still missed adenoviruses detected by the xTAG RVP or Prodesse ProAdeno+ Assay and LDT adenovirus singleplex [71,72].
Conclusions
Respiratory tract infections can be caused by a wide range of pathogens including viruses and bacteria. In addition, novel viruses, including bocavirus and novel CoVs, are increasingly being recognized as causes of respiratory tract illness. Given the non-specific nature of the respiratory symptoms, the ability to detect these novel viruses as well as other significant pathogens is paramount for optimal patient care. The FilmArray RP is the most extensive panel that is currently FDA cleared for rapid detection of respiratory tract pathogens. Although its adenovirus assays still require improvement, the overall clinical sensitivity and specificity of the FilmArray RP assay is better than conventional methods and comparable to other high complexity multiplexed molecular assays on the market.
Expert commentary
Multiplex molecular diagnostic assays are ideal for infections with a wide differential diagnosis. However, the design of broadly multiplexed assays is challenging as multiplex PCR assays are subject to decreased sensitivity due in part to, targets’ competition and primer dimers formation [64]. The overall sensitivity and specificity of the FilmArray RP in published reports varied between 85–100% and 100%, respectively with the sensitivity of the adenovirus assays remaining a challenge even in the new version of the assay. However, even with the lower sensitivity of the adenovirus, the FilmArray RP has drastically changed the diagnostic landscape and allowed implementation of a sensitive and rapid molecular assay in a wide range of diagnostic settings [73,74].
The FilmArray RP was the second highly multiplexed molecular assay to be cleared by the FDA and the first one to include bacterial pathogens in the panel. It is important to note however that as extensive as the FilmArray RP panel is, other important bacterial causes of pneumonia including S. pneumoniae and H. influenzae, are not included on the panel. When compared to other FDA cleared multiplexed assay (Table 1), the FilmArray RP has the greatest number of targets in its panel, the simplest workflow, the shortest turn-around-time and the highest reagent cost [59,65]. The true value of the FilmArray RP assay lies in its ability to provide actionable results to clinicians and to facilitate the flow of patients in the hospital by providing infection control staff with real-time information.
The only specimen type that is currently cleared by the FDA for testing is naso-pharyngeal swabs. However, other respiratory tract specimens are routinely submitted to the laboratory for testing including nasal swabs, nasal washes, throat swabs, bronchoalveolar lavage fluids and sputum. All studies included in this article evaluated the off-label use of FilmArray RP on other specimen types with sensitivity and specificity similar to that of NP swabs suggesting that RP would be useful for diagnosing both upper and lower respiratory tract infections.
Five-year view
Five years ago, the FDA cleared the xTAG RVP as the first broadly multiplexed molecular assay for the detection of respiratory viruses in nasopharyngeal swabs. Today there are four FDA-cleared broadly multiplexed assays, the xTAG RVP and the xTAG RVP FAST (Luminex Corp.), the eSensor RVP (GenMark inc.) and the FilmArray respiratory panel (RP) (BioFire Diagnostics). The FilmArray RP tests for a wide range of pathogens with high sensitivity and specificity and in a timely manner. As such, the FilmArray RP has raised the expectations of both the clinical laboratories and clinicians as to what is possible for the rapid and sensitive diagnosis of infectious diseases in general. The next 5 years should see the continued increase in the options for rapid, sensitive and simple to perform molecular assays for infectious disease diagnosis.
One of the limitations of the FilmArray RP is still its cost compared to traditional methods and other commercially available molecular diagnostic tools, although when considering workflow and labor costs, the overall cost of the FilmArray RP is comparable to other highly multiplexed assays (i.e., xTAG RVP and the eSensor RVP) [59]. In this era of health care reform, clinical laboratories and hospitals are constantly faced with the challenge of delivering the best patient care possible in the most cost-effective way. For the FilmArray RP to fully realize its potential, the cost of the assay will have to decrease so to be available to a wider range of patients.
The FilmArray RP is currently classified as a moderate complexity test and as such is subject to the requirements associated with performing moderate/high complexity tests including quality control and assessment and performance of the test in accredited laboratory settings [75]. It is possible that the FilmArray RP might eventually obtain a waived complexity category, which will allow its use in point of care settings like an emergency room or doctor’s office.
As antiviral drugs become available to treat viruses other than influenza and RSV, the ability of any molecular platform to rapidly diagnose the specific virus responsible for a respiratory syndrome will become even more important. Furthermore, quantitative or semi-quantitative assays that provide information on viral loads will be useful for monitoring of patient response to treatment or to gain a better understanding of the infectious doses for each of the viruses included in multiplexed panels. The next five years will see an emergence of outcome studies on the real impact and benefit of these assays on patient care and public health in general.
Finally, the impact of new and emerging infections cannot be denied. In the last year, the avian H7N9 influenza A virus and the Middle Eastern Syndrome Coronaviruses have reminded us of how quickly new and emerging viruses can appear and spread. The challenge for manufacturers of these molecular assays will be to design the assays to be sensitive and specific and yet broad enough to accommodate potential emerging pathogens.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Key issues
• Respiratory tract infections occur all year long with different pathogens peaking at different times but overlapping in their seasonal distributions.
• Clinical symptoms caused by respiratory pathogens, including bacteria and viruses are non-specific.
• There are currently four highly multiplexed respiratory panels that are US FDA approved for diagnosis of respiratory infections.
• The FilmArray Respiratory Panel is sensitive and specific, has the simplest workflow and fastest turn-around-time of all assays.
• The FilmArray Respiratory Panel is the only panel that includes both viral and bacterial causes of upper respiratory tract infections/lower respiratory tract infections.
References
- 1.Woodwell DA, Cherry DK. National ambulatory medical care survey: 2002 summary. Adv. Data. (346), 1–44 (2004). [PubMed]
- 2.Juven T, Mertsola J, Waris M et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr. Infect. Dis. J. 19(4), 293–298 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Koskenvuo M, Mottonen M, Rahiala J et al. Respiratory viral infections in children with leukemia. Pediatr. Infect. Dis. J. 27(11), 974–980 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Ginocchio CC, Zhang F, Manji R et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J. Clin. Virol. 45(3), 191–195 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JW, Kang HJ, Park JD, Shin HY, Ahn HS. Early pulmonary complications after hematopoietic stem cell transplantation in pediatric patients: association with cytomegalovirus infection. J. Pediatr. Hematol. Oncol. 31(8), 545–551 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 10(6), 589–597 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Mahony JB. Detection of respiratory viruses by molecular methods. Clin. Microbiol. Rev. 21(4), 716–747 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan SJ, Jacobson RM, Dowdle WR, Poland GA. 2009 H1N1 influenza. Mayo Clin. Proc. 85(1), 64–76 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br. J. Haematol. 143(4), 455–467 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am. J. Med. 102(3A), 27–30; discussion 42–23 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Champlin RE, Whimbey E. Community respiratory virus infections in bone marrow transplant recipients: the M.D. Anderson Cancer Center experience. Biol. Blood Marrow Transplant. 7(Suppl.), 8S–10S (2001). [DOI] [PubMed] [Google Scholar]
- 12.Chemaly RF, Ghosh S, Bodey GP et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 85(5), 278–287 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Ison MG, Hayden FG. Viral infections in immunocompromised patients: what's new with respiratory viruses? Curr. Opin. Infect. Dis. 15(4), 355–367 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Yorita KL, Holman RC, Sejvar JJ, Steiner CA, Schonberger LB. Infectious disease hospitalizations among infants in the United States. Pediatrics 121(2), 244–252 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Lieberman D. Management of respiratory infections in the elderly. Expert Rev. Anti Infect. Ther. 1(3), 505–516 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan A, Wang C, Yang J et al. Parainfluenza virus infections in children with hematologic malignancies. Pediatr. Infect. Dis. J. 30(10), 855–899 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan A, Wang C, Yang J, Shenep JL, Leung WH, Hayden RT. Symptomatic parainfluenza virus infections in children undergoing hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 17(10), 1520–1527 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renaud C, Campbell AP. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Curr. Opin. Infect. Dis. 24(4), 333–343 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iroh Tam PY. Approach to common bacterial infections: community-acquired pneumonia. Pediatr. Clin. North Am. 60(2), 437–453 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A revision of the systerm of nomenclature for influenza viruses: a WHO memorandum. Bull World Health Organ. 58(4), 585–591 (1980). [PMC free article] [PubMed] [Google Scholar]
- 21.Atmar RLaL S.E. Influenza Viruses. : Manual of Clinical Microbiology. Versalovic J. (). ASM Press, Washington DC, USA: (2011). [Google Scholar]
- 22.Palmer SM, Jr, Henshaw NG, Howell DN, Miller SE, Davis RD, Tapson VF. Community respiratory viral infection in adult lung transplant recipients. Chest 113(4), 944–950 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Vilchez RA, McCurry K, Dauber J et al. Influenza virus infection in adult solid organ transplant recipients. Am. J. Transplant. 2(3), 287–291 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Hall CB. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344(25), 1917–1928 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Hall CB, Weinberg GA, Iwane MK et al. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 360(6), 588–598 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols WG, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol. Blood Marrow Transplant. 7(Suppl) 11S–15S (2001). [DOI] [PubMed] [Google Scholar]
- 27.Henrickson KJ. Parainfluenza viruses. Clin. Microbiol. Rev. 16(2), 242–264 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chemaly RF, Hanmod SS, Rathod DB et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood 119(12), 2738–2745 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Vilchez RA, McCurry K, Dauber J et al. The epidemiology of parainfluenza virus infection in lung transplant recipients. Clin. Infect. Dis. 33(12), 2004–2008 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J. Infect. Dis. 187(5), 785–790 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Kamboj M, Gerbin M, Huang CK et al. Clinical characterization of human metapneumovirus infection among patients with cancer. J. Infect. 57(6), 464–471 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Kumar D, Humar A. Respiratory viral infections in transplant and oncology patients. Infect. Dis. Clin. North Am. 24(2), 395–412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larcher C, Geltner C, Fischer H, Nachbaur D, Muller LC, Huemer HP. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J. Heart Lung Transplant. 24(11), 1891–1901 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Weinberg A, Lyu DM, Li S, Marquesen J, Zamora MR. Incidence and morbidity of human metapneumovirus and other community-acquired respiratory viruses in lung transplant recipients. Transpl. Infect. Dis. 12(4), 330–335 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ison MG. Adenovirus infections in transplant recipients. Clin. Infect. Dis. 43(3), 331–339 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Bruno B, Gooley T, Hackman RC, Davis C, Corey L, Boeckh M. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol. Blood Marrow Transplant. 9(5), 341–352 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Ljungman P. Treatment of adenovirus infections in the immunocompromised host. Eur. J. Clin. Microbiol Infect. Dis. 23(8), 583–588 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Echavarria M. Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 21(4), 704–715 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmenberg AC, Spiro D, Kuzmickas R et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science 324(5923), 55–59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brownlee JW, Turner RB. New developments in the epidemiology and clinical spectrum of rhinovirus infections. Curr. Opin. Pediatr. 20(1), 67–71 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Jacobs SE, Lamson DM, St George K, Walsh TJ. Human rhinoviruses. Clin. Microbiol. Rev. 26(1), 135–162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraft CS, Jacob JT, Sears MH, Burd EM, Caliendo AM, Lyon GM. Severity of human rhinovirus infection in immunocompromised adults is similar to that of 2009 H1N1 influenza. J. Clin. Microbiol. 50(3), 1061–1063 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pabbaraju KaF J.D. Coronaviruses. : Manual of Clinical Microbiology. Versalovic J. (). ASM Press, Washington DC, USA: (2011). [Google Scholar]
- 44.Milano F, Campbell AP, Guthrie KA et al. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood 115(10), 2088–2094 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oosterhof L, Christensen CB, Sengelov H. Fatal lower respiratory tract disease with human corona virus NL63 in an adult haematopoietic cell transplant recipient. Bone Marrow Transplant. 45(6), 1115–1116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pene F, Merlat A, Vabret A et al. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin. Infect Dis. 37(7), 929–932 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunha BA. The atypical pneumonias: clinical diagnosis and importance. Clin. Microbiol. Infect. 12(Suppl. 12), 12–24 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Konig CH, Halperin S, Riffelmann M, Guiso N. Pertussis of adults and infants. Lancet Infect. Dis. 2(12), 744–750 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Baer G, Engelcke G, Abele-Horn M, Schaad UB, Heininger U. Role of Chlamydia pneumoniae and Mycoplasma pneumoniae as causative agents of community-acquired pneumonia in hospitalised children and adolescents. Eur. J. Clin. Microbiol. Infect. Dis. 22(12), 742–745 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Hallander HO, Gnarpe J, Gnarpe H, Olin P. Bordetella pertussis, Bordetella parapertussis, Mycoplasma pneumoniae, Chlamydia pneumoniae and persistent cough in children. Scand. J. Infect. Dis. 31(3), 281–286 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Ginocchio CC. Detection of respiratory viruses using non-molecular based methods. J. Clin. Virol. 40(Suppl. 1), S11–S14 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuypers J, Campbell AP, Cent A, Corey L, Boeckh M. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl. Infect. Dis. 11(4), 298–303 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Redelman-Sidi G, Sepkowitz KA, Huang CK et al. 2009 H1N1 influenza infection in cancer patients and hematopoietic stem cell transplant recipients. J. Infect. 60(4), 257–263 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Sails AD, Saunders D, Airs S, Roberts D, Eltringham G, Magee JG. Evaluation of the Cepheid respiratory syncytial virus and influenza virus A/B real-time PCR analyte specific reagent. J. Virol. Methods. 162(1–2), 88–90 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beck ET, Jurgens LA, Kehl SC et al. Development of a rapid automated influenza A, influenza B, and respiratory syncytial virus A/B multiplex real-time RT-PCR assay and its use during the 2009 H1N1 swine-origin influenza virus epidemic in Milwaukee, Wisconsin. J. Mol. Diagn. 12(1), 74–81 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murali S, Langston AA, Nolte FS, Banks G, Martin R, Caliendo AM. Detection of respiratory viruses with a multiplex polymerase chain reaction assay (MultiCode-PLx Respiratory Virus Panel) in patients with hematologic malignancies. Leuk. Lymphoma. 50(4), 619–624 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Hayden RT, Gu Z, Rodriguez A et al. Comparison of two broadly multiplexed PCR systems for viral detection in clinical respiratory tract specimens from immunocompromised children. J. Clin. Virol. 53(4), 308–313 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pierce VM, Hodinka RL. Comparison of the GenMark Diagnostics eSensor respiratory viral panel to real-time PCR for detection of respiratory viruses in children. J. Clin. Microbiol. 50(11), 3458–3465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Popowitch EB, O'Neill SS, Miller MB. Comparison of the Biofire Film Array RP, Genmark eSensor RVP, Luminex xTAG RVPv1, and Luminex xTAG RVP fast multiplex assays for detection of respiratory viruses. J. Clin. Microbiol. 51(5), 1528–1533 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merante F, Yaghoubian S, Janeczko R. Principles of the xTAG respiratory viral panel assay (RVP Assay). J. Clin. Virol. 40(Suppl. 40), S31–S35 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pabbaraju K, Wong S, Tokaryk KL, Fonseca K, Drews SJ. Comparison of the Luminex xTAG respiratory viral panel with xTAG respiratory viral panel fast for diagnosis of respiratory virus infections. J. Clin. Microbiol. 49(5), 1738–1744 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poritz MA, Blaschke AJ, Byington CL et al. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PLoS One 6(10), e26047 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.BioFire Diagnostics Inc. FilmArray® Operator’s Manual RUO. BioFire Diagnostics Inc., Salt Lake City UT, USA (2010)
- 64.Datta V, Hayden RT. In vitro nucleic acid amplification techniques. : Molecular Microbiology, Diagnostic Principles and Practice. Persing D. (). ASM Press, Washington DC, USA: 33–61 (2011). [Google Scholar]
- 65.Babady NE, Mead P, Stiles J et al. Comparison of the Luminex xTAG RVP Fast assay and the Idaho Technology FilmArray RP assay for detection of respiratory viruses in pediatric patients at a cancer hospital. J. Clin. Microbiol. 50(7), 2282–2288 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loeffelholz MJ, Pong DL, Pyles RB et al. Comparison of the FilmArray Respiratory Panel and Prodesse real-time PCR assays for detection of respiratory pathogens. J. Clin. Microbiol. 49(12), 4083–4088 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rand KH, Rampersaud H, Houck HJ. Comparison of two multiplex methods for detection of respiratory viruses: FilmArray RP and xTAG RVP. J. Clin. Microbiol. 49(7), 2449–2453 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pierce VM, Elkan M, Leet M, McGowan KL, Hodinka RL. Comparison of the Idaho Technology FilmArray system to real-time PCR for detection of respiratory pathogens in children. J. Clin. Microbiol. 50(2), 364–371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Renaud C, Crowley J, Jerome KR, Kuypers J. Comparison of FilmArray Respiratory Panel and laboratory-developed real-time reverse transcription-polymerase chain reaction assays for respiratory virus detection. Diagn. Microbiol. Infect. Dis. 74(4), 379–383 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hammond SP, Gagne LS, Stock SR et al. Respiratory virus detection in immunocompromised patients with FilmArray respiratory panel compared to conventional methods. J. Clin. Microbiol. 50(10), 3216–3221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bush JL, Zhang F, Elsayed H et al. Evaluation of the Biofire FilmArray Respiratory panel v1.6 and v1.7 for the detection of respiratory viruses. Presented at: 29th Clinical Virology Symposium. Daytonna Beach, FL, USA: 2013. [Google Scholar]
- 72.Mack K, Salamon D, Madison J et al. Evaluation of a modified version of the FilmArray Respiratory panel (RUO v1.7) for detection of adenovirus from nasopharyngeal specimens. Presented at: 29th Clinical Virology Symposium. Daytonna Beach, FL, USA: 2013. [Google Scholar]
- 73.Xu M, Qin X, Astion ML et al. Implementation of filmarray respiratory viral panel in a core laboratory improves testing turnaround time and patient care. Am. J. Clin. Pathol. 139(1), 118–123 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Couturier MR, Barney T, Alger G et al. Evaluation of the FilmArray(R) Respiratory Panel for clinical use in a large children's hospital. J. Clin. Lab Anal. 27(2), 148–154 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.US National Archives and Records Administration. Code of Federal Regulations. Title 42, CFR 493.17. Test Categorization. 1993. Washington, DC, USA. [Google Scholar]